Abstract
Bdellovibrio bacteriovorus is a microbial predator that offers promise as a living antibiotic for its ability to kill Gram-negative bacteria, including human pathogens. Even after six decades of study, fundamental details of its predation cycle remain mysterious. Here we used cryo-electron tomography to comprehensively image the lifecycle of B. bacteriovorus at nanometre-scale resolution. With high-resolution images of predation in a native (hydrated, unstained) state, we discover several surprising features of the process, including macromolecular complexes involved in prey attachment/invasion and a flexible portal structure lining a hole in the prey peptidoglycan that tightly seals the prey outer membrane around the predator during entry. Unexpectedly, we find that B. bacteriovorus does not shed its flagellum during invasion, but rather resorbs it into its periplasm for degradation. Finally, following growth and division in the bdelloplast, we observe a transient and extensive ribosomal lattice on the condensed B. bacteriovorus nucleoid.
Predation plays a crucial role in driving the evolution and energy flow in biological communities. In bacteria, different patterns of predatory behaviour have been described in which the predator either remains outside the prey or invades the prey’s cytoplasm or periplasm1. Since its description in 1962 as the first known bacterial parasite of bacteria2,3, Bdellovibrio bacteriovorus has been a paradigm of periplasmic predation, in which a small predator takes up residence in a larger Gram-negative prey’s periplasm4,5. The ability of B. bacteriovorus to invade other Gram-negative bacteria, including human pathogens, has rendered it a potential candidate as a living antibiotic or probiotic6–8. In addition, microbial predation has been suggested to have been involved in eukaryogenesis, the rise of multicellularity, and pathogenicity9–11.
The predatory lifecycle of B. bacteriovorus has been extensively studied using biochemical and microscopy approaches12–14. The free-living attack phase predator is transcriptionally streamlined, with a highly compacted spiral nucleoid15, and a vibrioid cell shape16. At one pole of the cell, a sheathed unipolar flagellum enables high-velocity17,18 collisions with prey, and attachment is mediated by type IV pili (T4P)19. After assessment of prey quality20–23, the process of invasion begins.
Prey invasion involves enzymatic modification of the prey cell wall by B. bacteriovorus at the predator–prey contact point13,24–27. Once B. bacteriovorus enters the prey’s periplasm, the entry pore is sealed and the modified prey is referred to as a bdelloplast28. Following prey entry, the flagella of B. bacteriovorus are also thought to be lost29,30. If sufficient nutrients are present in the bdelloplast31, B. bacteriovorus enters its growth phase. The predator consumes the cytoplasmic contents of the prey, and once available nutrients are exhausted, the predator septates synchronously and divides32,33. Finally, the progeny cells reset to the attack phase, lyse the prey cell wall34,35 and exit the bdelloplast to hunt new prey32.
Despite extensive study, the structural details of much of the invasion process remain unclear. In this Article, we used cryo-electron tomography (cryo-ET) to image the predation cycle of B. bacteriovorus invading different types of prey: Vibrio cholerae, and Escherichia coli minicells of various sizes. Our work reveals the macromolecular details of each stage of the B. bacteriovorus lifecycle and uncovers several unexpected features of the process, including absorption of the extracellular flagellum into the predator’s periplasm during attachment, a flexible portal structure associated with the prey peptidoglycan (PG) surrounding the predator during entry, and formation of a ribosome lattice around the predator’s nucleoid after the prey is consumed in the bdelloplast.
Results
Anatomy of the attack-phase B. bacteriovorus cell
Our cryo-tomograms of attack-phase B. bacteriovorus showed features previously described, including a compact spiral nucleoid occupying the centre of the cell (Fig. 1a)15,36. While the nucleoid excluded ribosomes, they were occasionally abundant at its periphery (Supplementary Fig. 1 and Supplementary Movie 1), as seen previously by cryo-ET15,36. Again consistent with previous cryo-ET of attack-phase cells36, we saw unidentified tubes (on average, two per cell) in the cytoplasm. The tubes had a uniform diameter of ~8 nm and were typically a few tens of nanometres in length (Supplementary Fig. 2 and Supplementary Movie 2). Each cell contained a single polar flagellum, sheathed in outer membrane (OM) (Fig. 1a), and subtomogram averaging of 79 particles revealed that the structure of the flagellar motor is similar to that recently published from a host-independent strain of B. bacteriovorus37 (Fig. 1b).
Fig. 1 |. Anatomy of attack-phase B. bacteriovorus.

a, A slice through an electron cryo-tomogram of an attack-phase B. bacteriovorus cell, with enlarged views of the flagellated (red) and biting (blue) poles. White rectangles highlight the flagellar motor (top) and non-piliated T4aP basal body (bottom), and the white ellipse highlights a rose-like complex. Question mark points to a cross-section through a periplasmic filamentous structure, and white arrow to a type IVa pilus. Scale bars, 50 nm. b–d, Central slices through subtomogram averages of the B. bacteriovorus flagellar motor (b, 79 particles), rose-like complex (c, 132 particles) and non-piliated T4aP basal body (d, 335 particles). White arrows in d point to the extracellular ring of the T4aP basal body. Scale bars, 20 nm. e,f, Central slices through subtomogram averages of non-piliated T4aP basal bodies with (left, white arrow) and without (right) the lower periplasmic ring. These particles were classified using the built-in PCA and k-means clustering functions of the PEET package85 (for more details, see the legend of Supplementary Fig. 6). Dashed black lines indicate a composite of slices through the tomogram at different z-heights (a), and a composite of subtomogram averages aligned on the outer and inner membrane, respectively (c). IM, inner membrane.
We also saw extracellular vesicles with a uniform diameter of ~25 nm near cells (Supplementary Fig. 3a). On the ‘biting’ pole opposite the flagellum, we noted several characteristic features that have not been described before. Rarely, we saw spherical and short filamentous structures in the periplasm (Fig. 1a and Supplementary Fig. 3b). We observed abundant, thin (~3–4 nm wide) fimbriae on the cell surface, with lengths ranging from ~50 to more than 100 nm (Supplementary Fig. 3a,c and Supplementary Movie 1). We also observed a complex, spanning the periplasm and with a prominent extracellular rosette of density. We call this unidentified structure the ‘rose-like complex’. On average, each cell had two to three rose-like complexes at the biting pole (Fig. 1a). Subtomogram averaging of 132 particles revealed a molecular complex spanning the entire periplasmic space with associated cytoplasmic densities (a ring ~17 nm in diameter) and extensive extracellular densities. Five distinct extracellular densities could be distinguished in cross-section: two stacked rings and a central cap (Fig. 1c).
We observed both piliated and non-piliated T4P basal bodies on the biting pole (Fig. 1a and Supplementary Figs. 4 and 5), with empty more abundant than piliated (Supplementary Table 2). On the basis of their defined localization at the biting pole, we imply that they are most likely the T4aP38. A subtomogram average of 335 non-piliated T4aP basal bodies revealed the architecture, including a distinctive extracellular ring present in both non-piliated and piliated basal bodies (Fig. 1d and Supplementary Fig. 4) that was not seen in subtomogram averages of T4P basal bodies in other species39–42. While all piliated basal bodies had the ring, not all non-piliated basal bodies did (Supplementary Fig. 5). This could be either because the external ring had disassembled or not yet assembled, or because these structures were not in fact T4aP but rather, for example, T4bP, which are also involved in adhesion to prey19. In addition, classifying the non-piliated T4aP using principal component analysis (PCA) revealed that ~1/2 of the particles had only the Secretin OM channel and extracellular ring and lacked the lower periplasmic ring (Fig. 1e,f and Supplementary Fig. 6). This might reflect different assembly stages, as it has been shown that T4P basal body in other species utilize an outside-in assembly pathway starting from the Secretin43.
Attachment of B. bacteriovorus to prey
We also observed attachment of B. bacteriovorus to V. cholerae and E. coli minicells (of various sizes). We identified B. bacteriovorus connected to prey by T4aP, with the tip of the extended pilus clearly in contact with the prey’s OM. At the resolution of our cryo-tomograms, no distinctive features were visible at the pilus–prey attachment point (Fig. 2a, Supplementary Fig. 7 and Supplementary Movies 3 and 4). Occasionally, we also saw thin fimbriae apparently contacting the OM of the prey cell (Supplementary Fig. 8).
Fig. 2 |. Attachment of B. bacteriovorus to prey.
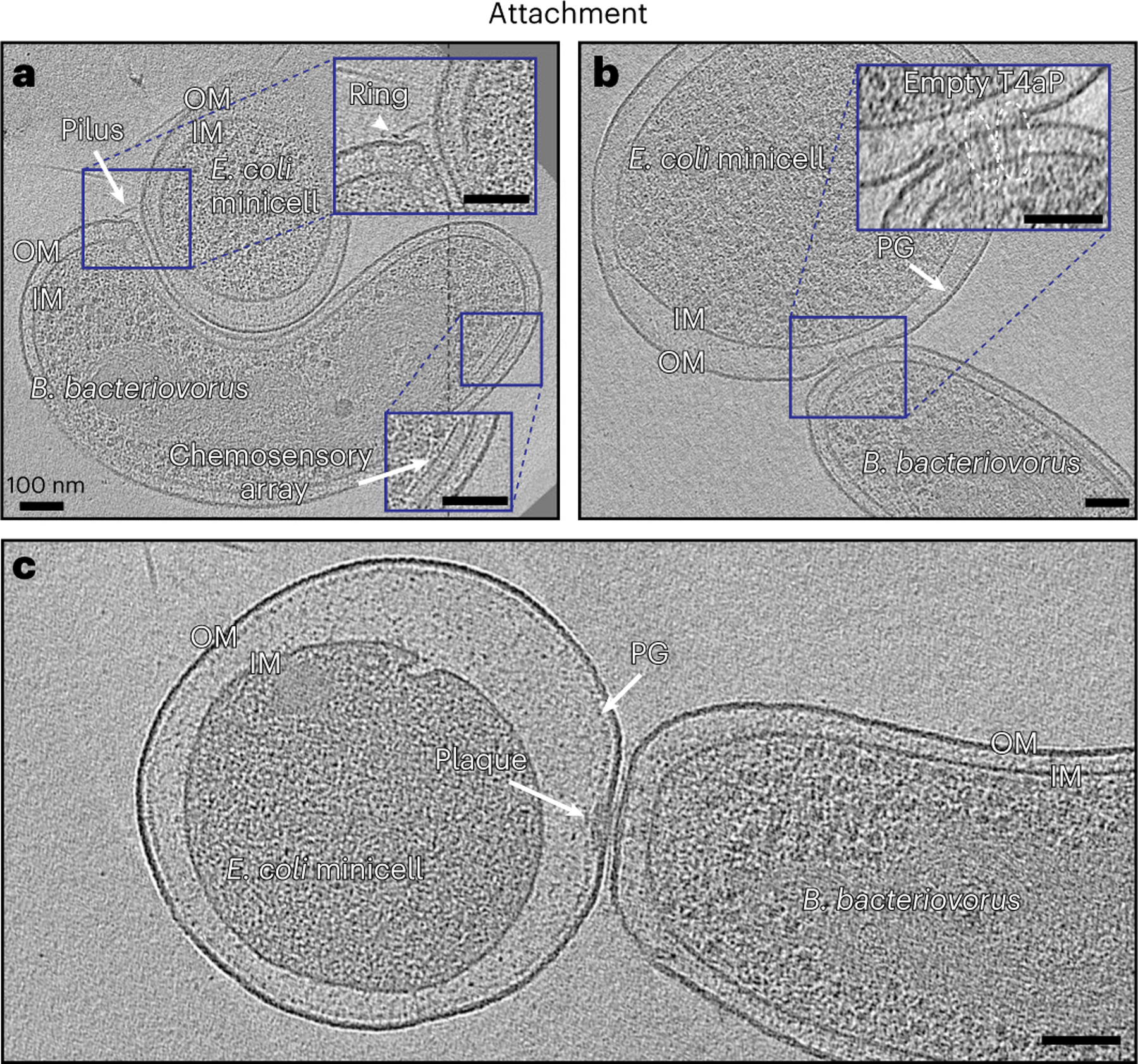
a, A slice through an electron cryo-tomogram showing a B. bacteriovorus cell attached to a prey (E. coli minicell) via T4aP. IM, inner membrane. b, A slice through an electron cryo-tomogram of B. bacteriovorus attached to prey (E. coli minicell) showing non-piliated T4aP basal bodies (white ellipses) penetrating to the prey’s PG layer. c, A slice through an electron cryo-tomogram of B. bacteriovorus attached to prey with a polar attachment plaque. Scale bars, 100 nm.
Additionally, we observed predator and prey in close proximity. In some cases, pilus attachments were still visible; in others the pili had retracted completely. In all cases, we observed non-piliated T4aP basal bodies aligned at the contact site, with rose-like complexes nearby (Supplementary Fig. 9 and Supplementary Movie 5). In several attachments to E. coli minicells, where finer detail could be resolved, we observed particles that look like the non-piliated T4aP basal bodies extended through the prey OM, with their distinctive extracellular rings apparently embedded in the prey PG (Fig. 2b and Supplementary Fig. 10). These particles have the same dimensions as T4aP basal bodies (Supplementary Fig. 11); however, they could still be not related to T4aP but to other systems like type II secretion system (T2SS) or a different form of the rose-like complex. At this stage, we also observed enlargement of the prey periplasm. In agreement with previous studies44, with rod-shaped prey the contact site was usually located on the side of the prey cell, and on the pole of the predator (Supplementary Fig. 12a). In some cases, particularly with smaller, spherical E. coli minicells, the attachment site was displaced slightly off the biting pole onto the side of the predator (Supplementary Fig. 12b–d).
In some cryo-tomograms, we observed an electron-dense but relatively unstructured plaque of material (henceforth referred to as the ‘attachment plaque’) at the contact point between the predator and the prey. The diameter of this plaque ranged from 15 to 70 nm, and the thickness usually extended from the predator’s OM to the prey’s PG cell wall (Fig. 2c and Supplementary Fig. 12c,d), suggesting a modification of the prey cell wall at the predator–prey contact point in agreement with previous reports13. We often observed the tentative non-piliated T4aP basal bodies near or in the plaque (Supplementary Fig. 12d), as well as rose-like complexes and, very occasionally, prey-attached pili nearby. While we sometimes observed two or even three B. bacteriovorus attached to the same prey cell with T4aP, only one ever formed an attachment plaque, consistent with the committed attachment observed in previous studies (Supplementary Fig. 13).
In the cryo-tomograms where the attachment plaque was formed at the biting pole, an unexpected process was seen at the other pole. The sheathed flagellum of the B. bacteriovorus was resorbed into the periplasmic space, wrapping around the inner membrane of the cell. The process seems to initiate with breakage of the flagellar motor at its OM-embedded ring (the lipopolysaccharide (L)-ring, Supplementary Fig. 14). The L-ring remained in the OM, which appeared more curved at this stage. Early in the process, the PG (P)-ring was still visible around the flagellar rod but was located more than 20 nm from the OM, compared with 10–11 nm in attack-phase cells (Supplementary Figs. 15 and 16 and Supplementary Movie 6). Consistent with a decoupling of the P- and L-rings, we observed two examples of B. bacteriovorus cells attached to prey with a plaque and with disrupted OM around the flagellum; in both cases, only the P-ring, and not the L-ring, was visible surrounding the rod of the motor (Supplementary Fig. 17).
In cells with larger portions of the flagellar filament resorbed into the periplasm, the motor (lacking the L-ring but including the P-ring and rod) was completely internalized to the periplasm and moved off the cell pole, with the hook and basal portion of the filament entering the periplasm (Fig. 3, Supplementary Figs. 18 and 19 and Supplementary Movie 7). The L-ring still encircled the filament at the junction of OM and flagellar sheath (Fig. 3, Supplementary Fig. 18 and Supplementary Movie 7). In other cells, most or all of the filament was internalized to the periplasm, wrapping around the cell (Supplementary Figs. 20 and 21 and Supplementary Movies 8–11). In some cases, we saw the motor further up the side of the cell. In other cases, we could not find the motor, either because it was degraded or because it was located in a part of the cell not visible due to the effect of the missing wedge of information in cryo-ET45. The exit point of the retracted flagellum sometimes shifted from the pole up the side of the cell (Supplementary Fig. 21 and Supplementary Movie 10). In cells with fully internalized filaments, we could no longer identify L-rings in the OM. Occasionally, the wrapped filament broke in the periplasm (Supplementary Movie 8). Supplementary Fig. 22 summarizes this absorption process on the basis of our cryo-ET data.
Fig. 3 |. B. bacteriovorus flagellar absorption.

a, A slice through an electron cryo-tomogram showing a B. bacteriovorus cell attached to a prey via an attachment plaque with its flagellum resorbing into the periplasm. Enlargements in the red-boxed areas highlight different parts of the absorbed flagellum. Scale bars, 50 nm (main panel) and 20 nm (enlargements). b, A 3D segmentation of a and an enlarged view illustrating different parts of the absorbed flagellum.
Chemosensory arrays remained visible in the cryo-tomograms of attachment phase. As in attack-phase cells, we observed small, uniformly sized membrane vesicles in the vicinity of the predator and attached prey (Supplementary Fig. 23). In some vesicles, densities were visible either inside and/or on the surface (Supplementary Fig. 23f). We also saw vesicles near the sheath of the flagellum during resorption (Supplementary Fig. 24).
Invasion of the prey periplasm
We captured 18 cryo-tomograms of B. bacteriovorus entering the periplasm of E. coli minicells, where only part of the predator fits inside the prey due to its small size. We call such events ‘non-productive’ invasions. Note that in these invasions we do not know how long before sample freezing a particular predator entered its prey or whether the non-permissive size of the small prey had incidental effects on the entry process. Still, this paradigm provided a unique opportunity to view short-lived stages of invasion at high resolution. In these cryo-tomograms of non-productive invasion, the attachment plaque at the contact site appeared to be replaced by a portal structure through which the predator entered the prey periplasm (Fig. 4, Supplementary Fig. 25 and Supplementary Movie 12). This portal ring, which bridged the OMs of predator and prey, appeared in cross-section as a thin (<5 nm), dark density extending from the prey’s PG layer to the outside of the cell, capping the open end of the prey OM (Fig. 4a and Supplementary Fig. 25a). The height of the portal in cross-section, on the order of a few tens of nanometres, varied between cells, and even on opposite sides of the same cell (for example, compare Fig. 4a and Supplementary Fig. 25a). In eight examples, we observed what appeared to be prey OM blebbing out from the portal (Supplementary Movie 13). Consistent with a tight seal model, the portal appeared to exert considerable force on the B. bacteriovorus cell, as previously observed46, reducing the distance between the outer and inner membranes by ~50% at the entry point compared with elsewhere in the cell, and constricting the deformation-resistant cell wall (Fig. 4b,c and Supplementary Fig. 25b,c). Consistent with previous reports of cell flexibility36, we observed that B. bacteriovorus could bend considerably to maximally occupy the prey periplasm (Fig. 4a).
Fig. 4 |. B. bacteriovorus prey invasion.
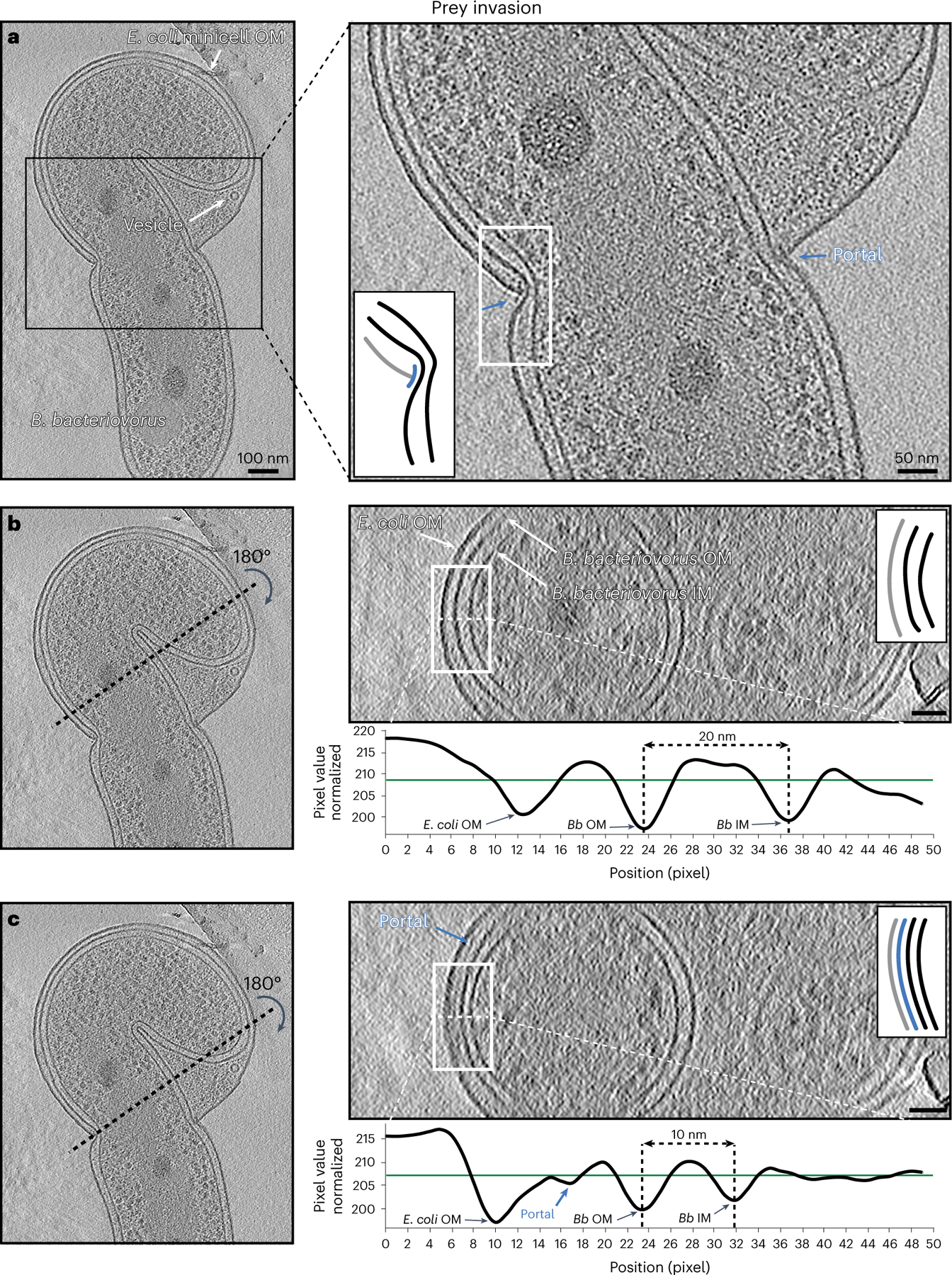
a, A slice through an electron cryo-tomogram (left) and enlarged view (right) showing a non-productive invasion by a B. bacteriovorus of an E. coli minicell. Blue arrows and inset schematic highlight the portal. b, Right (top): cross-section through the yz plane of the tomogram shown in a along the black dotted line indicated on the left. Note that this slice does not include the portal. Right (bottom): average density profile taken along the white dashed line (inside the white rectangle) in the top panel. The distance between the predator’s inner membrane (IM) and OM is indicated (20 nm). c, Similar to b but for a yz slice where the portal is visible. The distance between the predator’s IM and OM is indicated (10 nm). The schematics in the right panels of a–c represent the white-boxed areas in the corresponding slices, with the portal shown in blue, the prey OM in grey and the predator IM and OM in black. Scale bars, 100 nm (left panel of a) and 50 nm (other panels).
In all non-productive invasions, we observed that the B. bacteriovorus cells had fully degraded their absorbed periplasmic flagella and lacked rose-like complexes and fimbriae. Chemosensory arrays were also partially or completely degraded (Supplementary Figs. 26 and 27). Interestingly, while complexes morphologically similar to non-piliated T4aP basal bodies were still present in the predator, they lacked the characteristic extracellular ring found at earlier stages of invasion (Supplementary Fig. 28) and were less abundant than T4aP basal bodies in attack-phase cells. While we identified approximately three to six non-piliated basal bodies with extracellular rings in each attack-phase cell (171 cells in total), we identified only ~0.3 average particles lacking the extracellular ring in the cryo-tomograms of bdelloplasts and non-reproductive invasions of minicells (72 cells in total, Supplementary Tables 1 and 2). The extracellular ring could be absent in these particles either because it is lost during the invasion process or because these are different complexes, for example, the T2SS.
In two examples where a non-flagellated B. bacteriovorus cell was in the vicinity of a lysed prey, we observed knob-like densities on one of the predator’s poles (Supplementary Fig. 29 and Supplementary Movie 14). Given the lysed prey cell nearby and the lack of attack-phase structures such as flagella or chemosensory arrays in the B. bacteriovorus, one possibility is that these cells were pulled out of the prey post-invasion during sample preparation. However, few fimbrae could still be seen next to these knob-like structures (Supplementary Movie 14). When present, the knob-like structures could be abundant; we identified 23 examples on one cell (Supplementary Table 2 and Supplementary Movie 14). While leg-like densities could be seen extending from the extracellular domains to the PG layer in individual examples, subtomogram averaging failed to resolve a consistent structure, suggesting flexibility or differing stoichiometry.
Even though entry was incomplete, B. bacteriovorus in non-productive invasions of E. coli minicells showed signs of entering growth phase. Some predators had at least partially decondensed their nucleoids, and the prey cytoplasm was considerably reduced in size, presumably consumed by the predator. Perhaps related to this digestion, we observed multiple vesicles with a consistent size of ~25 nm in the prey periplasm (Fig. 4a and Supplementary Fig. 25a). We cannot tell whether they originated from prey or predator membrane, but their size is comparable to those we observed in the vicinity of isolated attack-phase and prey-attached B. bacteriovorus (see, for example, Supplementary Figs. 3a and 23).
Growth phase in the bdelloplast
We captured 54 cryo-tomograms of the bdelloplast stage from samples of B. bacteriovorus invading V. cholerae cells and E. coli minicells large enough to accommodate the entire predator. In these cryo-tomograms of bdelloplasts where the predator fully entered the prey’s periplasm, we observed a structure consisting of an extracellular bubble of what appeared to be membrane and an amorphous electron density associated with the prey OM and PG beneath it (Fig. 5, Supplementary Figs. 30 and 31 and Supplementary Movies 15–18). We always observed one such structure per bdelloplast, in both E. coli and V. cholerae bdelloplasts (Supplementary Figs. 32 and 33), and we hypothesize that this is the scar that seals the entry hole after predator entry because its dimensions (~200–300 nm) match what was previously reported using light fluorescence microscopy13. In invaded V. cholerae, the prey’s (non-functional) flagellum remained attached to the bdelloplast (Fig. 5f and Supplementary Fig. 30b,c). The flagellum remained connected to the part of the motor embedded in the OM and PG: the PL-rings and part of the rod. Presumably these parts were separated from the rest of the motor by periplasmic expansion. We did not observe any motor components still associated with the inner membrane. It is possible that flagellar relics remained only on V. cholerae bdelloplasts, and not E. coli, because the flagellar sheath aids in retention. In V. cholerae bdelloplasts, we sometimes also observed PL-subcomplexes (without associated filaments) resulting from previous flagellar loss events47–49 (Supplementary Fig. 30b).
Fig. 5 |. Anatomy of the bdelloplast.

a–c, Slices (at different z-levels) through an electron cryo-tomogram of a V. cholerae bdelloplast containing two B. bacteriovorus after predator division. d, A 3D segmentation of the bdelloplast shown in a–c. e, Enlargement (left) and 3D segmentation (right) of the white-boxed area in a, highlighting the features of the seal. f, Enlargement (left) and 3D segmentation (right) of the white-boxed area in c, highlighting the prey flagellar relic. Scale bars, 100 nm (a–c) and 50 nm (e and f).
In predators inside bdelloplasts, we saw neither chemosensory arrays nor any relics of flagella. Occasionally, we observed unidentified filamentous structures in the periplasm of the predators (Supplementary Fig. 34), which we also sometimes observed in (flagellated) attack-phase cells (Supplementary Fig. 3b). Interestingly, they were always located at the pole (the biting pole of attack-phase cells). These filamentous structures have the same diameter of flagellar filaments (~11–12 nm), and one possibility is that they are remnants of flagellar digestion. We also could not find any rose-like complexes or T4aP basal bodies with external rings in B. bacteriovorus inside bdelloplasts. As in non-productive invasions, we did identify putative non-piliated T4aP basal bodies lacking the external ring. Again, they were less abundant than in attack-phase cells; from 47 cryo-tomograms of non-productive invasions or early bdelloplasts, we identified 25 such particles (Supplementary Table 2). We did occasionally observe 8-nm-wide cytoplasmic tubes, as seen in other lifecycle stages (Supplementary Fig. 35). In addition, we saw variously sized spherical, nested and horseshoe-shaped vesicles in the predator’s cytoplasm, morphologically similar to those reported in other species50 (Supplementary Fig. 36).
Similar to non-productive invasions of E. coli minicells, we observed many uniformly sized (~25 nm) vesicles in the bdelloplast periplasm (Fig. 5, Supplementary Fig. 36 and Supplementary Movies 16–19). Consistent with active growth, B. bacteriovorus nucleoids were less condensed than in earlier stages and, in concert with the prey cytoplasm shrinking, the predator cell elongated and curled to fill most of the bdelloplast (Supplementary Fig. 37). Contrary to previous observations by traditional transmission electron microscopy46, we could not unambiguously identify a connection between the predator OM and prey inner membrane (Supplementary Figs. 31, 37 and 38 and Supplementary Movies 15 and 16).
In bdelloplasts where nearly all of the prey cytoplasm was consumed, the elongated B. bacteriovorus cell divided. The number of progeny depends on the size of the bdelloplast32, and we observed two or three progeny cells in E. coli and V. cholerae prey (for example, Fig. 5). In a few cases, division produced an extra, small spherical product, in accordance with previous reports51 (Supplementary Fig. 39). In some cases, bdelloplasts contained a very dense sphere of material, presumably containing the remnants of the prey cytoplasm (Fig. 5 and Supplementary Figs. 34 and 39). In other cases, not even this remained (a characteristic we use to define what we call an ‘end-stage bdelloplast’). The characteristic ~25 nm vesicles, however, were still present in end-stage bdelloplasts even after no prey cytoplasm remained.
In some elongated or divided B. bacteriovorus in end-stage bdelloplasts of E. coli minicells, we observed a remarkable hexagonal lattice of ribosomes coating the nucleoid (Fig. 6 and Supplementary Movies 18–22). The lattice spacing was ~20 nm, consistent with maximally dense packing of ribosomes (Supplementary Fig. 40). This arrangement was much more extensive than we and others observed in attack-phase cells15. We measured the distances from ribosomes to the apparent surface of the nucleoid in two tomograms. Of 2,304 ribosomes in one (Fig. 6) and 1,109 in the other (Supplementary Fig. 41), ~80% were located within 10 nm of the nucleoid surface. By comparison, in a simulation of the same number of randomly packed 20 nm spheres in the same tomographic volumes (Methods), only ~20–25% were expected to be located within 10 nm of the nucleoid surface (Fig. 6e and Supplementary Fig. 41), suggesting that the association we observed does not arise simply by chance. To investigate whether the ordered ribosomes shared the same orientation, we produced an ~4.7-nm-resolution subtomogram average of the ribosomes in a B. bacteriovorus cell and mapped it back into the tomographic volume using the positions and orientations determined during averaging. We found that individual particles were apparently randomly oriented on the nucleoid surface (Supplementary Fig. 42).
Fig. 6 |. Ribosomal nucleoid lattice in the end-stage bdelloplast.

a,b, Slices (at different z-levels) through an electron cryo-tomogram of an end-stage E. coli minicell bdelloplast containing two B. bacteriovorus cells after predator division, highlighting the hexagonal arrangement of ribosomes around the nucleoids. c,d, Rotated views of a 3D segmentation of the nucleoids and ribosomes of the cryo-tomogram shown in a and b. Scale bars, 50 nm. e, Distances of individual ribosomes from the nucleoid surface measured in the 3D segmentations of the cryo-tomogram shown in a and b (‘experiment’, solid line), compared with a simulation of randomly distributed 20-nm-wide spheres packed in the same segmented volume (‘random’, dashed line). See also Supplementary Movie 21.
Discussion
Here our cryo-ET imaging reveals the predation cycle of B. bacteriovorus in situ at nanometre-scale resolution and contextualizes decades of research on B. bacteriovorus predation, uncovering many surprising details of this process.
We observed several previously described unidentified structures, including cytoplasmic tubes. We also detected fimbriae on the biting pole of attack-phase cells, which could play a role in prey adhesion. The most intriguing structure we observed on attack-phase cells is the rose-like complex with elaborate extracellular domains extending nearly 20 nm out from the cell. The periplasmic portion of the rose-like complex is morphologically similar (at our resolution) to a recent structure of a tripartite efflux pump52, and since it was hypothesized that related B. bacteriovorus type I secretion systems (T1SS) might be involved in secreting enzymes that modify the prey during invasion18, it is possible that the rose-like complex is a T1SS. Consistent with a role in early invasion, we observed rose-like complexes on attack-phase cells and at prey contact sites, but not in cells during or after invasion.
Another machine with a notable extracellular domain is the tentative T4aP basal body. T4aP-like structures on the biting pole of B. bacteriovorus have been detected previously38,53, but our higher-resolution imaging revealed a unique extracellular ring surrounding the base of the pilus. The ring was also present in non-piliated basal bodies, indicating that it is stable in the absence of the pilus. It is possible that these complexes are not T4aP, but rather a related machine containing a Secretin pore in the OM, such as a T2SS, which is structurally similar54 and thought to be involved in B. bacteriovorus secretion18,55.
The role of T4aP in the predation process remains unresolved4. In our tomograms, multiple T4aP can be seen attached to prey cells and some appeared to be exerting force as they retracted, probably pulling the prey OM and PG closer to the predator. As the membranes were brought into contact, the shortening pili appeared fully disassembled, leaving empty basal bodies. Interestingly, these non-piliated basal bodies continued to mediate attachment and could be seen extending through the prey OM, with their extracellular rings located in the PG layer of the prey. Our results thus suggest that pili themselves do not drive entry, but rather force the initial connection or reorient the predator to the prey in a pattern akin to the upright reorientation of Caulobacter crescentus by their Tad pili during surface colonization56.
B. bacteriovorus typically lose their flagella when entering prey. Our images reveal a surprising mechanism: while attached to a prey cell, the flagellar motor is broken at the L-ring and the filament absorbed into the predator’s periplasm, where it is digested. We can speculate regarding the question of how B. bacteriovorus absorbs its flagellum into the periplasm. It is unlikely to be pulled from the motor, which we occasionally saw drift partway up the side of the cell before being fully degraded. Our observation of filaments partially absorbed up to a junction on the side of the cell suggests that the process involves zippering of the flagellar sheath and the OM. In other words, as the sheath becomes incorporated into the OM it simultaneously pushes the flagellar filament into the periplasm, which could also explain the curved OM visible during early stages of resorption. Perhaps this is related to the unique lipid composition of the sheath, which is predicted to be even more fluid than the rest of the OM57. Additionally, the fact that multiple flagellins make up distinct segments of the B. bacteriovorus flagellum58 might help in wrapping the filament around the cell similar to what has been described in other species59. The vesicles we observed in the vicinity of some absorbed flagella are consistent with previous reports that rotation of sheathed flagella can lead to shedding of OM vesicles60.
However, we do not know whether the vesicles formed because the motor was still rotating during absorption or due to the absorption process itself. It is also possible that these vesicles are a mere artefact of the sample procedure.
A major open question is the nature of the pore through which B. bacteriovorus enter their prey. Secreted PG-remodelling enzymes, presumably part of the dense plaque we observe at the attachment site that extends to the prey cell wall, are known to create, and subsequently seal, a reinforced circular porthole in the prey PG (see Figs. 2 and 3 in ref. 13). How the prey OM is modified remains more of a mystery. Prey cells remain intact and transcriptionally active throughout the initial entry process61, so it was proposed that the membranes of prey and predator must fuse62. Instead, we observed what seems to be a proteinaceous collar curving out from the prey PG to seal the hole in the OM and prevent interaction of the two membranes. Presumably this structure is associated with the PG-remodelling enzymes, and may even be a modified and reinforced extension of the cell wall, as suggested by ref. 46.
What provides the force for entry remains enigmatic. Over several decades, various models have been proposed, including flagellar rotation2, retraction of T4aP attached to the prey cell wall38,53, and attachment to the prey inner membrane as osmotic pressure rapidly expands the periplasm46. Our results rule out all of these models. We see that the B. bacteriovorus flagellum is broken, and at least partially absorbed, before entry. Similarly, pili appear to be fully disassembled before entry. Also, we see prey periplasmic expansion even before entry, presumably due to secreted enzymes that cleave Braun’s lipoprotein25, and de-crosslink and sculpt the prey PG during entry23, with no visible connection between the predator and the now-distant prey inner membrane, which is in accordance with a previous report where mutant predators could replicate while being between the prey’s cell wall and OM35. It is possible that an expanding periplasmic space provides a suction force. It is also possible that an active mechanism, such as a modified form of the gliding motility, walks the connection point with the portal down the B. bacteriovorus cell.
Following predator entry, we saw that the portal is sealed by a scar of electron-dense material, probably related to the resealing of the PG sacculus13,63, with a protruding bubble of what appears to be membrane that has not been observed previously. Given the blebs around the invasion portal we noticed on some prey cells in non-productive invasions, it may be prey OM. Alternatively, it may be OM pinched off from the end of the invading predator. We propose that the bubble must be somehow tethered to the seal, perhaps through membrane-embedded protein complexes.
While we did not see the connections to the prey cytoplasm during entry observed in reference46, we did see a few examples of the predator outer and prey inner membranes in close proximity in the bdelloplast. In most cases, however, we only observed vesicles in the bdelloplast, suggesting that this may be a mechanism for nutrient transfer. In our tomograms, we cannot tell which cell(s) are producing the vesicles in bdelloplasts. However, the vesicles seen near attack-phase cells or near B. bacteriovorus attached to a prey could be, unlike the vesicles seen inside the bdelloplast, unrelated to the predation process and merely a result of the sample preparation procedure. B. bacteriovorus is known to translocate a pore protein from its OM to the prey inner membrane64; perhaps similar transferred machinery produces vesicles to deliver prey cytoplasmic content, and lipids, to the growing predator inside the bdelloplast. It would be interesting to purify these vesicles from the bdelloplast for further characterization.
In cryo-tomograms where there is a complete consumption of the prey, we observed a striking hexagonal lattice of ribosomes on the surface of the B. bacteriovorus condensed nucleoid(s) when using E. coli minicells as a prey. One possibility for this ribosome lattice is that it is a depletion effect resulting from entropic forces encouraging large objects to aggregate on a surface65. However, we did not observe the effect on other cell surfaces such as the inner membrane, nor in other stages of the cell cycle, so we think this unlikely. Eukaryotic ribosomes have been observed to crystallize in hypothermic conditions66, but we see no relationship between the orientation of neighbouring particles consistent with a crystal, or with a polysome67. We think a more likely possibility is that the ribosomes are independently translating transcripts from the condensing nucleoid. The switch from growth phase to attack phase involves a transcriptional shift activating a few hundred genes, and inactivating many more68,69. The highly condensed attack-phase nucleoid excludes even small monomeric proteins33, so the ribosomes we observe on the surface may be, or recently have been, coupled to transcribing polymerases.
Together, our results provide the most complete view so far of the unique structures that enable the complex lifecycle of this bacterial predator, and raise new questions. We hope our work spurs further study of this fascinating process and informs potential future applications of bacterial predators as living antibiotics70.
Methods
Studying B. bacteriovorus predation with Cryo-ET
To visualize the predatory lifecycle of B. bacteriovorus in a near-native state, we applied cryo-ET imaging to samples of B. bacteriovorus HD100 at various timepoints, from 10 min to 48 h, after addition of prey (V. cholerae, or E. coli minicells of various sizes). Compared with thicker cells, the thinness of E. coli minicells yielded higher-resolution details about the predator–prey interaction. The small size of the minicells also prevented complete entry of the predator, allowing us to capture otherwise short-lived intermediates in the rapid invasion process. However, we note that we cannot preclude that the unique physiology of E. coli minicells, such as the frequent lack of a chromosome, might affect the behaviour of B. bacteriovorus during the predation process especially on the host nutrient consumption. Supplementary Table 1 lists the number of cryo-tomograms we acquired at each stage of the predatory lifecycle of B. bacteriovorus, and Supplementary Table 2 the number of examples we observed of each of the features described below. Finally, it is important to note that our cryo-tomograms do not have temporal information. In other words, when we observe a predator close to, or in direct contact with, a prey, or a predator penetrating a prey or completely inside it (that is, a bdelloplast), we do not know for how long any of these processes was happening before plunge-freezing the sample. Supplementary Movie 23 and Supplementary Fig. 43 offer an animated and schematic summary of all stages of the B. bacteriovorus predatory lifecycle that we observed in this study.
Strains and growth conditions
B. bacteriovorus HD100 cells were grown and prepared as described in ref. 71. To enrich for attack phase, B. bacteriovorus cells were grown in S17–1 prey solution at 30 °C, then filtered by a 0.45 μm filter. To enrich for attachment, B. bacteriovorus cells were grown in S17–1 prey solution at 30 °C, then filtered by a 0.45 μm filter and concentrated by centrifugation. E. coli WM3433 (minicell-producing)72 were cultured in LB medium at 30 °C. Minicells were enriched by two-step centrifugation (initially 3,000g for 3 min and subsequently 9,000g for 5 min). The two species were then mixed for 15 min. To enrich for non-productive invasions, the two species were mixed for 30 min. To enrich for bdelloplasts, B. bacteriovorus cells were grown in S17–1 prey solution at 30 °C, then filtered by a 0.45 μm filter. V. cholerae strain MKW 1383 was grown overnight in LB medium. Subsequently, B. bacteriovorus and V. cholerae were mixed and incubated in Ca-HEPES buffer at 30 °C for 16 h.
To enrich for end-stage bdelloplasts, a co-culture of B. bacteriovorus HD100 and E. coli WM3433 was incubated at 30 °C, 220 r.p.m. for 48 h, then was used to inoculate 100 ml HEPES buffer with 4 ml of overnight E. coli prey culture and grown for another 48 h. Cells were collected by centrifugation at 3,500g at 4 °C for 20 min. The pellet was resuspended in 1 ml ice-cold HG buffer (HEPES buffer + 10 mg ml−1 gelatin), loaded on a sucrose gradient (20% sucrose in HG buffer (40 ml), frozen at −20 °C and thawed overnight), and centrifuged at 2,000g with no brake at 4 °C for 30 min. The broad mid-band was collected and washed twice by centrifugation at 10,000g for 5 min before resuspension in HEPES buffer for vitrification.
Cryo-ET sample preparation and imaging
Samples were mixed with a solution of bovine serum albumin-treated 10 nm gold, and 3–4 μl were applied to freshly glow-discharged R2/2 carbon-coated 200 mesh copper Quantifoil grids (Quantifoil Micro Tools). Samples were plunge-frozen in a liquid ethane/propane mixture in a Vitrobot Mark III or Mark IV (FEI). For all samples except the end-stage E. coli minicell bdelloplast enrichment, imaging was performed with an FEI Tecnai G2 Polara 300 keV field emission gun transmission electron microscope (FEI Company) equipped with a Gatan energy filter and K2 Summit direct electron detector (Gatan) at Caltech or an FEI Titan Krios equipped with Gatan energy filter and Gatan K2 Summit direct detector at the Janelia Research Campus of HHMI. Tilt series were collected from −60° to +60° in 1° increments using UCSF Tomography software73, with a cumulative electron dose of 100 e− Å−2, a target defocus of −8 μm, and a pixel size of 3.3 Å, 3.9 Å, 4 Å or 4.2 Å.
End-stage bdelloplast-enriched samples were imaged using the fast-incremental single exposure method74,75 with SerialEM software76 using a dose-symmetric tilt scheme adapted from77. Data were collected using a Titan Krios 300 keV field emission gun transmission electron microscope (Thermo Fisher Scientific) equipped with a Gatan imaging filter and a K2 Summit direct detector in counting mode (Gatan) at Caltech. The tilt range was −60° to +60° with 3° tilt increment, target defocus of −8 μm, pixel size of 4.52 Å, frame rate of 0.05 s per frame and a total dose of 130 e− Å−2. Fast-incremental single exposure tilt series were gain normalized and motion corrected using the alignframes function of IMOD78.
Image processing and subtomogram averaging
Three-dimensional reconstructions of tilt series were performed either using the IMOD software package78, or automatically with RAPTOR through the Jensen Lab processing pipeline79. Subtomogram averaging was done using the PEET program80 with two-fold symmetrization applied along the particle Y axis. The number of particles averaged were: 132 particles for the rose-like complex, 335 particles for the non-piliated T4aP basal body and 79 particles for the flagellar motor.
For the subtomogram average of the ribosomes in end-stage bdelloplasts, the selected tilt series were aligned, contrast transfer function corrected, and reconstructed using EMAN2.91 (refs. 81,82). A total of 775 nucleoid-surrounding ribosomes from 2 tomograms were selected manually. The averaged structure of the ribosome was produced according to the ‘gold-standard’ protocol using a subtomogram refinement pipeline in EMAN2 (ref. 81). During alignment, particles shifts were limited to ~30 Å and a loose spherical mask was applied. The final, mask-corrected Fourier shell correlation was estimated in RELION3 using a soft-edge mask (Supplementary Fig. 42e)83. To analyse spatial relationships between nucleoid-coating ribosomes, the averaged map was placed back into particle locations in one of the original tomograms using EMAN2 (ref. 81). Ribosome maps were dusted, segmented and visualized in their original orientations within the tomogram using ChimeraX84.
To classify non-piliated T4aP particles on the basis of the presence/absence of the lower periplasmic ring, we used the built-in PCA and k-means clustering functions of the PEET package85. After an initial T4aP subtomogram average was obtained, a binary mask was drawn on the region of interest (the lower periplasmic ring) and used for PCA analysis. Subsequently, the particles were separated into two classes using 5–20 components and re-aligned on the basis of class number. The best clustering parameters were selected on the basis of the Akaike information criterion and Bayesian information criterion values and by visually inspecting the re-aligned particles.
Average density profiles for the portal were automatically calculated with a custom script, sideview-profile-average, written by Davi Ortega (https://www.npmjs.com/package/sideview-profile-average) and described in ref. 86.
Tomogram segmentations
Segmentations shown in Figs. 3 and 5, Supplementary Figs. 16, 20, 21 and 31 and Supplementary Movies 6, 7, 8, 12, 15 and 17 were produced manually using IMOD78. For Fig. 6, Supplementary Fig. 41 and Supplementary Movies 21 and 22, features were segmented automatically using the VISFD segmentation tools87 and PoissonRecon88,89, and edited and visualized using ChimeraX84. A tutorial is available demonstrating how to use VISFD to segment tomograms of B. bacteriovorus cells90. Note that manual corrections were often needed after the automatic segmentations.
To segment the nucleoid of B. bacteriovorus (Fig. 6, Supplementary Fig. 41 and Supplementary Movies 21 and 22), nucleoid density was inferred from the absence of other objects nearby such as ribosomes and proteins. For every voxel in the image, we calculated the fluctuations in brightness within a sphere of radius 20 nm (see the VISFD documentation for the ‘-fluct’ filter). To detect the nucleoid, we searched for regions in the image with relatively uniform brightness (where the fluctuations in nearby brightness were comparatively low). However, this simple method can mistake other homogeneous regions in the cell for nucleoid, such as regions above and below the cytoplasm in the Z direction (the upper and lower boundary of the cell is not visible due to the ‘missing wedge’ artefact). These regions were removed manually using the volume editing tools included with ChimeraX84.
Ribosomes were detected using the method of scale-free blob detection91, to detect dark blobs in the image of size between 120 Å and 215 Å (similar to the size of a ribosome). Dark blobs whose centres were closer than 12 nm to other blobs were discarded. Unfortunately, ribosomes are not sufficiently dark to be reliably detected using this method, especially in crowded environments. Furthermore, other dark objects of similar size can be mistaken for ribosomes. Hence, ribosomes that were not detected in the original image automatically were detected manually in IMOD78. Approximately 1/3 of the ribosomes were detected manually. Subsequently, ribosomes were approximated as spherical objects.
B. bacteriovorus membranes and their interior volumes were segmented automatically using VISFD using the ridge detection and tensor voting method92 to locate the visible surface of the membranes, followed by screened Poisson surface reconstruction88 to generate closed surfaces and their interior volumes. The details of how this was done are explained in the VISFD tutorial90. Manual corrections were applied when required using the volume editing tools included with ChimeraX84.
Plotting the distances between the ribosomes and the nucleoid surface
We first measured the distance from the centre of each ribosome to the nearest identified voxel in the nucleoid. Then we subtracted 10 nm from these distances, because each ribosome is approximately 20 nm wide, and plotted these numbers on the horizontal axis. The same distances were then measured using randomly generated spheres. To generate them, spheres 20 nm in diameter were added to the cytoplasmic volume at random locations. If a newly added sphere overlapped with the nucleoid or another sphere, or if it did not lie within the inner membrane, then it was discarded and a new randomly located sphere was generated. This process was repeated until the number of random spheres matched the number of ribosomes in the cytoplasmic volume for each tomogram (2,304 ribosomes for Fig. 6 and 1,109 for Supplementary Fig. 41).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
This project was funded by the National Institutes of Health (grant R01 AI127401 to G.J.J.) and a Baxter postdoctoral fellowship from Caltech to M.K. S.K. is supported by the Swedish Research Council (2019–06293). Cryo-ET work was performed in the Beckman Institute Resource Center for Transmission Electron Microscopy at the California Institute of Technology and the Howard Hughes Medical Institute Janelia Farm CryoEM Facility. We thank D. Villanueva Avalos for making the summary animation. We are deeply grateful to L. Sockett (University of Nottingham) for the gift of the B. bacteriovorus strain and helpful advice and comments.
Footnotes
Code availability
A tutorial (containing the codes) is available demonstrating how to use VISFD to segment tomograms of B. bacteriovorus cells (see jewettaij/visfd_tutorials: updated ‘STEP_0’ of the Bdellovibrio segmentation example (2021) https://doi.org/10.5281/ZENODO.5758691).
Competing interests
The authors declare no competing interests.
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41564-023-01401-2.
Peer review information Nature Microbiology thanks Andrew Lovering, Andrew Fenton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The full tomograms are available upon request.
References
- 1.Pérez J, Moraleda-Muñoz A, Marcos-Torres FJ & Muñoz-Dorado J Bacterial predation: 75 years and counting! Environ. Microbiol. 18, 766–779 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Stolp H & Starr MP Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie van Leeuwenhoek 29, 217–248 (1963). [DOI] [PubMed] [Google Scholar]
- 3.Stolp H & Petzold H Untersuchungen über einen obligat parasitischen Mikroorganismus mit lytischer Aktivität für Pseudomonas-Bakterien. J. Phytopathol. 45, 364–390 (1962). [Google Scholar]
- 4.Sockett RE Predatory lifestyle of Bdellovibrio bacteriovorus. Annu. Rev. Microbiol. 63, 523–539 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Laloux G Shedding light on the cell biology of the predatory bacterium Bdellovibrio bacteriovorus. Front. Microbiol. 10, 3136 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavallo FM, Jordana L, Friedrich AW, Glasner C & van Dijl JM Bdellovibrio bacteriovorus: a potential ‘living antibiotic’ to control bacterial pathogens. Crit. Rev. Microbiol. 47, 630–646 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Bonfiglio G et al. Insight into the possible use of the predator Bdellovibrio bacteriovorus as a probiotic. Nutrients 12, 2252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atterbury RJ et al. Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appl. Environ. Microbiol. 77, 5794–5803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidov Y & Jurkevitch E Predation between prokaryotes and the origin of eukaryotes. BioEssays 31, 748–757 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Erken M, Lutz C & McDougald D The rise of pathogens: predation as a factor driving the evolution of human pathogens in the environment. Microb. Ecol. 65, 860–868 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons NA & Kolter R On the evolution of bacterial multicellularity. Curr. Opin. Microbiol. 24, 21–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnham JC, Hashimoto T & Conti SF Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into Gram-negative bacterial hosts. J. Bacteriol. 96, 1366–1381 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuru E et al. Fluorescent D-amino-acids reveal bi-cellular cell wall modifications important for Bdellovibrio bacteriovorus predation. Nat. Microbiol 2, 1648–1657 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Said N, Chatzinotas A & Schmidt M Have an ion on it: the life‐cycle of Bdellovibrio bacteriovorus viewed by helium‐ion microscopy. Adv. Biosys. 3, 1800250 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Butan C et al. Spiral architecture of the nucleoid in Bdellovibrio bacteriovorus. J. Bacteriol. 193, 1341–1350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks EJ et al. Asymmetric peptidoglycan editing generates cell curvature in Bdellovibrio predatory bacteria. Nat. Commun. 13, 1509 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert C et al. Characterizing the flagellar filament and the role of motility in bacterial prey-penetration by Bdellovibrio bacteriovorus. Mol. Microbiol. 60, 274–286 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rendulic S et al. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303, 689–692 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Avidan O et al. Identification and characterization of differentially-regulated type IVb pilin genes necessary for predation in obligate bacterial predators. Sci. Rep. 7, 1013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobley L et al. Discrete cyclic di-GMP-dependent control of bacterial predation versus axenic growth in Bdellovibrio bacteriovorus. PLoS Pathog. 8, e1002493 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meek RW, Cadby IT, Moynihan PJ & Lovering AL Structural basis for activation of a diguanylate cyclase required for bacterial predation in Bdellovibrio. Nat. Commun. 10, 4086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caulton SG & Lovering AL Bacterial invasion and killing by predatory Bdellovibrio primed by predator prey cell recognition and self protection. Curr. Opin. Microbiol. 56, 74–80 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Lerner TR et al. Specialized peptidoglycan hydrolases sculpt the intra-bacterial niche of predatory Bdellovibrio and increase population fitness. PLoS Pathog. 8, e1002524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomashow MF & Rittenberg SC Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J. Bacteriol. 135, 998–1007 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomashow MF & Rittenberg SC Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: N-deacetylation of Escherichia coli peptidoglycan amino sugars. J. Bacteriol. 135, 1008–1014 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomashow MF & Rittenberg SC Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: attachment of long-chain fatty acids to escherichia coli peptidoglycan. J. Bacteriol. 135, 1015–1023 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tudor JJ, McCann MP & Acrich IA A new model for the penetration of prey cells by bdellovibrios. J. Bacteriol. 172, 2421–2426 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starr MP & Baigent NL Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J. Bacteriol. 91, 2006–2017 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shilo M in Current Topics in Microbiology and Immunology (eds Arber W et al. ) 174–204 (Springer, 1969). [DOI] [PubMed] [Google Scholar]
- 30.Thomashow MF & Rittenberg SC in Developmental Biology of Prokaryotes (ed Parish JH) 115–138 (Univ. California Press, 1979). [Google Scholar]
- 31.Rotem O et al. Cell-cycle progress in obligate predatory bacteria is dependent upon sequential sensing of prey recognition and prey quality cues. Proc. Natl Acad. Sci. USA 112, E6028–E6037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenton AK, Kanna M, Woods RD, Aizawa S-I & Sockett RE Shadowing the actions of a predator: backlit fluorescent microscopy reveals synchronous nonbinary septation of predatory Bdellovibrio inside prey and exit through discrete bdelloplast pores. J. Bacteriol. 192, 6329–6335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaljević J et al. Chromosome choreography during the non-binary cell cycle of a predatory bacterium. Curr. Biol. 31, 3707–3720 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding CJ et al. A lysozyme with altered substrate specificity facilitates prey cell exit by the periplasmic predator Bdellovibrio bacteriovorus. Nat. Commun. 11, 4817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert C et al. Interrupting peptidoglycan deacetylation during Bdellovibrio predator–prey interaction prevents ultimate destruction of prey wall, liberating bacterial-ghosts. Sci. Rep. 6, 26010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgnia MJ, Subramaniam S & Milne JLS Three-dimensional imaging of the highly bent architecture of Bdellovibrio bacteriovorus by using cryo-electron tomography. J. Bacteriol. 190, 2588–2596 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaban B, Coleman I & Beeby M Evolution of higher torque in Campylobacter-type bacterial flagellar motors. Sci. Rep. 8, 97 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahmoud KK & Koval SF Characterization of type IV pili in the life cycle of the predator bacterium Bdellovibrio. Microbiology 156, 1040–1051 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Chang Y-W et al. Architecture of the type IVa pilus machine. Science 351, aad2001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang Y-W et al. Architecture of the Vibrio cholerae toxin-coregulated pilus machine revealed by electron cryotomography. Nat. Microbiol. 2, 16269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold VA, Salzer R, Averhoff B & Kühlbrandt W Structure of a type IV pilus machinery in the open and closed state. eLife 4, e07380 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treuner-Lange A et al. PilY1 and minor pilins form a complex priming the type IVa pilus in Myxococcus xanthus. Nat. Commun. 11, 5054 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedrich C, Bulyha I & Søgaard-Andersen L Outside-in assembly pathway of the type IV pilus system in Myxococcus xanthus. J. Bacteriol. 196, 378–390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert C, Fenton AK, Hobley L & Sockett RE Predatory Bdellovibrio bacteria use gliding motility to scout for prey on surfaces. J. Bacteriol. 193, 3139–3141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumeister W Electron tomography of molecules and cells. Trends Cell Biol. 9, 81–85 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Abram D, Castro e Melo J & Chou D Penetration of Bdellovibrio bacteriovorus into host cells. J. Bacteriol. 118, 663–680 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan M Bacterial flagellar motor PL-ring disassembly subcomplexes are widespread and ancient. Proc. Natl Acad. Sci. USA 117, 8941–8947 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira JL et al. γ-proteobacteria eject their polar flagella under nutrient depletion, retaining flagellar motor relic structures. PLoS Biol. 17, e3000165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaplan M In situ imaging of the bacterial flagellar motor disassembly and assembly processes. EMBO J. 38, e100957 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobro MJ et al. Uncharacterized bacterial structures revealed by electron cryotomography. J. Bacteriol. 199, e00100–e00117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnham JC, Hashimoto T & Conti SF Ultrastructure and cell division of a facultatively parasitic strain of Bdellovibrio bacteriovorus. J. Bacteriol. 101, 997–1004 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alav I et al. Structure, assembly, and function of tripartite efflux and type 1 secretion systems in Gram-negative bacteria. Chem. Rev. 121, 5479–5596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans KJ, Lambert C & Sockett RE Predation by Bdellovibrio bacteriovorus HD100 requires type IV pili. J. Bacteriol. 189, 4850–4859 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghosal D et al. In vivo structure of the Legionella type II secretion system by electron cryotomography. Nat. Microbiol. 4, 2101–2108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dori-Bachash M, Dassa B, Pietrokovski S & Jurkevitch E Proteome-based comparative analyses of growth stages reveal new cell cycle-dependent functions in the predatory bacterium Bdellovibrio bacteriovorus. Appl. Environ. Microbiol. 74, 7152–7162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sangermani M, Hug I, Sauter N, Pfohl T & Jenal U Tad pili play a dynamic role in caulobacter crescentus surface colonization. mBio 10, e01237–19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomashow LS & Rittenberg SC Isolation and composition of sheathed flagella from Bdellovibrio bacteriovorus 109J. J. Bacteriol. 163, 1047–1054 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iida Y et al. Roles of multiple flagellins in flagellar formation and flagellar growth post bdelloplast lysis in Bdellovibrio bacteriovorus. J. Mol. Biol. 394, 1011–1021 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kühn MJ et al. Spatial arrangement of several flagellins within bacterial flagella improves motility in different environments. Nat. Commun. 9, 5369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aschtgen M-S et al. Rotation of Vibrio fischeri flagella produces outer membrane vesicles that induce host development. J. Bacteriol. 198, 2156–2165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lambert C, Ivanov P & Sockett RE A transcriptional ‘scream’ early response of E. coli prey to predatory invasion by Bdellovibrio. Curr. Microbiol. 60, 419–427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Negus D et al. Predator versus pathogen: how does predatory Bdellovibrio bacteriovorus interface with the challenges of killing gram-negative pathogens in a host setting? Annu. Rev. Microbiol. 71, 441–457 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Snellen JE & Starr MP Ultrastructural aspects of localized membrane damage in Spirillum serpens VHL early in its association with Bdellovibrio bacteriovorus 109D. Arch. Microbiol. 100, 179–195 (1974). [DOI] [PubMed] [Google Scholar]
- 64.Tudor JJ & Karp MA Translocation of an outer membrane protein into prey cytoplasmic membranes by bdellovibrios. J. Bacteriol. 176, 948–952 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocha B, Paul S & Vashisth H Role of entropy in colloidal self-assembly. Entropy 22, 877 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Byers B Structure and formation of ribosome crystals in hypothermic chick embryo cells. J. Mol. Biol. 26, 155–167 (1967). [DOI] [PubMed] [Google Scholar]
- 67.Brandt F et al. The native 3D organization of bacterial polysomes. Cell 136, 261–271 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Lambert C, Chang C-Y, Capeness MJ & Sockett RE The first bite—profiling the predatosome in the bacterial pathogen Bdellovibrio. PLoS ONE 5, e8599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karunker I, Rotem O, Dori-Bachash M, Jurkevitch E & Sorek R A global transcriptional switch between the attack and growth forms of Bdellovibrio bacteriovorus. PLoS ONE 8, e61850 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atterbury RJ & Tyson J Predatory bacteria as living antibiotics—where are we now?. Microbiology 167, 1–8 (2021). [DOI] [PubMed] [Google Scholar]
- 71.Lambert C & Sockett RE Laboratory maintenance of Bdellovibrio. Curr. Protoc. Microbiol. 9, 7B.2.1–7B.2.13 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Chen C-Y, Shiomi D, Niki H & Margolin W Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology 417, 304–311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng SQ et al. UCSF tomography: an integrated software suite for real-time electron microscopic tomographic data collection, alignment, and reconstruction. J. Struct. Biol. 157, 138–147 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Chreifi G, Chen S, Metskas LA, Kaplan M & Jensen GJ Rapid tilt-series acquisition for electron cryotomography. J. Struct. Biol. 205, 163–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisenstein F, Danev R & Pilhofer M Improved applicability and robustness of fast cryo-electron tomography data acquisition. J. Struct. Biol. 208, 107–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mastronarde DN Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Hagen WJH, Wan W & Briggs JAG Implementation of a cryo-electron tomography tilt-scheme optimized for high resolution subtomogram averaging. J. Struct. Biol. 197, 191–198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kremer JR, Mastronarde DN & McIntosh JR Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996). [DOI] [PubMed] [Google Scholar]
- 79.Ding HJ, Oikonomou CM & Jensen GJ The caltech tomography database and automatic processing pipeline. J. Struct. Biol. 192, 279–286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicastro D et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944–948 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Chen M et al. A complete data processing workflow for cryo-ET and subtomogram averaging. Nat. Methods 16, 1161–1168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang G et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Zivanov J et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pettersen EF et al. UCSF CHIMERAX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heumann JM, Hoenger A & Mastronarde DN Clustering and variance maps for cryo-electron tomography using wedge-masked differences. J. Struct. Biol. 175, 288–299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ortega DR et al. Repurposing a chemosensory macromolecular machine. Nat. Commun. 11, 2041 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jewett A jewettaij/visfd: fixed two bugs in the ‘crop_mrc’ program. Zenodo; 10.5281/ZENODO.5559243 (2021). [DOI] [Google Scholar]
- 88.Calakli F & Taubin G In Expanding the Frontiers of Visual Analytics and Visualization (eds Dill J et al. ) 323–338 (Springer, 2012). [Google Scholar]
- 89.Kazhdan M & Hoppe H Screened Poisson surface reconstruction. ACM Trans. Graph. 32, 1–13 (2013). [Google Scholar]
- 90.Jewett A jewettaij/visfd_tutorials: updated ‘STEP_0’ of the Bdellovibrio segmentation example. Zenodo; 10.5281/ZENODO.5758691 (2021). [DOI] [Google Scholar]
- 91.Lindeberg T Feature detection with automatic scale selection. Int. J. Comput. Vis. 30, 79–116 (1998). [Google Scholar]
- 92.Martinez-Sanchez A, Garcia I, Asano S, Lucic V & Fernandez J-J Robust membrane detection based on tensor voting for electron tomography. J. Struct. Biol. 186, 49–61 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full tomograms are available upon request.


