Abstract
Crown roots are the main components of root systems in cereals. Elucidating the mechanisms of crown root formation is instrumental for improving nutrient absorption, stress tolerance, and yield in cereal crops. Several members of the WUSCHEL-related homeobox (WOX) and lateral organ boundaries domain (LBD) transcription factor families play essential roles in controlling crown root development in rice (Oryza sativa). However, the functional relationships among these transcription factors in regulating genes involved in crown root development remain unclear. Here, we identified LBD16 as an additional regulator of rice crown root development. We showed that LBD16 is a direct downstream target of WOX11, a key crown root development regulator in rice. Our results indicated that WOX11 enhances LBD16 transcription by binding to its promoter and recruiting its interaction partner JMJ706, a demethylase that removes histone H3 lysine 9 dimethylation (H3K9me2) from the LBD16 locus. In addition, we established that LBD16 interacts with WOX11, thereby impairing JMJ706–WOX11 complex formation and repressing its own transcriptional activity. Together, our results reveal a feedback system regulating genes that orchestrate crown root development in rice, in which LBD16 acts as a molecular rheostat.
WOX11–JMJ706 helps remove histone H3 lysine 9 dimethylation from the LBD16 locus to increase LBD16; LBD16 proteins competitively interact with WOX11 against JMJ706 and interfere with LBD16 transactivation by WOX11/JMJ706.
IN A NUTSHELL.
Background: Crown roots (also called adventitious roots) are the main components of root systems in cereals and play essential roles in nutrient uptake, stress tolerance, and yield. WUSCHEL-related homeobox (WOX) and lateral organ boundaries domain (LBD) transcription factors regulate adventitious root morphogenesis in angiosperms. However, the molecular mechanisms by which these transcription factors regulate rice crown root development remain unclear.
Questions: Does LBD16 control crown root development in rice (Oryza sativa)? What is the relationship between LBD16 and WOX11 in governing rice crown root morphogenesis?
Findings: LBD16 promotes the emergence and outgrowth of crown roots in rice by influencing cell division. WOX11 recruits the histone demethylase JMJ706 that removes histone H3 lysine 9 dimethylation (H3K9me2) from the LBD16 promoter, thereby activating LBD16 transcription. More importantly, LBD16 competes with JMJ706 for interaction with WOX11 and represses its own upregulation by the WOX11–JMJ706 complex. These results reveal a feedback regulatory system controlling the expression of genes orchestrating crown root development in rice, in which LBD16 acts as a molecular rheostat.
Next steps: Root system improvement will enhance rice environmental adaptation and yield potential. Future research will focus on the underlying mechanisms mediating WOX11 recruitment of JMJ706 and removal of H3K9me2 leading to altered root architecture. Such advances would help breeders cultivate excellent varieties with ideal root systems.
Introduction
Roots are the main organs for anchoring the plant into the soil, absorbing water and nutrients, and responding to various stresses (Barberon et al. 2016; Karlova et al. 2021). In dicot plants, the primary root is the main root, and its branches form secondary and tertiary lateral roots. Some plant species can also develop adventitious roots (ARs) constitutively and/or inductively in response to environmental signals, such as mechanical damage, flooding, biotic stress, or plant hormones (Bellini et al. 2014; Druege et al. 2016; Lakehal and Bellini 2019). While in cereals such as rice (Oryza sativa), wheat (Triticum aestivum), and maize (Zea mays), the primary root degenerates shortly after germination (Marcon et al. 2013; Rogers and Benfey 2015). The ARs (known as crown roots [CRs]) are the main component of the root system and replenish throughout the whole life cycle (Hochholdinger et al. 2004, 2018; Gonin et al. 2019).
WUSCHEL-RELATED HOMEOBOX 11 (WOX11), as a determinant of root morphogenesis, regulates CR development by multiple mechanisms in rice. It is reported that WOX11 genetically and physically interacts with other regulators to promote CR development. For instance, WOX11 associates with transcription factor AP2/ETHYLENE-RESPONSIVE FACTOR 3 (ERF3), and the LATERAL ORGAN BOUNDARIES DOMAIN (LBD) protein CROWN ROOTLESS1 (CRL1) to promote CR growth or initiation through cytokinin pathway, while cytokinin in turn promotes WOX11 protein accumulation in the root apical meristem (Zhao et al. 2015; Geng et al. 2023). Moreover, WOX11 has been shown to recruit ADA2-GCN5 (TRANSCRIPTIONAL ADAPTOR PROTEINS 2-GENERAL CONTROL NON-REPRESSED PROTEIN 5) histone acetyltransferase complex to the root-specific gene loci, thus stimulating their transcriptional activation (Zhou et al. 2017). Recently, a report revealed that WOX11 regulates CR formation through reactive oxygen species-mediated protein lysine acetylation modification (Xu et al. 2023).
LBD proteins, also known as ASYMMETRIC LEAVES2-LIKE, are plant-specific transcription factors characterized by a highly conserved LOB domain in the N terminus (Chen et al. 2019). The LOB domain comprises a zinc finger-like motif required for DNA binding, a conserved glycine residue, and a leucine zipper-like sequence required for protein–protein interactions (Majer and Hochholdinger 2011). LBD family proteins are classified into 2 subclasses (Class I and Class II) (Chanderbali et al. 2015; Kong et al. 2017). Class I proteins that contain both the zinc finger-like and leucine zipper-like motifs can be grouped into 4 clades (IA, IB, IC, and IE), whereas Class II members that have only the zinc finger-like motif are divided into 2 clades (IIA and IIB) (Chanderbali et al. 2015; Kong et al. 2017). Among them, LBD IB subclass genes have conserved functions in regulating root development in different species (Taramino et al. 2007; Majer et al. 2012; Coudert et al. 2013; Muthreich et al. 2013; Chanderbali et al. 2015; Soyano et al. 2019; Zhang et al. 2020; Li et al. 2022; Omary et al. 2022; Mao et al. 2023). In rice, the LBD IB gene CRL1 was first discovered to control the formation of CRs (Inukai et al. 2005; Coudert et al. 2011, 2015; Gonin et al. 2022; Geng et al. 2023). It remains unknown whether other LBD members function in CR development in rice.
In this work, we showed that the rice LBD16 gene, another member of the LBD IB clade, is required for CR development and is a direct downstream target of WOX11. WOX11 interacts with histone demethylase JMJ706 to activate LBD16 expression. Furthermore, we found that LBD16 competes with JMJ706 for interaction with WOX11 and represses the transactivation of its own expression by the WOX11–JMJ706 complex. This work identifies LBD16 as a regulator of CR development, provides insight into chromatin mechanism of WOX11-mediated gene activation, and establishes a negative feedback system controlling CR development in rice.
Results
WOX11 directly activates LBD16 expression
In rice, most members of LBD IB subclass show high expression in roots (Supplementary Fig. S1). To study whether the root expression of rice LBD genes was dependent on WOX11, we analyzed the wox11 root transcriptome (Jiang et al. 2017) and found that LBD16 (LOC_02g57490) was downregulated in wox11 roots. To further confirm whether WOX11 was required for LBD16 expression, we analyzed LBD16 expression levels in CRs of WOX11-FLAG (OE-WOX11) and wox11 plants and found that the LBD16 transcript level was decreased by 10 times in wox11 but clearly increased by 100 times in OE-WOX11 plants compared with the wild type (WT) (Fig. 1A). Furthermore, in situ hybridization assays showed that LBD16 mRNA was located in CR primordia at the emergence and elongation stages where WOX11 is expressed (Supplementary Fig. S2A), with stronger hybridization signals detected in OE-WOX11 and weaker signals in wox11 CR primordia than that in WT plants (Fig. 1B). The results suggested that LBD16 is a downstream target activated by WOX11.
Figure 1.
WOX11 directly activates LBD16 expression. A) Relative transcript levels of LBD16 in CR tips of 7-d wox11, OE-WOX11 (overexpressing WOX11), and WT seedlings. Actin1 was used to normalize different samples. B) In situ hybridization detection of LBD16 transcripts in CR primordial of 4-d seedlings of WT (a), wox11 (b), and OE-WOX11 (c) with an antisense or sense probe (d). Arrows indicate emerging CR initials. Bar = 100 μm. C) WOX11 triggered the expression of ProLBD16:LUC in rice protoplast cells. Schemes of the constructs used in the cotransfection experiments are shown on the left. D) ChIP-qPCR assays of in vivo WOX11 binding to LBD16 in 7-d seedling CRs. Upper panel: schematic diagrams of the indicated binding loci. The transcribed regions (black boxes), translation start sites (ATG), and primer sets (Supplementary Figs. S1 to S3) used in ChIP experiments are indicated. The black triangle indicates the WOX11-binding site. Numbers indicate the distance of the binding site to ATG. Lower panel: ChIP assays with anti-FLAG in WT and OE-WOX11 (overexpressing WOX11) CRs. E) CR phenotypes of WT, wox11, and wox11 OE-LBD16 (overexpressing LBD16 in wox11, w11 OE16) at 7-d seedlings after germination. Bar = 1 cm. F) Toluidine blue-stained cross sections of coleoptilar nodes of WT, wox11, and wox11 OE-LBD16 (overexpressing LBD16 in wox11, w11 OE16) at 4 d after germination. Arrowheads indicate emerging CR initials. Bar = 100 μm. Data are shown as means ± Sd (n = 3 in A, C, and D and n = 12 in E). Student's t-test was used to calculate P-value (***P < 0.001; ****P < 0.0001; ns: not significant).
To confirm whether WOX11 functions as a direct regulator of LBD16, transient expression assays were performed and the results showed that WOX11 could directly activate LBD16 in rice protoplasts (Fig. 1C). Our previous studies revealed that WOX11 directly binds to the TTAATGG/C sequence in vitro and in vivo (Zhao et al. 2009, 2015). One WOX11-binding motif TTAATGC (−223 to −239 bp, S2 region) was found within the 2-kb proximal LBD16 promoter fragment (Fig. 1D, upper). To confirm the binding of WOX11 to LBD16 in planta, chromatin immunoprecipitation (ChIP) assays were performed using CRs of OE-WOX11 and WT plants. The S2 region including WOX11-binding site was highly enriched. By contrast, there was no obvious enrichment with the primers amplifying S1 and S3 regions. The results indicated that WOX11 directly binds to the LBD16 promoter region in vivo (Fig. 1D). In addition, we transferred LBD16-overexpressing vector driven by ubiquitin promoter (OE16) into wox11 background and found that LBD16 could also partially restore CR defective phenotype (Fig. 1, E and F). Taken together, these results indicated that WOX11 directly regulated LBD16 expression and was epistatic to LBD16.
LBD16 loss of function is strongly associated with defective CR development
To explore the function of LBD16 in CR development, 2 independent LBD16 knockout mutant alleles (lbd16-1 and lbd16-2) were generated using CRISPR/Cas9 technology (Supplementary Fig. S2B). Phenotypic analysis showed that the number of CRs in the mutants was significantly decreased compared with WT plants (Fig. 2A). Histological observations showed that the CR primordium initiation was retarded in the lbd16 plants (Fig. 2B). The results indicated that LBD16 promotes CR emergence.
Figure 2.
LBD16 loss of function is strongly associated with defective CR development. A) CR phenotypes of WT, lbd16-1, and lbd16-2 at 7-d seedlings after germination. Bar = 1 cm. B) Toluidine blue-stained cross sections of coleoptilar nodes of WT, lbd16-1, and lbd16-2 at 4 d after germination. Arrows indicate emerging CR initials. Bar = 100μm. C) Longitudinal view of EdU-labeled cells in 7-d seedling CR meristems of WT, lbd16-1, and lbd16-2. Numbers of EdU-labeled cells in root meristems were counted by ImageJ. Bar = 50 μm. Data are shown as means ± Sd (n = 10 in A and n = 3 in C). Student's t-test was used to calculate P-value (***P < 0.001; ****P < 0.0001).
Previous reports suggested that WOX11 regulates CR growth via promotion of cell division (Zhao et al. 2015; Zhou et al. 2017). This prompted us to investigate whether LBD16 promotes CR development by stimulating cell division. 5-Ethynyl-20-deoxyuridine (EdU), which labels newly replicated DNA, was used for detection of S-phase cell cycle progression in plant cells and tissues (Chehrehasa et al. 2009). The EdU signal was noticeably decreased in the root tips of 2 lbd16 lines versus WT (Fig. 2C). We also examined the expression levels of known auxin and cytokinin signaling pathway genes related to root development in the lbd16-1 and OE16 plants and found that expression levels of most genes were altered in these plants (Supplementary Fig. S3). These data suggest that the LBD16 controls CR emergence and elongation by regulating cell division through auxin and cytokinin signaling.
WOX11 interacts with histone demethylase JMJ706
Previous results indicated that LBD16 expression can be activated by the histone demethylase JMJ706 that removes H3K9 methylation from the LBD16 promoter (Sun and Zhou 2008). To study whether the WOX11-regulated LBD16 expression involved JMJ706, we performed yeast 2-hybrid assays and found that WOX11 and JMJ706 proteins could interact. Deletion analysis revealed that the JMJ706 C terminal (561 to 858 amino acids) has self-activation and the JMJ706 JmjN and JmjC region (1 to 432 amino acids) interacted with WOX11 (Supplementary Fig. S4 and Fig. 3A). The interaction was validated by pull-down assay with Escherichia coli-produced WOX11 tagged with glutathione S-transferase (GST) and the JmjN and JmjC region (1 to 432 amino acids) tagged with a 6 × His (Fig. 3B). Split-luciferase (LUC) complementation assays with JMJ706-N-terminal half of firefly LUC (nLUC) and C-terminal half of firefly LUC (cLUC)-WOX11 showed strong luminescence signals. Conversely, the coexpression of cLUC-WOX11 and nLUC, JMJ706-nLUC and cLUC or nLUC and nLUC, did not show any luminescence signals (Fig. 3C). These results confirmed the WOX11–JMJ706 interaction in plant cells. Furthermore, JMJ706 could be immunoprecipitated together with WOX11 in rice cells transfected with Pro35S:JMJ706-CFP and Pro35S:WOX11-FLAG or with Pro35S:WOX11-YFP and Pro35S:JMJ706-FLAG (Fig. 3D), demonstrating that WOX11 associated with JMJ706 in vivo.
Figure 3.
WOX11 interacts with histone demethylase JMJ706 in vitro and in vivo. A) Detection of WOX11 interaction with JMJ706 by yeast 2-hybrid assay (left). Schematic structures of full-length and truncated domains of JMJ706 and WOX11 were indicated on the right. B) Pull-down assay of WOX11 and JMJ706. JMJ706-6 × His (JmjN + JmjC) was incubated with GST or WOX11-GST in GST beads and was pulled down from the WOX11-GST conjugated GST beads. C) In vivo split-LUC complementation assay of the interaction between WOX11 and JMJ706 in N. benthamiana. Constructs encoding nLUC-tagged JMJ706 or nLUC alone were coinfiltrated into N. benthamiana leaves with cLUC-tagged WOX11 or cLUC alone. Infiltrated leaves were harvested and dark adapted for 5 min before detection of luminescence. D) Coimmunoprecipitation assay of WOX11 and JMJ706 interaction in rice cells. WOX11-FLAG or WOX11-YFP construct was cotransfected with JMJ706-CFP or JMJ706-FLAG, respectively.
JMJ706 functions in CR development
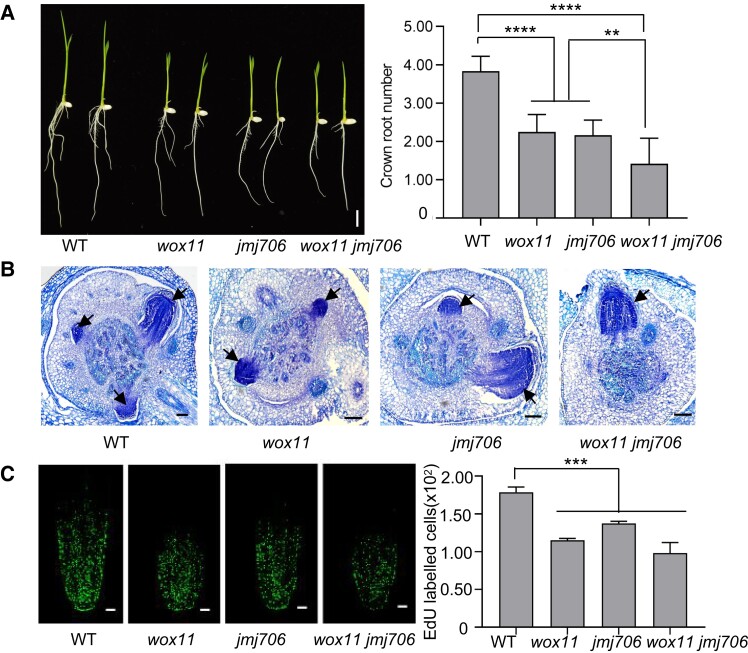
The above results suggested that JMJ706 was also involved in rice CR development. To test the hypothesis, we analyzed the root phenotype of a previously characterized T-DNA line in which the insertion is located in the promoter of JMJ706 (Sun and Zhou 2008). This mutant displayed fewer CRs at 7 d after germination compared with WT (Fig. 4A). Histological analysis showed that the initiation of CR primordium was retarded in jmj706 plants (Fig. 4B). Similarly, the cell division activity of root meristem was lower in jmj706 plants, as the EdU signal was noticeably decreased in jmj706 compared with WT (Fig. 4C). In situ hybridization analysis indicated that JMJ706 was expressed in CR primordia (Supplementary Fig. S5). These results confirm that JMJ706 plays a role in CR development.
Figure 4.
JMJ706 is involved in CR development. A) CR phenotypes of WT, wox11, jmj706, and wox11 jmj706 at 7 d after germination. Bar = 1 cm. B) Toluidine blue-stained cross sections of coleoptilar nodes of WT, wox11, jmj706, and wox11 jmj706 at 4 d after germination. Arrows indicate emerging CR initials. Bar = 100 μm. C) Longitudinal view of EdU-labeled cells in 7-d seedling CR meristems of WT, wox11, jmj706, and wox11 jmj706. Bar = 50 μm. Numbers of EdU-labeled cells in the CR tip were counted by ImageJ. Data are shown as means ± Sd (n = 12 in A and n = 3 in C). Student's t-test was used to calculate P-value (***P < 0.001; ****P < 0.0001).
To study the functional relationship between JMJ706 and WOX11 in CR development, we produced wox11 jmj706 double mutant by genetic crosses. The wox11 jmj706 double mutant plants exhibited more severe defects in CR numbers, CR primordium, and cell division activity compared with the wox11 or jmj706 single mutants (Fig. 4, A to C). In addition, compared with the wox11 and jmj706 single mutants, the expression levels of LBD16 were significantly reduced in the wox11 jmj706 double mutant (Supplementary Fig. S6). These results suggested that WOX11 and JMJ706 have a synergistic effect on CR development.
WOX11 is required for JMJ706 to upregulate LBD16 in CRs
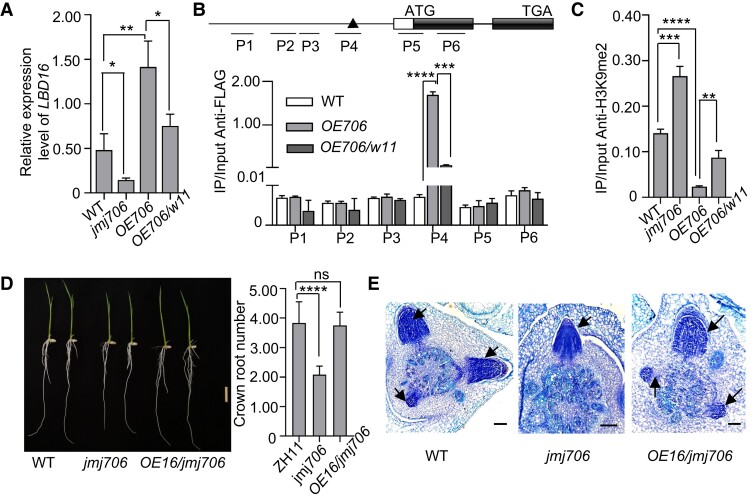
To validate the involvement of JMJ706 in LBD16 expression regulation during CR formation, we analyzed LBD16 transcript levels in CRs of WT, jmj706, and JMJ706-FLAG overexpression (OE706) lines. The results indicated that LBD16 expression was decreased in jmj706 but increased in OE706 plants compared with WT (Fig. 5A). Anti-FLAG ChIP-quantitative PCR (qPCR) analysis of OE706 CRs was performed to test JMJ706 binding to the LBD16 locus. As shown in Fig. 5B, JMJ706 was significantly enriched at P4 region of LBD6 locus covering the WOX11-binding motifs (TTAATGC), while no enrichment was detected in other tested regions in the LBD16 promoter and the coding sequence, suggesting that the enrichment of JMJ706 binding might be related to WOX11.
Figure 5.
WOX11 mediates the function of JMJ706 on LBD16. A)LBD16 expression level analysis by RT-qPCR in CR tips of 7-d seedlings of WT, jmj706, OE-JMJ706 (overexpressing JMJ706, OE706) and wox11 OE-JMJ706 (overexpressing JMJ706 in wox11, w11 OE706). B) In vivo binding of JMJ706 to LBD16 in 7-d seedling CRs detected by ChIP-qPCR assays. Upper panel: diagrams of the indicated binding loci. The transcribed regions (black boxes), translation start sites (ATG), and primer sets (P1 to P6) used in ChIP experiments are indicated. The black triangle indicates the binding site of WOX11. C) ChIP analysis of H3K9me2 enrichment on P4 loci within LBD16 promoter in WT, jmj706, OE706, (overexpressing JMJ706), and w11 OE706 (overexpressing JMJ706 in wox11). D) CR phenotypes of 7-d WT, jmj706, and jmj706 OE16 (overexpressing LBD16 in jmj706) seedlings. Bar = 1 cm. E) Toluidine blue-stained cross sections of coleoptilar nodes of 4-d WT, jmj706, and jmj706 OE16 (overexpressing LBD16 in jmj706) seedlings. Arrows indicate emerging CR initials. Bar = 100 μm. Data are shown as means ± Sd (n = 3 in A to C and n = 12 in D). Student's t-test was used to calculate P-value (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Given that JMJ706 is a histone demethylase of histone H3 lysine 9 dimethylation (H3K9me2), the H3K9me2 level at LBD16 locus was checked by ChIP experiments using H3K9me2 antibody in CRs of jmj706, OE706, and WT plants. H3K9me2 level at LBD16 locus was significantly enriched in jmj706 but clearly decreased in OE706 plants compared with WT (Fig. 5C). Besides, introducing LBD16-overexpressing vector (OE16) into jmj706 plants could restore CR-defective phenotype of jmj706 (Fig. 5, D and E). Together, the data indicate that JMJ706 controls LBD16 expression by removing H3K9me2 in CRs.
To test whether JMJ706-binding to LBD16 was dependent on WOX11, JMJ706-overexpression vector was transferred into wox11 plants (w11 OE706). ChIP-qPCR of OE706/w11 plants was performed to analyze whether the wox11 mutation affected JMJ706 binding to LBD16 promoter. The analysis revealed that the enrichment of JMJ706 at the P4 region of LBD16 promoter was clearly decreased in OE706/w11 compared with OE706 plants (Fig. 5B). Accordingly, H3K9me2 level at the LBD16 locus was higher in OE706/w11 than OE706 plants (Fig. 5C). Collectively, these results suggest that WOX11 recruits JMJ706 to LBD16 promoter to remove H3K9me2 to increase chromatin accessibility and LBD16 expression.
LBD16 interacts with WOX11 to inhibit its transcription
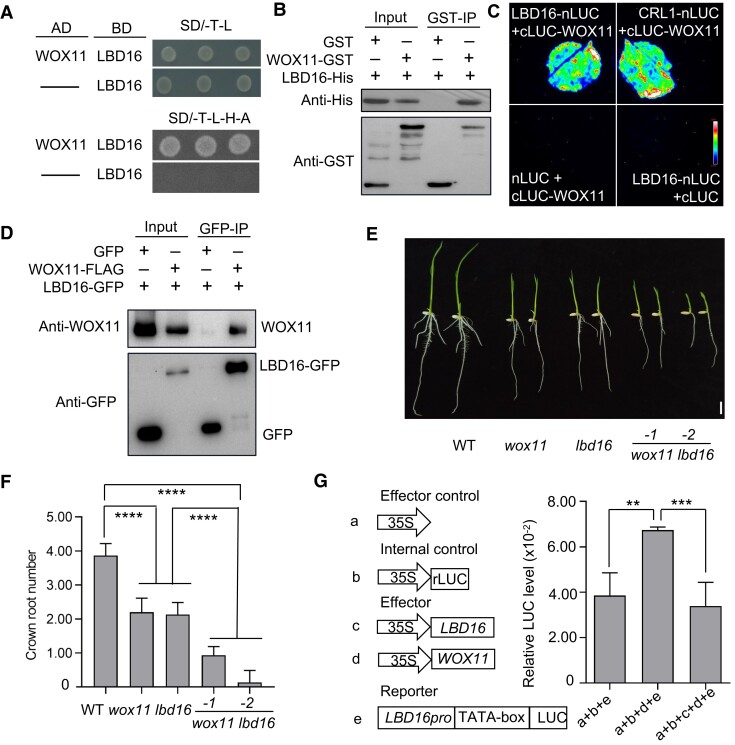
To further study the functional relationship between LBD16, WOX11, and JMJ706 in controlling CR growth, we analyzed the interaction between LBD16 and WOX11 or JMJ706 proteins. Yeast 2-hybrid assays showed that LBD16 could interact with WOX11 (Fig. 6A). In vitro GST pull-down and split-LUC complementation imaging assays confirmed the interaction between LBD16 and WOX11 (Fig. 6, B and C). Coimmunoprecipitation assays in transfected rice protoplasts further validated the interaction (Fig. 6D).
Figure 6.
LBD16 interacts with WOX11 to inhibit its transcription. A) Detection of LBD16 interaction with WOX11 by yeast 2-hybrid assay. B) Pull-down assay of association between LBD16 and WOX11. LBD16-6 × His was incubated with GST or WOX11-GST in GST beads and was pulled down from the WOX11-GST conjugated GST beads. C) In vivo split-LUC assay of LBD16 and WOX11 interaction in N. benthamiana leaves. D) Coimmunoprecipitation assay of LBD16 and WOX11 interaction in rice. E) CR phenotype of WT, wox11, lbd16, and wox11 lbd16 at 7-d seedlings. Bar = 1 cm. F) Statistical analysis for CR number in E). G) Relative LUC activities in rice protoplast cells that had been cotransfected with a reporter plasmid and an effector plasmid. Data are normalized to the internal LUC reference, which was cotransfected in the assay. Data are shown as means ± Sd (n = 15 in F and n = 3 in G). Student's t-test was used to calculate P-value (**P < 0.01; ***P < 0.001; ****P < 0.0001).
We subsequently generated wox11/lbd16 plants by CRISPR-Cas9 in the WT (Zhonghua11 [ZH11]) background (Supplementary Fig. S7). The wox11/lbd16 plants displayed a more severe phenotype compared with the wox11 and lbd16 single mutants (Fig. 6, E and F). This would suggest that LBD16 activation by WOX11 and repression by the WOX11–LBD16 complex operate to maintain an appropriate expression level of LBD16 during CR emergence and elongation. However, no interaction between LBD16 and JMJ706 was detected in Nicotiana benthamiana (Supplementary Fig. S8).
Next, using transcriptional activation assays, we analyzed the effect of the WOX11 and LBD16 interaction on LBD16 transcription. In the assays, the transformation of the Pro35S:WOX11 plasmid alone could activate the ProLBD16-LUC reporter gene. However, when both Pro35S:WOX11 and Pro35S:LBD16 plasmids were transferred together, the LBD16 promoter activity was reduced back to the control level (Fig. 6G). These results suggest that WOX11–LBD16 complex inhibits the transcription of the LBD16, suggesting a negative feedback mechanism for LBD16 regulation.
LBD16 and JMJ706 antagonize each other through competitive interaction with WOX11 protein
Yeast 3-hybrid assays showed that the 3 proteins WOX11, JMJ706, and LBD16 could not form a ternary complex (Supplementary Fig. S9). We next investigated whether LBD16 could inhibit the interaction between WOX11 and JMJ706 in the yeast 3-hybrid system with LBD16 under a Met25 promoter that is expressed only in the absence of methionine. The result showed that WOX11 and JMJ706 could interact each other (with growth on SD/-A-H-T-L medium), but induction of LBD16 led to a dramatic reduction of yeast growth on SD/-A-H-T-L-M medium (SD/-A-H-T-L-M) (Fig. 7A), indicating that LBD16 inhibited the formation of WOX11–JMJ706 complex. To confirm the observations, in vitro competitive pull-down assays were performed. The results demonstrated that increasing LBD16 amounts gradually increased the WOX11–LBD16 but decreased the WOX11–JMJ706 interaction (Fig. 7B), suggesting a competitive relationship between JMJ706 and LBD16 for interaction with WOX11. To further provide evidence for the competitive relationship, transient expression assays in protoplasts were performed. The transactivation of the ProLBD16-LUC reporter gene by the JMJ706-WOX11 complex was diminished by the presence of LBD16 (Fig. 7C). These results suggest the LBD16 competes with JMJ706 for interacting with WOX11, resulting in repression of its own expression.
Figure 7.
LBD16 and JMJ706 competitively interacted with WOX11. A) Yeast 3-hybrid assay showed that LBD16 inhibits the interaction between WOX11 and JMJ706. The AD-WOX11 and BD-JMJ706 were used as the control. B) In vitro pull-down assay showed the competitive binding between LBD16 with WOX11 against JMJ706. The JMJ706-His and LBD16-His are pulled down by WOX11-GST. Fixed amounts of WOX11-GST and JMJ706-N + C-His fusion proteins were incubated with increasing amount of LBD16-His fusion protein. C) Relative LUC activities in rice protoplast cells that had been cotransfected with a reporter plasmid and an effector plasmid. Data are shown as means ± Sd (n = 3). Student's t-test was used to calculate P-value (***P < 0.001). D) A proposed working model of JMJ706–WOX11–LBD16. WOX11 interacts with JMJ706 to remove H3K9me2 from the LBD16 locus and thus activating the expression of LBD16. Then, increased LBD16 proteins competitively interact with WOX11 against JMJ706 and interfere with LBD16 transactivation by the WOX11–JMJ706. Red circles label Me (H3K9me2). Aqua blue represents nucleosome; Orange denotes CR primordia upper and CRs (bottom), respectively.
Discussion
In this work, we show that LBD16 is a downstream target of WOX11 and is required for rice CR development. This is analogous to the observations in Arabidopsis (Arabidopsis thaliana) where AtLBD16 expression is activated by AtWOX11 during the initiation of axillary roots and callus formation mediated by auxin signaling (Liu et al. 2014; Sheng et al. 2017; Liu et al. 2018), suggesting that WOX11–LBD16 is an evolutionarily conserved regulatory module of axillary or CR formation. In addition, we have transferred LBD16-overexpressing vector driven by ubiquitin promoter (OE16) into wox11 background and found that LBD16 could also partially restore CR-defective phenotype (Fig. 1, E and F). These results suggest that LBD16 may also have a WOX11-independent function in CR development.
We show that WOX11 upregulates LBD16 expression by recruiting the histone demethylase JMJ706 in rice CRs, which is suppressed by LBD16 likely via disrupting the WOX11–JMJ706 complex. Alternatively, LBD16 may act as a DNA-binding transcriptional repressor. This possibility is supported by the presence of LBD protein-binding sites in the LBD16 locus (Supplementary Fig. S10) and by the observation that LBD16 suppresses WOX11-mediated activation of ProLBD16-LUC reporter gene in the transient expression system (Fig. 6G). Thus, the present work establishes a feedback regulatory system of gene expression controlling CR emergence and elongation, in which the WOX11–JMJ706 complex activates LBD16 transcription, leading to an accumulation of LBD16 that in turn competes with JMJ706 for WOX11 interaction or directly represses LBD16 transcription (Fig. 7D). This indicates that the LBD16 expression is tightly controlled, which highlights the importance of LBD16 in CR development.
There may be a threshold of LBD16 expression required to ensure the optimal root system architecture. This is supported by the observations that the overexpression of LBD16 produced no obvious CR phenotype (Supplementary Fig. S11). Excessive root system can lead to abnormal plant growth or reduced productivity. In rice, WOX11 overexpressing plants exhibit a large root system but poorly developed shoots (Zhao et al. 2009). Exogenous auxin treatment or overexpression of auxin biosynthesis genes increases root system volume but results in abnormal shoot growth (Zhang et al. 2018). The feedback regulation of LBD16 may provide an effective measure to balance root and shoot development.
It has been shown that the rice LBD protein CRL1 plays an essential role in CR initiation (Inukai et al. 2005). Previous work indicates that CRL1 functions synergistically with WOX11 to promote rice CR development (Geng et al. 2023). Likely, the functional interplay between WOX11 and LBD proteins constitutes an essential regulatory axis of CR development in rice. Because LBD proteins could form homo- or heterodimers (Lee et al. 2017), it remains unknown whether CRL1 and LBD16 interact to control LBD6 or CRL1 expression in CRs.
Epigenetic reprograming of chromatin modifications is essential for cell fate and development (Li 2002; Kouzarides 2007). It was shown that WOX11 recruits the histone acetyltransferase GCN5–ADA2 complex to enhance metabolic and cell division gene expression in CRs (Zhou et al. 2017). The present data showing that WOX11 recruits JMJ706 to demethylate H3K9me2 from the LBD16 locus reinforce the idea that WOX11 acts as a pioneer transcription factor to trigger epigenetic reprogramming of genes involved in CR initiation and development in rice.
The observation that WOX11 interacted with the N-terminal jmjN and jmjC region of JMJ706, which are conserved motifs in the jmjC demethylase family (Klose et al. 2006), suggests that WOX11 may potentially recruit other jmjC demethylases to its downstream target genes. In plants, histone H3K9me2 is a heterochromatin mark that mainly silences transposable element-associated sequences or genes, while H3K27me3 is a repressive mark in euchromatin regions known to maintain repressive state of developmental genes in organs/tissues where the genes are not expressed (McCarthy et al. 2021; Methot et al. 2021; Padeken et al. 2022). The regulation of LBD16 expression by H3K9me2 and the requirement of the H3K9me2 demethylase JMJ706 in various developmental processes shown in previous (Sun and Zhou 2008) and present work suggest that H3K9me2 also plays a role in the developmental switch of gene expression in rice.
It was reported that H3K9 demethylase INCREASE IN BONSAI METHYLATION 1 protects the transcripts of genes involved in meiosis and stomatal development from DNA methylation at CHG sites in Arabidopsis (Wang et al. 2016; Cheng et al. 2022). So far, no direct evidence supports the hypothesis that the key CR development regulatory factors WOX11, CRL1, and LBD16 are under DNA methylation control. Whether WOX11 recruitment of JMJ706 and removal of the H3K9me2 leading to changes in cell division are related to DNA methylation remains unclear and must be further elucidated.
Materials and methods
Plant materials
Rice (O. sativa ssp. japonica) varieties used in this study are ZH11 and Hwayoung. WOX11 T-DNA insertion knockout mutant (wox11) (Zhao et al. 2009), transgenic plants overexpressing WOX11 fused to FLAG tag (Cheng et al. 2018), and jmj706 (Sun and Zhou 2008) used in this study are described.
Two knockout lines for LBD16 (lbd16-1 and lbd16-2) and WOX11 and LBD16 double mutants (wox11 lbd16-1 and wox11 lbd16-2) were generated in ZH11 background using the CRISPR-Cas9 system (Gao and Zhao 2014). The CRISPR-P (http://crispr.hzau.edu.cn/CRISPR2/) program was used for designing of relevant sgRNAs. Specifically, the sgRNA AGTGCAGGCAAGCCAGTTCC was designed to generate the lbd16-1 and lbd16-2 single mutants and sgRNAs CATCTCGAGGCAGATGGGGT and AGTGCAGGCAAGCCAGTTCC were used to create the wox11 lbd16-1 and wox11 lbd16-2 double mutants. The Gibson Assembly method (Pme I digestion) was applied to assemble the sgRNA oligos into the CRISPR system.
For overexpressing LBD16 in WT (OE16), wox11 (wox11 OE16), and jmj706 (jmj706 OE16) background, overexpressing JMJ706 in WT (OE706) and wox11 background (w11 OE706) transgenic plants, both LBD16 and JMJ706 full-length cDNAs were amplified using the primer set LBD16-FLAG-F/R and JMJ706-FLAG-F/R (Supplementary Data Set S1), respectively, and then inserted into the overexpression vector pU2301-3 × FLAG (BamH I digestion) under the control of ubiquitin promoter (Cheng et al. 2018). Finally, the constructs were transformed into WT (ZH11) and wox11 mutant backgrounds, respectively.
Double mutant wox11 jmj706 was generated by genetic cross between wox11 with jmj706 plants and was selected by PCR using previously reported primers (Sun and Zhou 2008; Zhao et al. 2009).
Plant phenotype observation and growth conditions
Seeds were surface sterilized and germinated in media containing 0.3% (w/v) phytagel supplemented with 2% (w/v) sucrose at 28 °C (in light) and 24 °C (in dark) with a 16-h light (approximately 270 μmol·m−2·s−1)/8-h dark cycle (SANYO Versatile Environmental Test Chamber # MLR-351H). Seven days after germination on a square petri dish, seedlings with uniform growth were selected for counting the number of CRs. For growth in field, all indicated plants were grown in Wuhan in summer.
RNA extraction and RT-qPCR
The total RNA of rice root tips was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions followed by reverse transcription (1 µg RNA per reaction) using Vazyme mix (R312-01). Products were diluted by adding 80-µL ddH2O. RT-qPCR was performed using ChamQ SYBR qPCR Master Mix (Vazyme, Cat. No. Q311-02) on Applied Biosystems 7500 Real-Time PCR System. The following thermal profile was used for all reactions: 95 °C for 10 s and 42 cycles of 95 °C for 5 s and 60 °C for 30 s. The primers used are listed in Supplementary Data Set S1.
Transient reporter assays
The LUC activity assays were performed as previously described (Geng et al. 2023). Primers are listed in Supplementary Data Set S1.
ChIP-qPCR
ChIP experiments were performed as previously described (Liu et al. 2015). Briefly, about 0.5-g root tips of 7-d seedlings were cross-linked in 1% (w/v) formaldehyde for 30 min and used for chromatin extraction. After 20-min sonication (30-s on/30-s off, at high energy level) by Bioruptor Plus System (Diagenode), chromatin fragments were incubated with antibody-conjugated beads (5-μL anti-FLAG [Sigma F3165] coated onto protein G beads [Invitrogen 10003D], 5-μL anti-H3K9me2 [Abcam F3165] coated onto protein G beads [Invitrogen 10003D], and 5-μL anti-H3K9ac [Millipore F3165] coated onto protein G beads [Invitrogen 10003D]) overnight. After extensive washing, immunoprecipitated chromatin was reverse cross-linked and purified for qPCR. Specific primers were used for qPCR (Supplementary Data Set S1).
Yeast 2-hybrid assays
For yeast 2-hybrid, WOX11 cDNA was cloned into pGADT7 vector and JMJ706 and LBD16 cDNA were cloned into pGBKT7 vector, respectively. Truncated JMJ706 domains were obtained by PCR using specific primers (Supplementary Data Set S1) and tested by yeast 2-hybrid assays. The vectors pGADT7 and pGBKT7 were cotransformed into the yeast (Saccharomyces cerevisiae) strain AH109. The transformed yeasts were incubated on the SD/-Trp/-Leu medium at 28 °C. After 3 d, the clones were transferred to the SD/-Ade/- His/-Leu/-Trp medium for testing the interaction.
In vitro pull-down assays
GST, recombinant JMJ706-6 × His, LBD16-6 × His, and WOX11-GST fusion proteins were expressed in E. coli (strain BL21). JMJ706-6 × His or LBD16-6 × His was incubated with WOX11-GST or GST alone immobilized on GST beads and pulled down from the GST beads (GE Healthcare, Cat. No. 45-000-139). The proteins were subsequently analyzed by immunoblot with anti-GST antibody (Rabbit, Abcam Ab19256, 1:4,000 dilution) and anti-His antibody (Mouse, Abcam Ab9108, 1:4,000 dilution). Primers are listed in Supplementary Data Set S1.
Split-LUC complementation assays
JMJ706 and LBD16 cDNAs were cloned in-frame upstream of the sequence encoding the nLUC. WOX11 cDNA was cloned in-frame downstream of the sequence encoding the cLUC. Plasmids of different combinations were transiently coinfiltrated into N. benthamiana leaves. The infiltrated plants were kept in the dark for 3 d before being infiltrated with 0.15-mg/mL D-luciferin potassium salt as substrate. The leaves were harvested and kept in the dark for 5 min before determining luminescence signals by a CCD camera (TANAON 5200, Shanghai). Primers used in this study are listed in Supplementary Data Set S1.
Coimmunoprecipitation assays
For WOX11 and JMJ706 or LBD16 interaction, Pro35S:JMJ706-CFP with Pro35S:WOX11-FLAG or Pro35S:WOX11-YFP with Pro35S:JMJ706-FLAG or Pro35S:LBD16-GFP with Pro35S:LBD16-GFP and Pro35S: GFP were cotransfected into rice protoplasts, respectively. After 13-h incubation for transgene expression at room temperature, protoplasts were lysed in coimmunoprecipitation buffer (10 mm Tri-HCl, pH 7.5, 150 mm NaCl, 0.5 mm EDTA, 1% [v/v] Triton X-100, 1% [w/v] sodium deoxycholate, 0.1% [w/v] SDS, 1 mm DTT, 1 mm PMSF, and DNase I 15 U/mL) for 30 min, followed by centrifugation at 16,000 × g at 4 °C for 5 min to remove cellular debris. The supernatant was transferred to a new tube and incubated with GFP conjugated beads (Pierce, Cat. No. 2618) overnight and then precipitated and analyzed by immunoblot with anti-FLAG antibody (Mouse, Sigma F3165, 1:4,000 dilution) and anti-GFP antibody (Mouse, Abmart M20004, 1:4,000 dilution) to detect the WOX11 protein or JMJ706 protein. Primers are listed in Supplementary Data Set S1.
In situ hybridization and histological observation
All tested materials were fixed, dehydrated, embedded, sliced, and attached to slides as previously reported (Zhao et al. 2009). For preparation for the digoxigenin-labeled RNA probes, a specific coding region of genes was amplified via PCR using primers (Supplementary Data Set S1). The PCR products were used as templates for amplifying digoxigenin-labeled sense and antisense RNA probes. Tissue sections were cleared, dehydrated, dried, hybridized, and washed. The labeled probes were detected and images were photographed with a microscope (Nikon, ECLIPSE Ni-E). For histological analysis of CR primordia, 4-d seedlings were sampled. Images of CR primordia were photographed with a microscope (Nikon, ECLIPSE Ni-E); sense probes were used as a negative control.
EdU staining
EdU staining was performed as previously described (Li et al. 2015) using an EdU kit (C10310; Apollo 488) from Ribobio. Briefly, CRs of 7-d seedlings were immersed in 50 μm EdU solution for 3 h. After fixation for 30 min in 4% paraformaldehyde, a longitudinal vibration section was obtained and followed by treatment with Apollo. The fluorescence was detected with confocal microscopy (EGFP, Wavelength 488 nm, Voltage 563 V, OLYMPUS, FV1200).
Yeast 3-hybrid assays
Yeast 3-hybrid assays were performed using the MATCHMAKER GAL4 Two-Hybrid System. The full-length coding sequence of JMJ706 was cloned into the MCS I site of the pBridge vector (Clontech) and the coding sequence of LBD16 or WOX11 was inserted into the MCS II site as the bridge protein. The full-length coding sequence of WOX11 or LBD16 was cloned into the vector pGADT7. The vectors pGADT7 and pBridge were cotransformed into the yeast strain AH109. The transformed yeasts were incubated on the SD/-Trp/-Leu medium at 28 °C. After 3 d, the clones were transferred to the SD/-Ade/-His/-Leu/-Trp medium for testing the interaction. Alternatively, the clones were incubated on the plates containing SD/-Ade/-His/-Leu/-Trp/-Met to induce the LBD16 expression. All plates were incubated at 28 °C for 4 d. Primers are listed in Supplementary Data Set S1.
Statistical analysis
Statistical analyses were performed by using GraphPad Prism V7 (https://www.graphpad.com/scientific-software/prism/). Significances of differences between 2 groups were analyzed by Student's unpaired and 2-tailed t-tests with Welch's correction. The analysis results are provided in Supplementary Data Set S2.
Accession numbers
Sequence data from this article can be found in the Rice Genome Annotation Project website (http://rice.plantbiology.msu.edu/) under the following accession numbers: LBD16 (LOC_Os02g57490); WOX11 (LOC_Os07g48560); JMJ706 (LOC_Os10g42690); IAA3 (LOC_Os12g40900); IAA4 (LOC_Os01g08320); IAA5 (LOC_Os01g48450); IAA11 (LOC_Os03g43400); IAA31 (LOC_Os12g40900); ARF1 (LOC_Os01g13520); ARF12 (LOC_Os04g57610); ARF25 (LOC_Os12g41950); RR1(LOC_Os04g36070); RR2 (LOC_Os02g35180); RR3 (LOC_Os02g58350); and CKX4 (LOC_Os01g71310).
Supplementary Material
Acknowledgments
We are grateful to Prof. Xianghua Li for ordering reagents, Huazhi Song for helping in confocal microscopy, Shuangle Li for providing the pBridge vector, and Wentao Wang for providing the JMJ706-BD vector.
Contributor Information
Leping Geng, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Mingfang Tan, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Qiyu Deng, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Yijie Wang, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Ting Zhang, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Xiaosong Hu, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Miaomiao Ye, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Xingming Lian, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Dao-Xiu Zhou, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China; CNRS, INRAE, Institute of Plant Science Paris-Saclay (IPS2), University Paris-Saclay, Orsay 91405, France.
Yu Zhao, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Huazhong Agricultural University, Wuhan 430070, China.
Author contributions
L.G. performed most of the experiments described in this study, carried out data analyses, and prepared figures. M.T., Q.D., T.Z., X.H., and M.Y. contributed to the generation of the experimental materials. Y.W. performed yeast 2-hybrid assays. X.L. and D.-X.Z. revised the manuscript. Y.Z. supervised the project and wrote the manuscript with inputs from L.G. All authors read and approved the final manuscript.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1. Spatial expression profiles of LBD class IB genes in rice.
Supplementary Figure S2. Detection of LBD16 transcripts by in situ hybridization and generation of knockout alleles of LBD16.
Supplementary Figure S3. The expression levels of auxin and cytokinin signaling pathway genes in CR tips of 7-d lbd16-1 and OE16 (mixed samples of #2, #3, and #8) seedlings.
Supplementary Figure S4. Detection of JMJ706 C terminal (JMJ706-C) region self-activation activity by yeast 2-hybrid assay.
Supplementary Figure S5. Detection JMJ706 transcripts by in situ hybridization.
Supplementary Figure S6. Relative transcript levels of LBD16 in CR tips of 7-d wox11, jmj706, wox11 jmj706, and WT seedlings.
Supplementary Figure S7. Generation and characterization of CRISPR-Cas9 knockout lines of wox11 lbd16 in WT background.
Supplementary Figure S8. LBD16 and JMJ706 cannot interact in N. benthamiana cells.
Supplementary Figure S9. WOX11, LBD16, and JMJ706 cannot form trigonal complex.
Supplementary Figure S10. Determination of in vivo binding of LBD16 to LBD16 in the CRs of 7-d seedlings by ChIP-qPCR assays.
Supplementary Figure S11. CR phenotype and LBD16 expression levels in plants overexpressing LBD16 (OE16).
Supplementary Data Set S1. Primers used in this study.
Supplementary Data Set S2. Statistical analyses in this study.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31970806 and 31821005) and the Fundamental Research Funds for the Central Universities (2662023SKPY002).
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Barberon M, Vermeer JE, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell. 2016:164(3):447–459. 10.1016/j.cell.2015.12.021 [DOI] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014:65(1):639–666. 10.1146/annurev-arplant-050213-035645 [DOI] [PubMed] [Google Scholar]
- Chanderbali AS, He F, Soltis PS, Soltis DE. Out of the water: origin and diversification of the LBD gene family. Mol Biol Evol. 2015:32(8):1996–2000. 10.1093/molbev/msv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A. Edu, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009:177(1):122–130. 10.1016/j.jneumeth.2008.10.006 [DOI] [PubMed] [Google Scholar]
- Chen WF, Wei XB, Rety S, Huang LY, Liu NN, Dou SX, Xi XG. Structural analysis reveals a “molecular calipers” mechanism for a LATERAL ORGAN BOUNDARIES DOMAIN transcription factor protein from wheat. J Biol Chem. 2019:294(1):142–156. 10.1074/jbc.RA118.003956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Xu L, Bergér V, Bruckmann A, Yang C, Schubert V, Grasser KD, Schnittger A, Zheng B, Jiang H. H3k9 demethylases IBM1 and JMJ27 are required for male meiosis in Arabidopsis thaliana. New Phytol. 2022:235(6):2252–2269. 10.1111/nph.18286 [DOI] [PubMed] [Google Scholar]
- Cheng S, Tan F, Lu Y, Liu X, Li T, Yuan W, Zhao Y, Zhou DX. WOX11 recruits a histone H3K27me3 demethylase to promote gene expression during shoot development in rice. Nucleic Acids Res. 2018:46(5):2356–2369. 10.1093/nar/gky017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y, Bès M, Le TV, Pré M, Guiderdoni E, Gantet P. Transcript profiling of crown rootless1 mutant stem base reveals new elements associated with crown root development in rice. BMC Genom. 2011:12(1):387. 10.1186/1471-2164-12-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y, Dievart A, Droc G, Gantet P. ASL/LBD phylogeny suggests that genetic mechanisms of root initiation downstream of auxin are distinct in lycophytes and euphyllophytes. Mol Biol Evol. 2013:30(3):569–572. 10.1093/molbev/mss250 [DOI] [PubMed] [Google Scholar]
- Coudert Y, Le VAT, Adam H, Bès M, Vignols F, Jouannic S, Guiderdoni E, Gantet P. Identification of CROWN ROOTLESS1-regulated genes in rice reveals specific and conserved elements of postembryonic root formation. New Phytol. 2015:206(1):243–254. 10.1111/nph.13196 [DOI] [PubMed] [Google Scholar]
- Druege U, Franken P, Hajirezaei MR. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front Plant Sci. 2016:7:381. 10.3389/fpls.2016.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YB, Zhao YD. Self- processing of ribozyme- flanked RNAs into guide RNAs in vitro and in vivo for CRISPR- mediated genome editing. J Integr Plant Biol. 2014:56(4):343–349. 10.1111/jipb.12152 [DOI] [PubMed] [Google Scholar]
- Geng L, Li Q, Jiao L, Xiang Y, Deng Q, Zhou DX, Zhao Y. WOX11 and CRL1 act synergistically to promote crown root development by maintaining cytokinin homeostasis in rice. New Phytol. 2023:237(1):204–216. 10.1111/nph.18522 [DOI] [PubMed] [Google Scholar]
- Gonin M, Bergougnoux V, Nguyen TD, Gantet P, Champion A. What makes adventitious roots? Plants (Basel). 2019:8(7):240. 10.3390/plants8070240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonin M, Jeong K, Coudert Y, Lavarenne J, Hoang GT, Bes M, To HTM, Thiaw MN, Do TV, Moukouanga D, et al. CROWN ROOTLESS1 binds DNA with a relaxed specificity and activates OsROP and OsbHLH044 genes involved in crown root formation in rice. Plant J. 2022:111(2):546–566. 10.1111/tpj.15838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochholdinger F, Park WJ, Sauer M, Woll K. From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci. 2004:9(1):42–48. 10.1016/j.tplants.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Yu P, Marcon C. Genetic control of root system development in maize. Trends Plant Sci. 2018:23(1):79–88. 10.1016/j.tplants.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell. 2005:17(5):1387–1396. 10.1105/tpc.105.030981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhou S, Zhang Q, Song H, Zhou DX, Zhao Y. Transcriptional regulatory network of WOX11 is involved in the control of crown root development, cytokinin signals, and redox in rice. J Exp Bot. 2017:68(11):2787–2798. 10.1093/jxb/erx153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, Boer D, Hayes S, Testerink C. Root plasticity under abiotic stress. Plant Physiol. 2021:187(3):1057–1070. 10.1093/plphys/kiab392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006:7(9):715–727. 10.1038/nrg1945 [DOI] [PubMed] [Google Scholar]
- Kong Y, Xu P, Jing X, Chen L, Li L, Li X. Decipher the ancestry of the plant-specific LBD gene family. BMC Genom. 2017:18(S1):951. 10.1186/s12864-016-3264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007:128(4):693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Lakehal A, Bellini C. Control of adventitious root formation: insights into synergistic and antagonistic hormonal interactions. Physiol Plant. 2019:165(1):90–100. 10.1111/ppl.12823 [DOI] [PubMed] [Google Scholar]
- Lee HW, Kang NY, Pandey SK, Cho C, Lee SH, Kim J. Dimerization in LBD16 and LBD18 transcription factors is critical for lateral root formation. Plant Physiol. 2017:174(1):301–311. 10.1104/pp.17.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang J, Li L, Li J, Zhuang M, Li B, Li Q, Huang J, Du Y, Wang J, et al. TaMOR is essential for root initiation and improvement of root system architecture in wheat. Plant Biotechnol J. 2022:20(5):862–875. 10.1111/pbi.13765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002:3(9):662–673. 10.1038/nrg887 [DOI] [PubMed] [Google Scholar]
- Li J, Zhao Y, Chu H, Wang L, Fu Y, Liu P, Upadhyaya N, Chen C, Mou T, Feng Y, et al. SHOEBOX modulates root meristem size in rice through dose-dependent effects of gibberellins on cell elongation and proliferation. PLoS Genet. 2015:11(8):e1005464. 10.1371/journal.pgen.1005464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hu X, Qin P, Prasad K, Hu Y, Xu L. The WOX11–LBD16 pathway promotes pluripotency acquisition in callus cells during de novo shoot regeneration in tissue culture. Plant Cell Physiol. 2018:59(4):734–743. 10.1093/pcp/pcy010 [DOI] [PubMed] [Google Scholar]
- Liu J, Sheng L, Xu Y, Li J, Yang Z, Huang H, Xu L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014:26(3):1081–1093. 10.1105/tpc.114.122887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Zhou SL, Wang WT, Ye YR, Zhao Y, Xu QT, Zhou C, Tan F, Cheng SF, Zhou DX. Regulation of histone methylation and reprogramming of gene expression in the rice inflorescence meristem. Plant Cell. 2015:27(5):1428–1444. 10.1105/tpc.15.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer C, Hochholdinger F. Defining the boundaries: structure and function of LOB domain proteins. Trends Plant Sci. 2011:16(1):47–52. 10.1016/j.tplants.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Majer C, Xu C, Berendzen KW, Hochholdinger F. Molecular interactions of ROOTLESS CONCERNING CROWN AND SEMINAL ROOTS, a LOB domain protein regulating shoot-borne root initiation in maize (Zea mays L.). Philos Trans R Soc Lond B Biol Sci. 2012:367(1595):1542–1551. 10.1098/rstb.2011.0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Niu C, Li K, Fan L, Liu Z, Li S, Ma D, Tahir MM, Xing L, Zhao C, et al. Cytokinin-responsive MdTCP17 interacts with MdWOX11 to repress adventitious root primordium formation in apple rootstocks. Plant Cell. 2023:35(4):1202–1221. 10.1093/plcell/koac369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon C, Paschold A, Hochholdinger F. Genetic control of root organogenesis in cereals. Methods Mol Biol. 2013:959:69–81. 10.1007/978-1-62703-221-6_4 [DOI] [PubMed] [Google Scholar]
- McCarthy RL, Kaeding KE, Keller SH, Zhong Y, Xu LQ, Hsieh A, Hou Y, Donahue G, Becker JS, Alberto O, et al. Diverse heterochromatin-associated proteins repress distinct classes of genes and repetitive elements. Nat Cell Biol. 2021:23(11):1212–1212. 10.1038/s41556-021-00759-x [DOI] [PubMed] [Google Scholar]
- Methot SP, Padeken J, Brancati G, Zeller P, Delaney CE, Gaidatzis D, Kohler H, van Oudenaarden A, Grosshans H, Gasser SM. H3k9me selectively blocks transcription factor activity and ensures differentiated tissue integrity. Nat Cell Biol. 2021:23(11):1163–1175. 10.1038/s41556-021-00776-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthreich N, Majer C, Beatty M, Paschold A, Schutzenmeister A, Fu Y, Malik WA, Schnable PS, Piepho HP, Sakai H, et al. Comparative transcriptome profiling of maize coleoptilar nodes during shoot-borne root initiation. Plant Physiol. 2013:163(1):419–430. 10.1104/pp.113.221481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M, Gil-Yarom N, Yahav C, Steiner E, Hendelman A, Efroni I. A conserved superlocus regulates above- and belowground root initiation. Science. 2022:375(6584):eabf4368. 10.1126/science.abf4368 [DOI] [PubMed] [Google Scholar]
- Padeken J, Methot SP, Gasser SM. Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance. Nat Rev Mol Cell Bio. 2022:23(9):623–640. 10.1038/s41580-022-00483-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ED, Benfey PN. Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol. 2015:32:93–98. 10.1016/j.copbio.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Sheng L, Hu X, Du Y, Zhang G, Huang H, Scheres B, Xu L. Non-canonical WOX11-mediated root branching contributes to plasticity in Arabidopsis root system architecture. Development. 2017:144(17):3126–3133. 10.1242/dev.152132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Shimoda Y, Kawaguchi M, Hayashi M. A shared gene drives lateral root development and root nodule symbiosis pathways in Lotus. Science. 2019:366(6468):1021–1023. 10.1126/science.aax2153 [DOI] [PubMed] [Google Scholar]
- Sun Q, Zhou DX. Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc Natl Acad Sci U S A. 2008:105(36):13679–13684. 10.1073/pnas.0805901105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramino G, Sauer M, Stauffer JL, Multani D, Niu X, Sakai H, Hochholdinger F. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007:50(4):649–659. 10.1111/j.1365-313X.2007.03075.x [DOI] [PubMed] [Google Scholar]
- Wang Y, Xue X, Zhu JK, Dong J. Demethylation of ERECTA receptor genes by IBM1 histone demethylase affects stomatal development. Development. 2016:143(23):4452–4461. 10.1242/dev.129932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Yue Y, Chen Z, Zhou DX, Zhao Y. Transcription factor WOX11 regulates protein translation via ribosome protein acetylation in rice roots. Plant Physiol. 2023:191(4):2224–2228. 10.1093/plphys/kiad025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Li R, Xing J, Yan L, Wang R, Zhao Y. The YUCCA-Auxin-WOX11 module controls crown root development in rice. Front Plant Sci. 2018:9:523. 10.3389/fpls.2018.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Z, Ma B, Hou Q, Wan X. Phylogeny and functions of LOB domain proteins in plants. Int J Mol Sci. 2020:21(7):2278. 10.3390/ijms21072278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, Zhou DX. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell. 2015:27(9):2469–2483. 10.1105/tpc.15.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hu Y, Dai M, Huang L, Zhou DX. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009:21(3):736–748. 10.1105/tpc.108.061655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Jiang W, Long F, Cheng S, Yang W, Zhao Y, Zhou DX. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell. 2017:29(5):1088–1104. 10.1105/tpc.16.00908 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.