Abstract
Diabetes is a complex disease that affects a large percentage of the world's population, and it is associated with several risk factors. Self-management poses a significant challenge, but natural sources have shown great potential in providing effective glucose reducing solutions. Flavonoids, a class of bioactive substances found in different natural sources including medicinal plants, have emerged as promising candidates in this regard. Indeed, several flavonoids, including apigenin, arbutin, catechins, and cyanidin, have demonstrated remarkable anti-diabetic properties. The clinical effectiveness of these flavonoids is linked to their potential to decrease blood glucose concentration and increase insulin concentration. Thus, the regulation of certain metabolic pathways such as glycolysis and neoglycogenesis has also been demonstrated. In vitro and in vivo investigations revealed different mechanisms of action related to flavonoid compounds at subcellular, cellular, and molecular levels. The main actions reside in the activation of glycolytic signaling pathways and the inhibition of signaling that promotes glucose synthesis and storage. In this review, we highlight the clinical efficiency of natural flavonoids as well as the molecular mechanisms underlying this effectiveness.
Keywords: Diabetes, Natural products, Flavonoids, Medicine, Plant species, Mechanism of action, Metabolic diseases
1. Introduction
Diabetes is a complex metabolic disorder characterized by disrupted blood glucose regulation [1]. It has several risk factors, including genetics, eating patterns, microbial infections, oxidative stress, metabolism, and epigenetics [2,3]. Type 2 diabetes mellitus (T2DM) is the most common form and typically affects older adults [[4], [5], [6], [7]]. T2DM is initiated by hyperglycemia, which results in a rise in blood glucose concentration [8]. This leads to the glycosylation of proteins, impairing their function and causing several pathophysiological effects [[9], [10], [11]].
Effective diabetes management involves maintaining adequate food intake to stimulate cellular glucose breakdown. One way to achieve this is to inhibit key enzymes involved in the intestinal breakdown of complex sugars into simple sugars that are absorbable [[12], [13], [14]]. These key enzymes include α-amylase and α-glucosidase. Another approach is to stimulate glucose oxidation at the cellular level and its entry through the cell membrane [15,16]. Glucose transport is regulated by insulin, and the deregulation of insulin leads to hyperglycemia and an accumulation of glucose in the blood [17,18]. Thus, stimulating insulin and glucose receptors is a major therapeutic approach for the prevention and treatment of T2DM. Natural substances, particularly the secondary metabolites of medicinal plants, have garnered significant interest as potential antidiabetic drugs due to their rich bioactive molecules [[19], [20], [21], [22], [23], [24], [25], [26]]. Flavonoids are a diverse class of bioactive molecules found in plants that have demonstrated remarkable antidiabetic properties [[27], [28], [29]].
This paper aims to provide an updated overview of natural flavonoids as antidiabetic drugs, their major mechanisms of action, and their clinical applications. Flavonoids have been shown to improve glucose uptake, increase insulin sensitivity, inhibit carbohydrate-digesting enzymes, and decrease hepatic glucose production [[27], [28], [29]]. Furthermore, flavonoids have been reported to have protective effects against diabetes complications, such as nephropathy, neuropathy, and retinopathy [[27], [28], [29]]. Due to these promising findings, natural flavonoids have potential clinical applications as antidiabetic drugs.
2. Clinical investigations of antidiabetic properties of natural flavonoids
Various clinical studies have demonstrated the potential of natural flavonoids in managing diabetes. The findings of clinical trials investigating the antidiabetic effects of these bioactive compounds are summarized in Table 1. The main flavonoids that have been extensively studied in clinical settings include resveratrol, catechin, rutin, epicatechin, quercetin, hesperidin, and diosmin. In the subsequent sections, we will delve into the progress made in clinical investigations concerning these compounds.
Table 1.
Clinical trials of antidiabetic flavonoids.
| Molecules | Models | Experiment | Doses/Periods | Key findings | Authors |
|---|---|---|---|---|---|
| Resveratrol | 19 T2D patients | A randomized study Double-blind trial (Phase III) |
2 × 5 mg/day 4 weeks |
Decreased insulin resistance Improved insulin sensitivity |
[30] |
| 62 patients with T2D | A prospective, open-label, randomized, controlled trial | 250 mg/day 3 months |
Normalized glycaemia and HbA1c levels | [31] | |
| Non-obese postmenopausal women | A randomized, double-blind, placebo-controlled trial | 75 mg/day 12 weeks |
Increased resveratrol plasma concentration No effect on insulin sensitivity |
[32] | |
| 66 patients with type 2 diabetes mellitus (T2DM) | A randomized placebo-controlled double-blinded parallel clinical trial | 1 g/day 45 days |
Lowered fasting glycaemia levels, HbA1c, and resistance to insulin | [33] | |
| 12 participants with metabolic syndrome (MetS) | A randomized, double-blind, placebo-controlled clinical trial | 500 mg 3 times a day 90 days |
Decreased insulin AUC Decreased total insulin secretion |
[34] | |
| 8 overweight and sedentary men | A single dose (300 mg) on two separate occasions | Attenuated post-absorption insulin concentrations No changes in insulin signaling |
[35] | ||
| 60 non-alcoholic subjects with fatty liver disease (FLD) | A double-blind, randomized, placebo-controlled clinical study | 2,15 g (twice daily) 3 months |
Improved levels of glycaemia, LDL cholesterol, and total cholesterol | [36] | |
| 14 patients with T2D | A double-blind, randomized, crossover design | 500 mg (twice daily) 5 weeks |
No effect on postprandial and fasting glycaemia, or HbA1c | [37] | |
| 34 subjects suffering from polycystic ovary syndrome | A randomized double-blind, placebo-controlled trial | 1500 mg/day 3 months |
Lowered fasting insulin content (31.8 %) Augmented insulin sensitivity index (66.3 %) |
[38] | |
| 38 obese and overweight subjects | A randomized double-blind study | Resveratrol (80 mg/day) EGCG (282 mg/day) 12 weeks |
No impact on insulin-stimulated glucose disposal | [39] | |
| 17 subjects with T2D | A randomized double-blind crossover study | 150 mg/day 30 days |
No improvement in insulin sensitivity | [40] | |
| Middle-aged men suffering from metabolic syndrome | A randomized, placebo-controlled, double-blind, parallel group clinical trial | 150 and 1000 mg/day 16 weeks |
No improvement in glucose homeostasis | [41] | |
| 45 overweight or slightly obese volunteers | A Randomized placebo-controlled trial | 150 mg/day 4 weeks |
No effect on insulin and plasma glucose levels | [42] | |
| 13 men with T2D | A randomized, placebo controlled, cross-over trial | 150 mg/day 1 month |
No effect on insulin sensitivity | [43] | |
| 472 elderly diabetic patients | A single-blind randomized controlled clinical trial | 500 mg/day 6 months |
Improvemed insulin resistance Improvemed blood glucose parameters (decreased G6Pase and HbA1c) |
[44] | |
| 110 diabetic patients | A randomized, placebo-controlled trial | 200 mg/day 24 weeks |
Improved blood glucose as well as insulin synthesis and resistance levels | [45] | |
| Catechin | T2D patients not receiving insulin The participants ingested green tea containing either 582.8 mg of catechins (catechin group; n = 23) or 96.3 mg of catechins (control group; n = 20) per day |
A double-blind controlled study | 582.8 and 96.3 mg per day 12 weeks |
Augmented insulin and adiponectin production No effect on glucose and HbA1c |
[46] |
| Healthy postmenopausal women (Phase III) | 615 mg/350 mL per day 4 weeks |
Improved redox homeostasis Improved postprandial glycemic status |
[47] | ||
| Rutin | 50 participants suffering from T2DM | A double blind, placebo-controlled trial | 500 mg/day 3 months |
Lowered the levels of fasting glycaemia, insulin, insulin resistance, and HbA1c | [48] |
| 34 healthy adult participants | A randomized, placebo-controlled, double blind crossover study | 200 mg/day 3 weeks |
Lowered postprandial glycaemia levels | [49] | |
| Epicatechin | 37 healthy women and men | A randomized, double-blind, placebo-controlled study | 100 mg/day 4 weeks |
Improved insulin resistance Enhanced fasting plasma insulin |
[50] |
| Erythrocyte membrane AChE in normal and type 2 diabetic patients | – | Pronounced insulin-like effect | [51] | ||
| Quercetin | 37 healthy women and men | A randomized, double-blind, placebo-controlled study | 160 mg/day 4 weeks |
No impact on insulin resistance | [50] |
| Hesperidin and Diosmin | 127 diabetic patients with neuropathy and MetS | A randomized controlled trial | 1 g/day (for each) 12 weeks |
Improved glycaemia, LDL, and triglyceride rates Increased magnitude of enhancement when the two molecules are combined |
[52] |
2.1. Resveratrol
In a randomized, double-blind trial, Brasnyó et al. [30] investigated the potential of resveratrol in improving insulin sensitivity in T2D patients. Nineteen patients received a 2 × 5 mg daily dose of resveratrol orally for 4 weeks. The results showed a decrease in insulin resistance and an increase in insulin sensitivity and pAkt/Akt ratio. However, no significant effect on parameters associated with β-cell function was observed. In another clinical study conducted by Bhatt et al. [31], a 3-month oral treatment of 62 T2D patients with 250 mg/day of resveratrol normalized glycaemia, HbA1c, systolic blood pressure, total cholesterol, and total protein levels, without any effect on body weight or LDL and HDL cholesterol levels. These results are consistent with the anti-hyperglycemic and anti-hyper-lipidic effects of resveratrol revealed by other in vitro and in vivo studies.
However, a randomized, placebo-controlled trial conducted by Yoshino et al. [32] on non-obese postmenopausal women with normal GT (NGT) showed that a 12-week treatment with 75 mg/day of resveratrol did not improve insulin sensitivity of adipose tissue, skeletal muscle, or liver, nor did it affect inflammatory markers, plasma lipids, resting metabolic rates, or putative molecular targets (PPARGC1A, NAMPT, SIRT1, and AMPK). This indicates that resveratrol does not have positive metabolic effects in non-obese postmenopausal women with NGT.
Movahed et al. [33] confirmed the results obtained by Bhatt et al. [31] in 2013, showing that a single oral dose of resveratrol (1 g/day) for 45 days reduced fasting glycaemia levels, HbA1c, systolic blood pressure, insulin resistance, and increased HDL levels without affecting markers of renal and hepatic function. Two clinical studies conducted in 2014 also demonstrated the anti-diabetic activity of resveratrol. A three-month treatment of this flavonoid (50 mg) three times a day reduced BMI, weight, waist circumference (WC), fat mass, total insulin secretion, and area under the curve (AUC) of insulin [34]. Another study showed that single-dose supplementation with resveratrol (300 mg) attenuated post-absorption insulin concentrations with elevated p38 MAPK phosphorylation in skeletal muscle, but without any change in insulin signaling from adipose tissue or skeletal muscle [35]. Chen et al. [36]conducted a double-blind, randomized clinical study in 60 non-alcoholic subjects with fatty liver disease (FLD), showing that a three-month treatment with resveratrol (2 capsules, 150 mg) twice per day improved levels of LDL cholesterol, total cholesterol, blood glucose, transaminases, and HOMA-IR index, and augmented the level of adiponectin, with decreased TNF-α, FGF21, and cytokeratin 18 (CK-18) fragment levels. However, Thazhath et al. [37] found that supplementation with resveratrol (500 mg) twice a day for five weeks did not influence fasting and postprandial glycaemia, HbA1c, or total plasma GLP-1 in fourteen participants with T2D.
In a randomized, placebo-controlled study involving 34 subjects with polycystic ovary syndrome, a three-month oral treatment with 1500 mg/day of resveratrol resulted in a significant decrease (31.8 %) in fasting insulin levels and a significant increase (66.3 %) in the insulin sensitivity index. However, no effect was observed on endothelial function or inflammatory markers [38]. In contrast, a combination of resveratrol (80 mg) and epigallocatechin-3-gallate (EGCG) (282 mg) did not improve insulin-stimulated glucose disposal or endogenous glucose production inhibition in 38 obese and overweight subjects after 12 weeks of supplementation. Nonetheless, the combination was shown to enhance the oxidative ability of permeabilized muscle fibers [39]. These findings were consistent with those of Timmers et al. [40], who observed no improvement in hepatic or peripheral insulin sensitivity following one month of oral administration of resveratrol (150 mg/day). Additionally, a randomized, double-blind, clinical trial conducted with middle-aged men suffering from metabolic syndrome showed that daily intake of resveratrol (150 and 1000 mg) for 16 weeks did not improve glucose homeostasis, inflammatory status, or hepatic lipid content [41]. Finally, in a study involving 45 overweight or slightly obese volunteers receiving trans-resveratrol or placebo capsules for 4 weeks, separated by a washout period of at least 4 weeks, no changes were noted in fasting or postprandial inflammation, endothelial function, or plasma biomarkers following trans-resveratrol supplementation at a dose of 150 mg/day [42].
In 2018, a study investigated the effect of resveratrol on metabolic health in men at high risk of developing T2DM. After administering a daily oral dose of 150 mg for one month, this treatment did not improve insulin sensitivity but enhanced muscle mitochondrial function on a fatty acid-derived substrate [43].
More recently, a single-blind randomized clinical trial evaluated the effects of resveratrol on various parameters in 472 elderly type 2 diabetic patients. After six months of daily oral treatment with resveratrol, beneficial effects were observed, including improved insulin resistance, blood glucose parameters (reduced G6Pase and HbA1c), kidney function, inflammation (decreased pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α), and lipid profile (reduced triglycerides, total cholesterol, and HDL cholesterol) [44].
Another randomized clinical trial in 110 diabetic patients evaluated the effect of daily supplementation with resveratrol (200 mg) on glucose homeostasis and inflammation. After 24 weeks of treatment, significant improvements in glycaemia, insulin synthesis and resistance, TNF-α, and IL-6 levels were achieved [45].
2.2. Catechin
In a study conducted by Nagao et al. [46], the effects of green tea rich in catechins were investigated in patients with T2DM who were not receiving insulin therapy. The study involved a 12-week daily treatment with a beverage containing either 582.8 or 96.3 mg of catechins, which resulted in increased insulin and adiponectin secretion, but had no significant effect on glucose and HbA1c levels. These findings suggest that the consumption of catechin-rich foods by diabetics who are not on insulin therapy could potentially help prevent obesity, maintain low HbA1c levels, and improve insulin secretion capacity.
Similarly, Takahashi et al. [47] examined the effects of catechin-rich green tea on oxidative stress and postprandial hyperglycemia in healthy postmenopausal women. The study involved daily ingestion of catechin-rich green tea (615 mg/350 mL) for 4 weeks, which significantly improved redox homeostasis and postprandial glycemic status.
2.3. Epicatechin
A randomized, placebo-controlled study conducted by Dower et al. [50] aimed to evaluate the impact of pure epicatechin on the cardiometabolic health and vascular function of 37 healthy individuals, both male and female. After four weeks of treatment with 100 mg/day of pure epicatechin, a significant improvement in insulin resistance and fasting plasma insulin was observed, while fasting plasma glucose levels remained unaffected. However, no significant changes were detected in other health-related parameters such as NO levels, blood pressure, blood lipid profile, and flow-mediated dilation.
2.4. Quercetin
The study conducted by Dower et al. [50] also investigated the effects of daily supplementation with quercetin-3-glucoside (160 mg) on cardiovascular disease risk factors in 37 healthy individuals. The results revealed that four weeks of quercetin-3-glucoside supplementation did not lead to any significant changes in insulin resistance, flow-mediated dilation, or other cardiovascular disease risk factors.
2.5. Rutin
Several experiments, both in vitro and in vivo, have demonstrated the anti-diabetic potential of rutin. In line with these findings, clinical investigations have confirmed the efficacy of this flavonoid in treating diabetes. For instance, a placebo-controlled trial conducted on 50 individuals with T2DM showed that a 3-month administration of rutin (500 mg/day) significantly reduced fasting blood glucose, insulin, HbA1c, and insulin resistance levels [48]. Moreover, a recent study on 34 healthy adult participants who received rutin showed a decrease in postprandial glycaemia [49].
2.6. Hesperidin and diosmin
Experimental studies, both in vivo and in vitro, have demonstrated the anti-diabetic effects of hesperidin and diosmin. Recently, a randomized controlled trial was conducted to investigate the efficacy of these two flavones, administered alone or in combination (1 g/day for each), on 127 diabetic patients with neuropathy and metabolic syndrome (MetS). After 12 weeks of treatment, the separate administration of these flavones improved glycaemia, LDL, and triglyceride levels, with a greater magnitude of improvement observed when combined [52].
3. In vivo and in vitro anti-diabetic potential of flavonoids: mechanism insights
Flavonoids extracted and isolated from natural sources, particularly medicinal plants, showcase significant antidiabetic properties. Various in vitro and in silico studies, employing diverse experimental approaches, have been conducted. Table 2, Table 3 provide a comprehensive summary of previous research investigating the effects of flavonoids. In the subsequent section, we will elucidate the antidiabetic actions of each specific natural flavonoid.
Table 2.
In vitro/in silico anti-diabetic potential of flavonoids.
| Flavonoids | References | Models | Mechanisms |
|---|---|---|---|
| Apigenin | [53,54] | Assessment of high glucose (HG) and tumor necrosis factor α (TNFα) in endothelial cells (ECs) | Reduced the expression of glucose-induced LOX-1 and TNF-α, thereby preventing diabetes complications and mitigating their risk and severity |
| [55] | α-glucosidase assay | Inhibited α-glucosidase activity in a non-competitive manner through a monophasic kinetic process | |
| [56] | α-amylase assay | Inhibited Human and Aspergillus oryzae α-amylase activities in a competitive manner | |
| Arbutin | [57] | α-glucosidase and α-amylase assays | Inhibited (dose-dependently) α-amylase (81 %) and α-glucosidase (75 %) activity |
| [58] | L6 skeletal muscle cell line | Inhibited t-BHP-induced ROS generation Increased glucose uptake |
|
| Baicalin | [59] | Skeletal muscles of mice Myotubes of C2C12 cells |
Decreased NT-PGC-1α levels Enhanced GLUT4, PGC-1α, pP38MAPK, pAKT and pAS160 contents Increased GLUT4 mRNA, PGC-1α mRNA, PPARγ mRNA, GLUT1 mRNA expression |
| [60] | 3T3-L1 cells Adipocytes of DIO mice |
Decreased HOMA-IR and p-p38 MAPK and pERK levels Enhanced pAKT and PGC-1α contents Increased GLUT4 mRNA, PGC-1α mRNA expression Increased GLUT4 concentration in plasma membranes of adipocytes |
|
| [61] | Hepatocytes of high-fat diet (HFD)-induced obese mice | Decreased HOMA-IR Suppressed p-p38 MAPK, p-CREB, FoxO1, PGC-1α, PEPCK and G6Pase expression in liver of obese mice and hepatocytes Inhibited gluconeogenic genes by p38MAPK inhibitor in hepatocytes |
|
| [62] | Skeletal muscle and L6 myotubes | Elevated the levels of PGC-1α, GLUT4, p-p38MAPK, p-AKT and p-AS160 in skeletal muscle of obese mice Augmented the activity of PGC1α-GLUT4 axis in myotubes through activation of p38MAPK and AKT pathways |
|
| [63] | Insulin-resistant (IR)-HepG2 cells | Down-regulated IRS/PI3K/Akt signaling pathway Reduced GLUT4 expression and enhanced GSK-3β activity |
|
| Catechin | [64] | Human intestinal epithelial Caco-2 cells | Inhibited the intestinal absorption of glucose |
| [65] | α-glucosidase assay | Inhibited enzyme activity | |
| [66] | Hepa 1–6, L6 myoblasts, and 3T3-L1 | Activated, in combination with a gallocatechin moiety, LKB1/AMPK signaling pathway | |
| [67] | Inhibition of mammalian carbohydrate-degrading enzymes: - Rat intestinal maltase - Rabbit glycogen phosphorylase (GP) b |
EGCG, catechin 3-gallate (CG), gallocatechin 3-gallate (GCG), and epicatechin 3-gallate (ECG), were good inhibitors of maltase, with IC50 values of 16, 62, 67, and 40 μM, respectively GCG, ECG, EGCG, and CG inhibited GP b, with IC50 values of 6.3, 27, 34, and 35 μM, respectively |
|
| [68] | α-glucosidase and α-amylase assays | Inhibited α-glucosidase (IC50 = 31 μg/mL) Inhibited α-amylase (IC50 = 160 ± 6 μg/mL) |
|
| [69] | α-amylase assay | Inhibited α-amylase (IC50 = 637.5 ± 7.81 μmol/L) | |
| [70] | α-glucosidase assay | Inhibited α-glucosidase Accessed the active site of the α-glucosidase enzyme and bound to catalytic amino acid residues |
|
| [71] | In silico (software analysis) | Presented an optimal combination | |
| [72] | α-glycosidase assay | Inhibited the activity of α-glycosidase enzyme | |
| Cyanidin | [73] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 19.7 ± 0.24 μM) |
| [74] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 0.50 ± 0.05 mM) Synergistically inhibited intestinal α-glucosidase |
|
| [75] | Intestinal sucrose α-amylase assay |
Cyanidin-3-glucoside + Cyanidin-3-galactoside: Inhibited intestinal sucrase (IC50 = 0.50 ± 0.05 mM) Inhibited pancreatic α-amylase (IC50 = 0.30 ± 0.01 mM) |
|
| [76] | Intestinal maltase and sucrase | Inhibited intestinal maltase (IC50 = 2.323 ± 14.8 μM) Inhibited intestinal sucrase (IC50 = 250.2 ± 8.1 μM) |
|
| [77] | Adipocytes, 3T3-L1 cells | Decreased TNF-α concentration Activated adipocyte differentiation and insulin signaling |
|
| [78] | Human 3T3-L1 cells and omental adipocytes | Increased adipocyte glucose absorption Increased PPARγ activity Increased adiponectin production |
|
| [79] | Pancreatic β-cells, MIN6N | Decreased DNA fragmentation Reduced ROS generation Increased insulin secretion Prevented cell apoptosis |
|
| [80] | Pancreatic β-cells, MIN6N | Prevented oxidative stress-induced cell apoptosis Reduced H2O2-induced cell death |
|
| [81] | 3T3-Ll cells | Induced differentiation into smaller adipocytes Reduced TNF-α production Activated insulin signaling Enhanced glucose absorption Induced insulin-sensitive adipocytes |
|
| [82] | Hela cells and murine hepatocytes | Activated AMPK signaling pathway by suppressing its downstream kinase Improved GT |
|
| [83] | α-glucosidase and α-amylase assays | Inhibited α-amylase activity (IC50 = 7.5 μM) Inhibited α-glucosidase activity (IC50 = 13.72 μM) |
|
| [84] | 3T3-L1 adipocytes | Increased glucose absorption Enhanced GLUT4 membrane expression Enhanced phosphorylation of IRS-1 and Akt |
|
| [85] | Pancreatic INS-1 β-cells | Increased insulin synthesis Increased intracellular Ca2+ signals |
|
| [86] | α-glucosidase and DPP-4 assays | Inhibited α-glucosidase (IC50 = 479.8 μM) Inhibited DPP-4 (IC50 = 125.1 μM) |
|
| [87] | α-glucosidase assay | Inhibited α-glucosidase activity (IC50 = 22.7 ± 7.1 μmol/L) | |
| [88] | Human hepatocellular carcinoma cell HepG2 | Activated the AMPK pathway | |
| [89] | Liver HepG2 and L02 cells | Promoted glucose consumption via the regulation of the Wnt/β-catenin-WISP1 pathway | |
| [90] | Pancreatic cells (INS-1 cells) | Enhanced insulin synthesis by intracellular Ca2+ signalling Activated the PLC-IP3 pathway and the voltage-dependent Ca2+ channel |
|
| Delphinidin | [91] | Pancreatic cells (INS-1832/13) | Stimulated insulin synthesis |
| [92] | L6 myotubes | Increased the absorption of glucose | |
| [93] | Mouse jejunum samples and human intestinal cells (HT-29, Caco-2, and NCM460) | Decreased glucose uptake Affected the function of SGLT1 |
|
| [94] | α-amylase, α-glucosidase, and DPP-4 inhibition assays Glucose uptake in vitro |
Inhibited α-glucosidase (44.5 %) Inhibited α-amylase (24.2 %) Inhibited DPP-4 (78.8 %) Reduced glucose absorption (37.1 %) |
|
| [95] | Pancreatic RIN-m5F β-cells | Decreased cleaved caspase-3 level, adverse effects of oxidative stress, and apoptosis caused by high glucose concentrations Enhanced AMPKα Thr172 level phosphorylation |
|
| Epicatechin | [96] | Islets of Langerhans | Stimulated the secretion of insulin |
| [97] | Muscle, fat, and liver cells | Increased glycogen content, oxygen and insulin uptake | |
| [98] | Islets of Langerhans | Stimulated the conversion of (pro) insulin into insulin as well as its release | |
| [99] | Islets isolation | No impact on insulin release | |
| [100] | Ins-1E cells | Increased insulin synthesis Protected β-cells |
|
| [101] | L6 myoblasts | Promoted glucose absorption and GLUT4 translocation Activated PI3K signaling |
|
| [102] | HepG2 cells | (−)-epicatechin + β-glucan exhibited a synergistic effect on the Akt pathway | |
| [71] | In silico (software analysis) | Presented an optimal combination | |
| Hesperetin | [103] | 3T3-L1 cells | Inhibited the production of free fatty acids (FFA) stimulated by TNF-α Inactivated NF-κB and ERK pathways |
| [104] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 0.38 ± 0.05 mM) activity K slope = 0.23 ± 0.01 mM |
|
| Hesperidin | [105] | RAW 264.7 cells 3T3-L1 preadipocytes |
Normalized inflammation-induced insulin resistance (RAW 264.7 cells) Inhibited TNF-α-induced synthesis of interleukin-6 (IL-6) and prostaglandin E2 (PGE2) (3T3-L1 cells) |
| Kaempferol | [106] | Mature 3T3-L1 adipocytes | Enhanced insulin-stimulated glucose absorption |
| [107] | HIT-T15 cells | Protected HIT-T15 cells via interference with ROS metabolism | |
| [108] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 19.36 ± 2.43 μM) activity | |
| [109] | INS-1E cells Human pancreatic islets |
Protected β-cells and pancreatic human islets Enhanced insulin synthesis and secretory function |
|
| [110] | INS-1E cells Human pancreatic islets |
Inhibited cell apoptosis Decreased caspase-3 activity Improved insulin synthesis |
|
| [111] | Pancreatic cells, MIN6 | Improved the proliferation of β-cells | |
| [112] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 1.16 ± 0.04 × 10−5 mol/L) activity | |
| [113] | α-glucosidase and α-amylase assays | Inhibited α-amylase activity (IC50 = 51.24 μg/mL) Inhibited α-glucosidase activity (IC50 = 29.37 μg/mL) |
|
| [114] | α-glucosidase and α-amylase assays | Non-competitive α-glucosidase inhibition Competitive α-amylase inhibition |
|
| [115] | RIN-5F cells Pancreatic islets |
Increased anti-apoptotic activity and cell viability Stimulated autophagy Restored β-cell dysfunction |
|
| [116] | α-glucosidase and α-amylase assays | Inhibited the activity of α-amylase and α-glucosidase | |
| [115] | RIN-5F cell line | Restored β-cell dysfunction | |
| Luteolin | [117] | α-glucosidase and α-amylase assays | Blocked α-glucosidase activity (36 % at 0.5 mg/mL) (stronger than acarbose) Blocked α-amylase activity (less potent than acarbose) |
| [118] | Maltase, sucrose, and α-glucosidase activities | Blocked maltase activity (IC50 = 2.3 mM) At doses of 100 and 200 mg/kg: No effect was observed on other enzymes |
|
| [119] | 3T3-L1 adipocytes | Decreased TNF-α and IL-6 mRNA levels Increased glucose uptake response to insulin stimulation Enhanced Akt2 phosphorylation and PPARγ transcriptional activity |
|
| [120] | Endothelial cells | Increased insulin-dependent nitric oxid production | |
| [121] | Protein tyrosine phosphatase 1B (PTP1B) assay Aldose reductase (AR) assay |
Inhibited AR activities and the PTP1B effect | |
| [122] | MIN6 cells | Inhibited NF-κB activity Decreased NO production Stimulated insulin secretion |
|
| [123] | α-glucosidase assay | Blocked α-glucosidase (IC50 = 1.72 ± 0.05 × 10−4 mol/L) activity A single site of inhibition on the enzyme Ki = 1.40 ± 0.02 × 10−4 mol/L |
|
| [124] | 3T3-L1 cells and RAW264.7 macrophages | Suppressed macrophage cell infiltration | |
| Malvidin-3-O-glucoside | [125] | Caco-2 cells | Reduced the absorption of14C fructose (15 % for the highest concentration) |
| [94] | α-glucosidase, α-amylase, and DPP-4 assays Caco-2 cells Glucose absorption (in vitro) |
Inhibited α-amylase (29.6 %) activity Inhibited α-glucosidase (42.8 %) activity Inhibited DPP-4 (82.4 %) activity Decreased glucose absorption (55.2 %) |
|
| [126] | α-glucosidase, α-amylase, and DPP-4 assays Caco-2 cells |
Inhibited α-amylase (29.6 %) activity Inhibited α-glucosidase (42.8 %) activity Inhibited DPP-4 (82.4 %) activity |
|
| [127] | α-glucosidase and α-amylase assays | Inhibited α-glucosidase activity (IC50 = 55 μg/mL) | |
| [128] | α-glucosidase assay | Inhibited α-glucosidase activity in a reversible non-competitive way | |
| Myricetin | [129] | Adipocytes | Enhanced the insulin stimulatory effect Stimulated lipogenesis and uptake of both D-3-O-methyl-glucose and d-glucose Increased the Vmax of glucose transport |
| [130] | Rat adipocytes | Increased insulin-stimulated lipogenesis Stimulated glucose transport |
|
| [131] | Rat adipocytes | Inhibited glucose transport and the uptake of methylglucose | |
| [132] | C2C12 cells | Increased glucose absorption with AMPK and Akt activities Reduced insulin resistance |
|
| [133] | α-amylase and α-glucosidase inhibition assays 3T3-L1 cells |
Inhibited both α-glucosidase and α-amylase activities Enhanced glucose absorption Activated insulin-signaling pathway |
|
| [134] | α-glucosidase and α-amylase assays | Inhibited α-amylase (IC50 = 662 μg/mL) activity (reversible and competitive) Inhibited α-glucosidase (IC50 = 3 μg/mL) activity (reversible but non-competitive) |
|
| [135] | HepG2 cell line | Increased β-endorphin (BER) and adropin secretion Activated the Glucagon-like peptide-1 (GLP-1) receptor that modulates adropin expression |
|
| [136] | RAW 264.7 cells | Inhibited the expression levels of IFN-γ and IL-2 | |
| Naringenin | [137] | Preadipocytes | Stimulated glucose absorption (163 %) |
| [138] | α-glucosidase and 11β-HSD1 assay | Inhibited 11β-HSD1 activity (39.49 %) | |
| [103] | 3T3-L1 cells | Inhibited NF-κB and ERK pathway activation induced by TNF-α as well as the synthesis of Free Fatty Acids (FFA) induced by TNF-α | |
| [139] | L6 rat myotubes | Increased glucose absorption | |
| [140] | INS-1E cells | Induced glucose sensitivity Stimulated insulin synthesis Modified gene expression profiles |
|
| [141] | Porcine myotube cultures | Increased the phosphorylation of TBC1D1 by increasing translocation of GLUT4 and absorption of glucose | |
| [142] | α-glucosidase assay | Decreased postprandial glycaemia levels (in vitro) | |
| [143] | Molecular docking | Exhibited high binding affinity towards GLUT4 and PPARγ | |
| [144] | α-glucosidase assay | Exhibited an up-regulation of PPARγ receptors Exhibited potent anti-α-glucosidase activity |
|
| Naringin | [145] | Differentiated L6 myoblasts | Increased glucose absorption |
| [146] | RIN-5F cells | Prevented pancreatic β-cell dysfunction Reduced inhibition of insulin secretion |
|
| [147] | HepG2 cells | Stimulated glucose uptake independently of insulin stimulation Increased glucose uptake by inducing AMPK phosphorylation Bound to AMPK γ-subunit |
|
| Quercetin | [106] | Mature 3T3-L1 adipocytes | Improved insulin-stimulated glucose absorption |
| [148] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 0.017 mmol × L−1) activity | |
| [149] | C2C12 muscle cells | Improved glucose uptake, by stimulating AMPK pathway | |
| [150] | 11β-HSD1 assay | Inhibited 11β-HSD1 | |
| [151] | Embryonic fibroblasts | Prevented insulin sensitivity impairment | |
| [152] | INS-1 cells | Enhanced insulin secretion Increased β-cell function |
|
| [153] | C2C12 skeletal muscle cells | Improved insulin sensitivity Improved glucose absorption |
|
| [154] | L6 myoblasts | Reduced ROS production Normalized the level of GSH Increased glucose uptake via GLUT 4 translocation |
|
| [155] | H4IIE hepatocytes | Inhibited G6pase Activated the hepatic AMPK pathway |
|
| [134] | α-glucosidase and α-amylase assays | Inhibited α-amylase (IC50 = 770 μg/mL) activity (reversible and competitive) Inhibited α-glucosidase (IC50 = 32 μg/mL) activity (reversible but non-competitive) |
|
| [156] | L6 myoblasts | Involvement of the AMPK pathway and p38 MAPK in the uptake of 2-NBDG | |
| [157] | INS1 cells | Increased the levels of Sirtuin 3 (Sirt3), Catalase (CAT), and Superoxide Dismutase (SOD) | |
| [158] | α-glycosidase assay | Inhibited α-glycosidase activity | |
| [159] | α-amylase assay | Inhibited α-amylase (IC50 = 0.325 mg/mL) activity in a non-competitive way | |
| Quercitrin | [160] | Rat insulinoma (RINm5F) cells | Protected β-cells against cytokine-induced damage Improved glucose-stimulated insulin secretion (GSIS) Inhibited NF-κB translocation |
| Isoquercitrin | [161] | NCI–H716 cells | Stimulated GLP-1 production Inhibited DPP-4 competitively (IC50 = 96.8 mM) Ki = 236 mM |
| Rutin | [148] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 0.196 mmol × L−1) activity |
| [162] | Isolated soleus muscles from rats | Stimulated the uptake of14C glucose in diabetic rat soleus muscle | |
| [154] | L6 myoblasts | Increased glucose uptake, which was related to the translocation of GLUT4 | |
| [163] | C2C12 cells | Enhanced insulin receptor kinase (IRK) activity | |
| [164] | α-glucosidase and α-amylase assays | Blocked α-amylase (IC50 = 0.043 μM) activity Blocked α-glucosidase (IC50 = 0.037 μM) activity |
|
| [165] | 3T3-L1 and C2C12 mouse cell lines | Down-regulated the expression of protein tyrosine phosphatase-1B (PTP-1B) | |
| [166] | Human Amylin (hA) | Suppressed hA aggregations causing apoptosis in pancreatic cells | |
| [71] | In silico (software analysis) | Presented an optimal combination | |
| Strictinin ellagitannin | [167] | α-glucosidase assay | Inhibited α-glucosidase (IC50 = 2.4 μg/mL) activity |
| Petunidin | [91] | Pancreatic cells, INS-1832/13 | Increased insulin secretion |
| [168] |
In vitro (α-amylase assay) In silico (docking study) |
Inhibition enzyme activity Slowed glycaemia release |
Abbreviations 11β-HSD1: 11-beta-hydroxysteroid dehydrogenase type 1; AMPK pathway: Adenosine Monophosphate-activated Protein Kinase; AMPKα Thr172: Adenosine Monophosphate-activated Protein Kinase Alpha Threonine 172; BER: β-endorphin; CAT: Catalase; CG: Catechin 3-Gallate; DPP-4: Dipeptidyl peptidase-4; ECG: EpiCatechin 3-Gallate; EGCG: EpiGalloCatechin-3-Gallate; ERK: Extracellular Signal-regulated Kinase; FoxO1: Forkhead Box O1; FFA: Free Fatty Acids; GCG: GalloCatechin 3-Gallate; GLP-1: Glucagon-like peptide-1; GSIS: Glucose-Stimulated Insulin Secretion; Glucose Transporter 4: GLUT4; Glutathione: GSH; GP: Glycogen Phosphorylase; HG: High Glucose; hA: Human Amylin; IRK: Insulin Receptor Kinase; IRS-1: Insulin Receptor Substrate 1; IRS/PI3K/Akt: Insulin Receptor Substrate/Phosphoinositide 3-kinase/Akt; IFN-γ: Interferon gamma; IL: Interleukin; LOX-1: Lectin-like Oxidized low-density lipoprotein receptor-1; mRNA: messenger RNA; NF-κB: Nuclear Factor kappa B; NT-PGC-1α: N-terminal fragment of Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha; PPARγ: Peroxisome Proliferator-Activated Receptor Gamma; PPARγ: Peroxisome proliferator-activated receptor gamma; PEPCK: Phosphoenolpyruvate Carboxykinase; PLC-IP3 pathway: Phospholipase C-Inositol trisphosphate pathway; pAKT: Phosphorylated AKT (Protein Kinase B); pAS160: Phosphorylated AS160 (Akt Substrate of 160 kDa); p-CREB: Phosphorylated cAMP Response Element-Binding protein; p-P38MAPK: Phosphorylated P38 Mitogen-Activated Protein Kinase; PGE2: Prostaglandin E2; PTP1B: Protein Tyrosine Phosphatase 1B; PTP-1B: Protein Tyrosine Phosphatase-1B; ROS: Reactive Oxygen Species; SGLT1: Sodium/Glucose coTransporter 1; Sirt3: Sirtuin 3; SOD: Superoxide Dismutase; TBC1D 1: Tre-2/BUB2/CDC16 domain family member 1.
Table 3.
In vivo anti-diabetic potential of flavonoids.
| Flavonoids | References | Model | Mechanisms |
|---|---|---|---|
| Apigenin | [[169], [170], [171]] | Hyperglycemic rats | Stimulated insulin and glycogen synthesis Promoted glucose absorption Regulated key pathways involved in insulin signaling and glucose balance |
| [172,173] | Streptozotocin (STZ)-induced diabetic rats (IDR) | Stimulated insulin production Protected pancreatic β-cells Reduced hepatic G6Pase activity |
|
| [174] | STZ-IDR | Preserved pancreatic β-cells Promoted the translocation of GLUT4 to the cell membrane Decreased CD38 expression |
|
| [175] | High fat diet (HFD)-induced obese mice (IOM) | Apigenin improves metabolic disturbances by lowering fasting blood sugar and plasma insulin levels. Apigenin inhibits the inflammatory response mediated by NF-κB. | |
| [176] | HFD/STZ-IDR | Decreased insulin resistance and glycaemia content | |
| [177] | STZ-IDR | Decreased ROS levels Restored β-cell apoptosis |
|
| [178] | STZ-IDR | Improved biochemical parameters Repaired destroyed renal and hepatic architecture |
|
| Arbutin | [179] | Fasting and healthy dogs | Reduced glycaemia level |
| [180] | Alloxan (ALX)-IDR | Decreased insulin and serum glucose concentrations | |
| [181] | ALX-IDR | Increased glucagon-like peptide 1 (GLP-1) and GLP1R levels | |
| [182] | STZ-induced diabetic mice (IDM) | Apigenin inhibited increased blood sugar levels and prevented body weight loss. Apigenin activated antioxidant enzymes such as SOD, CAT, and GPX. |
|
| Baicalin | [59] | Diet-induced obese (DIO) mice | Decreased food intake and body weight Reversed high fat diet-induced glucose and insulin intolerance, hyperglycemia and insulin resistance |
| [60] | DIO mice | Decreased food intake and body weight Reversed HFD-induced glucose intolerance, hyperglycemia, and insulin resistance | |
| [61] | HFD-induced obese mice | Decreased body weight Alleviated HFD-induced glucose intolerance, hyperglycemia, and insulin resistance |
|
| [62] | Obese mice | Decreased hyperglycemia and insulin resistance Augmented glucose consumption |
|
| [63] | HFD-induced obese and pre-diabetic mice | Damaged the abilities of glycogen synthesis and glucose uptake Ameliorated hyperglycemia and dyslipidemia |
|
| Catechin | [183] | Saccharide-dosed rats | Increased insulin activity Inhibited intestinal sucrose and α-amylase activity |
| [184] | T2D rats | Improved glucose tolerance (GT) and oxidative status | |
| [65] | Rats receiving an oral dose of maltose (2 g/kg) | Reduced glycaemia | |
| [185] | Normal rats | Epigallocatechin-3-gallate (EGCG) increased glycaemia Reduced insulin-stimulated glucose absorption |
|
| [186] | STZ-IDM | Reduced glycaemia level Augmented tissue glycogen Enhanced GLUT4 mRNA |
|
| [187] | HFD-IDM | Stimulated insulin secretion | |
| [188] | HFD-IDM | Reduced the expression of certain markers of insulin resistance (IR-β and GLUT4) | |
| [189] | STZ-IDR | Activated insulin receptor (IR) Improved GT |
|
| [190] | STZ-IDM | Decreased glycaemia Protected against oxidative damage |
|
| [71] | ALX- IDM | Prevented hyperglycemia and hypoglycaemia | |
| Cyanidin | [191] | HFD-fed mice | Cyanidin exhibited beneficial effects on hyperinsulinemia, hyperglycemia, hyperleptinemia, and insulin sensitivity. It also reduced insulin resistance and the expression of TNF-α mRNA |
| [192] | Rats fed cyanidin-rich diets | Cyanidin decreased glycaemia and the expression levels of the G6Pase gene. It also increased insulin sensitivity, up-regulated GLUT4, and down-regulated RBP4. | |
| [193] | Diabetic BALB/c mice | Decreased glycated albumin (GA) (46.00 ± 2.50 %) Decreased glycated hemoglobin (HbA1c) (4.95 ± 0.20 %) |
|
| [81] | db/db mice | Activated insulin signaling Enhanced glucose absorption Induced insulin-sensitive adipocytes |
|
| [82] | Normal and obese mice | Increased sensitivity to insulin | |
| [194] | HFD-fed mice | Reduced resistance to insulin Enhanced sensitivity to insulin |
|
| [88] | HFD-fed mice | Inhibited gluconeogenesis | |
| [89] | Diabetic db/db mice | Up-regulated the expression of hepatic GLUT-1 | |
| Delphinidin | [195] | Diabetic C57b1/6J mice | No significant hypoglycaemic activity |
| [92] | Hyperglycemic obese mice | Lowered glucose production in hepatic cells Reduced fasting glycaemia levels |
|
| [193] | BALB/c mice | Reduced GA (30.50 ± 3.46 %) Decreased HbA1c (3.60 ± 0.25 %) |
|
| [194] | HFD-fed mice | Improved insulin sensitivity Decreased insulin resistance |
|
| Epicatechin | [196] | ALX-IDR | Decreased glycaemia Protected cells |
| [197] | ALX-IDR | Decreased glycaemia Regenerated cells |
|
| [198] | ALX-IDR | Decreased glycaemia | |
| [199] | STZ-IDR | Failed to reverse DM Failed to halt disease progression |
|
| [99] | STZ-induced β-cell damage Islets isolation |
Normalized glycaemia concentrations No impact on insulin release |
|
| [200] | HFD-fed mice | Reduced insulin resistance Enhanced insulin signaling pathway Decreased endoplasmic reticulum stress |
|
| [201] | HFD-fed mice | Decreased glycaemia and insulin contents Augmented blood leptin contents |
|
| [202] | HFD-fed mice | Improved sensitivity to insulin Decreased glycaemia |
|
| [203] | Nicotinamide (NA)/STZ-IDR | Improved insulin resistance and mRNA expression of GLUT4 Epicatechin + gallic acid improved the previous indices |
|
| [102] | Male Kunming mice | (−)-epicatechin + β-glucan exhibited a synergistic effect on the Akt pathway, subsequently enhancing glucose uptake | |
| [71] | ALX-IDM | Prevented hyperglycemia and hypoglycaemia | |
| Hesperetin | [204] | T2D Goto-Kakizaki (GK) rats | Normalized glucose-regulating enzyme activities Reduced serum and liver lipid levels |
| [205] | STZ-IDR | Improved glycaemia Reduced plasma glucose levels |
|
| [206] | STZ-IDR | Improved glycaemia and insulin levels Inhibited insulin resistance development Inhibited enzymes implicated in glucose metabolism |
|
| [207] | STZ-IDR | Improved plasma insulin and glycogen levels | |
| [208] | ALX-IDM | Restored glycaemia levels | |
| [209] | NA/STZ-IDR | Improved glucagon, serum glucose, and insulin Decreased activities of G6PD, glucose-6-phosphate (G6P), and fructose-1,6-bisphosphate (FBP) |
|
| Hesperidin | [210] | HFD-fed rats | Decreased blood glucose and G6Pase activities Increased glycogen concentration and plasma insulin |
| [204] | T2D GK rats | Normalized glucose-regulating enzyme activities Reduced serum and liver lipid levels |
|
| [211] | STZ-IDM | Decreased maternal glycaemia level | |
| [212] | STZ-induced marginal T1D rats | Decreased blood glucose Altered glucose-regulating enzyme activity |
|
| [213] | HFD/STZ-IDR | Increased serum insulin concentrations Decreased TNF-α expression |
|
| [214] | STZ-IDR | Normalized HbA1c, glucose, serum insulin, hepatic and muscle glycogen levels | |
| [215] | STZ-IDR | Decreased pancreatic cell degeneration Increased insulin concentrations |
|
| [216] | STZ-IDR | Decreased HbA1c, fructose-1,6-bisphosphatase (FBPase), and G6Pase Improved hepatic glycogen |
|
| [217] | HFD-IOM | Long-term daily treatment (11 weeks): Reduced glycaemia concentration Improved insulin resistance and glucose intolerance |
|
| [218] | HFD/ALX-induced insulin resistance | Improved fasting glycaemia Prevented impaired GT (IGT) development Regulated gluconeogenesis and glycolysis |
|
| [219] | STZ-IDR | Improved the levels of glycaemia, HbA1c, insulin, and lipid profile | |
| Kaempferol | [220] | ALX-IDR | Reduced hyperglycaemia |
| [221] | ALX-IDR (soleus muscle) | Increased muscle glycogen content Stimulated glucose absorption |
|
| [222] | Soleus muscle of male Wistar rats | Promoted glycogen synthesis | |
| [223] | Male Sprague–Dawley rats | Increased the KM Phlorizin + kaempferol 3-O-α-rhamnoside showed an additive inhibitory power on glucose intestinal absorption (GIA) |
|
| [224] | T2D KK-Ay mice | Decreased HbA1c and fasting glycaemia levels | |
| [225] | STZ-IDR | Reduced insulin resistance and fasting glycaemia Improved disorders related to glucose metabolism |
|
| [226] | HFD-IOM | Normalized the hyper-insulinemia, hyper-glycemia Improved insulin sensitivity Inhibited glycogen production and glucose uptake |
|
| [227] | HFD/STZ-IDR | Improved insulin resistance Reduced TNF-α and IL-6 levels |
|
| [228] | HFD-fed mice | Decreased HbA1c and fasting glycaemia levels Improved insulin resistance |
|
| [229] | STZ-IDM | Decreased hyperglycemia Decreased diabetes incidence Decreased liver glucose production Inhibited gluconeogenesis |
|
| [230] | HFD-IOM | Decreased diabetes incidence Decreased hyperglycemia and liver glucose production Improved gluconeogenesis |
|
| [231] | STZ-IDR | Reduced fasting glycaemia levels Increased fasting insulin levels |
|
| [232] | STZ-IDR | Kaempferol + myricetin normalized insulin and glucose levels, inflammatory cytokines, as well as lipid and liver enzymes | |
| Luteolin | [233] | STZ-IDR | Decreased glycaemia contents Increased blood insulin contents |
| [118] | Glycaemia determination in an animal model | At doses of 100 and 200 mg/kg: No effect was observed on glycaemia or other enzymes |
|
| [234] | Diabetic KK-Ay mice | Normalized HbA1c, glycaemia, and insulin levels | |
| [124] | HFD-IOM | Improved insulin resistance | |
| [235] | Type 2 diabetes mellitus (T2DM) mice | Normalized fasting blood glucose, glycated serum protein, and pancreatic islet function index Restored the pancreas |
|
| [236] | T2DM mice | Normalized pancreatic and hepatic functions, the modulation of intestinal microbiota composition, and the regulation of the PPAR signaling | |
| [237] | STZ-IDM | Luteolin + diosmin normalized glycaemia, insulin, HbA1c, and glycogen levels | |
| Malvidin-3-O-glucoside | [195] | Diabetic mice | Exerted a significant anti-hyperglycemic activity |
| [238] | HFD/STZ-IDR | Malvidin + metformin improved glucose and lipid metabolisms with inhibition of inflammation | |
| Myricetin | [130] | STZ-IDM | Reduced hyperglycemia (50 %) Increased the content of G6Pase, hepatic glycogen and glycogen synthase |
| [239] | STZ-IDM | Decreased plasma glucose concentrations Stimulated glucose storage in rat soleus muscles Increased expression of GLUT 4 |
|
| [240] | STZ-IDM | Decreased plasma glucose level Increased GLUT 4 expression Decreased PEPCK expression in liver |
|
| [241] | Obese rats | Improved insulin sensitivity via an important post-receptor insulin signaling | |
| [242] | HFD-fed rats | Decreased glycaemia levels Increased sensitivity to insulin Enhanced insulin action |
|
| [243] | Insulin-resistant rats | Decreased plasma glucose levels | |
| [244] | STZ-IDR | Decreased plasma glucose and HbA1c contents Increased plasma insulin and total haemoglobin contents |
|
| [245] | Rats fed a HF/HS diet | Decreased insulin and glycaemia levels Decreased HOMA-IR values and pro-inflammatory cytokine (TNF-α and IL-6) levels |
|
| [246] | STZ/cadmium-induced diabetic nephrotoxic rats | Ameliorated the levels of glucose, HbA1c, GP, and gluconeogenic enzymes Increased glycogen, GS, insulin, and the expression of insulin signaling molecules Protected pancreas |
|
| [247] | db/db mice | Blocked α-glucosidase activity Reduced levels of HbA1c and fasting blood glucose |
|
| [248] | Wistar rats | Exhibited glucoregulatory activity | |
| [249] | db/db mice | Increased adiponectin expression in brown adipose tissue (BAT) Improved insulin resistance by activating BAT |
|
| [135] | T1D rats | Increased β-endorphin (BER) and adropin secretion Decreased hyperglycemia |
|
| [136] | HFD-fed prediabetic mice | Exerted a remarkable hypoglycemic and hypolipidemic effect | |
| [232] | STZ-IDR | Myricetin + kaempferol normalized insulin and glucose and rates, inflammatory cytokines, as well as lipid and liver enzymes | |
| Naringenin | [138] | Non-insulin-dependent DM (NIDDM) | Reduced plasma glucose |
| [250] | NA/STZ-IDR | Reduced the levels of fasting glycaemia and HbA1c Increased serum insulin levels Protected pancreas |
|
| [251] | NA/STZ-IDR | Attenuated hematological values, inflammation proteins, and mRNA transcription | |
| [142] | HFD/STZ-IDR | Inhibited α-glucosidase (in vivo) activity | |
| [252] | HFD/STZ-IDR | Attenuated hyperinsulinemia and hyperglycemia Increased insulin sensitivity Modulated GLUT4 and TNF-α expressions |
|
| [176] | STZ-IDR | Reduced glycaemia and insulin resistance index | |
| [253] | STZ-IDM | Reduced glycaemia and HbA1c | |
| [254] | TSOD mice | Decreased hypoglycemic action of pioglitazone No effect on fasting glycaemia level |
|
| [255] | NA/STZ-IDR | Normalized reduced serum insulin concentrations Elevated GP and G6Pase activities |
|
| [143] | STZ-IDR | Attenuated glycaemia levels | |
| [256] | Insulin-deficient diabetic (IDD) mice induced by STZ | Naringenin + phytoestrogen 8-prenylnaringenin improved glucose homeostasis, STZ-induced disturbances in islet function, and insulin signaling defects | |
| Naringin | [210] | T2D male mice (C57BL/KsJ-db/db) | Attenuated glycaemia level Increased glycogen content Augmented plasma insulin and C-peptide |
| [257] | STZ-IDR | Decreased glycaemia and HbA1c Augmented plasma insulin level Decreased G6Pase and FBPase activities |
|
| [258] | NA/STZ-IDR | Decreased glycaemia Increased insulin level Decreased HbA1c Reduced FBPase and G6Pase activities |
|
| [213] | HFD/STZ-IDR | Decreased glycaemia level Increased serum insulin level |
|
| [259] | HFD/STZ-IDR | Decreased hyper-insulinemia, hyper-glycemia, insulin resistance, and TNF-α Increased β-cell function Increased PPARγ expression |
|
| [214] | HFD/STZ-IDR | Lowered elevated levels of HbA1c, glucose, serum insulin, hepatic, and muscle glycogen | |
| [260] | STZ-IDR | Exhibited hypoglycemic effects requiring insulin | |
| [261] | STZ-IDR | Decreased glycaemia level | |
| [255] | NA/STZ-IDR | Enhanced expression of adiponectin, IR subunit, and GLUT4 mRNA Reduced levels of hepatic glycogen, serum insulin, HbA1c, and G6Pase |
|
| [262] | HFD/STZ-IDR | Reduced HbA1c and glycaemia levels Augmented plasma insulin content Enhanced activities of carbohydrate metabolism key enzymes |
|
| [263] | STZ-IDM | Normalized hyperglycemia and islet dysfunction Protected β-cell apoptosis |
|
| Quercetin | [264] | STZ-IDR | Lowered plasma glucose content Improved GT test Regenerated pancreatic islets |
| [265] | STZ-IDR | Ameliorated diabetic status (25 %) | |
| [266] | STZ-IDR | Protected β-cells Decreased the levels of MDA and nitric oxide (NO) Preserved islet β-cells |
|
| [267] | STZ-IDR | Lowered glycaemia level Augmented insulin level Protected pancreatic β-cell structure |
|
| [268] | ALX-IDR | Reduced glucose level Increased insulin level Inhibited G6Pase activity |
|
| [269] | ALX-IDR | Prevented the rise in glycaemia | |
| [270] | High fructose diet (HFruD)-fed rats | Improved tyrosine phosphorylation Improved insulin sensitivity |
|
| [271] | STZ-IDM | Lowered glycaemia level Improved plasma insulin level Recovered cell proliferation |
|
| [272] | STZ-IDR | Decreased glycaemia levels Increased antioxidant enzyme activities |
|
| [150] | NA/STZ-IDR | Decreased glycaemia level | |
| [151] | HFD-fed rats | Inhibited PPARγ expression | |
| [273] | STZ-IDR | Attenuated glycaemia levels Reduced resistance to insulin |
|
| [274] | STZ-IDR db/db mice | Decreased glycaemia level Reduced HbA1c and plasma glucose rates Reduced intestinal maltase activity |
|
| [275] | STZ-IDR | Normalized postprandial hyperglycemia | |
| [276] | NA/STZ-IDR | Increased glycaemia absorption Reduced the activity of glucose transport |
|
| [277] | db/db mice | Reduced plasma glucose rates Decreased HOMA-IR |
|
| [278] | STZ-IDR | Reduced glycaemia levels Augmented β-cell number |
|
| [279] | ALX-IDM | Affected (positively) DNA damage Reduced hyperglycemia and enzyme markers (hepatic and renal) Increased expression levels of GLUT4 |
|
| [280] | Rats fed a HF/HS diet | Reduced glycaemia, HOMA-IR, and insulin levels | |
| [281] | STZ-IDR | Improved serum glycaemia levels Enhanced insulin levels Maintained glucose metabolic enzyme activities Preserved β-cell structure |
|
| [282] | Fructose/STZ-IDR | Decreased the levels of glycaemia, hepatic glycogen, and HbA1c Improved the activities of hexokinase and G6Pase |
|
| [283] | STZ-IDR | Exerted remarkable anti-diabetic effects on hyperglycemia | |
| [284] | STZ-IDR | Quercetin + EGCG restored pancreatic NIT-1 β-cell damage, which subsequently improved insulin secretion | |
| [157] | Diabetic db/db mice | Reduced elevated glycaemia and insulin rates | |
| [285] | STZ-IDR | Decreased glycaemia level | |
| [209] | NA/STZ-IDR | Improved serum glucose, glucagon, insulin, hepatic glycogen, and α-amylase Decreased activities of G6PD, FBP, G6P, and glucokinase Improved levels of GLUT2 and GLUT4 |
|
| [286] | Hypertensive rats | Reduced serum lipid peroxidation levels Increased insulin sensitivity Increased islet number per section and protein expression of CAT |
|
| Quercitrin | [287] | STZ-IDR | Lowered fasting glycaemia level Augmented insulin levels Reduced FBPase and G6Pase activities |
| [288] | STZ-IDR | Lowered fasting glycaemia level Augmented insulin contents Protected β-cells |
|
| [289] | STZ-IDR | Lowered fasting glycaemia and HbA1c levels Augmented insulin contents |
|
| [290] | STZ-IDR | Reduced glycaemia levels | |
| Isoquercitrin | [291] | Diabetic rats | Reduced hyperglycemia as a function of time Delayed the glycemic peak (to 30 min) |
| [292] | High-calorie diet and STZ-IDR | Improved fasting glycaemia levels, and GT | |
| [161] | STZ-IDM | Decreased fasting glycaemia, augmented serum insulin contents Inhibited variations in postprandial glycaemia |
|
| Rutin | [293] | STZ-IDR | Reduced fasting glycaemia and HbA1c levels Augmented C-peptide and insulin contents |
| [294] | STZ-IDR | Decreased fasting glycaemia level Decreased G6Pase and FBPase activities Augmented insulin and glycogen contents |
|
| [295] | STZ-IDR | Reduced fasting glycaemia in a concentration-dependent way | |
| [276] | NA/STZ-IDR | Decreased glycaemia Augmented glucose absorption Decreased glucose transport activity |
|
| [163] | Insulin resistance model and T2D | Induced a normoglycemic effect | |
| [296] | HFD/STZ-IDR | Reduced levels of glycaemia, HbA1c, and inflammatory mediators (TNF-α and IL-6) Preserved β-islet cell structure |
|
| [165] | T2D mouse model | Down-regulated the expression of protein tyrosine phosphatase-1B (PTP-1B) Lowered serum glucose contents (in vivo) |
|
| [166] | hA transgenic mice | Delayed the progression of diabetes | |
| [297] | HFD/STZ-IDM | Improved glycaemia and HbA1c levels, as well as pyruvate and GT | |
| [298] | STZ-IDR | Decreased fasting glycaemia levels Improved pancreatic tissue regeneration |
|
| [71] | ALX-IDM | Prevented hyperglycemia and hypoglycaemia | |
| [299] | STZ-IDR | Regulated HbA1c and total hemoglobin (tHb) levels Restored STZ-induced damages in pancreas |
|
| Strictinin ellagitannin | [167] | OSTT | Enhanced oral sucrose tolerance |
| Peonidin | [300] | Male Sprague−Dawley rats | Ponidin suppressed the rise in glycaemia, inhibited maltase activity (IC50 = 200 μM), and decreased the maximal glycaemia level by 16.5 %. |
Abbreviations: ALX: Alloxan; DIO: Diet-induced obese; FBP: Fructose-1,6-Bisphosphate; FBPase: Fructose-1,6-Bisphosphatase; G6P: Glucose-6-Phosphate; G6Pase: Glucose-6-Phosphatase; G6PD: Glucose-6-Phosphate Dehydrogenase; GIA: Glucose Intestinal Absorption; GK: Goto-Kakizaki; GA: Glycated Albumin; HbA1c: Glycated Hemoglobin; HFruD: High Fructose Diet; HFD: High Fat Diet; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; IDM: Induced Diabetic Mice; IDR: Induced Diabetic Rats; IOM: Induced Obese Mice; IR: Insulin Receptor; IDD: Insulin-Deficient Diabetic; NIDDM: Non-Insulin-Dependent DM; NO: Nitric Oxide; NA: Nicotinamide; PTP-1B: Protein Tyrosine Phosphatase-1B; STZ: Streptozotocin; tHb: total Hemoglobin.
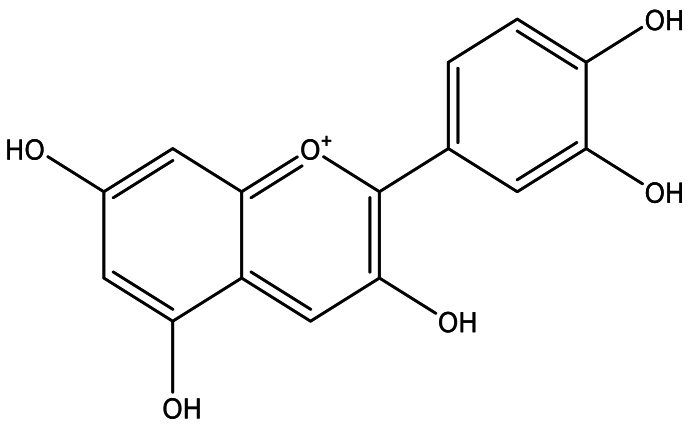
3.1. Apigenin
Numerous preclinical studies have demonstrated the anti-diabetic potential of apigenin (Fig. 1 showed the chemical structure of apigenin). In vivo hyperglycemia regulation of this compound was evaluated using an alloxan-induced diabetes (AID) mouse model [170,268,301]. Apigenin has been shown to exert various positive effects, including decreasing the activity of G-6-Pase and glucose concentration, as well as reducing the levels of serum insulin [172] and hepatic/muscle glycogen contents [301].
Fig. 1.
Chemical structure of apigenin.
In contrast to the aforementioned studies, Cazarolli et al. [170,171] demonstrated a significant anti-diabetic potential of apigenin in hyperglycemic rats. At doses of 50 and 100 μM, apigenin stimulated the synthesis of glycogen and insulin in the soleus muscle, resulting in an increase in the uptake of 14C-glucose in this tissue. Years later, Cazarolli et al. (2012) [169] elucidated the mechanism underlying the increased glucose uptake in the soleus muscle of hyperglycemic rats. They found that apigenin acts on insulin signaling pathways, including the tyrosine kinase receptor, atypical protein kinase C (aPKC), phosphatidylinositol 3-kinase (PI3K), and MEK.
Hossain et al. [174] investigated the mechanism underlying the anti-diabetic effects of apigenin in a streptozotocin (STZ)-induced diabetes (SID) rat model. They demonstrated that apigenin not only preserved pancreatic β-cells but also enhanced the translocation of GLUT4 in skeletal muscles and decreased the expression of the membrane glycoprotein CD38, thereby improving glucose homeostasis (as shown in Fig. 2). Ren et al. [176] confirmed these findings in T2DM rats caused by a low concentration of STZ as well as a high-fat diet (HFD). They observed a decrease in insulin resistance, glycaemia, and serum lipid levels with improved glucose tolerance (GT) upon treatment with apigenin.
Fig. 2.
Effects of apigenin on insulin synthesis and GLUT4 function.
Apigenin has also been shown to have several beneficial effects in HFD-induced obese mice. One study reported an increase in nitric oxide (NO) synthesis mediated by insulin, an improvement of vascular endothelial dysfunction, and inhibition of the inflammatory response associated with nuclear factor-κB (NF-κB). In addition, apigenin decreased the activity of liver enzymes glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK), leading to an improvement in metabolic disturbances such as decreased plasma insulin and fasting blood glucose concentrations [175].
Various in vitro methods have been employed to assess the anti-diabetic activity of apigenin, including the inhibition of carbohydrate-hydrolyzing enzymes [[53], [54], [55], [56],173,177]. Studies have shown that apigenin can inhibit the activity of α-glycosidase and human pancreatic α-amylase [55,56]. In addition, apigenin has been found to inhibit the expression of glucose-induced LOX-1 and TNF-α, which may help prevent diabetes complications such as arteriosclerosis by regulating NF-κB activity [53,54].
Furthermore, apigenin has been shown to protect pancreatic cells from oxidative stress induced by STZ and promote insulin production [173]. This effect on oxidative stress has also been confirmed through a reduction in ROS levels and the restoration of pancreatic β-cell apoptosis (Fig. 3) [177].
Fig. 3.
Protection of beta-cells by apigenin.
In a recent in vivo study, the potential hypoglycemic effect of apigenin was investigated using biochemical and histopathological parameters related to the liver and kidney. The results showed that oral administration of apigenin at a dose of 50 mg/kg/day improved the tested biochemical parameters and also exhibited a protective effect on the renal and hepatic architecture, as confirmed by histological examination. This suggests that apigenin may have therapeutic potential for the treatment of diabetes-associated organ damage [178].
3.2. Baicalin
Recently, several preclinical investigations have examined the effect of baicalin on diabetes and insulin resistance, as well as the underlying mechanisms (Table 2, Table 3). Indeed, these studies converge to suggest that baicalin has significant potential as a therapeutic agent for the treatment of obesity and insulin resistance. Their results revealed that this flavone acts through several molecular pathways, including the Akt/AS160/GLUT4 and P38MAPK/PGC1α/GLUT4 pathways, by accelerating the translocation of GLUT4 to the plasma membranes of adipocytes [59,60]. Additionally, baicalin showed an ability to suppress the expression of genes involved in gluconeogenesis and improve hepatic insulin resistance, mainly by inhibiting the p38 MAPK/PGC-1α signaling pathway [61,63]. Furthermore, other studies found that this compound protects against insulin resistance and metabolic dysfunction by activating the GALR2-GLUT4 signaling pathway, while attenuating oxidative stress and AGE production [62]. These results highlight the potential of baicalin as a promising natural treatment for metabolic disorders associated with obesity and prediabetes.
3.3. Arbutin
Arbutin, depicted in Fig. 4, has been investigated for its anti-diabetic properties since 1936, when Michel first reported on its potential therapeutic effects [179]. In vitro studies have shown that this flavonoid can dose-dependently inhibit α-amylase (81 %) and α-glucosidase (75 %) activities [57]. These findings were further confirmed in vivo using ALX-induced diabetic mice [180,181]. Oral administration of arbutin led to a significant increase in the levels of glucagon-like peptide 1 (GLP-1) and GLP1R, while decreasing serum insulin and glucose concentrations [180,181].
Fig. 4.
Chemical structure of arbutin.
In 2021, a team of Chinese researchers investigated the potential anti-diabetic effects of arbutin in diabetic mice induced by STZ Li et al. [182]. The study revealed that arbutin inhibited increased blood glucose levels and prevented weight loss in the animals, while also increasing plasma insulin concentrations and inducing the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX). These results suggest that arbutin may reduce diabetic symptoms through its antioxidant potential. A year later, Gholami Bahnemiri et al. [58] reported similar findings after pre-treating L6 skeletal muscle cells with arbutin (500 and 1000 μM) before inducing oxidative stress with tert-butyl hydroperoxide (t-BHP). Arbutin blocked ROS release and significantly increased glucose uptake, possibly by increasing the expression of glucose transporters GLUT1 and GLUT4 under oxidative stress.
3.4. Catechin
Numerous studies have demonstrated that catechin (Fig. 5) can enhance glucose homeostasis through multiple mechanisms.
Fig. 5.
Chemical structure of catechin.
Administering catechin orally (in vivo) led to a significant increase in 14C-glucose oxidation and a decrease in plasma glucose levels without altering C-peptide or plasma insulin. Additionally, catechin improved the antioxidant defense system and increased GLUT4 mRNA expression. It also restored the alterations of glycogen synthase (GS), G6Pase, glycogen phosphorylase (GP), and glucokinase, along with insulin receptor (IR) activation and improvement in glucose tolerance [186,189,190].
In rats, oral administration of this flavonoid increased insulin activity, decreased plasma glucose levels, and inhibited intestinal sucrose and α-amylase activities before administering soluble starch or sucrose [183]. Imada et al. [188] discovered that in animals made hyperglycemic by a HFD, catechins isolated from tea reduce some markers of insulin resistance. Similarly, in the same experimental protocol, oral administration of this compound stimulated insulin production and improved glucose tolerance (GT) [187]. In rats with T2DM, dietary intake of catechins was found to enhance GT and oxidative status [184].
In vitro tests, particularly those targeting digestive enzyme activity, have shown that catechin has a potent inhibition of α-glucosidase (IC50 = 31 μg/mL) and α-amylase (IC50 = 160 ± 6 μg/mL) [68]. A similar anti-α-amylase effect was observed in the study conducted by Xu et al. [69], with an IC50 value of 637.5 ± 7.81 μmol/L.
Studies using cultured cells have revealed significant anti-diabetic effects of catechin. In 2000, Shimizu et al. [64] demonstrated that catechin inhibits intestinal glucose uptake in intestinal epithelial cells (Caco-2). In 2009, Murase et al. [66] found that catechin activates the LKB1/AMPK pathway in vitro (using Hepa 1–6, L6 myoblasts, and 3T3-L1 cells) in combination with a gallocatechin moiety. Furthermore, catechin derivatives, such as gallocatechin 3-gallate (GCG), catechin 3-gallate (CG), EGCG, and epicatechin 3-gallate (ECG), inhibit maltase in vitro, with IC50 values of 6.3, 35, 34, and 27 μM, respectively [67]. In Caco-2 cells, EGCG inhibited maltase with an IC50 of 27 μM.
Recently, it has been discovered that catechins with a galloyl moiety (GM) have more potent inhibitory properties against α-glucosidase than those without GM [70]. GM was able to bind to catalytic amino acid residues of the α-glucosidase enzyme active site via hydrogen bonds and π-conjugations. In 2021, Mechchate et al. investigated the optimization of the anti-diabetic effects of certain plant flavonoids, including catechins, by developing a safe and potent multi-targeted mixture for the management of diabetes mellitus (DM) and its complications [71]. They found that a mixture containing all these molecules (catechin, epicatechin, and rutin) at 10 mg/kg will produce a new formulation with a powerful anti-hyperglycemic effect in combination, as confirmed in vivo (AID mice). Recently, Taslimi et al. [72] demonstrated the anti-diabetic potential of catechin 5-O-gallate on the activity of the α-glycosidase enzyme.
3.5. Epicatechin
Epicatechin (Fig. 6) has attracted the interest of many researchers for its potential anti-diabetic activity, both in vitro and in vivo studies [[96], [97], [98], [99], [100], [101],[196], [197], [198], [199], [200], [201], [202], [203]]. Regarding the in vivo evaluation of anti-diabetic activity, various animal models have been utilized, including STZ-induced diabetic (SID) mice. [99,199,203], ALX-induced diabetic mice [[196], [197], [198]], and HFD-fed mice [[200], [201], [202]].
Fig. 6.
Chemical structure of epicatechin.
In diabetic mice, treatment with epicatechin resulted in an improvement in blood glucose levels. This improvement was achieved through enhanced insulin signaling, decreased insulin resistance and endoplasmic reticulum stress, and increased levels of GLUT4, a glucose transporter protein (Fig. 7) and blood leptin concentrations [99,199,203]. Subsequent studies using oral administration of epicatechin to animals fed a high-fructose diet (HFruD) have also reported similar findings, which support the earlier results. These studies showed that epicatechin supplementation improved blood glucose levels and related parameters in animals on a high-fructose diet [[200], [201], [202]].
Fig. 7.
Antidiabetic mechanisms of epicatechin.
In contrast to the in vivo studies mentioned earlier, some researchers have utilized isolated islets of Langerhans for in vitro assessments to evaluate the potential anti-diabetic effects of epicatechin [[96], [97], [98]]. In the study by Ahmad et al. [97], epicatechin was found to increase glycogen content, oxygen uptake, and insulin uptake in muscle, fat, and liver cells. Another study by the same author reported that epicatechin stimulated the conversion of proinsulin into insulin and its secretion from Langerhans islets [98]. Hii and Howell [96]. demonstrated that epicatechin (1 mM) stimulated insulin secretion from isolated islets of Langerhans in vitro. In damaged Ins-1E cells, epicatechin (5–20 μM) increased insulin secretion and antioxidant enzyme levels [100]. In skeletal muscle cells, 3-O-acyl-epicatechin activated PI3K signaling, leading to increased glucose uptake and translocation of GLUT4 [101].
Furthermore, (−)-epicatechin has shown hypoglycemic effects in vivo by modulating glucose metabolism [102]. When combined with β-glucan, it exhibited a synergistic effect on the Akt pathway, enhancing glucose uptake. This synergistic effect was attributed to the inhibition of gluconeogenesis, down-regulation of glycogen synthase kinase-3β (GSK3β), enhancement of glycogen synthesis, and up-regulation of GLUT4. Epicatechin, either alone or in combination with catechin and rutin, has demonstrated a significant hypoglycemic effect, suggesting its potential as an effective anti-diabetic drug [71].
3.6. Cyanidin
The anti-diabetic activity of cyanidin (Fig. 8) has been investigated by several researchers [[73], [74], [75], [76], [77],[79], [80], [81], [82], [83], [84], [85], [86],[191], [192], [193], [194]]. In studies conducted by Daveri et al. and Tsuda et al. [191,193,194], the impact of cyanidin-based treatment on glycemic-related parameters was evaluated in different animal models. In HFD-fed mice, cyanidin improved hyperinsulinemia, hyperglycemia, hyperleptinemia, insulin sensitivity, and decreased insulin resistance and TNF-α mRNA levels [191]. Similarly, in STZ-induced diabetic mice, administration of cyanidin chloride at a dose of 100 mg/kg/day led to a decrease in glycated albumin (GA) levels and glycated hemoglobin (HbA1c) levels [193].
Fig. 8.
Chemical structure of cyanidin.
In rats fed cyanidin-rich diets, positive effects on glycemic control were also observed. These effects included a decrease in blood glucose levels, down-regulation of glucose-6-phosphatase (G6Pase) gene expression, improved insulin sensitivity, up-regulation of GLUT4 (glucose transporter 4) expression, and down-regulation of RBP4 (retinol-binding protein 4) in white adipose tissue [192]. These findings suggest that cyanidin has beneficial effects on glycemic control and insulin sensitivity in animal models of obesity and diabetes.
The anti-diabetic potential of cyanidin has been extensively studied both in vitro and in vivo. In in vitro experiments, cyanidin has shown inhibitory effects on enzymes involved in carbohydrate metabolism, including α-glucosidase, α-amylase, and dipeptidyl peptidase-4 (DPP-4). It has demonstrated inhibitory activity against α-glucosidase and pancreatic α-amylase, as well as sucrase and maltase [[73], [74], [75]]. Cyanidin-3-O-glucoside has also exhibited α-glucosidase and DPP-4 inhibition [86] and α-glucosidase and α-amylase inhibition [83].
In cell culture studies, cyanidin has shown positive effects on pancreatic β-cells, promoting cell survival, reducing apoptosis, and increasing insulin synthesis and secretion [80,85]. In adipocytes, cyanidin has been found to enhance glucose uptake, activate insulin signaling pathways, and improve insulin sensitivity [77,81,84]. In hepatocytes and other cells, cyanidin has been shown to activate the AMPK signaling pathway and improve glucose tolerance and insulin sensitivity [82].
Recent studies have further explored the anti-diabetic mechanisms of cyanidin. Fraisse et al. [87] demonstrated its potent inhibitory effect on α-glucosidase, surpassing the activity of acarbose. Jia et al. [88] revealed that cyanidin-3-O-glucoside exerts its anti-hyperglycemic effect by activating the AMPK pathway and inhibiting gluconeogenesis. Ye et al. [89] investigated the molecular mechanisms of cyanidin-3-O-glucoside in liver cells and diabetic mice, highlighting its hypoglycemic effects, up-regulation of liver GLUT-1 expression, and promotion of glucose consumption through the regulation of the Wnt/β-catenin-WISP1 signaling. Indeed, Kongthitilerd et al. [90] elucidated the mechanism of cyanidin-3-rutinoside on insulin secretion in rat pancreatic β-cells, showing that it improves insulin synthesis via intracellular Ca2+ signaling and activation of the PLC-IP3 pathway and voltage-dependent Ca2+ channel.
Overall, these studies provide evidence for the anti-diabetic potential of cyanidin through its effects on carbohydrate metabolism, insulin signaling, glucose uptake, and various cellular pathways involved in glucose homeostasis.
3.7. Delphinidin
Several preclinical investigators have tested the anti-diabetic potential of delphinidin (Fig. 9) [[91], [92], [93],95,126,193,194]. For this purpose, HFD-fed mice were used as an in vivo model [92,193,194]. In fact, in obese C57BL/6J mice, oral treatment of delphinidin 3-sambubioside-5-glucoside (D3S5G) diminished the production of glucose in hepatic cells as well as fasting glycaemia levels, and in parallel, it increased glucose absorption in L6 myotubes (skeletal muscle cells), dose-dependently [92]. A daily dose of delphinidin (100 mg/mL) was able to significantly reduce the values of GA (30.50 ± 3.46 %) and HbA1c (3.60 ± 0.25 %) [193]. Supplementation with this molecule also reduced resistance to insulin and improved its sensitivity [194].
Fig. 9.
Chemical structure of delphinidin.
In contrast, in vitro, delphinidin-3-glucoside remarkably stimulated insulin synthesis from INS-1832/13 cells (rodent pancreatic β-cells) [91]. In addition, delphinidin recorded other anti-diabetic effects in vitro such as inhibition of DPP-4 (34.4 %), α-amylase (35.6 %), α-glucosidase (37.8 %), and a decrease in ROS production (81.6 %) and glucose uptake [94]. This decrease in glucose uptake was confirmed in the same year by Hidalgo et al. [93] in mouse jejunum samples and intestinal cells (Caco-2, HT-29, and NCM460) by affecting sodium-glucose cotransporter 1 (SGLT1) function, a membrane protein involved in the transport of glucose. Moreover, in pancreatic RIN-m5F β-cells, Lai et al. [95] found that delphinidin decreases cleaved caspase-3 level, autophagy, adverse effects of oxidative stress, and apoptosis caused by high glucose concentrations, and increases the level of AMPKα Thr172 phosphorylation.
3.8. Hesperetin
Various methods were used to evaluate the anti-diabetic potential of hesperetin (Fig. 10) [103,104,[204], [205], [206], [207]]. Hesperetin has demonstrated its ability to improve glucose homeostasis in animal models of diabetes. Studies have shown that hesperetin treatment resulted in improvements in glucose levels, insulin levels, glycogen levels, and glucose metabolic enzymes, while also reducing insulin resistance [[205], [206], [207]]. Additionally, hesperetin normalized the activities of glucose-regulating enzymes and reduced serum and liver lipid levels, thereby improving glucose metabolism in vivo [204].
Fig. 10.
Chemical structure of hesperetin.
Furthermore, hesperetin has been found to inhibit the secretion of free fatty acids (FFA) stimulated by TNF-α and block the activation of the NF-κB and ERK signaling pathways, as demonstrated by Yoshida et al. [103]. It also exhibited inhibitory effects on α-glucosidase, with an IC50 value of 0.38 ± 0.05 mM [104]. These findings highlight the potential of hesperetin in improving glucose metabolism and its role in modulating key pathways involved in diabetes pathogenesis.
In recent studies, hesperetin has demonstrated its potential in restoring blood glucose levels in animal models of diabetes. In one study, hesperetin was administered to AID mice, resulting in a significant improvement in blood glucose levels [208]. Another study focused on the use of hesperetin extracted from Trifolium alexandrinum, a plant belonging to the Fabaceae family, for the treatment of T2DM in rats [209]. In this experiment, diabetic rats induced by NA/STZ were treated with 50 mg/kg of hesperetin for a duration of 4 weeks.
The treatment with hesperetin showed several beneficial effects in the rats. There was an improvement in serum glucose levels, as well as reductions in glucagon levels and the activities of hepatic glycogen, hepatic function enzymes, lipase enzymes, α-amylase, and lipid profiles. Additionally, the treatment increased the levels of antioxidant enzymes while decreasing the activities of G6PD, glucose-6-phosphate (G6P), fructose-1,6-bisphosphate (FBP), and glucokinase. The levels of GLUT2 and GLUT4, which are glucose transporters, were also improved. Furthermore, the expression levels of various proteins involved in glucose metabolism and signaling pathways were modulated by hesperetin treatment. This included changes in the expression levels of PI3K, AMPK, IR, IL-1β, and caspase-3. Overall, these recent in vivo experiments highlight the potential of hesperetin in improving blood glucose control and various metabolic parameters associated with diabetes.
3.9. Hesperidin
Several studies have experimentally evaluated the anti-diabetic activity of hesperidin (Fig. 11) [105,204,[210], [211], [212], [213], [214], [215], [216]]. In vivo evaluation was performed on STZ-induced T1D rats [211,212,215,216], and T2D rats [204,210,213,214].
Fig. 11.
Chemical structure of hesperedin
Hesperidin has been found to enhance glycemic metabolism through various mechanisms. These mechanisms include reducing glycaemia by lowering lipid levels in the blood and liver, modifying the activity of glucose regulatory enzymes [211,212], reducing degeneration of pancreatic cells and levels of TNF-α, increasing insulin concentrations [215], and decreasing fructose-1,6-bisphosphatase (FBPase), HbA1c, and G6Pase, while improving glycogen content in liver tissue [216]. Additionally, in GK rats with T2DM, hesperidin decreased hepatic lipid and serum levels and altered the activities of glucose regulatory enzymes [204].
In a model of T2DM, the administration of hesperidin demonstrated beneficial effects on serum insulin concentrations and TNF-α expression [213]. Another study revealed that oral treatment with hesperidin (at a dose of 50 mg/kg) normalized HbA1c levels, as well as serum insulin, glucose, hepatic and muscle glycogen levels. It also regulated resistin and adiponectin levels [214].
In rats fed a HFD, hesperidin was found to reduce blood glucose levels and the activity of G6Pase, while increasing glycogen concentration, hepatic glucokinase activity, C-peptide levels, and plasma insulin [210]. Furthermore, an in vitro evaluation of hesperidin's anti-diabetic activity utilized 3T3-L1 preadipocytes and RAW 264.7 cells. In RAW 264.7 cells, hesperidin dose-dependently reversed inflammation-induced insulin resistance induced by lipopolysaccharide (LPS), as evidenced by the inhibition of IL-6, TNF-α, and NO. In differentiated 3T3-L1 cells, it inhibited TNF-α-induced production of prostaglandin E2 (PGE2) and IL-6 [105].
Recent experiments investigated the impact of a water-soluble derivative of hesperidin called glucosyl hesperidin on hyperglycemia in HFD-induced obese mice. Long-term daily treatment (11 weeks) with this citrus flavonoid resulted in reduced blood glucose concentration, improved glucose intolerance, and insulin resistance. However, short-term treatment (2 weeks) did not affect blood glucose levels. These findings suggest that hesperidin could potentially be used for the prevention and/or treatment of obesity-related diabetes [217].
Using a rat model of HFD/alloxan-induced insulin resistance, Peng et al. [218] administered hesperidin orally for 35 days at a dose of 100 mg/kg. This treatment improved fasting blood glucose levels without affecting fasting insulin levels, and prevented impaired glucose tolerance (IGT) development. These results suggest that hesperidin can prevent the development of diabetes and insulin resistance by improving insulin sensitivity. The treatment also regulated gluconeogenesis and glycolysis by inducing phosphorylation of the insulin receptor (IR) and increasing glucokinase activity, while decreasing the activities of G6Pase and phosphoenolpyruvate carboxykinase (GTP).
To enhance the sustained release and potency of hesperidin as a potential anti-diabetic agent compared to conventional treatments, a new hesperidin nano-carrier was prepared and characterized. When administered to diabetic rats, this formulation exhibited prolonged production of hesperidin and resulted in improvements in glycemia, HbA1c levels, insulin levels, and lipid profile [219].
3.10. Kaempferol
Regarding the anti-diabetic potential of natural substances, kaempferol (Fig. 12) was one of the most investigated molecules [[106], [107], [108], [109],[112], [113], [114], [115],115,116,[220], [221], [222], [223], [224], [225], [226], [227], [228], [229], [230]]. In rats with diabetes induced by ALX (alloxan), oral administration of increasing doses of kaempferitrin (50, 100, and 200 mg/kg) resulted in a significant reduction in hyperglycemia [220]. This effect was further elucidated in a study by Zanatta et al. [221], where administration of kaempferol 3-neohesperidoside (at a dose of 100 mg/kg) via gavage showed increased muscle glycogen content. Additionally, it stimulated glucose uptake in the soleus muscle through the activation of the PI3K (phosphoinositide 3-kinase) and PKC (protein kinase C) pathways.
Fig. 12.
Chemical structure of kaempferol.
Various studies have utilized the STZ-induced diabetic animal model to evaluate the anti-diabetic properties of kaempferol [225,227,229]. In one study, oral administration of kaempferol (at doses of 50, 100, and 200 mg/kg) reduced insulin resistance and fasting blood glucose levels, improving glucose metabolism disorders [227]. Another study demonstrated that kaempferol improved insulin resistance, decreased IL-6 and TNF-α levels, and reduced the incidence of hyperglycemia, diabetes, and hepatic glucose synthesis. It also enhanced hexokinase activity in the liver and skeletal muscle, as well as improved gluconeogenesis and hepatic pyruvate carboxylase activity [230]. Furthermore, oral administration of kaempferol (at a daily dose of 50 mg/kg) to rats fed a HFD improved glycemic control by reducing G6Pase and pyruvate carboxylase activity in the liver, increasing protein kinase B (PKB) and hexokinase activity, and enhancing insulin sensitivity [230].
Using the same experimental protocol, dietary intake of kaempferol glycoside (at a concentration of 0.15 %) decreased fasting blood glucose levels, HbA1c, and the expression of sterol regulatory element-binding protein (SREBP-1c) and peroxisome proliferator-activated receptor (PPAR-γ), while improving insulin resistance [228]. Similarly, in obese mice fed a HFD, dietary kaempferol intake (at a concentration of 0.05 %) significantly normalized hyperinsulinemia, circulating lipid profile, and hyperglycemia. This was accompanied by improved insulin sensitivity, altered expression of AMPK and GLUT4 in adipose and muscle tissues, and inhibition of glycogen synthesis and glucose uptake [226]. Kaempferol 3-neohesperidoside promoted glycogen synthesis in rat soleus muscle [222] and reduced HbA1c and fasting blood glucose levels in mice with T2DM [224].
Various in vitro methods have been employed to assess the anti-diabetic activity of kaempferol, including tests for α-glucosidase and α-amylase inhibition. Studies have shown that kaempferol exhibits strong inhibitory activity against these digestive enzymes [114,116]. It has been found to possess potent inhibitory activity against α-glucosidase, with IC50 values ranging from 19.36 ± 2.43 μM [108] to 1.16 ± 0.04 × 10-5 mol/L (Peng et al., 2016). Additionally, it demonstrates significant inhibitory potential against α-glycosidase and α-amylase, with IC50 values of 29.37 and 51.24 μg/mL, respectively [113].
Several research teams have employed cell culture assays to investigate the mechanisms of kaempferol's anti-diabetic action. In 3T3-L1 adipocytes, this flavonol acts as a weak partial agonist and significantly enhances insulin-stimulated glucose uptake [106]. Studies conducted on INS-1E β-cells and human pancreatic islets have utilized various tests, including measuring caspase-3 activity, cell apoptosis, insulin secretion, and the expression of relevant proteins. These studies have shown that kaempferol reduces caspase-3 activity, inhibits cell apoptosis, improves insulin secretion, and increases the expression of anti-apoptotic proteins such as Bcl-2, Akt, and pancreatic/duodenal homeobox-1 (PDX-1) [109,110]. Moreover, in murine pancreatic islets and RIN-5F cells, kaempferol exhibits anti-apoptotic effects, enhances cell viability, stimulates autophagy via the AMPK/mTOR pathway, and restores β-cell function [115]. It is worth noting that the protective potential of kaempferol on β-cells has been observed in various studies. For instance, kaempferol has been shown to protect HIT-T15 cells against oxidative damage induced by 2-deoxy-d-ribose (dRib) by interfering with ROS metabolism [107].
Recent studies have also demonstrated the hypoglycemic effect of kaempferol in vivo, with a reduction in fasting glucose levels and an increase in fasting insulin levels observed in a rat model of diabetes [231]. In the same animal model, a recent study reported that a combination of kaempferol and myricetin can normalize glucose levels, inflammatory cytokines, insulin levels, lipid and liver enzymes, as well as oxidative stress markers in diabetic animals [232].
3.11. Luteolin
Luteolin (Fig. 13) is a flavonoid present in several medicinal plants with anti-diabetic activity. In vitro and in vivo experiments showed its anti-diabetic potential [117,119,120,[122], [123], [124],233,234,245,300]. In an in vitro study conducted by Kim et al. (2000) [117], luteolin (0.5 mg/mL) demonstrated superior inhibitory effects (36 %) on α-glucosidase compared to α-amylase and the positive control, acarbose. The inhibitory activity of luteolin on α-glucosidase was further confirmed by Yan et al. [123], who reported a dose-dependent inhibition with an IC50 value of 1.72 ± 0.05 × 10−4 mol/L. Additionally, Yan et al. [123] revealed that luteolin acts as a non-competitive inhibitor (NCI) with a unique binding site on the α-glucosidase enzyme, with a Ki value of 1.40 ± 0.02 × 10−4 mol/L.
Fig. 13.
Chemical structure of Luteolin).
Luteolin demonstrated significant anti-diabetic effects in cell culture models. In 3T3-L1 adipocytes, it was found to reduce mRNA levels of TNF-α and IL-6, enhance glucose uptake in response to insulin, promote Akt2 phosphorylation, and increase PPARγ transcriptional activity, indicating its positive impact on glucose metabolism [119]. In endothelial cells, luteolin was shown to improve insulin-dependent nitric oxide production, highlighting its potential in enhancing endothelial function [120]. The compound also exhibited inhibitory effects on aldose reductase (AR) activities and protein tyrosine phosphatase 1B (PTP1B), both of which play significant roles in regulating glucose metabolism [121]. In MIN6 cells, luteolin inhibited NF-κB activity, reduced nitric oxide production, and stimulated insulin synthesis, suggesting its potential in preserving β-cell function [122]. Additionally, in an in vitro (3T3-L1 cells and RAW264.7 macrophages) and in vivo (HFD-fed mice) study, luteolin suppressed macrophage infiltration and polarization in adipocytes, leading to improved insulin resistance through the activation of the AMPKα1 pathway [124].
Luteolin has been extensively studied for its anti-diabetic effects in various experimental models. In an animal model, it showed significant inhibition of maltase activity, although it did not have an effect on glycaemia or other enzymes such as sucrase and α-glucosidase [300]. In diabetic rats, luteolin decreased blood glucose levels, increased insulin levels, and improved pancreatic function [233]. Another study in diabetic rats reported reduced expression of TNF-α and IL-6 genes, improved insulin-mediated endothelial-dependent relaxation, and restored insulin signaling [120]. Luteolin administration to diabetic KK-Ay mice normalized insulin, glycaemia, and HbA1c levels, demonstrating its anti-hyperglycemic effects [234]. To enhance the anti-hyperglycemic effects of luteolin in a T2DM model, researchers modified its structure and synthesized compounds such as 6,8-(1,3-diaminoguanidine) luteolin (DAGL) and its chromium complex (DAGL·Cr), as well as 6,8-guanidyl luteolin quinone-chromium (GLQ·Cr) [235,236]. DAGL and DAGL·Cr exhibited beneficial effects, including normalization of fasting glycaemia, body weight, glycated serum protein, and pancreatic islet function, with a restorative capacity in the pancreas. The hypoglycemic mechanism was attributed to the regulation of the PI3K/AKT-1 signaling pathway. GLQ·Cr attenuated hyperglycemic symptoms by normalizing pancreatic and hepatic functions, modulating intestinal microbiota composition, and regulating the PPAR signaling pathway. In a recent study, luteolin was combined with another flavone, diosmin, in selenium nanoparticles (SeNPs) to improve diabetes management [237]. The nanospheres containing both flavonoids showed normalization of blood glucose, insulin, HbA1c, lipid profile, and glycogen levels in mice with diabetes. These nanospheres also exhibited antioxidant potential and protective effects against liver damage. Overall, luteolin has demonstrated promising anti-diabetic effects through various mechanisms and has been explored for structural modifications and combination strategies to enhance its therapeutic potential in the management of diabetes.
3.12. Malvidin-3-O-glucoside
The compound malvidin-3-O-glucoside has shown potential anti-diabetic effects in both in vivo and in vitro studies. In a study by Grace et al. [195] (Fig. 14), malvidin-3-O-glucoside exhibited significant anti-hyperglycemic activity in diabetic mice when administered at a dose of 300 mg/kg. This compound also demonstrated inhibition of α-glucosidase and α-amylase activity in in vitro experiments [127].
Fig. 14.
Chemical structure of malvidin-3-O-glucoside.
In an in vitro cell culture model using Caco-2 cells, Mojica et al. [94,126] investigated the molecular markers associated with diabetes and the effects of malvidin-3-O-glucoside. Treatment with a 100 μM dose of malvidin-3-O-glucoside resulted in the inhibition of α-glucosidase (42.8 %), α-amylase (29.6 %), and DPP-IV (82.4 %) activities. Additionally, it reduced glucose uptake by 55.2 % in Caco-2 cells. Another study on the same cell line demonstrated that malvidin-3-O-glucoside reduced 14C fructose absorption by 15 % at the highest concentration tested [125].
A recent in vitro study conducted by Xue et al. [128] investigated the interaction between malvidin-3-O-glucoside and α-glucosidase, revealing valuable insights into its mechanism of action. The study reported that malvidin-3-O-glucoside acts as a reversible non-competitive inhibitor of α-glucosidase. Notably, the binding of malvidin-3-O-glucoside to the enzyme induced structural modifications in α-glucosidase, resulting in the regulation of specific amino acid residues and their microenvironment.
Furthermore, a recent study by Zou et al. [238], explored the effects of combined therapy involving malvidin and metformin in a rat model of T2DM induced by a HFD and STZ. The findings demonstrated that the combination therapy of malvidin with metformin exhibited significant improvements in glucose and lipid metabolism. Moreover, the treatment showed efficacy in inhibiting inflammation, suggesting a potential multifaceted therapeutic approach.
3.13. Naringin
Using animal models of SID, several in vivo studies have evaluated the anti-hyperglycemic activity of naringin (Fig. 15). Naringin, a molecule found in certain foods, has been shown to have several beneficial effects on glucose regulation and pancreatic beta-cell health. Studies conducted [257,261,263] have demonstrated that this molecule can lower plasma glucose levels in a dose-dependent manner.
Fig. 15.
Chemical structure of naringin.
Furthermore, naringin has been found to protect beta-cells from apoptosis, which is the programmed cell death, by inhibiting both the extrinsic pathway (mediated by death receptors) and the intrinsic pathway (mediated by mitochondria). In insulin-deficient mice, it has also been observed to improve abnormalities in pancreatic islets.
The specific beneficial effects of naringin on T2DM as opposed to type 1 diabetes (T1D) have been attributed to the requirement of insulin presence, as explained by Xulu and Oroma Owira [260].
In addition, when administered orally to diabetic rats in high concentrations along with vitamin C, naringin has been found to reduce blood glucose levels, HbA1c (glycated hemoglobin), and the activities of enzymes involved in gluconeogenesis (G6Pase and FBPase) in the kidneys and liver. It also increased plasma insulin levels, hepatic glycogen content, and the activity of the enzyme hexokinase, which is involved in glucose metabolism [257].
Oral administration of naringin has been found to have several positive effects, in induced diabetes by nicotinamide (NA) and STZ. These include reducing HbA1c (glycated hemoglobin), blood glucose levels, and the activities of enzymes involved in gluconeogenesis (G6Pase and FBPase). Naringin treatment also stimulated insulin secretion and increased the activities of glucose-6-phosphate dehydrogenase (G6PD) and glucokinase, which are enzymes involved in glucose metabolism [258].
Supplementing naringin to rats with T2DM resulted in enhanced expression of adiponectin, insulin receptor (IR) subunit, and glucose transporter 4 (GLUT4) mRNA. It also reduced hepatic glycogen levels, C-peptides, serum insulin, HbA1c, and G6Pase activity [255]. Similar results were observed in another study using male mice with T2DM (C57BL/KsJ-db/db). Naringin supplementation increased serum insulin levels and decreased glucose, resistin, TNF-α, and free fatty acid (FFA) levels [210].
Many researchers have employed the HFD and STZ-induced diabetic rat model in their experiments. Naringin treatment in these rats resulted in increased serum insulin levels and reduced levels of glucose, resistin, TNF-α, and FFA [213]. In a subsequent study, (Ahmed et al. [214] demonstrated that naringin lowered elevated levels of HbA1c, serum insulin, glucose, hepatic and muscle glycogen, resistin, and adiponectin.
Furthermore, in diabetic rats, naringin was able to decrease HbA1c and blood glucose levels and increase plasma insulin levels in a dose-dependent manner. It also normalized the levels of altered liver enzymes (G6Pase, FBPase, G6PD, GP, hexokinase, and GS), restored the number of insulin-immunoreactive β-cells, and improved glycogen content [262].
In a study conducted by Kumar Sharma et al. [259] on rats with T2DM, naringin exhibited anti-diabetic efficacy. The researchers observed a decrease in hyperglycemia (high blood sugar), hyperinsulinemia (elevated insulin levels), and insulin resistance. Naringin also provided protection to beta-cells in the pancreas by regulating oxidative stress and inflammation (specifically, IL-6 and TNF-α) and influencing the production of dysregulated adipocytokines. The study found that naringin increased the expression of HSP-27, PPARγ, and HSP-72, which are proteins involved in cellular stress response and inflammation regulation.
The anti-diabetic effects of naringin have also been demonstrated in vitro studies using cell cultures. Nzuza et al. [146] conducted research on RIN-5F cells, which are pancreatic beta-cell lines, and found that naringin prevented pancreatic beta-cell dysfunction, leading to the reduction of insulin secretion inhibition [145]. investigated the effects of naringin on L6 myoblasts, a type of muscle cell, and observed that naringin increased glucose uptake in differentiated L6 myoblasts, indicating improved glucose utilization by the cells.
Furthermore, Dayarathne et al. [147] studied the relationship between AMPK phosphorylation (activation) and glucose uptake in HepG2 cells, a human liver cell line, treated with high concentrations of glucose. They examined the effects of naringin and its aglycone form, naringenin, both derived from citrus, on glucose uptake. The study revealed that these flavonoids stimulated glucose uptake independently of insulin stimulation by inducing AMPK phosphorylation. Naringin and naringenin bound to the AMPK γ-subunit with high affinities, suggesting their ability to positively modulate AMPK activation and enhance glucose uptake without relying on insulin secretion.
Collectively, these studies highlight the ability of naringin to regulate glucose metabolism, improve insulin sensitivity, protect beta-cells, and enhance glucose uptake, indicating its potential as a therapeutic agent for diabetes management.
3.14. Naringenin
Naringenin (Fig. 16), similar to other compounds, has been extensively studied for its anti-diabetic activity using experimentally induced diabetes in animals. Ortiz-Andrade et al. [138] conducted both in vitro and in vivo tests to evaluate the effects of naringenin. In in vitro tests, they found that naringenin inhibited the activity of 11β-HSD1 enzyme (by 39.49 %), which is involved in glucocorticoid metabolism, without affecting the activity of α-glucosidase enzyme. In their in vivo study using diabetic rats, oral treatment with naringenin (50 mg/kg) for 21 days resulted in reduced fasting blood glucose levels, HbA1c (glycated hemoglobin), and increased serum insulin concentrations. It also demonstrated protective effects on pancreatic beta-cells [251]. In a subsequent study by the same authors using the same experimental protocol, they observed that naringenin attenuated hematological abnormalities, reduced inflammation proteins, and modulated mRNA transcription [251].
Fig. 16.
Chemical structure of Naringenin.
Furthermore, Priscilla et al. [142,252] administered naringenin orally to rats with diabetes induced by a HFD and STZ. Their research showed that naringenin attenuated hyperglycemia (high blood sugar) and hyperinsulinemia (elevated insulin levels). It competitively inhibited α-glucosidase, an enzyme involved in carbohydrate digestion, and modulated the expressions of TNF-α (tumor necrosis factor-alpha) and GLUT4 (glucose transporter 4) proteins. Naringenin also improved insulin sensitivity and restored abnormalities in pancreatic tissues.
Multiple studies have confirmed the hypoglycemic efficacy of naringenin in animal models of diabetes induced by STZ. These studies have shown that naringenin reduces HbA1c levels, blood glucose levels, and the insulin resistance index while improving glucose tolerance [143,176,253]. Indeed, Singh et al. [143] conducted molecular studies and reported that naringenin activates the GLUT4/PPARγ pathways, with strong binding affinity to GLUT4 and PPARγ receptors. Additionally, naringenin normalized reduced C-peptide and serum insulin concentrations, elevated GP and G6Pase activities, and restored hepatic glycogen content [255].
In vitro studies have also been conducted to investigate the anti-diabetic effects of naringenin on cultured cells. For instance, naringenin induced glucose uptake (163 %) in rat adipocytes through various assays, including lipolysis, lipogenesis, and glucose uptake assays [137]. Treatment of 3T3-L1 cells with naringenin inhibited the activation of the NF-κB and ERK pathways induced by TNF-α, as well as the synthesis of free fatty acids (FFAs) induced by TNF-α [103]. Naringenin also increased glucose absorption in muscle cells (L6) by phosphorylating/activating the AMPK pathway [139]. In INS-1E cells, naringenin induced glucose sensitivity, stimulated insulin secretion, and altered gene expression profiles [140]. Another study using porcine myotube cultures showed that naringenin increased the phosphorylation of TBC1D1, leading to GLUT4 translocation and glucose uptake, in a TBC1D1-dependent manner [141].
Recently, Park et al. [256] investigated the anti-diabetic potential of a derivative of naringenin called 8-prenylnaringenin (8-PN) in insulin-deficient diabetic (IDD) mice induced by STZ. Oral treatment with 8-PN and naringenin improved glucose homeostasis, restored islet function, and corrected insulin signaling defects. In the pancreas and liver, 8-PN increased the expression levels of estrogen receptor-alpha (ERα) and fibroblast growth factor 21 (FGF21) specifically in the liver.
In a recent in vitro study by Prasad and Srinivasan [144], naringenin was isolated from the ethanolic extract of Tinospora sinensis stems, and its effect on activating PPARγ receptors and inhibiting α-glucosidase enzyme was evaluated. Naringenin demonstrated potent anti-α-glucosidase activity and up-regulation of PPARγ receptors.
These findings collectively demonstrate the hypoglycemic effects of naringenin, both in vivo and in vitro, and suggest its potential as an anti-diabetic agent. Furthermore, the derivative 8-PN and the identification of naringenin from plant extracts highlight the ongoing research to explore the anti-diabetic properties of these compounds.
3.15. Quercitrin
In studies conducted with STZ-induced diabetic (SID) rats, quercitrin (Fig. 17) has been investigated for its anti-hyperglycemic effects. These studies have shown that quercitrin reduces fasting blood glucose levels and HbA1c, while increasing C-peptide and plasma insulin levels [[287], [288], [289]]. Babujanarthanam et al. [287] observed increased hexokinase activity and glycogen content, decreased G6Pase and FBPase activities, and protection of pancreatic cells with reduced fatty infiltrates and expansion of islets.
Fig. 17.

Chemical structure of Quercitrin.
In rat insulinoma (RINm5F) cells, quercitrin has shown protective effects on β-cells against cytokine-induced damage, improved glucose-stimulated insulin secretion (GSIS), and inhibition of NF-κB translocation [160].
3.16. Isoquercitrin
Another flavonoid (Fig. 18) has also been studied for its anti-hyperglycemic activity. Paulo et al. [291] conducted the first study on the topic and found that an administered dose of 100 mg/kg of isoquercitrin reduced hyperglycemia in diabetic rats and delayed the glycemic peak. Huang et al. [292] demonstrated that daily oral treatment with isoquercitrin (10 and 30 mg/kg) for 21 days improved fasting blood glucose levels, glucose tolerance, and clinical symptoms in a dose-dependent manner in Wistar rats rendered diabetic by a high-calorie diet and STZ injection.
Fig. 18.
Chemical structure of Isoquercitrin.
Inhibition of dipeptidyl peptidase-4 (DPP-4) by isoquercitrin has also been observed. Zhang et al. [161] reported a high inhibition of isoquercitrin on DPP-4 competitively, with Ki and IC50 values of 236 and 96.8 mM, respectively. In an experimental in vitro (NCI–H716 cells) and in vivo (SID mice) model, isoquercitrin stimulated GLP-1 production in vitro and decreased fasting blood glucose levels, increased serum insulin and GLP-1 levels, and inhibited postprandial glycemia variations in a concentration-dependent manner [161].
These studies highlight the potential of quercitrin and isoquercitrin as anti-hyperglycemic agents, with effects on blood glucose levels, insulin secretion, pancreatic cell protection, and DPP-4 inhibition.
3.17. Rutin
Rutin has been extensively studied for its potential as an anti-diabetic agent (Fig. 19). In studies conducted with STZ-induced diabetic (SID) rats, oral administration of rutin resulted in significant decreases in fasting blood glucose levels and HbA1c, as well as increases in C-peptide and insulin levels [293,294]. These studies also reported improvements in G6Pase and FBPase activities, glycogen content, and hexokinase activity, as well as protection of the pancreas through expansion of islets and reduction of fat infiltration.
Fig. 19.
Chemical structure of Rutin.
Rutin has shown inhibitory effects on α-glucosidase in vitro, with an IC50 value of 0.196 mmol/L [148]. In SID rats, oral administration of rutin resulted in a concentration-dependent decrease in fasting blood glucose levels [295]. Another study on NA/STZ-induced diabetic rats demonstrated a significant reduction in glycaemia with rutin treatment, along with improvements in glucose uptake and inhibition of glucose transport [276]. The mechanism of action for glucose uptake stimulation by rutin was studied and found to involve the mitogen-activated protein kinase (MAPK), aPKC, and PI3K pathways [162].
Combining rutin with quercetin has shown synergistic inhibitory effects on α-glucosidase and α-amylase activities [164]. Rutin alone exhibited strong inhibition against both enzymes, and its combination with quercetin further enhanced the inhibitory effects. Oral treatment with rutin in HFD/STZ-induced type 2 diabetic rats resulted in reduced glycaemia, HbA1c, and inflammatory mediators, as well as preservation of β-islet cell histological structure and reversal of hepatocyte enlargement [296].
In vitro and in vivo studies have demonstrated the anti-diabetic potential of rutin. It has been shown to enhance insulin-dependent translocation of GLUT4 and increase insulin receptor kinase (IRK) activity [163]. Rutin has also been found to increase glucose uptake in L6 myoblasts through GLUT4 translocation under oxidative stress conditions [154]. Other studies have indicated that rutin down-regulates protein tyrosine phosphatase-1B (PTP-1B) expression levels, enhances insulin signaling pathways, and decreases serum glucose levels in animal models [165,166]. Rutin supplementation has also been shown to improve blood glucose levels, HOMA-B%, HbA1c levels, and pancreatic tissue regeneration in diabetic animal models [297].
To improve the bioavailability and stability of rutin, researchers have explored encapsulating the molecule in nanophytosomes. In SID rats, the encapsulated rutin formulation demonstrated greater efficacy than free rutin in regulating HbA1c and total hemoglobin levels, as well as restoring pancreatic damage induced by STZ [299].
Overall, these studies highlight the anti-diabetic potential of rutin, including its effects on glucose metabolism, insulin secretion, pancreatic protection, and inhibition of key enzymes involved in carbohydrate digestion. Encapsulation techniques may further enhance the therapeutic efficacy of rutin in diabetes treatment.
3.18. Resveratrol
Resveratrol is a natural phytoalexin with multiple health benefits (Fig. 20), including anti-diabetic activities. In various in vivo studies, it has shown promising effects in lowering glycaemia levels and improving insulin resistance.
Fig. 20.
Chemical structure of Resveratrol.
In experimental models of diabetes, including SID rats and NA/STZ-induced diabetic rats, resveratrol demonstrated a concentration-dependent reduction in plasma glucose levels [302]. It also promoted glycogen production in hepatocytes, stimulated glucose uptake, and delayed insulin resistance.
In insulin-deficient diabetic rats, resveratrol improved glucose uptake in skeletal muscle through the PI3K-Akt pathway and lowered plasma glucose levels via insulin-dependent and insulin-independent mechanisms. It also increased the expression of GLUT4 in the soleus muscle and stimulated insulin synthesis [303].
A 30-day oral treatment with resveratrol was found to lower glycaemia levels and HbA1c and normalize plasma insulin contents and certain biochemical parameters [304]. Additionally, resveratrol-induced GLUT4 translocation in the myocardium of SID animals was proposed to be insulin-independent, with increased AMPK phosphorylation, GLUT4 expression, and glucose uptake in myoblastic cells [305].
Resveratrol treatment for 30 days also improved enzyme activities related to carbohydrate metabolism and glucose storage in renal and hepatic tissues. It reduced insulin, HbA1c, and glycaemia levels and improved the activities of FBPase, G6Pase, G6PD, GS, GP, hexokinase, and pyruvate kinase (PK) [306].
Resveratrol has been extensively studied for its anti-diabetic effects, and numerous in vivo studies have demonstrated its potential in improving various aspects of diabetes. In male C57BL/6 mice with diet-induced diabetes, long-term intracerebroventricular infusion of resveratrol normalized hyperglycemia and hyperinsulinemia while improving hypothalamic inflammatory NF-κB signaling [307]. Similarly, in diabetic rats, a 30-day oral treatment with resveratrol improved hyperglycemia, insulin secretion, HbA1c, and the expression of pro-inflammatory cytokines [308]. Resveratrol also exhibited antioxidant effects by reducing hydroperoxide, lipid peroxide, and protein carbonyl levels and modulating the activity of antioxidant enzymes (CAT, GPX, SOD, and GST). It protected β-cells from oxidative damage in these studies. In a mouse model of HFD-induced diabetes, resveratrol administration for 35 days decreased glucose intolerance and increased GLP-1 and insulin concentrations, improving insulin sensitivity and reducing β-cell apoptosis [309]. Additionally, resveratrol improved insulin resistance, hyperglycemia, lipid peroxidation, and hepatic steatosis in Wrn mutant mice, with modulation of genes involved in GSH metabolism and the insulin-signaling pathway [310]. Resveratrol treatment in non-obese diabetic (NOD) mice decreased the expression of CCR6, a chemokine receptor, in splenocytes [311]. It also demonstrated anti-diabetic effects by reducing glycaemia levels, increasing glucose uptake, and protecting β-cells in in vitro and in vivo tests [312]. In various animal models of diabetes, resveratrol normalized dyslipidemia, reduced serum glucose levels, and improved body weight loss [313]. It also improved insulin levels and glucose excursion in an oral glucose tolerance test [314]. The anti-diabetic effects of resveratrol were mediated through activation of the AMPK pathway and its downstream targets, leading to improvements in glycaemia, lipid profiles, adiponectin levels, and insulin sensitivity [315]. Resveratrol exhibited protective effects against apoptosis and oxidative stress in pancreatic cells, preventing diabetes in animal models [316]. It enhanced glucose tolerance, increased β-cell mass, and reduced islet fibrosis and oxidative damage in diabetic mice [317]. Furthermore, resveratrol supplementation showed significant anti-diabetic and antioxidant effects, relieving damage to the pancreas, kidneys, and liver in mice treated with alloxan [318]. It also improved insulin resistance induced by methylglyoxal, reducing serum glucose levels and TNF-α content while increasing insulin and p-Nrf 2 protein expressions [319].
In studies conducted in 2015, the combination of resveratrol with vitamin C was found to have beneficial effects in diabetic animals. It improved body weight and restored levels of blood glucose, total protein, MDA, LH, and antioxidant enzymes [320]. Another study in the same year investigated the effect of resveratrol on a mouse model of gestational diabetes mellitus (GDM). Resveratrol at 10 mg/kg improved insulin tolerance and glucose metabolism by activating AMPK and reducing G6Pase production [321]. Resveratrol also showed the ability to reduce glycaemia and HbA1c levels, stimulate insulin synthesis, and protect β-cells in diabetic rats [322].
In 2018, two experiments explored the combined effect of resveratrol with other compounds on diabetic animals induced by alloxan and STZ. The results showed that resveratrol alone or in combination with vitamin E or quercetin improved insulin sensitivity, normalized blood glucose levels, and preserved pancreatic cell structure [281,323].
Resveratrol has also been nano-encapsulated for the preparation of functional snacks, which exhibited enhanced anti-diabetic properties compared to snacks without or with free resveratrol [324]. In another study, resveratrol derivatives, including trans-ε-viniferin and vateriferol, demonstrated significant hypoglycemic effects and inhibited α-glucosidase activity in diabetic mice and in vitro experiments [325].
Recent studies in 2021 investigated the impact of resveratrol on insulin production and resistance, GLP-1, and oxidative stress in a rat model of T2DM induced by NA/STZ. The findings showed reductions in insulin resistance, increases in GLP-1, insulin levels, and total antioxidant capacity, along with improvements in intestinal and pancreatic histological alterations [326,327].
3.19. Quercetin
Quercetin (Fig. 21), a natural plant pigment, has shown beneficial effects in various models of diabetes. In a study by Shetty et al. [265] using SID rats, a diet containing quercetin (1 g/kg) improved 25 % of the diabetic state in the animals. One year later, Coskun et al. [266] observed a protective effect on β-cells by measuring antioxidant enzyme activities (CAT, SOD, and GPX) in diabetic rats. Injection of quercetin (15 mg/kg) in diabetic animals increased enzyme activities, reduced levels of MDA and nitric oxide (NO), and preserved β-cells.
Fig. 21.
Chemical structure of Quercetin.
In ALX-induced diabetic rats, oral administration of quercetin-3-O-glucoside (15 mg/kg/day) for 10 days increased insulin levels, decreased serum glucose concentrations, and inhibited G6Pase activity [268]. Similar results were seen in Lukačínová et al. [269], where oral treatment with quercetin (50 and 100 mg/kg) for 7 days prevented the rise in blood glucose. In an in vitro study on mature 3T3-L1 adipocytes, quercetin improved glucose absorption stimulated by insulin, inhibited NO production in macrophage cells, and acted as a partial agonist of PPARγ [106]. Kannappan and Anuradha [270]. showed that a 60-day treatment with quercetin (50 mg/kg) improved tyrosine phosphorylation and insulin sensitivity in an HFruD-induced insulin resistance model.
Kobori et al. [271] evaluated the protective effect of quercetin on BALB/c mice with SID. Rats given a diet containing quercetin (0.5 %) for 14 days showed decreased glycemia, improved plasma insulin levels, and enhanced cell proliferation through the inhibition of Cdkn1a expression, leading to improved liver and pancreas function. In vitro studies on enzyme kinetics by Li et al. [148] confirmed the inhibitory effect of quercetin on α-glucosidase compared to acarbose.
In SID rats, treatment with quercetin (15 mg/kg) for 25 days resulted in decreased glycemia levels and increased antioxidant enzyme activities [272]. In an in vitro study on C2C12 muscle cells, Eid et al. [149] found that quercetin 3-O-glycosides and quercetin improved glucose uptake in the absence of insulin through the stimulation of the AMPK signaling pathway. Torres-Piedra et al. [150] reported a range of beneficial effects following five days of oral treatment with quercetin (50 mg/kg) in diabetic animals, including the reduction of triglycerides, LDL, HDL, and total cholesterol levels.
In a diabetic rat model, El-Baky [273]. administered quercetin (20 mg/kg) for 8 weeks, resulting in significantly lower glycemia, insulin resistance, NO, and MDA levels. Insulin levels and antioxidant enzyme activities were increased, and β-cell function was improved. Similarly, in an STZ-induced diabetic model, oral administration of quercetin (100 mg/kg) reduced plasma glucose and HbA1c levels [274].
Hussain et al. [275] found that oral doses of quercetin (300 and 600 mg/kg) improved postprandial hyperglycemia in SID rats, with reductions of 32.0 % and 64.0 %, respectively, compared to the positive control acarbose. It also lowered triglyceride and total cholesterol levels compared to the control group [276]. Mechanistically, quercetin reduced glucose transport activity and increased glucose absorption by the hemidiaphragm in vivo.
In a C57BL/KsJ-db/db mouse model of T2DM, Jeong et al. [277] demonstrated the hypoglycemic, antioxidant, and hypolipidemic effects of quercetin. After 6 weeks of a quercetin-based diet (at 0.04 % and 0.08 %), plasma glucose, HOMA-IR, triglyceride levels, and TBARS levels were reduced, while HDL-cholesterol and plasma adiponectin were increased. Insulin levels showed no significant effect. Quercetin also reversed pancreatic morphological alterations induced by STZ, established connections between certain islets and pancreatic ducts, decreased iNOS and caspase 3 immunoreactivity in β-cells, and increased the number of these cells [278].
In C2C12 skeletal muscle cells, quercetin improved glucose uptake in a concentration-dependent manner and attenuated TNF-α-induced insulin resistance by activating the Akt and AMPK pathways [160]. Alam et al. [279] investigated the effect of quercetin on DNA damage and hyperglycemia in alloxan-induced T2D mice. The results showed positive effects on DNA damage, reduced hyperglycemia, enzyme markers, and TBARS levels, and increased expression levels of GLUT4.
Arias et al. [280] administered quercetin (30 mg/kg) for 6 weeks to rats fed a high-fat/high-sucrose (HF/HS) diet and found reduced glycaemia, HOMA-IR, and insulin levels without affecting lipoprotein lipase and lipogenic enzyme activities. In L6 myoblasts, quercetin reduced ROS production and normalized GSH levels, leading to increased glucose uptake via the GLUT4 translocation pathway under TBHP-induced oxidative stress [154]. In H4IIE hepatocytes, quercetin (50 μM) inhibited G6pase and activated the hepatic AMPK pathway, and similar effects were observed in L6 muscle cells and HepG2 hepatocytes [155].
Dhanya et al. [156] elucidated the molecular mechanisms of quercetin's action against T2DM in L6 myotubes. They found involvement of the AMPK pathway and its downstream target, p38 MAPK, in the uptake of 2-NBDG.
Quercetin has been found to inhibit the activity of α-amylase and α-glucosidase, with IC50 values of 770 μg/mL and 32 μg/mL, respectively [134]. The inhibition of these enzymes by quercetin was reversible for α-amylase and competitive, while it was non-competitive for α-glucosidase.
In an animal model of T2D, a 28-day daily treatment with quercetin (25 and 50 mg/kg) reduced glycaemia, hepatic glycogen levels, and HbA1c, while improving hexokinase and G6Pase activities [282]. It also enhanced the activity of antioxidant enzymes (CAT, SOD, and GSH) and decreased TBARS levels. Quercetin restored hepatic and pancreatic damage induced by fructose-STZ in diabetic animals.
Recent studies have shown the anti-diabetic effects of quercetin in SID animals. A 28-day oral treatment with quercetin (100 mg/kg) significantly reduced hyperglycemia [283]. In another study, the combination therapy of quercetin with EGCG protected against β-cell damage in SID animals, improving insulin synthesis through up-regulation of BCL-2 expression and down-regulation of miR-16-5p [284]. Quercetin treatment in INS1 cells and diabetic db/db mice reduced glycaemia and insulin levels, increased the levels of Sirt3, CAT, and SOD, and decreased cleaved caspase-3 levels and the Bax/Bcl-2 ratio, indicating protection against oxidation-induced apoptosis in T2D via Sirt3 [157].
A self-emulsifying drug delivery system containing quercetin and other flavonoids has been developed to optimize the concentration of quercetin for effective reduction of glycaemia in diabetes [285]. This formulation showed a greater hypoglycemic effect compared to the reference drug glibenclamide, potentially due to increased insulin production.
Similar to hesperetin, quercetin extracted from T. alexandrinum exhibited beneficial anti-diabetic effects in vitro and in vivo study [209]. Another study investigated the antioxidant effects of quercetin on insulin production, signaling, and action in hypertensive rats, showing reduced serum lipid peroxidation rates, improved insulin sensitivity, and increased expression of CAT, VEGF, and M3R [286].
Recent in vitro studies have investigated the effects of quercetin on α-glucosidase and α-amylase enzyme activities. Qu et al. [158] found that quercetin and its derivatives extracted from Potentilla bifurca exhibited inhibitory effects on α-glucosidase. However, glycosylation of quercetin weakened this inhibitory effect.
In another study by Shen et al. [159]., the inhibition of α-amylase by quercetin was explored using multi-spectroscopic and molecular docking analyses to examine the structure-activity relationships. The results showed that quercetin inhibited α-amylase activity in a non-competitive manner, with an IC50 value of 0.325 mg/mL. Furthermore, quercetin altered the microenvironment of aromatic amino acid residues in the enzyme. Molecular docking analysis revealed that quercetin formed hydrogen bonds with key active site residues (Asp 300, Glu 233, and Asp 197) of the enzyme.
3.20. Myricetin
In an earlier in vitro study conducted by Ong and Khoo [129], the impact of myricetin (Fig. 22) on glucose transport and lipogenesis was evaluated using various tests. The study involved determining d-glucose transport, lipogenesis, and 3-O-methylglucose transport. The results indicated that myricetin enhanced the insulin-stimulatory effect, stimulating lipogenesis and the uptake of both D-3-O-methyl-glucose and d-glucose in rat adipocytes. Additionally, myricetin increased the maximum velocity (Vmax) of glucose transport.
Fig. 22.

Chemical structure of Myricetin.
In a subsequent study by Ong and Khoo [130], diabetic mice were intraperitoneally injected with myricetin for four days. The treatment resulted in a 50 % reduction in hyperglycemia in the animals. Furthermore, myricetin increased insulin-stimulated lipogenesis and stimulated glucose transport in adipocytes. Regarding glycogen metabolism, myricetin increased the content of G6Pase, hepatic glycogen synthase, and hepatic glycogen without affecting the total glycogen synthase content.
To investigate the impact of myricetin on lowering plasma glucose levels in diabetes, Liu et al. [239,240] conducted a study where diabetic rats were treated with a daily dose of myricetin for two weeks. The results revealed that myricetin decreased plasma glucose concentrations in a concentration-dependent manner and promoted glucose storage in the soleus muscles of diabetic rats. This effect was accompanied by an increased expression of GLUT4, a glucose transporter protein.
To elucidate the underlying mechanism of this process in vitro, rat adipocytes were isolated and exposed to myricetin in a study by Strobel et al. [131]. The findings demonstrated that myricetin inhibited glucose transport and the uptake of methylglucose by these cells.
In contrast, a study conducted by Liu et al. [241] investigated the improvement in insulin sensitivity in obese rats. These rats were given intravenous injections of myricetin three times a day for one week. The treatment resulted in enhanced insulin sensitivity through significant changes in post-receptor insulin signaling.
To further examine the insulin-resistant model, the same research team fed rats a high-fructose diet (HFruD) for six weeks. Oral glucose tolerance test (OGTT) results demonstrated that myricetin supplementation reduced blood glucose levels and improved insulin resistance by increasing insulin sensitivity. The activation of insulin receptors was also assessed, revealing that myricetin enhanced insulin sensitivity by improving the activities of GLUT4 and IRS-1-associated PI3-kinase in the rats' soleus muscles [242].
Insulin-resistant rats were subjected to intravenous injections of myricetin (1 mg/kg) three times a day for a duration of two weeks. The treatment resulted in reduced plasma glucose levels accompanied by increased concentrations of plasma β-endorphin [243]. Moreover, an investigation into the activation of insulin receptors (IRs) demonstrated improvements in the signaling intermediates downstream of IRs.
Furthermore, in a study involving C2C12 cells, treatment with myricetin was found to enhance glucose uptake and increase the activities of AMPK and Akt. This led to improved insulin sensitivity by reducing insulin resistance [132].
Kandasamy and Ashokkumar [244,246], conducted studies in which they administered a daily oral dose of myricetin (1 mg/kg) to SID rats. This treatment resulted in several beneficial effects, including reduced blood glucose levels, normalization of carbohydrate metabolic markers (such as HbA1c, gluconeogenic enzymes, and GP), increased insulin levels, and improved expression of glycogen, GS, and insulin signaling molecules (IRS-1, IRS-2, PKB, GLUT2, and GLUT4). Furthermore, histopathological evaluation of pancreatic cells showed that myricetin exerted a protective effect against damage caused by STZ.
In db/db mice, myricetin demonstrated inhibition of the digestive enzyme α-glucosidase and reduced levels of HbA1c, fasting blood glucose, and intestinal maltase activity [247].
In a study conducted by Ha-Neul Choi et al. [245], rats were fed a high-fat/high-sucrose (HF/HS) diet supplemented with 0.12 % myricetin for a period of 12 weeks. The myricetin supplementation resulted in decreased levels of insulin and blood glucose, as well as reduced HOMA-IR values, compared to the control group. Additionally, the myricetin-treated group exhibited lower levels of pro-inflammatory cytokines (TNF-α and IL-6).
Meng et al. [134] discovered that myricetin, like quercetin mentioned earlier, inhibits the activity of α-glucosidase and α-amylase. The IC50 values for myricetin were found to be 3 μg/mL for α-glucosidase and 662 μg/mL for α-amylase. The inhibitory effect of myricetin on these enzymes was reversible, but it acted competitively on α-amylase and non-competitively on α-glucosidase. Similarly, Arumugam et al. [133] also observed this inhibitory effect of myricetin on both enzymes involved in carbohydrate hydrolysis.
Furthermore, myricetin has demonstrated insulin-like activity in 3T3-L1 cells. It enhances glucose uptake, lipid accumulation, and adiponectin production by activating the insulin-signaling pathway [248]. In addition, Hu et al. [249] found that myricetin increases adiponectin expression in brown adipose tissue (BAT) and improves insulin resistance by activating BAT, highlighting its glucoregulatory properties.
Li et al. [135] conducted experiments involving both acute and chronic treatments with myricetin to assess its hypoglycemic effect in animals with T1D in vivo and to understand its mechanism of action in the HepG2 cell line in vitro. In the acute treatment, myricetin demonstrated a concentration-dependent increase in β-endorphin (BER) and adropin secretion, leading to a reduction in hyperglycemia. This effect was attributed to the activation of the GLP-1 receptor, which in turn regulated the expression of adropin. It was deduced that the observed rise in plasma adropin levels was mediated by endogenous β-endorphin subsequent to GLP-1 receptor activation.
Early management of predisposition to diabetes and prediabetes is crucial. A recent study by Yang et al. [136], examined the impact of myricetin on prediabetes both in vivo and in vitro. In RAW 264.7 cells exposed to high glucose levels, a treatment of 10 μM myricetin decreased the expression levels of IL-2 and interferon-gamma (IFN-γ) and reversed the immunosuppressive effects induced by elevated glucose. In prediabetic mice fed a HFD, oral administration of myricetin demonstrated significant hypoglycemic and hypolipidemic effects, and it restored their innate and adaptive immune functions.
Furthermore, as noted above, co-treatment of myricetin and kaempferol has synergistic therapeutic potential in the management of diabetes [232].
4. Conclusion and perspectives
In recent years, there has been a surge in interest surrounding the potential of natural flavonoids as antidiabetic agents. These bioactive compounds, abundant in fruits, vegetables, and other plant sources, have garnered attention for their reported benefits in regulating glucose levels and enhancing insulin sensitivity. Flavonoids, with their diverse biological activities, offer promising antidiabetic properties by modulating key enzymes in glucose metabolism, improving insulin signaling, and reducing oxidative stress associated with diabetes.
Recent scientific literature highlights specific flavonoids like quercetin, resveratrol, and epigallocatechin gallate, showcasing their potential in lowering blood glucose levels, enhancing insulin sensitivity, and protecting pancreatic beta cells. Their natural origin and generally favorable safety profile make them attractive alternatives to conventional antidiabetic medications, particularly in resource-limited settings where they are readily available and affordable.
Despite the promising outlook, further research is necessary to elucidate the mechanisms of action, optimal dosages, and long-term effects of flavonoids in diabetes management. Rigorous preclinical and clinical studies are crucial to establish their efficacy, safety, and potential interactions with other medications.
In conclusion, while there is optimism regarding the potential of flavonoids as complementary or alternative therapies for diabetes, continued research efforts and clinical trials are imperative to fully harness their therapeutic benefits and integrate them into mainstream diabetes care.
CRediT authorship contribution statement
Abdelhakim Bouyahya: Writing – original draft, Supervision, Resources, Methodology. Abdelaali Balahbib: Writing – review & editing, Methodology, Investigation. Asaad Khalid: Writing – original draft, Validation, Resources. Hafiz A. Makeen: Writing – original draft, Software, Resources, Project administration. Hassan A. Alhazmi: Writing – review & editing, Resources, Project administration, Methodology. Mohammed Albratty: Writing – original draft, Software. Andi Hermansyah: Writing – review & editing, Supervision, Software, Resources, Methodology. Long Chiau Ming: Writing – original draft, Supervision, Resources, Project administration. Khang Wen Goh: Writing – original draft, Validation, Supervision, Resources, Methodology. Nasreddine El Omari: Writing – original draft, Validation, Software, Resources, Project administration, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their thanks to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research through the project number (ISP23-81).
Contributor Information
Asaad Khalid, Email: akahmed@jazanu.edu.sa.
Andi Hermansyah, Email: andi-h@ff.unair.ac.id.
Long Chiau Ming, Email: chiaumingl@sunway.edu.my.
References
- 1.Al-Ishaq R.K., Abotaleb M., Kubatka P., Kajo K., Büsselberg D. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9:430. doi: 10.3390/biom9090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds M.A. Modifiable risk factors in periodontitis: at the intersection of aging and disease. Periodontol. 2014;64:7–19. doi: 10.1111/prd.12047. 2000. [DOI] [PubMed] [Google Scholar]
- 3.Alemany-Cosme E., Sáez-González E., Moret I., Mateos B., Iborra M., Nos P., Sandoval J., Beltrán B. Oxidative stress in the pathogenesis of Crohn's disease and the interconnection with immunological response, microbiota, external environmental factors, and epigenetics. Antioxidants. 2021;10:64. doi: 10.3390/antiox10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellot M., Jawal L., Morel T., Fournier J.-P., Tubach F., Cadwallader J.-S., Christiaens A., Zerah L. Barriers and Enablers for Deprescribing glucose-lowering treatment in older adults: a Systematic review. J. Am. Med. Dir. Assoc. 2024;25:439–447.e18. doi: 10.1016/j.jamda.2023.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Yakaryılmaz F.D., Öztürk Z.A. Treatment of type 2 diabetes mellitus in the elderly. World J. Diabetes. 2017;8:278–285. doi: 10.4239/wjd.v8.i6.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunstan D.W., Daly R.M., Owen N., Jolley D., De Courten M., Shaw J., Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–1736. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 7.Castaneda C., Layne J.E., Munoz-Orians L., Gordon P.L., Walsmith J., Foldvari M., Roubenoff R., Tucker K.L., Nelson M.E. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 8.Meyerovitch J., Farfel Z., Sack J., Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J. Biol. Chem. 1987;262:6658–6662. [PubMed] [Google Scholar]
- 9.Rahman S., Rahman T., Ismail A.A.-S., Rashid A.R.A. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes. Metab. 2007;9:767–780. doi: 10.1111/j.1463-1326.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 10.Kranstuber A.L., Del Rio C., Biesiadecki B.J., Hamlin R.L., Ottobre J., Gyorke S., Lacombe V.A. Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front. Physiol. 2012;3:292. doi: 10.3389/fphys.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur. J. Clin. Invest. 2004;34:785–796. doi: 10.1111/j.1365-2362.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 12.Scheen A.J. Is there a role for α-glucosidase inhibitors in the prevention of type 2 diabetes mellitus? Drugs. 2003;63:933–951. doi: 10.2165/00003495-200363100-00002. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan V., Rani R., Awana M., Pitale D., Kulshreshta A., Sharma S., Bollinedi H., Singh A., Singh B., Singh A.K. Role of nutraceutical starch and proanthocyanidins of pigmented rice in regulating hyperglycemia: enzyme inhibition, enhanced glucose uptake and hepatic glucose homeostasis using in vitro model. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127505. [DOI] [PubMed] [Google Scholar]
- 14.Williams B.A., Verstegen M.W., Tamminga S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001;14:207–228. doi: 10.1079/NRR200127. [DOI] [PubMed] [Google Scholar]
- 15.Bouche C., Serdy S., Kahn C.R., Goldfine A.B. The cellular fate of glucose and its relevance in type 2 diabetes. Endocr. Rev. 2004;25:807–830. doi: 10.1210/er.2003-0026. [DOI] [PubMed] [Google Scholar]
- 16.Gray L.R., Tompkins S.C., Taylor E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014;71:2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson J.W. Metabolic stress in insulin's target cells leads to ROS accumulation–a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581:3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Gannon N.P., Schnuck J.K., Vaughan R.A. BCAA metabolism and insulin sensitivity–Dysregulated by metabolic status? Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700756. [DOI] [PubMed] [Google Scholar]
- 19.Anwer T., Safhi M.M., Makeen H.A., Alshahrani S., Siddiqui R., Sivakumar S.M., Shaheen E.S., Alam M.F. Antidiabetic potential of Moringa oleifera Lam. leaf extract in type 2 diabetic rats, and its mechanism of action. Trop. J. Pharm. Res. 2021;20:95–103. doi: 10.4314/tjpr.v20i1.15. [DOI] [Google Scholar]
- 20.Anwer T., Sharma M., Khan G., Alam M.F., Alam N., Ali M.S., Alam M.S. Preventive role of Withania somnifera on hyperlipidemia and cardiac oxidative stress in streptozotocin induced type 2 diabetic rats. Trop. J. Pharm. Res. 2017;16:119–125. doi: 10.4314/tjpr.v16i1.15. [DOI] [Google Scholar]
- 21.Moni S.S., Alam M.F., Makeen H.A., Jabeen A., Sanobar S., Siddiqui R., Moochikkal R., Fouda S. Therapeutic potential of oleic acid nanovesicles prepared from petroleum ether extract of Sargassum binderi in streptozotocin–induced diabetic wound in Wistar rats. Trop. J. Pharm. Res. 2018;17:2123–2128. doi: 10.4314/tjpr.v17i11.2. [DOI] [Google Scholar]
- 22.Zafar A., Alruwaili N.K., Panda D.S., Imam S.S., Alharbi K.S., Afzal M., Shalaby K., Kazmi I., Alshehri S. Potential of natural bioactive compounds in management of diabetes: review of preclinical and clinical evidence. Curr. Pharmacol. Rep. 2021;7:107–122. [Google Scholar]
- 23.Fatiha M., Fatma B., Awatif B., Nesrine A., Noureddine D. Antidiabetic bioactive compounds from plants, Med. Technol. J. 2018;2:199–214. [Google Scholar]
- 24.Yogeswari S., Bindu K.H., Kamalraj S., Ashokkumar V., Jayabaskaran C. Antidiabetic, Antithrombin and Cytotoxic bioactive compounds in five cultivars of Piper betle L. Environ. Technol. Innov. 2020;20 [Google Scholar]
- 25.Agarwal S., Singh V., Chauhan K. Antidiabetic potential of seaweed and their bioactive compounds: a review of developments in last decade. Crit. Rev. Food Sci. Nutr. 2021:1–32. doi: 10.1080/10408398.2021.2024130. [DOI] [PubMed] [Google Scholar]
- 26.Bouyahya A., El Omari N., Elmenyiy N., Guaouguaou F.-E., Balahbib A., Belmehdi O., Salhi N., Imtara H., Mrabti H.N., El-Shazly M. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci. Technol. 2021;115:147–254. [Google Scholar]
- 27.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Savych A., Milian I. Total flavonoid content in the herbal mixture with antidiabetic activity. Pharmacologyonline. 2021;2:68–75. [Google Scholar]
- 29.Xiao J., Capanoglu E., Jassbi A.R., Miron A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016;56:S29–S45. doi: 10.1080/10408398.2015.1067595. [DOI] [PubMed] [Google Scholar]
- 30.Brasnyó P., Molnár G.A., Mohás M., Markó L., Laczy B., Cseh J., Mikolás E., Szijártó I.A., Mérei Á., Halmai R., Mészáros L.G., Sümegi B., Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 31.Bhatt J.K., Thomas S., Nanjan M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Yoshino J., Conte C., Fontana L., Mittendorfer B., Imai S., Schechtman K.B., Gu C., Kunz I., Fanelli F.R., Patterson B.W., Klein S. Resveratrol supplementation does not improve metabolic function in Nonobese women with normal glucose tolerance. Cell Metabol. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movahed A., Nabipour I., Lieben Louis X., Thandapilly S.J., Yu L., Kalantarhormozi M., Rekabpour S.J., Netticadan T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013;2013:1–11. doi: 10.1155/2013/851267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Méndez-del Villar M., González-Ortiz M., Martínez-Abundis E., Pérez-Rubio K.G., Lizárraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014;12:497–501. doi: 10.1089/met.2014.0082. [DOI] [PubMed] [Google Scholar]
- 35.Williams C.B., Hughes M.C., Edgett B.A., Scribbans T.D., Simpson C.A., Perry C.G.R., Gurd B.J. An Examination of Resveratrol's mechanisms of action in human tissue: impact of a single dose in vivo and dose responses in skeletal muscle ex vivo. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S., Zhao X., Ran L., Wan J., Wang X., Qin Y., Shu F., Gao Y., Yuan L., Zhang Q., Mi M. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig. Liver Dis. 2015;47:226–232. doi: 10.1016/j.dld.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Thazhath S.S., Wu T., Bound M.J., Checklin H.L., Standfield S., Jones K.L., Horowitz M., Rayner C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. Am. J. Clin. Nutr. 2016;103:66–70. doi: 10.3945/ajcn.115.117440. [DOI] [PubMed] [Google Scholar]
- 38.Banaszewska B., Wrotyńska-Barczyńska J., Spaczynski R.Z., Pawelczyk L., Duleba A.J. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2016;101:4322–4328. doi: 10.1210/jc.2016-1858. [DOI] [PubMed] [Google Scholar]
- 39.Most J., Timmers S., Warnke I., Jocken J.W., van Boekschoten M., de Groot P., Bendik I., Schrauwen P., Goossens G.H., Blaak E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: a randomized controlled trial. Am. J. Clin. Nutr. 2016;104:215–227. doi: 10.3945/ajcn.115.122937. [DOI] [PubMed] [Google Scholar]
- 40.Timmers S., de Ligt M., Phielix E., van de Weijer T., Hansen J., Moonen-Kornips E., Schaart G., Kunz I., Hesselink M.K.C., Schrauwen-Hinderling V.B., Schrauwen P. Resveratrol as Add-on therapy in subjects with well-controlled type 2 diabetes: a randomized controlled trial. Diabetes Care. 2016;39:2211–2217. doi: 10.2337/dc16-0499. [DOI] [PubMed] [Google Scholar]
- 41.Kjær T.N., Ornstrup M.J., Poulsen M.M., Stødkilde-Jørgensen H., Jessen N., Jørgensen J.O.L., Richelsen B., Pedersen S.B. No beneficial effects of resveratrol on the metabolic syndrome: a randomized placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2017;102:1642–1651. doi: 10.1210/jc.2016-2160. [DOI] [PubMed] [Google Scholar]
- 42.Made S., Plat J., Mensink R. Trans-resveratrol supplementation and endothelial function during the fasting and postprandial Phase: a randomized placebo-controlled trial in overweight and slightly obese participants. Nutrients. 2017;9:596. doi: 10.3390/nu9060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Ligt M., Bruls Y.M.H., Hansen J., Habets M.-F., Havekes B., Nascimento E.B.M., Moonen-Kornips E., Schaart G., Schrauwen-Hinderling V.B., van Marken Lichtenbelt W., Schrauwen P. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol. Metab. 2018;12:39–47. doi: 10.1016/j.molmet.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma N., Zhang Y. Effects of resveratrol therapy on glucose metabolism, insulin resistance, inflammation, and renal function in the elderly patients with type 2 diabetes mellitus: a randomized controlled clinical trial protocol. Medicine (Baltim.) 2022;101 doi: 10.1097/MD.0000000000030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahjabeen W., Khan D.A., Mirza S.A. Role of resveratrol supplementation in regulation of glucose hemostasis, inflammation and oxidative stress in patients with diabetes mellitus type 2: a randomized, placebo-controlled trial, Complement. Ther. Med. 2022;66 doi: 10.1016/j.ctim.2022.102819. [DOI] [PubMed] [Google Scholar]
- 46.Nagao T., Meguro S., Hase T., Otsuka K., Komikado M., Tokimitsu I., Yamamoto T., Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2009;17:310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi M., Miyashita M., Suzuki K., Bae S., Kim H.-K., Wakisaka T., Matsui Y., Takeshita M., Yasunaga K. Acute ingestion of catechin-rich green tea improves postprandial glucose status and increases serum thioredoxin concentrations in postmenopausal women. Br. J. Nutr. 2014;112:1542–1550. doi: 10.1017/S0007114514002530. [DOI] [PubMed] [Google Scholar]
- 48.Bazyar H., Moradi L., Zaman F., Zare Javid A. The effects of rutin flavonoid supplement on glycemic status, lipid profile, atherogenic index of plasma, brain-derived neurotrophic factor (BDNF), some serum inflammatory, and oxidative stress factors in patients with type 2 diabetes mellitus: a double-blind, placebo-controlled trial. Phytother Res. 2022;37:271–284. doi: 10.1002/ptr.7611. [DOI] [PubMed] [Google Scholar]
- 49.Hashizume Y., Tandia M. Suppressive effect of a single dose of monoglucosyl rutin on postprandial blood glucose elevation: a randomized, placebo-controlled, double-blind crossover study, Funct. Foods Health Dis. 2021;11:270–282. [Google Scholar]
- 50.Dower J.I., Geleijnse J.M., Gijsbers L., Zock P.L., Kromhout D., Hollman P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015;101:914–921. doi: 10.3945/ajcn.114.098590. [DOI] [PubMed] [Google Scholar]
- 51.Rizvi S.I., Zaid M.A. Insulin-like effect of (-)Epicatechin on Erythrocyte membrane Acetylcholinesterase activity in type 2 diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2001;28:776–778. doi: 10.1046/j.1440-1681.2001.03513.x. [DOI] [PubMed] [Google Scholar]
- 52.Osama H., Hamed E.O., Mahmoud M.A., Abdelrahim M.E. The effect of hesperidin and diosmin individually or in combination on metabolic profile and neuropathy among diabetic patients with metabolic syndrome: a randomized controlled trial. J. Diet. Suppl. 2022:1–14. doi: 10.1080/19390211.2022.2107138. [DOI] [PubMed] [Google Scholar]
- 53.Yamagata K., Miyashita A., Chino M., Matsufuji H. Apigenin inhibits tumor necrosis factor alpha plus high glucose-induced LOX-1 expression in human endothelial cells. Microvasc. Res. 2011;81:60–67. doi: 10.1016/j.mvr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Yamagata K., Miyashita A., Matsufuji H., Chino M. Dietary flavonoid apigenin inhibits high glucose and tumor necrosis factor α-induced adhesion molecule expression in human endothelial cells. J. Nutr. Biochem. 2010;21:116–124. doi: 10.1016/j.jnutbio.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Zeng L., Zhang G., Lin S., Gong D. Inhibitory mechanism of apigenin on α-glucosidase and Synergy analysis of flavonoids. J. Agric. Food Chem. 2016;64:6939–6949. doi: 10.1021/acs.jafc.6b02314. [DOI] [PubMed] [Google Scholar]
- 56.Sahnoun M., Saibi W., Brini F., Bejar S. Apigenin isolated from A. americana encodes Human and Aspergillus oryzae S2 α-amylase inhibitions: credible approach for antifungal and antidiabetic therapies. J. Food Sci. Technol. 2018;55:1489–1498. doi: 10.1007/s13197-018-3065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yousefi F., Mahjoub S., Pouramir M., Khadir F. Hypoglycemic activity of Pyrus biossieriana Buhse leaf extract and arbutin: inhibitory effects on alpha amylase and alpha glucosidase. Casp. J. Intern. Med. 2013;4:763. [PMC free article] [PubMed] [Google Scholar]
- 58.Gholami Bahnemiri M., Nouri H.R., Zabihi E., Sadeghi F., Pouramir M. Effects of arbutin on glucose uptake by glucose transporter 4 (GLUT4) and its cytoprotective properties in L6 skeletal muscle cell line. Cell Biochem. Funct. 2022;40:417–425. doi: 10.1002/cbf.3706. [DOI] [PubMed] [Google Scholar]
- 59.Fang P., Yu M., Zhang L., Wan D., Shi M., Zhu Y., Bo P., Zhang Z. Baicalin against obesity and insulin resistance through activation of AKT/AS160/GLUT4 pathway. Mol. Cell. Endocrinol. 2017;448:77–86. doi: 10.1016/j.mce.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 60.Fang P., Yu M., Min W., Han S., Shi M., Zhang Z., Bo P. Beneficial effect of baicalin on insulin sensitivity in adipocytes of diet-induced obese mice. Diabetes Res. Clin. Pract. 2018;139:262–271. doi: 10.1016/j.diabres.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Fang P., Sun Y., Gu X., Shi M., Bo P., Zhang Z., Bu L. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1α pathway. Phytomedicine. 2019;64 doi: 10.1016/j.phymed.2019.153074. [DOI] [PubMed] [Google Scholar]
- 62.Yu M., Han S., Wang M., Han L., Huang Y., Bo P., Fang P., Zhang Z. Baicalin protects against insulin resistance and metabolic dysfunction through activation of GALR2/GLUT4 signaling. Phytomedicine. 2022;95 doi: 10.1016/j.phymed.2021.153869. [DOI] [PubMed] [Google Scholar]
- 63.Miao L., Zhang X., Zhang H., Cheong M.S., Chen X., Farag M.A., Cheang W.S., Xiao J. Baicalin ameliorates insulin resistance and regulates hepatic glucose metabolism via activating insulin signaling pathway in obese pre-diabetic mice. Phytomedicine. 2024;124 doi: 10.1016/j.phymed.2023.155296. [DOI] [PubMed] [Google Scholar]
- 64.Shimizu M., Kobayashi Y., Suzuki M., Satsu H., Miyamoto Y. Regulation of intestinal glucose transport by tea catechins. Biofactors. 2000;13:61–65. doi: 10.1002/biof.5520130111. [DOI] [PubMed] [Google Scholar]
- 65.Matsui T., Tanaka T., Tamura S., Toshima A., Tamaya K., Miyata Y., Tanaka K., Matsumoto K. α-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007;55:99–105. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- 66.Murase T., Misawa K., Haramizu S., Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem. Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 67.Kamiyama O., Sanae F., Ikeda K., Higashi Y., Minami Y., Asano N., Adachi I., Kato A. In vitro inhibition of α-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010;122:1061–1066. doi: 10.1016/j.foodchem.2010.03.075. [DOI] [Google Scholar]
- 68.Yilmazer-Musa M., Griffith A.M., Michels A.J., Schneider E., Frei B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agric. Food Chem. 2012;60:8924–8929. doi: 10.1021/jf301147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu Y., Zhang Z., Li L., Joshi M.K., Huang N., Niu J., Lu Y. Catechins play key role in green tea extract–induced postprandial hypoglycemic potential in vitro. Eur. Food Res. Technol. 2013;237:89–99. doi: 10.1007/s00217-013-1945-6. [DOI] [Google Scholar]
- 70.Sun L., Song Y., Chen Y., Ma Y., Fu M., Liu X. The galloyl moiety enhances the inhibitory activity of catechins and theaflavins against α-glucosidase by increasing the polyphenol–enzyme binding interactions. Food Funct. 2021;12:215–229. doi: 10.1039/d0fo02689a. [DOI] [PubMed] [Google Scholar]
- 71.Mechchate H., Es-Safi I., Haddad H., Bekkari H., Grafov A., Bousta D. Combination of Catechin, Epicatechin, and Rutin: optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2021;88 doi: 10.1016/j.jnutbio.2020.108520. [DOI] [PubMed] [Google Scholar]
- 72.Taslimi P., Kocyigit U.M., Tüzün B., Kirici M. Biological effects and molecular docking studies of Catechin 5-O-gallate: antioxidant, anticholinergics, antiepileptic and antidiabetic potentials. J. Biomol. Struct. Dyn. 2022;40:2489–2497. doi: 10.1080/07391102.2020.1840440. [DOI] [PubMed] [Google Scholar]
- 73.Adisakwattana S., Ngamrojanavanich N., Kalampakorn K., Tiravanit W., Roengsumran S., Yibchok-Anun S. Inhibitory activity of cyanidin-3-rutinoside on α-glucosidase. J. Enzyme Inhib. Med. Chem. 2004;19:313–316. doi: 10.1080/14756360409162443. [DOI] [PubMed] [Google Scholar]
- 74.Adisakwattana S., Charoenlertkul P., Yibchok-anun S. α-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J. Enzyme Inhib. Med. Chem. 2009;24:65–69. doi: 10.1080/14756360801906947. [DOI] [PubMed] [Google Scholar]
- 75.Akkarachiyasit S., Charoenlertkul P., Yibchok-anun S., Adisakwattana S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010;11:3387–3396. doi: 10.3390/ijms11093387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adisakwattana S., Yibchok-Anun S., Charoenlertkul P., Wongsasiripat N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011;49:36–41. doi: 10.3164/jcbn.10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inaguma T., Han J., Isoda H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. BMC Proc. 2011;5:P21. doi: 10.1186/1753-6561-5-S8-P21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scazzocchio B., Vari R., Filesi C., D'Archivio M., Santangelo C., Giovannini C., Iacovelli A., Silecchia G., Volti G.L., Galvano F., Masella R. Cyanidin-3-O- -glucoside and protocatechuic acid exert insulin-like effects by upregulating PPAR activity in human Omental adipocytes. Diabetes. 2011;60:2234–2244. doi: 10.2337/db10-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J.S., Kim Y.R., Park J.M., Kim Y.E., Baek N.I., Hong E.K. Cyanidin-3-glucoside isolated from mulberry fruits protects pancreatic β-cells against glucotoxicity-induced apoptosis. Mol. Med. Rep. 2015;11:2723–2728. doi: 10.3892/mmr.2014.3078. [DOI] [PubMed] [Google Scholar]
- 80.Lee J.S., Kim Y.R., Song I.G., Ha S.-J., Kim Y.E., Baek N.-I., Hong E.K. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. Int. J. Mol. Med. 2015;35:405–412. doi: 10.3892/ijmm.2014.2013. [DOI] [PubMed] [Google Scholar]
- 81.Matsukawa T., Inaguma T., Han J., Villareal M.O., Isoda H. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. J. Nutr. Biochem. 2015;26:860–867. doi: 10.1016/j.jnutbio.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 82.Talagavadi V., Rapisarda P., Galvano F., Pelicci P., Giorgio M. Cyanidin-3- O -β-glucoside and protocatechuic acid activate AMPK/mTOR/S6K pathway and improve glucose homeostasis in mice. J. Funct.Foods. 2016;21:338–348. doi: 10.1016/j.jff.2015.12.007. [DOI] [Google Scholar]
- 83.Choi Kyungha, Choi S.-I., Park M.H., Han J.-S. Cyanidin-3-O-glucoside ameliorates postprandial hyperglycemia in diabetic mice. J. Life Sci. 2017;27:32–37. doi: 10.5352/JLS.2017.27.1.32. [DOI] [Google Scholar]
- 84.Choi Kyungha, Lee H.A., Park M.H., Han J.-S. Cyanidin-3-rutinoside increases glucose uptake by activating the PI3K/Akt pathway in 3T3-L1 adipocytes. Environ. Toxicol. Pharmacol. 2017;54:1–6. doi: 10.1016/j.etap.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Suantawee T., Elazab S., Hsu W., Yao S., Cheng H., Adisakwattana S. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of l-type voltage-dependent Ca2+ channels. Nutrients. 2017;9:814. doi: 10.3390/nu9080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cásedas G., Les F., González-Burgos E., Gómez-Serranillos M.P., Smith C., López V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes, South Afr. J. Bot., Le. 2019;120:241–246. doi: 10.1016/j.sajb.2018.07.001. [DOI] [Google Scholar]
- 87.Fraisse D., Bred A., Felgines C., Senejoux F. Impact of simulated gastrointestinal conditions on antiglycoxidant and α-glucosidase inhibition capacities of cyanidin-3-O-glucoside. Antioxidants. 2021;10:1670. doi: 10.3390/antiox10111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jia Y., Wu C., Rivera-Piza A., Kim Y.-J., Lee J.H., Lee S.-J. Mechanism of action of cyanidin 3-O-glucoside in gluconeogenesis and oxidative stress-induced cancer cell senescence. Antioxidants. 2022;11:749. doi: 10.3390/antiox11040749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ye X., Chen W., Tu P., Jia R., Liu Y., Tang Q., Chen C., Yang C., Zheng X., Chu Q. Antihyperglycemic effect of an anthocyanin, cyanidin-3-O-glucoside, is achieved by regulating GLUT-1 via the Wnt/β-catenin-WISP1 signaling pathway. Food Funct. 2022;13:4612–4623. doi: 10.1039/d1fo03730g. [DOI] [PubMed] [Google Scholar]
- 90.Kongthitilerd P., Thilavech T., Marnpae M., Rong W., Yao S., Adisakwattana S., Cheng H., Sauntawee T. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca2+ channels and the PLC-IP3 pathway in pancreatic β-cells, Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112494. [DOI] [PubMed] [Google Scholar]
- 91.Jayaprakasam B., Vareed S.K., Olson L.K., Nair M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 92.Rojo L.E., Ribnicky D., Logendra S., Poulev A., Rojas-Silva P., Kuhn P., Dorn R., Grace M.H., Lila M.A., Raskin I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis) Food Chem. 2012;131:387–396. doi: 10.1016/j.foodchem.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hidalgo J., Teuber S., Morera F., Ojeda C., Flores C., Hidalgo M., Núñez L., Villalobos C., Burgos R. Delphinidin reduces glucose uptake in mice jejunal tissue and human intestinal cells lines through FFA1/GPR40. Int. J. Mol. Sci. 2017;18:750. doi: 10.3390/ijms18040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mojica L., Berhow M., Gonzalez de Mejia E. Food Chem. 2017;229:628–639. doi: 10.1016/j.foodchem.2017.02.124. [DOI] [PubMed] [Google Scholar]
- 95.Lai D., Huang M., Zhao L., Tian Y., Li Y., Liu D., Wu Y., Deng F. Delphinidin-induced autophagy protects pancreatic β cells against apoptosis resulting from high-glucose stress via AMPK signaling pathway. Acta Biochim. Biophys. Sin. 2019;51:1242–1249. doi: 10.1093/abbs/gmz126. [DOI] [PubMed] [Google Scholar]
- 96.Hii C.S., Howell S.L. Effects of epicatechin on rat islets of Langerhans. Diabetes. 1984;33:291–296. doi: 10.2337/diab.33.3.291. [DOI] [PubMed] [Google Scholar]
- 97.Ahmad F., Khalid P., Khan M.M., Rastogi A.K., Kidwai J.R. Insulin like activity in (−) epicatechin. Acta Diabetol. Lat. 1989;26:291–300. doi: 10.1007/BF02624640. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad F. Effect of (-) epicatechin on cAMP content, insulin release and conversion of proinsulin to insulin in immature and mature rat islets in vitro. Indian J. Exp. Biol. 1991;29:516–520. [PubMed] [Google Scholar]
- 99.Kim M.-J., Ryu G.R., Chung J.-S., Sim S.S., Min D.S., Rhie D.-J., Yoon S.H., Hahn S.J., Kim M.-S., Jo Y.-H. Protective effects of epicatechin against the toxic effects of streptozotocin on rat pancreatic islets: in vivo and in vitro. Pancreas. 2003;26:292–299. doi: 10.1097/00006676-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 100.Martín M.Á., Fernández-Millán E., Ramos S., Bravo L., Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014;58:447–456. doi: 10.1002/mnfr.201300291. [DOI] [PubMed] [Google Scholar]
- 101.Ueda-Wakagi M., Mukai R., Fuse N., Mizushina Y., Ashida H. 3-O-Acyl-epicatechins increase glucose uptake activity and GLUT4 translocation through activation of PI3K signaling in skeletal muscle cells. Int. J. Mol. Sci. 2015;16:16288–16299. doi: 10.3390/ijms160716288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Z.-H., Li B. (-)-Epicatechin and β-glucan from highland barley grain modulated glucose metabolism and showed synergistic effect via Akt pathway. J. Funct.Foods. 2021;87 [Google Scholar]
- 103.Yoshida H., Takamura N., Shuto T., Ogata K., Tokunaga J., Kawai K., Kai H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-α in mouse adipocytes. Biochem. Biophys. Res. Commun. 2010;394:728–732. doi: 10.1016/j.bbrc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 104.Gong Y., Qin X.-Y., Zhai Y.-Y., Hao H., Lee J., Park Y.-D. Inhibitory effect of hesperetin on α-glucosidase: molecular dynamics simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2017;101:32–39. doi: 10.1016/j.ijbiomac.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 105.Chae B.S., Shin T.Y. Articels: hesperidin ameliorates TNF-α-mediated insulin resistance in differentiated 3T3-L1 cells. Nat. Prod. Sci. 2012;18:254–260. [Google Scholar]
- 106.Fang X.-K., Gao J., Zhu D.-N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82:615–622. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 107.Lee Y.J., Suh K.S., Choi M.C., Chon S., Oh S., Woo J.-T., Kim S.-W., Kim J.-W., Kim Y.S. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytother Res. 2010;24:419–423. doi: 10.1002/ptr.2983. [DOI] [PubMed] [Google Scholar]
- 108.Habtemariam S. α-Glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat. Prod. Commun. 2011;6 doi: 10.1177/1934578X1100600211. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y., Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011;670:325–332. doi: 10.1016/j.ejphar.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Zhen W., Maechler P., Liu D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. J. Nutr. Biochem. 2013;24:638–646. doi: 10.1016/j.jnutbio.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li H., Ji H.-S., Kang J.-H., Shin D.-H., Park H.-Y., Choi M.-S., Lee C.-H., Lee I.-K., Yun B.-S., Jeong T.-S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic β-cell function and suppressing hepatic lipid accumulation in db/db mice. J. Agric. Food Chem. 2015;63:7198–7210. doi: 10.1021/acs.jafc.5b01639. [DOI] [PubMed] [Google Scholar]
- 112.Peng X., Zhang G., Liao Y., Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016;190:207–215. doi: 10.1016/j.foodchem.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 113.Ibitoye O.B., Uwazie J.N., Ajiboye T.O. Bioactivity-guided isolation of kaempferol as the antidiabetic principle from Cucumis sativus L. fruits. J. Food Biochem. 2018;42 doi: 10.1111/jfbc.12479. [DOI] [Google Scholar]
- 114.Sheng Z., Ai B., Zheng L., Zheng X., Xu Z., Shen Y., Jin Z. Inhibitory activities of kaempferol, galangin, carnosic acid and polydatin against glycation and α-amylase and α-glucosidase enzymes. Int. J. Food Sci. Technol. 2018;53:755–766. doi: 10.1111/ijfs.13579. [DOI] [Google Scholar]
- 115.Varshney R., Gupta S., Roy P. Cytoprotective effect of kaempferol against palmitic acid-induced pancreatic β-cell death through modulation of autophagy via AMPK/mTOR signaling pathway. Mol. Cell. Endocrinol. 2017;448:1–20. doi: 10.1016/j.mce.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 116.Yin P., Yang L., Xue Q., Yu M., Yao F., Sun L., Liu Y. Identification and inhibitory activities of ellagic acid- and kaempferol-derivatives from Mongolian oak cups against α-glucosidase, α-amylase and protein glycation linked to type II diabetes and its complications and their influence on HepG2 cells' viability. Arab. J. Chem. 2018;11:1247–1259. doi: 10.1016/j.arabjc.2017.10.002. [DOI] [Google Scholar]
- 117.Kim J.-S., Kwon C.-S., SoN K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000;64:2458–2461. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- 118.Matsui T., Kobayashi M., Hayashida S., Matsumoto K. Luteolin, a flavone, does not suppress postprandial glucose absorption through an inhibition of α-glucosidase action. Biosci. Biotechnol. Biochem. 2002;66:689–692. doi: 10.1271/bbb.66.689. [DOI] [PubMed] [Google Scholar]
- 119.Ding L., Jin D., Chen X. Luteolin enhances insulin sensitivity via activation of PPARγ transcriptional activity in adipocytes. J. Nutr. Biochem. 2010;21:941–947. doi: 10.1016/j.jnutbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 120.Deqiu Z., Kang L., Jiali Y., Baolin L., Gaolin L. Luteolin inhibits inflammatory response and improves insulin sensitivity in the endothelium. Biochimie. 2011;93:506–512. doi: 10.1016/j.biochi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 121.Choi Jae Sue, Islam MdN., Ali MdY., Kim Y.M., Park H.J., Sohn H.S., Jung H.A. The effects of C-glycosylation of luteolin on its antioxidant, anti-Alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Arch Pharm. Res. (Seoul) 2014;37:1354–1363. doi: 10.1007/s12272-014-0351-3. [DOI] [PubMed] [Google Scholar]
- 122.Ding Y., Shi X., Shuai X., Xu Y., Liu Y., Liang X., Wei D., Su D. Luteolin prevents uric acid-induced pancreatic β-cell dysfunction. J. Biomed. Res. 2014;28:292. doi: 10.7555/JBR.28.20130170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan J., Zhang G., Pan J., Wang Y. α-Glucosidase inhibition by luteolin: kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014;64:213–223. doi: 10.1016/j.ijbiomac.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 124.Zhang L., Han Y.-J., Zhang X., Wang X., Bao B., Qu W., Liu J. Luteolin reduces obesity-associated insulin resistance in mice by activating AMPKα1 signalling in adipose tissue macrophages. Diabetologia. 2016;59:2219–2228. doi: 10.1007/s00125-016-4039-8. [DOI] [PubMed] [Google Scholar]
- 125.Andrade N., Araújo J.R., Correia-Branco A., Carletti J.V., Martel F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct.Foods. 2017;36:429–439. doi: 10.1016/j.jff.2017.07.032. [DOI] [Google Scholar]
- 126.Mojica L., Berhow M., De Mejia E.G. Black bean coat anthocyanin-rich extracts and pure anthocyanins modulated molecular markers of diabetes. FASEB J. 2017;31:646. 38. [Google Scholar]
- 127.Rodriguez A.G., Karakayaa S. 2017. In Vitro α–amylase Inhibition and α–glucosidase Inhibition Activities of Biscuit Enriched with Anthocyanin and Docosahexaenoic Acid. [Google Scholar]
- 128.Xue B., Tian J., Wang Y., Jin B., Deng H., Gao N., Xie X., Tang S., Li B. Mechanism underlying the interaction of malvidin-3-O-galactoside with protein tyrosine phosphatase-1B and α-glucosidase. J. Mol. Struct. 2022;1253 [Google Scholar]
- 129.Ong K.C., Khoo H.-E. Insulinomimetic effects of myricetin on lipogenesis and glucose transport in rat adipocytes but not glucose transporter translocation. Biochem. Pharmacol. 1996;51:423–429. doi: 10.1016/0006-2952(95)02195-7. [DOI] [PubMed] [Google Scholar]
- 130.Ong K.C., Khoo H.-E. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000;67:1695–1705. doi: 10.1016/S0024-3205(00)00758-X. [DOI] [PubMed] [Google Scholar]
- 131.Strobel P., Allard C., Perez-Acle T., Calderon R., Aldunate R., Leighton F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005;386:471–478. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ding Y., Dai X., Zhang Z., Li Y. Myricetin attenuates hyperinsulinemia-induced insulin resistance in skeletal muscle cells. Eur. Food Res. Technol. 2012;234:873–881. doi: 10.1007/s00217-012-1701-3. [DOI] [Google Scholar]
- 133.Arumugam B., Palanisamy U.D., Chua K.H., Kuppusamy U.R. Potential antihyperglycaemic effect of myricetin derivatives from Syzygium malaccense. J. Funct.Foods. 2016;22:325–336. doi: 10.1016/j.jff.2016.01.038. [DOI] [Google Scholar]
- 134.Meng Y., Su A., Yuan S., Zhao H., Tan S., Hu C., Deng H., Guo Y. Evaluation of total flavonoids, myricetin, and quercetin from hovenia dulcis thunb. As inhibitors of α-amylase and α-glucosidase. Plant Foods Hum. Nutr. 2016;71:444–449. doi: 10.1007/s11130-016-0581-2. [DOI] [PubMed] [Google Scholar]
- 135.Li Y.-X., Cheng K.-C., Liu I.-M., Niu H.-S. Myricetin increases circulating adropin level after activation of glucagon-like peptide 1 (GLP-1) receptor in type-1 diabetic rats. Pharmaceuticals. 2022;15:173. doi: 10.3390/ph15020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang L., Gao Y., Gong J., Wang H., Farag M.A., Simal-Gandara J., Zhao Y., Nie S., Xiao J. Myricetin ameliorated prediabetes via immunomodulation and gut microbiota interaction. Food Front. 2022;3:749–772. [Google Scholar]
- 137.Lim S., Soh K., Kuppusamy U. Effects of naringenin on lipogenesis, lipolysis and glucose uptake in rat adipocyte primary culture: a natural antidiabetic agent. Internet J. Alternative Med. 2008;5:11. 11. [Google Scholar]
- 138.Ortiz-Andrade R.R., Sánchez-Salgado J.C., Navarrete-Vázquez G., Webster S.P., Binnie M., García-Jiménez S., León-Rivera I., Cigarroa-Vázquez P., Villalobos-Molina R., Estrada-Soto S. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes. Metab. 2008;10:1097–1104. doi: 10.1111/j.1463-1326.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 139.Zygmunt K., Faubert B., MacNeil J., Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010;398:178–183. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 140.Bhattacharya S., Oksbjerg N., Young J.F., Jeppesen P.B. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes. Metab. 2014;16:602–612. doi: 10.1111/dom.12236. [DOI] [PubMed] [Google Scholar]
- 141.Bhattacharya S., Rasmussen M.K., Christensen L.P., Young J.F., Kristiansen K., Oksbjerg N. Naringenin and falcarinol stimulate glucose uptake and TBC1D1 phosphorylation in porcine myotube cultures. J. Biochem. Pharmacol. Res. 2014;2:91–98. [Google Scholar]
- 142.Priscilla D.H., Roy D., Suresh A., Kumar V., Thirumurugan K. Naringenin inhibits α-glucosidase activity: a promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014;210:77–85. doi: 10.1016/j.cbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 143.Singh A.K., Raj V., Keshari A.K., Rai A., Kumar P., Rawat A., Maity B., Kumar D., Prakash A., De A., Samanta A., Bhattacharya B., Saha S. Isolated mangiferin and naringenin exert antidiabetic effect via PPAR γ/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018;280:33–44. doi: 10.1016/j.cbi.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 144.Prasad B.S., Srinivasan K.K. Nat. Prod. Exp. Drug Discov. Springer; 2023. α-Glucosidase inhibition and upregulation of PPARγ by flavonoid naringenin from Tinospora sinensis stem, a possible mechanism of antidiabetic activity; pp. 267–289. [Google Scholar]
- 145.Dhanya R., Arun K.B., Nisha V.M., Syama H.P., Nisha P., Santhosh Kumar T.R., Jayamurthy P. Preconditioning L6 muscle cells with naringin ameliorates oxidative stress and increases glucose uptake. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nzuza S., Ndwandwe D.E., Owira P.M.O. Naringin protects against HIV-1 protease inhibitors-induced pancreatic β-cell dysfunction and apoptosis. Mol. Cell. Endocrinol. 2016;437:1–10. doi: 10.1016/j.mce.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 147.Dayarathne L.A., Ranaweera S.S., Natraj P., Rajan P., Lee Y.J., Han C.-H. The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose treated HepG2 cells. J. Vet. Sci. 2021;22 doi: 10.4142/jvs.2021.22.e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li Y.Q., Zhou F.C., Gao F., Bian J.S., Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009;57:11463–11468. doi: 10.1021/jf903083h. [DOI] [PubMed] [Google Scholar]
- 149.Eid H.M., Martineau L.C., Saleem A., Muhammad A., Vallerand D., Benhaddou-Andaloussi A., Nistor L., Afshar A., Arnason J.T., Haddad P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010;54:991–1003. doi: 10.1002/mnfr.200900218. [DOI] [PubMed] [Google Scholar]
- 150.Torres-Piedra M., Ortiz-Andrade R., Villalobos-Molina R., Singh N., Medina-Franco J.L., Webster S.P., Binnie M., Navarrete-Vázquez G., Estrada-Soto S. A comparative study of flavonoid analogues on streptozotocin–nicotinamide induced diabetic rats: quercetin as a potential antidiabetic agent acting via 11β-Hydroxysteroid dehydrogenase type 1 inhibition. Eur. J. Med. Chem. 2010;45:2606–2612. doi: 10.1016/j.ejmech.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 151.Wein S., Behm N., Petersen R.K., Kristiansen K., Wolffram S. Quercetin enhances adiponectin secretion by a PPAR-γ independent mechanism. Eur. J. Pharm. Sci. 2010;41:16–22. doi: 10.1016/j.ejps.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 152.Youl E., Bardy G., Magous R., Cros G., Sejalon F., Virsolvy A., Richard S., Quignard J., Gross R., Petit P., Bataille D., Oiry C. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway: quercetin effects on pancreatic β-cells. Br. J. Pharmacol. 2010;161:799–814. doi: 10.1111/j.1476-5381.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dai X., Ding Y., Zhang Z., Cai X., Bao L., Li Y. Quercetin but not quercitrin ameliorates tumor necrosis factor-alpha-induced insulin resistance in C2C12 skeletal muscle cells. Biol. Pharm. Bull. 2013;36:788–795. doi: 10.1248/bpb.b12-00947. [DOI] [PubMed] [Google Scholar]
- 154.Dhanya R., Arun K.B., Syama H.P., Nisha P., Sundaresan A., Santhosh Kumar T.R., Jayamurthy P. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 2014;158:546–554. doi: 10.1016/j.foodchem.2014.02.151. [DOI] [PubMed] [Google Scholar]
- 155.Eid H., Nachar A., Thong F., Sweeney G., Haddad P. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Phcog. Mag. 2015;11:74. doi: 10.4103/0973-1296.149708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dhanya R., Arya A.D., Nisha P., Jayamurthy P. Quercetin, a lead compound against type 2 diabetes ameliorates glucose uptake via AMPK pathway in skeletal muscle cell line. Front. Pharmacol. 2017;8:336. doi: 10.3389/fphar.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J.-Y., Nie Y.-X., Dong B.-Z., Cai Z.-C., Zeng X.-K., Du L., Zhu X., Yin X.-X. Quercetin protects islet β-cells from oxidation-induced apoptosis via Sirt3 in T2DM, Iran. J. Basic Med. Sci. 2021;24:629. doi: 10.22038/ijbms.2021.52005.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Qu X., Li J., Yan P., Wang G., Liu W., Zeng Y., Liu L. Quercetin of Potentilla bifurca 3-glycosylation substitution impact the inhibitory activity on α-glucosidase. Phcog. Mag. 2022;18:458. [Google Scholar]
- 159.Shen H., Wang J., Ao J., Hou Y., Xi M., Cai Y., Li M., Luo A. Structure-activity relationships and the underlying mechanism of α-amylase inhibition by hyperoside and quercetin: multi-spectroscopy and molecular docking analyses. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2023;285 doi: 10.1016/j.saa.2022.121797. [DOI] [PubMed] [Google Scholar]
- 160.Dai X., Ding Y., Zhang Z., Cai X., Li Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2013;31:265–271. doi: 10.3892/ijmm.2012.1177. [DOI] [PubMed] [Google Scholar]
- 161.Zhang L., Zhang S.-T., Yin Y.-C., Xing S., Li W.-N., Fu X.-Q. Hypoglycemic effect and mechanism of isoquercitrin as an inhibitor of dipeptidyl peptidase-4 in type 2 diabetic mice. RSC Adv. 2018;8:14967–14974. doi: 10.1039/C8RA00675J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kappel V.D., Cazarolli L.H., Pereira D.F., Postal B.G., Zamoner A., Reginatto F.H., Silva F.R.M.B. Involvement of GLUT-4 in the stimulatory effect of rutin on glucose uptake in rat soleus muscle: effect of rutin on glucose uptake. J. Pharm. Pharmacol. 2013;65:1179–1186. doi: 10.1111/jphp.12066. [DOI] [PubMed] [Google Scholar]
- 163.Hsu C.-Y., Shih H.-Y., Chia Y.-C., Lee C.-H., Ashida H., Lai Y.-K., Weng C.-F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014;58:1168–1176. doi: 10.1002/mnfr.201300691. [DOI] [PubMed] [Google Scholar]
- 164.Oboh G., Ademosun A.O., Ayeni P.O., Omojokun O.S., Bello F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015;24:1103–1110. doi: 10.1007/s00580-014-2040-5. [DOI] [Google Scholar]
- 165.Lee D.-G., Jang I.S., Yang K.E., Yoon S.-J., Baek S., Lee J.Y., Suzuki T., Chung K.-Y., Woo S.-H., Choi J.-S. Effect of rutin from tartary buckwheat sprout on serum glucose-lowering in animal model of type 2 diabetes. Acta Pharm. 2016;66:297–302. doi: 10.1515/acph-2016-0021. [DOI] [PubMed] [Google Scholar]
- 166.Aitken J.F., Loomes K.M., Riba-Garcia I., Unwin R.D., Prijic G., Phillips A.S., Phillips A.R.J., Wu D., Poppitt S.D., Ding K., Barran P.E., Dowsey A.W., Cooper G.J.S. Rutin suppresses human-amylin/hIAPP misfolding and oligomer formation in-vitro , and ameliorates diabetes and its impacts in human-amylin/hIAPP transgenic mice. Biochem. Biophys. Res. Commun. 2017;482:625–631. doi: 10.1016/j.bbrc.2016.11.083. [DOI] [PubMed] [Google Scholar]
- 167.Guo Yuchen. Degree Thesis of Institute of Biotechnology. ZTE University; 2019. Strictinin isolated from Raw Pu-erh tea, a potent aphla-glucosidase inhibitor, reduces postprandial blood glucose in C57BL/6J mice.https://www.airitilibrary.com/Publication/alDetailedMesh?docid=U0005-1607201918071000 [Google Scholar]
- 168.Vellingiri V., Ganesh S., Venkat M., Pemaiah B. Docking studies on antidiabetic molecular targets of phytochemical compounds of syzygium cumini (l.) Skeels. Asian J. Pharm. Clin. Res. 2016;9:287. doi: 10.22159/ajpcr.2016.v9s3.14920. [DOI] [Google Scholar]
- 169.Cazarolli L.H., Kappel V.D., Pereira D.F., Moresco H.H., Brighente I.M.C., Pizzolatti M.G., Silva F.R.M.B. Anti-hyperglycemic action of apigenin-6-C-β-fucopyranoside from Averrhoa carambola. Fitoterapia. 2012;83:1176–1183. doi: 10.1016/j.fitote.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 170.Cazarolli L.H., Folador P., Moresco H.H., Brighente I.M.C., Pizzolatti M.G., Silva F.R.M.B. Mechanism of action of the stimulatory effect of apigenin-6-C-(2″-O-α-l-rhamnopyranosyl)-β-l-fucopyranoside on 14C-glucose uptake. Chem. Biol. Interact. 2009;179:407–412. doi: 10.1016/j.cbi.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 171.Cazarolli L.H., Folador P., Moresco H.H., Brighente I.M.C., Pizzolatti M.G., Silva F.R.M.B. Stimulatory effect of apigenin-6-C-β-l-fucopyranoside on insulin secretion and glycogen synthesis. Eur. J. Med. Chem. 2009;44:4668–4673. doi: 10.1016/j.ejmech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 172.Panda S., Kar A. Apigenin (4‘,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan-induced diabetic mice. J. Pharm. Pharmacol. 2007;59:1543–1548. doi: 10.1211/jpp.59.11.0012. [DOI] [PubMed] [Google Scholar]
- 173.Esmaeili M.A., Sadeghi H. Pancreatic Β-cell protective effect of rutin and apigenin isolated from Teucrium polium. Pharmacol. Online. 2009;2:341–353. [Google Scholar]
- 174.Hossain C.M., Ghosh M.K., Satapathy B.S., Dey N.S., Mukherjee B. Apigenin causes biochemical modulation, GLUT4 and Cd38 alterations to improve diabetes and to protect damages of some vital organs in experimental diabetes. Am. J. Pharmacol. Toxicol. 2014;9:39–52. [Google Scholar]
- 175.Jung U., Cho Y.-Y., Choi M.-S. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients. 2016;8:305. doi: 10.3390/nu8050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ren B., Qin W., Wu F., Wang S., Pan C., Wang L., Zeng B., Ma S., Liang J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur. J. Pharmacol. 2016;773:13–23. doi: 10.1016/j.ejphar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 177.Wang N., Yi W.J., Tan L., Zhang J.H., Xu J., Chen Y., Qin M., Yu S., Guan J., Zhang R. Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cell. Dev. Biol. Anim. 2017;53:554–563. doi: 10.1007/s11626-017-0135-4. [DOI] [PubMed] [Google Scholar]
- 178.Anandan S., Urooj A. Hypoglycemic effects of apigenin from morus indica in streptozotocin induced diabetic rats. Int J Cur Res Rev. 2021;13:100. [Google Scholar]
- 179.Michel F.Y. Arbutin diabetes. Exp. Biol. Med. 1936;35:62–64. doi: 10.3181/00379727-35-8858P. [DOI] [Google Scholar]
- 180.Azarbayjani M.A., Shirkhani S., Pouramir M. The effect of a swim workout program along with the use of arbutin on glucose and insulin levels in rats with hyperglycemia. Int. J. Biosci. 2014;IJB 4:292–297. [Google Scholar]
- 181.Farzanegi P. The effects of aerobic training and arbutin on GLP1 and GLP1R in diabetes Rats. Indian J Fundam Appl Life Sci. 2014;4:2231–6345. [Google Scholar]
- 182.Li H., Cao W., Wei L.-F., Xia J.-Q., Gu Y., Gu L.-M., Pan C.-Y., Liu Y.-Q., Tian Y.-Z., Lu M. Arbutin alleviates diabetic symptoms by attenuating oxidative stress in a mouse model of type 1 diabetes. Int. J. Diabetes Dev. Ctries. 2021;41:586–592. [Google Scholar]
- 183.Matsumoto N., Ishigaki F., Ishigaki A., Iwashina H., Hara Y. Reduction of blood glucose levels by tea catechin. Biosci. Biotechnol. Biochem. 1993;57:525–527. doi: 10.1271/bbb.57.525. [DOI] [Google Scholar]
- 184.Igarashi K., Honma K., Yoshinari O., Nanjo F., Hara Y. Effects of dietary catechins on glucose tolerance, blood pressure and oxidative status in goto-kakizaki rats. J. Nutr. Sci. Vitaminol. 2007;53:496–500. doi: 10.3177/jnsv.53.496. [DOI] [PubMed] [Google Scholar]
- 185.Park J., Jin J., Baek W., Park S., Sung H., Kim Y., Lee J., Song D. Ambivalent role of gallated catechins in glucose tolerance in humans: a novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J. Physiol. Pharmacol. 2009;60:101–109. [PubMed] [Google Scholar]
- 186.Daisy P., Balasubramanian K., Rajalakshmi M., Eliza J., Selvaraj J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine. 2010;17:28–36. doi: 10.1016/j.phymed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 187.Huang C.F., Chen Y.W., Yang C.Y., Lin H.Y., Way T.D., Chiang W., Liu S.H. Extract of Lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J. Agric. Food Chem. 2011;59:1087–1094. doi: 10.1021/jf103382h. [DOI] [PubMed] [Google Scholar]
- 188.Imada S., Tanaka A., Nishiumi S., Ashida H. Concentration of catechins and caffeine in black tea affects suppression of fat accumulation and hyperglycemia in high-fat diet-fed mice. Food Sci. Technol. Res. 2011;17:353–359. doi: 10.3136/fstr.17.353. [DOI] [Google Scholar]
- 189.Pitchai D., Manikkam R. Hypoglycemic and insulin mimetic impact of catechin isolated from Cassia fistula: a substantiate in silico approach through docking analysis. Med. Chem. Res. 2012;21:2238–2250. doi: 10.1007/s00044-011-9722-1. [DOI] [Google Scholar]
- 190.Samarghandian S., Azimi-Nezhad M., Farkhondeh T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose Response. 2017;15 doi: 10.1177/1559325817691158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Nutrient-gene interactions-dietary cyanidin 3-ObD-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 192.Sasaki R., Nishimura N., Hoshino H., Isa Y., Kadowaki M., Ichi T., Tanaka A., Nishiumi S., Fukuda I., Ashida H., Horio F., Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007;74:1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 193.Gharib A., Faezizadeh Z., Godarzee M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013;79:1599–1604. doi: 10.1055/s-0033-1350908. [DOI] [PubMed] [Google Scholar]
- 194.Daveri E., Cremonini E., Mastaloudis A., Hester S.N., Wood S.M., Waterhouse A.L., Anderson M., Fraga C.G., Oteiza P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018;18:16–24. doi: 10.1016/j.redox.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grace M.H., Ribnicky D.M., Kuhn P., Poulev A., Logendra S., Yousef G.G., Raskin I., Lila M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chakravarthy B., Gupta S., Gambhir S., Gode K. The prophylactic action of (-)-epicatechin against alloxan induced diabetes in rats. Life Sci. 1981;29:2043–2047. doi: 10.1016/0024-3205(81)90660-3. [DOI] [PubMed] [Google Scholar]
- 197.Chakravarthy B.K., Gupta S., Gode K.D. Functional beta cell regeneration in the islets of pancreas in alloxan induced diabetic rats by (−)-epicatechin. Life Sci. 1982;31:2693–2697. doi: 10.1016/0024-3205(82)90713-5. [DOI] [PubMed] [Google Scholar]
- 198.Sheehan E.W., Stiff D.D., Duah F., Slatkin D.J., Schiff P.L., Zemaitis M.A. The lack of effectiveness of (−)-epicatechin against alloxan induced diabetes in Wistar rats. Life Sci. 1983;33:593–597. doi: 10.1016/0024-3205(83)90246-1. [DOI] [PubMed] [Google Scholar]
- 199.bone A.J., Hii C.S.T., Brown D., Smith W., Howell S.L. Assessment of the antidiabetic activity of epicatechin in streptozotocin-diabetic and spontaneously diabetic BB/E rats. Biosci. Rep. 1985;5:215–221. doi: 10.1007/BF01119590. [DOI] [PubMed] [Google Scholar]
- 200.Bettaieb A., Vazquez Prieto M.A., Rodriguez Lanzi C., Miatello R.M., Haj F.G., Fraga C.G., Oteiza P.I. (−)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014;72:247–256. doi: 10.1016/j.freeradbiomed.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shih C.-C., Wu J.-B., Jian J.-Y., Lin C.-H., Ho H.-Y. (−)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, prevents diabetes and hyperlipidemia by regulation of glucose transporter 4 and AMP-activated protein kinase phosphorylation in high-fat-fed mice. Int. J. Mol. Sci. 2015;16:24983–25001. doi: 10.3390/ijms161024983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cremonini E., Bettaieb A., Haj F.G., Fraga C.G., Oteiza P.I. (-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016;599:13–21. doi: 10.1016/j.abb.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ibrahim G., Ahmed O., Abbas N., El Fateh M. Evaluation of the anti-diabetic effects of epicatechin and/or gallic acid in STZ/NA- induced diabetic Wister rats. Res. J. Appl. Biotechnol. 2018;4:87–104. doi: 10.21608/rjab.2018.57527. [DOI] [Google Scholar]
- 204.Akiyama S., Katsumata S., Suzuki K., Nakaya Y., Ishimi Y., Uehara M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in goto-kakizaki rats with type 2 diabetes. Biosci. Biotechnol. Biochem. 2009;73:2779–2782. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 205.Revathy J., Abdullah S.S. Influence of hesperetin on glycoprotein components in diabetic rats. Int. J. Sci. Eng. Res. 2016;7:214–220. [Google Scholar]
- 206.Revathy J. The role of hesperetin in the management of diabetes mellitus and its complications. J. Cancer Treat. Res. 2017;5:1. doi: 10.11648/j.jctr.20170501.11. [DOI] [Google Scholar]
- 207.Revathy J., Subramani S., Sheik Abdullah S.H., Udaiyar M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018;97:98–106. doi: 10.1016/j.biopha.2017.10.102. [DOI] [PubMed] [Google Scholar]
- 208.Nerdy N., Meliala L., Barus B.R., Lestari P., Ginting S., Ariani P., Mierza V., Bakri T.K. Effect of hesperetin treatment on blood glucose level, spermatozoa quality, and spermatozoa quantity in alloxan-induced diabetic mice. J. Kedokt. Hewan-Indones. J. Vet. Sci. 2021;15:1–6. [Google Scholar]
- 209.Abdou H.M., Hamaad F.A., Ali E.Y., Ghoneum M.H. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112838. [DOI] [PubMed] [Google Scholar]
- 210.Jung U.J., Lee M.-K., Jeong K.-S., Choi M.-S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 211.Toumi M.L., Merzoug S., Boutefnouchet A., Tahraoui A., Ouali K., Guellati M.A. Hesperidin, a natural citrus flavanone, alleviates hyperglycaemic state and attenuates embryopathies in pregnant diabetic mice. J. Med. Plants Res. 2009;3:862–869. [Google Scholar]
- 212.Akiyama S., Katsumata S., Suzuki K., Ishimi Y., Wu J., Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2009;46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Abdel-Moneim A., Ashour M., Mahmoud A., Ahmed O. Insulin sensitizing effects of hesperidin and naringin in experimental model of induced type 2 diabetes in rats: focus on tumor necrosis factor-alpha and resistin. Nat. Sci. 2011;7:134–141. [Google Scholar]
- 214.Ahmed O.M., Mahmoud A.M., Abdel-Moneim A., Ashour M.B. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012;41 doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 215.Dokumacioglu E., Iskender H., Sen T.M., Ince I., Dokumacioglu A., Kanbay Y., Erbas E., Saral S. The effects of hesperidin and quercetin on serum tumor necrosis factor-alpha and interleukin-6 levels in streptozotocin-induced diabetes model. Phcog. Mag. 2018;14:167. doi: 10.4103/pm.pm_41_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sundaram R., Nandhakumar E., Haseena Banu H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods. 2019;29:644–653. doi: 10.1080/15376516.2019.1646370. [DOI] [PubMed] [Google Scholar]
- 217.Yoshida H., Tsuhako R., Sugita C., Kurokawa M. Glucosyl hesperidin has an anti-diabetic effect in high-fat diet-induced obese mice. Biol. Pharm. Bull. 2021;44:422–430. doi: 10.1248/bpb.b20-00849. [DOI] [PubMed] [Google Scholar]
- 218.Peng P., Jin J., Zou G., Sui Y., Han Y., Zhao D., Liu L. Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway. Exp. Ther. Med. 2021;21:1. doi: 10.3892/etm.2020.9485. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.El-Shahawy A.A., Abdel-Moneim A., Ebeid A.S., Eldin Z.E., Zanaty M.I. A novel layered double hydroxide-hesperidin nanoparticles exert antidiabetic, antioxidant and anti-inflammatory effects in rats with diabetes. Mol. Biol. Rep. 2021;48:5217–5232. doi: 10.1007/s11033-021-06527-2. [DOI] [PubMed] [Google Scholar]
- 220.de Sousa E., Zanatta L., Seifriz I., Creczynski-Pasa T.B., Pizzolatti M.G., Szpoganicz B., Silva F.R.M.B. Hypoglycemic effect and antioxidant potential of kaempferol-3,7- O -(α)-dirhamnoside from bauhiniaforficata leaves. J. Nat. Prod. 2004;67:829–832. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 221.Zanatta L., Rosso Â., Folador P., Figueiredo M.S., Pizzolatti M.G., Leite L.D., Silva F.R. Insulinomimetic effect of kaempferol 3-neohesperidoside on the rat soleus muscle. J. Nat. Prod. 2008;71:532–535. doi: 10.1021/np070358+. [DOI] [PubMed] [Google Scholar]
- 222.Cazarolli L.H., Folador P., Pizzolatti M.G., Mena Barreto Silva F.R. Signaling pathways of kaempferol-3-neohesperidoside in glycogen synthesis in rat soleus muscle. Biochimie. 2009;91:843–849. doi: 10.1016/j.biochi.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 223.Rodríguez P., González-Mujica F., Bermúdez J., Hasegawa M. Inhibition of glucose intestinal absorption by kaempferol 3-O-α-rhamnoside purified from Bauhinia megalandra leaves. Fitoterapia. 2010;81:1220–1223. doi: 10.1016/j.fitote.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 224.Zang Y., Sato H., Igarashi K. Anti-diabetic effects of a kaempferol glycoside-rich fraction from unripe soybean (edamame, Glycine max L. Merrill. ‘Jindai’) leaves on KK- Ay mice. Biosci. Biotechnol. Biochem. 2011;75:1677–1684. doi: 10.1271/bbb.110168. [DOI] [PubMed] [Google Scholar]
- 225.Liu G., Liu Y., Sun C., Bao H., Yu J. Effects of kaempferol on glycolipid metabolism and insulin resistance in rats with type 2 diabetes. J. Clin. Med. Pract. 2012;9 http://en.cnki.com.cn/Article_en/CJFDTotal-XYZL201209002.htm [Google Scholar]
- 226.Alkhalidy H., Moore W., Zhang Y., McMillan R., Wang A., Ali M., Suh K.-S., Zhen W., Cheng Z., Jia Z., Hulver M., Liu D. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β -cell mass in middle-aged obese diabetic mice. J. Diabetes Res. 2015;2015:1–14. doi: 10.1155/2015/532984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 227.Luo C., Yang H., Tang C., Yao G., Kong L., He H., Zhou Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015;28:744–750. doi: 10.1016/j.intimp.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 228.Zang Y., Zhang L., Igarashi K., Yu C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015;6:834–841. doi: 10.1039/C4FO00844H. [DOI] [PubMed] [Google Scholar]
- 229.Alkhalidy H., Moore W., Wang Y., Luo J., McMillan R., Zhen W., Zhou K., Liu D. The flavonoid kaempferol ameliorates streptozotocin-induced diabetes by suppressing hepatic glucose production. Molecules. 2018;23:2338. doi: 10.3390/molecules23092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Alkhalidy H., Moore W., Wang A., Luo J., McMillan R.P., Wang Y., Zhen W., Hulver M.W., Liu D. Kaempferol ameliorates hyperglycemia through suppressing hepatic gluconeogenesis and enhancing hepatic insulin sensitivity in diet-induced obese mice. J. Nutr. Biochem. 2018;58:90–101. doi: 10.1016/j.jnutbio.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Alshehri A.S., El-Kott A.F., Eleawa S.M., El-Gerbed M.S.A., Khalifa H.S., El-Kenawy A.E., Albadrani G.M., Abdel-Daim M.M. Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J. Physiol. Pharmacol. 2021;72 doi: 10.26402/jpp.2021.3.04. [DOI] [PubMed] [Google Scholar]
- 232.Al-Abbasi F.A., Kazmi I. 2022. Therapeutic Role of Kaempferol and Myricetin in Streptozotocin Induced Diabetes Synergistically via Modulation in Pancreatic Amylase, Glycogen Storage and Insulin Secretion. [DOI] [PubMed] [Google Scholar]
- 233.Zarzuelo A., Jimenez I., Gamez M., Utrilla P., Fernadez I., Torres M., Osuna I. Effects of luteolin 5-O-β-rutinoside in streptozotocin-induced diabetic rats. Life Sci. 1996;58:2311–2316. doi: 10.1016/0024-3205(96)00231-7. [DOI] [PubMed] [Google Scholar]
- 234.Zang Y., Igarashi K., Li Y. Anti-diabetic effects of luteolin and luteolin-7-O-glucoside on KK-Ay mice. Biosci. Biotechnol. Biochem. 2016;80:1580–1586. doi: 10.1080/09168451.2015.1116928. [DOI] [PubMed] [Google Scholar]
- 235.Ge X., He X., Lin Z., Zhu Y., Jiang X., Zhao L., Zeng F., Chen L., Xu W., Liu T. 6, 8-(1, 3-Diaminoguanidine) luteolin and its Cr complex show hypoglycemic activities and alter intestinal microbiota composition in type 2 diabetes mice. Food Funct. 2022;13:3572–3589. doi: 10.1039/d2fo00021k. [DOI] [PubMed] [Google Scholar]
- 236.Ge X., He X., Liu J., Zeng F., Chen L., Xu W., Shao R., Huang Y., Farag M.A., Capanoglu E. Amelioration of type 2 diabetes by the novel 6, 8-guanidyl luteolin quinone-chromium coordination via biochemical mechanisms and gut microbiota interaction. J. Adv. Res. 2022;46:173–188. doi: 10.1016/j.jare.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gutiérrez R.M.P., Gómez J.T., Urby R.B., Soto J.G.C., Parra H.R. Evaluation of diabetes effects of selenium nanoparticles synthesized from a mixture of luteolin and diosmin on streptozotocin-induced type 2 diabetes in mice. Molecules. 2022;27:5642. doi: 10.3390/molecules27175642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zou W., Zhang C., Gu X., Li X., Zhu H. Metformin in combination with malvidin prevents progression of non-alcoholic fatty liver disease via improving lipid and glucose metabolisms, and inhibiting inflammation in type 2 diabetes rats. Drug Des. Devel. Ther. 2021;15:2565. doi: 10.2147/DDDT.S307257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liu I.-M., Liou S.-S., Lan T.-W., Hsu F.-L., Cheng J.-T. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2005;71:617–621. doi: 10.1055/s-2005-871266. [DOI] [PubMed] [Google Scholar]
- 240.Liu I.-M., Liou S.-S., Cheng J.-T. Mediation of β-endorphin by myricetin to lower plasma glucose in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2006;104:199–206. doi: 10.1016/j.jep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 241.Liu I.-M., Tzeng T.-F., Liou S.-S., Lan T.-W. Improvement of insulin sensitivity in obese zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007;73:1054–1060. doi: 10.1055/s-2007-981577. [DOI] [PubMed] [Google Scholar]
- 242.Liu I.-M., Tzeng T.-F., Liou S.-S., Lan T.-W. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007;81:1479–1488. doi: 10.1016/j.lfs.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 243.Tzeng T.-F., Liou S.-S., Liu I.-M. Myricetin ameliorates Defective post-receptor insulin signaling via β-endorphin signaling in the skeletal muscles of fructose-fed rats. Evid. Based Complement. Alternat. Med. 2011;2011:1–9. doi: 10.1093/ecam/neq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kandasamy N., Ashokkumar N. Myricetin, a natural flavonoid, normalizes hyperglycemia in streptozotocin-cadmium-induced experimental diabetic nephrotoxic rats. Biomed. Prev. Nutr. 2012;2:246–251. doi: 10.1016/j.bionut.2012.04.003. [DOI] [Google Scholar]
- 245.Choi Ha-Neul, Kang M.-J., Lee S.-J., Kim J.-I. Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet. Nutr. Res. Pract. 2014;8:544. doi: 10.4162/nrp.2014.8.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kandasamy N., Ashokkumar N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin–cadmium induced diabetic nephrotoxic rats. Toxicol. Appl. Pharmacol. 2014;279:173–185. doi: 10.1016/j.taap.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 247.Kang S.-J., Park J.-H.Y., Choi H.-N., Kim J.-I. α-glucosidase inhibitory activities of myricetin in animal models of diabetes mellitus. Food Sci. Biotechnol. 2015;24:1897–1900. doi: 10.1007/s10068-015-0249-y. [DOI] [Google Scholar]
- 248.Li Y., Zheng X., Yi X., Liu C., Kong D., Zhang J., Gong M. Myricetin: a potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. FASEB J. 2017;31:2603–2611. doi: 10.1096/fj.201601339R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hu T., Yuan X., Wei G., Luo H., Lee H.J., Jin W. Myricetin-induced brown adipose tissue activation prevents obesity and insulin resistance in db/db mice. Eur. J. Nutr. 2018;57:391–403. doi: 10.1007/s00394-017-1433-z. [DOI] [PubMed] [Google Scholar]
- 250.Annadurai T., Muralidharan A.R., Joseph T., Hsu M.J., Thomas P.A., Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin–nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012;68:307–318. doi: 10.1007/s13105-011-0142-y. [DOI] [PubMed] [Google Scholar]
- 251.Annadurai T., Thomas P.A., Geraldine P. Ameliorative effect of naringenin on hyperglycemia-mediated inflammation in hepatic and pancreatic tissues of Wistar rats with streptozotocin- nicotinamide-induced experimental diabetes mellitus. Free Radic. Res. 2013;47:793–803. doi: 10.3109/10715762.2013.823643. [DOI] [PubMed] [Google Scholar]
- 252.Priscilla D.H., Jayakumar M., Thirumurugan K. Flavanone naringenin: an effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct.Foods. 2015;14:363–373. doi: 10.1016/j.jff.2015.02.005. [DOI] [Google Scholar]
- 253.Sharma A., Patar A.K., Bhan S. Cytoprotective, antihyperglycemic and antioxidative effect of naringenin on liver and kidneys of Swiss diabetic mice. Int. J. Health Sci. 2016;14 [Google Scholar]
- 254.Yoshida H., Tsuhako R., Atsumi T., Narumi K., Watanabe W., Sugita C., Kurokawa M. Naringenin interferes with the anti-diabetic actions of pioglitazone via pharmacodynamic interactions. J. Nat. Med. 2017;71:442–448. doi: 10.1007/s11418-016-1063-4. [DOI] [PubMed] [Google Scholar]
- 255.Ahmed O.M., Hassan M.A., Abdel-Twab S.M., Abdel Azeem M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017;94:197–205. doi: 10.1016/j.biopha.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 256.Park S., Sim K.-S., Hwangbo Y., Park S.-J., Kim Y.-J., Kim J.-H. Naringenin and phytoestrogen 8-prenylnaringenin protect against islet dysfunction and inhibit apoptotic signaling in insulin-deficient diabetic mice. Molecules. 2022;27:4227. doi: 10.3390/molecules27134227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Punithavathi V.R., Anuthama R., Prince P.S.M. Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male Wistar rats. J. Appl. Toxicol. 2008;28:806–813. doi: 10.1002/jat.1343. [DOI] [PubMed] [Google Scholar]
- 258.Pari L., Suman S. Efficacy of naringin on hepatic enzymes of carbohydrate metabolism in streptozotocin-nicotinamide induced type 2 diabetic rats. Int J Pharm Biol Arch. 2010;1:280–286. [Google Scholar]
- 259.Kumar Sharma A., Bharti S., Ojha S., Bhatia J., Kumar N., Ray R., Kumari S., Singh Arya D. Up-regulation of PPARγ, heat shock protein-27 and -72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. Br. J. Nutr. 2011;106:1713–1723. doi: 10.1017/S000711451100225X. [DOI] [PubMed] [Google Scholar]
- 260.Xulu S., Oroma Owira P.M. Naringin ameliorates atherogenic dyslipidemia but not hyperglycemia in rats with type 1 diabetes. J. Cardiovasc. Pharmacol. 2012;59:133–141. doi: 10.1097/FJC.0b013e31823827a4. [DOI] [PubMed] [Google Scholar]
- 261.Al-Kurdy M.J.J. Hypoglycemic and hypolipidimic effect of naringin in diabetic male rats. Al-Qadisiyah J. Vet. Med. Sci. 2014;13:43. doi: 10.29079/vol13iss1art276. [DOI] [Google Scholar]
- 262.Pari L., Chandramohan R. Modulatory effects of naringin on hepatic key enzymes of carbohydrate metabolism in high-fat diet/low-dose streptozotocin-induced diabetes in rats. Gen. Physiol. Biophys. 2017;36:343–352. doi: 10.4149/gpb_2016055. [DOI] [PubMed] [Google Scholar]
- 263.Lim Y.J., Kim J.H., Pan J.H., Kim J.K., Park T.-S., Kim Y.J., Lee J.H., Kim J.H. Naringin protects pancreatic β-cells against oxidative stress-induced apoptosis by inhibiting both intrinsic and extrinsic pathways in insulin-deficient diabetic mice. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700810. [DOI] [PubMed] [Google Scholar]
- 264.Vessal M., Hemmati M., Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2003;135:357–364. doi: 10.1016/S1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 265.Shetty A.K., Rashmi R., Rajan M.G.R., Sambaiah K., Salimath P.V. Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr. Res. 2004;24:373–381. doi: 10.1016/j.nutres.2003.11.010. [DOI] [Google Scholar]
- 266.Coskun O., Kanter M., Korkmaz A., Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and ?-cell damage in rat pancreas. Pharmacol. Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 267.Adewole S.O., Caxton-Martins E.A., Ojewole J.A. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats, Afr. J. Tradit. Complement. Altern. Med. 2007;4:64–74. doi: 10.4314/ajtcam.v4i1.31196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Panda S., Kar A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. Biofactors. 2007;31:201–210. doi: 10.1002/biof.5520310307. [DOI] [PubMed] [Google Scholar]
- 269.Lukačínová A., Mojžiš J., Beňačka R., Keller J., Maguth T., Kurila P., Vaško L., Racz O., Ništiar F. others, Preventive effects of flavonoids on alloxan-induced diabetes mellitus in rats. Acta Vet. Brno. 2008;77:175–182. [Google Scholar]
- 270.Kannappan S., Anuradha C.V. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. INDIAN J MED RES. 2009:9. [PubMed] [Google Scholar]
- 271.Kobori M., Masumoto S., Akimoto Y., Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol. Nutr. Food Res. 2009;53:859–868. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- 272.Abdelmoaty M.A., Ibrahim M.A., Ahmed N.S., Abdelaziz M.A. Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J. Clin. Biochem. 2010;25:188–192. doi: 10.1007/s12291-010-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.El-Baky A.E.A. Quercetin protective action on oxidative stress, sorbitol, insulin risistance and β-cells function in expermintal diabetic rats. Int J Pharm Stud Res. 2011:11–18. [Google Scholar]
- 274.Kim J.-H., Kang M.-J., Choi H.-N., Jeong S.-M., Lee Y.-M., Kim J.-I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011;5:107. doi: 10.4162/nrp.2011.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hussain S.A., Ahmed Z.A., Mahwi T.O., Aziz T.A. Effect of quercetin on postprandial glucose excursion after mono- and disaccharides challenge in normal and diabetic rats. J. Diabetes Mellit. 2012;2:82–87. doi: 10.4236/jdm.2012.21013. [DOI] [Google Scholar]
- 276.Jadhav R., Puchchakayala G. Hypoglycemic and antidiabetic activity of flavonoids: boswellic acid, ellagic acid, quercetin, rutin on streptozotocin-nicotinamide induced type 2 diabetic rats. Int. J. Pharm. Pharm. Sci. 2012;1 [Google Scholar]
- 277.Jeong S.-M., Kang M.-J., Choi H.-N., Kim J.-H., Kim J.-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012;6:201. doi: 10.4162/nrp.2012.6.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rifaai R.A., El-Tahawy N.F., Ali Saber E. Effect of quercetin on the Endocrine pancreas of the experimentally induced diabetes in male Albino rats: a histological and Immunohistochemical study. J. Diabetes Metab. 2012:3. doi: 10.4172/2155-6156.1000182. [DOI] [Google Scholar]
- 279.Alam MdM., Meerza D., Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109:8–14. doi: 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 280.Arias N., Macarulla M.T., Aguirre L., Martínez-Castaño M.G., Portillo M.P. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014;9:361. doi: 10.1007/s12263-013-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yang D.K., Kang H.-S. Anti-diabetic effect of Cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018;26:130–138. doi: 10.4062/biomolther.2017.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Oyedemi S.O., Nwaogu G., Chukwuma C.I., Adeyemi O.T., Matsabisa M.G., Swain S.S., Aiyegoro O.A. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: in silico studies of molecular interaction of quercetin with hexokinase and catalase. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13127. [DOI] [PubMed] [Google Scholar]
- 283.H Bakr E.-S. Comparative study on antidiabetic effects of Coenzyme Q10 and quercetin on streptozotocin-induced hyperglycemia in rats. مجلة الاقتصاد المنزلي. 2021;37:157–176. [Google Scholar]
- 284.Liu H., Wang L., Li F., Jiang Y., Guan H., Wang D., Sun-Waterhouse D., Wu M., Li D. The synergistic protection of EGCG and quercetin against streptozotocin (STZ)-induced NIT-1 pancreatic β cell damage via upregulation of BCL-2 expression by miR-16-5p. J. Nutr. Biochem. 2021;96 doi: 10.1016/j.jnutbio.2021.108748. [DOI] [PubMed] [Google Scholar]
- 285.Shaikhomar O.A., Bahattab O.S. Physiological effect of quercetin as a natural flavonoid to be used as hypoglycemic agent in diabetes mellitus type II rats. Saudi J Biomed Res. 2021;6:10–17. [Google Scholar]
- 286.Serra C.A., Dos Reis A.F., Calsa B., Bueno C.S., Helaehil J.V., de Souza S.A.R., de Oliveira C.A., Vanzella E.C., do Amaral M.E.C. Quercetin prevents insulin dysfunction in hypertensive animals. J. Diabetes Metab. Disord. 2022:1–11. doi: 10.1007/s40200-022-00987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Babujanarthanam R., Kavitha P., Pandian M.R. Quercitrin, a bioflavonoid improves glucose homeostasis in streptozotocin-induced diabetic tissues by altering glycolytic and gluconeogenic enzymes: quercitrin improves glucose homeostasis in diabetic tissues. Fundam. Clin. Pharmacol. 2009;24:357–364. doi: 10.1111/j.1472-8206.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- 288.Babujanarthanam R., Kavitha P., Mahadeva Rao U.S., Pandian M.R. Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin: induced diabetic rat tissues. Mol. Cell. Biochem. 2011;358:121–129. doi: 10.1007/s11010-011-0927-x. [DOI] [PubMed] [Google Scholar]
- 289.Babujanarthanam R., Kavitha P., Rajalakshmi G. Antihyperglycaemic and antioxidant role of quercitrin, a Bio- flavonoid, in streptozotocin-induced DiabeticWistar rat tissues. J. Pharm. Res. 2011:5. [Google Scholar]
- 290.Us M.R. Effects of quercitrin on diabetic Physiological criterions and hematological parameters studied in diabetic rats. Int. Med. J. 2019;26 [Google Scholar]
- 291.Paulo A., Martins S., Branco P., Dias T., Borges C., Rodrigues A.I., do Céu Costa M., Teixeira A., Mota-Filipe H. The opposing effects of the flavonoids isoquercitrin and Sissotrin, isolated fromPterospartum tridentatum, on oral glucose tolerance in rats. Phytother Res. 2008;22:539–543. doi: 10.1002/ptr.2403. [DOI] [PubMed] [Google Scholar]
- 292.Huang X.-L., He Y., Ji L.-L., Wang K.-Y., Wang Y.-L., Chen D.-F., Geng Y., OuYang P., Lai W.-M. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget. 2017;8 doi: 10.18632/oncotarget.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kamalakkannan N., Prince P.S.M. Antihyperglycaemic and antioxidant effect of rutin, a Polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar rats. Basic Clin. Pharmacol. Toxicol. 2006;98:97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- 294.Prince P.S.M., Kamalakkannan N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006;20:96–102. doi: 10.1002/jbt.20117. [DOI] [PubMed] [Google Scholar]
- 295.Hunyadi A., Martins A., Hsieh T.-J., Seres A., Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Niture N.T., Ansari A.A., Naik S.R. Anti-hyperglycemic activity of Rutin in streptozotocin-induced diabetic rats: an effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J. Exp. Biol. 2014:8. [PubMed] [Google Scholar]
- 297.Lee L.-C., Hou Y.-C., Hsieh Y.-Y., Chen Y.-H., Shen Y.-C., Lee I.-J., Shih M.-C.M., Hou W.-C., Liu H.-K. Dietary supplementation of rutin and rutin-rich buckwheat elevates endogenous glucagon-like peptide 1 levels to facilitate glycemic control in type 2 diabetic mice. J. Funct.Foods. 2021;85 [Google Scholar]
- 298.Rakhmat I.I., Yuslianti E.R., Koswara T. 12th Annu. Sci. Meet. Med. Fac. Univ. Jenderal Achmad Yani Int. Symp. Emerg. Prep. Disaster Response COVID 19 PandemicASMC 2021. Atlantis Press; 2021. Flavonoid-rutin effect to blood glucose level and pancreas regeneration in diabetic rats; pp. 64–66. [Google Scholar]
- 299.Amjadi S., Shahnaz F., Shokouhi B., Azarmi Y., Siahi-Shadbad M., Ghanbarzadeh S., Kouhsoltani M., Ebrahimi A., Hamishehkar H. Nanophytosomes for enhancement of rutin efficacy in oral administration for diabetes treatment in streptozotocin-induced diabetic rats. Int. J. Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121208. [DOI] [PubMed] [Google Scholar]
- 300.Matsui T., Ebuchi S., Kobayashi M., Fukui K., Sugita K., Terahara N., Matsumoto K. Anti-hyperglycemic effect of Diacylated anthocyanin derived from Ipomoea batatas Cultivar Ayamurasaki can Be achieved through the r-glucosidase inhibitory action. J. Agric. Food Chem. 2002;50:7244–7248. doi: 10.1021/jf025913m. [DOI] [PubMed] [Google Scholar]
- 301.Osigwe C.C., Akah P.A., Nworu C.S., Okoye F.B. Apigenin: a methanol fraction component of Newbouldia laevis leaf, as a potential antidiabetic agent. J Phytopharm. 2017;6:38–44. [Google Scholar]
- 302.Su H.-C., Hung L.-M., Chen J.-K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 303.Chi T.-C., Chen W.-P., Chi T.-L., Kuo T.-F., Lee S.-S., Cheng J.-T., Su M.-J. Phosphatidylinositol-3-kinase is involved in the antihyperglycemic effect induced by resveratrol in streptozotocin-induced diabetic rats. Life Sci. 2007;80:1713–1720. doi: 10.1016/j.lfs.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 304.Palsamy P., Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed. Pharmacother. 2008;62:598–605. doi: 10.1016/j.biopha.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 305.Penumathsa S.V., Thirunavukkarasu M., Zhan L., Maulik G., Menon V.P., Bagchi D., Maulik N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell Mol. Med. 2008;12:2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Palsamy P., Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin–nicotinamide-induced diabetic rats. Chem. Biol. Interact. 2009;179:356–362. doi: 10.1016/j.cbi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 307.Ramadori G., Gautron L., Fujikawa T., Vianna C.R., Elmquist J.K., Coppari R. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150:5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Palsamy P., Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic β-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J. Cell. Physiol. 2010;224:423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 309.Dao T.-M.A., Waget A., Klopp P., Serino M., Vachoux C., Pechere L., Drucker D.J., Champion S., Barthélemy S., Barra Y., Burcelin R., Sérée E. Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which Contributes to the glycemic control. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Labbé A., Garand C., Cogger V.C., Paquet E.R., Desbiens M., Le Couteur D.G., Lebel M. Resveratrol improves insulin resistance hyperglycemia and Hepatosteatosis but not Hypertriglyceridemia, inflammation, and Life span in a mouse model for Werner syndrome. J. Gerontol. Ser. A. 2011;66A:264–278. doi: 10.1093/gerona/glq184. [DOI] [PubMed] [Google Scholar]
- 311.Lee S.-M., Yang H., Tartar D.M., Gao B., Luo X., Ye S.Q., Zaghouani H., Fang D. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia. 2011;54:1136–1146. doi: 10.1007/s00125-011-2064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Minakawa M., Kawano A., Miura Y., Yagasaki K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J. Clin. Biochem. Nutr. 2011;48:237–244. doi: 10.3164/jcbn.10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mohamad Shahi M., Haidari F., Shiri M.R. Comparison of effect of resveratrol and vanadium on diabetes related dyslipidemia and hyperglycemia in streptozotocin induced diabetic rats. Adv. Pharm. Bull. EISSN. 2011;2251–7308 doi: 10.5681/APB.2011.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Sharma S., Misra C.S., Arumugam S., Roy S., Shah V., Davis J.A., Shirumalla R.K., Ray A. Antidiabetic activity of resveratrol, a known SIRT1 activator in a genetic model for type-2 diabetes. Phytother Res. 2011;25:67–73. doi: 10.1002/ptr.3221. [DOI] [PubMed] [Google Scholar]
- 315.Do G.-M., Jung U.J., Park H.-J., Kwon E.-Y., Jeon S.-M., McGregor R.A., Choi M.-S. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 2012;56:1282–1291. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- 316.Ku C.R., Lee H.J., Kim S.K., Lee E.Y., Lee M.-K., Lee E.J. Resveratrol prevents streptozotocin-induced diabetes by inhibiting the apoptosis of pancreatic β-cell and the cleavage of poly (ADP-ribose) polymerase. Endocr. J. 2011:1111040641. doi: 10.1507/endocrj.ej11-0194. 1111040641. [DOI] [PubMed] [Google Scholar]
- 317.Lee Y.-E., Kim J.-W., Lee E.-M., Ahn Y.-B., Song K.-H., Yoon K.-H., Kim H.-W., Park C.-W., Li G., Liu Z., Ko S.-H. Chronic resveratrol treatment protects pancreatic islets against oxidative stress in db/db mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ramar M., Manikandan B., Raman T., Priyadarsini A., Palanisamy S., Velayudam M., Munusamy A., Marimuthu Prabhu N., Vaseeharan B. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012;690:226–235. doi: 10.1016/j.ejphar.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 319.Cheng A.-S., Cheng Y.-H., Lee C.-Y., Chung C.-Y., Chang W.-C. Resveratrol protects against methylglyoxal-induced hyperglycemia and pancreatic damage in vivo. Nutrients. 2015;7:2850–2865. doi: 10.3390/nu7042850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lalitha V., Korah M.C., Sengottuvel S., Sivakumar T. Antidiabetic and antioxidant activity of resveratrol and Vitamin-C combination on streptozotocin induced diabetic rats. Int J Pharm Pharm Sci. 2015;7:455–458. [Google Scholar]
- 321.Yao L., Wan J., Li H., Ding J., Wang Y., Wang X., Li M. Resveratrol relieves gestational diabetes mellitus in mice through activating AMPK. Reprod. Biol. Endocrinol. 2015;13:118. doi: 10.1186/s12958-015-0114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kaur G., Padiya R., Adela R., Putcha U.K., Reddy G.S., Reddy B.R., Kumar K.P., Chakravarty S., Banerjee S.K. Garlic and resveratrol attenuate diabetic complications, loss of β-cells, pancreatic and hepatic oxidative stress in streptozotocin-induced diabetic rats. Front. Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rehman K., Saeed K., Munawar S.M., Akash M.S.H. Resveratrol regulates hyperglycemia-induced modulations in experimental diabetic animal model. Biomed. Pharmacother. 2018;102:140–146. doi: 10.1016/j.biopha.2018.03.050. [DOI] [PubMed] [Google Scholar]
- 324.Ahmad M., Gani A. Development of novel functional snacks containing nano-encapsulated resveratrol with anti-diabetic, anti-obesity and antioxidant properties. Food Chem. 2021;352 doi: 10.1016/j.foodchem.2021.129323. [DOI] [PubMed] [Google Scholar]
- 325.Wang W., Liu Z., Kong F., He L., Fang L., Shu Q. Quantitative analysis of resveratrol derivatives in the seed coats of tree peonies and their hypoglycemic activities in vitro/vivo. Food Funct. 2022;13:846–856. doi: 10.1039/d1fo03412j. [DOI] [PubMed] [Google Scholar]
- 326.Pegah A., Khodadadi I., Mirzaei F., Tayebinia H., Abbasi E. Simultaneous effect of resveratrol and Probiotics on insulin resistance and glucagon-like peptide (GLP-1) levels in diabetic rats. J. Babol Univ. Med. Sci. 2021;23:337–344. [Google Scholar]
- 327.Pegah A., Abbasi-Oshaghi E., Khodadadi I., Mirzaei F., Tayebinia H. Probiotic and resveratrol normalize GLP-1 levels and oxidative stress in the intestine of diabetic rats. Metab. Open. 2021;10 doi: 10.1016/j.metop.2021.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]