ABSTRACT
Autophagy, an important cellular stress response mechanism, is often exploited by a variety of cancer cells to sustain rapid growth under stresses such as nutrient deprivation and hypoxia. Autophagy also plays a key role in tumor resistance to chemotherapy, radiotherapy or targeted therapy. Inhibition of autophagy is therefore a promising tumor treatment strategy. However, there is still a lack of effective autophagy inhibitors suitable for clinical use. Most drug development has focused on enzymes like the VPS34 and ULK1 kinases, or the cysteine protease ATG4B, which plays different roles in autophagy. We discovered a drug molecule Eltrombopag that inhibits the expression of autophagic lysosomal genes at the stage of transcriptional level, where the synthesis of these proteins has not really begun, by directly inhibiting the TFEB (transcription factor EB). This drug can improve the therapeutic effect of Temozolomide on glioblastoma treatment, further confirming the value of inhibiting autophagy in the treatment of cancer.
Abbreviation: VPS34: vacuolar protein sorting 34; ULK1: unc-51 like autophagy activating kinase 1; TFEB: transcription factor EB; MITF: microphthalmia-associated transcription factor; TFE3: transcription factor E3; EO: Eltrombopag; ITC: isothermal titration calorimetry; bHLH-LZ: basic helix-loop-helix leucine zipper; LAMP1: lysosomal-associated membrane protein 1; CTSF: cathepsin F; HEXA: hexosaminidase subunit alpha.
KEYWORDS: Autophagy inhibition, Eltrombopag, glioblastoma, the transcription factor EB (TFEB), cancer therapy
TFEB (transcription factor EB) is a central transcriptional regulator of autophagic responses. By binding to a palindromic GTCACGTGAC motif present in the promoter region of most known lysosomal genes, also known as CLEAR element, TFEB up-regulates the transcriptional expression of genes involved in lysosomal biogenesis and function. With its systematic and upstream roles in autophagy regulation, TFEB is an ideal drug target for autophagy modulation. Indeed, trehalose, a low molecular disaccharide, appears to induce TFEB dephosphorylation and nuclear translocation, which in turn leads to autophagy activation. An international multicenter Phase III trial has been initiated to evaluate the safety and efficacy of trehalose in treating adults with spinocerebellar ataxia type 3. Activation of TFEB is an emerging therapeutic strategy to treat a number of lysosome storage disorders. However, little is known about how to directly and efficiently inhibit TFEB by small molecules that may further improve the efficacy of multiple cancer therapies.
Most transcription factors lack well-defined pockets allowing small-molecule binding that could be used for drug design. Therefore, these transcription factors have traditionally been considered “undruggable”. Our laboratory has been studying the MiT/TFE transcription factor family, which include TFEB, MITF (microphthalmia-associated transcription factor) and TFE3 (transcription factor E3). In collaboration with the Fisher’s, Chen’s and Guo’s groups, we discovered a small symmetric molecule, TT-012, which disrupts the MITF dimer formation and potently inhibits MITF activity providing a potential strategy for melanoma treatment. In our recently published work, in collaboration with the Feng’s group, we reported a direct and potent TFEB small molecule inhibitor [1].
Through a fluorescence anisotropy-based screening assay that monitoring the interaction between the TFEB and the fluorophore labeled CLEAR DNA, we identified an FDA-approved small-molecule drug, Eltrombopag (EO), which efficiently inhibits TFEB-CLEAR DNA interaction both in vitro and in cells (Figure 1). Due to the limited solubility of EO, conventional methods such as ITC (isothermal titration calorimetry) are not compatible to quantify the interaction between EO and TFEB. Thus, we conjugated EO with biotin (EO-Biotin). EO-Biotin maintained the TFEB-CLEAR DNA disrupting ability and by immobilizing it on a chip via the biotin tag, we found that using plasmon resonance the bHLH-LZ (basic helix-loop-helix leucine zipper) domain of TFEB interacts with EO with a Kd of 345.7 nM.
Figure 1.
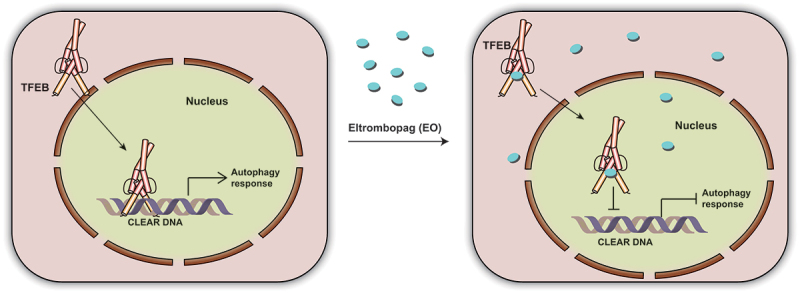
A schematic cartoon showing that EO binds to TFEB to interfere CLEAR DNA recognition and inhibits the autophagic response.
Mechanistic studies suggest that EO affects TFEB recognition of DNA by binding to the bHLH-LZ domain of TFEB, especially at the bottom surface of the HLH domain to hinder DNA recognition. A basic residue on the flexible loop region of TFEB, R271, which is conserved in multiple b-HLH-LZ and b-HLH transcription factors, was shown to be significantly involved in the interaction with EO. Whether this region represents a potential drug design pocket for this transcription factor family, however, will require further investigation. EO inhibits the expression of TFEB downstream genes by directly preventing TFEB from binding to DNA, but not by altering its phosphorylation level and subcellular localization. EO selectively inhibits the transcriptional activity of TFEB at the genomic scale and efficiently suppresses autophagy. Importantly, EO increases the sensitivity of glioblastoma to Temozolomide treatment in vitro and in vivo. In particular, EO decreased the protein levels of the TFEB target genes LAMP1, CTSF and HEXA, and inhibited autophagy levels in tumors from a glioblastoma xenograft mouse model. In combination with Temozolomide, EO reduced the tumor proliferation rate and prolonged the survival time of glioblastoma xenografted mice, indicating that EO could be used as an effective autophagy inhibitor to enhance the effects of chemotherapy in glioblastomas.
As an FDA-approved drug, EO has long been used to treat chronic immune thrombocytopenia. The proven safety of EO is a significant advantage that strongly facilitates its clinical application as a novel autophagy inhibitor. The combination of EO with various targeted or chemotherapeutic agents may provide new therapeutic option for the treatment of multiple types of tumors.
Overall, our findings highlight that the central transcriptional regulator of autophagy TFEB is a druggable target for the development of autophagy inhibitors. By targeting TFEB, autophagy can be inhibited systematically at the transcriptional level, providing a new therapeutic modality. Thus, EO is a drug with great clinical potential to treat a wide range of cancers.
Funding Statement
The work was supported by the National Natural Science Foundation of China [22277134, 21977108, 21977107, 22277132, 21621002]
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- [1].Lin Y, Shi Q, Yang G, et al. A small-molecule drug inhibits autophagy gene expression through the central regulator TFEB. Proc Natl Acad Sci USA. 2023;120:e2213670120. [DOI] [PMC free article] [PubMed] [Google Scholar]


