Abstract
Objective: Precise assessment of spinal instability is critical before and after radiotherapy (RT) for evaluating the effectiveness of RT. Therefore, we retrospectively evaluated the efficacy of RT in spinal instability over a period of 6 months after RT, utilizing the spinal instability neoplastic score (SINS) in patients with painful spinal metastasis. We retrospectively evaluated 108 patients who received RT for painful vertebral metastasis in our institution. Mechanical pain at metastatic vertebrae, radiological responses of irradiated vertebrae, and spinal instability were assessed. Follow-up assessments were done at the start of and at intervals of 1, 2, 3, 4, and 6 months after RT, with the pain disappearing in 67%, 85%, 93%, 97%, and 100% of the patients, respectively. The median SINS were 8, 6, 6, 5, 5, and 4 at the beginning and after 1, 2, 3, 4, and 6 months of RT, respectively. Multivariate analysis revealed that posterolateral involvement of spinal elements (PLISE) was the only risk factor for continuous potentially unstable/unstable spine at 1 month. In conclusion, there was improvement of pain, and recalcification results in regaining spinal stability over time after RT although vertebral body collapse and malalignment occur in some irradiated vertebrae. Clinicians should pay attention to PLISE in predicting continuous potentially unstable/unstable spine.
Keywords: spinal metastases, spinal instability neoplastic score, radiotherapy, posterolateral involvement of spinal elements, risk factor
Introduction
It has been reported that 60%–70% of patients with advanced cancer have spinal metastasis.1,2 Spinal metastases are most common in breast, lung, and prostate cancers. 3 Brihaye et al. reported that symptomatic spine metastases arose in 16.5%, 15.6%, and 9.2% of patients with breast cancer, lung cancer, and prostate cancer, respectively. 4 The number of patients with spinal metastasis is expected to increase as the advancement of cancer treatment improves the survival of patients. As bone metastasis progresses and the vertebral bodies and arches become more destroyed, vertebral body fractures occur.1,2 In addition, the enlarged tumor invades the spinal canal and compresses the spinal cord in an oncological phenomenon called malignant spinal cord compression. As a result, pain and paralysis appear and the patient’s quality of life decreases.5,6 In addition, when performance status decreases, chemotherapy may not be available, which negatively affects the prognosis. The goals of the treatment for patients with spine metastases are palliative, including preservation of neurologic function, maintenance of spinal stability, local tumor control, and improved quality of life. The treatment modalities include radiotherapy (RT), surgery, bone modifying agents (BMAs), thermal ablation techniques, and radiopharmaceuticals.5,6 Recently, minimally invasive spine stabilization using percutaneous pedicle screws and stereotactic radiosurgery have been developed for the management of spinal metastasis.7,8 BMAs include zoledronic acid, a bisphosphonate, and denosumab, a monoclonal antibody directed against the receptor activator of nuclear factor kappa-B ligand. They have been shown to decrease and delay the occurrence of skeletal-related events in metastatic solid tumors and are approved in many countries.9,10 These options are selected as the multidisciplinary approach in consideration of the prognosis and performance status of the patients.
In the treatment of spinal metastasis, it is important to assess spinal instability to choose the most ideal treatment regimen. A review of the literature shows that the predictors of spinal instability include spine location, tumor size, bone quality (BQ), and spinal deformity.11,12 There are some studies which tried to evaluate spinal instability.13,14 However, there are no studies which used validated assessment tools for precise evaluation and classification of spinal instability. In 2010, the Spinal Oncology Study Group formalized the spinal instability neoplastic score (SINS) to assess spinal instability. 15 SINS was developed to help physicians to investigate more precise spinal instability. 15 It has been reported to improve communication within a multidisciplinary team and facilitate appropriate referrals between different medical specialists (oncologists, radiologists, and spine surgeons), which enables to implement prompt and appropriate treatment.16,17 SINS was also proved to be a reliable tool for predicting adverse events related to RT for spinal metastasis.15,18-20 In 2013, the American Academy of Orthopedic Surgeons introduced SINS as a classification system of spinal instability in an instructional course lecture for general practitioners. 21 The SINS assesses spinal instability by a sum of 6 component scores: spine location, pain, BQ, radiographic alignment, vertebral body collapse (VBC), and posterolateral involvement of spinal elements (PLISE). Since its introduction, many authors have reported the usefulness of diagnosing spinal instability and the value of pretreatment SINS to predict pain response, as well as the need for post-RT irradiation.15,18-20 Furthermore, its usefulness in predicting the development of VBC after RT in spinal metastasis has been demonstrated in a meta-analysis and systematic review.22,23
Although percutaneous minimally invasive techniques have developed for spinal metastasis, conservative treatment with RT is the most common treatment for it. It is known that bone formation appears after RT even in lesions that were osteolytic at RT. This reparative process is called re-ossification.24-26 However, no studies have evaluated spinal instability over time after RT. Therefore, we evaluated spinal instability over a period of 6 months after RT, utilizing the SINS in patients with painful spinal metastasis without paralysis. We also examined their risk factors for continuous spinal instability. We hypothesized that stability would be achieved over time by the improvement of pain and bone formation.
Patients and Methods
Inclusion Criteria
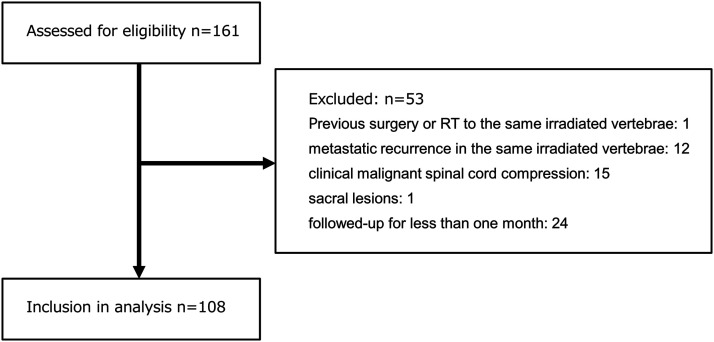
We retrospectively evaluated the file records of patients who received RT for palliation of painful vertebral bone metastasis without paralysis in our institution between July 2012 and June 2016. We excluded patients who had received previous surgery or RT to the same irradiated vertebrae, as well as those who had metastatic recurrence in the same irradiated vertebrae, clinical malignant spinal cord compression, sacral lesions, and those who were followed-up for less than 1 month. Thus, 108 patients (56 men and 52 women) were included in this study (Figure 1). Their median age was 66 years (range, 30-91 years). The primary tumor sites were lung (39), breast (20), prostate (15), colorectum (12), stomach (7), liver (4), pancreas (3), and others (8). The locations were the cervical spine (10), thoracic spine (49), and lumbar spine (49).
Figure 1.
Schematic diagram showing inclusion criteria for the present study. Note. We retrospectively evaluated the file records of patients who received RT for palliation of painful vertebral bone metastasis without paralysis in our institution between July 2012 and June 2016. We excluded patients who had received previous surgery or RT to the same irradiated vertebrae, as well as those who had metastatic recurrence in the same irradiated vertebrae, clinical malignant spinal cord compression, sacral lesions, and those who were followed-up for less than 1 month. Thus, 108 patients were included in this study.
Treatment
Our treatment policy was to perform RT normally in the absence of paralysis and to add surgery in cases of pain or spinal instability that were difficult to control. In addition, if paralysis appeared, decompression would be performed urgently. In this study, the target included patients without paralysis. All patients underwent conventional RT. 27 The choice of RT treatment regimen dose fractionation was determined by the treating radiation oncologist. RT was performed with 6- to 10-MV X-ray of linear accelerators, and the doses of the target volumes were prescribed to be ≥90% of the EBRT dose, in principle. The biologically effective dose (BED) was calculated to compare the various fractionated schedules. The BED10 (BED calculated using an α/β of 10 Gy) was calculated by nd (1 + d/(α/β)), where d is the fraction dose, n is the number of fractions, and α/β is 10 Gy. RT of BED10 (fraction schedules) were as follows: 14.4 Gy (1 × 8 Gy) in 1 patient, 30.0 Gy (=4 × 5 Gy) in 14 patients, 35.1 Gy (=9 × 3 Gy) in 1 patient, 39 Gy (=10 × 3 Gy) in 80 patients, 46.8 Gy (=12 × 3 Gy) in 2 patients, and 56 Gy (=10 × 4 Gy) in 10 patients. Systemic anticancer agents (endocrine therapy, molecular targeted therapy, and cytotoxic chemotherapy) were administered to 70 patients (65%) after RT. All patients were treated conservatively, with bracing in patients with spinal instability.
Pain Assessment
We evaluated the pain at the time of movement (mechanical pain) at metastatic vertebrae using the numerical rating scale (NRS), which is validated and recommended by International Bone Metastases Consensus Working Party guidelines.28,29 This patient-based assessment tool evaluates the pain intensity on a scale of 0 (no pain) to 10 (worst pain). We assessed pain over time, at the beginning of RT and at 1, 2, 3, 4, and 6 months after RT. 30
Radiological Assessment
The status of the vertebral bone was evaluated by computed tomography (CT) (Aquilion, Canon) at 120 kV and a slice thickness of 5 mm. All images were viewed with routine bone window settings (window level 200HU, window width 2000HU) with axial, coronal, and sagittal planes. We evaluated the individual component scores of SINS: spine location, BQ, VBC, radiographic alignment, and PLISE.
We performed radiological evaluations over time, at the beginning of RT and at 1, 2, 3, 4, and 6 months after RT. BQ was classified into 3 categories for the evaluation of SINS; lytic, mixed, or blastic. Radiological responses of irradiated vertebrae with lytic and mixed lesions after RT were assessed as follows; blastic change was defined as the complete fill-in or sclerosis of an initially lytic or mixed lesion and mixed change was defined as the development of a sclerotic rim or partial fill-in or sclerosis of an initially lytic lesion. 31 VBC was defined as a reduction in the vertebral body height compared to the height of the upper and lower vertebral bodies. The degree of collapse was scored as 3 (> 50% collapse), 2 (< 50% collapse), 1 (no collapse with >50% body involved), or 0 (none of the above) based on the SINS criteria. 15
Assessment of Spinal Instability
For assessing spinal instability, we used a reliable and validated tool, SINS. Its usefulness in the diagnosis of spinal instability, the value of predicting pain response, and the need for post-RT irradiation has been reported by many authors. Its excellent intra- and inter-observer reliability for evaluating instability of spinal metastasis was reported in a systematic review and meta-analysis.14-16 The SINS assesses spinal instability by a sum of 6 components scores: 1 clinical component (pain) and 5 radiographic components (spine location, pain, BQ, radiographic alignment, VBC, and PLISE) (Table 1). 15 In this scoring system, the minimum score is 0, and the maximum is 18. The total score is divided in 3 categories of stability: stable (0-6 points), potentially unstable (7-12 points), and unstable (13-18 points). In this study, we divided the total score in 2 categories: stable spine (< 7) and potentially unstable/unstable spine (≥7). SINS was independently evaluated by 2 experienced observers (EN and HK), and consensus was reached by discussion to determine the final score when scores differed.
Table 1.
Spinal Instability Neoplastic Score.
| Score | |
|---|---|
| Location | |
| Junctional (occiput-C2, C7-T2, T11-L1, L5-S1) | 3 |
| Mobile spine (C3-C6, L2-L4) | 2 |
| Semirigid (T3-T10) | 1 |
| Rigid (S2-S5) | 0 |
| Pain a | |
| Yes | 3 |
| Occasional pain but not mechanical | 1 |
| Pain-free lesion | 0 |
| Bone lesion | |
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Subluxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse with >50% body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement of spinal elements b | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
aPain improvement with recumbency and/or pain with movement/loading of spine.
bFacet, pedicle, or costovertebral joint fracture or replacement with tumor.
Statistical Analysis
Overall survival (OS) of patients with stable spine (SINS <7) or potentially unstable/unstable spine (SINS ≥7) at the beginning of RT was evaluated by Kaplan-Meier method.
Clinical data were assessed to evaluate the risk factors for spinal instability at 1 month after RT in patients with potentially unstable/unstable spine (SINS ≥7) at the time of the RT, including the following: age, gender, primary cancer site, radiation site, chemotherapy after RT, BMAs (denosumab or zoledronic acid), RT of BED10, NRS, and some components of the SINS (BQ, radiographic spinal alignment, VBC, and PLISE).
Univariate analysis was performed using chi-square test and multivariate analysis was performed using logistic regression. For all analyses, associations were considered significant if the associated P value was <.05. All statistical analyses were performed with the statistical computing software BellCurve for Excel (Social Survey Research Information Co., Tokyo, Japan). Regression analysis was additionally performed for the continuous variables. The reporting of this study conforms to STROBE guidelines. 32
Results
Tumor Control in the Irradiated Vertebra
Recurrence of the spinal metastases in the same irradiated vertebra occurred in 7 patients (6%). Median time to retreatment was 8 months (range 3-24 months); 3 patients within 6 months and 4 patients after 6 months of RT. The primary tumors were 3 patients with colon cancer, and 1 each with gastric cancer, breast cancer, pancreatic cancer, and Paget disease. Five patients received re-irradiation and 1 patient received laminectomy.
Among patients with recurrence of spinal metastases within 6 months after RT, 1 patient with colorectal cancer achieved no pain at 1 month, but it appeared at 3 months. Although temporary improvement of pain was achieved with re-irradiation, increase of pain was noted with the occurrence of paralysis 5 months after the first RT. In the other patient with colorectal cancer, neurological pain was noted in the femur without pain in the irradiated vertebra at 3 months by tumor regrowth, which stopped temporarily with reirradiation. In the patient with gastric cancer, neurological pain was noted in the right buttock without pain in the irradiated vertebra at 4 months, which disappeared by re-irradiation.
Overall Survival
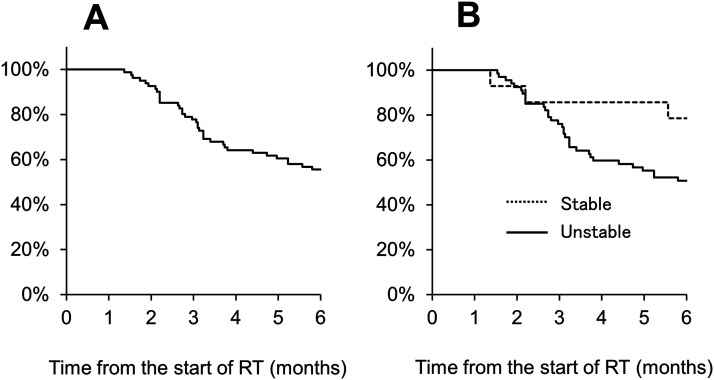
Among the patients, 44 patient had dead during 6 months and 11 patients had lost 1 month after RT due to the difficulty of evaluation by their worse general condition. The number of evaluated patients was 108, 84, 70, 59, and 46 at 1, 2, 3, 4, and 6 months, respectively. At 3 and 6 months after RT, the OS rates were 75% and 57%, respectively (Figure 2(A)). At 3 and 6 months after RT, the OS rates were 89% and 82% in patients with stable spine and 72% and 52% in patients with unstable spine, respectively (Figure 2(B)). There was no association between the OS and SINS (stable spine [< 7] or unstable spine [≥7]) (P = .74).
Figure 2.
Overall survival assessed by using the Kaplan-Meier method. (A) At 3 and 6 months after RT, the OS rates were 78% and 56%. (B) At 3 and 6 months after RT, the OS rates were 86% and 79% in patients with stable spine and 76% and 51% in patients with unstable spine, respectively, which was not significant.
Pain Response
At each of 1 to 6 months, pain disappeared in 72 (67%), 71 (85%), 65 (93%), 57 (97%), and 46 (100%) of patients. Median NRS scores were 5 before RT and 0, 0, 0, 0, and 0 at 1, 2, 3, 4, and 6 months, respectively, representing a significant decrease over time (P < .001). None of the patients had pain which was difficult to control by means of conservative treatment, and none of the patients required surgery.
Radiological Assessment
At the beginning of RT, 42, 46, and 20 patients had lytic, mixed, and blastic lesions, respectively (Table 2). The number of patients with lytic lesion was 36, 18, 4, 3, and 2 at 1, 2, 3, 4, and 6 months, respectively. The number of patients with mixed lesion was 50, 40, 37, 28, and 12 at 1, 2, 3, 4, and 6 months, respectively. The number of patients with blastic lesion was 22, 26, 29, 28, and 32 at 1, 2, 3, 4, and 6 months, respectively. The rates of achievement of blastic change were 0%, 0%, 13%, 22%, and 43% in patients with lytic lesion and 4%, 24%, 36%, 43%, and 76% in patients with mixed lesion at 1, 2, 3, 4, and 6 months, respectively. At the beginning of RT, spinal deformity (kyphosis) was seen in 7 patients (7%). New deformity (kyphosis) occurred in 3 patients (3%) at 1 month after RT. At the beginning of RT, 6, 64, 30 and 8 patients were >50% collapse, <50% collapse, no collapse with >50% body involved, and no collapse with ≤50% body involved, respectively. The new VBC and progression of VBC, which led to the increase in SINS scores, occurred in 5, and 7 patients, respectively after the initiation of RT until a median of 1 month. At the beginning of RT, destruction of posterolateral elements of the spine was seen in 32 patients (30%), unilateral in 26 patients (24%) and bilateral in 6 patients (6%). In 9 patients, it was repaired by re-calcification after RT.
Table 2.
Bone Quality at the Vertebral Bone Metastases.
| Before RT | 1M | 2 M | 3M | 4 M | 6M | |
|---|---|---|---|---|---|---|
| Lytic (n = 42) | Lytic | 36 | 18 | 4 | 3 | 2 |
| Mixed | 6 | 13 | 16 | 13 | 7 | |
| Blastic | 0 | 0 | 3 | 3 | 6 | |
| Mixed (n = 46) | Lytic | 0 | 0 | 0 | 0 | 0 |
| Mixed | 44 | 27 | 21 | 15 | 5 | |
| Blastic | 2 | 9 | 11 | 12 | 16 | |
| Blastic (n = 20) | Blastic | 20 | 17 | 15 | 13 | 10 |
| Total | 108 | 84 | 70 | 59 | 46 | |
Spinal Instability Neoplastic Score
Spinal instability neoplastic score was independently evaluated by 2 experienced observers and consensus was reached by discussion to determine the final score when scores differed. The median SINS was 8 (range, 5-13) at the beginning of RT. The number of patients of stable, potentially unstable, and unstable were 19 (18%), 84 (78%), and 5 (4%), respectively. There were 19 and 89 patients with stable spine (< 7) and unstable spine (≥7), respectively.
The number of patients with stable spine were 56 (52%), 54 (64%), 56 (80%), 49 (83%), and 42 (91%) at 1, 2, 3, 4, and 6 months after RT, respectively (Table 3). Patients with stable spine remained so until the last follow-up. The score of SINS increased in 5 patients (5%) due to the progression of the collapse and/or occurrence of deformity.
Table 3.
The Change of Spinal Instability Neoplastic Score.
| Before RT | 1M | 2 M | 3M | 4 M | 6M | |
|---|---|---|---|---|---|---|
| Stable (n = 14) | Stable | 14 | 11 | 10 | 10 | 8 |
| Medium | 0 | 0 | 0 | 0 | 0 | |
| Unstable | 0 | 0 | 0 | 0 | 0 | |
| Medium (n = 64) | Stable | 25 | 32 | 34 | 30 | 25 |
| Medium | 38 | 23 | 10 | 6 | 2 | |
| Unstable | 1 | 0 | 0 | 0 | 0 | |
| Unstable (n = 3) | Stable | 0 | 0 | 0 | 0 | 0 |
| Medium | 1 | 0 | 2 | 2 | 1 | |
| Unstable | 2 | 2 | 0 | 0 | 0 | |
| Total | 81 | 68 | 56 | 48 | 36 | |
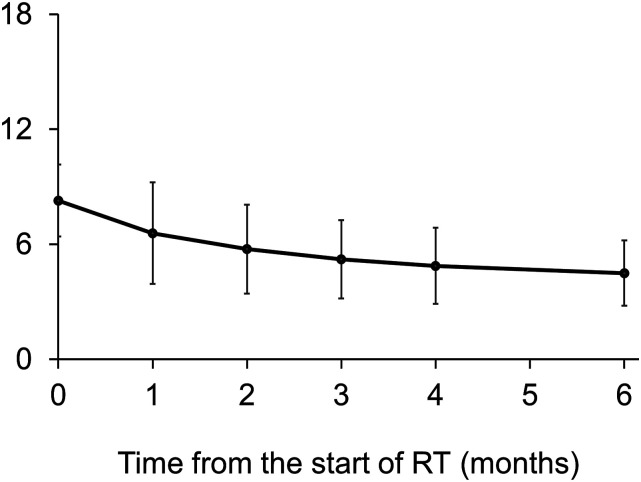
The median SINS were 8, 6, 6, 5, 5, and 4 at the beginning of RT and after 1, 2, 3, 4, and 6 months, respectively. This suggested a significant decrease of SINS after RT compared to that of before RT (P < .001) (Figure 3).
Figure 3.
The median SINS at the beginning and after RT. The median SINS significant decreased over time (P < .001).
Univariate analysis revealed that VBC and PLISE were the risk factors for potentially unstable/unstable spine at 1 month (Table 4). Multivariate analysis revealed that PLISE (relative risk, 3.5; 95% confidence interval, 1.27 to 9.51; P < .05) was the only risk factor for potentially unstable/unstable spine at 1 month. At 1 month, spinal instability was seen in 78% and 47% of the patients with and without PLISE, respectively, and this was significant (P < .01). Regression analysis was conducted on “age,”" RT of BED10,” and “NRS,” resulting in coefficients of determination (R2) of .00045, .0064, and .039, respectively.
Table 4.
Risk Factors for Spinal Instability at 1 Month After RT.
| Covariates | Patients, No. | P-Value | |
|---|---|---|---|
| Patients without Instability (n = 37) | Patients with Instability (n = 52) | ||
| Age, years | |||
| <65 | 14 | 23 | |
| ≥65 | 23 | 29 | .66 |
| Gender | |||
| Male | 15 | 30 | |
| Female | 22 | 22 | .13 |
| Primary cancer site | |||
| Lung | 13 | 23 | |
| Others | 24 | 29 | .51 |
| Radiation site | |||
| Cervical spine | 3 | 7 | |
| Thoracic spine | 13 | 23 | |
| Lumbar spine | 21 | 22 | .36 |
| Chemotherapy after RT | |||
| Yes | 24 | 31 | |
| No | 13 | 21 | .66 |
| Bone modifying agent | |||
| Yes | 33 | 48 | |
| No | 4 | 4 | .71 |
| RT dose (BED10) | |||
| <39 | 11 | 3 | |
| ≥39 | 26 | 49 | .003 |
| NRS | |||
| <4 | 18 | 20 | |
| ≥4 | 19 | 32 | .39 |
| Bone quality | |||
| Lytic | 14 | 27 | |
| Mixed or blastic | 23 | 25 | .20 |
| Radiographic spinal alignment | |||
| Normal alignment | 36 | 46 | |
| De novo deformity or subluxation/translation present | 1 | 6 | .23 |
| Vertebral body collapse | |||
| No collapse | 15 | 10 | |
| Collapse | 22 | 42 | .03 |
| Posterolateral involvement of spinal elements | |||
| Bilateral/Unilateral | 7 | 25 | |
| No involvement | 30 | 27 | .006 |
Discussion
In this study, we first showed the over-time improvement of spinal instability after RT, utilizing SINS. Precise assessment of spinal instability is critical at the start of RT to evaluate the effectiveness of RT as well as for deciding which patients require surgical intervention.5,6 There are several tools for evaluating the spinal instability.5,6 However, none of these tools have been completely validated or widely used in a clinical setting. Several studies utilizing Taneichi score reported that among patients who were classified unstable prior to RT, 17%–19% and 24%–32% of patients were classified as stable at 3 and 6 months after RT, respectively.33,34 However, this tool is limited to be utilized for lytic thoracolumbar lesion. In this study, we decided to utilize SINS to evaluate over-time instability after RT.
We first used SINS as an assessment tool for spinal instability in the course of RT. Although only 18% of patients were diagnosed with stable spines, its rate gradually increased and 80% of the patients were diagnosed with stable spine at 3 months after RT and the SINS increased in only 4% of the patients.
SINS was determined by the over-time antagonism between the improving factors (pain, BQ, and PLISE) and the exacerbating factors (radiographic alignment and VBC). We found that the value of the SINS decreased in many cases due to the disappearance of pain and bone formation of the vertebral body, even if vertebral body collapse or misalignment occurs during the course. Mechanical pain at the metastatic vertebrae can be a good indicator of spinal instability. Previous studies showed that pain decreased in 71%–75% of patients at 3 months after RT.35,36 In this study, pain disappeared in 93% of patients at 3 months after RT. In evaluating BQ, a lytic lesion means there is a lack of mineralization, which is a strong indicator of developing VBC. However, sclerotic changes appear in irradiated vertebrae when RT has been effective.24-26 In this study, over time CT showed that re-ossification was gradually obtained in the irradiated vertebrae; the rates of achievement of blastic changes were 13% and 36% in patients with lytic and mixed lesion at 3 months, respectively. Multivariate analysis revealed that PLISE was the only risk factor for continuous potentially unstable/unstable spine at 1 month. PLISE consists of the facet, pedicle, and costovertebral joint and plays an important role in the stability of the vertebral bone.11,12 Taneichi et al. reported that the risk factors for vertebral body fractures were costovertebral joint destruction in the thoracic region (T1-T10) and pedicle destruction in the thoracolumbar and lumbar region (T10-L5) in patients with lytic vertebral metastasis. 32 In this study, destruction of posterolateral elements of the spine was seen in 30% of patients, which would lead to the spinal instability. However, in 1 third of the patients, this was repaired by re-calcification after RT. Therefore, clinicians should pay attention to not only BQ and VBC but also PLISE.
It should be noted that exacerbating factors (radiographic alignment and VBC) worsened the SINS over time after RT. Radiographic spinal alignment is 1 of the most important parameters indicating spinal instability.11,12 The subluxation or translation had a score of 4, the highest score among SINS. In this study, kyphosis developed in a total of 10% (7% at the beginning of RT and 3% at 1 month after RT) of the patients. Another worsening factor, VBC had already developed in 65% of patients at the start of RT and it was seen in an additional 5% of patients until a median of 1 month. These 2 factors never improve by means of conservative treatment. As a result of combining these 2 contradictory factors, SINS led to significant improvement over time by disappearance of pain and re-calcification, though VBC and malalignment progressed in some patients. Therefore, SINS is also useful for assessing spinal instability over time.
There are several limitations in this study. Since this study is a retrospective study, no comparison was made with surgical cases. In this study, 5 patients had spinal instability (SINS≥13), though they need no surgery. However, this can be because of the short prognosis of patients with bone metastasis. Although percutaneous minimally invasive techniques have developed for spinal metastasis, their indication had not been established. Then, it is impossible to discuss which cases can be treated conservatively and which cases require surgery. In the future, we think it is necessary to conduct a randomized study to compare the treatment outcome of surgery and conservative treatment to establish the standard treatment for spinal instability (SINS≥13). Another limitation was patients’ loss of follow-up. This is also reported in several randomized studies evaluating the utility of RT for bone metastasis. Hartsell reported that in a phase III randomized study of comparing the effect of palliative RT with 8 Gy/1 fraction and 30 Gy/10 fractions, 32% of patients were lost or died before 3 months. 34 Howell reported that in a randomized study of comparing the effect of palliative RT with 8 Gy/1 fraction and 30 Gy/10 fractions, 35% of patients were lost at 3 months. 35 This is also common in the study for patients with bone metastasis, given their relatively short survival. As the other limitation of the study, we did not evaluate the bone mineral density of the enrolled patients before and after the treatment, the Body Mass Index, the blood levels of vitamin D, parathyroid hormone, and calcium. Furthermore, we do not routinely perform MRI. Therefore, it is not possible to examine the association of the cancer types (breast cancer, lung cancer, and prostate cancer) and the nature of their bone metastasis.
Conclusions
In conclusion, although VBC and malalignment occur in some irradiated vertebrae, improvement of pain and re-calcification results in regaining spinal stability over time after RT. PLISE was proven to be the risk factor for continuous spinal instability. Therefore, clinicians should pay attention to not only BQ and VBC but also PLISE.
Acknowledgments
We are grateful to all patients for the study.
Appendix.
Abbreviations
- BMAs
Bone modifying agents
- BQ
Bone quality
- CT
Computed tomography
- NRS
Numerical rating scale
- OS
Overall survival
- PLISE
Posterolateral involvement of spinal elements
- RT
Radiotherapy
- SINS
Spinal instability neoplastic score
- VBC
Vertebral body collapse
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by JSPS KAKENHI (grant no. 22K09401, 23K08678, and 23K08698).
Ethical Statement
Ethical Approval
This study was approved by the Ethics Review Board of Shikoku Cancer Center (location of the review board; Ko-160, Minamiumemoto-cho, Matsuyama city, approval number; 2017-26, approval day; September 6, 2017) and conducted in accordance with the World Medical Association Declaration of Helsinki.
Informed Consent
As this study is a retrospective study, patient consent was obtained in an opt-out consent method.
ORCID iD
Eiji Nakata https://orcid.org/0000-0002-5971-4142
References
- 1.Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010;13:94-108. [DOI] [PubMed] [Google Scholar]
- 2.Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer. 2010;46:2696-2707. [DOI] [PubMed] [Google Scholar]
- 3.Gouveia AG, Chan DCW, Hoskin PJ, et al. Advances in radiotherapy in bone metastases in the context of new target therapies and ablative alternatives: a critical review. Radiother Oncol. 2021;29:55-67. [DOI] [PubMed] [Google Scholar]
- 4.Porras JL, Pennington Z, Hung B, et al. Radiotherapy and surgical advances in the treatment of metastatic spine tumors: a narrative review. World Neurosurg. 2021;151:147-154. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson MB, Glaenzer B, Malamis A. Percutaneous minimally invasive techniques in the treatment of spinal metastases. Curr Treat Options Oncol. 2016;17:56. [DOI] [PubMed] [Google Scholar]
- 6.Soltys SG, Grimm J, Milano MT, et al. Stereotactic body radiation therapy for spinal metastases: tumor control probability analyses and recommended reporting standards. Int J Radiat Oncol Biol Phys. 2021;110:112-123. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro CL. Bone-modifying agents (BMAs) in breast cancer. Clin Breast Cancer. 2021;21:e618-e630. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Zhou L, Liu X, Wen X, Li H, Li W. Meta-analysis of clinical trials to assess denosumab over zoledronic acid in bone metastasis. Int J Clin Pharm. 2021;43:2-10. [DOI] [PubMed] [Google Scholar]
- 9.Leone A, Cianfoni A, Zecchi V, Cortese MC, Rumi N, Colosimo C. Instability and impending instability in patients with vertebral metastatic disease. Skeletal Radiol. 2019;48:195-207. [DOI] [PubMed] [Google Scholar]
- 10.Weber MH, Burch S, Buckley J, et al. Instability and impending instability of the thoracolumbar spine in patients with spinal metastases: a systematic review. Int J Oncol. 2011;38:5-12. [PubMed] [Google Scholar]
- 11.Sprave T, Hees K, Bruckner T, et al. The influence of fractionated radiotherapy on the stability of spinal bone metastases: a retrospective analysis from 1047 cases. Radiat Oncol. 2018;13:134.E.l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foerster R, Habermehl D, Bruckner T, et al. Spinal bone metastases in gynecologic malignancies: a retrospective analysis of stability, prognostic factors and survival. Radiat Oncol. 2014;9:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35:1221-1229. [DOI] [PubMed] [Google Scholar]
- 14.Versteeg AL, van der Velden JM, Verkooijen HM, et al. The effect of introducing the spinal instability neoplastic score in routine clinical practice for patients with spinal metastases. Oncol. 2016;21:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versteeg AL, Verlaan JJ, Sahgal A, et al. The spinal instability neoplastic score: impact on oncologic decision-making. Spine. 2016;41:S231-S237. [DOI] [PubMed] [Google Scholar]
- 16.Shi DD, Hertan LM, Lam TC, et al. Assessing the utility of the spinal instability neoplastic score (SINS) to predict fracture after conventional radiation therapy (RT) for spinal metastases. Pract Radiat Oncol. 2018;8:e285-e294. [DOI] [PubMed] [Google Scholar]
- 17.van der Velden JM, Versteeg AL, Verkooijen HM, et al. Prospective evaluation of the relationship between mechanical stability and response to palliative radiotherapy for symptomatic spinal metastases. Oncol. 2017;22:972-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallizia E, Apicella G, Cena T, Di Genesio PM, Deantonio L, Krengli M. The spine instability neoplastic score (SINS) in the assessment of response to radiotherapy for bone metastases. Clin Transl Oncol. 2017;19:1382-1387. [DOI] [PubMed] [Google Scholar]
- 19.Quinn RH, Randall RL, Benevenia J, Berven SH, Raskin KA. Contemporary management of metastatic bone disease: tips and tools of the trade for general practitioners. J Bone Joint Surg Am. 2013;95:1887-1895. [DOI] [PubMed] [Google Scholar]
- 20.Kim YR, Lee CH, Yang SH, et al. Accuracy and precision of the spinal instability neoplastic score (SINS) for predicting vertebral compression fractures after radiotherapy in spinal metastases: a meta-analysis. Sci Rep. 2021;11:5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CH, Hong JT, Lee SH, et al. Korean spine Oncology Research society. Is the spinal instability neoplastic score accurate and reliable in predicting vertebral compression fractures for spinal metastasis? A systematic review and qualitative analysis. J Korean Neurosurg Soc. 2021;64:4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamaoka T, Costelloe CM, Madewell JE, et al. Tumour response interpretation with new tumour response criteria vs the World Health Organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer. 2010;102:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soliman M, Taunk NK, Simons RE, et al. Anatomic and functional imaging in the diagnosis of spine metastases and response assessment after spine radiosurgery. Neurosurg Focus. 2017;42:E5. [DOI] [PubMed] [Google Scholar]
- 24.Kouloulias V, Liakouli Z, Zygogianni A, Mystakidou K, Kouvaris JR. Bone density as a marker of response to radiotherapy in bone metastatic lesions: a review of the published data. Int J Mol Sci. 2016;17:1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennington Z, Ahmed AK, Cottrill E, Westbroek EM, Goodwin ML, Sciubba DM. Intra- and interobserver reliability of the Spinal Instability Neoplastic Score system for instability in spine metastases: a systematic review and meta-analysis. Ann Transl Med. 2019;7:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rief H, Bischof M, Bruckner T, et al. The stability of osseous metastases of the spine in lung cancer--a retrospective analysis of 338 cases. Radiat Oncol. 2013;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makita K, Hamamoto Y, Kanzaki H, et al. Local control of bone metastases treated with external beam radiotherapy in recent years: a multicenter retrospective study. Radiat Oncol. 2021;16:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow E, Hoskin P, Mitera G, et al. International bone metastases consensus working party. Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82:1730-1737. [DOI] [PubMed] [Google Scholar]
- 29.Fallon M, Hoskin PJ, Colvin LA, et al. Randomized double-blind trial of pregabalin versus placebo in conjunction with palliative radiotherapy for cancer-induced bone pain. J Clin Oncol. 2016;20(34):550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakata E, Sugihara S, Kataoka M, et al. Early response assessment of palliative conventional radiotherapy for painful uncomplicated vertebral bone metastases. J Orthop Sci. 2018;23:912-917. [DOI] [PubMed] [Google Scholar]
- 31.Nakata E, Sugihara S, Kataoka M, et al. Early response assessment of re-ossification after palliative conventional radiotherapy for vertebral bone metastases. J Orthop Sci. 2019;24:332-336. [DOI] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 33.Taneichi H, Kaneda K, Takeda N, Abumi K, Satoh S. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine. 1997;22:239-245. [DOI] [PubMed] [Google Scholar]
- 34.Westhoff PG, de Graeff A, Monninkhof EM, Dutch Bone Metastasis Study Group , et al. Quality of life in relation to pain response to radiation therapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 2015;93:694-701. [DOI] [PubMed] [Google Scholar]
- 35.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798-804. [DOI] [PubMed] [Google Scholar]
- 36.Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119:888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]