Key Points
Question
What are the prescription and dispensation patterns of QT-prolonging medications with known torsades de pointes risk and selected interacting medications for people receiving hemodialysis?
Findings
This cross-sectional study of 20 761 adults 60 years or older found that QT-prolonging medications for individuals with dialysis-dependent kidney failure were commonly prescribed by nonnephrology clinicians and from nonacute settings. Prescriptions for potentially interacting medications often originated from different prescribers.
Meaning
The findings of this study suggest that clinician- and health system–level strategies aimed at minimizing high-risk medication–prescribing practices in the population undergoing dialysis are needed.
Abstract
Importance
Individuals with dialysis-dependent kidney failure have numerous risk factors for medication-related adverse events, including receipt of care by multiple clinicians and initiation of some QT-prolonging medications with known risk of torsades de pointes (TdP), which is associated with higher risk of sudden cardiac death. Little is known about the prescription and dispensation patterns of QT-prolonging medications among people receiving dialysis, hindering efforts to reduce drug-related harm from these and other medications in this high-risk population.
Objective
To examine prescription and dispensation patterns of QT-prolonging medications with known TdP risk and selected interacting medications prescribed to individuals receiving hemodialysis.
Design, Setting, and Participants
This cross-sectional study included patients 60 years or older who were enrolled in Medicare Parts A, B, and D receiving in-center hemodialysis from January 1 to December 31, 2019. Analyses were conducted from October 20, 2022, to June 16, 2023.
Exposures
New-user prescriptions for the 7 most frequently filled QT-prolonging medications characterized by the timing of the new prescription relative to acute care encounters, the type of prescribing clinician and pharmacy that dispensed the medication, and concomitant use of selected medications known to interact with the 7 most frequently filled QT-prolonging medications with known TdP risk.
Main Outcomes and Measures
The main outcomes were the frequencies of the most commonly filled and new-use episodes of QT-prolonging medications; the timing of medication fills relative to acute care events; prescribers and dispensing pharmacy characteristics for new use of medications; and the frequency and types of new-use episodes with concurrent use of potentially interacting medications.
Results
Of 20 761 individuals receiving hemodialysis in 2019 (mean [SD] age, 74 [7] years; 51.1% male), 10 992 (52.9%) filled a study drug prescription. Approximately 80% (from 78.6% for odansetron to 93.9% for escitalopram) of study drug new-use prescriptions occurred outside of an acute care event. Between 36.8% and 61.0% of individual prescriptions originated from general medicine clinicians. Between 16.4% and 26.2% of these prescriptions occurred with the use of another QT-prolonging medication. Most potentially interacting drugs were prescribed by different clinicians (46.3%-65.5%).
Conclusions and Relevance
In this cross-sectional study, QT-prolonging medications for individuals with dialysis-dependent kidney failure were commonly prescribed by nonnephrology clinicians and from nonacute settings. Prescriptions for potentially interacting medications often originated from different prescribers. Strategies aimed at minimizing high-risk medication-prescribing practices in the population undergoing dialysis are needed.
This cross-sectional study of older adults with kidney failure receiving hemodialysis examines prescription and dispensation patterns of QT-prolonging medications with known torsades de pointes risk and selected interacting medications prescribed to this population.
Introduction
There are approximately 500 000 people with hemodialysis-dependent kidney failure in the US,1 and these individuals have a tremendous burden of comorbid disease that often requires care by multiple clinicians and treatment with numerous medications. On average, patients receiving hemodialysis are cared for simultaneously by clinicians from 5 different specialties2,3 and are prescribed 10 to 12 daily medications.4,5 Evidence from other chronic disease populations shows that individuals with more (vs fewer) medication prescribers have higher risks of drug interactions,6,7 receipt of inappropriate medications,8 and occurrence of adverse drug events.9 Findings are similar among patients who receive prescriptions from acute (vs ambulatory) care settings and/or clinicians not involved in their long-term care.8
Individuals with dialysis-dependent kidney failure may be particularly vulnerable to medication-related harms, as the impacts of kidney dysfunction on drug pharmacokinetics (ie, absorption, distribution, metabolism, and excretion)10 and pharmacodynamics (ie, the physiologic drug effects)11 can increase the risk of drug-related adverse events. For example, compared with clinically relevant comparator medications, QT-prolonging medications with known risks of torsades de pointes (TdP), such as respiratory fluoroquinolones, azithromycin, ondansetron, and certain selective serotonin reuptake inhibitors, are associated with higher risks of sudden cardiac death in people receiving hemodialysis.12,13,14,15 This risk is further elevated in the setting of concomitant use of other QT-prolonging medications with known risk of TdP than in the setting of stand-alone use.12,14 Prescriber education programs,16,17 medication reconciliation,18,19,20 and electronic health record (EHR) supports21,22,23 reduce unsafe prescribing practices in populations not undergoing dialysis. However, the absence of data on the origins of prescriptions for high-risk medications among people receiving dialysis hinders targeted implementation of such risk-mitigation strategies in the population undergoing dialysis.
To address this knowledge gap, we undertook this study to investigate the origins of prescriptions for QT-prolonging medications with known risks of TdP. We also selected interacting medications in a sample of older individuals with hemodialysis-dependent kidney failure in the Medicare fee-for-service (FFS) and Part D programs.
Methods
Study Design, Data Source, and Cohort
The University of North Carolina at Chapel Hill Institutional Review Board reviewed this study. A waiver of the requirement to obtain informed consent was granted because of the study’s large size, data anonymity, and retrospective nature. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.24
We conducted a cross-sectional study using the Medicare Master Beneficiary Summary File, as well as institutional, clinician, and prescription drug claims (Parts A, B, and D, respectively) from a random 20% sample of Medicare FFS beneficiaries 60 years or older (ages available in data source) to identify individuals receiving in-center hemodialysis as of January 1 (the study index date) through December 31, 2019. For cohort inclusion, we required that individuals have a diagnosis code for end-stage kidney disease (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision code N18.6) and 1 or more claims for outpatient dialysis treatment with Medicare as the primary payer in the 6-month baseline period (July 1 through December 31, 2018) (eTable 1 in Supplement 1). We excluded beneficiaries who received home dialysis or hospice care, underwent a kidney transplant, died, enrolled in Medicare Advantage, or did not have continuous Medicare Part A, B, or D coverage either during the baseline period or on January 1, 2019. After the index date, we stopped observing individuals’ medication use at the onset of any of the exclusionary events listed above.
QT-Prolonging Medication Use
We used the CredibleMeds website to compile a list of medications that can cause QT prolongation and/or TdP (eTable 2 in Supplement 1).25 CredibleMeds is an online resource that relies on up-to-date information from published literature, medication package inserts, the US Food and Drug Administration’s Adverse Event Reporting System, and other sources to classify QT-prolonging medications as having a known, possible, or conditional risk of TdP.25 We used Medicare Part D claims to ascertain each beneficiary’s outpatient use of a QT-prolonging medication with known TdP risk during 2019.
We then created separate new-user cohorts of the 7 most frequently filled QT-prolonging medications with known TdP risk in 2019 (hereinafter described as the 7 new-user cohorts): azithromycin, ondansetron, levofloxacin, ciprofloxacin, amiodarone, escitalopram, and fluconazole (eTable 3 in Supplement 1). The focus on a limited number of high-risk medications allowed for more detailed, medication-specific descriptions of prescription and dispensation patterns of medications shown to be associated with a higher risk of sudden cardiac death among people receiving maintenance hemodialysis.12,13,14,15 We defined new use as a prescription fill for a study medication after a 180-day washout period free of prescription fills for the study drug of interest. Studying new use supported reliable ascertainment of the origin of study prescription medications (ie, prescriber and dispenser) and captured the point in time when the prescriber made the prescribing decision and might have considered medications that were already prescribed to the patient in their decision to prescribe the study medication.
Cohort Characterization
Covariates were ascertained in the baseline period using the Medicare Master Beneficiary Summary File and claims data and included demographics, comorbid conditions, concomitant medication use, and health care utilization metrics (eTable 1 in Supplement 1). Race and ethnicity categories included Black, Hispanic, non-Hispanic White, and other (Asian or Pacific Islander, American Indian or Alaska Native, other, and unknown). Race and ethnicity were ascertained by Medicare enrollment data and were included in the study to characterize cohort demographics. Groups in the other category were collapsed due to small individual sample sizes. Comorbid conditions were considered present if there was an applicable diagnosis code located in any position on 1 or more inpatient claims or on 2 or more outpatient claims during the 180-day baseline period. Concomitant medication use was determined on January 1, 2019, using Part D prescription drug claims. Polypharmacy was defined as use of 5 or more prescribed medications; hyperpharmacy was defined as use of 10 or more prescribed medications.
QT-Prolonging Medication Prescription Characterization
Within each of the 7 QT-prolonging medication new-user cohorts, we characterized the timing of the newly prescribed QT-prolonging medication relative to acute care encounters and identified the type of clinician who prescribed the medication and the type of pharmacy that dispensed the medication. First, we examined whether the study medications were newly prescribed after acute care encounters, including urgent care or emergency department visits or hospitalizations. Eligible urgent care visits were those occurring within 1 week before the QT-prolonging medication new use. Eligible emergency department visits were those occurring within 1 week before the QT-prolonging medication new use when the QT-prolonging medication was prescribed by an emergency department clinician. Eligible hospitalizations were inpatient hospital stays with discharge dates falling within 1 week before the QT-prolonging medication new use.
Second, we used the prescriber and pharmacy characteristics in Medicare Part D claims files to characterize the prescriber and dispensing pharmacy of the QT-prolonging medications. Prescriber credentials and specialty type were ascertained from clinician specialty and credentials reported in the prescriber characteristics file and defined according to the National Uniform Claim Committee’s Health Care Provider Taxonomy codes.26 Pharmacies were categorized as either commercial or retail, institutional (eg, hospital or skilled nursing facilities), mail order, or other based on data in the Centers for Medicare & Medicaid Services pharmacy characteristics file.27
Third, we characterized the concomitant use of selected medications known to interact with the 7 most frequently filled QT-prolonging medications with known TdP risk. We used Medicare Part D claims to ascertain each beneficiary’s outpatient use of a potentially interacting medication of interest on the date of the new-use prescription fill for the study medication. Pharmacodynamically interacting drugs of interest included other QT-prolonging medications with a known risk of TdP (eTable 2 in Supplement 1). Drugs known to inhibit the metabolism of the new-use study medications (ie, cytochrome P450 [CYP] 3A4 and CYP2C19 isoenzyme inhibitors) were considered as potential pharmacokinetic interactors (eTable 4 in Supplement 1). We used the University of Washington Drug Interaction Database28 to compile the list of relevant CYP inhibitors.
Statistical Analysis
We described baseline characteristics of the cohort of Medicare FFS beneficiaries receiving outpatient hemodialysis by their use (vs nonuse) status of any QT-prolonging medication with known TdP risk in 2019. Within the 7 new-user cohorts, we reported the numbers and frequencies of new-use episodes of study drugs relative to the presence (vs the absence) of acute care events as well as the prescribers and dispensing pharmacies for these new-use episodes. To assess the prevalence of concomitant use of medications that may have potentially interacted with a newly prescribed QT-prolonging medication with known TdP risk, we described the number and percentage of new-use episodes of known TdP risk medications during which patients were taking a medication with potential to pharmacodynamically and, separately, pharmacokinetically interact with the new-use medication on the date of the new-use fill. For each interacting drug pair, we described the frequency of pairs prescribed by different clinicians or the same clinician (by specialty) and those dispensed by different pharmacies or the same pharmacy (by type). All statistical analyses were performed from October 20, 2022, to June 16, 2023, using SAS, version 9.4 (SAS Institute Inc).
Results
Overall Study Population
Of the 20 761 individuals receiving in-center maintenance hemodialysis on January 1, 2019 (mean [SD] age, 74 [7] years; 10 150 [48.9%] female and 10 611 [51.1%] male), 10 992 (52.9%) filled an outpatient prescription for a QT-prolonging medication with known TdP risk in 2019 (eFigure in Supplement 1). Table 1 displays baseline characteristics of the cohort. Among the 10 992 users of medication with known TdP risk in the cohort, the mean (SD) age was 74 (7) years; 56.2% had a low-income subsidy; and 28.0% were Black, 6.4% were Hispanic, 56.0% were non-Hispanic White, and 9.7% were of other race or ethnicity. Compared with nonusers, users of QT-prolonging medications with known TdP risk were more frequently female (52.6% vs 44.7%) and non-Hispanic White (56.0% vs 47.9%) and had higher rates of heart failure (53.1% vs 44.2%), arrhythmias (42.4% vs 33.2%), depression (26.8% vs 16.1%), polypharmacy (65.0% vs 49.6%), and hyperpharmacy (17.7% vs 8.8%).
Table 1. Baseline Patient Characteristics by Use and Nonuse of QT-Prolonging Medications With Known TdP Risk in 2019a.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Overall (N = 20 761) | Use of medication with known TdP risk (n = 10 992)b | Nonuse of medication with known TdP risk (n = 9769)b | |
| Demographic and clinical characteristics | |||
| Age, mean (SD), yc | 74 (7) | 74 (7) | 74 (7) |
| Sex | |||
| Female | 10 150 (48.9) | 5784 (52.6) | 4366 (44.7) |
| Male | 10 611 (51.1) | 5208 (47.4) | 5403 (55.3) |
| Race and ethnicity | |||
| Black | 6459 (31.1) | 3073 (28.0) | 3386 (34.7) |
| Hispanic | 1300 (6.3) | 699 (6.4) | 601 (6.2) |
| Non-Hispanic White | 10 834 (52.2) | 6155 (56.0) | 4679 (47.9) |
| Otherd | 2168 (10.4) | 1065 (9.7) | 1103 (11.3) |
| Social factors | |||
| Low-income subsidy | 11 305 (54.5) | 6173 (56.2) | 5132 (52.5) |
| Alcohol or drug use or dependence | 669 (3.2) | 378 (3.4) | 291 (3.0) |
| Tobacco use | 1690 (8.1) | 956 (8.7) | 734 (7.5) |
| History of noncompliance | 1738 (8.4) | 981 (8.9) | 757 (7.7) |
| Selected comorbid conditions | |||
| Diabetes | 15 294 (73.7) | 8276 (75.3) | 7018 (71.8) |
| Ischemic heart disease | 10 891 (52.5) | 6193 (56.3) | 4698 (48.1) |
| Heart failure | 10 154 (48.9) | 5839 (53.1) | 4315 (44.2) |
| Peripheral artery disease | 8053 (38.8) | 4569 (41.6) | 3484 (35.7) |
| Arrhythmia | 7906 (38.1) | 4664 (42.4) | 3242 (33.2) |
| Gastroesophageal reflux disease | 6627 (31.9) | 4002 (36.4) | 2625 (26.9) |
| Valvular disease | 5323 (25.6) | 2965 (27.0) | 2358 (24.1) |
| Stroke | 5097 (24.6) | 2902 (26.4) | 2195 (22.5) |
| Depression | 4521 (21.8) | 2944 (26.8) | 1577 (16.1) |
| Anxiety | 3515 (16.9) | 2297 (20.9) | 1218 (12.5) |
| Conduction disorder | 3190 (15.4) | 1810 (16.5) | 1380 (14.1) |
| Cardiac defibrillator or pacemaker | 2663 (12.8) | 1581 (14.4) | 1082 (11.1) |
| Liver disease | 2322 (11.2) | 1253 (11.4) | 1069 (10.9) |
| Gastrointestinal bleed | 1979 (9.5) | 1132 (10.3) | 847 (8.7) |
| Peptic ulcer | 272 (1.3) | 164 (1.5) | 108 (1.1) |
| Health care utilization, prior 6 mo | |||
| No. of hospitalizationse | |||
| 0 | 12 136 (58.5) | 5986 (54.5) | 6150 (63.0) |
| 1 | 4405 (21.2) | 2537 (23.1) | 1868 (19.1) |
| ≥2 | 4220 (20.3) | 2469 (22.5) | 1751 (17.9) |
| Use of ≥1 QT-prolonging medication according to TdP riskb,f | |||
| Any | 16 168 (77.9) | 9545 (86.8) | 6623 (67.8) |
| Possible | 4692 (22.6) | 2915 (26.5) | 1777 (18.2) |
| Conditional | 13 350 (64.3) | 7755 (70.6) | 5595 (57.3) |
| Known | 8511 (41.0) | 6335 (57.6) | 2176 (22.3) |
| General outpatient medication usef | |||
| Any use of prescribed medications | 19 348 (93.2) | 10 469 (95.2) | 8879 (90.9) |
| Polypharmacy | 11 996 (57.8) | 7149 (65.0) | 4847 (49.6) |
| Hyperpharmacy | 2806 (13.5) | 1945 (17.7) | 861 (8.8) |
| Total unique prescribers, mean (SD) | 2.4 (1.5) | 2.5 (1.5) | 2.2 (1.4) |
Abbreviation: TdP, torsades de pointe.
Characteristics were ascertained in the 180-day baseline period (July 1 through December 31, 2018).
Prior use of a QT-prolonging medication with TdP risk is defined as the use of specified medications in the 180-day baseline period. CredibleMeds classifies medications that can prolong the QT interval as having a known, possible, or conditional TdP risk.25 Drugs with known TdP risk are those that prolong the QT interval and are clearly associated with a known risk of TdP, even when taken as recommended. Drugs with possible TdP risk are those that can cause QT prolongation but currently lack evidence for a risk of TdP when taken as recommended. Drugs with conditional TdP risk are those that are associated with TdP only under certain conditions (eg, excessive dose, in patients with conditions such as hypokalemia, or when taken with interacting drugs) or drugs that create conditions that facilitate or induce TdP (eg, cause an electrolyte disturbance that induces TdP). Lists of medications falling into each category are provided in eTable 2 in Supplement 1.
Age as of January 1, 2019.
Other race and ethnicity includes Asian or Pacific Islander, American Indian or Alaska Native, other, and unknown. This category was combined because of small individual sample sizes.
Hospitalizations within the previous 6 months (July 1 through December 31, 2018).
Use of specified medication (or medications) as of January 1, 2019. Polypharmacy (use of ≥5 prescribed medications) and hyperpharmacy (use of ≥10 prescribed medications) were not mutually exclusive.
Use of QT-Prolonging Medications With Known TdP Risk
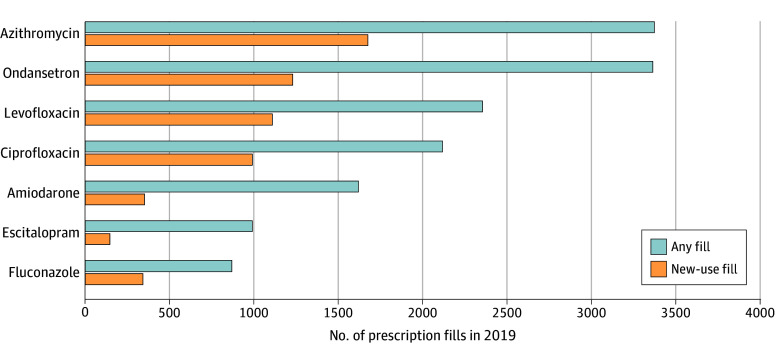
Among the 10 992 Medicare beneficiaries with 1 or more prescription fills of a medication with QT-prolonging potential in 2019, 57.6% were prescribed medications with known TdP risk, and 86.8% were prescribed medications with any (ie, known, possible, or conditional) TdP risk during baseline (Table 1). During 2019, users of QT-prolonging medications with known TdP risk had a mean (SD) of 5.5 (6.1) fills of such medications and 14.8 (15.5) fills of medications with any TdP risk (eTable 5 in Supplement 1). The majority of people receiving known TdP risk medications were prescribed antibacterials or antifungals (azithromycin [30.7%], levofloxacin [21.4%], ciprofloxacin [19.3%], fluconazole [7.9%], ondansetron [30.6%], amiodarone [14.7%], or escitalopram [9.0%]) (eTable 3 in Supplement 1). As shown in Figure 1, these medications were among the most frequently filled overall (including repeat fills among existing users) and also the most common QT-prolonging medications with new-use episodes.
Figure 1. Frequently Filled QT-Prolonging Medications With Known Torsades de Pointes (TdP) Risk.
The 7 QT-prolonging medications with known TdP risk were the most frequently filled by Medicare fee-for-service and Part D beneficiaries receiving maintenance hemodialysis in 2019. Any fill includes repeat fills among existing users, and new-use fill indicates a prescription for the specified medication without evidence of a prior prescription for the same medication in the preceding 180 days.
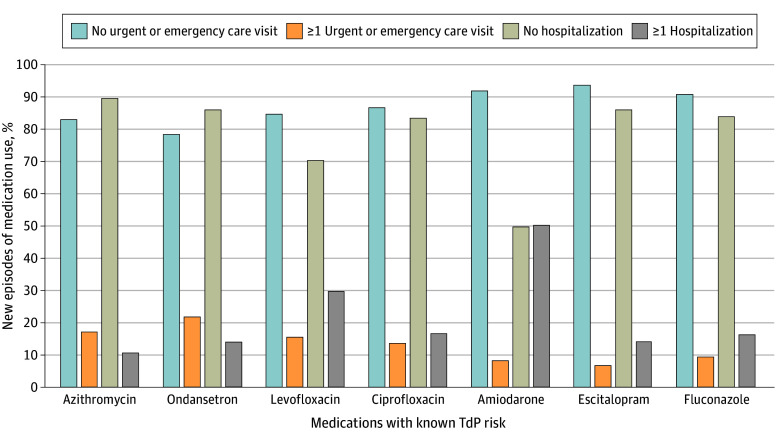
Approximately 80% (from 78.6% for odansetron to 93.9% for escitalopram) of new-use episodes of any QT-prolonging medication with known TdP risk occurred outside temporal proximity to an acute care event. Fewer than one-quarter (from 6.1% for escitalopram to 21.4% for odansetron) of new-use episodes of the study medications occurred within 1 week of an urgent care or emergency department visit, and for new-use episodes occurring within 1 week of a hospitalization, percentages ranged from 10.3% (for azithromycin) to 50.2% (for amiodarone) (Figure 2).
Figure 2. Frequency of Acute Care Events Preceding New Use of QT-Prolonging Medications With Known Torsades de Pointes (TdP) Risk by Medication.
Timing of medication fills, relative to acute care events, of the 7 QT-prolonging medications with known TdP risk most frequently filled by Medicare fee-for-service and Part D beneficiaries receiving maintenance hemodialysis in 2019. Acute care events were captured using Medicare Parts A and B claims for urgent care visits, emergency department visits, and hospitalizations (including observation stays). Hospitalizations and urgent care or emergency department visits were not mutually exclusive.
Prescriber and Pharmacy Characteristics of QT-Prolonging Medications With Known TdP Risk
Users of QT-prolonging medication had a mean (SD) number of different prescribers of outpatient medications of 2.5 (1.5) in use on January 1, 2019 (Table 1). Of new-use prescriptions for the study QT-prolonging medications with known TdP risk during 2019, most (80.2%) came from nonnephrologists (Table 2). Whereas 10.5% to 19.8% of prescriptions for QT-prolonging medications with known TdP risk originated from nephrologists, 36.8% to 61.0% of such prescriptions originated from general medicine clinicians. Over three-quarters (75.3%) of all new-use prescriptions for QT-prolonging medications with known TdP risk in 2019 were ordered by medical doctors or doctors of osteopathy, followed by advanced practice clinicians (13.6%-19.6% of new prescriptions written by advanced practice clinicians). Most new-use prescriptions for QT-prolonging medications with known TdP risk (across the 7 new-user cohorts) were dispensed by commercial retail pharmacies (72.3%-90.1%).
Table 2. Origin of Prescriptions for New Use of QT-Prolonging Medications With Known TdP Risk in 2019 by Medicationa.
| Prescription origin | Medication, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Azithromycin | Ondansetron | Levofloxacin | Ciprofloxacin | Amiodarone | Escitalopram | Fluconazole | |
| Medication use, No. | |||||||
| New-use episodes | 3168 | 2546 | 2203 | 1875 | 638 | 310 | 682 |
| First new-use episodes | 3018 | 2463 | 2130 | 1816 | 634 | 310 | 665 |
| First new-use episodes with no hospitalization 1 wk prior to fill | 2708 | 2123 | 1501 | 1518 | 316 | 267 | 559 |
| Pharmacy characteristics | |||||||
| Commercial or retail | 2439 (90.1) | 1588 (74.8) | 1224 (81.5) | 1324 (87.2) | 250 (79.1) | 193 (72.3) | 456 (81.6) |
| Hospital or institutional | 263 (9.7) | 519 (24.4) | 275 (18.3) | 190 (12.5) | 64 (20.3) | 70 (26.2) | 102 (18.2) |
| Mail order | SC | SC | SC | SC | SC | SC | SC |
| Other | SC | SC | SC | SC | SC | SC | SC |
| Prescriber specialty | |||||||
| Emergency medicine | 196 (7.2) | 235 (11.1) | 96 (6.4) | 91 (6.0) | SC | SC | 13 (2.3) |
| Medicine | 1979 (73.1) | 1443 (68.0) | 1033 (68.8) | 944 (62.2) | 251 (79.4) | 210 (78.7) | 405 (72.5) |
| Cardiology | NR | NR | SC | SC | 99 (31.3) | SC | SC |
| Infectious diseases | SC | SC | NR | NR | SC | SC | SC |
| Nephrology | 536 (19.8) | 242 (11.4) | 201 (13.4) | 249 (16.4) | NR | 28 (10.5) | 67 (12.0) |
| General medicine | 1152 (42.5) | 1001 (47.2) | 698 (46.5) | 559 (36.8) | 120 (38.0) | 163 (61.0) | 271 (48.5) |
| Other medicineb | 261 (9.6) | 186 (8.8) | 114 (7.6) | 111 (7.3) | 20 (6.3) | 18 (6.7) | 60 (10.7) |
| Hospitalist | 16 (0.6) | 20 (0.9) | 23 (1.5) | 11 (0.7) | SC | SC | SC |
| Obstetrics and gynecology | SC | SC | SC | SC | SC | SC | 18 (3.2) |
| Other specialtyc | 37 (1.4) | 16 (0.8) | 63 (4.2) | 81 (5.3) | SC | SC | 17 (3.0) |
| Pain, palliative care, and anesthesia | SC | SC | SC | SC | SC | SC | SC |
| Psychiatry | SC | SC | SC | SC | SC | 13 (4.9) | SC |
| Surgery | 16 (0.6) | 56 (2.6) | 71 (4.7) | 152 (10.0) | SC | SC | 15 (2.7) |
| Unknown | 457 (16.9) | 345 (16.3) | 210 (14.0) | 234 (15.4) | 49 (15.5) | 40 (15.0) | 85 (15.2) |
| Prescriber credentials | |||||||
| Physiciand | 2046 (75.6) | 1607 (75.7) | 1202 (80.1) | 1208 (79.6) | 259 (82.0) | 212 (79.4) | 421 (75.3) |
| Advanced practice cliniciane | 530 (19.6) | 402 (18.9) | 225 (15.0) | 240 (15.8) | 43 (13.6) | 41 (15.4) | 109 (19.5) |
| Anesthesia | SC | SC | SC | SC | SC | SC | SC |
| Midwife or registered nurse | 34 (1.3) | 15 (0.7) | SC | 12 (0.8) | SC | SC | SC |
| Unknown | 95 (3.5) | 96 (4.5) | 66 (4.4) | 57 (3.8) | 13 (4.1) | 12 (4.5) | 18 (3.2) |
Abbreviations: NR, nonreported number; SC, small cell size; TdP, torsades de pointe.
The table reports SC (n = <11) and selected cell sizes (NR; 11 to 30) for data that may be identifiable, according to the Centers for Medicare & Medicaid Services’ data-use reporting requirements.
Includes medical genetics and allergy and immunology.
Includes podiatry, physical medicine and rehabilitation, otolaryngology, dentistry, dermatology, radiology, legal medicine, neuromusculoskeletal medicine, nuclear medicine, optometry, and pathology.
Includes medical doctor and doctor of osteopathy.
Includes nurse practitioner and physician assistant.
Prescriptions for Medications With Potential for Drug-Drug Interactions
Between 6.1% and 37.1% of new-use prescriptions for QT-prolonging medications with known TdP risk were filled in the setting of an existing prescription for a potentially interacting medication (Table 3 and eTable 6 in Supplement 1). For example, between 16.4% and 26.2% of new QT-prolonging medication prescriptions with known TdP risk occurred with the use of another pharmacodynamically interacting QT-prolonging medication. Of new ondansetron prescriptions, 23.6% occurred when patients had an existing prescription for a drug with pharmacodynamic-interaction potential (ie, another medication with known TdP risk) on the date of ondansetron new use (Table 3), and 37.1% of new escitalopram prescriptions occurred when patients were taking a study CYP2C19 inhibitor (ie, a drug with potential pharmacokinetic-interaction risk) on the date of escitalopram new use (eTable 6 in Supplement 1).
Table 3. Practitioner Characteristics of Common Pharmacodynamically Interacting Medication Pairs Among New Users of QT-Prolonging Medications With Known TdP Risk in 2019a.
| Characteristic | Medication, No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Azithromycin | Ondansetron | Levofloxacin | Ciprofloxacin | Amiodarone | Escitalopram | Fluconazole | |
| New-use episodes, No. | 3168 | 2546 | 2203 | 1875 | 638 | 310 | 682 |
| First new episodes, No. | 3018 | 2463 | 2130 | 1816 | 634 | 310 | 665 |
| Concurrent use with another QT-prolonging medication with known TdP riskb | 516 (17.1) | 582 (23.6) | 463 (21.7) | 367 (20.2) | 104 (16.4) | 54 (17.4) | 174 (26.2) |
| Pharmacies | |||||||
| Different | 83 (16.1) | 64 (11.0) | 68 (14.7) | 48 (13.1) | 15 (14.4) | SC | 16 (9.2) |
| Same | 433 (83.9) | 518 (89.0) | 395 (85.3) | 319 (86.9) | 89 (85.6) | 51 (94.4) | 158 (90.8) |
| Commercial or retail | 363 (83.8) | 359 (69.3) | 298 (75.4) | 268 (84.0) | 67 (75.3) | 31 (60.8) | 125 (79.1) |
| Institutionalc | 69 (15.9) | 153 (29.5) | 93 (23.5) | 47 (14.7) | 20 (22.5) | 19 (37.3) | 30 (19.0) |
| Other | SC | SC | SC | SC | SC | SC | SC |
| Mail order | SC | SC | SC | SC | SC | SC | SC |
| Prescribers | |||||||
| Different | 338 (65.5) | 315 (54.1) | 292 (63.1) | 234 (63.8) | 64 (61.5) | 25 (46.3) | 96 (55.2) |
| Same | 178 (34.5) | 267 (45.9) | 171 (36.9) | 133 (36.2) | 40 (38.5) | 29 (53.7) | 78 (44.8) |
| General medicined | 121 (68.0) | 195 (73.0) | 130 (76.0) | 82 (61.7) | 30 (75.0) | 25 (86.2) | 51 (65.4) |
| Nephrology | 15 (8.4) | 11 (4.1) | 11 (6.4) | 16 (12.0) | SC | SC | SC |
| Othere | 26 (14.6) | 36 (13.5) | 17 (9.9) | 20 (15.0) | SC | SC | 13 (16.7) |
| Unknown | 16 (9.0) | 25 (9.4) | 13 (7.6) | 15 (11.3) | SC | SC | SC |
Abbreviations: SC, small cell size; TdP, torsades de pointes.
The table reports SC (<11) for data that may be identifiable, according to the Centers for Medicare & Medicaid Services’ data-use reporting requirements.
Denominators for pharmacy and prescriber data.
Pharmacy within long-term care facility.
Includes internal medicine, family medicine, and general practice.
Includes emergency medicine, cardiology, infectious diseases, hospitalist medicine, psychiatry, surgery, medical genetics, allergy, immunology, podiatry, physical medicine and rehabilitation, otolaryngology, dentistry, dermatology, radiology, legal medicine, neuromusculoskeletal medicine, nuclear medicine, optometry, and pathology.
We observed different prescribing patterns across pharmacodynamically and pharmacokinetically interacting medications. Roughly half of new prescriptions for study QT-prolonging medications occurring in the setting of concomitant use of another QT-prolonging medication with known TdP risk were written by different prescribers (46.3%-65.5%), and most were dispensed by the same pharmacies (83.9%-94.4%) (Table 3 and eTable 6 in Supplement 1). When 2 drugs with potential for pharmacodynamic interaction were dispensed by the same pharmacy, the dispensing pharmacy was most often a commercial or retail pharmacy. When 2 drugs with potential for pharmacodynamic interaction were prescribed by the same clinician, the clinician was most often a nonnephrologist. In contrast, more than half (53.9%) of new-use escitalopram prescriptions in the setting of concurrent use of a study CYP2C19 inhibitor (drugs with potential for pharmacokinetic interaction) were dispensed by the same commercial or retail pharmacy (89.8%) and written by different prescribers (53.3%) (eTable 6 in Supplement 1).
Discussion
In this cross-sectional study, the findings suggest that Medicare beneficiaries aged 60 years or older who have hemodialysis-dependent kidney failure were commonly prescribed QT-prolonging medications with known TdP risk by nonnephrology clinicians and that such prescriptions often originated from nonacute care settings. The findings also suggest that up to one-third of new-use prescriptions for the most commonly prescribed QT-prolonging medications were filled in the setting of concomitant use of a potentially interacting medication and that prescriptions for the medications with potential for interaction often originated from different prescribers.
Existing data demonstrate that individuals with dialysis-dependent kidney failure are prescribed QT-prolonging medications at higher rates than people without kidney failure and that patients receiving dialysis who have risk factors for drug-induced QT prolongation (ie, female sex, advanced age, comorbid heart failure, and concurrent use of ≥1 QT-prolonging medication) are exposed to medications with known TdP risk more often than individuals without such risk factors.29 Moreover, comparative safety studies of some QT-prolonging medications with known TdP risk (eg, citalopram, escitalopram, and levofloxacin) show that, compared with use of clinically relevant comparator medications, use of QT-prolonging medications with known TdP risk is associated with a higher risk of sudden cardiac death, a potential drug-related complication.12,14 Furthermore, prior studies have shown elevated sudden cardiac death risk when patients are prescribed QT-prolonging drugs in the setting of concomitant use of drugs with potential for pharmacodynamic and pharmacokinetic interaction.12,30 Together with our findings, these data underscore the importance of identifying strategies to mitigate unsafe medication-prescribing practices for people receiving hemodialysis.
To our knowledge, there is no published research on the origins of prescriptions for people receiving maintenance hemodialysis. Our data provide new insights on the origins and contexts of high-risk medications among patients receiving hemodialysis. We found that less than one-quarter of prescriptions for QT-prolonging medications with known TdP risk occurred within 1 week of an acute care encounter. Instead, the majority of these medications were prescribed from nonhospital settings, suggesting that hospital-based medication-monitoring systems, on their own, may be inadequate to mitigate harm from unsafe medication-prescribing practices.
We focused our analysis on medications with known TdP risk because we sought to examine medications relevant to sudden cardiac death, the leading cause of death in the population undergoing hemodialysis. Identifying potentially harmful prescription patterns is essential, as medication use is a potentially modifiable risk factor for sudden cardiac death, unlike risk factors more challenging to modify (eg, change in ventricular architecture, fluid and electrolyte shifts inherent to dialysis procedures). However, it is likely that our study’s findings are a microcosm of a larger issue of risky prescription practices among individuals receiving maintenance hemodialysis. Investigation of the prescription and dispensation patterns of other potentially harmful medications (eg, opioids,31,32 benzodiazepines,32 sedative hypnotics,33,34 and muscle relaxants35) is warranted.
Our findings highlight opportunities for the uptake of strategies targeting outpatient prescribers and dispensing pharmacies to reduce drug-related harms of high-risk medications for clinically complex patients. In the population undergoing dialysis, educating nonnephrology clinicians about medication safety concepts specific to kidney failure is one potential approach to reducing medication-related complications.36 However, this approach is unlikely to be successful on its own due to the diversity of clinicians contributing to the care of patients receiving dialysis.37,38,39 Other strategies shown to be effective in the outpatient setting include pharmacist-led medication reconciliation and medication management programs40,41,42,43,44 as well as interoperable EHRs and clinical decision support systems.36,37,45,46 Pharmacist-led medication reconciliation, the process of generating an accurate and complete list of patient medications, inclusive of prescription and over-the-counter medications, herbals, and supplements,44 can be effective for reducing medication-related harm.40,41,42,43 The process falls short when clinicians lack up-to-date information on patient comorbid conditions and medication use. Gaps in information are particularly common in dialysis care because the majority of US dialysis clinics use EHR systems that do not communicate with local health system EHRs.36,47 Such challenges with interoperable EHRs and clinical decision support systems45,46 and missteps in cross-clinician communication have been associated with reliance on the dialysis clinic for detection of potentially dangerous prescribing practices.37
In 2022, the Centers for Medicare & Medicaid Services introduced a medication reconciliation measure to the End-Stage Renal Disease Quality Incentive Program, a Medicare pay-for-performance program. The safety measure requires dialysis clinics to attest to providing monthly medication reconciliation for all patients under their care. For medication reconciliation to be effective at reducing drug-related complications, it must be performed in close proximity to the unsafe prescribing event. While existing evidence supports acute care encounters as events that should trigger medication reconciliation, our finding that QT-prolonging medications with known TdP risk most often originate from nonacute ambulatory settings suggests that outpatient clinician visits should also be triggers for medication reconciliation. Areas ripe for future investigation include identification of the optimal timing, frequency, and setting for medication reconciliation as well as adoption of integrated communication systems aimed at minimizing harm from care fragmentation.38,39
Within the system of care, the dispensing pharmacy and prescription drug plan could play important roles in medication safety checks. We found that QT-prolonging medications with known TdP risk and their interacting medications were most commonly ordered by different prescribers but filled at the same commercial or retail pharmacy. As such, pharmacies may be well-positioned to identify potentially risky medication prescribing. Existing commercial and institutional pharmacy software systems detect prescriptions for contraindicated and/or incorrectly dosed medications as well as medication combinations with potential for interaction. However, these systems are effective only to the extent that pharmacy-ordering systems can cross-check for interacting medications at the time of prescription fill and that this cross-check occurs within and across affiliated pharmacy sites (ie, chain retail pharmacies), when the data underlying the alerts are accurate, and when dispensing pharmacists act on the triggered alerts. Pharmacy systems, especially in community settings, may not be connected to EHR systems and thus lack up-to-date clinical information.36,48 However, prescription drug plans may be better positioned in this regard, as they collect prescription drug information at the point of pharmacy purchase. To maximize the potential effectiveness of medication-monitoring systems and reconciliation among individuals with dialysis-dependent kidney failure, as well as patients more broadly, information systems that integrate data from care settings, dispensing pharmacies, and prescription drug plans are needed.
Limitations
Our findings should be considered in the context of study limitations. First, we described prescription medication fills, which may not have reflected patients’ actual medication use. Similarly, we were unable to account for prescription adjustments initiated by clinicians in response to a perceived change in risk-benefit that might have occurred after prescription fill (ie, verbally communicated and not documented). Second, our data source captured medications obtained under Medicare prescription drug plans and did not include medications purchased without insurance, under other insurance plans (eg, Medicare Advantage in 2019,49 private insurance), or over the counter. The validity of claims’ file prescriber and pharmacy data was unknown; misclassification of clinician specialty, credentials, or pharmacy characteristics was possible. Last, our analyses were descriptive; we did not investigate potential associations of prescription origins with risks of adverse clinical outcomes.
Conclusions
In this cross-sectional study, Medicare beneficiaries with kidney failure receiving hemodialysis were commonly prescribed QT-prolonging medications with known TdP risk maintenance by nonnephrology clinicians and from nonacute settings. Prescriptions for potentially interacting medications often originated from different prescribers. These findings highlight the need to better coordinate medication management at the clinician and health system levels and suggest that both prescriber- and pharmacy-targeted strategies may be important to minimize drug-related complications in the high-risk population undergoing dialysis.
eFigure. Cohort Sampling Scheme
eTable 1. Data Sources: Diagnosis Codes, Data Files for Social Factors, Comorbid Conditions, and Health Care Utilization
eTable 2. List of QT-Prolonging Medications With a Known, Possible, and Conditional Risk of TdP
eTable 3. Most Frequent QT-Prolonging Medications With Known TdP Risk Filled in 2019
eTable 4. Medications in CYP Inhibitor Classes
eTable 5. QT-Prolonging Medication Use in 2019
eTable 6. Pharmacy and Prescriber Characteristics of the Most Common Study Pharmacokinetic Medications Interacting With New-Use Escitalopram in 2019
Data Sharing Statement
References
- 1.Volume 2: end stage renal disease. Am J Kidney Dis. 2023;81(3)(suppl 10):S224-S674. doi: 10.1053/j.ajkd.2022.12.003 [DOI] [Google Scholar]
- 2.Rifkin DE, Laws MB, Rao M, Balakrishnan VS, Sarnak MJ, Wilson IB. Medication adherence behavior and priorities among older adults with CKD: a semistructured interview study. Am J Kidney Dis. 2010;56(3):439-446. doi: 10.1053/j.ajkd.2010.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonelli M, Wiebe N, Manns BJ, et al. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1(7):e184852. doi: 10.1001/jamanetworkopen.2018.4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frament J, Hall RK, Manley HJ. Medication reconciliation: the foundation of medication safety for patients requiring dialysis. Am J Kidney Dis. 2020;76(6):868-876. doi: 10.1053/j.ajkd.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St Peter WL. Management of polypharmacy in dialysis patients. Semin Dial. 2015;28(4):427-432. doi: 10.1111/sdi.12377 [DOI] [PubMed] [Google Scholar]
- 6.Andersson ML, Böttiger Y, Kockum H, Eiermann B. High prevalence of drug-drug interactions in primary health care is caused by prescriptions from other healthcare units. Basic Clin Pharmacol Toxicol. 2018;122(5):512-516. doi: 10.1111/bcpt.12939 [DOI] [PubMed] [Google Scholar]
- 7.Bjerrum L, Gonzalez Lopez-Valcarcel B, Petersen G. Risk factors for potential drug interactions in general practice. Eur J Gen Pract. 2008;14(1):23-29. doi: 10.1080/13814780701815116 [DOI] [PubMed] [Google Scholar]
- 8.Holmes HM, Luo R, Kuo YF, Baillargeon J, Goodwin JS. Association of potentially inappropriate medication use with patient and prescriber characteristics in Medicare Part D. Pharmacoepidemiol Drug Saf. 2013;22(7):728-734. doi: 10.1002/pds.3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green JL, Hawley JN, Rask KJ. Is the number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother. 2007;5(1):31-39. doi: 10.1016/j.amjopharm.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM. Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol. 2018;13(7):1085-1095. doi: 10.2215/CJN.00340118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller F, Hann A. Clinical pharmacodynamics: principles of drug response and alterations in kidney disease. Clin J Am Soc Nephrol. 2018;13(9):1413-1420. doi: 10.2215/CJN.10960917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assimon MM, Brookhart MA, Flythe JE. Comparative cardiac safety of selective serotonin reuptake inhibitors among individuals receiving maintenance hemodialysis. J Am Soc Nephrol. 2019;30(4):611-623. doi: 10.1681/ASN.2018101032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assimon MM, Pun PH, Wang L, et al. Azithromycin use increases the risk of sudden cardiac death in patients with hemodialysis-dependent kidney failure. Kidney Int. 2022;102(4):894-903. doi: 10.1016/j.kint.2022.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assimon MM, Pun PH, Wang LC, et al. Analysis of respiratory fluoroquinolones and the risk of sudden cardiac death among patients receiving hemodialysis. JAMA Cardiol. 2022;7(1):75-83. doi: 10.1001/jamacardio.2021.4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ismail S, Funk MJ, Flythe JE. Ondansetron and the risk of sudden cardiac death among individuals receiving maintenance hemodialysis. J Am Soc Nephrol. Published online February 27, 2024. doi: 10.1681/ASN.0000000000000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins RE, Bui T, Magliano D, Arnold C, Dooley M. Prescriber education interventions to optimize opioid prescribing in acute care: a systematic review. Pain Physician. 2019;22(6):E551-E562. doi: 10.36076/ppj/2019.22.E551 [DOI] [PubMed] [Google Scholar]
- 17.Trivalle C, Cartier T, Verny C, et al. ; IMEPAG GROUP . Identifying and preventing adverse drug events in elderly hospitalised patients: a randomised trial of a program to reduce adverse drug effects. J Nutr Health Aging. 2010;14(1):57-61. doi: 10.1007/s12603-010-0010-4 [DOI] [PubMed] [Google Scholar]
- 18.Ciapponi A, Fernandez Nievas SE, Seijo M, et al. Reducing medication errors for adults in hospital settings. Cochrane Database Syst Rev. 2021;11(11):CD009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killin L, Hezam A, Anderson KK, Welk B. Advanced medication reconciliation: a systematic review of the impact on medication errors and adverse drug events associated with transitions of care. Jt Comm J Qual Patient Saf. 2021;47(7):438-451. doi: 10.1016/j.jcjq.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 20.Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003. doi: 10.1136/bmjopen-2015-010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Awdishu L, Coates CR, Lyddane A, et al. The impact of real-time alerting on appropriate prescribing in kidney disease: a cluster randomized controlled trial. J Am Med Inform Assoc. 2016;23(3):609-616. doi: 10.1093/jamia/ocv159 [DOI] [PubMed] [Google Scholar]
- 22.Skalafouris C, Blanc AL, Grosgurin O, et al. Development and retrospective evaluation of a clinical decision support system for the efficient detection of drug-related problems by clinical pharmacists. Int J Clin Pharm. 2023;45(2):406-413. doi: 10.1007/s11096-022-01505-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skalafouris C, Reny JL, Stirnemann J, et al. Development and assessment of PharmaCheck: an electronic screening tool for the prevention of twenty major adverse drug events. BMC Med Inform Decis Mak. 2022;22(1):146. doi: 10.1186/s12911-022-01885-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 25.Woosley R, Heise C, Romero K. QTDrugs list. Updated May 26, 2022. Accessed June 3, 2022. https://crediblemeds.org
- 26.National Uniform Claim Committee. Health care provider taxonomy, Version 22.1. July 2022. Accessed December 14, 2022. https://www.nucc.org/images/stories/PDF/taxonomy_22_1.pdf
- 27.Research Data Assistance Center (ResDAC). Data documentation: Part D pharmacy characteristics file. University of Minnesota. Accessed December 14, 2022. https://resdac.org/cms-data/files/part-d-pharmacy-characteristics/data-documentation
- 28.Certara. Drug interaction solutions. DIDB—The Drug Interaction Database. Accessed July 10, 2019. https://www.druginteractionsolutions.org/solutions/drug-interaction-database/
- 29.Assimon MM, Wang L, Pun PH, Winkelmayer WC, Flythe JE. Use of QT prolonging medications by hemodialysis patients and individuals without end-stage kidney disease. J Am Heart Assoc. 2020;9(13):e015969. doi: 10.1161/JAHA.120.015969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assimon MM, Pun PH, Al-Khatib SM, et al. Proton pump inhibitors may enhance the risk of citalopram- and escitalopram-associated sudden cardiac death among patients receiving hemodialysis. Pharmacoepidemiol Drug Saf. 2022;31(6):670-679. doi: 10.1002/pds.5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol. 2018;13(5):746-753. doi: 10.2215/CJN.09910917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyne A, Rai R, Cuerden M, Clark WF, Suri RS. Opioid and benzodiazepine use in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2011;6(2):326-333. doi: 10.2215/CJN.04770610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assimon MM, Flythe JE. Zolpidem versus trazodone initiation and the risk of fall-related fractures among individuals receiving maintenance hemodialysis. Clin J Am Soc Nephrol. 2020;16(1):88-97. doi: 10.2215/CJN.10070620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Psychoactive medications and adverse outcomes among older adults receiving hemodialysis. J Am Geriatr Soc. 2019;67(3):449-454. doi: 10.1111/jgs.15740 [DOI] [PubMed] [Google Scholar]
- 35.Mina D, Johansen KL, McCulloch CE, Steinman MA, Grimes BA, Ishida JH. Muscle relaxant use among hemodialysis patients: prevalence, clinical indications, and adverse outcomes. Am J Kidney Dis. 2019;73(4):525-532. doi: 10.1053/j.ajkd.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 36.Hall RK, Rutledge J, Lucas A, et al. Stakeholder perspectives on factors related to deprescribing potentially inappropriate medications in older adults receiving dialysis. Clin J Am Soc Nephrol. 2023;18(10):1310-1320. doi: 10.2215/CJN.0000000000000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beben T, Rifkin DE. Recognizing our limits: deficiencies in end-of-life education for nephrology trainees. Am J Kidney Dis. 2015;65(2):209-210. doi: 10.1053/j.ajkd.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 38.Sloan CE, Zhong J, Mohottige D, et al. Fragmentation of care as a barrier to optimal ESKD management. Semin Dial. 2020;33(6):440-448. doi: 10.1111/sdi.12929 [DOI] [PubMed] [Google Scholar]
- 39.Wang V, Diamantidis CJ, Wylie J, Greer RC. Minding the gap and overlap: a literature review of fragmentation of primary care for chronic dialysis patients. BMC Nephrol. 2017;18(1):274. doi: 10.1186/s12882-017-0689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alshogran OY, Hajjar MH, Muflih SM, Alzoubi KH. The role of clinical pharmacist in enhancing hemodialysis patients’ adherence and clinical outcomes: a randomized-controlled study. Int J Clin Pharm. 2022;44(5):1169-1178. doi: 10.1007/s11096-022-01453-0 [DOI] [PubMed] [Google Scholar]
- 41.Burgess LH, Kramer J, Castelein C, et al. Pharmacy-led medication reconciliation program reduces adverse drug events and improves satisfaction in a community hospital. HCA Healthc J Med. 2021;2(6):411-421. doi: 10.36518/2689-0216.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pai AB, Boyd A, Depczynski J, Chavez IM, Khan N, Manley H. Reduced drug use and hospitalization rates in patients undergoing hemodialysis who received pharmaceutical care: a 2-year, randomized, controlled study. Pharmacotherapy. 2009;29(12):1433-1440. doi: 10.1592/phco.29.12.1433 [DOI] [PubMed] [Google Scholar]
- 43.Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16(3):185-195. doi: 10.18553/jmcp.2010.16.3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wigneswaran J, St Peter WL, Nissenson AR, et al. Redefining medication management in dialysis: a kidney pharmacy quality pyramid. Kidney Med. 2019;1(5):307-314. doi: 10.1016/j.xkme.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Earl TR, Katapodis ND, Schneiderman SR. Reducing adverse drug events in older adults. In: Hall KK, Shoemaker-Hunt S, Hoffman L, et al, eds. Making Healthcare Safer III: A Critical Analysis of Existing and Emerging Patient Safety Practices (Internet). Agency for Healthcare Research and Quality; March 2020;9-1-9-19. Accessed February 22, 2024. https://www.ncbi.nlm.nih.gov/books/NBK555524/ [PubMed]
- 46.Li E, Clarke J, Ashrafian H, Darzi A, Neves AL. The impact of electronic health record interoperability on safety and quality of care in high-income countries: systematic review. J Med Internet Res. 2022;24(9):e38144. doi: 10.2196/38144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beben T, Rifkin DE. The life-changing magic of tidying up the medication list. Clin J Am Soc Nephrol. 2023;18(10):1254-1256. doi: 10.2215/CJN.0000000000000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hudson JQ, Maxson R, Barreto EF, et al. Education standards for pharmacists providing comprehensive medication management in outpatient nephrology settings. Kidney Med. 2022;4(8):100508. doi: 10.1016/j.xkme.2022.100508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen KH, Oh EG, Meyers DJ, Kim D, Mehrotra R, Trivedi AN. Medicare Advantage enrollment among beneficiaries with end-stage renal disease in the first year of the 21st Century Cures Act. JAMA. 2023;329(10):810-818. doi: 10.1001/jama.2023.1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Cohort Sampling Scheme
eTable 1. Data Sources: Diagnosis Codes, Data Files for Social Factors, Comorbid Conditions, and Health Care Utilization
eTable 2. List of QT-Prolonging Medications With a Known, Possible, and Conditional Risk of TdP
eTable 3. Most Frequent QT-Prolonging Medications With Known TdP Risk Filled in 2019
eTable 4. Medications in CYP Inhibitor Classes
eTable 5. QT-Prolonging Medication Use in 2019
eTable 6. Pharmacy and Prescriber Characteristics of the Most Common Study Pharmacokinetic Medications Interacting With New-Use Escitalopram in 2019
Data Sharing Statement