Abstract
Roses are among the most popular ornamental plants cultivated worldwide for their great economic, symbolic, and cultural importance. Nevertheless, rapid petal senescence markedly reduces rose (Rosa hybrida) flower quality and value. Petal senescence is a developmental process tightly regulated by various phytohormones. Ethylene accelerates petal senescence, while gibberellic acid (GA) delays this process. However, the molecular mechanisms underlying the crosstalk between these phytohormones in the regulation of petal senescence remain largely unclear. Here, we identified SENESCENCE-ASSOCIATED F-BOX (RhSAF), an ethylene-induced F-box protein gene encoding a recognition subunit of the SCF-type E3 ligase. We demonstrated that RhSAF promotes degradation of the GA receptor GIBBERELLIN INSENSITIVE DWARF1 (RhGID1) to accelerate petal senescence. Silencing RhSAF expression delays petal senescence, while suppressing RhGID1 expression accelerates petal senescence. RhSAF physically interacts with RhGID1s and targets them for ubiquitin/26S proteasome-mediated degradation. Accordingly, ethylene-induced RhGID1C degradation and RhDELLA3 accumulation are compromised in RhSAF-RNAi lines. Our results demonstrate that ethylene antagonizes GA activity through RhGID1 degradation mediated by the E3 ligase RhSAF. These findings enhance our understanding of the phytohormone crosstalk regulating petal senescence and provide insights for improving flower longevity.
An ethylene-induced F-box protein, RhSAF, accelerates petal senescence by destabilizing the gibberellic acid receptor RhGID1.
IN A NUTSHELL.
Background: Rose (Rosa hybrida) is the queen of flowers, cultivated worldwide for its great economic, symbolic, and cultural importance. Petal senescence is vital for ensuring optimal offspring production, but delaying petal senescence and extending a flower's longevity have applications in the ornamental flower industry. The timing of petal senescence determines flower longevity and is regulated by phytohormones. Ethylene is the major phytohormone promoting petal senescence, while gibberellic acid (GA) represses this process. The molecular mechanisms underlying the crosstalk between these phytohormones in regulating rose petal senescence remain largely unclear.
Question: How does ethylene antagonize the effects of GA to accelerate petal senescence?
Findings: The ethylene-induced F-box protein SENESCENCE-ASSOCIATED F-BOX (RhSAF) accelerates petal senescence by repressing GA signaling in rose. At the early stages of flower opening, the RhGID1 GA receptors are stabilized, as low ethylene levels in petals fail to induce RhSAF expression. During the late stages of flower opening, ethylene levels increase and upregulate RhSAF expression. RhSAF then recognizes RhGID1s and triggers their ubiquitin-mediated degradation through the 26S proteasome, which attenuates GA signaling and accelerates petal senescence.
Next steps: The present work indicates that additional proteins are RhSAF substrates. It will be interesting to explore their function in phytohormone crosstalk and in regulating petal senescence.
Introduction
Senescence is a general feature of all living organisms and is critical for plant fitness. As the final stage of plant development, senescence involves the gradual and orderly degeneration of macromolecules and organelles, with the remobilization of nutrients from senescing organs to developing tissues and organs (Woo et al. 2019; Chen et al. 2021; Sun et al. 2021). These active and programmed processes are coordinately regulated by various internal and external signals, such as environmental stresses, developmental status, and phytohormones (Guo et al. 2021).
Ethylene is a gaseous hormone and a positive regulator of plant organ senescence, including the senescence of leaves and flowers, and fruit ripening (Iqbal et al. 2017). In ethylene-sensitive plants, such as rose (Rosa hybrida), ethylene production increases during flower senescence (Pei et al. 2013). Exogenous ethylene treatments accelerate petal senescence, while the application of ethylene biosynthesis or activity inhibitors delays petal senescence (Serek et al. 1995; Cuquel et al. 2007; Huang et al. 2017; Ha et al. 2019). Genetic approaches have identified many ethylene-related genes involved in senescence processes (Woo et al. 2019; Guo et al. 2021; Sun et al. 2021); for example, the constitutive ethylene response mutant ctr1 (constitutive triple response 1) exhibits early senescence and flower abscission, whereas the ethylene-insensitive mutants etr1 (ethylene response 1) and ein2 (ethylene insensitive 2) exhibit a delayed leaf or flower senescence phenotype (Li et al. 2013; Kim et al. 2014; Xu et al. 2014). In rose, ethylene employs several protein kinases and transcriptional factors, including RhCIPK3/6, RhHB1/6, RhMYB108, RhERF113, and RhWRKY33, to promote petal senescence (Lü et al. 2014; Wu et al. 2017, 2022; Khaskheli et al. 2018; Zhang et al. 2019; Jing et al. 2021; Chen et al. 2023).
Like ethylene, gibberellic acid (GA) also regulates senescence processes (Woo et al. 2019). GAs are a large group of tetracyclic diterpenoids generally acknowledged as senescence-impeding hormones. GA derepresses its signaling cascade by inducing the degradation of nuclear proteins DELLAs, the GA signaling master repressors (Achard and Genschik 2009; Davière and Achard 2013). GA binds to its soluble nuclear receptor, GIBBERELLIN INSENSITIVE DWARF1 (GID1), which triggers its interaction with transcriptional repressor DELLAs. This GA–GID1–DELLA interaction promotes the binding of an SCF ubiquitin ligase to DELLAs. Subsequently, the DELLAs are polyubiquitinated and degraded by the 26S proteasome (Davière and Achard 2013; Bao et al. 2020).
The content of GAs gradually decreases during senescence. The external application of GAs could retard the leaf senescence of dandelion (Taraxacum ojficinale), banana (Musa cavendishii Lamb.), and Chinese flowering cabbage (Brassica rapa var. parachinensis), as well as petal senescence of Gerbera (Gerbera jamesonii) and petunia (Petunia hybrida) and rose (Rosa hybrida) (Fletcher and Osborne 1965; Whyte and Luckwill 1966; Fischer 2012; Fan et al. 2018; Ma et al. 2018). In rose, exogenously applied paclobutrazol (PAC), a gibberellin biosynthesis inhibitor, hastens petal senescence, and silencing of GA biosynthesis gene GIBBERELLIN 20-OXIDASE 1 (RhGA20ox1) promoted petal senescence, demonstrating that GA retards rose petal senescence (Lü et al. 2014).
Ethylene and GAs function antagonistically to regulate petal senescence (Ma et al. 2018; Sarwat and Tuteja 2019; Sun et al. 2021). In rose (R. hybrida), the ethylene-induced transcriptional factor HOMEOBOX 1 (RhHB1) positively regulates petal senescence by directly inhibiting the expression of RhGA20ox1 (Lü et al. 2014). In petunia, the ectopic expression of the ethylene-induced gene FLOWERING bHLH 1 (PhFBH4) accelerates flower senescence, and the transcript level of the GA catabolic gene GIBBERELLIN 2-OXIDASE 3 (GA2ox3) is increased, indicating the antagonistic interaction between ethylene and GAs in flower senescence (Yin et al. 2015). These discoveries reveal that ethylene antagonizes the effects of GAs by regulating their accumulation. Ethylene regulates Arabidopsis (Arabidopsis thaliana) development by inhibiting GA signaling (Achard et al. 2003, 2007; Vriezen et al. 2004), whether and how ethylene antagonizes GA signaling in rose petal senescence remain largely unknown at the molecular level.
The ubiquitin-proteasome system (UPS) plays an important role in various biological processes, including the regulation of phytohormone signaling (Kelley 2018). Briefly, free ubiquitin proteins are attached to target proteins in sequential reactions performed by three main enzymes: ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3). The E3 ligases define substrate specificity and are further divided into four main types: HECT, RING, U-box, and cullin-RING ligases (CRLs) (Vierstra 2009). In the S-PHASE KINASE-ASSOCIATED PROTEIN 1 (SKP1)–CUL1-F-box (SCF) complexes, the F-box proteins are responsible for specifically recognizing and recruiting target proteins (Sharma et al. 2016). Several E3 ubiquitin ligases involved in plant organ senescence have been identified. In Arabidopsis, the mutation of an F-box protein gene, ORESARA 9 (ORE9), which was later characterized as MORE AXILLARY GROWTH LOCUS 2 (MAX2) and shown to be involved in strigolactone signaling, resulted in delayed leaf senescence (Woo et al. 2001; Ueda and Kusaba 2015). ORESARA 1 (ORE1) is also a key transcription factor promoting leaf senescence in Arabidopsis, while NITROGEN LIMITATION ADAPTATION (NLA) encodes an E3 ubiquitin ligase and delays leaf senescence by destabilizing ORE1 through the UPS (Park et al. 2018); however, whether F-box protein genes regulate petal senescence remains unclear.
Here, we identify an F-box domain-containing protein, RhSAF, which interacts with RhGID1B/C. We show that RhSAF functions as an E3 ubiquitin ligase and mediates RhGID1B/C ubiquitination and degradation. The RNA interference (RNAi) of RhSAF resulted in plants with delayed petal senescence and elevated RhGID1C protein levels. Furthermore, ethylene-induced RhGID1C degradation and RhDELLA3 accumulation are compromised in RhSAF-RNAi plants. Thus, we propose a model in which RhSAF mediates the antagonistic effects between ethylene and GA signaling in petal senescence.
Results
Silencing RhSAF delays petal senescence
Our earlier studies indicated that protein ubiquitination plays a critical role in petal senescence (Lu et al. 2019). The F-box protein of the SCF E3s is responsible for recognizing specific substrates for ubiquitination. To identify potential F-box protein genes that may regulate rose (R. hybrida) petal senescence, we analyzed the rose petal transcriptome data generated previously (Pei et al. 2013). We first analyzed the ethylene-responsive genes and found that 19 F-box protein genes were upregulated upon ethylene treatment (Supplementary Fig. S1A; Supplementary Table S1). The F-box protein gene (unigene ID: RU20110) was particularly interesting among these genes because its expression level increased during flower opening and senescence (Supplementary Fig. S1B; Supplementary Table S2); thus, this F-box protein gene was termed as RhSAF (SENESCENCE-ASSOCIATED F-BOX) and subjected to further investigation.
The expression profile of RhSAF was further verified using reverse transcription quantitative PCR (RT-qPCR). Consistent with the transcriptome data, the transcript level of RhSAF increased gradually during petal opening and senescence (Fig. 1A). Notably, RhSAF was induced upon ethylene treatment and repressed by treatment with ethylene perception inhibitor, 1-methylcyclopropene (1-MCP) (Fig. 1B). To explore whether RhSAF protein level is modulated during senescence or by ethylene, we produced antibodies against RhSAF to detect its protein abundance. In line with RT-qPCR results, RhSAF abundance gradually increased during flower opening and peaked at stage 5 (Fig. 1C), and ethylene promoted the accumulation of RhSAF, whereas 1-MCP inhibited it (Fig. 1D).
Figure 1.
Silencing RhSAF delays petal senescence. A) RT-qPCR showing relative expression of RhSAF (n = 3) in petals of different opening stages. Mean values ± Sd are shown. B) Flowers at stage 2 were treated with air, ethylene or 1-MCP for 24 h. RhSAF transcript was detected by RT-qPCR. C) RhSAF protein accumulated during flower opening. Total protein was extracted from petals at different opening stages (stages 1 to 5). D) RhSAF protein increased upon ethylene treatment and decreased upon 1-MCP treatment. Flowers at stage 2 were treated with air, ethylene, or 1-MCP for 24 h before sampling. For (C) and (D) immunoblotting analysis was performed using anti-RhSAF antibodies, and Tubulin was used as an internal control. E) Petal senescence phenotype of WT and RhSAF-RNAi (RNA interference) plants was recorded daily after stage 5. Scale bars: 4 cm. F) RT-qPCR analyses of RhSAF in petals of WT and RhSAF-RNAi plants. G) Length of senescence phase (from fully open flowers to occurrence of senescence), each flower represented a biological replicate. H, I) relative expression of RhSAG12 (H) and (I) ion leakage in petals of WT and RhSAF-RNAi plants. For A-B and F to I, mean values ± Sd are shown from three biological replicates (n = 3). For A to D, F, H, and I, the outmost layer petals of three flowers were pooled together as one biological replicate. For A, the significance of differences at each stage was tested using the one-way ANOVA with LSD comparisons test (P < 0.05); for B and F to I, asterisks represent statistically significant differences (**P < 0.01), as determined by Dunnett test (B) and the t-test (F to I). IB, immunoblot.
To assess the function of RhSAF in rose petal senescence, we silenced its expression in rose petals using virus-induced gene silencing (VIGS) (Tian et al. 2014). We measured the flower life span and defined the senescence phase as the period from stage 5 to the emergence of petal wilting or abscission. Compared with the control plants transformed with a TRV empty vector, the TRV-RhSAF-silenced plants showed significantly delayed petal senescence (Supplementary Fig. S2, A and B). An RT-qPCR analysis indicated that RhSAF expression was considerably repressed in the TRV-RhSAF petals relative to the TRV-control petals (Supplementary Fig. S2C). Correspondingly, the expression of the senescence marker gene SENESCENCE-ASSOCIATED GENE 12 (RhSAG12) was significantly suppressed in the RhSAF-silenced petals relative to the control petals (Supplementary Fig. S2D).
To further confirm the function of RhSAF in petal senescence, we obtained rose transgenic RNAi lines generated using an Agrobacterium tumefaciens-mediated transformation of somatic embryos (Liu et al. 2021) (Fig. 1E). The expression of RhSAF was significantly suppressed in the RhSAF-RNAi plants relative to the wild type (WT) (Fig. 1F). Like the TRV-RhSAF-silenced plants, the RhSAF-RNAi plants also displayed significantly delayed petal senescence compared with the WT (Fig. 1, E and G). Consistent with this, the senescence marker gene RhSAG12 and ion leakage were significantly repressed in the petals of RhSAF-RNAi relative to the WT petals (Fig. 1, H and I). Collectively, these results indicated that RhSAF positively regulates rose petal senescence.
RhSAF is a component of the SCF E3 ligase complex
A search of the protein domain analysis database SMART revealed that RhSAF contains a conversed F-box domain at its N-terminus, a nuclear localization signal (NLS), and a transmembrane domain located at the C-terminus (Fig. 2A; Supplementary Fig. S3). To verify its subcellular localization, we transiently co-expressed RhSAF–GFP with the plasma membrane marker PM-mcherry (Liang et al. 2020) or the nucleus marker NF-YA4-mcherry (Zhang et al. 2019) in Nicotiana benthamiana leaves. A confocal microscopy analysis revealed that RhSAF-GFP co-localized with PM-mcherry and NF-YA4-mcherry (Fig. 2B). To confirm it is a nucleus protein, we co-expressed the truncated RhSAF1–315 lacking the transmembrane domain with the nucleus marker NF-YA4-mcherry in N. benthamiana leaves. The results showed that RhSAF1–315 mainly localized to the nucleus (Fig. 2B), indicating that RhSAF mainly localized both to the nucleus and the plasma membrane.
Figure 2.
RhSAF is a component of the SCF E3 ligases complex. A) Predicted diagram of RhSAF full-length protein. The RhSAF protein contains an F-box domain (26–65 a.a.), a nuclear localization signal (NLS, 278–287 a.a.), and a transmembrane domain (316–339 a.a.). B) Subcellular localization of RhSAF in N. benthamiana leaves. RhSAF-GFP was co-expressed with the plasma membrane marker PM-mcherry (top panel) or the nucleus marker NF-YA4-mcherry (middle panel). The truncated RhSAF1–315 lacking the transmembrane domain was co-expressed with the nucleus marker NF-YA4-mcherry (bottom panel). Scale bars: 20 μm. C, D) Y2H assay revealing the interaction between RSKs and RhSAF1–315 (C) or RhSAF1–315ΔF (D). RhSAF1–315 represents truncated RhSAF lacking the transmembrane domain. RhSAF1–315ΔF represents truncated RhSAF lacking the F-box and the transmembrane domain. Yeast cells were grown on a control medium (SD/-Leu/-Trp) or a selective medium (SD/-Leu/-Trp/-His + 0.5 mm 3-AT). SD, synthetic dropout medium. AD, GAL4 activation domain. BD, GAL4 DNA-binding domain. 3-AT, 3-amino-1,2,4-triazole.
RhSAF encodes an F-box protein, which prompted us to assess whether RhSAF is a component of an SCF-type E3 ligase complex. A total of 21 SKP1-like genes were identified in a search of the rose genome (Raymond et al. 2018). According to phylogenetic analyses with SKP1s from Arabidopsis (ASKs), those rose SKP1-like proteins were then named as RSK1 to RSK21 (RosaSKP1-like) according to their homology to SKP1 domain in ASK1 (Supplementary Fig. S4).
Full-length of RSK1, 2, 4, 5, 7, 12, 15, 16, 18, 20 were successfully cloned from petal cDNA and used to test interaction with RhSAF. The truncated version of RhSAF1–315 lacking the transmembrane domain was fused with the GAL4 DNA-binding domain (BD), and RSK1s were fused to the GAL4 activation domain (AD). Among those RSK1s, only RSK4/7/12/20 interact with RhSAF (Fig. 2C). Furthermore, another truncated RhSAF, RhSAF1–315ΔF, lacking the F-box domain and the transmembrane domain, did not interact with the RSKs (Fig. 2D), indicating that the F-box domain is essential for the interaction between RhSAF and the RSKs. Together, these results suggest that RhSAF is a component of the SCF complex in rose.
RhSAF mediates the ubiquitination and degradation of RhGID1B/C
To decipher the RhSAF-mediated mechanism that accelerates petal senescence, we performed a yeast two-hybrid (Y2H) screening to identify the potential interacting partners of this protein. The truncated RhSAF protein lacking the transmembrane domain (RhSAF1–315), was used as bait for Y2H screening with the prey library constructed from petal cDNA. Through Y2H screening, we identified a number of proteins that interact with RhSAF (Supplementary Table S3). The GA receptor protein, RhGID1C, was of particular interest and was examined further. Since RhGID1C and RhGID1B are homologs, the interactions between RhSAF and RhGID1B/C were verified in Y2H assays, revealing that RhSAF1–315 interacts with RhGID1B and RhGID1C in yeast (Saccharomyces cerevisiae) (Fig. 3A).
Figure 3.
RhSAF interacts with RhGID1B/C. A) Y2H assays showing the interactions between RhSAF and RhGID1s. Yeast cells were grown on a control medium (SD/-Leu/-Trp) or a selective medium (SD/-Leu/-Trp/-His + 0.5 mm 3-AT + X-α-gal). B) SFLC assay showing the interactions between RhSAF and RhGID1s in N. benthamiana epidermal cells. RhSAF fused with the C-terminus of LUC (cLUC-RhSAF) was co-expressed with RhGID1B/C fused with the N-terminus of LUC (RhGID1B/C-nLUC) by co-infiltrating Agrobacterium carrying different plasmids into N. benthamiana leaves. Images were collected 3 d after infiltration. C) BiFC showing the interactions in the nucleus between RhSAF and RhGID1s. RhSAF was fused to the N-terminal of YFP and RhGID1B/C to the C-terminal of YFP. A combination of nYFP-RhEBF1 and RhEIN3-cYFP was used as a positive control, while the combinations of nYFP-RhSAF and RhEIN3-cYFP, nYFP-RhEBF1 and RhGID1B/C-cYFP1 were used as negative controls. Constructions for each combination and nucleus marker NF-YA4-mcherry were transiently co-expressed in Arabidopsis mesophyll protoplasts. Scale bars = 30 μm. D) Co-IP assay showing the interactions between RhSAF and RhGID1s. Agrobacterium carrying HA-SAF and RhGID1B-GFP or RhGID1C-GFP were co-infiltrated in N. benthamiana leaves. Co-IP was carried out with GFP-trap agarose beads from total crude proteins, and immunoblotting analysis was done with anti-HA antibodies and anti-GFP antibodies. SD, synthetic dropout medium. AD, GAL4 activation domain. BD, GAL4 DNA-binding domain. 3-AT, 3-amino-1,2,4-triazole. LUC, firefly luciferase. IB, immunoblot.
We performed a split-luciferase complementation (SFLC) assay to validate the interactions between RhSAF with RhGID1C and RhGID1B in vivo. The cLUC-RhSAF and RhGID1B-nLUC or RhGID1B-nLUC constructs were transiently co-expressed in N. benthamiana leaves, with both combinations generating luciferase signals that were not observed in the control cells (Fig. 3B). To investigate the subcellular localization of the interaction between RhGID1s and RhSAF, we conducted a bimolecular fluorescence complementation (BiFC) assay. RhSAF-nYFP and cYFP-RhGID1B/C were transiently co-expressed in Arabidopsis mesophyll protoplasts. The combination of RhEBF1 (EIN3-BINDING F-BOX PROTEIN 1)-nYFP and cYFP-RhEIN3 (ETHYLENE INSENSITIVE 3) was used as a positive control. YFP signals were observed within the nucleus when RhSAF and RhGID1B/C were co-expressed, while no signals were detected in negative controls (Fig. 3C).
We further verified the interactions of RhSAF with RhGID1B or RhGID1C in vivo using a co-immunoprecipitation (Co-IP) assay. The HA-RhSAF and RhGID1B-GFP or RhGID1C-GFP fusion proteins were transiently co-expressed in N. benthamiana leaves. The RhGID1B-GFP or RhGID1C-GFP protein complexes were immunoprecipitated with the GFP-trap agarose beads, and their Co-IP partners were then detected using anti-HA antibodies. As shown in Fig. 3D, HA-RhSAF co-immunoprecipitated with RhGID1B/C-GFP, but not with the GFP control. These results indicate that RhSAF physically interacts with RhGID1B and RhGID1C.
To determine whether RhSAF regulates RhGID1B/C abundance, we co-expressed RhGID1B/C-FLAG with GFP-RhSAF or GFP-RhSAFΔF in N. benthamiana leaves. GFP-RhSAFΔF is a truncated protein lacking the F-box domain, which cannot form an SCF complex (Fig. 2D), and was thus used as a control. The RhGID1B/C-FLAG protein abundances were determined using immunoblots. Compared with GFP-RhSAFΔF, the overexpression of GFP-RhSAF substantially reduced RhGID1B/C-FLAG abundance (Fig. 4A), indicating that RhSAF promotes RhGID1B/C degradation. To determine which pathway mediates RhGID1B/C proteins degradation, we treated the above N. benthamiana leaves with the 26S proteasome inhibitor MG132. The addition of MG132 increased RhGID1B/C protein levels (Fig. 4B), indicating that RhSAF regulates RhGID1B/C stability through the 26S proteasome degradation pathway.
Figure 4.
RhSAF mediates the ubiquitination and degradation of RhGID1B/C. A) RhSAF promotes the degradation of RhGID1s in N. benthamiana leaves. Agrobacterium carrying GFP-RhSAF/GFP-RhSAFΔF, RhGID1B/C-FLAG, and GFP-FLAG plasmids were co-infiltrated in N. benthamiana leaves, respectively. Immunoblotting analysis was performed using anti-GFP antibodies or anti-FLAG antibodies. B) Treatment with the 26S proteasome inhibitor MG132 increased the level of RhGID1B and RhGID1C. Agrobacterium carrying HA-RhSAF and RhGID1B-FLAG or RhGID1C-FLAG plasmids were co-infiltrated in N. benthamiana leaves. Sixty hours after Agrobacterium infiltration, the infiltrated leaves were treated with 60 μM CHX (cycloheximide) and with or without 25 μM MG132 for 12 h. Immunoblotting analysis was performed using anti-GFP antibodies or anti-FLAG antibodies. C) Ubiquitination of RhGID1B and RhGID1C is mediated by RhSAF. Agrobacterium carrying GFP-RhSAF, HA-ubiquitin, and RhGID1B-FLAG or RhGID1C-FLAG plasmids were co-infiltrated in N. benthamiana leaves. Sixty hours after Agrobacterium infiltration, the leaves were treated with 25 μM MG132 for 12 h. Ubiquitinated proteins were purified using TUBE2-conjugated agarose. Immunoprecipitated proteins were analyzed by immunoblotting analysis with anti-FLAG antibodies to detect ubiquitinated RhGID1B/C-FLAG (top panel) and anti-ubiquitin antibodies as loading controls. For input, anti-GFP and anti-FLAG were used to detect the expression of individual proteins. Rubisco large subunit (rbcL) stained by Ponceau S was used as the loading control. IP, immunoprecipitation. IB, immunoblot.
Given that RhSAF encodes the F-box subunit of an SCF E3 ligase, we examined whether RhSAF could mediate the ubiquitination of RhGID1B/C in vivo. RhGID1B/C-FLAG was co-expressed with HA-UBQ and GFP-RhSAF or GFP-RhSAFΔF in N. benthamiana leaves. After incubation with MG132, we used the Tandem Ubiquitin-Binding Entities 2 (TUBE2) to pull down the polyubiquitinated proteins, and polyubiquitinated RhGID1B/C-FLAG was detected by immunoblot using anti-FLAG antibodies. As shown in Fig. 4C, much more polyubiquitinated RhGID1B/C-FLAG was observed when it was expressed with GFP-RhSAF than with GFP-RhSAFΔF. Taken together, these results indicate that RhSAF promotes the ubiquitination and degradation of the RhGID1B/C proteins.
Silencing RhGID1B/C accelerates petal senescence
Previously, we showed that GA treatment delayed rose petal senescence (Lü et al. 2014). To further confirm the inhibitory effect of endogenous GAs on petal senescence, we treated the rose plants with GA3 or GA biosynthesis inhibitor PAC. GA3 treatment delayed petal senescence dramatically, whereas PAC treatment accelerated petal senescence (Supplementary Fig. S5, A and B). In line with the senescence syndrome, GA treatment significantly repressed RhSAG12 expression and ion leakage, while PAC treatment exhibited the opposite effects compared to the mock (Supplementary Fig. S5, C and D). Therefore, GA functions to delay petal senescence.
We next assessed whether RhGID1B/C play a role in petal senescence. We silenced RhGID1B or RhGID1C in rose plants using VIGS; however, this did not alter flower senescence (Supplementary Fig. S6), indicating potential functional redundancy between RhGID1B and RhGID1C. To overcome this redundancy, we generated a TRV-RhGID1B/C construct to simultaneously silence RhGID1B and RhGID1C in rose petals. An RT-qPCR analysis indicated that RhGID1B and RhGID1C expression levels were significantly repressed in the TRV-RhGID1B/C petals relative to the TRV-control petals (Fig. 5A). As shown in Fig. 5, B and C, silencing both RhGID1B and RhGID1C significantly accelerated petal senescence. Accordingly, the expression of the senescence marker gene RhSAG12 was increased in the RhGID1B/C-silenced petals compared with the control petals (Fig. 5D).
Figure 5.
Silencing RhGID1B/c accelerates petal senescence. A) Relative expression levels of RhGID1B and RhGID1C in petals of TRV control and RhGID1B, RhGID1C double silenced (TRV-RhGID1B/C) plants. B) Petal senescence phenotype of TRV control and TRV-RhGID1B/C plants. Scale bars: 4 cm. C, D) Length of senescence phase (C) and relative expression of RhSAG12 (D) in petals of TRV control and TRV-RhGID1B/C plants. E) Relative expression levels of RhSAF, RhGID1B and RhGID1C in petals of TRV control and RhSAF, RhGID1B, RhGID1C triple silenced (TRV-RhSAF/RhGID1B/C) plants. F) Length of senescence phase of TRV control and TRV-RhSAF/RhGID1B/C plants. G) The petal senescence phenotype of TRV control and TRV-RhSAF/RhGID1B/C plants was recorded daily after stage 5. Scale bars: 4 cm. For A, C, D, E and F, mean values ± Sd are shown from five biological replicates (n = 5). For A, D, and E, the outmost layer petals of three flowers at stage 5 were pooled together as one biological replicate. Asterisks represent statistically significant differences between TRV and silenced plants (**P < 0.01) and n.s. represents not statistically significant, as determined by the t-test. TRV, tobacco rattle virus.
RhSAF associates with and destabilizes RhGID1B/C, with RhSAF and RhGID1B/C accelerating and delaying petal senescence, respectively. This led us to explore whether the delayed senescence phenotype of RhSAF-RNAi petals is due to increased RhGID1B/C level. To test this hypothesis, we used the VIGS approach to silence both RhSAF and RhGID1B/C (TRV-RhSAF + RhGID1B/C) in rose plants. The RT-qPCR assay showed that expressions of RhSAF and RhGID1B/C were significantly reduced in TRV-RhSAF + RhGID1B/C plants (Fig. 5E). Notably, in comparison with the control plants transformed with the TRV empty vector, silencing RhSAF and RhGID1B/C did not alter the timing of flower senescence (Fig. 5, F and G). Together, these results indicate that RhSAF accelerates petal senescence by destabilizing RhGID1B/C.
Ethylene promotes the degradation of RhGID1s through RhSAF
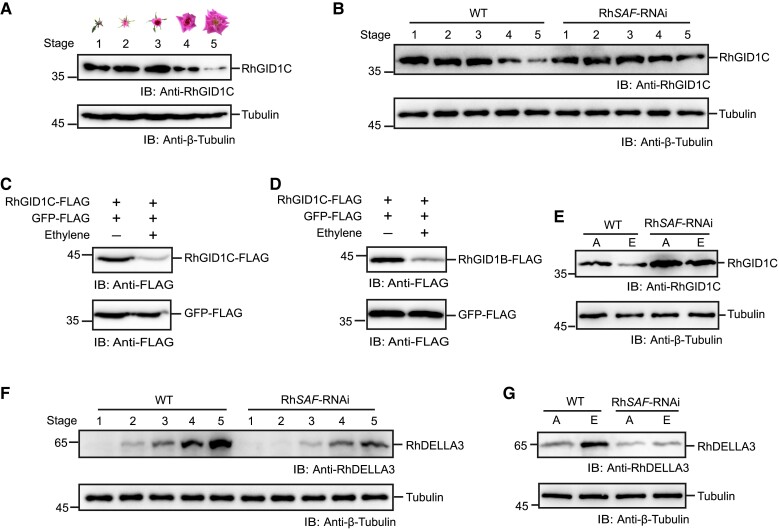
To detect the endogenous RhGID1B/C protein, we tried to produce antibodies against RhGID1B and RhGID1C; however, only anti-RhGID1C antibodies were successfully generated. Using the anti-RhGID1C antibodies, we were able to detect RhGID1C protein accumulation in rose petals during flower opening and senescence. As shown in Fig. 6A, the RhGID1C protein levels dramatically declined from stage 3 to stage 5. To assess whether the reduced accumulation of RhGID1C was caused by the altered transcription of RhGID1C during petal development, we analyzed the RhGID1C transcripts using RT-qPCR. The expression of RhGID1C was slightly altered during petal development (Supplementary Fig. S7A), indicating that the reduced RhGID1C accumulation is mainly regulated at the protein level.
Figure 6.
Ethylene promotes RhGID1s degradation and RhDELLA accumulation through RhSAF. A) accumulation of RhGID1C substantially declined at stage 5. Total protein was extracted from petals at different opening stages (stages 1 to 5) and immunoblotting analysis was performed using anti-RhGID1C antibodies. Detection of Tubulin was used as an internal control. B) Immunoblot assay showing the RhGID1C protein abundance in WT and RhSAF-RNAi petals during flower opening. C, D) Immunoblot assay showing that ethylene treatment promotes the degradation of RhGID1C (C) and RhGID1B (D). Agrobacterium carrying GFP-FLAG and RhGID1B-FLAG or RhGID1C-FLAG plasmids were co-infiltrated in rose petals. After 48 h, petals were treated with 10 ppm ethylene for 24 h. The abundance of RhGID1B, RhGID1C, and GFP was detected by anti-FLAG antibodies by immunoblotting. E) Ethylene-induced degradation of RhGID1C was impeded in RhSAF-RNAi plants. Flowers at stage 2 were treated with air (A) or ethylene (E) for 24 h. The abundance of RhGID1C was detected by immunoblotting using anti-RhGID1C antibodies. F) Immunoblot blot assay showing the endogenous RhDELLA3 abundance in WT and RhSAF-RNAi petals during flower opening, the RhDELLA3 was detected by anti-RhDELLA3 antibodies. G) Ethylene-induced RhDELLA3 accumulation was compromised in RhSAF-RNAi plants. Flowers at stage 2 were treated with air (A) or 10 μL L–1 ethylene (E) for 24 h, and the endogenous RhDELLA3 abundance was detected by immunoblot using the anti-RhDELLA3 antibodies. Tubulin was used as an internal control. RNAi, RNA interference. IB, immunoblot.
We further determined whether the protein accumulation of RhGID1C is affected by RhSAF. The abundance of RhGID1C in WT and RhSAF-RNAi petals was detected using immunoblots, revealing substantially higher levels in the RhSAF-RNAi petals, especially from stage 4 to stage 5 (Fig. 6B). To study whether the increased RhGID1C protein levels were caused by a higher transcription of RhGID1C in RhSAF-RNAi lines, we analyzed the RhGID1C transcripts; however, no obvious differences in RhGID1C expression were detected between the WT and RhSAF-RNAi lines (Supplementary Fig. S7B), indicating that the RhSAF protein destabilizes RhGID1C.
Since RhSAF expression is induced upon ethylene treatment (Fig. 1, B and D), and RhSAF encodes an E3 ligase that mediates the ubiquitination and degradation of RhGID1B/C (Fig. 4), we next investigated how the ethylene signal regulates the protein level of RhGID1B/C. RhGID1B/C-FLAG were transiently expressed in rose petals and treated with ethylene, and the corresponding protein levels were determined using immunoblots with anti-FLAG antibodies. Ethylene was found to substantially promote the degradation of RhGID1B/C (Fig. 6, C and D).
To further test whether RhSAF participates in the ethylene-induced protein degradation of RhGID1C, we treated the WT and RhSAF-RNAi plants with ethylene. The RhGID1C protein level was determined using immunoblots with anti-RhGID1C antibodies. Ethylene substantially promoted the degradation of RhGID1C in the WT, but only a slight decrease was observed in the RhSAF-RNAi plants (Fig. 6E). RhGID1C transcript levels did not change after ethylene treatment, however (Supplementary Fig. S7B), indicating that the degradation of RhGID1C promoted by ethylene is dependent on RhSAF.
DELLA proteins are commonly acknowledged as the master suppressors of GA signaling and GID1 is required for their degradation (Sun and Gubler 2004; Ueguchi-Tanaka et al. 2007). Given that ethylene promotes the degradation of RhGID1s, we speculated that ethylene enhances the stability of rose DELLA proteins. To test this, we first searched the rose genome and identified three DELLA proteins (Supplementary Fig. S8). Thus, specific peptide of these three proteins was used to produce polyclonal antibodies and only anti-RhDELLA3 antibodies were successfully generated. The abundance of endogenous RhDELLA3 was determined by immunoblot with anti-RhDELLA3 antibodies. Similar to RhSAF, RhDELLA3 gradually accumulated in petals during flowering opening and peaked at stage 5 (Fig. 6F), which was in contrast to RhGID1C (Fig. 6A). Compared with WT, the accumulation of RhDELLA3 was reduced in RhSAF-RNAi plants (Fig. 6F). In addition, ethylene promoted the accumulation of RhDELLA3 in WT while this accumulation was weakened in RhSAF-RNAi petals (Fig. 6G). In summary, the above results indicated that RhSAF-mediated the ethylene-induced degradation of RhGID1, thereby leading to the accumulation of DELLA proteins and the suppression of the GA signaling.
RhSAF-RhGID1s module is required for ethylene-induced petal senescence
The above molecular and biochemical data prompted us to investigate whether the RhSAF-RhGID1s module plays a role in ethylene-induced petal senescence. We treated the WT, RhSAF-RNAi, TRV-RhGID1B/C, and TRV-RhSAF/RhGID1B/C lines flowers at stage 2 with ethylene or air (control) to determine whether senescence sensitivity to ethylene was affected. In the presence of ethylene, the senescence phase of WT was significantly reduced (Fig. 7, A and B). However, the senescence phase of RhSAF-RNAi reduced much less in response to ethylene, suggesting that RhSAF is essential for ethylene-induced senescence. Similarly, the ethylene-induced petal senescence was compromised in TRV-RhGID1B/C and TRV-RhSAF/RhGID1B/C plants as well (Fig. 7, A and B), suggesting that RhSAF-RhGID1s module is required for ethylene-induced petal senescence.
Figure 7.
Silencing of RhGID1B/C and RhSAF reduced sensitivity to ethylene. A) phenotypes of WT control, TRV control, RhGID1B/C, and RhSAF-silenced flowers with air or ethylene treatment were recorded daily. Scale bars: 4 cm. B) length of the senescence phase (from fully open flowers to occurrence of senescence) of WT control, TRV control, RhGID1B/C, and RhSAF-silenced flowers with air or ethylene treatment. Mean values ± Sd are shown from five biological replicates (n = 5). The significance of differences at each stage was tested using the one-way ANOVA with the Tukey comparisons test (P < 0.05). RNAi, RNA interference. TRV, tobacco rattle virus.
Discussion
GA signaling is vital for petal senescence regulation
Petal senescence is an evolutionarily acquired strategy critical for plant reproduction and fitness. The crosstalk between ethylene and GA metabolism has previously been shown to play a crucial role in petal senescence (Lü et al. 2014; Yin et al. 2015; Sun et al. 2021). In this study, we demonstrated that GAs act downstream of ethylene, and that ethylene antagonizes the action of the GAs by destabilizing RhGID1s during petal senescence.
GAs appear to have a dual and contradictory role, both delaying and accelerating senescence, depending on the species, tissue type, developmental stage, and environmental conditions (Shamsi et al. 2019; Woo et al. 2019). In Arabidopsis, GAs are considered to promote leaf senescence. Exogenous treatment with GA3 accelerates leaf senescence, a process that is retarded in the ga1-3 mutant lacking proper DELLA protein activity and GA biosynthesis (Chen et al. 2014). By contrast, leaf senescence occurred earlier in the Q-DELLA/ga1-3 mutant (Chen et al. 2014). Recently, WRKY45 and WRKY6 transcription factors were characterized to interact with the DELLA proteins in GAs-mediated leaf senescence, supporting the positive role of GAs in leaf senescence (Chen et al. 2017a; Zhang et al. 2018).
In contrast with the results of the Arabidopsis studies, GA functions to delay rose petal senescence. Our previous studies showed that the exogenous application of GAs delays petal senescence, and represses the expression of the GA biosynthesis gene RhGA20ox1, resulting in the acceleration of petal senescence (Lü et al. 2014), demonstrating that GA metabolism plays essential roles in rose petal senescence. In the present study, we investigate the function of GA signaling in petal senescence. During petal senescence, the amount of the GA receptor, RhGID1C, was substantially reduced, while the abundance of the core GA signaling repressor, RhDELLA3, markedly increased (Fig. 6, B and F). The silencing of RhGID1s genes accelerates the senescence of rose petals (Fig. 5B), demonstrating that GA signaling is also critical for the regulation of petal senescence.
RhGID1s are degraded by a SCF-type E3 ligase during petal senescence
The involvement of the UPS in phytohormone signaling is well documented (Vierstra 2009), with several critical components involved in phytohormone signaling being targeted by various E3 ligases under different conditions. The clade A protein phosphatase 2C (PP2C) ABI1 (ABA-INSENSITIVE 1) works as a coreceptor of abscisic acid (ABA) to control multiple ABA responses (Raghavendra et al. 2010); however, ABA also triggers the degradation of ABI1 through two U-box E3 ligases, PUB12/13 (Kong et al. 2015). Likewise, the CRL3 E3 ligase BTB/POZ AND MATH DOMAIN proteins (BPMs) and RING E3 ligase ABA-insensitive RING protein3 (AIRP3) were found to interact with and promote the ubiquitination and degradation of ABI1 (Julian et al. 2019; Pan et al. 2020). BRI1-EMS-SUPPRESSOR1 (BES1) is the master transcription factor controlling the expression of many brassinosteroid (BR)-responsive genes (Kim et al. 2011). The F-box protein crl recognizes BES1 and mediates its ubiquitination in response to strigolactone (Wang et al. 2013). Furthermore, the RING-type E3 ligase SINA of A. thaliana (SINAT) and F-box protein BES1-ASSOCIATED F-BOX1 (BAF1) were found to target BES1 for degradation in the light and during sucrose starvation, respectively (Yang et al. 2017; Wang et al. 2021).
A GA RECEPTOR RING E3 UBIQUITIN LIGASE (GARU), mediates the degradation of GID1 via the ubiquitin-proteasome pathway and thus negatively regulates the GA response (Nemoto et al. 2017). The SA receptor, NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1), was reported to act as a recognition subunit of Cullin3-RING E3 ligase to degrade GID1 in response to salicylic acid (Yu et al. 2022). Nevertheless, it remained unclear whether there existed another route to modulate GID1s homeostasis. Here, we demonstrated that the rose SCF-type E3 ligase, RhSAF, promotes the ubiquitination and degradation of the RhGID1s through its F-box domain. We therefore speculate that, for an important component involved in multiple biological processes, plants require multiple E3 ligases to ensure protein stability properly regulated in response to changes in complex environments and developmental signals, which may be a common mechanism by which plants improve their fitness (Ali et al. 2020; Nolan et al. 2020).
SCF complexes are the largest family of E3 ligases, with hundreds of F-box protein genes identified in plants (Xu et al. 2009). It has been hypothesized that sessile plants evolved many more F-box proteins than other species to respond to various stimuli rapidly and precisely, because they lack the option to change their location (Skaar et al. 2013). Indeed, F-box proteins were reported to regulate many aspects of plant growth and development (Abd-Hamid et al. 2020); however, only a few F-box protein genes have been reported to regulate organ senescence. In Arabidopsis, the mutation of an F-box protein gene, ORE9, which was later identified to be MAX2 in strigolactone signaling, resulted in delayed leaf senescence (Woo et al. 2001). In rice (Oryza sativa), an F-BOX PROTEIN CONTAINING A KELCH REPEAT MOTIF (OsFBK12), interacts with and destabilizes S-ADENOSYL-L-METHIONINE SYNTHETASE1 (SAMS1) to regulate leaf senescence (Chen et al. 2013). In petunia, an ANKYRIN REPEAT RING ZINC FINGER PROTEIN (PhXB3), was thought to promote petal senescence (Xu et al. 2007). However, F-box protein genes regulating petal senescence have remained elusive. Previous proteomics studies showed that the protein ubiquitination level is dramatically changed during petal senescence, indicating that F-box proteins might play an important role in this process (Guo et al. 2017; Lu et al. 2019). Here, we identified an F-box protein, RhSAF, which accelerates petal senescence through its E3 ubiquitin ligase activity.
Ethylene antagonizes the effects of GA in petal senescence
Plants have evolved sophisticated mechanisms to coordinate hormone activities in response to developmental and environmental fluctuations. Crosstalk between the ethylene and GA pathways has been widely studied, and their relationship can be either synergistic or antagonistic depending on their plant species, biological processes, and target cell types (Fu et al. 2015; Qin et al. 2022). These two hormones act synergistically in regulating seed germination, apical hook development, and hypocotyl elongation in Arabidopsis, while antagonistically in flowering, root growth, and petal senescence (Fu et al. 2015; Ma et al. 2018). Specifically, it was found that DELLA mediates ethylene-induced inhibition of root growth, and RGA (REPRESSOR OF ga1-3) was stabilized in ctr1 mutant upon ethylene treatment (Achard et al. 2003). However, even decades later, the mechanism by which ethylene stabilizes the DELLA proteins remains unknown. In the present study, RhDELLA3 and RhGID1C show antagonistic expression patterns during flower opening and upon ethylene treatment (Fig. 6). Further investigations revealed that RhSAF is required for ethylene-induced RhDELLA3 stabilization and RhGID1C degradation (Fig. 6E), revealing a mechanism by which ethylene antagonizes GA action through degrading RhGID1s and stabilizing RhDELLA3.
Efficient communication between different cellular compartments is vital for cells to respond and adapt to developmental and environmental changes. The ethylene signaling coordinates intracellular compartment communications to regulate plant development through its multilocation signal components. The ethylene is perceived by the endoplasmic reticulum (ER)-localized receptors (Zhao et al. 2021). Ethylene perception triggers the translocation of the ER-localized CTR1 and EIN2-CEND to the nucleus (Park et al. 2023). Subsequently, the nucleus-localized EIN3 is stabilized and activates the transcription of ethylene-responsive genes (Zhao et al. 2021). Among these ethylene-responsive genes, the ubiquitin-proteasome genes execute their missions by destabilizing specific proteins. Here, we reveal that the F-box protein, RhSAF, responds to ethylene signals from ER and destabilizes the nucleus-localized GA receptors, RhGID1s.
Molecular and biochemical evidence demonstrates that RhGID1s are targets of RhSAF, indicating that RhGID1s act downstream of RhSAF. However, RhSAF and RhGID1s double silenced flowers did not senesce as early as the RhGID1s silenced flowers (Fig. 5G). One possibility is that besides RhGID1s, RhSAF relies on additional proteins to promote petal senescence. The subcellular localization results support this possibility. RhSAF localizes both to the nucleus and the plasma membrane (Fig. 2B), but the interaction between RhSAF and RhGID1s only occurred in the nucleus (Fig. 3C), we speculate that RhSAF may target additional plasma membrane-associated or nuclear proteins to regulate senescence. Mining the whole substrates of RhSAF will help to reveal the mechanisms underlying RhSAF in petal senescence regulation. This issue remains to be elucidated in the future. Taken together, these results reveal a mechanism by which ethylene promotes rose petal senescence. We propose a model in which ethylene promotes the degradation of RhGID1s by activation of E3 ligase, RhSAF, thus inhibiting the negative effects of GA during petal senescence (Fig. 8).
Figure 8.
Schematic model for RhSAF-RhGID1s regulatory module in petal senescence. At the early stages of flower opening, RhGID1s are stabilized since the low amount of ethylene present in petals fails to induce the expression of RhSAF, thus promoting GA signaling and preventing petal senescence (up panel). At the late stages of flower opening (down panel), the expression of RhSAF is induced by the increased amount of ethylene. RhSAF then assembles into the SCF complex and recognizes RhGID1s and mediates their ubiquitination and degradation through the 26S proteasome, which attenuates GA signaling and accelerates petal senescence. The thickness of the arrows indicates the amount of ethylene or RhSAF. CUL1, Cullin 1 protein; RSK, rose SKP1 protein; RBX1, RING-box protein 1; E2, ubiquitin-conjugating enzyme; Ub, ubiquitin; 26S, 26S proteasome.
Materials and methods
Plant materials and growth conditions
The rose (R. hybrida) cultivar Samantha was used as the WT control. The rose plantlets were propagated by tissue culture, as described previously (Wu et al. 2017). Briefly, rose stems with one node were grown in MS medium (4.4 g L–1 MS, 30 g L–1 sucrose, 1.0 mg L–1 6-BA, 0.05 mg L–1 NAA, 1.0 mg L–1 GA3 [pH 5.9], and 5.5 g L–1 agar) for 45 d, and then transferred to rooting medium (4.4 g L–1 MS, 0.1 mg L–1 NAA [pH 5.9], and 6.5 g L–1 agar) for 30 d. The rooted plants were transplanted into pots (9 cm diameter) containing a mixture of nutritive soil and vermiculite (1:1) and cultured at a 16 h light/8 h dark photoperiod, by white fluorescent lamps (intensity of 150 μmol m−2 s−1, SINOL, SN-T5, 16 W, Dongguan, China), with a relative humidity of ∼60% and a 22 °C ambient temperature. Growth conditions for N. benthamiana are the same as those for rose plants.
Senescence phenotyping
Flower opening stages are defined as follows: stage 1, unopened bud with visible petals; stage 2, completely opened bud with loose outer layer petals; stage 3, partially opened flower; stage 4, flower with outer layer fully opened; stage 5, fully opened flower. We evaluated the senescence by integrating morphological, molecular, and physiological indicators. At the morphological level, we defined the flower senescence phase as the period from the development of fully open flowers to the emergence of these senescence symptoms including petal wilting or abscission. At the molecular and physiological levels, we measure the expression of the senescence marker gene RhSAG12 and ion leakage levels.
Ion leakage measurement
Rose petals were immersed in 20 mL of distilled water and placed in a constant temperature oscillator at 25 °C and 200 rpm for 30 min. The initial conductivity was measured using a conductivity meter (Mettler-Toledo FiveGo F3). Subsequently, the solution was boiled in a water bath for 15 min, and the total conductivity was measured after cooling to room temperature. Ion leakage was calculated by dividing the initial conductivity by the total conductivity.
RNA extraction and RT-qPCR
The total RNA was extracted using the hot borate method (Gao et al. 2016), and the first-strand cDNA was synthesized with HiScript II Q Select RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China). An RT-qPCR was performed using the Step One Plus Applied Biosystems real-time PCR system (ABI, Thermo Fisher Scientific) with 2×Realtime PCR Supermix (SYBR green, with anti-Tag) (Mei5 Biotechnology, Beijing, China). RhUBI2 (UBIQUITIN 2) was used as the reference gene (Meng et al. 2013). All experiments were performed in triplicate. Data were calculated using the 2−ΔΔCt quantification method (Livak and Schmittgen 2001). The primers used for the PCR are provided in Supplementary Table S4.
VIGS
VIGS was performed as previously described (Wu et al. 2017). Gene-specific fragments of RhSAF (400 bp), RhGID1B (400 bp), and RhGID1C (400 bp) were obtained in a PCR and then inserted into the pTRV2 vector (Ma et al. 2008) to generate the constructs pTRV2-RhSAF, pTRV2-RhGID1B, and pTRV2-RhGID1C. For the double silencing of RhGID1B and RhGID1C, the gene-specific fragments (400 bp) for RhGID1B and RhGID1C were obtained using PCR and then jointly inserted into the pTRV2 vector. Similarly, the RhSAF, RhGID1B, and RhGID1C triple-silencing construct was generated using the above method. The constructs were transformed into Agrobacterium (A. tumefaciens) strain GV3101. The phenotypes of all transformed rose flowers were observed and photographed daily.
Subcellular localization
The subcellular localization of RhSAF was detected in N. benthamiana leaves. The coding sequence (CDS) of RhSAF was cloned and inserted into the Super1300-GFP vector (Sun et al. 2022) to generate the ProSuper:GFP-RhSAF construct. Pro35S:PM-mcherry (CD3-1007) was used as a plasma membrane marker (Liang et al. 2020) and Pro35S:NF-YA4-mcherry was used as the nuclear marker (Zhang et al. 2019). Agrobacterium carrying ProSuper:GFP-RhSAF and Pro35S:PM-mcherry or Pro35S:NF-YA4-mcherry were mixed in a 1:1 ratio and infiltrated into the N. benthamiana leaves. Three days after infiltration, the fluorescence signals were observed using confocal microscopy (Olympus FluoView FV1000). The GFP and mCherry fluorescent proteins were excited with 488 and 543 nm laser lines (laser intensity 35%), respectively. The fluorescence emissions were collected as below: GFP (506 to 538 nm, gain 550) and mCherry (575 to 630 nm, gain 680), respectively.
Identification of RSKs and RhDELLAs in the rose genome
The whole Rosa protein sequence was downloaded from the genome annotation database of R. chinensis “Old Blush” (https://lipm-browsers.toulouse.inra.fr/pub/RchiOBHm-V2/) (Raymond et al. 2018). To identify the rose SKP1 and DELLA candidates, we downloaded the Hidden Markov Model (HMM) profile of Skp1 (PF01466) and Skp1_POZ (PF03931), GRAS (PF03514), DELLA (PF12041) from Pfam protein family database (http://pfam.xfam.org/). HMMER 3.0 (https://www.ebi.ac.uk/Tools/hmmer/) was used to search the protein sequences with an E-value of 1e−3 as a minimum. All candidate protein sequences were examined using a BLASTP-algorithm-based search and Arabidopsis (A. thaliana) (http://www.arabidopsis.org/) SKP1 amino acid sequences as queries was conducted with an e-value ≤ 1e−3. After removing all of the redundant sequences, the output putative protein sequences were submitted to CDD (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi), Pfam and SMART (http://smart.embl-heidelberg.de/) to confirm the conserved domain. The predicted protein sequences without the relevant domain were excluded.
Phylogenetic analysis
The amino acid sequences were aligned using the ClustalW (https://www.genome.jp/tools-bin/clustalw). The alignment result was then used to construct a phylogenetic tree using the neighbor-joining method with MEGA version 11 software (Tamura et al. 2021) using the following parameters: 1,000 bootstrap replicates and the following parameters: Poisson correction, pairwise deletion, and uniform rates. Tree files and alignments are provided in Supplementary Files S1 to S4.
Y2H assay
Y2H assays were performed using as previously described (Chen et al. 2021). Briefly, RhGID1B/C were cloned into pGADT7 to be fused with GAL-AD fusion and RhSAF was cloned into pGBKT7 to be fused with GAL-BD. The yeast (S. cerevisiae) strain Y2H Gold was co-transformed with the above two plasmids using PEG/LiAc methods and plated on a selection medium lacking tryptophan and leucine (SD-Trp-Leu) at 28 °C for 3 d. The transformants were then diluted and dropped onto SD-Trp-Leu and SD-Trp-Leu-His + 0.5 mm 3-AT + X-gal plates for further selection.
Split-luciferase complementation (SFLC) assay
The SFLC was performed as previously described (Chen et al. 2017b). Briefly, the CDS of RhSAF was cloned into pCAMBIA1300-cLUC, and RhGID1B/C and RhSKP1s were introduced into pCAMBIA1300-nLUC (Kong et al. 2015). The constructs were transformed into A. tumefaciens EHA105. The transformed A. tumefaciens cells were collected by centrifugation at 4,000 × g for 8 min, and then resuspended with infiltration buffer (0.2 mm acetosyringone, 10 mm MgCl2, 10 mm MES, pH 5.6) to a final OD600 of ∼1.5. Agrobacterium cells carrying the nLUC or cLUC constructs were mixed in a 1:1 ratio and infiltrated into N. benthamiana leaves. After 3 d, the LUC signals were detected using a charge-coupled device camera (CHEMIPROHT 1300B/LND, 16 bits; Roper Scientific, Sarasota, FL, USA).
Bimolecular fluorescence complementation assays
Full-length cDNA fragments of RhSAF, RhGID1B, RhGID1C, RhEBF1, and RhEIN3 were PCR-amplified and cloned into pSAT6-neYFP or pSAT6-ceYFP (Citovsky et al. 2006). Isolation and transformation of Arabidopsis mesophyll protoplasts were performed as previously described (Yoo et al. 2007). Fluorescence signals were observed using a Zeiss confocal laser scanning microscope (LSM 980). The GFP and mCherry fluorescent proteins were excited with 488 and 561 nm (laser intensity 10%), respectively, and fluorescence emissions were collected as below: GFP (495 to 562 nm, gain 700) and mCherry (580 to 633 nm, gain 710), respectively.
Co-IP assay
Four-week-old N. benthamiana leaves were co-infiltrated with Agrobacterium carrying the plasmid ProSuper:3×HA-RhSAF and ProSuper:RhGID1B/C-GFP or ProSuper:GFP. After 3 d, the N. benthamiana leaves were collected for Co-IP assays. The total proteins were extracted with IP buffer (50 mm Tris-HCl [pH 7.5], 150 mm NaCl, 1 mm EDTA, 0.5% (v/v) Triton X-100, 20% (v/v) glycerol, 10 mm NaF, 1 mm DTT, and Roche cOmplete ULTRA EDTA-free proteinase inhibitor cocktail, Mannheim, Germany). The extracts were incubated with GFP-Trap (ChromoTek, Martinsried, Germany) for 2 h and washed in IP buffer five times. The beads were mixed with SDS sample buffer (100 mm Tris-HCl [pH 6.8], 4% (w/v) SDS, 0.2% (v/v) bromophenol blue, 20% (v/v) glycerol, and 200 mm DTT) and heated at 95 °C for 10 min. The IP products were detected using immunoblots with anti-GFP (1:3000, Abclonal AE012, Wuhan, China) and anti-HA (1:2000, Roche 11867423001, Mannheim, Germany) antibodies.
In vivo degradation assay
The CDS of RhSAF, RhSAFΔF, RhGID1B/C, and GFP were cloned into the Super1300 vector to generate the constructs ProSuper:GFP-RhSAF, ProSuper:GFP-RhSAFΔF, ProSuper:3×HA-RhSAF, ProSuper:RhGID1B/C-3×FLAG, and ProSuper:GFP-3×FLAG, respectively. Agrobacterium cells carrying the above plasmids were infiltrated into N. benthamiana leaves. GFP-FLAG was used as an internal control. At 2.5 d after the infiltration, 60 μM cycloheximide (CHX) was infiltrated into the same leaves for 12 h, after which the samples were harvested. The proteins were extracted with SDS sample buffer in boiling water for 10 min, then the samples were detected using immunoblots with anti-GFP (1:3000, Abclonal AE012, Wuhan, China) and anti-DDDDK tag (Binds to FLAG tag sequence) antibodies (1:5000, Abclonal AE005, Wuhan, China).
In vivo ubiquitination assay
Agrobacteria carrying the ProSuper:RhGID1B/C-3×FLAG, ProSuper:UBQ14-3×HA, and ProSuper:GFP-RhSAF (or ProSuper:GFP-RhSAFΔF as a negative control) were mixed in a 1:1:1 ratio and infiltrated into N. benthamiana leaves. At 2.5 d after the infiltration, 25 μM MG132 was infiltrated into the same leaves, and samples were harvested 12 h after the MG132 treatment. The N. benthamiana leaves were collected for IP assays. The total proteins were extracted with IP buffer (50 mm Tris-HCl [pH 7.5], 150 mm NaCl, 1 mm EDTA, 0.5% (v/v) Triton X-100, 20% (v/v) glycerol, 10 mm NaF, 1 mm DTT, Roche cOmplete ULTRA EDTA-free proteinase inhibitor cocktail, and 50 μM MG132). Next, the lysates were centrifuged at 16,200 × g for 10 min. For purification of the ubiquitinated protein, the supernatant for each sample was incubated with 30 μL TUBE2 agarose (LifeSensors, UM402, Philadelphia, USA) at 4 °C for 5 h. The beads were washed in IP buffer five times and the proteins were eluted using SDS sample buffer. The IP products were detected using immunoblots with the anti-DDDDK (1:5000, Binds to FLAG tag sequence, Abclonal AE005, Wuhan, China) and anti-ubiquitin (1:2000, Santa Cruz Biotechnology SC-8017, Dallas, United States) antibodies.
Hormone treatment
The ethylene and GA3 treatment assays were performed when the rose flower buds were grown to stage 2. For ethylene and 1-MCP treatment, the rose plants were sealed in a 40 L chamber with 10μL L–1 ethylene or air as a control, then incubated at 22 ± 1 °C for 24 h. 1 m NaOH was added to the chamber to prevent more CO2 accumulating. For GA3 and PAC treatment, two leaflets beneath the flower buds were immersed in a solution containing 100 μM GA3 or PAC solution. They were then subjected to a vacuum infiltration to allow the solution to permeate the leaves.
Antibodies generation and specificity validation
For the generation of the RhGID1C antibody, synthetic KLH-conjugated peptides CMEDNNEVNEVDLTDS were used as an immunogen to produce polyclonal antiserum in mice (Animal Experimental Center, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China). The polyclonal antibodies against RhSAF (ABclonal, Wuhan, China) and RhDELLA3 (Abmart, Shanghai, China) were generated through immunizing rabbits with synthetic KLH-conjugated peptides SVSDERVEGIISTC (For RhSAF) and RVIYDDNSEYDLRAIPGIAAYPQSQLQTATEINSKRLKC (For RhDELLA3), respectively. To validate the specificity and functionality of these above antibodies, we transiently expressed the RhGID1C-FLAG, GFP-RhSAF, RhDELLA3-FLAG, or empty vector (negative control) in N. benthamiana. The total protein of N. benthamiana leaves was extracted and subjected to immunoblotting. Anti-DDDDK or anti-GFP tag antibodies were used to indicate the size of the target proteins (Supplementary Fig. S9).
Petal protein extraction and immunoblotting
Total protein of petals was extracted with lysis buffer (50 mm Tris-HCl pH 7.5, 150 mm NaCl, 20 mm DTT, 1 mm EDTA (pH 8.0), 0.5% (w/v) PVP40, 0.5% (v/v) Triton X-100 (Sigma, Saint Louis, United States), 0.5% (v/v) NP40, 20% (v/v) glycerol, 10 mm NaF, Roche cOmplete ULTRA EDTA-free proteinase inhibitor cocktail). The samples were separated by tris-glycine gel and analyzed by immunoblot using anti-GFP (1:3,000, EASYBIO, BE2001, Beijing, China), anti-DDDDK tag (Binds to FLAG tag sequence, 1:5,000, ABclonal, AE005, Wuhan, China), and anti-Tubulin (Abmart, 1:5,000, M20005, Shanghai, China), anti-RhSAF (1:1,000), anti-RhDELLA3 (1:1,000), anti-RhGID1C (1:1,000) antibodies.
Statistical analysis
Data are presented as the mean ± standard deviation (Sd). Statistical analysis was performed with SPSS software. Comparisons between two groups of data were calculated by two-sided Student's t-test (*P < 0.05; **P < 0.01). One-way ANOVA with Tukey comparisons test was used for multiple comparisons and a value of P < 0.05 was considered to be statistically significant. Statistical data are shown in Supplementary Data Set 1.
Accession numbers
The rose gene sequences from this article can be found in GenBank (http://www.ncbi.nlm.nih.gov) under the following accession numbers: RhSAF (XM_024303861), RhGID1B (XM_024307000), RhGID1C (XM_024332999), and RhDELLA3 (XM_024332768), RhEIN3 (XM_024342597), RhEBF1 (XM_024306661).
Supplementary Material
Acknowledgments
We thank Dr. Zhizhong Gong (China Agricultural University) for providing the pSuper1300 vector and Dr. Yule Liu (Tsinghua University) for providing the TRV vector.
Contributor Information
Jingyun Lu, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Guifang Zhang, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Chao Ma, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Yao Li, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Chuyan Jiang, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Yaru Wang, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Bingjie Zhang, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Rui Wang, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Yuexuan Qiu, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Yanxing Ma, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Yangchao Jia, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Cai-Zhong Jiang, Crops Pathology and Genetic Research Unit, United States Department of Agriculture, Agricultural Research Service, Davis, CA 95616, USA; Department of Plant Sciences, University of California at Davis, Davis, CA 95616, USA.
Xiaoming Sun, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Nan Ma, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Yunhe Jiang, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Junping Gao, Beijing Key Laboratory of Development and Quality Control of Ornamental Crops, Department of Ornamental Horticulture, College of Horticulture, China Agricultural University, Beijing 100193, China.
Author contributions
Y. Jiang, C.M., and J.G. conceived and designed the research. J.L., G.Z., Y.L., C.J., Y.W., B.Z., R.W., Y.Q., Y.M., and Y. Jia performed the experiments. C.-Z.J., X.S., and N.M. provided technical support and conceptual advice. J.L., G.Z., Y. Jiang, and J.G. analyzed the data. Y. Jiang., G.Z., J.L., and J.G. wrote the manuscript with input from all co-authors.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1. Expression of the F-box protein genes induced by ethylene and age.
Supplementary Figure S2. RhSAF promotes petal senescence.
Supplementary Figure S3. RhSAF protein sequence feature.
Supplementary Figure S4. Phylogenetic analysis of RSKs in rose.
Supplementary Figure S5. GA treatment delays petal senescence.
Supplementary Figure S6. RhGID1B and RhGID1C show functional redundancy in petal senescence.
Supplementary Figure S7. Expression profiles of RhGID1C.
Supplementary Figure S8. Phylogenetic analysis of RhDELLAs in rose.
Supplementary Figure S9. Validation of the specificity of RhGID1C, RhSAF, and RhDELLA3 antibodies.
Supplementary Table S1. Ethylene-responsive F-box protein genes.
Supplementary Table S2. The expression level of ethylene-responsive F-box protein genes during flower opening and senescence.
Supplementary Table S3. RhSAF-interacting proteins identified by Y2H screen.
Supplementary Table S4. Sequences of the primers.
Supplementary Data Set 1. Summary of statistical tests.
Supplementary File 1. Newick file of phylogenetic tree of DELLAs.
Supplementary File 2. Newick file of phylogenetic tree of SKP1.
Supplementary File 3. Alignment of DELLA sequences.
Supplementary File 4. Alignment of SKP1 sequences.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 32230094 and 32172620) and the National Key Research and Development Program of China (grant no. 2018YFD1000400).
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Abd-Hamid N-A, Ahmad-Fauzi M-I, Zainal Z, Ismail I. Diverse and dynamic roles of F-box proteins in plant biology. Planta. 2020:251(3):68. 10.1007/s00425-020-03356-8 [DOI] [PubMed] [Google Scholar]
- Achard P, Baghour M, Chapple A, Hedden P, Straeten DVD, Genschik P, Moritz T, Harberd NP. The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc Natl Acad Sci USA. 2007:104(15):6484–6489. 10.1073/pnas.0610717104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard P, Genschik P. Releasing the brakes of plant growth: how GAs shutdown DELLA proteins. J Exp Bot. 2009:60(4):1085–1092. 10.1093/jxb/ern301 [DOI] [PubMed] [Google Scholar]
- Achard P, Vriezen WH, Van Der Straeten D, Harberd NP. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell. 2003:15(12):2816–2825. 10.1105/tpc.015685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Pardo JM, Yun DJ. Desensitization of ABA-signaling: the swing from activation to degradation. Front Plant Sci. 2020:11:379. 10.3389/fpls.2020.00379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Hua C, Shen L, Yu H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J Integr Plant Biol. 2020:62(1):118–131. 10.1111/jipb.12892 [DOI] [PubMed] [Google Scholar]
- Chen C, Ma Y, Zuo L, Xiao Y, Jiang Y, Gao J. The CALCINEURIN B- LIKE 4/CBL-INTERACTING PROTEIN 3 module degrades repressor JAZ5 during rose petal senescence. Plant Physiol. 2023:193(2):1605–1620. 10.1093/plphys/kiad365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Li Y, Li Y, Wang Y, Jiang C, Choisy P, Xu T, Cai Y, Pei D, et al. AUXIN RESPONSE FACTOR 18-HISTONE DEACETYLASE 6 module regulates floral organ identity in rose (Rosa hybrida). Plant Physiol. 2021:186(2):1074–1087. 10.1093/plphys/kiab130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Leng WB, Li DZ, Xia HW, Ren M, Tang QL, Gong QY, Gao FB, Bi F. Noninvasive imaging of ras activity by monomolecular biosensor based on split-luciferase complementary assay. Sci Rep. 2017b:7(1):9945. 10.1038/s41598-017-08358-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Xiang S, Chen Y, Li D, Yu D. Arabidopsis WRKY45 interacts with the DELLA protein RGL1 to positively regulate age-triggered leaf senescence. Mol Plant. 2017a:10(9):1174–1189. 10.1016/j.molp.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Chen M, Maodzeka A, Zhou L, Ali E, Wang Z, Jiang L. Removal of DELLA repression promotes leaf senescence in Arabidopsis. Plant Sci. 2014:219–220:26–34. 10.1016/j.plantsci.2013.11.016 [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu Y, Luo W, Li W, Chen N, Zhang D, Chong K. The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes, including leaf senescence. Rice Plant Physiol. 2013:163(4):1673–1685. 10.1104/pp.113.224527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Lee L-Y, Vyas S, Glick E, Chen M-H, Vainstein A, Gafni Y, Gelvin SB, Tzfira T. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol. 2006:362(5):1120–1131. 10.1016/j.jmb.2006.08.017 [DOI] [PubMed] [Google Scholar]
- Cuquel FL, Drefahl A, Garrett Dronk A. Enhancing vase life of rose with 1-MCP. Acta Hortic. 2007(751):455–458. 10.17660/ActaHortic.2007.751.57 [DOI] [Google Scholar]
- Davière JM, Achard P. Gibberellin signaling in plants. Development. 2013:140(6):1147–1151. 10.1242/dev.087650 [DOI] [PubMed] [Google Scholar]
- Fan Z-Q, Tan X-L, Shan W, Kuang J-F, Lu W-J, Chen J-Y. Characterization of a transcriptional regulator, BrWRKY6, associated with gibberellin-suppressed leaf senescence of Chinese flowering cabbage. J Agric Food Chem. 2018:66(8):1791–1799. 10.1021/acs.jafc.7b06085 [DOI] [PubMed] [Google Scholar]
- Fischer AM. The complex regulation of senescence. CRC Crit Rev Plant Sci. 2012:31(2):124–147. 10.1080/07352689.2011.616065 [DOI] [Google Scholar]
- Fletcher RA, Osborne DJ. Regulation of protein and nucleic acid synthesis by gibberellin during leaf senescence. Nature. 1965:207(5002):1176–1177. 10.1038/2071176a0 [DOI] [Google Scholar]
- Fu X, Gao X, Liu X. Integration of ethylene and gibberellin signaling. In: Wen C-K, editor. Ethylene in plants. Dordrecht: Springer Netherlands; 2015. p. 153–173. [Google Scholar]
- Gao Y, Liu C, Li X, Xu H, Liang Y, Ma N, Fei Z, Gao J, Jiang C-Z, Ma C. Transcriptome profiling of petal abscission zone and functional analysis of an aux/IAA family gene RhIAA16 involved in petal shedding in rose. Front Plant Sci. 2016:7:1375. 10.3389/fpls.2016.01375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liu J, Wei Q, Wang R, Yang W, Ma Y, Chen G, Yu Y. Proteomes and ubiquitylomes analysis reveals the involvement of ubiquitination in protein degradation in petunias. Plant Physiol. 2017:173(1):668–687. 10.1104/pp.16.00795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ren G, Zhang K, Li Z, Miao Y, Guo H. Leaf senescence: progression, regulation, and application. Mol Hortic. 2021:1(1):5. 10.1186/s43897-021-00006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha STT, Lim J-H, In B-C. Extension of the vase life of cut roses by both improving water relations and repressing ethylene responses. Hortic Sci Technol. 2019:37(1):65–77. 10.12972/kjhst.20190007 [DOI] [Google Scholar]
- Huang S, Gong B, Wei F, Ma H. Pre-harvest 1-methylcyclopropene application affects post-harvest physiology and storage life of the cut rose cv. Carola. Hortic Environ Biotechnol. 2017:58(2):144–151. 10.1007/s13580-017-0081-9 [DOI] [Google Scholar]
- Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan MIR. Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci. 2017:8:475. 10.3389/fpls.2017.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing W, Zhao Q, Zhang S, Zeng D, Xu J, Zhou H, Wang F, Liu Y, Li Y. RhWRKY33 positively regulates onset of floral senescence by responding to wounding- and ethylene-signaling in rose plants. Front Plant Sci. 2021:12:726797. 10.3389/fpls.2021.726797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian J, Coego A, Lozano-Juste J, Lechner E, Wu Q, Zhang X, Merilo E, Belda-Palazon B, Park SY, Cutler SR, et al. The MATH-BTB BPM3 and BPM5 subunits of cullin3-RING E3 ubiquitin ligases target PP2CA and other clade A PP2Cs for degradation. Proc Natl Acad Sci U S A. 2019:116(31):15725–15734. 10.1073/pnas.1908677116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DR. E3 ubiquitin ligases: key regulators of hormone signaling in plants. Mol Cell Proteom. 2018:17(6):1047–1054. 10.1074/mcp.MR117.000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaskheli AJ, Ahmed W, Ma C, Zhang S, Liu Y, Li Y, Zhou X, Gao J. RhERF113 functions in ethylene-induced petal senescence by modulating cytokinin content in rose. Plant Cell Physiol. 2018:59(12):2442–2451. 10.1093/pcp/pcy162 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hong SH, Kim YW, Lee IH, Jun JH, Phee B-K, Rupak T, Jeong H, Lee Y, Hong BS, et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J Exp Bot. 2014:65(14):4023–4036. 10.1093/jxb/eru112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-W, Guan S, Burlingame AL, Wang Z-Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol Cell. 2011:43(4):561–571. 10.1016/j.molcel.2011.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Cheng J, Zhu Y, Ding Y, Meng J, Chen Z, Xie Q, Guo Y, Li J, Yang S, et al. Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat Commun. 2015:6(1):8630. 10.1038/ncomms9630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Peng J, Wen X, Guo H. ETHYLENE-INSENSITIVE3 Is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell. 2013:25(9):3311–3328. 10.1105/tpc.113.113340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Jiang C, Liu Y, Gao Y, Lu J, Aiwaili P, Fei Z, Jiang CZ, Hong B, Ma C, et al. Auxin regulates sucrose transport to repress petal abscission in rose (Rosa hybrida). Plant Cell. 2020:32(11):3485–3499. 10.1105/tpc.19.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan Y, Jiang H, Bao Y, Ning G, Zhao L, Zhou X, Zhou H, Gao J, Ma N. Agrobacterium tumefaciens-mediated transformation of modern rose (Rosa hybrida) using leaf-derived embryogenic callus. Hortic Plant J. 2021:7(4):359–366. 10.1016/j.hpj.2021.02.001 [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001:25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu J, Xu Y, Fan Y, Wang Y, Zhang G, Liang Y, Jiang C, Hong B, Gao J, Ma C. Proteome and ubiquitome changes during rose petal senescence. Int J Mol Sci. 2019:20(24):6108. 10.3390/ijms20246108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü P, Zhang C, Liu J, Liu X, Jiang G, Jiang X, Khan MA, Wang L, Hong B, Gao J. RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J. 2014:78(4):578–590. 10.1111/tpj.12494 [DOI] [PubMed] [Google Scholar]
- Ma N, Ma C, Liu Y, Shahid MO, Wang C, Gao J. Petal senescence: a hormone view. J Exp Bot. 2018:69(4):719–732. 10.1093/jxb/ery009 [DOI] [PubMed] [Google Scholar]
- Ma N, Xue J, Li Y, Liu X, Dai F, Jia W, Luo Y, Gao J. Rh-PIP2; 1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol. 2008:148(2):894–907. 10.1104/pp.108.120154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng YL, Li N, Tian J, Gao JP, Zhang CQ. Identification and validation of reference genes for gene expression studies in postharvest rose flower (Rosa hybrida). Sci Hortic-Amsterdam. 2013:158:16–21. 10.1016/j.scienta.2013.04.019 [DOI] [Google Scholar]
- Nemoto K, Ramadan A, Arimura G-I, Imai K, Tomii K, Shinozaki K, Sawasaki T. Tyrosine phosphorylation of the GARU E3 ubiquitin ligase promotes gibberellin signalling by preventing GID1 degradation. Nat Commun. 2017:8(1):1004. 10.1038/s41467-017-01005-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 2020:32(2):295–318. 10.1105/tpc.19.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Lin B, Yang X, Liu L, Xia R, Li J, Wu Y, Xie Q. The UBC27-AIRP3 ubiquitination complex modulates ABA signaling by promoting the degradation of ABI1 in Arabidopsis. Proc Natl Acad Sci U S A. 2020:117(44):27694–27702. 10.1073/pnas.2007366117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Yao T, Seo JS, Wong ECC, Mitsuda N, Huang C-H, Chua N-H. Arabidopsis NITROGEN LIMITATION ADAPTATION regulates ORE1 homeostasis during senescence induced by nitrogen deficiency. Nat Plants. 2018:4(11):898–903. 10.1038/s41477-018-0269-8 [DOI] [PubMed] [Google Scholar]
- Park HL, Seo DH, Lee HY, Bakshi A, Park C, Chien Y-C, Kieber JJ, Binder BM, Yoon GM. Ethylene-triggered subcellular trafficking of CTR1 enhances the response to ethylene gas. Nat Commun. 2023:14(1):365. 10.1038/s41467-023-35975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H, Ma N, Tian J, Luo J, Chen J, Li J, Zheng Y, Chen X, Fei Z, Gao J. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals. Plant Physiol. 2013:163(2):775–791. 10.1104/pp.113.223388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Pandey BK, Li Y, Huang G, Wang J, Quan R, Zhou J, Zhou Y, Miao Y, Zhang D, et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell. 2022:34(4):1273–1288. 10.1093/plcell/koac008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends Plant Sci. 2010:15(7):395–401. 10.1016/j.tplants.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Raymond O, Gouzy J, Just J, Badouin H, Verdenaud M, Lemainque A, Vergne P, Moja S, Choisne N, Pont C, et al. The Rosa genome provides new insights into the domestication of modern roses. Nat Genet. 2018:50(6):772–777. 10.1038/s41588-018-0110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwat M, Tuteja N, editors. Chapter 13—flower senescence: present status and future aspects. In: Senescence signalling and control in plants. London: Academic Press; 2019. p. 211–225. [Google Scholar]
- Serek M, Sisler EC, Reid MS. Effects of 1-MCP on the vase life and ethylene response of cut flowers. Plant Growth Regul. 1995:16(1):93–97. 10.1007/BF00040512 [DOI] [Google Scholar]
- Shamsi IH, Sagonda T, Zhang X, Zvobgo G, Joan HI. Chapter 6—the role of growth regulators in senescence. In: Sarwat M, Tuteja N, editors. Senescence signalling and control in plants. London: Academic Press; 2019. p. 99–110. [Google Scholar]
- Sharma B, Joshi D, Yadav PK, Gupta AK, Bhatt TK. Role of ubiquitin-mediated degradation system in plant biology. Front Plant Sci. 2016:7:806–806. 10.3389/fpls.2016.00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol. 2013:14(6):369–381. 10.1038/nrm3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol. 2004:55:197–420. [DOI] [PubMed] [Google Scholar]
- Sun X, Qin M, Yu Q, Huang Z, Xiao Y, Li Y, Ma N, Gao J. Molecular understanding of postharvest flower opening and senescence. Mol Hortic. 2021:1(1):7. 10.1186/s43897-021-00015-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Feng Z, Ding Y, Qi Y, Jiang S, Li Z, Wang Y, Qi J, Song C, Yang S, et al. RAF22, ABI1 and OST1 form a dynamic interactive network that optimizes plant growth and responses to drought stress in Arabidopsis. Mol Plant. 2022:15(7):1192–1210. 10.1016/j.molp.2022.06.001 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11 molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021:38(7):3022–3027. 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Pei H, Zhang S, Chen J, Chen W, Yang R, Meng Y, You J, Gao J, Ma N. TRV-GFP: a modified tobacco rattle virus vector for efficient and visualizable analysis of gene function. J Exp Bot. 2014:65(1):311–322. 10.1093/jxb/ert381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Kusaba M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol. 2015:169(1):138–147. 10.1104/pp.15.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Nakajima M, Motoyuki A, Matsuoka M. Gibberellin receptor and its role in gibberellin signaling in plants. Annu Rev Plant Biol. 2007:58(1):183–198. 10.1146/annurev.arplant.58.032806.103830 [DOI] [PubMed] [Google Scholar]
- Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009:10(6):385–397. 10.1038/nrm2688 [DOI] [PubMed] [Google Scholar]
- Vriezen WH, Achard P, Harberd NP, Van Der Straeten D. Ethylene-mediated enhancement of apical hook formation in etiolated Arabidopsis thaliana seedlings is gibberellin dependent. Plant J. 2004:37(4):505–516. 10.1046/j.1365-313X.2003.01975.x [DOI] [PubMed] [Google Scholar]
- Wang P, Nolan TM, Clark NM, Jiang H, Montes-Serey C, Guo H, Bassham DC, Walley JW, Yin Y. The F-box E3 ubiquitin ligase BAF1 mediates the degradation of the brassinosteroid-activated transcription factor BES1 through selective autophagy in Arabidopsis. Plant Cell. 2021:33(11):3532–3554. 10.1093/plcell/koab210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun S, Zhu W, Jia K, Yang H, Wang X. Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev Cell. 2013:27(6):681–688. 10.1016/j.devcel.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Whyte P, Luckwill LC. A sensitive bioassay for gibberellins based on retardation of leaf senescence in Rumex obtusifolius (L). Nature. 1966:210(5043):1360–1360. 10.1038/2101360a0 [DOI] [Google Scholar]
- Woo HR, Chung KM, Park J-H, Oh SA, Ahn T, Hong SH, Jang SK, Nam HG. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001:13(8):1779–1790. 10.1105/TPC.010061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HR, Kim HJ, Lim PO, Nam HG. Leaf senescence: systems and dynamics aspects. Annu Rev Plant Biol. 2019:70(1):347–376. 10.1146/annurev-arplant-050718-095859 [DOI] [PubMed] [Google Scholar]
- Wu L, Ma N, Jia Y, Zhang Y, Feng M, Jiang C-Z, Ma C, Gao J. An ethylene-induced regulatory module delays flower senescence by regulating cytokinin content. Plant Physiol. 2017:173(1):853–862. 10.1104/pp.16.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zuo L, Ma Y, Jiang Y, Gao J, Tao J, Chen C. Protein kinase RhCIPK6 promotes petal senescence in response to ethylene in rose (Rosa Hybrida). Genes (Basel). 2022:13(11):1989. 10.3390/genes13111989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Zhang W, Wen C-K. ENHANCING ctr1–10 ETHYLENE RESPONSE2 is a novel allele involved in CONSTITUTIVE TRIPLE-RESPONSE1-mediated ethylene receptor signaling in Arabidopsis. BMC Plant Biol. 2014:14(1):48–48. 10.1186/1471-2229-14-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Ma H, Nei M, Kong H. Evolution of F-box genes in plants: different modes of sequence divergence and their relationships with functional diversification. Proc Natl Acad Sci U S A. 2009:106(3):835–840. 10.1073/pnas.0812043106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Jiang C-Z, Donnelly L, Reid MS. Functional analysis of a RING domain ankyrin repeat protein that is highly expressed during flower senescence. J Exp Bot. 2007:58(13):3623–3630. 10.1093/jxb/erm212 [DOI] [PubMed] [Google Scholar]
- Yang M, Li C, Cai Z, Hu Y, Nolan T, Yu F, Yin Y, Xie Q, Tang G, Wang X. SINAT e3 ligases control the light-mediated stability of the brassinosteroid-activated transcription factor BES1 in Arabidopsis. Dev Cell. 2017:41(1):47–58.e44. 10.1016/j.devcel.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Chang XX, Kasuga T, Bui M, Reid MS, Jiang CZ. A basic helix-loop-helix transcription factor, PhFBH4, regulates flower senescence by modulating ethylene biosynthesis pathway in petunia. Hortic Res. 2015:2(1):9. 10.1038/hortres.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007:2(7):1565–1572. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- Yu X, Cui X, Wu C, Shi S, Yan S. Salicylic acid inhibits gibberellin signaling through receptor interactions. Mol Plant. 2022:15(11):1759–1771. 10.1016/j.molp.2022.10.001 [DOI] [PubMed] [Google Scholar]
- Zhang S, Feng M, Chen W, Zhou X, Lu J, Wang Y, Li Y, Jiang C-Z, Gan S-S, Ma N. In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat Plants. 2019:5(3):290–299. 10.1038/s41477-019-0376-1 [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhao Q, Zeng D, Xu J, Zhou H, Wang F, Ma N, Li Y. RhMYB108, an R2R3-MYB transcription factor, is involved in ethylene- and JA-induced petal senescence in rose plants. Hortic Res. 2019:6(1):131–142. 10.1038/s41438-019-0221-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Z, Wang X, Wang J, Fan K, Li Z, Lin W. DELLA proteins negatively regulate dark-induced senescence and chlorophyll degradation in Arabidopsis through interaction with the transcription factor WRKY6. Plant Cell Rep. 2018:37(7):981–992. 10.1007/s00299-018-2282-9 [DOI] [PubMed] [Google Scholar]
- Zhao H, Yin C-C, Ma B, Chen S-Y, Zhang J-S. Ethylene signaling in rice and Arabidopsis: new regulators and mechanisms. J Integr Plant Biol. 2021:63(1):102–125. 10.1111/jipb.13028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon reasonable request.