Abstract
Purpose
The increasing use of computed tomography (CT) imaging has led to the detection of more ground-glass nodules (GGNs) and subsolid nodules (SSNs), which may be malignant and require a biopsy for proper diagnosis. Approximately 75% of persistent GGNs can be attributed to adenocarcinoma in situ or minimally invasive adenocarcinoma. A CT-guided biopsy has been proven to be a reliable procedure with high diagnostic performance. However, the diagnostic accuracy and safety of a CT-guided biopsy for GGNs and SSNs with solid components ≤6 mm are still uncertain. The aim of this study is to assess the diagnostic accuracy of a CT-guided core needle biopsy (CNB) for GGN and SSNs with solid components ≤6 mm.
Methods
This is a retrospective study of patients who underwent CT-guided CNB for the evaluation of GGNs and SSNs with solid components ≤6 mm between February 2020 and January 2023. Biopsy findings were compared to the final diagnosis determined by definite histopathologic examination and clinical course.
Results
A total of 22 patients were enrolled, with a median age of 74 years (IQR: 68-81). A total of 22 nodules were assessed, comprising 15 (68.2%) SSNs with a solid component measuring ≤6 mm and seven (31.8%) pure GGNs. The histopathological examination revealed that 12 (54.5%) were diagnosed as malignant, nine (40.9%) as benign, and one (4.5%) as non-diagnostic. The overall diagnostic accuracy and sensitivity for malignancy were 86.36% and 85.7%, respectively.
Conclusion
A CT-guided CNB for GGNs and SSNs with solid components measuring ≤6 mm appears to have a high diagnostic accuracy.
Keywords: computed tomography-guided core needle biopsy, diagnostic accuracy, lung cancer, subsolid nodule, ground-glass nodule
Introduction
A pulmonary nodule is defined as a small (≤3 cm in diameter) and focal radiographic opacity that is surrounded by lung parenchyma [1]. Based on appearance, pulmonary nodules are categorized as solid and subsolid. Subsolid pulmonary nodules (SSNs) include pure ground-glass nodules (GGNs) and part-solid nodules. A GGN is defined as a hazy attenuation of a lung with visible bronchial and vascular structures [2]. Part-solid nodules are the nodules with both ground-glass and solid components [2].
SSNs, including those composed solely of GGNs, are being more commonly recognized in clinical practice due to the increased utilization and enhanced resolution of computed tomography (CT) imaging. The incidence of a subsolid nodule is reported to be 4.2% in baseline screening for lung cancer and 0.7% in annual repeat screening [3]. Approximately 80% of persistent GGNs can be attributed to adenocarcinoma in situ or minimally invasive adenocarcinoma [4]. Adenocarcinoma in situ refers to adenocarcinoma consisting entirely of the lepidic/bronchioloalveolar pattern, while minimally invasive adenocarcinoma refers to an invasive adenocarcinoma with a predominantly lepidic growth pattern and a maximum invasive size of 3 cm or less [5]. However, it should be noted that the differential diagnosis of GGN is not limited to malignancy alone, as it may also comprise premalignant lesions such as atypical adenomatous hyperplasia and non-neoplastic conditions such as focal fibrosis and inflammation [6]. Biopsy and tissue sample acquisition are imperative to rule out malignancy and to determine the nature of the lesion.
A CT-guided core needle biopsy (CNB) is a minimally invasive procedure for the diagnosis of thoracic lesions. It is an essential tool for confirming the neoplastic nature of the lesion and characterizing its genotypic and molecular profile. A CT-guided biopsy has been proven a reliable procedure, with reported achieved diagnostic accuracy rates of 93% for SSNs with solid components of any size [7]. However, the diagnostic accuracy and safety of a CT-guided biopsy for GGNs and SSNs with solid components ≤6 mm are still uncertain when compared to solid nodules.
The aim of this study was to assess the diagnostic accuracy of a CT-guided CNB for GGNs and SSNs with solid components ≤6 mm.
Materials and methods
This is a retrospective analysis conducted at Mayo Clinic, Florida, United States, on patients who underwent a CT-guided CNB for the evaluation of pulmonary nodules between February 2020 and January 2023. The inclusion criteria were the presence of GGNs or SSNs with a solid component measuring ≤6 mm. We recorded patients' baseline characteristics, procedure details, assessment of the nodules, and complications encountered during the biopsy. The data were encrypted and stored in a secure database. The study received approval from the Institutional Review Board (IRB # 22-010644). The primary outcome was to determine the efficacy and diagnostic performance of a CT-guided CNB for diagnosing GGNs and SSNs. The secondary goals were to evaluate the safety of this procedure, as determined by the incidence of complications that occurred following the biopsy.
CT-guided CNB
Siemens Healthineers' SOMATOM scanners (Siemens Healthineers; Siemens Medical Solutions, Malvern, PA) were used for the CT-guided CNB procedures. Prior to the biopsy, a thorough analysis of pre-procedure CT scans determined the optimal sampling route considering nodule location, patient positioning, and needle entry site and angle to avoid fissured areas, bullae, and substantial aerated pulmonary parenchyma. Anesthesia was administered locally with 1% lidocaine solution, and needle sizes for biopsy ranged from 18G to 20G. All patients underwent a tissue core biopsy with a coaxial technique, followed by rapid on-site evaluation (ROSE) for sample adequacy and preliminary diagnosis. Additional passes were made if necessary. Post-procedure, chest radiography was performed within two to four hours to assess pneumothorax. Patients followed the institution's recovery protocol before discharge.
Complications
Adverse events associated with the intervention were documented within a seven-day post-procedural period. The two most common complications were pneumothorax and hemoptysis. Pneumothorax was considered an adverse effect if it led to hospitalization for observation or required chest tube placement, as defined by established criteria [8]. Hemoptysis was categorized using the Nashville Bleeding Scale, where bleeding was labeled as mild, moderate, or severe according to escalating interventions to control bleeding, from suctioning to advanced ventilatory support, transfusion, or resuscitation, and procedure abortion [9].
Histopathological diagnosis and follow-up
All procedures in this study included ROSE to assess sample adequacy, with a cytotechnologist present for immediate pathological interpretation. Subsequently, biopsy findings were cross-referenced with the conclusive diagnosis through definitive histopathologic examination, immunohistochemical staining, and progression of the clinical course. In instances where an initial definite diagnosis could not be established, secondary procedures such as re-intervention, surgical resection, alterations in CT, or if there was evidence of a malignant clinical progression provided the final diagnosis.
Statistical analysis
Clinicodemographic information was presented using medians and interquartile ranges for quantitative data values, while categorical variables were expressed as counts and percentages. Descriptive analysis of the data was conducted using Microsoft Excel and SPSS Statistics (version 28.0). Diagnostic accuracy was evaluated as follows: biopsy samples were categorized as true positives (TP) if they resulted in a definitive malignant diagnosis or suspicion of malignancy confirmed through a subsequent procedure or demonstrated malignant progression - growth in size or number - at six months follow-up or later. True negatives (TN) were assigned to cases with a conclusive benign diagnosis or those negative for malignancy, subsequently confirmed as benign through a secondary procedure or exhibiting benign clinical progression, including lesion resolution, size reduction, or stability, at six months follow-up or later. False positives (FP) were characterized by initial malignant diagnosis, but subsequent investigation or clinical course revealed benign disease at six months follow-up or later. False negatives (FN) comprised cases with a benign pathology or negative for malignancy that, following a secondary procedure or clinical progression, were confirmed as malignant at six months follow-up or later. The results were tabulated in a 2 x 2 table for sensitivity, specificity, positive predictive value, and negative predictive value estimations.
Results
In this study, a total of 22 patients were enrolled, with a median age of 74 years (IQR, 68-81) and a median BMI of 25.5 kg/m2 (IQR: 21.2-27.8). Of the participants, 16 (72.7%) were female, and 10 (45.5%) had a personal history of cancer, but none were lung cancer. General demographic information, along with patients’ smoking history, is presented in Table 1.
Table 1. Demographic and clinical characteristics.
Quantitative data are presented as median and interquartile range.
| Demographic and Clinical Characteristics | CT-Guided CNB (N=22) |
| Age | 74 (68, 81) |
| BMI | 25.5 (21.2, 27.8) |
| Gender | |
| Female | 16 (72.27%) |
| Male | 6 (27.3%) |
| Personal History of Cancer | |
| No | 12 (54.5%) |
| Yes | 10 (45.5%) |
| Personal History of Lung Cancer | |
| No | 22 (100%) |
| Yes | 0 (0%) |
| Smoking History | |
| Former | 18 (81.8%) |
| Never | 3 (13.6%) |
| Current | 1 (4.5%) |
| Pack-years | 26.3 (18,38) |
A total of 22 nodules were assessed, comprising 15 (68.2%) SSNs, with a solid component measuring ≤6 mm (Figure 1), and seven (31.8%) pure GGNs (Figure 2, Table 2). The majority of target nodules (9, 40.9%) were located in the left upper lobe. The nodules had a median maximum cross-sectional diameter of 1.4 cm (IQR: 1.1-1.6), and a minimum cross-sectional diameter of 0.9 cm (IQR: 0.7-1.1). SSNs had a median maximum diameter of solid components of 0.6 cm (IQR: 0.5-0.6) and a minimum diameter of 0.35 cm (IQR: 0.3-0.43). The median distance from the skin to the nodule was 6.53 cm (IQR: 5.94-7.6), while the median distance from the pleura was 1.04 cm (IQR: 0.4-1.8).
Table 2. Ground-glass opacities and subsolid nodules characteristics.
Quantitative data are presented as median and interquartile range.
| Nodule Characteristics | CT-Guided CNB (N=22) |
| Nodule Location | |
| LUL | 9 (40.9%) |
| RUL | 7 (31.8%) |
| RLL | 5 (22.7%) |
| RML | 1 (4.5%) |
| LLL | 0 (0%) |
| Nodule Type | |
| Part solid | 15 (68.2%) |
| Ground-glass | 7 (31.8%) |
| Nodule size maximum (cm) | 1.4 (1.1, 1.6) |
| Nodule size minimum (cm) | 0.9 (0.7, 1.1) |
| Solid component size maximum (cm) | 0.6 (0.5, 0.3) |
| Solid component size minimum (cm) | 0.35 (0.3, 0.43) |
| Distance from skin (cm) | 6.53 (5.94, 7.6) |
| Distance to pleura (cm) | 1.04 (0.4, 1.8) |
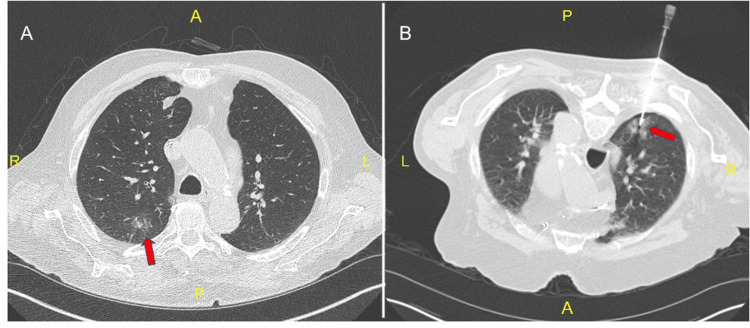
Figure 1. A) Chest computed tomography axial view showing a 20 x 14 mm sub-solid nodule with solid components measuring 4 mm in the right upper lobe. B) Computed tomography image obtained during a CT-guided biopsy, showing a biopsy needle targeting a subsolid nodule in the right upper lobe.
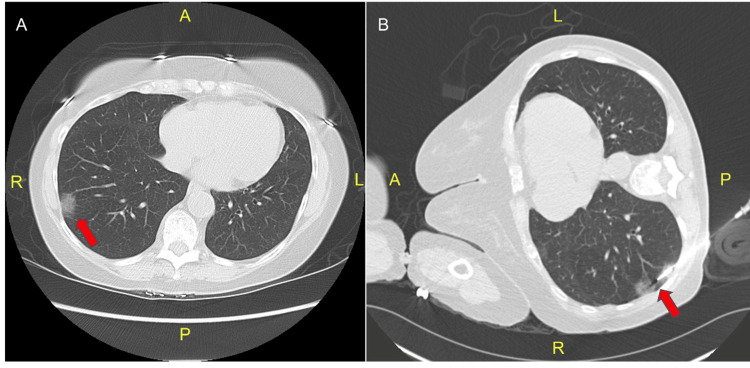
Figure 2. A) Chest computed tomography axial view showing a 23 x 14 mm ground-glass nodule in the right lower lobe. B) Computed tomography image obtained during a CT-guided biopsy, showing a biopsy needle targeting the ground-glass nodule in the right lower lobe.
The median duration of the procedure was 25.5 minutes (IQR: 20-36). Among the complications encountered, pneumothorax was the most common (seen in nine (40.9%) patients), followed by bleeding (seen in two (9.1%) patients). A total of 5 (22.7%) patients required admission for pneumothorax, of which two required chest tubes (Table 3).
Table 3. CT-guided core needle biopsy procedure details.
Quantitative data are presented as median and interquartile range.
| CT-Guided CNB Procedure Details | CT-Guided CNB (N=22) | |
| Biopsy Needle Size | ||
| 18G | 19 (86.4%) | |
| 20G | 3 (13.6%) | |
| Number of Needle Passes | ||
| 1 | 3 (15.8%) | |
| 2 | 10 (52.6%) | |
| 3 | 3 (15.8%) | |
| 4 | 2 (10.5%) | |
| 5 | 1 (4.5%) | |
| Duration of the Procedure in Minutes | 26.5 (20, 36) | |
| Complications | ||
| Pneumothorax | 9 (40.9%) | |
| Hemorrhage | 2 (9.1%) | |
| Others | 0 (0%) | |
| Chest Tube Placement | ||
| No | 20 (90.9%) | |
| Yes | 2 (9.1%) | |
| Hospital Admission | ||
| No | 17 (77.3%) | |
| Yes | 5 (22.7%) | |
| Length of stay in hospital, days | 0 (0,0) | |
The histopathological examination of 22 biopsy samples revealed that 12 (54.5%) were diagnosed as malignant, nine (40.9%) as benign, and one (4.5%) as non-diagnostic. For detecting malignancy, overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy were found to be 85.7%, 100%, 100%, 77.7%, and 86.36%, respectively (Table 4).
Table 4. Diagnostic performance of a CT-guided core needle biopsy for ground-glass opacity and subsolid nodules.
| Pathology Report | |
| Malignant | 12 (54.5%) |
| Benign | 9 (40.9%) |
| Non-diagnostic | 1 (4.5%) |
| Malignancy diagnosis | |
| True Positive | 12 |
| False Positive | 0 |
| True Negative | 7 |
| False Negative | 2 |
| Diagnostic Accuracy | |
| Sensitivity | 85.7% |
| Specificity | 100% |
| Positive Predictive Value | 100% |
| Negative Predictive Value | 77.7% |
Discussion
The diagnostic performance of a CT-guided CNB was assessed in 22 SSNs, consisting of seven GGNs and 15 part-solid nodules with a solid component measuring ≤6 mm, at our tertiary care referral center. Although a CT-guided CNB has been established as a reliable and highly accurate diagnostic method for lung nodules, the diagnostic sensitivity for SSNs was found to be relatively low when compared to solid nodules [10]. This may be attributed to the lower cellular density of SSNs compared to solid nodules [10]. Furthermore, fewer studies have been conducted on nodules ≤1 cm [11,12], and no clear data are available for diagnostic accuracy of part-solid nodules with solid components ≤6 mm using a CT-guided CNB.
Recent advancements in low-dose CT screening protocols have led to the detection of an increasing number of SSNs and smaller nodules, resulting in the diagnosis of cancer in smaller nodules [13,14]. It has been shown that the size of the pulmonary nodule is a determining factor for the diagnostic accuracy of a CT-guided CNB [15].
The present study revealed an overall sensitivity of 85.7%, specificity of 100%, PPV of 100%, NPV of 77.7%, and a diagnostic yield of 86.36%. These findings were comparable to earlier studies that used a CT-guided CNB as the diagnostic tool [7,15,16]. However, in our study, the nodules were smaller, and we were able to preserve a high diagnostic accuracy despite specifically targeting pure GGNs and SSNs with solid components ≤6 mm. Previous research by Shimizu et al. indicated that a CT-guided aspiration biopsy had lower diagnostic yields for GGN-dominant lesions when compared to solid lesions [17]. Additionally, Hur et al. reported that diagnostic accuracy was notably decreased by the presence of a larger GG component [18]. The observed differences in accuracy could potentially be attributed to the lower cellularity of aspirates obtained from part-solid lesions [19]. Our study found a higher diagnostic yield despite we performed biopsies on GG-predominant lesions. It is noteworthy that our study employed core CNB, which is capable of obtaining adequate core specimens, while previous studies that reported lower diagnostic yields for lesions with a predominant GGN component utilized a fine needle aspiration biopsy [17,18]. The use of CNB may have contributed to the higher diagnostic accuracy observed in our study by enabling the acquisition of larger and more representative tissue samples.
The diagnostic accuracy of CNB for lesions with varying GG component proportions remains a subject of debate in the literature. For instance, Yamagami et al. reported that the diagnostic accuracy of CNB differed significantly with the proportion of GG component, even though CNB was used [20]. In contrast, Kim et al. reported conflicting results, indicating that the diagnostic accuracy of CNB was not influenced by the proportion of GG components. Both studies analyzed the differences in diagnostic accuracy according to the GG component proportion of part-solid lesions [16]. The conflicting results of these studies suggest that further research is needed to clarify the diagnostic accuracy of CNB for lesions with varying GG component proportions.
In the present study, the most frequently encountered complication was pneumothorax, which occurred in 40.9% of cases. The incidence of pneumothorax reported in previous studies varied considerably, ranging from 15% to 50% [21,22], regardless of the nature of the nodule. Among the cases of pneumothorax observed in our study, only two required chest tube insertion, while the remaining cases resolved spontaneously. Hemorrhage was the second most common complication, occurring in two cases. The severity of the hemorrhage was low and did not need further interventions.
The study presented several limitations that should be taken into consideration when interpreting the results. Firstly, it was a retrospective study, which might have introduced selection bias. To minimize this bias, future studies should consider using a prospective design. Secondly, the study was conducted at a single center, which may limit the generalizability of our findings. It is possible that the patient population at this center may differ from other centers, which could affect the diagnostic yield and complication rates. Therefore, future studies should involve multiple centers to increase the external validity of the results or compare them with other alternative sampling strategies such as robotic-assisted bronchoscopy. Thirdly, the study had a relatively small sample size as we focused only on GGNs and had a strict cut-off for solid components, which may limit the statistical power of the analysis. As a result, the study may not have been able to detect small but clinically significant differences in diagnostic yield or complication rates. To address this limitation, future studies should aim to enroll larger sample sizes.
Conclusions
Our study assessing the diagnostic performance of CT CNB for small SSNs with solid components ≤6 mm demonstrated a notable overall sensitivity of 85.7% and diagnostic yield of 86.36%. Despite the inherent challenges posed by smaller nodules, our results were comparable to prior studies using CNB for larger lesions. Particularly, our focus on pure GGNs and SSNs with a small solid component did not compromise diagnostic accuracy. Further studies comparing alternative approaches are needed.
The authors have declared that no competing interests exist.
Author Contributions
Acquisition, analysis, or interpretation of data: Anoop Koratala, Nikitha C. Chandra, Prasanth Balasubramanian, Alejandra Yu Lee-Mateus, Alanna Barrios-Ruiz, Ana Garza-Salas
Drafting of the manuscript: Anoop Koratala, Nikitha C. Chandra, Alejandra Yu Lee-Mateus, Alanna Barrios-Ruiz, Ana Garza-Salas
Critical review of the manuscript for important intellectual content: Prasanth Balasubramanian, Andrew Bowman, Rolf Grage, Sebastian Fernandez-Bussy, David Abia-Trujillo
Concept and design: Andrew Bowman, Rolf Grage, Sebastian Fernandez-Bussy, David Abia-Trujillo
Supervision: Sebastian Fernandez-Bussy, David Abia-Trujillo
Human Ethics
Consent was obtained or waived by all participants in this study. Mayo Clinic Institutional Review Board issued approval 22-010644. The above-referenced application was reviewed by expedited review procedures and is determined to be exempt from the requirement for IRB approval (45 CFR 46.104d, category 4).
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.ACR appropriateness Criteria® radiographically detected solitary pulmonary nodule. Kanne JP, Jensen LE, Mohammed TL, et al. J Thorac Imaging. 2013;28:0–3. doi: 10.1097/RTI.0b013e31827657c8. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. MacMahon H, Naidich DP, Goo JM, et al. Radiology. 2017;284:228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 3.CT screening for lung cancer: nonsolid nodules in baseline and annual repeat rounds. Yankelevitz DF, Yip R, Smith JP, et al. Radiology. 2015;277:555–564. doi: 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. Lee HY, Choi YL, Lee KS, Han J, Zo JI, Shim YM, Moon JW. AJR Am J Roentgenol. 2014;202:0–33. doi: 10.2214/AJR.13.11819. [DOI] [PubMed] [Google Scholar]
- 5.The 2021 WHO classification of lung tumors: impact of advances since 2015. Nicholson AG, Tsao MS, Beasley MB, et al. J Thorac Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 6.The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. Travis WD, Asamura H, Bankier AA, et al. J Thorac Oncol. 2016;11:1204–1223. doi: 10.1016/j.jtho.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Diagnostic accuracy and complications of CT-guided core needle lung biopsy of solid and part-solid lesions. Yun S, Kang H, Park S, Kim BS, Park JG, Jung MJ. Br J Radiol. 2018;91:20170946. doi: 10.1259/bjr.20170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Loh SE, Wu DD, Venkatesh SK, et al. https://www.annals.edu.sg/pdf/42VolNo6Jun2013/V42N6p285.pdf. Ann Acad Med Singap. 2013;42:285–290. [PubMed] [Google Scholar]
- 9.Standardized definitions of bleeding after transbronchial lung biopsy: a Delphi consensus statement from the Nashville working group. Folch EE, Mahajan AK, Oberg CL, et al. Chest. 2020;158:393–400. doi: 10.1016/j.chest.2020.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comparison of CT-guided core needle biopsy in pulmonary ground-glass and solid nodules based on propensity score matching analysis. An W, Zhang H, Wang B, Zhong F, Wang S, Liao M. Technol Cancer Res Treat. 2022;21 doi: 10.1177/15330338221085357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Percutaneous CT-guided aspiration and core biopsy of pulmonary nodules smaller than 1 cm: analysis of outcomes of 305 procedures from a tertiary referral center. Choi SH, Chae EJ, Kim JE, Kim EY, Oh SY, Hwang HJ, Lee HJ. AJR Am J Roentgenol. 2013;201:964–970. doi: 10.2214/AJR.12.10156. [DOI] [PubMed] [Google Scholar]
- 12.Diagnostic feasibility and safety of CT-guided core biopsy for lung nodules less than or equal to 8 mm: a single-institution experience. Chang YY, Chen CK, Yeh YC, Wu MH. Eur Radiol. 2018;28:796–806. doi: 10.1007/s00330-017-5027-1. [DOI] [PubMed] [Google Scholar]
- 13.Early Lung Cancer Action Project: overall design and findings from baseline screening. Henschke CI, McCauley DI, Yankelevitz DF, et al. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 14.Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, Heindel W. Radiology. 2002;222:773–781. doi: 10.1148/radiol.2223010490. [DOI] [PubMed] [Google Scholar]
- 15.Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. Tsukada H, Satou T, Iwashima A, Souma T. AJR Am J Roentgenol. 2000;175:239–243. doi: 10.2214/ajr.175.1.1750239. [DOI] [PubMed] [Google Scholar]
- 16.Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. Kim TJ, Lee JH, Lee CT, Jheon SH, Sung SW, Chung JH, Lee KW. AJR Am J Roentgenol. 2008;190:234–239. doi: 10.2214/AJR.07.2441. [DOI] [PubMed] [Google Scholar]
- 17.Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Shimizu K, Ikeda N, Tsuboi M, Hirano T, Kato H. Lung Cancer. 2006;51:173–179. doi: 10.1016/j.lungcan.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. Hur J, Lee HJ, Nam JE, Kim YJ, Kim TH, Choe KO, Choi BW. AJR Am J Roentgenol. 2009;192:629–634. doi: 10.2214/AJR.08.1366. [DOI] [PubMed] [Google Scholar]
- 19.Lung cancer cytology: potential pitfalls and mimics - a review. Idowu MO, Powers CN. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2872744/ Int J Clin Exp Pathol. 2010;3:367–385. [PMC free article] [PubMed] [Google Scholar]
- 20.Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CT-fluoroscopic guidance for ground-glass opacity lesions. Yamagami T, Yoshimatsu R, Miura H, Yamada K, Takahata A, Matsumoto T, Hasebe T. Br J Radiol. 2013;86 doi: 10.1259/bjr.20120447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Complications of CT-guided lung biopsy with a non-coaxial semi-automated 18 gauge biopsy system: Frequency, severity and risk factors. Elshafee AS, Karch A, Ringe KI, et al. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Eur Radiol. 2017;27:138–148. doi: 10.1007/s00330-016-4357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]