Abstract
Background
The unprecedented emergence of the COVID-19 pandemic necessitated the development and global distribution of vaccines, making the understanding of global vaccine acceptance and hesitancy crucial to overcoming barriers to vaccination and achieving widespread immunization.
Objective
This umbrella review synthesizes findings from systematic reviews and meta-analyses to provide insights into global perceptions on COVID-19 vaccine acceptance and hesitancy across diverse populations and regions.
Methods
We conducted a literature search across major databases to identify systematic reviews and meta-analysis that reported COVID-19 vaccine acceptance and hesitancy. The AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews) criteria were used to assess the methodological quality of included systematic reviews. Meta-analysis was performed using STATA 17 with a random effect model. The data synthesis is presented in a table format and via a narrative.
Results
Our inclusion criteria were met by 78 meta-analyses published between 2021 and 2023. Our analysis revealed a moderate vaccine acceptance rate of 63% (95% CI 0.60%-0.67%) in the general population, with significant heterogeneity (I2 = 97.59%). Higher acceptance rates were observed among health care workers and individuals with chronic diseases, at 64% (95% CI 0.57%-0.71%) and 69% (95% CI 0.61%-0.76%), respectively. However, lower acceptance was noted among pregnant women, at 48% (95% CI 0.42%-0.53%), and parents consenting for their children, at 61.29% (95% CI 0.56%-0.67%). The pooled vaccine hesitancy rate was 32% (95% CI 0.25%-0.39%) in the general population. The quality assessment revealed 19 high-quality, 38 moderate-quality, 15 low-quality, and 6 critically low-quality meta-analyses.
Conclusions
This review revealed the presence of vaccine hesitancy globally, emphasizing the necessity for population-specific, culturally sensitive interventions and clear, credible information dissemination to foster vaccine acceptance. The observed disparities accentuate the need for continuous research to understand evolving vaccine perceptions and to address the unique concerns and needs of diverse populations, thereby aiding in the formulation of effective and inclusive vaccination strategies.
Trial Registration
PROSPERO CRD42023468363; https://tinyurl.com/2p9kv9cr
Keywords: COVID-19, vaccine acceptance, vaccine hesitancy, umbrella review, systematic review, meta-analysis, vaccine, hesitancy, global perceptions, perception, random effect model, synthesis, healthcare workers, patients, patient, chronic disease, pregnant women, parents, child, children
Introduction
The global health landscape has been profoundly altered by the emergence of COVID-19, triggered by SARS-CoV-2. First identified in Wuhan, China, in December 2019, this virulent pathogen swiftly traversed continents, leading the World Health Organization (WHO) to categorize the situation as both a pandemic and public health emergency of international concern. The repercussions of this pandemic have been multifaceted, with a staggering death toll and profound impact on socioeconomic structures worldwide [1]. In the face of this unprecedented challenge, the global community recognized the pressing need for effective countermeasures. Although therapeutic interventions were explored, the primary focus shifted to preventive strategies, with vaccines against COVID-19 emerging as the most promising solution [2]. The efficacy of this approach, however, is contingent not just on the scientific success of vaccine development but equally on the global populace's acceptance of these vaccines [3].
By the midpoint of 2022, the scientific community had successfully developed, trialed, and secured emergency use authorization for several vaccines [4]. However, the distribution of these vaccines unveiled pronounced disparities [5]. Higher-income nations, with their robust health care infrastructures and financial resources, rapidly initiated vaccination drives. In stark contrast, many resource-limited countries faced challenges ranging from limited vaccine access to infrastructural constraints [6]. A more insidious challenge that emerged globally, irrespective of a country's economic status, was vaccine hesitancy. Rooted in a complex interplay of factors, including safety apprehensions, distrust toward health advisories, cultural nuances, and the deluge of misinformation, vaccine hesitancy has been observed across diverse geographies, from Africa and Europe to North America [3].
Empirical studies conducted across various regions have painted a mixed picture of vaccine acceptance [3,7]. Although certain demographics exhibited a commendable eagerness to embrace vaccination, others displayed pronounced skepticism [3,8]. These disparities in vaccine acceptance, if unchecked, have the potential to impede global strides toward achieving herd immunity, a critical milestone in the fight against the pandemic. Recognizing the pivotal role of vaccine acceptance in the trajectory of the pandemic, it becomes imperative to understand the nuances of global vaccine perceptions. Numerous systematic reviews and meta-analyses have been published, shedding light on factors that are driving vaccine hesitancy and acceptance [8-17].
In this context, our umbrella review sought to collate and synthesize findings from these diverse studies, aiming to present a holistic understanding of global COVID-19 vaccine acceptance rates and hesitancy rates. This approach offers a comprehensive overview of existing evidence, highlighting consistencies and discrepancies across studies. By assessing the quality and breadth of current research, umbrella reviews identify knowledge gaps and inform evidence-based decision-making. They serve as a valuable tool for policymakers, clinicians, and researchers, providing a holistic understanding of a topic without the need for sifting through numerous individual studies. Through this study, we aspired to provide valuable insights that can steer future vaccination strategies, ensuring they are both effective and inclusive. Our umbrella review aimed to collate and synthesize findings from these diverse studies to present a comprehensive understanding of global COVID-19 vaccine acceptance and hesitancy rates.
Methods
The method for conducting this umbrella review was based on the framework set forth by the Joanna Briggs Institute [18]. This study adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline [19] (Multimedia Appendix 1). This study was registered in PROSPERO.
Inclusion Criteria
We specifically targeted meta-analyses of epidemiological studies that investigated either the acceptance/willingness or hesitancy toward the COVID-19 vaccine. Our scope was global, encompassing studies from all geographical locations without any specific focus on a particular population. This inclusivity ensured that our review captured a diverse range of perspectives and settings. However, to maintain the rigor and specificity of our review, we excluded certain types of publications. Specifically, narrative or systematic reviews that did not include a meta-analysis, conference abstracts, and letters to the editors were not considered. In essence, our inclusion criteria were centered on meta-analyses of prospective, retrospective, or cross-sectional studies that evaluated rates of vaccine acceptance or hesitancy (Table S1 in Multimedia Appendix 2).
Literature Search
We conducted a comprehensive literature search across 4 major databases: PubMed, Scopus, Embase, and the Cochrane Database of Systematic Reviews. The search spanned from the inception of each database until August 20, 2023. To ensure a thorough retrieval of relevant articles, “keyword search” and “textword search” were used, and different search phrases were combined using Boolean and proximity operators. Specifically, we used the terms (“meta-analysis” OR “systematic review”) AND (Acceptance OR willingness OR hesitancy OR intention OR unwillingness) AND (“COVID-19” OR “Sars-cov-2” OR “corona*”). To further enhance the robustness of our search, we also manually screened the reference lists of the identified articles. This step ensured that we did not overlook any pertinent studies that might not have been captured through the database search. For transparency and replicability, the complete search strategy, including all terms and combinations used, is documented in Table S2 in Multimedia Appendix 2. Importantly, we did not impose any filters or restrictions during our search. This means that articles of any type, published in any year, and in any language were considered, ensuring a broad and inclusive search scope.
Screening
The screening process for this systematic review was conducted by 2 independent authors, structured into 2 sequential steps to ensure unbiased selection and comprehensive coverage of relevant studies. The first step, primary screening, involved scrutinizing the titles and abstracts of identified articles to shortlist those potentially relevant to topic. Subsequently, in the second step, articles that passed the primary screening were subjected to a thorough full-text review. During this stage, the authors carefully evaluated the complete content of each article, focusing on the removal of duplicates and a more detailed assessment of each study's relevance and alignment with the review's scope and objectives. To enhance the precision and efficiency of our screening process, we used the specialized software Nested Knowledge with its AutoLit function, instrumental in streamlining our workflow and improving the accuracy of article selection. In cases of disagreement or uncertainty between the 2 reviewers, a third reviewer with senior expertise was engaged to mediate and provide decisive judgment, ensuring a consensus-based approach to the final selection of studies.
Data Extraction
During the data extraction process, 2 independent authors systematically reviewed each study that met our inclusion criteria. From each eligible meta-analysis, they gathered a comprehensive set of details. This included the first author's name, year of publication, type of study design, total number of participants, type of population, and the date when the database search was conducted. Additionally, they extracted the pooled acceptance rate for each subgroup and specific number of participants within these subgroups, accompanied by their 95% CIs. Furthermore, they documented the P values for pooled effects, the results from the Egger or Beggs test (which measures publication bias), and the I2 statistics, which offer insights into the heterogeneity of the studies. Any associated P value for significance was also recorded. Given the complexity of the data and the importance of accuracy, any discrepancies or disagreements that arose between the 2 primary authors were diligently addressed. They consulted a third, senior reviewer to ensure consistency and precision in the data extraction process.
Assessment of Methodological Quality
To ensure the rigor and reliability of our review, the methodological quality of each included meta-analysis was meticulously evaluated. This assessment was jointly undertaken by 2 authors using the well-established AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews) tool [20], which is recognized for its robustness in appraising systematic reviews. Based on the criteria set by AMSTAR-2, studies were categorized into 1 of 4 methodological quality grades: high, moderate, low, or critically low. A study was deemed to be of high quality if it exhibited no flaws or only a single minor defect. In contrast, a moderate quality designation was given to studies that presented multiple minor defects. The distinction between minor and major defects was made based on the guidelines provided by the AMSTAR-2 tool. Any disagreements or uncertainties regarding the quality grading were discussed and resolved collaboratively between the 2 authors to ensure a consistent and objective assessment.
Data Analysis
Data analysis was conducted using STATA version 17. Proportions, along with their 95% CIs, were pooled from all included eligible meta-analyses for each outcome and based on the population [21]. We used a random effects model to compute the combined effect sizes, recognizing the inherent variability among the studies and providing a more conservative estimate of the overall effect. The degree of variability or heterogeneity in outcomes across studies was quantified using the I2 metric. Values for I2 can range from 0% to 100%, with higher values indicating greater heterogeneity. We predetermined specific thresholds to assess the statistical significance of the observed heterogeneity. The 95% prediction interval provides a more comprehensive understanding of the range in which the true effect size lies, considering the observed heterogeneity. A P value <0.05 was considered statistically significant.
Results
Search Results
We identified a total of 662 articles through the primary search, of which 263 duplicates were eliminated, leaving 399 for title and abstract screening. In the primary screening (title and abstract), 214 articles were excluded, leaving 185 articles for full-text screening (Figure 1). We excluded 108 articles for various reasons, including only systematic reviews without a meta-analysis and the incorrect population, outcomes, or study design. As a result, 77 articles fulfilled the eligibility criteria. Additionally, we conducted a citation search to maintain the rigor of the review and found 4 relevant articles, of which 1 was included. This umbrella review ultimately identified 78 meta-analyses that met the inclusion criteria.
Figure 1.
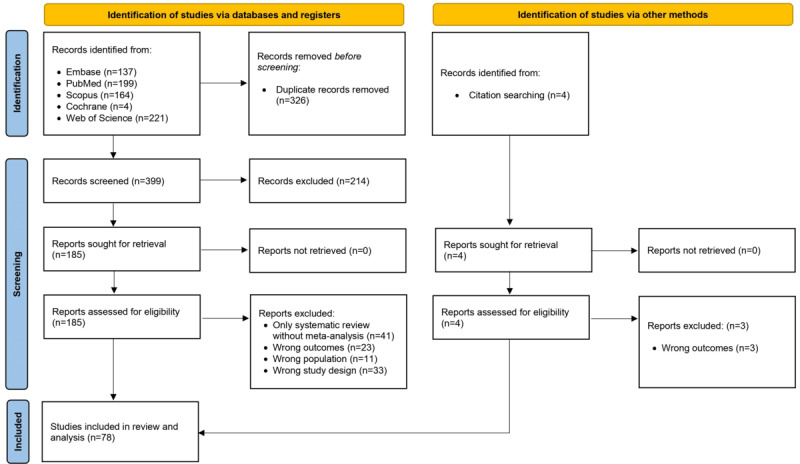
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram illustrating the screening and selection process.
Characteristics of Meta-Analyses and Quality Assessment
Table S3 in Multimedia Appendix 2 [9-17,22-90] presents an overview of all included meta-analyses published between 2021 and 2023. These studies looked at different groups, such as the general population; health care workers; parents; pregnant women; migrant workers; Black or African communities; Chinese communities; and people with specific health conditions such as cancer, HIV, diabetes, inflammatory bowel disease, and epilepsy. The focus of these meta-analyses was on outcomes such as the rates of vaccine acceptance, hesitance, willingness, uncertainty, and unwillingness, as well as the intention to receive vaccines. Most of the meta-analyses included articles from around the world, while some concentrated on specific countries.
The quality of these meta-analyses was evaluated using the AMSTAR-2 criteria. Among the meta-analyses examined, 19 meta-analyses were rated as being of high quality; 38 meta-analyses received a moderate quality rating; 15 meta-analyses received a low quality rating, which points to potential limitations in their methodology; and 6 meta-analyses were classified as having critically low quality, implying significant concerns about their methods and the reliability of their findings (Table S4 in Multimedia Appendix 2 [9-17,22-90]).
Vaccine Acceptance and Hesitancy Among Populations
Table 1 provides a summary of vaccine acceptance and hesitancy rates among different populations. Acceptance rates were studied in 58 systematic reviews and meta-analyses, and 12 distinct reviews reported on hesitancy rates (Table 2).
Table 1.
Summary of vaccine acceptance rates across different populations.
| Studies by population | Acceptance rate, % (95% CI) | Heterogeneity (I2), % | Overall acceptance rate, % (95% CI) | ||||||
| General population | 97.59 | 0.63 (0.60-0.67) | |||||||
|
|
Wang et al, 2021 [22] | 0.74 (0.71-0.77) |
|
|
|||||
|
|
Alimohamadi et al, 2022 [10] | 0.67 (0.62-0.74) |
|
|
|||||
|
|
Abdelmoneim et al, 2022 [23] | 0.81 (0.75-0.85) |
|
|
|||||
|
|
Nehal et al, 2021 [15] | 0.66 (0.6-0.7) |
|
|
|||||
|
|
Khabour, 2022 [24] | 0.39 (0.33-0.46) |
|
|
|||||
|
|
Sahile et al, 2022 [25] | 0.57 (0.47-0.67) |
|
|
|||||
|
|
Norhayati et al, 2021 [12] | 0.61 (0.59-0.64) |
|
|
|||||
|
|
Wake, 2021 [26] | 0.48 (0.39-0.58) |
|
|
|||||
|
|
Alarcón-Braga et al, 2022 [27] | 0.78 (0.74-0.82) |
|
|
|||||
|
|
Mekonnen and Mengistu, 2022 [28] | 0.56 (0.47-0.64) |
|
|
|||||
|
|
Mengistu et al, 2022 [29] | 0.64 (0.6-0.69) |
|
|
|||||
|
|
Gudayu and Mengistie, 2023 [30] | 0.68 (0.67-0.68) |
|
|
|||||
|
|
Kumar et al, 2023 [16] | 0.62 (0.55-0.69) |
|
|
|||||
|
|
Alemayehu et al, 2022 [31] | 0.60 (0.52-0.67) |
|
|
|||||
|
|
Kawuki et al, 2023 [32] | 0.58 (0.49-0.67) |
|
|
|||||
|
|
Wang et al, 2021 [22] | 0.67 (0.67-0.68) |
|
|
|||||
|
|
Belay et al, 2022 [33] | 0.51 (0.43-0.58) |
|
|
|||||
|
|
Robinson et al, 2021 [34] | 0.72 (0.66-0.78) |
|
|
|||||
|
|
Mahmud et al, 2022 [35] | 0.62 (0.58-0.66) |
|
|
|||||
|
|
Azanaw et al, 2022 [36] | 0.55 (0.47-0.62) |
|
|
|||||
|
|
Terry et al, 2022 [37] | 0.73 (0.64-0.81) |
|
|
|||||
|
|
Yenew et al, 2023 [38] | 0.67 (0.60-0.74) |
|
|
|||||
|
|
Kukreti et al, 2022 [39] | 0.60 (0.51-0.68) |
|
|
|||||
|
|
Nnaemeka et al, 2023 [40] | 0.52 (0.46-0.57) |
|
|
|||||
|
|
Akem Dimala et al, 2021 [41] | 0.71 (0.66-0.76) |
|
|
|||||
|
|
Renzi et al, 2022 [42] | 0.66 (0.61-0.71) |
|
|
|||||
|
|
Kazeminia et al, 2022 [43] | 0.63 (0.59-0.68) |
|
|
|||||
|
|
Mose et al, 2022 [44] | 0.51 (0.43-0.59) |
|
|
|||||
|
|
Yanto et al, 2022 [9] | 0.71 (0.69-0.74) |
|
|
|||||
| Chronic disease | 87.50 | 0.69 (0.61-0.76) | |||||||
|
|
Wang et al, 2021 [22] | 0.85 (0.82-0.88) |
|
|
|||||
|
|
Yazdani et al, 2022 [45] | 0.76 (0.67-0.85) |
|
|
|||||
|
|
Zhao et al, 2023 [46] | 0.65 (0.59-0.72) |
|
|
|||||
|
|
Lin et al, 2022 [47] | 0.58 (0.45-0.75) |
|
|
|||||
|
|
Meybodi et al, 2022 [48] | 0.59 (0.39-0.79) |
|
|
|||||
|
|
Ejamo et al, 2023 [49] | 0.62 (0.56-0.69) |
|
|
|||||
|
|
Ekpor and Akyirem, 2023 [50] | 0.76 (0.66-0.83) |
|
|
|||||
|
|
Prabani et al, 2022 [51] | 0.59 (0.52-0.67) |
|
|
|||||
| Health care workers | 91.72 | 0.64 (0.57-0.71) | |||||||
|
|
Wang et al, 2021 [22] | 0.65 (0.55-0.75) |
|
|
|||||
|
|
Luo et al, 2021 [11] | 0.51 (0.41-0.62) |
|
|
|||||
|
|
Alimohamadi et al, 2022 [10] | 0.55 (0.47-0.64) |
|
|
|||||
|
|
Shui et al, 2022 [52] | 0.78 (0.73-0.83) |
|
|
|||||
|
|
Lin et al, 2022 [85] | 0.81 (0.72-0.89) |
|
|
|||||
|
|
Ackah et al, 2022 [53] | 0.46 (0.37-0.54) |
|
|
|||||
|
|
Moltot et al, 2023 [54] | 0.54 (0.42-0.66) |
|
|
|||||
|
|
Ulbrichtova et al, 2022 [55] | 0.71 (0.67-0.75) |
|
|
|||||
|
|
Politis et al, 2023 [56] | 0.64 (0.55-0.72) |
|
|
|||||
|
|
Wang et al, 2022 [13] | 0.66 (0.61-0.67) |
|
|
|||||
| Pregnant women | 74.20 | 0.48 (0.42-0.53) | |||||||
|
|
Sarantaki et al, 2022 [57] | 0.53 (0.44-0.61) |
|
|
|||||
|
|
Nikpour et al, 2022 [58] | 0.54 (0.45-0.62) |
|
|
|||||
|
|
Nassr et al, 2022 [59] | 0.47 (0.38-0.57) |
|
|
|||||
|
|
Halemani et al, 2022 [60] | 0.54 (0.46-0.61) |
|
|
|||||
|
|
Shamshirsaz et al, 2022 [61] | 0.47 (0.38-0.57) |
|
|
|||||
|
|
Galanis et al, 2022 [62] | 0.27 (0.18-0.37) |
|
|
|||||
|
|
Bhattacharya et al, 2022 [63] | 0.49 (0.42-0.56) |
|
|
|||||
|
|
Worede et al, 2023 [14] | 0.42 (0.28-0.56) |
|
|
|||||
|
|
Azami et al, 2022 [64] | 0.53 (0.47-0.59) |
|
|
|||||
| Parents regarding vaccinating their children | 50.29 | 0.61 (0.56-0.67) | |||||||
|
|
Wang et al, 2022 [65] | 0.58 (0.28-0.98) |
|
|
|||||
|
|
Galanis et al, 2022 [66] | 0.6 (0.517-0.68) |
|
|
|||||
|
|
Chen et al, 2022 [67] | 0.61 (0.53-0.68) |
|
|
|||||
|
|
Ma et al, 2022 [68] | 0.7 (0.62-0.78) |
|
|
|||||
|
|
Alimoradi et al, 2023 [69] | 0.57 (0.52-0.62) |
|
|
|||||
| Migrants and refugees | 74.04 | 0.69 (0.56-0.82) | |||||||
|
|
Alimoradi et al, 2023 [70] | 0.7 (0.62-0.77) |
|
|
|||||
|
|
Hajissa et al, 2023 [71] | 0.56 (0.449-0.685) |
|
|
|||||
| Chinese community residents | N/Aa | 0.80 (0.72-0.88) | |||||||
|
|
Xu and Zhu, 2022 [72] | 0.8 (0.71-0.87) |
|
|
|||||
aN/A: not applicable.
Table 2.
Summary of vaccine hesitancy rates across different populations.
| Studies by population | Hesitancy rate, % (95% CI) | Heterogeneity (I2), % | Overall hesitancy rate, % (95% CI) | |||
| General population | 73.90 | 0.32 (0.25-0.39) | ||||
|
|
Patwary et al, 2022 [73] | 0.382 (0.272-0.497) |
|
|
||
|
|
Islam et al, 2023 [17] | 0.265 (0.22-0.31) |
|
|
||
|
|
Kawuki et al, 2023 [32] | 0.29 (0.18-0.43) |
|
|
||
|
|
Fajar et al, 2022 [74] | 0.25 (0.19-0.32) |
|
|
||
|
|
Cénat et al, 2022 [75] | 0.423 (0.337-0.51) |
|
|
||
| Older adults | N/Aa | 0.27 (0.16-0.39) | ||||
|
|
Veronese et al, 2021 [76] | 0.27 (0.15-0.38) |
|
|
||
| Black/African American people | N/A | 0.35 (0.25-0.44) | ||||
|
|
Ripon et al, 2022 [77] | 0.35 (0.26-0.45) |
|
|
||
| People with diabetes | N/A | 0.27 (0.14-0.40) | ||||
|
|
Bianchi et al, 2023 [84] | 0.27 (0.156-0.419) |
|
|
||
| Health care students | N/A | 0.26 (0.18-0.33) | ||||
|
|
Patwary et al, 2022 [79] | 0.258 (0.185-0.338) |
|
|
||
| Pregnant and breastfeeding women | N/A | 0.48 (0.43-0.53) | ||||
|
|
Bianchi et al, 2022 [80] | 0.484 (0.434-0.534) |
|
|
||
| Parents regarding vaccinating their children | 95.79 | 0..39 (0.07-0.70) | ||||
|
|
Bianchi et al, 2023 [81] | 0.55 (0.43-0.66) |
|
|
||
|
|
Galanis et al, 2022 [66] | 0.229 (0.173-0.29) |
|
|
||
| Migrants | N/A | 0.31 (0.21-0.41) | ||||
|
|
Hajissa et al, 2023 [71] | 0.31 (0.215-0.42) |
|
|
||
| Health care workers | 97.30 | 0.29 (0.18-0.33) | ||||
|
|
Bianchi et al, 2022 [78] | 0.13 (0.069-0.209) |
|
|
||
|
|
Kigongo et al, 2023 [82] | 0.46 (0.38-0.54) |
|
|
||
aN/A: not applicable.
Vaccine Acceptance
We synthesized findings from 29 systematic reviews to assess the vaccine acceptance rate in the general population. The pooled acceptance rates ranged from 51% to 81%. Our meta-analysis revealed a consolidated vaccine acceptance rate of 63% (95% CI 0.60%-0.67%). Notably, a high level of heterogeneity was observed, with an I2 of 97.59% (Figure 2). The 8 systematic reviews focused on individuals with chronic diseases reported a pooled acceptance rate of 69% (95% CI 0.61%-0.76%) and an I2 of 87.5% (Multimedia Appendix 3). Health care workers were the focus of 10 systematic reviews and meta-analyses, indicating a 64% acceptance rate (95% CI 0.57%-0.71%) and a heterogeneity I2 of 91.72% (Multimedia Appendix 4). Vaccine acceptance was comparatively lower among pregnant women, as depicted by 9 systematic reviews, showing a rate of 48% (95% CI 0.42%-0.53%) and an I2 of 74.2% (Multimedia Appendix 5). Similarly, 5 systematic reviews presented a 61.29% acceptance rate (95% CI 0.56%-0.67%) with 50% heterogeneity for parents consenting for their children (Multimedia Appendix 6). Vaccine acceptance among migrants and refugees was investigated by 2 reviews, showing a prevalence of 69% (95% CI 0.56%-0.82%) with an I2 of 74%, and 1 review focused on the Chinese community, reporting an 80% acceptance rate (95% CI 0.72%-0.88%; Multimedia Appendix 7).
Figure 2.

Forest plot depicting the pooled acceptance rates for COVID-19 vaccines in the general population. REML: restricted maximum likelihood.
Vaccine Hesitancy
Figure 3 shows the forest plot of COVID-19 vaccine hesitancy for different populations. Vaccine hesitancy in the general population was reported by 5 systematic reviews, with the observed hesitancy varying between 25% and 42%. Our meta-analysis showed a pooled vaccine hesitancy rate of 32% (95% CI 0.25%-0.39%), with a high level of heterogeneity (I2=73.90%). In older adults, 1 review reported a hesitancy rate of 27% (95% CI 0.15%-0.38%). For Black people or African Americans, vaccine hesitancy was 35% (95% CI 0.26%-0.45%) in another review. Pregnant or breastfeeding women exhibited a higher hesitancy rate of 48.4% (95% CI 0.43%-0.53%), as reported by another systematic review. Results on the hesitancy rates among parents considering vaccinating their children were provided by 2 reviews, revealing a pooled hesitancy rate of 39% (95% CI 0.07%-0.70%), accompanied by a high level of heterogeneity (I2=95.7%). Migrant workers exhibited a hesitancy rate of 31% (95% CI 0.21%-0.41%) according to 1 study. Last, health care workers showed a rate of 29% (95% CI 0.18%-0.33%), as concluded from 2 systematic reviews.
Figure 3.

Forest plot showing COVID-19 vaccine hesitancy rates for different populations. REML: restricted maximum likelihood.
Discussion
Our umbrella review synthesized findings from numerous systematic reviews and meta-analyses, providing insights into global vaccine acceptance and hesitancy rates across diverse populations and geographies. The consolidated acceptance rate of 63% in the general population indicates a moderate level of willingness to receive the vaccine.
The emergence of the COVID-19 pandemic necessitated the prompt development and distribution of vaccines to curb the spread of the virus and mitigate its adverse impacts on global health, economies, and societies [91]. As of the midpoint of 2021, several vaccines had received emergency use authorization, signifying a milestone in the fight against the pandemic. However, the realization of the potential of these vaccines is significantly influenced by the global population's acceptance and willingness to get vaccinated [92,93]. However, the significant heterogeneity observed in this and other populations studied underscores the diverse and complex landscape of vaccine perceptions globally [53]. The vaccine acceptance rates among health care workers and individuals with chronic diseases were relatively higher, possibly reflecting a better understanding of the disease's risk and the vaccine's benefits by these populations [94,95]. However, the observed heterogeneity suggests diverse opinions and possibly varied information dissemination within these groups. The disparities in vaccine acceptance and hesitancy across populations are emblematic of the intricate tapestry of perceptions, beliefs, and information access that characterize the global populace. For instance, the lower acceptance rates observed among pregnant women and parents consenting for their children are likely influenced by concerns about vaccine safety in these vulnerable groups, emphasizing the need for targeted communication strategies addressing these concerns [96].
The pronounced disparities in vaccine acceptance across different populations highlight the urgent need for tailored, population-specific intervention strategies [97]. A one-size-fits-all approach may not address the unique concerns, misconceptions, and information needs of different demographic groups. For example, pregnant women exhibited lower acceptance and higher hesitancy rates, possibly due to concerns regarding the vaccine's impact on pregnancy and the fetus [98,99]. Addressing such specific concerns through targeted awareness campaigns and counseling can enhance vaccine acceptance in this group. Similarly, the lower acceptance rates in parents consenting for their children necessitate interventions addressing parental concerns about vaccine safety and efficacy in children [100]. Engaging pediatricians and child health care providers in vaccine advocacy can potentially alleviate parental apprehensions and foster trust. The high heterogeneity observed across studies denotes the existence of multiple influencing factors, including cultural, socioeconomic, educational, and individual beliefs, which vary extensively within and across populations [101,102]. The variations may also reflect the differences in study designs, populations, and time frames, emphasizing the need for standardization in future research to facilitate comparability and generalizability [103].
The emergence of vaccine hesitancy as a global phenomenon irrespective of a country's economic status underscores the influential role of information dissemination and public perception in shaping vaccine-related behaviors [104,105]. Misinformation and distrust in health advisories have been pivotal in fostering hesitancy, indicating the need for credible, clear, and consistent communication from health authorities and governments [106-110]. Addressing misinformation necessitates a multifaceted approach involving the collaboration of health care providers, public health officials, social media platforms, and community leaders. The propagation of accurate, comprehensible, and transparent information regarding vaccine development, efficacy, and safety can contribute to counteracting misinformation. Health care workers, with an acceptance rate of 64%, play a crucial role in shaping public perceptions and behaviors regarding vaccination [111]. As trusted sources of health information, health care providers can effectively address concerns, clarify misconceptions, and advocate for the benefits of vaccination [63,68]. Their interactions with patients and communities can significantly influence vaccine acceptance, especially in populations with high hesitancy levels, such as pregnant women and parents. However, the hesitancy rate of 29% among health care workers is concerning, as it can potentially impact their vaccine advocacy efforts. Addressing the concerns and information needs of health care workers is imperative to fostering confidence in vaccines and enhancing their role as vaccine advocates.
The acceptance and hesitancy rates in specific communities, such as Black or African American and Chinese communities, underscore the impact of cultural and community nuances on vaccine perceptions [112,113]. Culturally sensitive approaches, community engagement, and addressing systemic barriers are essential to enhancing vaccine acceptance in such communities [114,115]. The 80% acceptance rate in Chinese communities may be indicative of the influence of community norms, government policies, and public health campaigns in shaping vaccine perceptions. Understanding the sociocultural dynamics and leveraging community influences can be instrumental in developing effective strategies to enhance vaccine acceptance in different cultural contexts.
This umbrella review, although offering insights into global vaccine acceptance and hesitancy, does possess limitations inherent to the studies included. The substantial heterogeneity across these studies hinders the ability to draw definitive conclusions and underscores the necessity for a cautious interpretation of the findings. Variations in study designs, targeted populations, time frames, and geographic locations highlight the need for standardization in future research to improve comparability and generalizability. Our review only included articles published in English. The overlap of the same primary studies is inevitable; different systematic reviews might have included the same primary studies.
Future research should focus on exploring the underlying factors influencing vaccine acceptance and hesitancy in diverse populations and contexts. Qualitative studies can provide in-depth insights into individual beliefs, perceptions, and information needs, enabling the development of targeted interventions. Longitudinal studies can assess the temporal variations in vaccine perceptions and the impact of evolving information landscapes on vaccine-related behaviors.
Abbreviations
- AMSTAR
A Measurement Tool to Assess Systematic Reviews
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- WHO
World Health Organization
PRISMA Checklist.
Supplementary tables.
Forest plot illustrating the pooled vaccine acceptance rate for individuals with chronic diseases.
Forest plot depicting the vaccine acceptance rate among health care workers.
Forest plot depicting the vaccine acceptance rate among pregnant women.
Pooled vaccine acceptance rate of parents consenting for their children.
Vaccine acceptance rates for Chinese community residents, migrants, and refugees.
Footnotes
Authors' Contributions: TAR, PS, RI, and AD conceived of or designed the study. AAR, HAA, MFAS, NAAK, MA, AAALI, HAA, IHN, SR, and NK collected the data. KALM, MNK, SG, and QSZ analyzed the data. PS, TAR, RI, and AD drafted the manuscript. KALM, MNK, SG, and QSZ critically revised the manuscript. All authors gave final approval for submission. All authors attest they meet the ICMJE criteria for authorship.
Conflicts of Interest: None declared.
References
- 1.Park JJH, Mogg R, Smith GE, Nakimuli-Mpungu E, Jehan F, Rayner CR, Condo J, Decloedt EH, Nachega JB, Reis G, Mills EJ. How COVID-19 has fundamentally changed clinical research in global health. The Lancet Global Health. 2021 May;9(5):e711–e720. doi: 10.1016/s2214-109x(20)30542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omer SB, Benjamin RM, Brewer NT, Buttenheim AM, Callaghan T, Caplan A, Carpiano RM, Clinton C, DiResta R, Elharake JA, Flowers LC, Galvani AP, Lakshmanan R, Maldonado YA, McFadden SM, Mello MM, Opel DJ, Reiss DR, Salmon DA, Schwartz JL, Sharfstein JM, Hotez PJ. Promoting COVID-19 vaccine acceptance: recommendations from the Lancet Commission on Vaccine Refusal, Acceptance, and Demand in the USA. The Lancet. 2021 Dec;398(10317):2186–2192. doi: 10.1016/s0140-6736(21)02507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarus JV, Wyka K, White TM, Picchio CA, Gostin LO, Larson HJ, Rabin K, Ratzan SC, Kamarulzaman A, El-Mohandes A. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat Med. 2023 Feb 09;29(2):366–375. doi: 10.1038/s41591-022-02185-4.10.1038/s41591-022-02185-4 [DOI] [PubMed] [Google Scholar]
- 4.National Academies of Sciences, Engineering, and Medicine. Health and Medicine Division. Board on Health Sciences Policy. Board on Population Health and Public Health Practice. Committee on Equitable Allocation of Vaccine for the Novel Coronavirus. Gayle H, Foege W, Brown L, Kahn B. Framework for equitable allocation of COVID-19 vaccine. National Academies Press. 2020. [2024-03-09]. https://nap.nationalacademies.org/catalog/25917/framework-for-equitable-allocation-of-covid-19-vaccine . [PubMed]
- 5.Gozzi N, Chinazzi M, Dean NE, Longini IM, Halloran ME, Perra N, Vespignani A. Estimating the impact of COVID-19 vaccine inequities: a modeling study. Nat Commun. 2023 Jun 06;14(1):3272. doi: 10.1038/s41467-023-39098-w. doi: 10.1038/s41467-023-39098-w.10.1038/s41467-023-39098-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez I, Dickson S, Tang S, Gabriel N, Berenbrok LA, Guo J. Disparities in distribution of COVID-19 vaccines across US counties: A geographic information system-based cross-sectional study. PLoS Med. 2022 Jul 28;19(7):e1004069. doi: 10.1371/journal.pmed.1004069. https://dx.plos.org/10.1371/journal.pmed.1004069 .PMEDICINE-D-21-04494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macharia JM, Gakenye GW, Rozmann N, Onchonga D, Mwangi RW, Kaposztas Z, Mathenge JM, Pusztai D, Pinter M, Sugar M, Raposa BL. An empirical assessment of the factors influencing acceptance of COVID-19 vaccine uptake between Kenyan and Hungarian residing populations: A cross-sectional study. Sci Rep. 2022 Dec 23;12(1):22262. doi: 10.1038/s41598-022-26824-5. doi: 10.1038/s41598-022-26824-5.10.1038/s41598-022-26824-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel) 2021 Feb 16;9(2):1. doi: 10.3390/vaccines9020160. https://www.mdpi.com/resolver?pii=vaccines9020160 .vaccines9020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanto TA, Lugito NPH, Hwei LRY, Virliani C, Octavius GS. Prevalence and determinants of COVID-19 vaccine acceptance in South East Asia: a systematic review and meta-analysis of 1,166,275 respondents. Trop Med Infect Dis. 2022 Nov 09;7(11):361. doi: 10.3390/tropicalmed7110361. https://www.mdpi.com/resolver?pii=tropicalmed7110361 .tropicalmed7110361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alimohamadi Y, Hosamirudsari H, Hesari E, Sepandi M. Global COVID-19 vaccine acceptance rate: a systematic review and meta-analysis. Z Gesundh Wiss. 2022 Sep 26;31(11):1–13. doi: 10.1007/s10389-022-01757-5. https://europepmc.org/abstract/MED/36188446 .1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo C, Yang Y, Liu Y, Zheng D, Shao L, Jin J, He Q. Intention to COVID-19 vaccination and associated factors among health care workers: A systematic review and meta-analysis of cross-sectional studies. Am J Infect Control. 2021 Oct;49(10):1295–1304. doi: 10.1016/j.ajic.2021.06.020. https://linkinghub.elsevier.com/retrieve/pii/S0196-6553(21)00460-0 .S0196-6553(21)00460-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norhayati MN, Che Yusof R, Azman YM. Systematic review and meta-analysis of COVID-19 vaccination acceptance. Front Med (Lausanne) 2021 Jan 27;8:783982. doi: 10.3389/fmed.2021.783982. https://europepmc.org/abstract/MED/35155467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Wang Y, Cheng X, Li X, Yang Y, Li J. Acceptance of coronavirus disease 2019 (COVID-19) vaccines among healthcare workers: A meta-analysis. Front Public Health. 2022;10:881903. doi: 10.3389/fpubh.2022.881903. https://europepmc.org/abstract/MED/36187624 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worede DT, Kassahun M, Endalew B. COVID-19 vaccine acceptance and predictors among pregnant women in Ethiopia: Systematic Review and Meta-Analysis. Public Health Pract (Oxf) 2023 Jun;5:100386. doi: 10.1016/j.puhip.2023.100386. https://linkinghub.elsevier.com/retrieve/pii/S2666-5352(23)00032-0 .S2666-5352(23)00032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehal KR, Steendam LM, Campos Ponce M, van der Hoeven M, Smit GSA. Worldwide vaccination willingness for COVID-19: a systematic review and meta-analysis. Vaccines (Basel) 2021 Sep 24;9(10):1. doi: 10.3390/vaccines9101071. https://www.mdpi.com/resolver?pii=vaccines9101071 .vaccines9101071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar G, Jena S, Snigdha NT, Basha S, Narayanan JK, Luke AM. Acceptance of COVID-19 vaccines in India: a systematic review and meta-analysis. Vaccines (Basel) 2023 May 09;11(5):964. doi: 10.3390/vaccines11050964. https://www.mdpi.com/resolver?pii=vaccines11050964 .vaccines11050964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam MM, Yunus MY, Akib MS, Iqbal MR, Khan M. Prevalence of COVID-19 vaccine hesitancy in South Asia: a systematic review and meta-analysis. JPSS. 2023 Mar 29;31:587–611. doi: 10.25133/JPSSv312023.033. [DOI] [Google Scholar]
- 18.Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015 Sep;13(3):132–40. doi: 10.1097/XEB.0000000000000055.01787381-201509000-00004 [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021 Mar 29;372:n160. doi: 10.1136/bmj.n160. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=33781993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. doi: 10.1136/bmj.j4008. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=28935701 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushi G, Shabil M, Padhi BK, Ahmed M, Pandey P, Satapathy P, Rustagi S, Pradhan KB, Al-Qaim ZH, Sah R. Prevalence of acute kidney injury among dengue cases: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2024 Jan 02;118(1):1–11. doi: 10.1093/trstmh/trad067.7272638 [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Yang L, Jin H, Lin L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prev Med. 2021 Sep;150:106694. doi: 10.1016/j.ypmed.2021.106694. https://europepmc.org/abstract/MED/34171345 .S0091-7435(21)00263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelmoneim SA, Sallam M, Hafez DM, Elrewany E, Mousli HM, Hammad EM, Elkhadry SW, Adam MF, Ghobashy AA, Naguib M, Nour El-Deen AE, Aji N, Ghazy RM. COVID-19 vaccine booster dose acceptance: systematic review and meta-analysis. Trop Med Infect Dis. 2022 Oct 13;7(10):1. doi: 10.3390/tropicalmed7100298. https://www.mdpi.com/resolver?pii=tropicalmed7100298 .tropicalmed7100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khabour O. The COVID-19 vaccine acceptance in Jordan: a meta-analysis and review of the literature. Eur Rev Med Pharmacol Sci. 2022 Nov;26(21):8188–8196. doi: 10.26355/eurrev_202211_30172. https://www.europeanreview.org/article/30172 .30172 [DOI] [PubMed] [Google Scholar]
- 25.Sahile A, Gizaw G, Mgutshini T, Gebremariam Z, Bekele G. COVID-19 Vaccine Acceptance Level in Ethiopia: A Systematic Review and Meta-Analysis. Can J Infect Dis Med Microbiol. 2022;2022:2313367. doi: 10.1155/2022/2313367. doi: 10.1155/2022/2313367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wake AD. The acceptance rate toward COVID-19 vaccine in Africa: a systematic review and meta-analysis. Global Pediatric Health. 2021 Sep 30;8:2333794X2110487. doi: 10.1177/2333794x211048738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alarcón-Braga EA, Hernandez-Bustamante EA, Salazar-Valdivia FE, Valdez-Cornejo VA, Mosquera-Rojas MD, Ulloque-Badaracco JR, Rondon-Saldaña JC, Zafra-Tanaka JH. Acceptance towards COVID-19 vaccination in Latin America and the Caribbean: A systematic review and meta-analysis. Travel Med Infect Dis. 2022 Sep;49:102369. doi: 10.1016/j.tmaid.2022.102369. https://europepmc.org/abstract/MED/35680058 .S1477-8939(22)00115-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mekonnen BD, Mengistu BA. COVID-19 vaccine acceptance and its associated factors in Ethiopia: A systematic review and meta-analysis. Clin Epidemiol Glob Health. 2022 Mar;14:101001. doi: 10.1016/j.cegh.2022.101001. https://linkinghub.elsevier.com/retrieve/pii/S2213-3984(22)00042-2 .S2213-3984(22)00042-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mengistu DA, Demmu YM, Asefa YA. Global COVID-19 vaccine acceptance rate: Systematic review and meta-analysis. Front Public Health. 2022 Dec 8;10:1044193. doi: 10.3389/fpubh.2022.1044193. https://europepmc.org/abstract/MED/36568768 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudayu TW, Mengistie HT. COVID-19 vaccine acceptance in sub-Saharan African countries: A systematic review and meta-analysis. Heliyon. 2023 Feb;9(2):e13037. doi: 10.1016/j.heliyon.2023.e13037. https://linkinghub.elsevier.com/retrieve/pii/S2405-8440(23)00244-X .S2405-8440(23)00244-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alemayehu A, Demissie A, Yusuf M, Gemechu Lencha A, Oljira L. Covid-19 vaccine acceptance and determinant factors among general public in East Africa: a systematic review and meta-analysis. Health Serv Res Manag Epidemiol. 2022;9:23333928221106269. doi: 10.1177/23333928221106269. https://journals.sagepub.com/doi/abs/10.1177/23333928221106269?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_23333928221106269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawuki J, Chen S, Fang Y, Liang X, Chan PS, Wang Z. COVID-19 vaccine acceptance, attitude and perception among slum and underserved communities: a systematic review and meta-analysis. Vaccines (Basel) 2023 Apr 23;11(5):1. doi: 10.3390/vaccines11050886. https://www.mdpi.com/resolver?pii=vaccines11050886 .vaccines11050886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belay GM, Alemu TG, Techane MA, Wubneh CA, Assimamaw NT, Tamir TT, Muhye AB, Kassie DG, Wondim A, Terefe B, Tarekegn BT, Ali MS, Fentie B, Gonete AT, Tekeba B, Kassa SF, Desta BK, Ayele AD, Dessie MT, Atalell KA. COVID-19 vaccine acceptance rate and its predictors in Ethiopia: A systematic review and meta-analysis. Hum Vaccin Immunother. 2022 Nov 30;18(6):2114699. doi: 10.1080/21645515.2022.2114699. https://europepmc.org/abstract/MED/36094824 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson E, Jones A, Lesser I, Daly M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021 Apr 08;39(15):2024–2034. doi: 10.1016/j.vaccine.2021.02.005. https://europepmc.org/abstract/MED/33722411 .S0264-410X(21)00140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmud S, Mohsin M, Hossain S, Islam MM, Muyeed A. The acceptance of COVID-19 vaccine at early stage of development and approval: A global systematic review and meta-analysis. Heliyon. 2022 Sep;8(9):e10728. doi: 10.1016/j.heliyon.2022.e10728. https://linkinghub.elsevier.com/retrieve/pii/S2405-8440(22)02016-3 .S2405-8440(22)02016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azanaw J, Endalew M, Zenbaba D, Abera E, Chattu VK. COVID-19 vaccine acceptance and associated factors in 13 African countries: A systematic review and meta-analysis. Front Public Health. 2022 Jan 24;10:1001423. doi: 10.3389/fpubh.2022.1001423. https://europepmc.org/abstract/MED/36761336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry E, Cartledge S, Damery S, Greenfield S. Factors associated with COVID-19 vaccine intentions during the COVID-19 pandemic; a systematic review and meta-analysis of cross-sectional studies. BMC Public Health. 2022 Sep 02;22(1):1667. doi: 10.1186/s12889-022-14029-4. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-022-14029-4 .10.1186/s12889-022-14029-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yenew C, Dessie AM, Gebeyehu AA, Genet A. Intention to receive COVID-19 vaccine and its health belief model (HBM)-based predictors: A systematic review and meta-analysis. Hum Vaccin Immunother. 2023 Dec 31;19(1):2207442. doi: 10.1080/21645515.2023.2207442. https://europepmc.org/abstract/MED/37170620 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kukreti S, Rifai A, Padmalatha S. Willingness to obtain COVID-19 vaccination in general population: A systematic review and meta-analysis. Journal of Global Health. 2022;12:05006. doi: 10.7189/jogh.12.05006. https://jogh.org/wp-content/uploads/2022/02/jogh-11-05006.pdf . [DOI] [Google Scholar]
- 40.Nnaemeka V, Okafor N, Orababa O. COVID-19 vaccine acceptance in Nigeria: A rapid systematic review and meta-analysis. MedRxiv. Preprint posted online on February 16, 2023. 2023 doi: 10.1101/2023.02.16.23286008. doi: 10.1101/2023.02.16.23286008. [DOI] [Google Scholar]
- 41.Akem Dimala C, Kadia BM, Nguyen H, Donato A. Community and provider acceptability of the COVID-19 vaccine: a systematic review and meta-analysis. Advances in Clinical Medical Research and Healthcare Delivery. 2021 Nov 12;1(3):1. doi: 10.53785/2769-2779.1076. [DOI] [Google Scholar]
- 42.Renzi E, Baccolini V, Migliara G, Bellotta C, Ceparano M, Donia P, Marzuillo C, De Vito C, Villari P, Massimi A. Mapping the prevalence of COVID-19 vaccine acceptance at the global and regional level: a systematic review and meta-analysis. Vaccines (Basel) 2022 Sep 07;10(9):1. doi: 10.3390/vaccines10091488. https://www.mdpi.com/resolver?pii=vaccines10091488 .vaccines10091488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazeminia M, Afshar ZM, Rajati M, Saeedi A, Rajati F. Evaluation of the acceptance rate of Covid-19 vaccine and its associated factors: a systematic review and meta-analysis. J Prev (2022) 2022 Aug 10;43(4):421–467. doi: 10.1007/s10935-022-00684-1. https://europepmc.org/abstract/MED/35687259 .10.1007/s10935-022-00684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mose A, Wasie A, Shitu S, Haile K, Timerga A, Melis T, Sahle T, Zewdie A. Determinants of COVID-19 vaccine acceptance in Ethiopia: A systematic review and meta-analysis. PLoS One. 2022 Jun 3;17(6):e0269273. doi: 10.1371/journal.pone.0269273. https://dx.plos.org/10.1371/journal.pone.0269273 .PONE-D-22-04079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yazdani A, Mirmosayyeb O, Ghaffary EM, Hashemi MS, Ghajarzadeh M. COVID-19 vaccines and patients with multiple sclerosis: willingness, unwillingness and hesitancy: a systematic review and meta-analysis. Neurol Sci. 2022 Jul 05;43(7):4085–4094. doi: 10.1007/s10072-022-06051-6. https://europepmc.org/abstract/MED/35381877 .10.1007/s10072-022-06051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Du J, Li Z, Xu Z, Wu Y, Duan W, Wang W, Zhang T, Xu J, Wu H, Huang X. It is time to improve the acceptance of COVID-19 vaccines among people with chronic diseases: A systematic review and meta-analysis. J Med Virol. 2023 Feb;95(2):e28509. doi: 10.1002/jmv.28509. [DOI] [PubMed] [Google Scholar]
- 47.Lin K, Huang H, Fang S, Zheng G, Fu K, Liu N, Du H. Should patients with epilepsy be vaccinated against coronavirus disease 2019? A systematic review and meta-analysis. Epilepsy Behav. 2022 Sep;134:108822. doi: 10.1016/j.yebeh.2022.108822. https://europepmc.org/abstract/MED/35853315 .S1525-5050(22)00271-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meybodi MA, Rotundo L, Shakoor D, Brackett A, Sharifian M, Ahlawat S. Tu1547: COVID-19 vaccine in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastroenterology. 2022 May;162(7):S-1005–S-1006. doi: 10.1016/s0016-5085(22)62385-0. [DOI] [Google Scholar]
- 49.Ejamo JY, Legese GL, Tesfaye YA, Liben FE. COVID-19 vaccine acceptance among people living with HIV: A systematic review and meta-analysis. Trop Med Int Health. 2023 Aug;28(8):601–611. doi: 10.1111/tmi.13908. [DOI] [PubMed] [Google Scholar]
- 50.Ekpor E, Akyirem S. Global acceptance of COVID-19 vaccine among persons with diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2023 Jul;201:110731. doi: 10.1016/j.diabres.2023.110731. https://europepmc.org/abstract/MED/37236364 .S0168-8227(23)00494-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prabani KIP, Weerasekara I, Damayanthi HDWT. COVID-19 vaccine acceptance and hesitancy among patients with cancer: a systematic review and meta-analysis. Public Health. 2022 Nov;212:66–75. doi: 10.1016/j.puhe.2022.09.001. https://europepmc.org/abstract/MED/36244261 .S0033-3506(22)00256-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shui X, Wang F, Li L, Liang Q. COVID-19 vaccine acceptance among healthcare workers in China: A systematic review and meta-analysis. PLoS One. 2022;17(8):e0273112. doi: 10.1371/journal.pone.0273112. https://dx.plos.org/10.1371/journal.pone.0273112 .PONE-D-22-16803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ackah M, Ameyaw L, Gazali Salifu M, Afi Asubonteng DP, Osei Yeboah C, Narkotey Annor E, Abena Kwartemaa Ankapong E, Boakye H. COVID-19 vaccine acceptance among health care workers in Africa: A systematic review and meta-analysis. PLoS One. 2022;17(5):e0268711. doi: 10.1371/journal.pone.0268711. https://dx.plos.org/10.1371/journal.pone.0268711 .PONE-D-21-31691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moltot T, Lemma T, Silesh M, Sisay M, Shewangizaw A, Getaneh T, Tsegaw B. COVID-19 vaccine acceptance among health care professionals in Ethiopia: A systematic review and meta-analysis. Hum Vaccin Immunother. 2023 Dec 31;19(1):2188854. doi: 10.1080/21645515.2023.2188854. https://europepmc.org/abstract/MED/36949629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulbrichtova R, Svihrova V, Svihra J. Prevalence of COVID-19 vaccination among medical students: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022 Mar 29;19(7):1. doi: 10.3390/ijerph19074072. https://www.mdpi.com/resolver?pii=ijerph19074072 .ijerph19074072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Politis M, Sotiriou S, Doxani C, Stefanidis I, Zintzaras E, Rachiotis G. Healthcare workers' attitudes towards mandatory COVID-19 vaccination: a systematic review and meta-analysis. Vaccines (Basel) 2023 Apr 21;11(4):880. doi: 10.3390/vaccines11040880. https://www.mdpi.com/resolver?pii=vaccines11040880 .vaccines11040880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarantaki A, Kalogeropoulou VE, Taskou C, Nanou C, Lykeridou A. COVID-19 vaccination and related determinants of hesitancy among pregnant women: a systematic review and meta-analysis. Vaccines (Basel) 2022 Nov 30;10(12):1. doi: 10.3390/vaccines10122055. https://www.mdpi.com/resolver?pii=vaccines10122055 .vaccines10122055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikpour M, Sepidarkish M, Omidvar S, Firouzbakht M. Global prevalence of acceptance of COVID-19 vaccines and associated factors in pregnant women: a systematic review and meta-analysis. Expert Rev Vaccines. 2022 Jun 22;21(6):843–851. doi: 10.1080/14760584.2022.2053677. [DOI] [PubMed] [Google Scholar]
- 59.Nassr AA, Hessami K, Morain S, Afshar Y, Arian S, Mesh N, Aagaard KM, Shamshirsaz AA. Intention to receive COVID-19 vaccine during pregnancy: A systematic review and meta-analysis. American Journal of Obstetrics and Gynecology. 2022 Jan;226(1):S173–S174. doi: 10.1016/j.ajog.2021.11.303. [DOI] [PubMed] [Google Scholar]
- 60.Halemani K, Dhiraaj S, Latha T, Mishra P, Issac A. The prevalence of COVID vaccine acceptance among pregnant women: A systematic review and meta-analysis. Clinical Epidemiology and Global Health. 2022 Sep;17:101144. doi: 10.1016/j.cegh.2022.101144. doi: 10.1016/j.cegh.2022.101144. [DOI] [Google Scholar]
- 61.Shamshirsaz AA, Hessami K, Morain S, Afshar Y, Nassr AA, Arian SE, Asl NM, Aagaard K. Intention to receive COVID-19 vaccine during pregnancy: a systematic review and meta-analysis. Am J Perinatol. 2022 Apr;39(5):492–500. doi: 10.1055/a-1674-6120. [DOI] [PubMed] [Google Scholar]
- 62.Galanis P, Vraka I, Siskou O, Konstantakopoulou O, Katsiroumpa A, Kaitelidou D. Uptake of COVID-19 vaccines among pregnant women: a systematic review and meta-analysis. Vaccines (Basel) 2022 May 12;10(5):1. doi: 10.3390/vaccines10050766. https://www.mdpi.com/resolver?pii=vaccines10050766 .vaccines10050766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhattacharya O, Siddiquea BN, Shetty A, Afroz A, Billah B. COVID-19 vaccine hesitancy among pregnant women: a systematic review and meta-analysis. BMJ Open. 2022 Aug 18;12(8):e061477. doi: 10.1136/bmjopen-2022-061477. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=35981769 .bmjopen-2022-061477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azami M, Nasirkandy MP, Esmaeili Gouvarchin Ghaleh H, Ranjbar R. COVID-19 vaccine acceptance among pregnant women worldwide: A systematic review and meta-analysis. PLoS One. 2022;17(9):e0272273. doi: 10.1371/journal.pone.0272273. https://dx.plos.org/10.1371/journal.pone.0272273 .PONE-D-22-08719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Chen S, Fang Y. Parental willingness and associated factors of pediatric vaccination in the era of COVID-19 pandemic: a systematic review and meta-analysis. Vaccines (Basel) 2022 Sep 02;10(9):1453. doi: 10.3390/vaccines10091453. https://www.mdpi.com/resolver?pii=vaccines10091453 .vaccines10091453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galanis P, Vraka I, Siskou O, Konstantakopoulou O, Katsiroumpa A, Kaitelidou D. Willingness, refusal and influential factors of parents to vaccinate their children against the COVID-19: A systematic review and meta-analysis. Prev Med. 2022 Apr;157:106994. doi: 10.1016/j.ypmed.2022.106994. https://europepmc.org/abstract/MED/35183597 .S0091-7435(22)00042-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen F, He Y, Shi Y. Parents' and guardians' willingness to vaccinate their children against COVID-19: a systematic review and meta-analysis. Vaccines (Basel) 2022 Jan 24;10(2):1. doi: 10.3390/vaccines10020179. https://www.mdpi.com/resolver?pii=vaccines10020179 .vaccines10020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma Y, Ren J, Zheng Y, Cai D, Li S, Li Y. Chinese parents' willingness to vaccinate their children against COVID-19: A systematic review and meta-analysis. Front Public Health. 2022;10:1087295. doi: 10.3389/fpubh.2022.1087295. https://europepmc.org/abstract/MED/36590001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alimoradi Z, Lin C, Pakpour AH. Worldwide estimation of parental acceptance of COVID-19 vaccine for their children: a systematic review and meta-analysis. Vaccines (Basel) 2023 Feb 24;11(3):1. doi: 10.3390/vaccines11030533. https://www.mdpi.com/resolver?pii=vaccines11030533 .vaccines11030533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alimoradi Z, Sallam M, Jafari E, Potenza MN, Pakpour AH. Prevalence of COVID-19 vaccine acceptance among migrant and refugee groups: A systematic review and -analysis. Vaccine X. 2023 Aug;14:100308. doi: 10.1016/j.jvacx.2023.100308. https://linkinghub.elsevier.com/retrieve/pii/S2590-1362(23)00049-9 .S2590-1362(23)00049-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hajissa K, Mutiat H, Kaabi NA, Alissa M, Garout M, Alenezy AA, Almaghrabi RH, Alrasheed HA, Al-Subaie MF, Alhani HM, Alshehri AA, Almazni IA, Alqahtani AS, Bahwerth FS, Alqethami NH, Alzayer AA, Rabaan AA. COVID-19 vaccine acceptance and hesitancy among migrants, refugees, and foreign workers: a systematic review and meta-analysis. Vaccines (Basel) 2023 Jun 06;11(6):1. doi: 10.3390/vaccines11061070. https://www.mdpi.com/resolver?pii=vaccines11061070 .vaccines11061070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu B, Zhu Y. A systematic review and meta-analysis of the factors associating the willingness of Chinese community residents to receive COVID-19 vaccine. Ann Palliat Med. 2022 Nov;11(11):3483–3493. doi: 10.21037/apm-22-1099. doi: 10.21037/apm-22-1099. [DOI] [PubMed] [Google Scholar]
- 73.Patwary MM, Alam MA, Bardhan M, Disha AS, Haque MZ, Billah SM, Kabir MP, Browning MHEM, Rahman MM, Parsa AD, Kabir R. COVID-19 vaccine acceptance among low- and lower-middle-income countries: a rapid systematic review and meta-analysis. Vaccines (Basel) 2022 Mar 11;10(3):1. doi: 10.3390/vaccines10030427. https://www.mdpi.com/resolver?pii=vaccines10030427 .vaccines10030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fajar JK, Sallam M, Soegiarto G, Sugiri YJ, Anshory M, Wulandari L, Kosasih SAP, Ilmawan M, Kusnaeni K, Fikri M, Putri F, Hamdi B, Pranatasari ID, Aina L, Maghfiroh L, Ikhriandanti FS, Endiaverni WO, Nugraha KW, Wiranudirja O, Edinov S, Hamdani U, Rosyidah L, Lubaba H, Ariwibowo R, Andistyani R, Fitriani R, Hasanah M, Nafis FAD, Tamara F, Latamu FO, Kusuma HI, Rabaan AA, Alhumaid S, Mutair AA, Garout M, Halwani MA, Alfaresi M, Al Azmi R, Alasiri NA, Alshukairi AN, Dhama K, Harapan H. Global prevalence and potential influencing factors of COVID-19 vaccination hesitancy: a meta-analysis. Vaccines (Basel) 2022 Aug 19;10(8):1356. doi: 10.3390/vaccines10081356. https://www.mdpi.com/resolver?pii=vaccines10081356 .vaccines10081356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cénat JM, Noorishad P, Moshirian Farahi SMM, Darius WP, Mesbahi El Aouame A, Onesi O, Broussard C, Furyk SE, Yaya S, Caulley L, Chomienne M, Etowa J, Labelle PR. Prevalence and factors related to COVID-19 vaccine hesitancy and unwillingness in Canada: A systematic review and meta-analysis. J Med Virol. 2023 Jan 03;95(1):e28156. doi: 10.1002/jmv.28156. https://europepmc.org/abstract/MED/36114154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veronese N, Saccaro C, Demurtas J, Smith L, Dominguez LJ, Maggi S, Barbagallo M. Prevalence of unwillingness and uncertainty to vaccinate against COVID-19 in older people: A systematic review and meta-analysis. Ageing Res Rev. 2021 Dec;72:101489. doi: 10.1016/j.arr.2021.101489. https://europepmc.org/abstract/MED/34662744 .S1568-1637(21)00236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ripon RK, Motahara U, Alam A, Ishadi KS, Sarker MS. A meta-analysis of COVID-19 vaccines acceptance among black/African American. Heliyon. 2022 Dec;8(12):e12300. doi: 10.1016/j.heliyon.2022.e12300. https://linkinghub.elsevier.com/retrieve/pii/S2405-8440(22)03588-5 .S2405-8440(22)03588-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bianchi FP, Stefanizzi P, Brescia N, Lattanzio S, Martinelli A, Tafuri S. COVID-19 vaccination hesitancy in Italian healthcare workers: a systematic review and meta-analysis. Expert Rev Vaccines. 2022 Sep 30;21(9):1289–1300. doi: 10.1080/14760584.2022.2093723. [DOI] [PubMed] [Google Scholar]
- 79.Patwary MM, Bardhan M, Haque MZ, Sultana R, Alam MA, Browning MHEM. COVID-19 vaccine acceptance rate and its factors among healthcare students: a systematic review with meta-analysis. Vaccines (Basel) 2022 May 19;10(5):1. doi: 10.3390/vaccines10050806. https://www.mdpi.com/resolver?pii=vaccines10050806 .vaccines10050806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bianchi FP, Stefanizzi P, Di Gioia MC, Brescia N, Lattanzio S, Tafuri S. COVID-19 vaccination hesitancy in pregnant and breastfeeding women and strategies to increase vaccination compliance: a systematic review and meta-analysis. Expert Rev Vaccines. 2022 Oct 20;21(10):1443–1454. doi: 10.1080/14760584.2022.2100766. [DOI] [PubMed] [Google Scholar]
- 81.Bianchi FP, Stefanizzi P, Cuscianna E, Riformato G, Di Lorenzo A, Giordano P, Germinario CA, Tafuri S. COVID-19 vaccination hesitancy among Italian parents: A systematic review and meta-analysis. Hum Vaccin Immunother. 2023 Dec 31;19(1):2171185. doi: 10.1080/21645515.2023.2171185. https://europepmc.org/abstract/MED/36698309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kigongo E, Kabunga A, Tumwesigye R, Musinguzi M, Izaruku R, Acup W. Prevalence and predictors of COVID-19 vaccination hesitancy among healthcare workers in Sub-Saharan Africa: A systematic review and meta-analysis. PLoS One. 2023;18(7):e0289295. doi: 10.1371/journal.pone.0289295. https://dx.plos.org/10.1371/journal.pone.0289295 .PONE-D-22-29316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Q, Hu S, Du F, Zang S, Xing Y, Qu Z, Zhang X, Lin L, Hou Z. Mapping global acceptance and uptake of COVID-19 vaccination: A systematic review and meta-analysis. Commun Med (Lond) 2022;2:113. doi: 10.1038/s43856-022-00177-6. doi: 10.1038/s43856-022-00177-6.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bianchi FP, Stefanizzi P, Martinelli A, Brescia N, Tafuri S. COVID-19 vaccination hesitancy in people affected by diabetes and strategies to increase vaccine compliance: A systematic narrative review and meta-analysis. Vaccine. 2023 Feb 10;41(7):1303–1309. doi: 10.1016/j.vaccine.2023.01.036. https://linkinghub.elsevier.com/retrieve/pii/S0264-410X(23)00058-0 .S0264-410X(23)00058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin GSS, Lee HY, Leong JZ, Sulaiman MM, Loo WF, Tan WW. COVID-19 vaccination acceptance among dental students and dental practitioners: A systematic review and meta-analysis. PLoS One. 2022;17(4):e0267354. doi: 10.1371/journal.pone.0267354. https://dx.plos.org/10.1371/journal.pone.0267354 .PONE-D-22-00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abu El Kheir-Mataria W, Saleh BM, El-Fawal H, Chun S. COVID-19 vaccine hesitancy among parents in low- and middle-income countries: a meta-analysis. Front Public Health. 2023;11:1078009. doi: 10.3389/fpubh.2023.1078009. https://europepmc.org/abstract/MED/36923043 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geng H, Cao K, Zhang J, Wu K, Wang G, Liu C. Attitudes of COVID-19 vaccination among college students: A systematic review and meta-analysis of willingness, associated determinants, and reasons for hesitancy. Hum Vaccin Immunother. 2022 Nov 30;18(5):2054260. doi: 10.1080/21645515.2022.2054260. https://europepmc.org/abstract/MED/35438612 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nindrea RD, Djanas D, Darma IY, Hendriyani H, Sari NP. The risk factors and pregnant women's willingness toward the SARS-CoV-2 vaccination in various countries: A systematic review and meta-analysis. Clin Epidemiol Glob Health. 2022;14:100982. doi: 10.1016/j.cegh.2022.100982. https://linkinghub.elsevier.com/retrieve/pii/S2213-3984(22)00022-7 .S2213-3984(22)00022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nindrea RD, Usman E, Katar Y, Sari NP. Acceptance of COVID-19 vaccination and correlated variables among global populations: A systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;12:100899. doi: 10.1016/j.cegh.2021.100899. https://linkinghub.elsevier.com/retrieve/pii/S2213-3984(21)00207-4 .S2213-3984(21)00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Kuang K. Predictors of COVID-19 vaccination hesitancy in China: a meta-analysis. Public Health. 2023 Jul;220:135–141. doi: 10.1016/j.puhe.2023.05.009. https://europepmc.org/abstract/MED/37320944 .S0033-3506(23)00157-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li M, Wang H, Tian L, Pang Z, Yang Q, Huang T, Fan J, Song L, Tong Y, Fan H. COVID-19 vaccine development: milestones, lessons and prospects. Signal Transduct Target Ther. 2022 May 03;7(1):146. doi: 10.1038/s41392-022-00996-y. doi: 10.1038/s41392-022-00996-y.10.1038/s41392-022-00996-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guidry JPD, Laestadius LI, Vraga EK, Miller CA, Perrin PB, Burton CW, Ryan M, Fuemmeler BF, Carlyle KE. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control. 2021 Feb;49(2):137–142. doi: 10.1016/j.ajic.2020.11.018. https://europepmc.org/abstract/MED/33227323 .S0196-6553(20)31002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wake AD. The willingness to receive COVID-19 vaccine and its associated factors: "Vaccination Refusal Could Prolong the War of This Pandemic" - a systematic review. Risk Manag Healthc Policy. 2021;14:2609–2623. doi: 10.2147/RMHP.S311074. https://europepmc.org/abstract/MED/34188572 .311074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nemr N, Kishk RM, Soliman NH, Farghaly RM, Kishk SM, Louis N. Perception of COVID-19 and vaccine acceptance among healthcare workers. Int J Microbiol. 2022;2022:1607441. doi: 10.1155/2022/1607441. doi: 10.1155/2022/1607441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swarup SS, Padhi BK, Satapathy P, Shabil M, Bushi G, Gandhi AP, Khatib MN, Gaidhane S, Zahiruddin QS, Rustagi S, Barboza JJ, Sah R. Cardiovascular consequences of financial stress: A systematic review and meta-analysis. Curr Probl Cardiol. 2024 Feb;49(2):102153. doi: 10.1016/j.cpcardiol.2023.102153.S0146-2806(23)00570-4 [DOI] [PubMed] [Google Scholar]
- 96.Wang M, Wen W, Wang N, Zhou M, Wang C, Ni J, Jiang J, Zhang X, Feng Z, Cheng Y. COVID-19 vaccination acceptance among healthcare workers and non-healthcare workers in China: a survey. Front Public Health. 2021;9:709056. doi: 10.3389/fpubh.2021.709056. https://europepmc.org/abstract/MED/34409011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benham JL, Atabati O, Oxoby RJ, Mourali M, Shaffer B, Sheikh H, Boucher J, Constantinescu C, Parsons Leigh J, Ivers NM, Ratzan SC, Fullerton MM, Tang T, Manns BJ, Marshall DA, Hu J, Lang R. COVID-19 vaccine-related attitudes and beliefs in Canada: national cross-sectional survey and cluster analysis. JMIR Public Health Surveill. 2021 Dec 23;7(12):e30424. doi: 10.2196/30424. https://publichealth.jmir.org/2021/12/e30424/ v7i12e30424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fieselmann J, Annac K, Erdsiek F, Yilmaz-Aslan Y, Brzoska P. What are the reasons for refusing a COVID-19 vaccine? A qualitative analysis of social media in Germany. BMC Public Health. 2022 Apr 27;22(1):846. doi: 10.1186/s12889-022-13265-y. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-022-13265-y .10.1186/s12889-022-13265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shabil M, Bushi G, Beig MA, Rais MA, Ahmed M, Padhi BK. Cardiovascular manifestation in tuberculosis cases: a systematic review and meta-analysis. Curr Probl Cardiol. 2023 Jul;48(7):101666. doi: 10.1016/j.cpcardiol.2023.101666.S0146-2806(23)00083-X [DOI] [PubMed] [Google Scholar]
- 100.Mudhune V, Ondeng'e K, Otieno F, Otieno DB, Bulinda CM, Okpe I, Nabia S, Bar-Zeev N, Otieno O, Wonodi C. Determinants of COVID-19 vaccine acceptability among healthcare workers in Kenya-a mixed methods analysis. Vaccines (Basel) 2023 Jul 27;11(8):1. doi: 10.3390/vaccines11081290. https://www.mdpi.com/resolver?pii=vaccines11081290 .vaccines11081290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Figa Z, Temesgen T, Zemeskel AG, Ganta M, Alemu A, Abebe M, Ashuro Z. Acceptance of COVID-19 vaccine among healthcare workers in Africa, systematic review and meta-analysis. Public Health Pract (Oxf) 2022 Dec;4:100343. doi: 10.1016/j.puhip.2022.100343. https://linkinghub.elsevier.com/retrieve/pii/S2666-5352(22)00119-7 .S2666-5352(22)00119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shabil M, Bushi G, Bodige PK, Maradi PS, Patra BP, Padhi BK, Khubchandani J. Effect of fenugreek on hyperglycemia: a systematic review and meta-analysis. Medicina (Kaunas) 2023 Jan 27;59(2):248. doi: 10.3390/medicina59020248. https://www.mdpi.com/resolver?pii=medicina59020248 .medicina59020248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koh SWC, Liow Y, Loh VWK, Liew SJ, Chan Y, Young D. COVID-19 vaccine acceptance and hesitancy among primary healthcare workers in Singapore. BMC Prim Care. 2022 Apr 15;23(1):81. doi: 10.1186/s12875-022-01693-z. https://europepmc.org/abstract/MED/35421920 .10.1186/s12875-022-01693-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao Y, Chai R, Yang J, Zhang X, Huang X, Yu M, Fu G, Lan G, Qiao Y, Zhou Q, Li S, Xu J. Reasons for COVID-19 vaccine hesitancy among Chinese people living with HIV/AIDS: structural equation modeling analysis. JMIR Public Health Surveill. 2022 Jun 30;8(6):e33995. doi: 10.2196/33995. https://publichealth.jmir.org/2022/6/e33995/ v8i6e33995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang Y, Zhang L, Fu J, Wu Y, Wang H, Xiao W, Xin Y, Dai Z, Si M, Chen X, Jia M, Leng Z, Cui D, Su X. COVID-19 vaccine hesitancy among patients recovered from COVID-19 infection in Wuhan, China: cross-sectional questionnaire study. JMIR Public Health Surveill. 2023 Jul 03;9:e42958. doi: 10.2196/42958. https://publichealth.jmir.org/2023//e42958/ v9i1e42958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hou Z, Tong Y, Du F, Lu L, Zhao S, Yu K, Piatek SJ, Larson HJ, Lin L. Assessing COVID-19 vaccine hesitancy, confidence, and public engagement: a global social listening study. J Med Internet Res. 2021 Jun 11;23(6):e27632. doi: 10.2196/27632. https://www.jmir.org/2021/6/e27632/ v23i6e27632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao S, Hu S, Zhou X, Song S, Wang Q, Zheng H, Zhang Y, Hou Z. The prevalence, features, influencing factors, and solutions for COVID-19 vaccine misinformation: systematic review. JMIR Public Health Surveill. 2023 Jan 11;9:e40201. doi: 10.2196/40201. https://publichealth.jmir.org/2023//e40201/ v9i1e40201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Su Z, McDonnell D, Cheshmehzangi A, Li X, Maestro D, Šegalo S, Ahmad J, Hao X. With great hopes come great expectations: access and adoption issues associated with COVID-19 vaccines. JMIR Public Health Surveill. 2021 Aug 04;7(8):e26111. doi: 10.2196/26111. https://publichealth.jmir.org/2021/8/e26111/ v7i8e26111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shabil M, Murti K, Kumar VU, Kumar R, Kumar N, Dhingra S, Parihar VK, Ravichandiran V, Pandey K. Older PLHIV are at higher cardiovascular risk with poor quality of life. Curr HIV Res. 2023;21(6):354–360. doi: 10.2174/011570162X277586231218104922.CHR-EPUB-136779 [DOI] [PubMed] [Google Scholar]
- 110.Shabil M, Kumar VU, Dhingra S, Ravichandiran V, Parihar VK, Kumar N, Pandey K, Murti K. Current scenario and strategies to tackle cardiovascular disease risk in HIV geriatrics. Curr. Pharmacol. Rep. 2023 Sep 05;9(6):523–539. doi: 10.1007/s40495-023-00332-0. [DOI] [Google Scholar]
- 111.Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, Barrett E, Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines (Basel) 2021 Feb 03;9(2):119. doi: 10.3390/vaccines9020119. https://www.mdpi.com/resolver?pii=vaccines9020119 .vaccines9020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Budhwani H, Maragh-Bass AC, Tolley EE, Comello MLG, Stoner MCD, Adams Larsen M, Brambilla D, Muessig KE, Pettifor A, Bond CL, Toval C, Hightow-Weidman LB. Tough Talks COVID-19 digital health intervention for vaccine hesitancy among Black young adults: protocol for a hybrid Type 1 effectiveness implementation randomized controlled trial. JMIR Res Protoc. 2023 Feb 13;12:e41240. doi: 10.2196/41240. https://www.researchprotocols.org/2023//e41240/ v12i1e41240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bachtiger P, Adamson A, Chow J, Sisodia R, Quint JK, Peters NS. The impact of the COVID-19 pandemic on the uptake of influenza vaccine: UK-wide observational study. JMIR Public Health Surveill. 2021 Apr 14;7(4):e26734. doi: 10.2196/26734. https://publichealth.jmir.org/2021/4/e26734/ v7i4e26734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mayer MA, Vidal-Alaball J, Puigdellívol-Sánchez A, Marín Gomez FX, Leis A, Mendioroz Peña J. Clinical characterization of patients with COVID-19 in primary care in Catalonia: retrospective observational study. JMIR Public Health Surveill. 2021 Feb 08;7(2):e25452. doi: 10.2196/25452. https://publichealth.jmir.org/2021/2/e25452/ v7i2e25452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sigalo N, Frias-Martinez V. Using COVID-19 vaccine attitudes found in tweets to predict vaccine perceptions in traditional surveys: infodemiology study. JMIR Infodemiology. 2023 Nov 30;3:e43700. doi: 10.2196/43700. https://infodemiology.jmir.org/2023//e43700/ v3i1e43700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
Supplementary tables.
Forest plot illustrating the pooled vaccine acceptance rate for individuals with chronic diseases.
Forest plot depicting the vaccine acceptance rate among health care workers.
Forest plot depicting the vaccine acceptance rate among pregnant women.
Pooled vaccine acceptance rate of parents consenting for their children.
Vaccine acceptance rates for Chinese community residents, migrants, and refugees.


