Abstract
Introduction
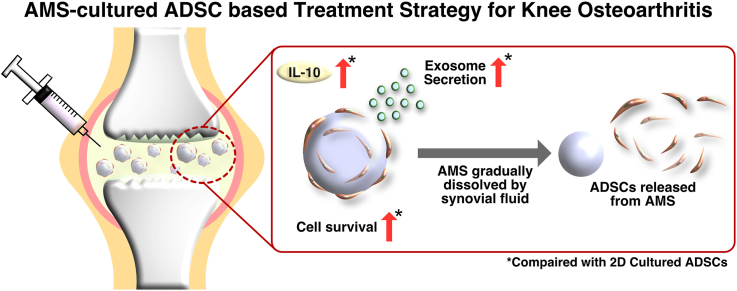
Administration of adipose-derived stem cells (ADSCs) into the joint cavity has been shown to alleviate the symptoms of knee osteoarthritis (OA) by releasing exosomes and anti-inflammatory cytokines. However, the therapeutic effect of these cells is limited by their rapid disappearance after administration. Thus, it is necessary to prolong cell survival in the joint cavity. This study aimed to investigate the potential application of ADSCs adhered to atelocollagen microspheres (AMSs) for cell therapy of knee OA.
Methods
ADSCs were cultured for 2, 4, and 7 days in AMS suspension or adherent culture dishes. The supernatants were analyzed for IL-10 and exosome secretion via enzyme-linked immunosorbent assay and Nanosight. The effect of AMS was compared with that of adherent-cultured ADSCs (2D-cultured ADSCs) using transcriptome analysis. Moreover, the solubility of AMS and viability of ADSCs were evaluated using synovial fluid (SF) from patients with knee OA.
Results
Compared with 2D-cultured ADSCs, AMS-cultured ADSCs exhibited a significant increase in secretion of exosomes and IL-10, and the expression of several genes involved in extracellular matrix and immune regulation were altered. Furthermore, when AMS-cultured ADSCs were cultured in SF from knee OA patients to mimic the intra-articular environment, the SF dissolved the AMSs and released viable ADSCs. In addition, AMS-cultured ADSCs showed significantly higher long-term cell viability than 2D-cultured ADSCs.
Conclusion
Increased survival of AMS-adhered ADSCs was observed in the intra-articular environment, and AMSs were found to gradually dissipate. These results suggest that AMS-adhered ADSCs are promising source for cell therapy of knee OA.
Keywords: Adipose-derived stem cell, Atelocollagen microsphere, Knee osteoarthritis, Exosome, IL-10, RNA-seq
Graphical abstract
1. Introduction
Minor knee osteoarthritis (OA) is mainly treated via a nonsurgical (conservative) approach, which emphasizes on lifestyle changes, such as weight loss and structured exercise, as well as the use of nonsteroidal anti-inflammatory drugs and acetaminophen for pain management. However, the use of nonsteroidal anti-inflammatory drugs for pain management in knee OA may be associated with an increased risk of cardiovascular events. In cases where the disease has advanced and symptoms remain unalleviated, surgical treatment approaches, such as total knee arthroplasty and osteotomy, might be pursued [1]. However, they are more invasive than conservative management. Therefore, in recent years, there has been growing interest and advancement in nonsurgical treatments, such as stem cell therapy. The intra-articular administration of stem cells has several advantages, such as pain relief, inhibition of disease progression, and lower invasiveness than surgical treatment.
Adipose-derived stem cells (ADSCs) are one of the prominent cell therapy options for knee OA. Numerous clinical trials have been conducted for knee OA [2], and factors such as exosomes, anti-inflammatory cytokines, and growth factors have been reported to contribute to the therapeutic efficacy of ADSCs [3,4]. In general, ADSCs are primarily isolated from adipose tissue using enzymatic methods, expanded under culture conditions, suspended in saline, and administered via intra-articular injection [5,6]. Various studies have suggested that the efficacy of administered ADSCs may be limited due to their reduced survival as a result of environmental stress at the site of administration [[7], [8], [9], [10]]. In addition, Toupet et al. reported that more than 90% of the cells disappeared within 10 days when ADSCs were administered into the knee joints of rats with knee OA [11].
To enhance the therapeutic efficacy of ADSCs against knee OA, it is essential to maintain the viability of ADSCs within the joint cavity. Various methods have been developed to prolong the survival of stem cells at the site of administration, including the use of spheroid culture techniques [12,13] and improvement of the culture medium [14,15]. Among these methods, microspheres have recently emerged as a highly promising culture tool [16,17]. Microspheres are primarily composed of collagen and offer distinct advantages, such as a large surface area, excellent cell adhesion and proliferation properties, and biodegradability within the body [18]. The use of microspheres enables short-term, high-density, and large-scale culture of various cells, including bone marrow-derived stem cells, ADSCs, umbilical cord-derived stem cells, and chondrocytes [19]. In addition, depending on the material of microspheres, direct administration of microsphere-adhered cells to disease sites is feasible. The high-density cell culture environment created by microspheres has been reported to enhance intercellular interactions and influence cell proliferation and differentiation [20,21]. In the present study, we focused on atelocollagen, one of the microsphere materials.
Unlike conventional collagen, atelocollagen lacks telopeptides at the N- and C-termini, resulting in reduced antigenicity and suppressed immune activation [22,23]. In a previous clinical trial, intra-articular administration of atelocollagen was reported to improve knee pain in patients with knee OA [24]. Therefore, combining atelocollagen with ADSC therapy has the potential to enhance therapeutic efficacy. By adhering ADSCs to atelocollagen-based therapeutic microspheres, they can be retained in the joint cavity for long time. The low antigenicity of atelocollagen may escape from the activation of immune cells within the joint cavity. In addition, the release of exosomes and anti-inflammatory cytokines by ADSCs is expected to enhance the resolution of knee OA.
This study evaluated the characteristics of ADSCs cultured on atelocollagen microspheres (AMSs) in vitro. Furthermore, the effect of SF from patients with knee OA on ADSCs was assessed to understand the influence of the joint cavity environment on these cells. Overall, this study aimed to evaluate the potential application of ADSCs adhered to AMS for cell therapy of knee OA.
2. Method
2.1. Microsphere preparation
The AMSs used in this study were manufactured from collagen derived from bovine skin and had a diameter of approximately 100–400 μm (KOKEN Co., Ltd., Tokyo, Japan). AMS solution was centrifuged to remove the supernatant and washed with phosphate-buffered saline (PBS). Finally, the microspheres were suspended in a serum-free medium, ADSC-4 (Kohjin Bio Co., Ltd., Sakado, Japan), and incubated overnight at 4 °C before being used in the experiments.
2.2. ADSC loading onto AMS for stirred culture
Human-derived ADSCs (Lonza K.K., Basel, Switzerland) [25] were cultured in KBM ADSC-4 medium (Kohjin Bio) and incubated at 37 °C in 5% CO2. ADSCs were allowed to adhere to AMS in ultralow attachment 6-well plate (Corning Inc., Corning, NY, USA) according to the manufacturer's instructions (KOKEN Co., Ltd.). Most of the living ADSCs were found to adhere to AMS (Fig. S1a and b). Subsequently, the 6-well plate was placed on a continuous-action shaker (Wave-PR, Taitec Corp., Saitama, Japan) set at a speed of 15 rpm and a shaking angle of 6°. The cultures were maintained for 1 week, with 1 mL of ADSC-4 medium being added every 3 days.
2.3. Cell viability assay using trypan blue staining
ADSCs cultured on adherent culture dishes (BM Equipment Co., Ltd., Tokyo, Japan) and on AMSs for 2, 4, and 7 days (AMS culture) were washed with PBS. The ADSCs were then detached using 0.25% trypsin–ethylenediaminetetraacetic acid (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan); detachment was confirmed under the microscope (Fig. S2). Cell viability was then assessed using trypan blue staining.
2.4. ADSC-derived exosome preparation and analysis with Nanosight
The supernatants of the 2D and AMS cultures of ADSCs were collected, and exosomes were isolated using the MagCapture Exosome Isolation Kit (FUJIFILM Wako Pure Chemical) according to the manufacturer's instructions. The size distribution of ADSC-derived exosomes was determined using the NanoSight NS 300 instrument (Malvern Panalytical Ltd., Malvern, UK) according to the manufacturer's instructions. For analysis, each sample was diluted with PBS in a 10-fold dilution and then analyzed using NTA Version 3.0, with a camera level of 15, a detection threshold of 1, an automatic minimum expected particle size, and an automatic jump distance. A 60-s recording was performed five times, and the acquired data were used for quantitative measurements.
2.5. Exosome characterization
Exosomes in the supernatants of the 2D and AMS cultures of ADSCs were isolated using the PS Capture TM Exosome Flow Cytometry Kit (FUJIFILM Wako Pure Chemical) following the kit's protocol. Surface marker expression analysis of ADSC-derived exosomes was performed for each sample of ADSC-derived exosomes based on a previous study [26]. Briefly, the following mouse IgG anti-human monoclonal antibodies (mAbs) were added: FITC-conjugated anti-CD9 mAbs (BioLegend, Inc., San Diego, CA, USA), PE-conjugated anti-CD63 mAbs (BioLegend, Inc) and PE-conjugated anti-CD81 mAbs (BioLegend, Inc). All analyses were performed with a flow cytometer (FACS Canto II, BD Biosciences, San Jose, CA, USA). The acquired data were analyzed using Flowjo software (BD Biosciences).
2.6. Enzyme immunoassay for interleukin 10 (IL-10) in the culture supernatant
IL-10 concentrations in the culture supernatant were measured using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The assays were performed in duplicate.
2.7. RNA-seq analysis
Total RNA was extracted from the samples using the RNeasy Mini Kit (Qiagen NV, Venlo, Netherlands). The integrity of the RNA was assessed using Agilent Technologies’ 2100 Bioanalyzer and RNA 6000 Nano Kit, which confirmed that all samples had an RNA integrity number of >9. The quality of the RNA was further assessed using the 5200 Fragment Analyzer System and the Agilent HS RNA Kit (Agilent Technologies). Subsequently, the DNA libraries were assembled using the MGIEasy RNA Directional Library Prep Set (MGI Tech). The quality of these libraries was assessed using the 5200 Fragment Analyzer System and the dsDNA 915 Reagent Kit (Agilent Technologies). The libraries were then circularized and converted into DNA nanoballs using the MGIEasy Circularization Kit and DNBSEQ-G400RS High-throughput Sequencing Kit (MGI Tech). Finally, sequencing was performed on a DNBSEQ-G400 instrument (MGI Tech) with a read length of 2 × 100 bp.
2.8. Bioinformatics
The quality of the sequencing data was assessed using FastQC (Version 0.12.1). To ensure data integrity, adapter sequences and low-quality reads (quality score <30, read length <30, and N > 5) were removed using fastp (Version 0.23.3). The resulting purified reads were then mapped to the human reference sequence (GRCh38.p13) using STAR (Version 2.7.10b). Gene expression levels were determined using RSEM (Version 1.3.1). Differentially expressed genes were identified using the Wald test in the DESeq2 R package. Gene ontology (GO) analysis was conducted using the clusterProfiler R package.
2.9. SF collection
Following approval by the Ethics Review Committee of Kanazawa Medical School (approval number I583), SF was collected from the knee joints of patients with knee OA (Kellgren–Laurence [K–L] classification Grades 2–3) according to previous reports [27,28]. All patients provided informed consent prior to SF collection.
2.10. AMS dissolution by SF obtained from patients with knee OA
Approximately 100 μL of AMS was suspended in 900 μL of 0.9% saline solution. The resulting suspension was aliquoted into 1.5-mL tubes and centrifuged at 300 × g for 5 min. The supernatant was carefully removed, and 200 μL of SF at various concentrations (0%, 20%, 40%, 60%, 80%, and 100%) was added. The AMSs mixed with SF were then seeded into 96-well plates and incubated for 24 and 48 h. The entire well was captured using a microscope (EVOS® FL Cell Imaging System; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and the AMS cross-sectional area and number per well were evaluated using ImageJ.
2.11. Effect of SF on the viability of 2D- and AMS-cultured ADSCs
After 7 days of culture, 2D-cultured ADSCs were harvested using 0.25% trypsin–ethylenediaminetetraacetic acid (FUJIFILM Wako Pure Chemical). AMS-cultured ADSCs were collected along with the medium and centrifuged at 300 × g for 5 min. The culture supernatant was discarded, and the cells were resuspended in a 0.9% saline solution and aliquoted into tubes. After centrifugation, the supernatant was discarded, and 200 μL of saline containing 0% or 80% SF was added to each well of a 96-well plate. Cell viability by percentage of relative light units (RLUs) in each well was measured using a GloMax 96 microplate luminometer (Promega Corp., Madison, WI, USA) after 24 and 48 h of incubation according to previous study [27].
2.12. Statistical analysis
Statistical analysis was performed using one-way analysis of variance followed by Tukey's multiple comparison test to evaluate cell viability, exosome concentration, and exosome secretion in ADSCs (Fig. 1, Fig. 2c). Cell number and cell viability by trypan blue staining were analyzed by Student's t-test (Fig. S1b). Cytokine secretion ability (Fig. 3, Fig. 5d) and exosome marker expression (Fig. S3) were analyzed using Student's t-test. For gene expression analysis from RNA-seq data, the Wald test was used for significance testing (Fig. S4). AMS degradation by synovial fluid (SF) (Fig. 4) and cell viability in 2D- and AMS-cultured ADSCs exposed to SF (Fig. 5b) were analyzed using one-way analysis of variance followed by Tukey's multiple comparison test. The ratio of RLUs in three ADSC states were analyzed using a two-way repeated measure analysis of variance followed by Tukey's multiple comparison test (Fig. 5c). All data are presented as means ± standard deviations. All statistical analyses were conducted using GraphPad Prism software (Version 9.4.1; GraphPad Software Inc., San Francisco, CA, USA) or R (Version 4.3.0).
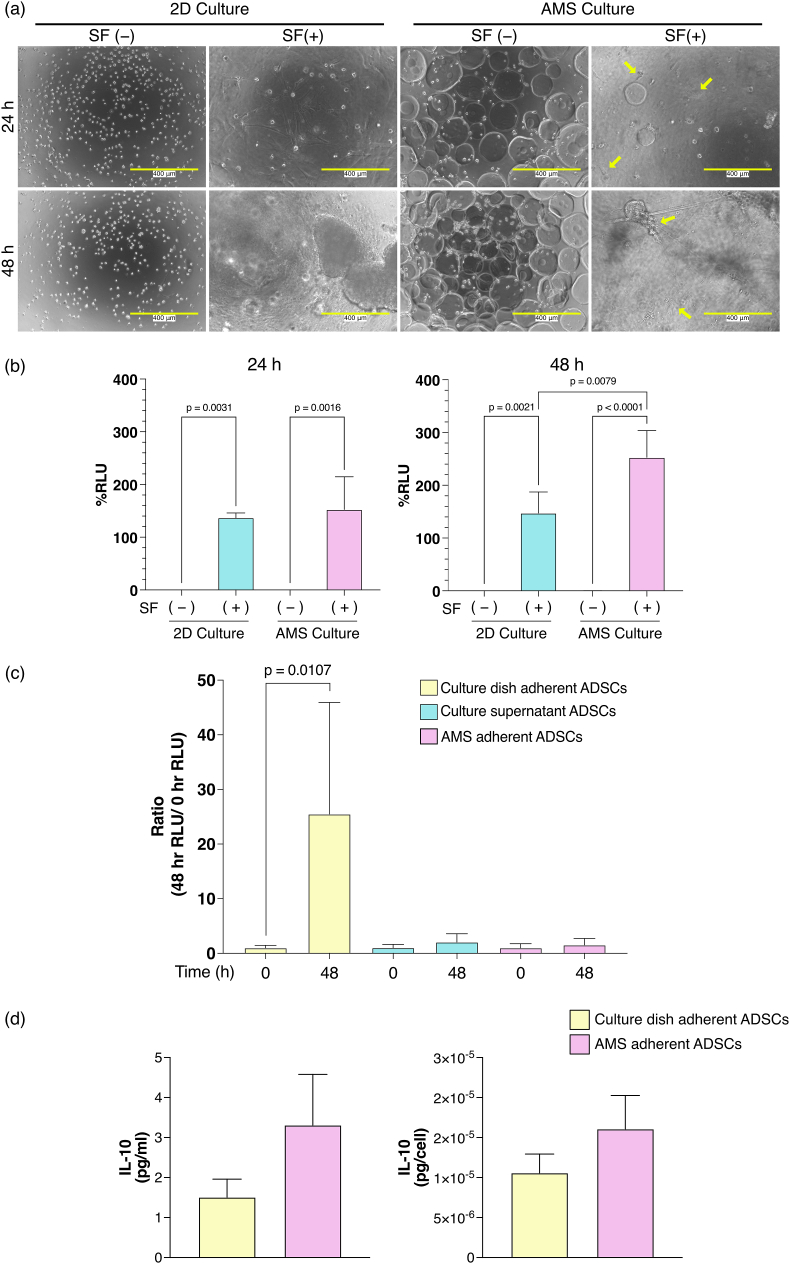
Fig. 1.
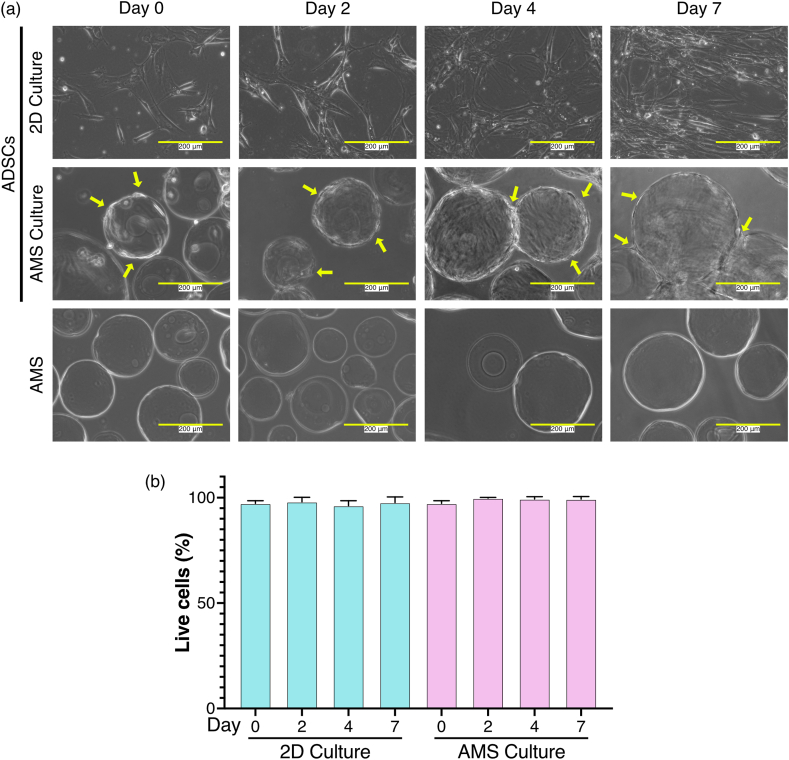
Temporal evaluation of 2D- and AMS-cultured ADSC morphology and viability. (a) Cellular images of ADSCs cultured in adherent culture and stirred culture with AMSs. White scale bars indicate 200 μm, and yellow arrows show ADSCs adhering to the AMSs. (b) Quantification of cell viability using trypan blue staining for both 2D and AMS cultures (n = 3). After staining with trypan blue, the cells were counted with a hemocytometer. The mean number of cells was 44. Values are expressed as a percentage, representing the number of viable cells relative to the total number of cells. Bar graphs display means ± standard deviations.
Fig. 2.
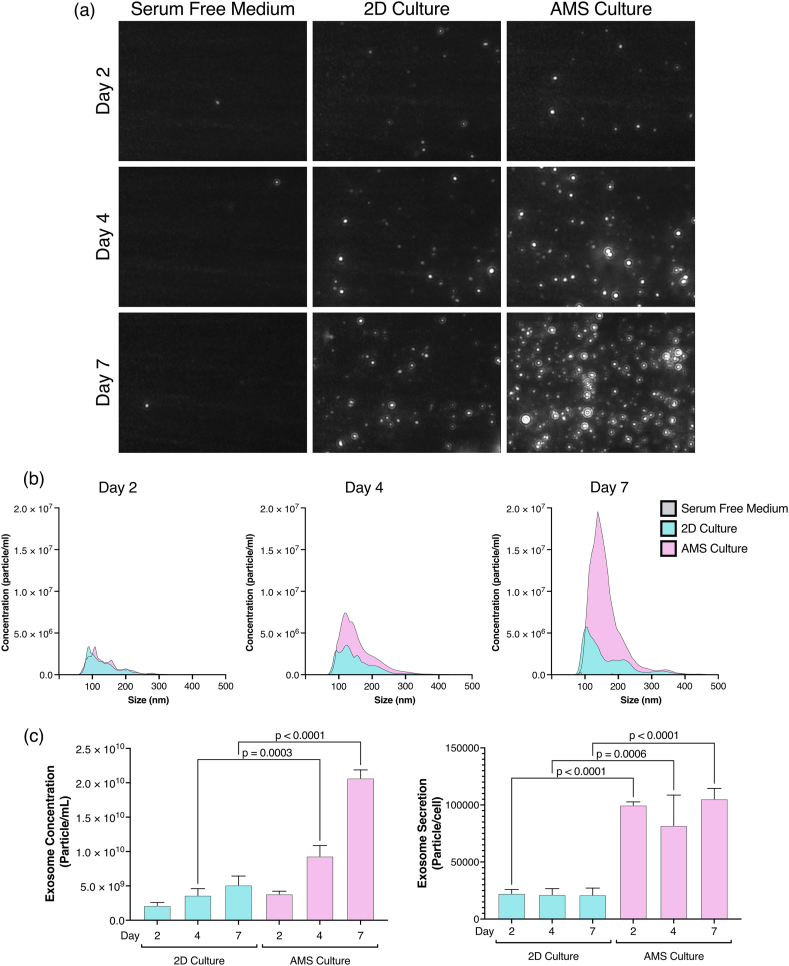
Characterization of exosomes secreted by 2D- and AMS-cultured ADSCs. (a) Particle visualization and (b) size distribution of ADSC-derived exosomes in 2D and AMS cultures, as determined through nanoparticle tracking analysis (n = 3). (c) The concentration of exosome and the number of exosomes secreted per single cell in the culture supernatant. The number of exosomes secreted per cell was calculated by multiplying the exosome concentrations obtained from nanoparticle tracking analysis by the culture medium volume and dividing by the number of cells (n = 3). P-values were obtained from one-way analysis of variance followed by Tukey's multiple comparison test. Data are represented as means ± standard deviations. AMS: atelocollagen microspheres; ADSCs: adipose-derived stem cells.
Fig. 3.
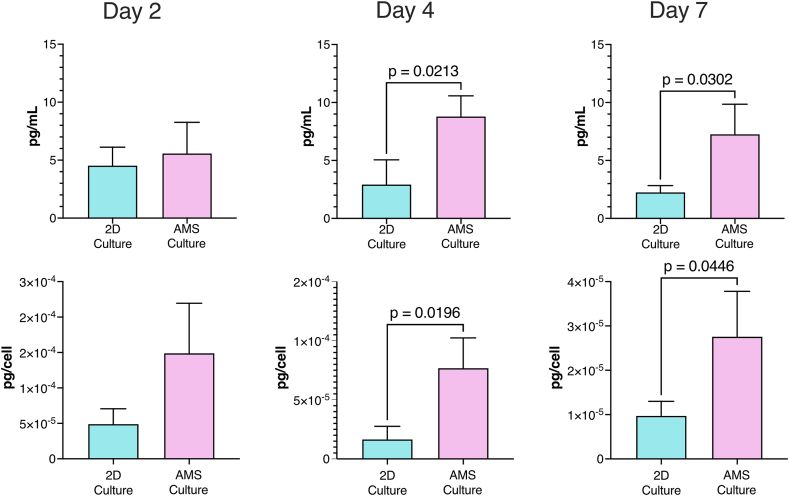
ADSC cytokine release in AMS and 2D monolayer culture supernatants. ADSCs were incubated in AMS and 2D monolayer cultures for 2, 4, and 7 days. Supernatants were then collected to determine IL-10 concentrations using an enzyme-linked immunosorbent assay. Bar graph depicts IL-10 concentrations in the culture supernatant on days 2, 4, and 7. After the cells were trypsinized and collected, cell counts in AMS and monolayer culture cells were estimated by trypan blue staining. Upper layer shows the concentration in medium and lower layer shows cytokine concentration per living cells. Data are presented as means ± standard deviations. P-values were obtained using the Student's t-test. AMS: atelocollagen microspheres; ADSCs: adipose-derived stem cells.
Fig. 5.
Viability of 2D- and atelocollagen microsphere (AMS)-cultured adipose-derived stem cells after exposure to synovial fluid (SF) from three patients with knee osteoarthritis. (a) Cell morphology at different time points after the addition of 80% SF to fresh 2D- and AMS-cultured ADSCs. The yellow arrow indicates that the AMSs were dissolved by SF, leading to the release of ADSCs from the AMSs and adhered to the bottom of the culture dish. (b) Bar graph shows cell viability percentage at each time point after exposure to 80% SF for fresh 2D- and AMS-cultured ADSCs. Relative light unit percentage (%RLU) at 0 h is presented as 100% (n = 3). White scale bars represent 400 μm. P-values were obtained using one-way analysis of variance followed by Tukey's multiple comparison test. Data are presented as means ± standard deviations. (c) The bar graph shows the released live cells from AMS after 48 h of incubation in AMS-cultured ADSCs with 80% SF. The ratio of RLUs at 0 and 48 h are shown for the three ADSC states (n = 3). The RLUs in the samples at each time point were calculated by subtracting the RLU of 80% SF as blank. The RLU ratio at 0 h was determined by dividing the 0 h RLU of each sample by the average 0 h RLU. Similarly, the RLU ratio at 48 h was calculated by dividing the 48 h RLU of each sample by its corresponding 0 h RLU. “Culture dish adherent ADSCs” indicates ADSCs adhered to the bottom of the culture dish (yellow bar). “Culture supernatant ADSCs” indicates ADSCs released from AMS by SF remaining in the supernatant (blue bar). “AMS-adherent ADSCs” indicates ADSCs that remain adhered to AMS (red bar). P-values were obtained using a two-way repeated measure analysis of variance followed by Tukey's multiple comparison test. AMS: atelocollagen microspheres; ADSCs: adipose-derived stem cells. (d) The bar graph depicts the secretion capacity of IL-10 in AMS-adherent ADSCs and culture dish adherent ADSCs (n = 3). After culturing ADSCs on the AMS in 80% SF for 48 h, ADSCs that adhered to the bottom of the culture dish and those that remained attached to the AMS were collected. These cells were then cultured in serum-free medium for 7 days, and the IL-10 concentration in the culture supernatant was measured.
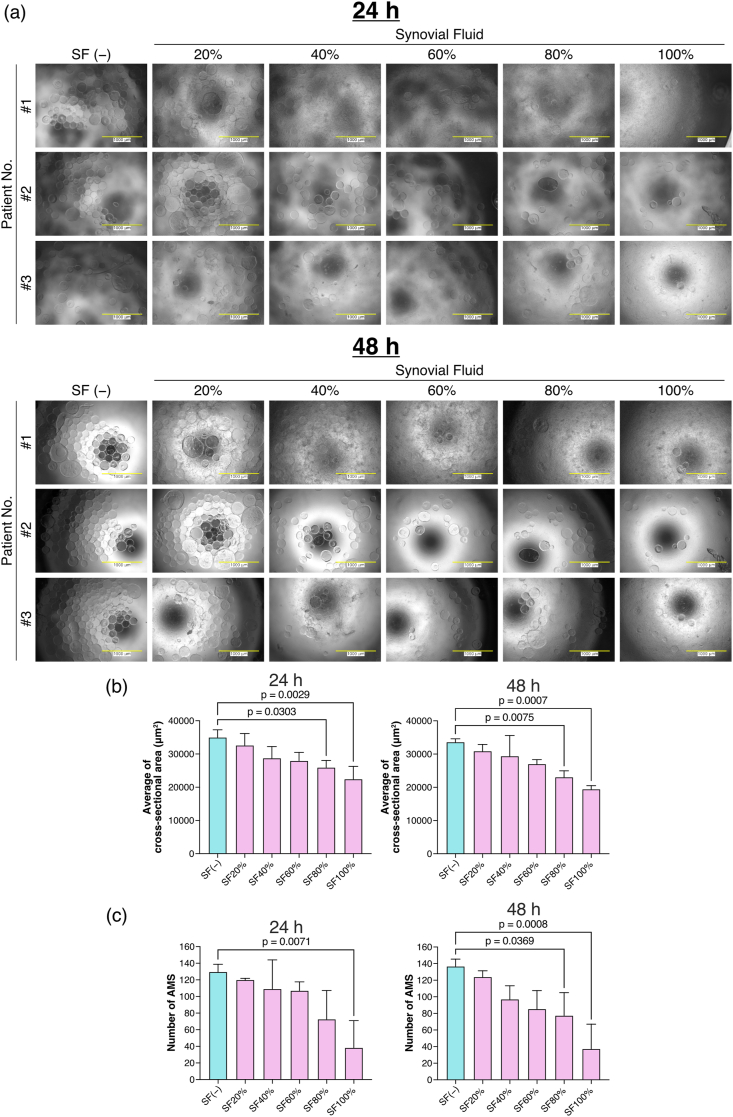
Fig. 4.
Decomposition of atelocollagen microspheres (AMSs) in synovial fluid (SF). (a) Culture dishes seeded with AMSs were supplemented with each SF concentration. AMSs were incubated with SFs from three patients at 37 °C for 24 and 48 h and then examined using phase contrast microscopy (n = 3). White scale bars represent 1000 μm. (b) AMS cross-sectional area and (c) number per well for each SF concentration were determined (n = 3). P-values were obtained using one-way analysis of variance followed by Tukey's multiple comparison test. Data are presented as means ± standard deviations. AMS: atelocollagen microspheres; ADSCs: adipose-derived stem cells.
3. Results
3.1. Evaluation of the 2D- and AMS-cultured ADSC morphology over time
Since the use of ADSCs cultured in serum-free media has been recommended for the treatment of knee OA [29], ADSCs were cultured in a serum-free medium in the present study. With the aim of clinical applications, the suitability of AMS-adherent ADSCs for culture in serum-free medium was evaluated over time using microscopic imaging. As shown in Fig. 1a, both 2D- and AMS-cultured ADSCs exhibited cell proliferation over time. The yellow arrows in Fig. 1a indicate the adherence of ADSCs to AMSs. To confirm the detachment of ADSCs from AMSs using trypsin, the viability of AMS-adhered ADSCs was assessed using trypan blue staining (Fig. S2). The detached 2D- and AMS-cultured ADSCs showed over 90% viability (Fig. 1b). There were no significant differences in survival rate between the two groups. These findings indicate that AMS-cultured ADSCs can be successfully cultured in a serum-free medium.
3.2. ADSCs adhered to AMS and cultured in stirred culture show increased secretion of exosomes
Huang et al. reported that variations in gravitational environments could influence the secretion capabilities of cytokines and growth factors from MSCs [30]. This finding suggests that differences in gravitational environments may affect stem cell function. In the case of ADSCs, those adhered to culture dishes (referred to as 2D-cultured ADSCs) are primarily affected by the downward force of gravity throughout the culture period. On the other hand, ADSCs adhered to AMSs are affected by the multidirectional force of gravity due to stirred culture [31].
A large number of particles in the 70–150 nm range were observed in the culture supernatant of AMS-cultured ADSCs compared with that of 2D-cultured ADSCs (Fig. 2a and b). Furthermore, the concentration of exosomes in the culture supernatant and the number of exosomes released from the cells were significantly higher in AMS-cultured ADSCs than in 2D-cultured ADSCs (Fig. 2c). The purity of the isolated exosomes in the culture supernatant was assessed by evaluating the surface expression of the exosome markers CD9, CD63, and CD81 using flow cytometry (Fig. S3a). The expression of CD63 and CD81 was confirmed in particles derived from both 2D- and AMS-cultured ADSCs and clearly indicated the release of exosomes (Fig. S3b). These results indicated that ADSCs adhered to AMS and cultured in stirred culture exhibited an enhanced exosome secretion capacity due to the culture environment.
3.3. Secretion capacity of IL-10 in 2D- and AMS-cultured ADSCs
ADSCs are well-known for their potent immunomodulatory properties, primarily attributed to their secretion of a diverse range of immunoregulatory and growth factors [32]. Thus, we evaluated the secretion of the representative anti-inflammatory cytokine IL-10 derived from ADSCs. Compared with 2D-cultured ADSCs, the supernatant of AMS-cultured ADSCs exhibited a significant increase in IL-10 production at both 4 and 7 days (Fig. 3). These findings indicated that AMS culture can enhance the secretory capacity of the anti-inflammatory cytokine IL-10 in ADSCs.
3.4. Comprehensive gene expression analysis using RNA-seq
In the present study, AMS-cultured ADSCs showed an increased secretion of exosomes and cytokines compared with 2D-cultured ADSCs. To investigate the underlying genetic factors associated with these altered properties, total RNA was extracted from 2D- and AMS-cultured ADSCs, and RNA-seq analysis was performed (n = 3). First, principal component analysis was performed on the obtained RNA-seq results to assess the gene expression patterns between the two sample groups. The gene expression profiles of 2D- and AMS-cultured ADSCs were found to be closely positioned (Fig. S4a). The genes with significantly altered expression between the two groups were then extracted, considering a p-value of <0.05 and a log₂ fold change >2. A total of 105 genes demonstrated significant variability in their expression. Among these, 50 genes were significantly upregulated in AMS-cultured ADSCs, whereas 55 genes were significantly downregulated (Fig. S4b, Table S1).
Furthermore, GO analysis using RNA-seq results was performed on the differentially expressed genes, and the findings indicated that genes involved in extracellular matrix, immune regulation, and differentiation were altered (Table 1). In particular, the expression of IL-32, known to induce the expression of the inflammatory cytokines IL-1β and IL-6, which contribute to knee OA, was significantly reduced. In addition, the expression of PRG4, a gene reported to influence the therapeutic effect on knee OA, was significantly upregulated in AMS-cultured ADSCs.
Table 1.
Gene ontology (GO) analysis of the RNA-seq data.
| GO analysis of upregulated genes (50 genes) in AMS-cultured ADSCs | |||
|---|---|---|---|
| ID | Description | p.adjust | #Molecules |
| GO:0062023 |
collagen-containing extracellular matrix |
0.0019 |
PRG4, APOE, TINAGL1, SFRP2, SERPINA1, VIT, COL28A1 |
| GO analysis of downregulated genes (55 genes) in AMS-cultured ADSCs | |||
| ID |
Description |
p.adjust |
#Molecules |
| GO:0032330 | regulation of chondrocyte differentiation | 0.037 | SCIN, RFLNA, ZNF664-RFLNA |
| GO:0043931 | ossification involved in bone maturation | 0.024 | DCHS1, RFLNA, ZNF664-RFLNA |
| GO:0005125 | cytokine activity | 0.00018 | IL32, LIF, INHBB, CXCL3, CXCL1, CXCL8, WNT7B |
| GO:0050900 | leukocyte migration | 0.037 | ICAM1, SLAMF8, CXCL3, CXCL1, VEGFD, CXCL8 |
| GO:1990266 | neutrophil migration | 0.037 | SLAMF8, CXCL3, CXCL1, CXCL8 |
3.5. AMS was dissolved in SF derived from patients with knee OA
ADSCs are exposed to SF when AMS-cultured ADSCs are administered into the knee joint cavity. Therefore, the evaluation of influence of SF exposure on AMS-cultured ADSCs is important. In addition, several studies have reported the presence of collagenases in SF, which are enzymes catalyze the degradation of collagen [33,34]. Therefore, in the present study, we confirmed whether AMSs without ADSCs were dissolved by SF. SF from three patients with knee OA was used herein (patient information is provided in Table S2). After addition of SF, a concentration-dependent reduction in the number of AMSs was observed at both 24 and 48 h (Fig. 4a). Furthermore, when the number and cross-sectional area of AMSs were quantified based on the microscopic images of AMSs treated with different concentrations of SF, a significant concentration-dependent decrease was observed (Fig. 4b and c). These findings strongly suggest that AMSs can be dissolved in SF, indicating that AMSs may disappear when administered into the knee joint cavity of patients with OA.
3.6. Effect of SF on the viability of AMS-adhered ADSCs
Assessing the impact of SF on ADSCs released from degraded AMS is also vital, as these cells have potential in treating knee OA as stem cells. To investigate the release of ADSCs from AMSs after SF treatment, 2D-cultured ADSCs were exposed to 80% SF under specific culture conditions. In microscopic images, 2D-cultured ADSCs were observed to adhere to the bottom of the culture dish after 24 h and aggregate after 48 h. In contrast, AMS-cultured ADSCs showed that the AMSs were dissolved by SF, resulting in the release of ADSCs from the AMSs and their adherence to the bottom surface after 24 and 48 h (Fig. 5a; yellow arrow). Interestingly, the RLU percentage for AMS-cultured ADSCs was significantly higher at 48 h than that for 2D-cultured ADSCs (Fig. 5b). Furthermore, the supernatant was collected after 48 h and separated into ADSCs adhering to AMS, ADSCs released from AMS, and ADSCs adhering to the bottom of the culture dish. A significant increase in the RLU ratio was observed at 48 h than at 0 h in ADSCs released from AMS and adhered to the bottom of the culture dish (Fig. 5c). This indicates that the live ADSCs can be gradually released from the AMS by SF. Furthermore, ADSCs that adhered to the bottom of the culture dish due to AMS dissolution by SF and those that remained attached to AMS can secrete IL-10 (Fig. 5d). These findings suggest that AMS-cultured ADSCs have a longer survival time than 2D-cultured ADSCs and be released from AMSs and secrete IL-10 when administered into the knee joint cavity of patients with OA.
4. Discussion
To the best of our knowledge, this is the first report to evaluate the characteristics and utility of AMS-adhered ADSCs, with the aim of improving the therapeutic efficacy of ADSCs for knee OA. Atelocollagen without telopeptides, unlike conventional collagen, has reduced antigenicity [24]. Therefore, administration of atelocollagen may reduce intra-articular immune cell activation more effectively than the use of conventional collagen. In addition, clinical trials have reported pain relief in patients with knee OA following intra-articular administration of atelocollagen [25]. Given these advantages, the combination of ADSC-based cell therapy and AMSs holds promise for improving therapeutic outcomes in knee OA.
ADSCs have demonstrated anti-inflammatory and cytoprotective effects on chondrocytes. Furthermore, the release of exosomes from these cells has been reported to contribute to these effects through paracrine mechanisms [35]. Therefore, exosomes released from ADSCs are recognized for their therapeutic potential in knee OA [36]. Furthermore, several factors have been reported to alter exosome secretion, such as cellular stress and culture environment [37,38]. Therefore, it is suggested that the cellular stress induced by the agitated culture of AMS-adhered ADSCs may affect the upregulated exosome release process (Fig. 2). As shown Fig. S3, expression patterns of CD9 and CD63 differ between the 2D and AMS-cultured ADSC-derived exosomes. Mashiko et al. reported that adhesion of ADSCs to AMS increased the percentage of stage-specific embryonic antigen-3 (SSEA-3)-positive cells with superior pluripotency [31]. Therefore, AMS culture environment can alter cell populations, and AMS-based culture may be an effective method for producing more functional and homogeneous cell populations. Further studies should be performed to elucidate the effect of increased SSEA-3-positive cells in AMS-cultured ADSCs on the therapeutic efficacy of knee OA.
On the other hand, ADSCs possess a high immunoregulatory capacity as a result of their secretion of immunomodulatory and growth factors [39]. In a previous study, we generated ADSC spheroids using a specialized culture device and demonstrated an increased secretion of the representative anti-inflammatory cytokine IL-10 compared with 2D cultures [28]. IL-10 has inhibitory effects on intra-articular inflammation in patients with knee OA [40,41]. In this study, the IL-10 secretion capacity of AMS-cultured ADSCs was evaluated, and a significant increase in the IL-10 secretion capacity was observed compared with that of 2D-cultured ADSCs (Fig. 3). Cytokine secretion by ADSCs is influenced by variations in the culture environment, with hypoxic conditions and spheroid cultures promoting IL-10 secretion by ADSCs [28,40,42]. Therefore, it is suggested that stirred culture with AMS-adherent ADSCs may also impact the ability of ADSCs to secrete IL-10. Based on these findings, AMS-adhered ADSCs are expected to secrete a large number of exosomes and IL-10, thereby improving their therapeutic effect against knee OA. Future studies are required to investigate how the culture environment affects the secretion of not only IL-10 but also other cytokines associated with knee OA, such as IL-32 and transforming growth factor beta.
A comprehensive gene expression analysis was conducted on 2D- and AMS-cultured ADSCs using RNA-seq. The results revealed that 50 genes were significantly upregulated, whereas 55 genes were significantly downregulated in AMS-cultured ADSCs compared with those in 2D-cultured ADSCs (Table S1). GO analysis using RNA-seq data showed that the expression levels of many genes involved in extracellular matrix and immune regulation were altered (Table 1). These results suggest that some of the changes in gene expression in AMS-cultured ADSCs may lead to improved therapeutic efficacy.
Notably, the expression of PRG4 was significantly upregulated in AMS-cultured ADSCs compared with that in 2D-cultured ADSCs (Fig. S4). Abubacker et al. reported that recombinant human proteoglycan 4 (PRG4) protein injection into the knee joints of mice with knee OA, induced by PRG4 knockout, slowed down cartilage degeneration [43]. Furthermore, the expression of PRG4 in the joint cavity of knee OA has been reported to exhibit potential therapeutic implications [44]. Therefore, it is suggested that AMS-cultured ADSCs may enhance therapeutic effects against knee OA by releasing PRG4 and potentially preventing cartilage degeneration.
IL-32 is known to induce the production of inflammatory cytokines, such as tumor necrosis factor-alpha, IL-1β, and IL-6, which are the main causes of arthritis, by immune cells, such as macrophages and monocytes [45]. Furthermore, the administration of human IL-32 into the knee joint cavity of mice has been shown to induce arthritis [46]. Therefore, the expression of IL-32 is thought to promote the activation of immune cells within the joint and potentially exacerbate inflammation. Interestingly, in the present study, IL-32 expression was significantly lower in AMS-cultured ADSCs than in 2D-cultured ADSCs (Fig. S4). These findings suggest that the use of AMS-cultured ADSCs may suppress the activation of immune cells in the joint cavity and subsequently attenuate arthritis. Further research is needed to fully understand the relationship between these changes in gene expression and the therapeutic effects on knee OA.
In the treatment of knee OA using ADSCs, the prolonged presence of ADSCs within the knee joint is believed to contribute to sustained therapeutic effects [47]. SF contains various matrix metalloproteinases that are considered important mediators of cartilage degradation [48,49]. As shown in Fig. 4, the major component of AMSs is collagen, which is progressively degraded by the collagenase present in SF. As a result, AMS-adhered ADSCs are gradually released into the joint cavity. Furthermore, compared with 2D-cultured ADSCs, AMS-cultured ADSCs exhibited a significant increase in the living ADSC percentage after 48 h of exposure to SF, suggesting that AMSs may improve the tissue retention of living ADSCs in the SF-containing joint cavity (Fig. 5a and b). Interestingly, the time-dependent increase in the RLU percentage observed in AMS-cultured ADSCs in the SF suggests that AMS-cultured ADSCs may undergo cell proliferation in the SF (Fig. 5b). As shown in Fig. 5c and d, live ADSCs were released by the gradual degradation of AMS by SF, and these ADSCs could secrete IL-10. Therefore, an approach using AMSs that avoids cell-damaging procedures, such as trypsinization, may be beneficial in maintaining ADSC viability and functionality in the joint cavity.
5. Conclusions
In conclusion, the use of AMS-cultured ADSCs represents a promising approach for ADSC-based cell therapy in patients with knee OA. This approach is expected to improve therapeutic efficacy by promoting the sustained release of exosomes and anti-inflammatory cytokines as well as improving cell survival in the joint cavity. Direct injection of AMS-cultured ADSCs can be prepared without trypsinization, thereby simplifying the procedure and facilitating clinical application. Importantly, our findings suggest that AMSs can promote ADSC proliferation and activation in an environment mimicking the intra-articular environment with the addition of SF. Furthermore, the gradual dissolution of AMSs in SF suggests that ADSCs with promoted activity may be released over time into the joint cavity, resulting in a sustained therapeutic effect. In the future, optimization of the number of ADSCs attached to the microspheres as well as the size and shape of the microspheres will be important considerations for clinical applications.
Author contributions
A.F., Y.I. (Yasuhito Ishigaki), and T.I., Conception and design; T.S., T.H., H.K., H.H., and Y.T., Analysis and interpretation of the data; A.F., T.S., and N.K., Obtaining of funding; T.S., T.H., H.K., Y.N., and A.F., Collection and assembly of data; Y.I. (Yasuo Iida), Statistical expertise; I.T., H.S., Y.S., and S.Y., Administrative, technical, or logistic support; Y.N., Provision of study materials; N.Y., Y.S., T.I., A.K., S.O., and N.K., Supervision; T.S., Drafting of the article; A.F. and T.I., Final approval of the article; All authors, Critical revision of the article for important intellectual content.
Role of the funding source
The present study was funded by the JSPS KAKENHI grant numbers 22K16779, 23K14363, 22K20726 and the Private University Research Branding Project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. This work was supported by a Grant for Collaborative Research from Kanazawa Medical University (C2021-3 and C2022-1) and the Shibuya Science Culture and Sports Foundation (2021).
Data availability
The data supporting the findings of this study are available from the corresponding author on request. The RNA-seq data used in this study have been deposited in the DNA Data Bank of Japan under the accession number DRA016914.
Ethical statements
This study was conducted in accordance with the Declaration of Helsinki and approved by the Kanazawa Medical University Specified Certified Regenerative Medicine Committee, the Institutional Review Board for Genetic Analysis Research (protocol code G129 and 17 April 2017 of approval, protocol code G173 and 23 June 2021 of approval), and the Kanazawa Medical University Medical Ethics Review Board (protocol code I583 and 24 March 2021 of approval).
Declaration of competing interest
The authors declared that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
The authors thank the staff at the clinical research support office and the center for regenerative medicine at Kanazawa Medical University. We also appreciate Shigetaka Shimodaira, Department of Regenerative Medicine, Kanazawa Medical University, for the technical assistance in this work. Authors also express thanks to Ms. Ayane Kuwano in Kanazawa Institute of Technology, Japan. RNA-seq was performed by Bioengineering Lab. Co., Ltd. (Kanagawa, Japan). The supercomputing resource was provided by Human Genome Center, Institute of Medical Science, University of Tokyo.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2024.04.010.
Contributor Information
Takuya Sakamoto, Email: taku0731@kanazawa-med.ac.jp.
Atsushi Fuku, Email: f-29@kanazawa-med.ac.jp.
Tetsuhiro Horie, Email: horie-te@kanazawa-med.ac.jp.
Hironori Kitajima, Email: kuppy@kanazawa-med.ac.jp.
Yuka Nakamura, Email: yuka-n@kanazawa-med.ac.jp.
Ikuhiro Tanida, Email: tanida@neptune.kanazawa-it.ac.jp.
Hiroshi Sunami, Email: sunami@med.u-ryukyu.ac.jp.
Hiroaki Hirata, Email: hiro6246@kanazawa-med.ac.jp.
Yoshiyuki Tachi, Email: y-t-s17@kanazawa-med.ac.jp.
Yasuo Iida, Email: yiida@kanazawa-med.ac.jp.
Sohsuke Yamada, Email: sohsuke@kanazawa-med.ac.jp.
Naoki Yamamoto, Email: naokiy@fujita-hu.ac.jp.
Yusuke Shimizu, Email: yyssprs@gmail.com.
Yasuhito Ishigaki, Email: ishigaki@kanazawa-med.ac.jp.
Toru Ichiseki, Email: tsy-ichi@kanazawa-med.ac.jp.
Ayumi Kaneuji, Email: kaneuji@kanazawa-med.ac.jp.
Satoshi Osawa, Email: osawa@neptune.kanazawa-it.ac.jp.
Norio Kawahara, Email: kawa@kanazawa-med.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Arden N.K., Perry T.A., Bannuru R.R., Bruyère O., Cooper C., Haugen I.K., et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol. 2021;17:59–66. doi: 10.1038/s41584-020-00523-9. [DOI] [PubMed] [Google Scholar]
- 2.Zelinka A., Roelofs A.J., Kandel R.A., De Bari C. Cellular therapy and tissue engineering for cartilage repair. Osteoarthritis Cartilage. 2022;30:1547–1560. doi: 10.1016/j.joca.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S., Chu W.C., Lai R.C., Lim S.K., Hui J.H.P., Toh W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135–2140. doi: 10.1016/j.joca.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Kuroda K., Kabata T., Hayashi K., Maeda T., Kajino Y., Iwai S., et al. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression orthopedics and biomechanics. BMC Muscoskel Disord. 2015;16:1–10. doi: 10.1186/s12891-015-0701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K Il, Lee W.S., Kim J.H., Bae J.K., Jin W. Safety and efficacy of the intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritic knee: a 5-year follow-up study. Stem Cells Transl Med. 2022;11:586–596. doi: 10.1093/stcltm/szac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwasawa A., Okazaki K., Noda K., Fukushima T., Nihei K. Intra-articular injection of culture-expanded adipose tissue-derived stem cells for knee osteoarthritis: assessments with clinical symptoms and quantitative measurements of articular cartilage volume. J Orthop Sci. 2023;28:408–415. doi: 10.1016/j.jos.2021.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Hodgetts S.I., Beilharz M.W., Scalzo A.A., Grounds M.D. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or NK1.1+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- 8.Oh J.S., Kim K.N., An S.S., Pennan W.A., Kim H.J., Gwak S.J., et al. Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant. 2011;20:837–849. doi: 10.3727/096368910X539083. [DOI] [PubMed] [Google Scholar]
- 9.Mingliang R., Bo Z., Zhengguo W. Stem cells for cardiac repair: status, mechanisms, and new strategies. Stem Cell Int. 2011;2011 doi: 10.4061/2011/310928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing D., Liu W., Wang B., Li J.J., Zhao Y., Li H., et al. Intra-articular injection of cell-laden 3D microcryogels empower low-dose cell therapy for osteoarthritis in a rat model. Cell Transplant. 2020;29:1–12. doi: 10.1177/0963689720932142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toupet K., Maumus M., Luz-Crawford P., Lombardo E., Lopez-Belmonte J., Van Lent P., et al. Survival and biodistribution of xenogenic adipose mesenchymal stem cells is not affected by the degree of inflammation in arthritis. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Białkowska K., Komorowski P., Bryszewska M., Miłowska K. Spheroids as a type of three-dimensional cell cultures-examples of methods of preparation and the most important application. Int J Mol Sci. 2020;21:1–17. doi: 10.3390/ijms21176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J., Mineda K., Wu S.H., Mashiko T., Doi K., Kuno S., et al. An injectable non-cross-linked hyaluronic-acid gel containing therapeutic spheroids of human adipose-derived stem cells. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-01528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brindo da Cruz I.C., Velosa A.P.P., Carrasco S., dos Santos Filho A., Tomaz de Miranda J., Pompeu E., et al. Post-adipose-derived stem cells (ADSC) stimulated by collagen type v (col v) mitigate the progression of osteoarthritic rabbit articular cartilage. Front Cell Dev Biol. 2021;9:1–11. doi: 10.3389/fcell.2021.606890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koellensperger E., Bollinger N., Dexheimer V., Gramley F., Germann G., Leimer U. Choosing the right type of serum for different applications of human adipose tissue-derived stem cells: influence on proliferation and differentiation abilities. Cytotherapy. 2014;16:789–799. doi: 10.1016/j.jcyt.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Leslie S.K., Cohen D.J., Hyzy S.L., Dosier C.R., Nicolini A., Sedlaczek J., et al. Microencapsulated rabbit adipose stem cells initiate tissue regeneration in a rabbit ear defect model. J Tissue Eng Regen Med. 2018;12:1742–1753. doi: 10.1002/term.2702. [DOI] [PubMed] [Google Scholar]
- 17.Shikani A.H., Fink D.J., Sohrabi A., Phan P., Polotsky A., Hungerford D.S., et al. Propagation of human nasal chondrocytes in microcarrier spinnre culture. Am J Rhinol. 2004;18:105–112. [PubMed] [Google Scholar]
- 18.Yao L., Phan F., Li Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res Ther. 2013;4 doi: 10.1186/scrt320. 0-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao S., Meng H., Li J., Zhao J., Xu Y., Wang A., et al. Potential and recent advances of microcarriers in repairing cartilage defects. J Orthop Translat. 2021;27:101–109. doi: 10.1016/j.jot.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X.Y., Chen J.Y., Tong X.M., Mei J.G., Chen Y.F., Mou X.Z. Recent advances in the use of microcarriers for cell cultures and their ex vivo and in vivo applications. Biotechnol Lett. 2020;42 doi: 10.1007/s10529-019-02738-7. [DOI] [PubMed] [Google Scholar]
- 21.Jin G.Z., Park J.H., Wall I., Kim H.W. Isolation and culture of primary rat adipose derived stem cells using porous biopolymer microcarriers. Tissue Eng Regen Med. 2016;13:242–250. doi: 10.1007/s13770-016-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynn A.K., Yannas I.V., Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 23.Holmes R., Kirk S., Tronci G., Yang X., Wood D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater Sci Eng C. 2017;77:823–827. doi: 10.1016/j.msec.2017.03.267. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.S., Oh K.J., Moon Y.W., In Y., Lee H.J., Kwon S.Y. Intra-articular injection of type I atelocollagen to alleviate knee pain: a double-blind, randomized controlled trial. Cartilage. 2021;13:342S. doi: 10.1177/1947603519865304. 50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taki Y., Fuku A., Nakamura Y., Koya T., Kitajima H., Tanida I., et al. A morphological study of adipose-derived stem cell sheets created with temperature-responsive culture dishes using scanning electron microscopy. Med Mol Morphol. 2022;55:187–198. doi: 10.1007/s00795-022-00319-8. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto T., Koya T., Togi M., Yoshida K., Kato T., Ishigaki Y., et al. Different in vitro-generated MUTZ-3-derived dendritic cell types secrete dexosomes with distinct phenotypes and antigen presentation potencies. Int J Mol Sci. 2022;23:8362. doi: 10.3390/ijms23158362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitajima H., Sakamoto T., Horie T., Kuwano A., Fuku A., Taki Y., et al. Synovial fluid derived from human knee osteoarthritis increases the viability of human adipose-derived stem cells through upregulation of FOSL1. Cells. 2023;12:330. doi: 10.3390/cells12020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuku A., Taki Y., Nakamura Y., Kitajima H., Takaki T., Koya T., et al. Evaluation of the usefulness of human adipose-derived stem cell spheroids formed using SphereRing® and the lethal damage sensitivity to synovial fluid in vitro. Cells. 2022;11:337. doi: 10.3390/cells11030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrikoski M., Juntunen M., Boucher S., Campbell A., Vemuri M.C., Mannerström B., et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4:27. doi: 10.1186/scrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang P., Russell A.L., Lefavor R., Durand N.C., James E., Harvey L., et al. Feasibility, potency, and safety of growing human mesenchymal stem cells in space for clinical application. NPJ Microgravity. 2020;6:16. doi: 10.1038/s41526-020-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mashiko T., Kanayama K., Saito N., Shirado T., Asahi R., Mori M., et al. Selective proliferation of highly functional adipose-derived stem cells in microgravity culture with stirred microspheres. Cells. 2021;10:1–19. doi: 10.3390/cells10030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuhashi K., Tsuboi N., Shimizu A., Katsuno T., Kim H., Saka Y., et al. Serum-starved adipose-derived stromal cells ameliorate crescentic GN by promoting immunoregulatory macrophages. J Am Soc Nephrol. 2013;24:587–603. doi: 10.1681/ASN.2012030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gysen P., Malaise M., Caspar S., Franchimont P. Measurement of proteoglycans, elastase, collagenase and protein in synovial fluid in inflammatory and degenerative arthropathies. Clin Rheumatol. 1985;4:39–50. doi: 10.1007/BF02032316. [DOI] [PubMed] [Google Scholar]
- 34.Sorsa T., Konttinen Y., Lindy O., Ritchlin C., Saari H., Suomalainen K., et al. Collagenase in synovitis of rheumatoid arthritis. Semin Arthritis Rheum. 1992;22:44–53. doi: 10.1016/0049-0172(92)90048-i. [DOI] [PubMed] [Google Scholar]
- 35.Miao C., Zhou W., Wang X., Fang J. The research progress of exosomes in osteoarthritis, with particular emphasis on the mediating roles of miRNAs and lncRNAs. Front Pharmacol. 2021;12:1–14. doi: 10.3389/fphar.2021.685623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taghiyar L., Jahangir S., Khozaei Ravari M., Shamekhi M.A., Eslaminejad M.B. Cartilage repair by mesenchymal stem cell-derived exosomes: preclinical and clinical trial update and perspectives. Adv Exp Med Biol. 2021;1326:73–93. doi: 10.1007/5584_2021_625. [DOI] [PubMed] [Google Scholar]
- 37.Debbi L., Guo S., Safina D., Levenberg S. Boosting extracellular vesicle secretion. Biotechnol Adv. 2022;59:2023. doi: 10.1016/j.biotechadv.2022.107983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha J.M., Shin E.K., Sung J.H., Moon G.J., Kim E.H., Cho Y.H., et al. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci Rep. 2018;8:1–16. doi: 10.1038/s41598-018-19211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Ghadban S., Artiles M., Bunnell B.A. Adipose stem cells in regenerative medicine: looking forward. Front Bioeng Biotechnol. 2022;9:1–10. doi: 10.3389/fbioe.2021.837464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L., Wang X., Wang J., Liu D., Wang Y., Huang Z., et al. Hypoxia-induced secretion of IL-10 from adipose-derived mesenchymal stem cell promotes growth and cancer stem cell properties of Burkitt lymphoma. Tumour Biol. 2016;37:7835–7842. doi: 10.1007/s13277-015-4664-8. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L., Zhou X., Huang C., Bao J., Li J., Xu K., et al. The elevated expression of IL-38 serves as an anti-inflammatory factor in osteoarthritis and its protective effect in osteoarthritic chondrocytes. Int Immunopharm. 2021;94 doi: 10.1016/j.intimp.2021.107489. [DOI] [PubMed] [Google Scholar]
- 42.Vu B.T., Le H.T., Nguyen K.N., Van Pham P. Hypoxia, serum starvation, and TNF-α can modify the immunomodulation potency of human adipose-derived stem cells. Adv Exp Med Biol. 2021;11:3–18. doi: 10.1007/5584_2021_672. [DOI] [PubMed] [Google Scholar]
- 43.Abubacker S., Premnath P., Shonak A., Leonard C., Shah S., Zhu Y., et al. Absence of proteoglycan 4 (Prg4) leads to increased subchondral bone porosity which can be mitigated through intra-articular injection of PRG4. J Orthop Res. 2019;37:2077–2088. doi: 10.1002/jor.24378. [DOI] [PubMed] [Google Scholar]
- 44.Ruan M.Z.C., Erez A., Guse K., Dawson B., Bertin T., Chen Y., et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albuquerque R., Komsi E., Starskaia I., Ullah U., Lahesmaa R. The role of Interleukin-32 in autoimmunity. Scand J Immunol. 2021;93:1–9. doi: 10.1111/sji.13012. [DOI] [PubMed] [Google Scholar]
- 46.Joosten L.A., Netea M.G., Kim S.H., Yoon D.Y., Oppers-Walgreen B., Radstake T.R., et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhattacharjee M., Escobar Ivirico J.L., Kan H., Shah S., Otsuka T., Bordett R., et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2120968119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tchetverikov I., Lohmander L.S., Verzijl N., Huizinga T.W., TeKoppele J.M., Hanemaaijer R., et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64:694–698. doi: 10.1136/ard.2004.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rengel Y., Ospelt C., Gay S. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther. 2007;9:221. doi: 10.1186/ar2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author on request. The RNA-seq data used in this study have been deposited in the DNA Data Bank of Japan under the accession number DRA016914.