Abstract
In this article, we show that passage in SCID mice rendered a human CD4+ T-cell line (CEM cells) highly susceptible to infection by macrophage-tropic (M-tropic) strains and primary clinical isolates of human immunodeficiency virus type 1 (HIV-1). This in vivo-acquired permissiveness of CEM cells was associated with the induction of a CD45RO+ phenotype as well as of some β-chemokine receptors. Regulated upon activation, normal T-cell expressed and secreted chemokine entirely inhibited the ability of M-tropic HIV-1 strains to infect these cells. These findings may lead to new approaches in investigating in vivo the capacity of different HIV strains to exploit chemokine receptors in relation to the dynamics of the activation and/or differentiation state of human CD4+ T cells.
Understanding the scenario of in vivo interactions between human immunodeficiency virus type 1 (HIV-1) and target cells is an issue of crucial importance in AIDS research. However, progress in this area has been hampered by the problems in reconciling the results obtained in studies using in vitro cell systems with the events occurring under in vivo conditions and, possibly, with those observed in HIV-1-infected patients. In particular, overestimating the significance of in vitro virus-target cell assays for determining viral tropism and pathogenicity may lead to misleading conceptions about HIV-1 pathogenesis, if it is not sufficiently taken into account that the phenotypes of both virus and target cells can significantly change in the course of in vivo infection. This obviously has profound implications for viral transmission, pathogenesis, and disease progression. For this reason and due to the general confusion created by the present classification systems, some authors have recently proposed that a new HIV-1 classification based on the coreceptor usage rather than in vitro assays is needed (2).
The susceptibility to infection with different HIV-1 strains is related to the expression of various chemokine receptors on T-lymphocyte subsets (1–4, 7, 8, 14). In fact, CXCR4 (the principal coreceptor for T-cell tropic [T-tropic] HIV-1 strains) is mainly expressed on naive CD4+ T lymphocytes (CD45RA), while CCR5 (the principal coreceptor for macrophage-tropic [M-tropic] HIV-1 strains) is predominantly expressed on memory CD4+ T lymphocytes (CD45RO) (3). Although some studies have suggested that the progressive differentiation of human CD4+ T cells toward a memory phenotype is associated with an increased susceptibility to HIV-1 infection (21, 24, 27), there is no direct in vivo evidence on the relationships between T-cell differentiation and the importance of coreceptor usage for HIV-1 cell tropism and HIV-1 induced disease.
Human-severe combined immunodeficient (SCID) mouse xenografts represent unique and practical in vivo models with which to study the early events triggered by the interaction of HIV-1 with the human immune system (11–13, 15–18). In the present study, we investigated the possible changes in the permissiveness to various HIV-1 strains of a human CD4+ T-cell line (CEM-SS) (19) after transplantation into SCID mice.
Acquired susceptibility to the M-tropic HIV-1 strain SF162 by CEM cells grown in SCID mice.
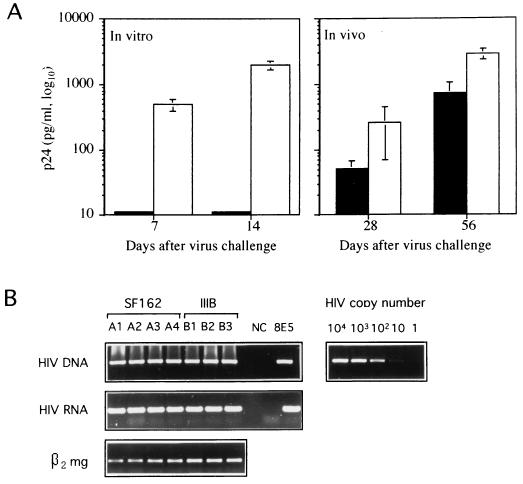
We first compared the abilities of syncytium-inducing T-tropic (IIIB) and non-syncytium-inducing M-tropic (SF162) strains of HIV-1 to infect CEM cells in vitro and after transplantation into SCID mice. CB.17 SCID/SCID female mice were injected subcutaneously (s.c.) in the shoulder with 20 × 106 uninfected CEM-SS cells resuspended in 0.2 ml of RPMI 1640 (22). SCID mice were treated with a monoclonal anti-mouse granulocyte antibody to deplete animals of some residual reactivity, as previously described (22). The in vivo HIV-1 infection of CEM-SCID mice was performed by a simultaneous s.c. injection of 20 × 106 uninfected CEM-SS cells (American Type Culture Collection, Rockville, Md.) with 106 50% tissue culture infective doses of cell-free virus (10). The virus stocks were derived from clarified culture medium of phytohemagglutinin-interleukin 2-stimulated HIV-1-infected peripheral blood mononuclear cells, frozen at −140°C. Titers were determined by standard end-point dilution methods. The viral strains used in these experiments were HIV-1 IIIB and HIV-1 SF162. Under all conditions, the HIV-1-infected chimeras were sacrificed when the tumors reached 20- to 25-mm mean diameter and analyzed for the virus replication at the tumor site and p24 antigenemia. At sacrifice, the CEM cell tumors were excised, and single-cell suspension was obtained as described (22). Cell suspensions were subjected to HIV-1 DNA PCR, as described (20), and HIV-1 reverse transcription-PCR (RT-PCR) with specific primers was performed to detect all viral RNAs, as reported elsewhere (10). Sera of infected animals were tested for HIV p24 antigen by an antigen capture enzyme-linked immunosorbent assay (Dupont, Bruxelles, Belgium). For HIV-1 in vitro infection, cells were pelleted and incubated with the virus inoculum at multiplicity of infection of 0.1 for 1 h at 37°C, washed three times, and cultured in complete medium. As shown in Fig. 1A (left), HIV-1 SF162 did not infect the parental CEM cells maintained under in vitro condition up to 2 weeks after challenge, while these cells were fully permissive to HIV-1 IIIB. In contrast to the results of in vitro experiments, the in vivo infection induced a productive infection with both HIV-1 strains. In fact, high levels of p24 antigenemia (Fig. 1A, right) were detected in the sera of xenografted animals infected with both SF162 and III-B HIV-1 strains up to 2 months after the in vivo virus challenge. Moreover, the DNA PCR and RT-PCR analyses of CEM cells obtained from s.c. tumors showed high levels of HIV-1 infection with both HIV-1 strains (Fig. 1B). These results strongly suggested that the SCID mouse environment had induced important changes in CEM cells, rendering them permissive to a broader spectrum of HIV-1 strains.
FIG. 1.
Acquired susceptibility to infection with the SF162 strain of HIV-1 by CEM cells grown in SCID mice. (A) p24 antigen levels in the supernatants of CEM cells infected in vitro with either IIIB (white columns) or SF162 (black columns) strain (left) and in sera of SCID mice transplanted s.c. with CEM cells and simultaneously infected with either IIIB (white columns) or SF162 (black columns) (right). Histograms represent the means ± standard errors for six samples. (B) HIV-1 infection of CEM cell tumors grown in SCID mice. DNA PCR for gag-specific sequences (top row). The detection range of proviral copy number was determined in parallel by amplifying known amounts of 8E5 cell line DNA. RT-PCR (middle row) was performed with specific primers to detect all viral RNAs. β2-Microglobulin (β2 mg) RT-PCR (bottom row) was run in parallel to normalize the levels of human RNA in all the samples. Single animals (A1 through A4 and B1 through B3), negative control (NC), and positive control (8E5) are indicated.
Characterization of CEM cell phenotype in SCID mice.
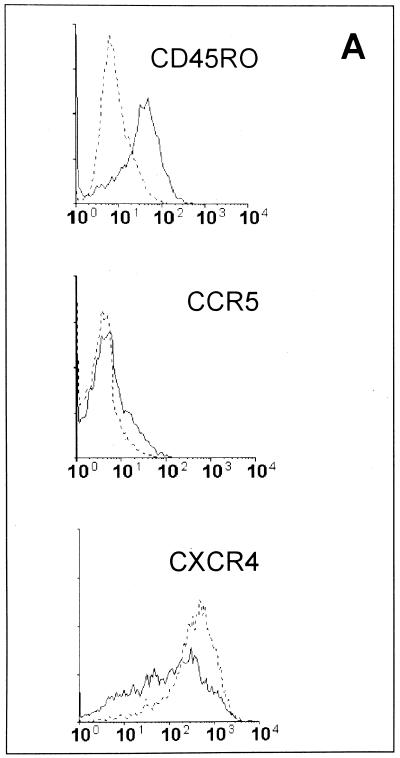
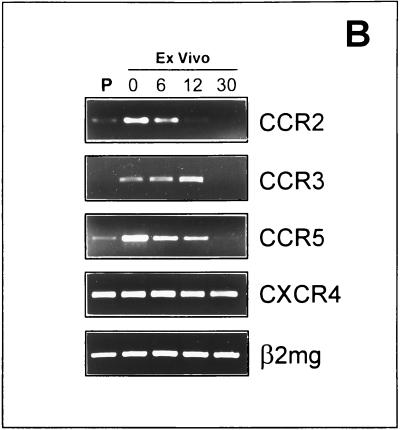
We next characterized the phenotype of ex vivo CEM cells as compared to the parental cells. CEM cells obtained from uninfected s.c. tumors were analyzed by flow cytometry by using the following monoclonal antibodies: anti-CD45RA fluorescein isothiocyanate and anti-CD45RO phycoerythrin (PE) (Becton Dickinson, San José, Calif.), anti-CXCR4 PE, and anti-CCR5 PE (Pharmingen, San Diego, Calif.). Cells were fixed with 2% paraformaldehyde and analyzed on a FACSORT cytometer (Becton Dickinson) equipped with a 488-nm argon laser. Data were recorded and analyzed by using LYSIS II software (Becton Dickinson). The results, summarized in Fig. 2A, show that CEM cells obtained from the s.c. tumors grown in SCID mice (ex vivo CEM cells) differed significantly from the parental CEM cells. In particular, (i) ex vivo CEM cells were CD45RO+ while the parental cells were CD45RO−, (ii) the ex vivo CEM cells expressed low level of CCR5 on the cell membrane while the parental CEM cells were all negative for CCR5 (staining with control PE-conjugated antibodies did not show nonspecific binding on ex vivo CEM cells [data not shown]), and (iii) the percentages of CXCR4+ cells did not show significant differences between ex vivo and parental CEM cells. CD45RA, CD3, and CD4 were equally expressed in both the parental and ex vivo CEM cells (data not shown). To further explore the phenotype of ex vivo CEM cells we used RT-PCR analysis. mRNAs coding for human chemokine receptors (CCR2, CCR3, CXCR4, and CCR5) were detected in CEM cells by amplifying the RNA isolated as previously described (20) with specific primer pairs. The following primer sequences were used for CXCR4: 5′ TGCTGTATGTCTCGTGGTAGG and 3′ TGTAGGTGCTGAAATCAACCC. Primers specific for CCR5, CCR2, and CCR3 are reported elsewhere (6, 9). The samples were amplified for 30 to 35 cycles under the following conditions: 94°C for 40 s, 62°C for 40 s, and 72°C for 40 s. β2-Microglobulin RT-PCR (20) was run in parallel to normalize the levels of human RNA in all the samples. Preliminary experiments were performed to verify that the human primers used did not cross-react with murine sequences. All RT-PCR products were in the linear range of amplification (data not shown). The results of the RT-PCR analysis of the HIV-1 coreceptors were consistent with the flow cytometry results. In fact, a marked upregulation of CCR2, CCR3, and CCR5 mRNAs occurred in the ex vivo CEM cells as compared to the parental cells, while the mRNA for CXCR4 was equally expressed in the two cell types (Fig. 2B). Notably, RT-PCR analysis showed that the ex vivo CEM cells progressively lost their new phenotype when maintained under in vitro conditions for a few weeks. In fact, at 30 days of in vitro culture, the expression of CCR2, CCR3, and CCR5 mRNAs of these cells was virtually identical to that of the original parental cells (Fig. 2B), while the expression of CXCR4 mRNA did not change during the culture period (Fig. 2B). These changes paralleled a progressive loss of the CD45RO phenotype for ex vivo CEM cells occurring at 2 to 3 weeks of in vitro culture (data not shown). Taken together, these data indicated that the phenotype of ex vivo CEM cells markedly differed from that of parental cells. This could be the result of either a progressive differentiation of CEM cells due to the stimuli present in the mouse environment or an expansion of a very small fraction of CEM cells (expressing the CD45RO phenotype and higher levels of chemokine receptors) in SCID mice. This hypothesis may appear possible given the results for CCR5, which is detectable (at the mRNA level) in parental CEM cells. However, it seems unlikely in the view of the fact that CD45RO and the other chemokine receptors (with the exception of CXCR4) are not expressed in parental CEM cells.
FIG. 2.
Time course of phenotypic changes of ex vivo CEM cells. (A) Flow cytometric analysis of parental CEM cells (dotted lines) and of CEM cells obtained from s.c. tumors grown in SCID mice (ex vivo CEM cells) (solid lines) labeled with anti-CD45RO, anti-CCR5, and anti-CXCR4 antibodies. (B) RT-PCR in parental (P) and ex vivo CEM cells. The results of one representative experiment are shown. Ex vivo CEM cells were collected and analyzed immediately after harvesting (0) and at 6, 12, and 30 days of in vitro culture. RNA was amplified with primer pairs for CCR2, CCR3, CXCR4, and CCR5.
HIV-1 infection of ex vivo CEM cells and role of β-chemokine receptors.
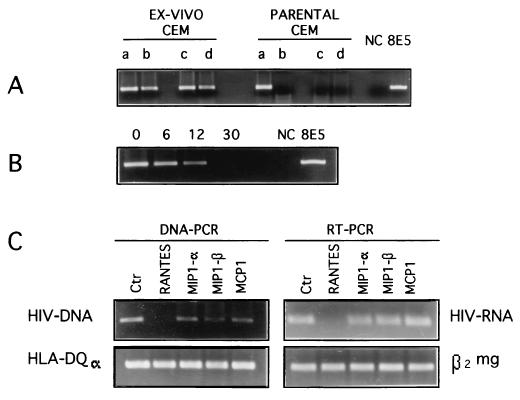
We then characterized the spectrum of permissiveness of ex vivo CEM cells to HIV-1 infection, by challenging these cells with SF162, IIIB, and two primary clinical isolates (G and PD, kindly provided by Maria Capobianchi, Institute of Virology, University “La Sapienza,” Rome, Italy), as compared to the parental CEM cells. Ex vivo CEM cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum. Cells were seeded at 2 × 105/ml and passaged every three days. For HIV-1 in vitro infection, parental and ex vivo CEM cells were pelleted and incubated with the virus inoculum at multiplicity of infection of 0.1 for 1 h at 37°C, washed three times, and cultured in complete medium. The results of these experiments were consistent with the in vivo findings illustrated in Fig. 1, in that the ex vivo CEM cells remained highly permissive to both SF162 and IIIB strains whereas the parental CEM cells did not efficiently integrate and replicate the non-syncytium-inducing M-tropic HIV-1 strain SF162, even at 12 days after the challenge (Fig. 3A). Moreover, the ex vivo CEM cells were efficiently infected by both the primary clinical isolates (Fig. 3A). Notably, the finding that no proviral HIV-1 copies were detected after infection of parental CEM cells with HIV-1 SF-162 (Fig. 3A) renders it unlikely that our data simply reflect an in vivo selection of a small fraction of preexisting CEM cells permissive for M-tropic HIV-1 strains. As shown in Fig. 3B, the permissiveness of the ex vivo CEM cells to SF162 progressively declined during in vitro culture. These results suggested that the acquired permissiveness of ex vivo CEM cells was dependent on the HIV-1 coreceptors gained in vivo, in that both the newly acquired coreceptor phenotype and the permissiveness to the M-tropic strain were progressively lost during in vitro culture. To verify whether the upregulation of the β-chemokine receptors on the ex vivo CEM cells had a role in their acquired permissiveness to M-tropic HIV-1 strains, we tested whether the β-chemokines regulated upon activation, normal T-cell expressed and secreted chemokine (RANTES), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and monocyte chemotactic protein 1 (MCP-1) could interfere with the entry, integration, and replication of SF162 in these cells. For these experiments, recombinant human RANTES, MIP-1α, MIP-1β (R&D Systems, Minneapolis, Minn.), and MCP-1 (PeproTech, EC, Ltd., London, United Kingdom) were each added at 300 ng/ml to the cells together with the virus inoculum and at every passage in culture. Controls were cultured in complete medium without supplements. PCR analysis clearly showed that RANTES exerted an early inhibitory effect on viral entry. Treatment with this β-chemokine resulted in undetectable levels of HIV-1 proviral copies in ex vivo CEM cells (Fig. 3C, left). MIP-1β exerted a slight inhibitory effect on SF162 infection, while little or no effect was observed with either MIP-1α or the CCR2 ligand MCP-1 (Fig. 3C, left). RT-PCR analysis of HIV-1 RNA confirmed that RANTES virtually abolished SF162 replication in the ex vivo CEM cells (Fig. 3C, right). These results confirmed that the slight increase in CCR5 expression in ex vivo CEM cells shown by flow cytometry (Fig. 2) was key in rendering these cells permissive to HIV-1 SF162 strain.
FIG. 3.
Permissiveness of ex vivo CEM cells to infection with M-tropic HIV-1 strains and effects by β-chemokines. (A) PCR analysis of HIV-1 proviral DNA in parental and ex vivo CEM cells. Parental and ex vivo CEM cells were infected in vitro with the HIV-1 IIIB (lane a) or SF162 (lane c) strain or with each of two primary clinical isolates (lanes b and d). DNA samples were analyzed for HIV-1 proviral DNA at 12 days after the virus challenge. Negative controls (NC) and positive controls (8E5) were also processed. (B) Time course of permissiveness of ex vivo CEM cells to the HIV-1 SF162 strain. Ex vivo CEM cells were infected with HIV-1 SF162 immediately after harvesting (0) and after 6, 12, and 30 days of in vitro culture and analyzed for the presence of proviral DNA by PCR at 3 days after virus challenge. (C) Effects of the various chemokines on the infection of ex vivo CEM cells with the HIV-1 SF162 strain. Ex vivo CEM cells were infected in vitro with HIV-1 SF162 and cultured with and without (Ctr) the addition of the chemokines RANTES, MIP-1α, MIP-1β, and MCP-1. Samples were analyzed for the presence of HIV-1 proviral DNA (left) and RNA (right) by PCR at 3 days after the infection. HLA-DQα DNA PCR and β2-microglobulin (β2 mg) RT-PCR were run in parallel to normalize the levels of human DNA and RNA, respectively, in all the samples. The results are representative of four separate experiments.
Some studies have shown that CD4+ T lymphocytes expressing the memory phenotype are infectable with a broad spectrum of HIV-1 strains, while naive CD4+ T cells are exclusively permissive to T-tropic strains of HIV-1 and require cellular activation signals for productive infection (21, 23, 24, 27). This phenomenon has recently been elucidated by the demonstration that naive T cells predominantly express CXCR4 HIV-1 coreceptor while memory T cells express CCR5 (3). In vitro studies have largely confirmed that human CD4+ T-cell lines are exclusively permissive to T-tropic HIV-1 strains through CXCR4 usage (2). The data presented here indicate that the passage of a human CD4+ T-cell line into SCID mice markedly changed the phenotype of these cells, rendering them permissive to both an M-tropic strain and primary clinical isolates of HIV-1, which did not enter the parental cells maintained in vitro. This permissiveness was associated with the acquisition of a memory phenotype and was mostly dependent on the availability of the CCR5 HIV-1 coreceptor, since the occupancy of this receptor by RANTES and MIP-1β, its natural ligands, markedly inhibited the SF162 infection. Thus, the changes induced by the SCID mouse environment were responsible for the acquired permissiveness of CEM cells to the M-tropic HIV-1 strain, SF162. This suggests that the dynamics of activation and differentiation of CD4+ T cells, induced by antigen stimulation or merely maintained by environmental factors (20, 25, 26), may continuously influence the emergence of different viral phenotypes during the development of HIV-1 infection and the progression to AIDS (3, 21, 23, 24, 27). Notably, different from peripheral blood lymphocytes, the great majority of CD4+ T cells in the mucosal tissues exhibit the memory phenotype (5) and, therefore, are potentially susceptible to the infection with a broad spectrum of HIV-1 strains. In this study, we have shown that a specific environmental factors of SCID mice may induce differentiation stimuli even for a T-cell line, rendering these cells permissive to the so-called R5 viruses (2) through the specific upregulation of CCR5 HIV-1 coreceptor. These data emphasize the need for a new classification of HIV-1 strains based on coreceptor use (2) rather than on other parameters such as the cell target (M-tropic or T-tropic), the capacity to induce syncytia in cell lines (syncytium inducing or non-syncytium inducing), or the growth kinetics in culture (slow/low level or rapid/high level).
New concepts regarding both the early events of HIV-1 infection and the mechanisms leading to the CD4+ T-cell depletion occurring in AIDS patients may stem from experiments performed with human/SCID mouse xenograft models. Our model offers new opportunities for a practical in vivo investigation, under highly controlled conditions, of mechanisms of HIV-1 infection in relation to the dynamics of activation and differentiation of human CD4+ T cells.
Acknowledgments
We are indebted to Angela Lippa for secretarial assistance.
This work was supported by grants from the Italian Ministry of Health (Progetto di Ricerca sull’AIDS 1997, 10A/L).
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Berger E A, Doms R W, Fenyo E M, Korber B T M, Littman D, Moore J P, Sattentau Q J, Schuitemaker H, Sodrosky J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapham P R. HIV and chemokines: ligands sharing cell-surface receptors. Trends Cell Biol. 1997;7:264–268. doi: 10.1016/S0962-8924(97)01075-1. [DOI] [PubMed] [Google Scholar]
- 5.De Maria R, Fais S, Silvestri M, Frati L, Pallone F, Santoni A, Testi R. Continuous in vivo activation and transient hyporesponsiveness to TcR/CD3 triggering of human gut lamina propria lymphocytes. Eur J Immunol. 1993;23:3104–3108. doi: 10.1002/eji.1830231209. [DOI] [PubMed] [Google Scholar]
- 6.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 7.Doranz B J, Rucker J, Yi Y, Smith R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactor. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza M P, Harden V A. Chemokines and HIV-1 second receptors. Nat Med. 1996;12:1293–1300. doi: 10.1038/nm1296-1293. [DOI] [PubMed] [Google Scholar]
- 9.Frade J M R, Llorente M, Mellado M, Alcamì J, Gutiérrez-Ramos J C, Zaballos A, del Real G, Martìnez-A C. The amino-terminal domain of the CCR2 chemokine receptor acts as a coreceptor for HIV-1 infection. J Clin Investig. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapenta C, Fais S, Rizza P, Spada M, Logozzi M, Parlato S, Santini S M, Belardelli F, Proietti E. U937-SCID mouse xenografts: a new model for acute in vivo HIV-1 infection suitable to test antiviral strategies. Antivir Res. 1997;36:81–90. doi: 10.1016/s0166-3542(97)00041-7. [DOI] [PubMed] [Google Scholar]
- 11.McCune J M. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;24:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 12.McCune J M, Namikawa R, Shih C C, Rabin L, Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990;247:564–566. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- 13.McCune J M. Animal models of HIV-1 disease. Science. 1997;278:2141–2142. doi: 10.1126/science.278.5346.2141. [DOI] [PubMed] [Google Scholar]
- 14.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 15.Mosier D E, Gulizia R J, Baird S M, Wilson D B. Transfer of functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 16.Mosier D E, Gulizia R J, Baird S M, Wilson D B, Spector D H, Spector S A. Human immunodeficiency virus infection of human PBL-SCID mice. Science. 1991;25:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 17.Mosier D E, Gulizia R J, MacIsaac P D, Torbett B E, Levy J A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 18.Namikawa R, Kanemisha H, Lieberman M, Weissman I L, McCune J M. Infection of the SCID-hu mouse by HIV-1. Science. 1991;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 19.Nara P L, Hatch W C, Dunlop N M, Robey W G, Arthur L O, Gonda M A, Fischinger P J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987;3:283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- 20.Rizza P, Santini S M, Logozzi M, Lapenta C, Sestili P, Gherardi G, Lande R, Spada M, Parlato S, Belardelli F, Fais S. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J Virol. 1996;70:7958–7964. doi: 10.1128/jvi.70.11.7958-7964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roederer M, Raju P A, Mitra D K, Herzenberg L A, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Investig. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santini S M, Spada M, Parlato S, Logozzi M, Lapenta C, Proietti E, Belardelli F, Fais S. Treatment of severe combined immunodeficiency (SCID) mice with anti-murine granulocyte monoclonal antibody improves human leukocyte xenotransplantation. Transplantation. 1998;65:416–420. doi: 10.1097/00007890-199802150-00022. [DOI] [PubMed] [Google Scholar]
- 23.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Investig. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tary-Lehmann M, Saxon A, Lehmann P V. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529–533. doi: 10.1016/0167-5699(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 26.Uittembogaart C H, Anisman D J, Tary-Lehmann M, Vollger L W, Breit T M, Van Dongen J J M, Saxon A. The SCID mouse environment causes immunophenotypic changes in human immature T-cell lines. Int J Cancer. 1994;56:546–551. doi: 10.1002/ijc.2910560414. [DOI] [PubMed] [Google Scholar]
- 27.Wood T C, Roberts B D, Butera S T, Folks T M. Loss of inducible virus in CD45RA naive cells after human immunodeficiency virus-1 entry accounts for preferential viral replication in CD45RO memory cells. Blood. 1997;89:1635–1641. [PubMed] [Google Scholar]