Abstract
Background/Aim
Surgical outcomes of colorectal cancer (CRC) in patients with renal failure (RF) remain to be clarified. The objective of this research was to investigate how RF impacts the surgical outcomes in patients with CRC.
Patients and Methods
A retrospective analysis was performed on clinical data from 633 patients who underwent colorectal resection for CRC between January 2017 and December 2021. Outcomes of the patients with and without RF were compared. RF was defined as estimated Glomerular Filtration Rate less than 30.
Results
Forty-five (7%) patients with RF were identified. RF was a significant risk factor for postoperative complications after colorectal cancer surgery (odds ratio=2.19, 95% confidence interval=1.08-4.42, p=0.0284). The patients with RF had significantly more comorbidity (p=0.016), and higher American Society of Anesthesiologists physical status (p<0.01). Hemoglobin level (p<0.01) and PNI (p<0.01) were significantly lower in those with RF. Postoperative complications were significantly higher (p=0.016), and the postoperative hospital stay was significantly longer (p<0.01) among patients with RF compared to those without RF. Patients with RF, excluding those undergoing hemodialysis, had significantly more complications compared to those without RF (p=0.004).
Conclusion
Careful attention should be paid to perioperative management in RF colorectal cancer patients.
Keywords: Renal dysfunction, hemodialysis, colorectal cancer, surgery
Colorectal cancer (CRC), among the most common diagnosed neoplasms in both Eastern and Western countries (1), recently became the leading cause of cancer-related death in women in Japan (2). Laparoscopic surgery for CRC, along with a recovery program during the perioperative period, reportedly improves surgical outcomes (3), and previous studies have identified the risk factors for postoperative complications and prognostic predictors after curative surgery in patients with CRC (4).
Chronic kidney disease (CKD) accounts for 9.1% of the world’s population, and there are more people diagnosed with CKD (5). As one ages, the risk of developing cancer in Japan and Western countries has gradually increased (1,2). CKD has been reported to be associated with the development of CRC (6), and thus, the number of CRC cases among CKD patients has also increased. Although postoperative outcomes of hemodialysis (HD) patients have been reported for colorectal cancer (7,8), there are limited studies on the results of colorectal surgery for CRC among patients with renal failure (RF). The purpose of this study was to identify the feasibility, and safety in patients with RF after colorectal surgery in comparison to those without RF.
Patients and Methods
Patients and clinicopathological characteristics. This study was approved by the Ethics Committee of the Japanese Red Cross Fukuoka Hospital (no. 622), and it conformed to the provisions of the Declaration of Helsinki. As this study design was retrospective, written informed consent was obtained using the opt-out method.
This study analyzed 708 consecutive patients with CRC who underwent surgical treatments at our institution between January 2017 and December 2021. Subsequently, those who underwent colostomy or ileostomy, and staging laparoscopy were excluded. Ultimately, a total of 633 patients were registered in this study. The patients’ clinicopathological data were gathered from their medical records. The following information was also obtained: age, sex, body mass index, comorbidities (including diabetes mellitus, cardiac disease, cerebrovascular disease, respiratory disease, and chronic renal failure), American Society of Anesthesiologists physical status (ASA-PS), preoperative hemoglobin (Hb), preoperative serum levels of blood urea nitrogen (BUN) and creatinine (Cr), preoperative differential leukocyte count, and prognostic nutritional index (PNI), which was calculated as follows: 10×serum albumin [g/dl]+0.005×total lymphocyte count in peripheral blood [/mm3], C-reactive protein-to-albumin ratio (CAR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), tumor location, level of lymph node dissection, operative time, estimated blood loss volume, performance of blood transfusion, length of resected specimen, postoperative complications, length of postoperative stay, and Union for International Cancer Control stage (9). Estimated Glomerular Filtration Rate (eGFR) (ml/min/1.73 m2) was calculated as follows: 194×Cr [mg/dl]–1.094×age–0.287 (male), and 194×Cr [mg/dl] –1.094×age–0.287×0.739(female). RF was defined as an eGFR less than 30.
Perioperative management. All patients underwent rehabilitation after admission, and early mobilization was performed the day after surgery in accordance with the clinical path. For mechanical bowel preparation, patients with RF received a polyethylene glycol electrolyte lavage solution containing ascorbic acid the day before surgery, while the non-RF group received magnesium citrate. Chemical bowel preparation using kanamycin and metronidazole was performed. All patients underwent standard colorectal surgery, and blood flow evaluation using indocyanine green was conducted before anastomosis. HD was administered on the day before surgery and every two-three days postoperatively by a nephrologist. After hospital discharge, all patients underwent blood testing every three or six months, contrast-enhanced computed tomography scans every six months, and colonoscopy every two years to check for recurrence.
Statistical analysis. The statistical analysis was conducted using JMP® statistical software, version 16.1.0 (SAS Institute Inc., Cary, NC, USA). The analysis of continuous variables between two groups was conducted using the Mann-Whitney U-test, whereas categorical variables were conducted using the χ2 test or Fisher’s exact test. Simple logistic regression analysis was used for the univariate analysis. The clinicopathological factors with a significant difference in the univariate analysis were applied for multivariate analysis using a logistic regression to identify factors that were independently associated with postoperative complications. A statistically significant difference was defined as a p<0.05.
Results
Characteristics of patients. The study included 339 male and 294 female patients with a median age of 72 years. The median BMI was 21.8 kg/m2. Among them, 232 patients (37%) had comorbidities, and forty-five (7%) with RF were identified. A low ASA-PS (<3) was present in 544 patients (86%). The median levels of Hb, BUN, Cr, PNI, CAR, NLR, PLR, and LMR were 12.1 (5.4-17.5), 14.2 (3.1-75.6), 0.77 (0.24-12.2), 46.46 (20.46-120.98), 0.047 (0-14.06), 2.39 (0.36-22.2), 168.25 (10.73-14,230.77), and 4.54 (0.61-28.5), respectively. Colon cancer was diagnosed in 472 patients (75%), and rectal cancer in 161 (25%). Laparoscopic surgery was performed in 552 patients (87%), with central node dissection in 524 (83%). The median operation time and estimated blood loss were 293 minutes and 24 ml, respectively. Transfusion was performed in 66 patients (10%). Stage I or II was the final diagnosis in 379 patients (59%). Among the 633 patients, 45 (7%) had RF, including 18 HD patients.
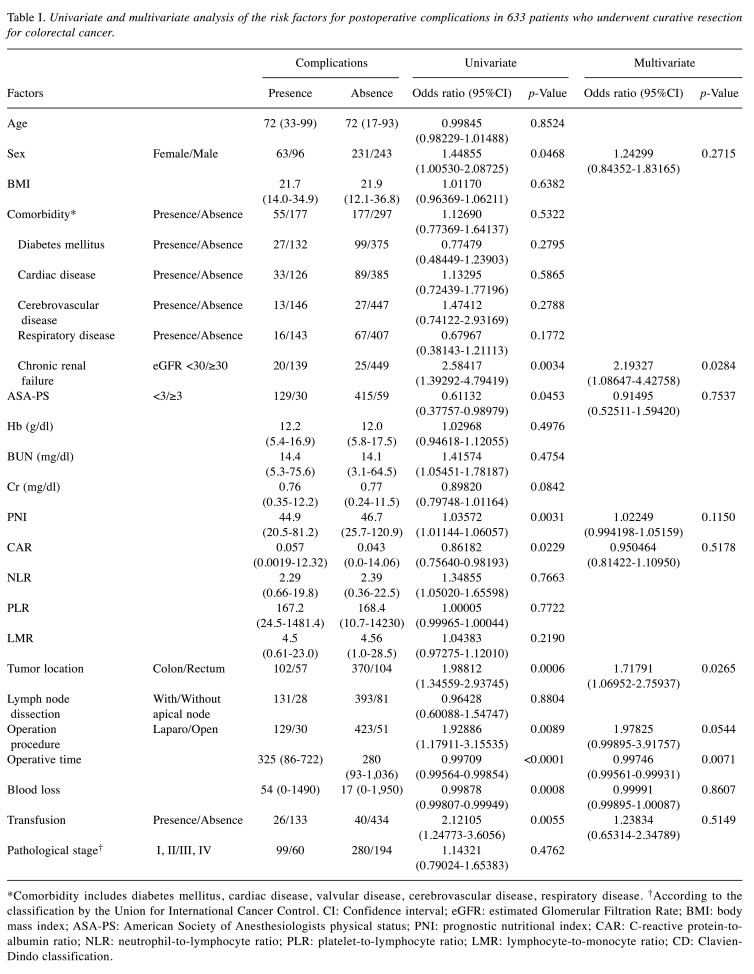
Risk factor for postoperative complications after colorectal cancer surgery. The univariate analysis showed that sex, eGFR <30, ASA-PS, PNI, CAR, tumor location, operation procedure, operative time, blood loss, and transfusion were significantly associated with the incidence of postoperative complications, defined by Clavien-Dindo classification (CD) (≥2). In a multivariate analysis, eGFR <30 [odds ratio (OR)=2.19, 95% confidence interval (CI)=1.08-4.42, p=0.0284], tumor location (OR=1.71, 95%CI=1.06-2.75, p=0.0265), and operative time (OR=0.997, 95%CI=0.995-0.999, p=0.0071) were identified as significant factors indicating postoperative complications (Table I).
Table I. Univariate and multivariate analysis of the risk factors for postoperative complications in 633 patients who underwent curative resection for colorectal cancer.
*Comorbidity includes diabetes mellitus, cardiac disease, valvular disease, cerebrovascular disease, respiratory disease. †According to the classification by the Union for International Cancer Control. CI: Confidence interval; eGFR: estimated Glomerular Filtration Rate; BMI: body mass index; ASA-PS: American Society of Anesthesiologists physical status; PNI: prognostic nutritional index; CAR: C-reactive protein-toalbumin ratio; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio; CD: Clavien- Dindo classification.
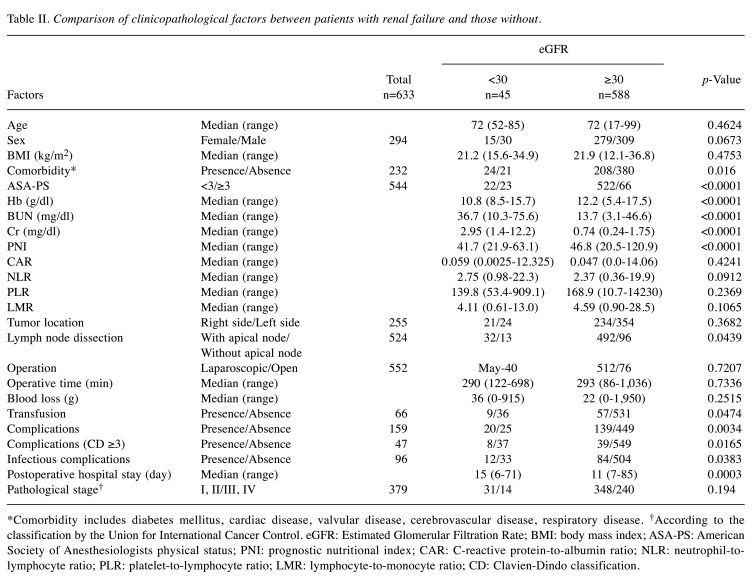
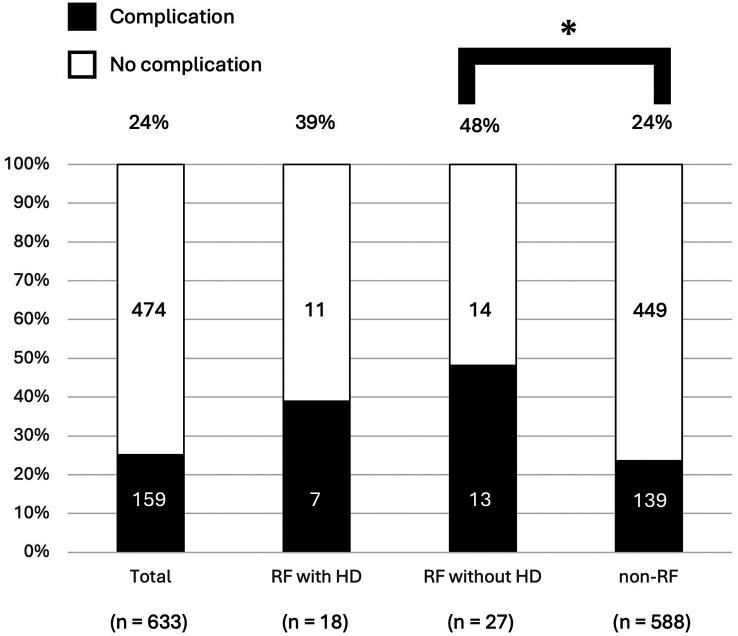
Characteristics and short-term outcomes after colorectal surgery in the patients with RF. The patients with RF had significantly more comorbidity (p=0.016), and higher American Society of Anesthesiologists physical status (p<0.0001). Hemoglobin levels (p<0.0001), BUN (p<0.0001), Cr (p<0.0001), and PNI (p<0.0001) were significantly lower in patients with RF. Transfusion was more frequently performed (p=0.0474). Postoperative complications were significantly higher (p=0.016), and the postoperative hospital stay was significantly longer (p<0.01) among RF compared to non-RF patients. The RF patients had more severe complications (CD≥3) and infectious complications (p=0.0165 and p=0.0383, respectively) (Table II). No significant difference in postoperative complications (CD≥2) was identified between HD patients and non-RF patients (p=0.136). Nonetheless, the patients with RF, excluding those on HD, had significantly more complications than non-RF patients (p=0.004) (Figure 1).
Table II. Comparison of clinicopathological factors between patients with renal failure and those without.
*Comorbidity includes diabetes mellitus, cardiac disease, valvular disease, cerebrovascular disease, respiratory disease. †According to the classification by the Union for International Cancer Control. eGFR: Estimated Glomerular Filtration Rate; BMI: body mass index; ASA-PS: American Society of Anesthesiologists physical status; PNI: prognostic nutritional index; CAR: C-reactive protein-to-albumin ratio; NLR: neutrophil-tolymphocyte ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio; CD: Clavien-Dindo classification.
Figure 1. Postoperative complications rate after colorectal surgery for colorectal cancer. A comparison of the postoperative complications (Clavien-Dindo ≥2) rate among three cohorts, including patients with renal failure (RF) excluding hemodialysis (HD), HD patients, and non-RF patients, revealed that RF patients without HD had significantly more complications compared to those without RF (*p=0.004).
Discussion
The following findings were revealed in this study: 1) among comorbidities, only renal dysfunction was a significant risk factor for postoperative complications after colorectal cancer surgery; 2) the rate of postoperative complications for colorectal cancer surgery in severe RF patients without HD were 48%, significantly higher than that in non-RF patients.
Most RF patients, including those undergoing HD, tend to have comorbid diseases, such as cardiovascular disease, atherosclerotic disease, metabolic disease, and diabetes mellitus (7,10). Additionally, they face many disadvantages, such as organ vulnerability, delayed healing, increased susceptibility to bleeding, and a higher risk of infection, leading to elevated perioperative complications (8). In our study, patients with RF exhibited significant differences in postoperative complications compared to those without RF. Previous studies reported complication rates ranging from 36.8 to 50% in HD patients after CRC surgery (7,8). The postoperative complication rate (CD ≥2) was 44.4% in this study with a severe complication rate of 17.7%, and there were no patient deaths within 30 days after surgery. In contrast to RF patients without HD, the absence of differences in the postoperative complication rate between HD and non-RF patients can be attributed to the following reasons: 1) the final judgement of surgery in HD patients with especially low activities of daily living was made in the preoperative conference with surgeons, radiologists, nephrologists, and nurses; and 2) all HD patients underwent colorectal surgery and lymphadenectomy with standardized procedures within the team consisting of the surgeon who meets the requirements of the Japan Society for Endoscopic Surgery (JSES) endoscopic surgical skill qualification system. Moreover, HD was performed by specialists in CKDs and end-stage renal diseases at our institution. Additionally, RF patients without HD may exhibit a severe uremic state similar to HD patients, potentially leading to delayed wound healing processes. In fact, Abe et al. reported that uremia had a significant impact on delaying the healing of infections and wounds in HD patients (11). These results suggest that careful perioperative management should also be undertaken for severe RF patients without HD.
The median postoperative hospital stay was longer in RF patients compared to non-RF patients. Poor wound healing and slow recovery after surgery were observed in HD patients due to lower PNI and nutritional status, resulting in a longer hospital stay. Tominaga et al. (12) indicated that a low preoperative PNI was correlated with delayed bowel recovery and extended postoperative hospitalization after laparoscopic colectomy. Thus, the improvement of PNI might lead to a shorter hospital stay, and strategies for nutrition improvement, such as nutrition and rehabilitation support, were established during the perioperative phases.
In comparison to conventional open surgery, laparoscopic colorectal surgery is considered to provide improved surgical visualization, less blood loss, reduced would size, lower pain levels, less impaired respiratory or cardiac functions, fewer complications, and shorter hospitalization (4,13). These benefits could be expected to be advantageous for patients with RF. In addition, previous reports have demonstrated that laparoscopic surgery in HD patients is associated with improved postoperative complications, reduce mortality rates, and shorter hospital stays (8,14). In our study, despite the majority of RF patients undergoing laparoscopic surgery (89%), postoperative complications after colorectal surgery did not decrease. Although changing the body position is crucial in laparoscopic surgery, blood pressure fluctuations due to these positional changes might have an adverse effect. Further alternative strategies should be considered.
Study limitations. First, this study was a small and retrospective analysis of medical records from a single institution. Second, eGFR measurement was a simple but less accurate evaluation method than other methods such as inulin clearance. Third, the operability of the patients with surgery was not compared to those without surgery. Therefore, the actual effects of RF including HD in patients with CRC remain unclear.
Conclusion
The rate of postoperative complications after surgical treatment for RF patients with CRC was high. Especially, careful attention should be paid for perioperative management in RF patients without HD. Further investigations are needed to establish the treatment strategies for colorectal surgery among RF patients with CRC.
Funding
Funding was not received for this study.
Conflicts of Interest
All Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Takaaki Fujimoto performed the data acquisition, analysis, and interpretation and wrote the article. Shigetaka Inoue performed the data acquisition and revised the manuscript. Taketo Matsunaga performed the data acquisition, and interpretation. Toru Shumizu, Haruka Mitsubuchi, Takahito Matsuyoshi, Kaou Matsuda, Soshi Terasaka, Takaharu Yasui, Chizu Kameda, Yasuhiro Ogura, and Junji Ueda performed the data acquisition. Kentaro Nakai, and Masanori Tokumoto identified and quantified the data analysis for renal function, and revised the manuscript. Kenichi Nishiyama examined the histopathological assessment. Eishi Nagai revised the manuscript. Kentaro Motoyama identified and quantified the data analysis for renal function, and revised the manuscript. Yuji Nakafusa revised and finally approved the manuscript.
Acknowledgements
The Authors would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Kamo KI, Fukui K, Ito Y, Nakayama T, Katanoda K. How much can screening reduce colorectal cancer mortality in Japan? Scenario-based estimation by microsimulation. Jpn J Clin Oncol. 2022;52(3):221–226. doi: 10.1093/jjco/hyab195. [DOI] [PubMed] [Google Scholar]
- 3.Ni X, Jia D, Chen Y, Wang L, Suo J. Is the enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J Gastrointest Surg. 2019;23(7):1502–1512. doi: 10.1007/s11605-019-04170-8. [DOI] [PubMed] [Google Scholar]
- 4.Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of randomized clinical trial. Lancet Oncol. 2009;10(1):44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 5.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 6.Kozlowski L, Bielawska K, Zhymaila A, Malyszko J. Chronic kidney disease prevalence in patients with colorectal cancer undergoing surgery. Diagnostics (Basel) 2022;12(9):2137. doi: 10.3390/diagnostics12092137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neki K, Takeda Y, Kosuge M, Ohkuma M, Yatabe S, Sugano H, Kumamoto T, Dairaku K, Eto K. Short-term postoperative outcomes of colorectal cancer patients with chronic renal failure on dialysis. In Vivo. 2022;36(5):2461–2464. doi: 10.21873/invivo.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraishi T, Tominaga T, Nonaka T, Hashimoto S, Hamada K, Araki M, Sumida Y, Takeshita H, Fukuoka H, Wada H, To K, Yamashita M, Tanaka K, Sawai T, Nagayasu T. Effect of hemodialysis on short-term outcomes after colon cancer surgery. PLoS One. 2022;17(1):e0262531. doi: 10.1371/journal.pone.0262531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brierly JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. New York, NY, USA, Wiley. 2016 [Google Scholar]
- 10.Mayanagi S, Oba K, Aoyama T, Tanaka K, Kanda M, Honda M, Maeda H, Kashiwabara K, Muto M, Sakamoto J, Yamagishi H, Yoshikawa T. Feasibility and safety of adjuvant chemotherapy for resected colorectal cancer in patients with renal insufficiency: a pooled analysis of individual patient data from five Japanese large-scale clinical trials. Anticancer Res. 2023;43(7):3089–3095. doi: 10.21873/anticanres.16480. [DOI] [PubMed] [Google Scholar]
- 11.Abe H, Mafune K. Risk factors for maintenance hemodialysis patients undergoing elective and emergency abdominal surgery. Surg Today. 2014;44(10):1906–1911. doi: 10.1007/s00595-013-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tominaga T, Nagasaki T, Akiyoshi T, Fukunaga Y, Honma S, Nagaoka T, Matsui S, Minami H, Miyanari S, Yamaguchi T, Ueno M. Prognostic nutritional index and postoperative outcomes in patients with colon cancer after laparoscopic surgery. Surg Today. 2020;50(12):1633–1643. doi: 10.1007/s00595-020-02050-2. [DOI] [PubMed] [Google Scholar]
- 13.Shiroshita H, Inomata M, Akira S, Kanayama H, Yamaguchi S, Eguchi S, Wada N, Kurokawa Y, Uchida H, Seki Y, Ieiri S, Iwazaki M, Sato Y, Kitamura K, Tabata M, Mimata H, Takahashi H, Uemura T, Akagi T, Taniguchi F, Miyajima A, Hashizume M, Matsumoto S, Kitano S, Watanabe M, Sakai Y. Current status of endoscopic surgery in Japan: The 15th National Survey of Endoscopic Surgery by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg. 2022;15(2):415–426. doi: 10.1111/ases.13012. [DOI] [PubMed] [Google Scholar]
- 14.Hu WH, Cajas-Monson LC, Eisenstein S, Parry L, Ramamoorthy S. Association of dialysis with adverse postoperative outcomes in colorectal cancer – an analysis of ACS-NSQIP. Int J Colorectal Dis. 2015;30(11):1557–1562. doi: 10.1007/s00384-015-2347-y. [DOI] [PubMed] [Google Scholar]