Abstract
Exposure to phthalates, used as plasticizers and solvents in consumer products, is ubiquitous. Despite growing concerns regarding their neurotoxicity, brain differences associated with gestational exposure to phthalates are understudied. We included 775 mother-child pairs from Generation R, a population-based pediatric neuroimaging study with prenatal recruitment, who had data on maternal gestational phthalate levels and T1-weighted magnetic resonance imaging in children at age 10 years. Maternal urinary concentrations of phthalate metabolites were measured at early, mid-, and late pregnancy. Child IQ was assessed at age 14 years. We investigated the extent to which prenatal exposure to phthalates is associated with brain volumetric measures and whether brain structural measures mediate the association of prenatal phthalate exposure with IQ. We found that higher maternal concentrations of monoethyl phthalate (mEP, averaged across pregnancy) were associated with smaller total gray matter volumes in offspring at age 10 years (β per log10 increase in creatinine adjusted mEP=−10.7, 95%CI: −18.12, −3.28). Total gray matter volumes partially mediated the association between higher maternal mEP and lower child IQ (β for mediated path =−0.31, 95%CI: −0.62, 0.01, p = 0.05, proportion mediated = 18%). An association of higher monoisobutyl phthalate (mIBP) and smaller cerebral white matter volumes was present only in girls, with cerebral white matter volumes mediating the association between higher maternal mIBP and lower IQ in girls. Our findings suggest the global impact of prenatal phthalate exposure on brain volumetric measures that extends into adolescence and underlies less optimal cognitive development.
Introduction
Mental health problems and intellectual disability are a leading cause of health loss among children and adolescents in high income countries.1 A combination of genetic predisposition, social determinants, environmental factors, and stochastic processes affect the developing brain and contribute to cognitive impairments and mental health problems in children. Given the rapid developmental changes in the brain and immaturity of metabolic pathways, the fetal period is a critical window of susceptibility for environmental insults.2 Toxic environmental chemicals receive particular attention because their contribution is potentially preventable.3 One group of such chemicals that presents a concern are phthalates, which are ubiquitously used as plasticizers and solvents in a wide range of commercial products, such as personal care products, food packaging, and vinyl flooring. In recent years the use of certain phthalates (e.g., di-2-ethylhexyl phthalate [DEHP]) in consumer products have been regulated under European Union Regulations4 and their use are restricted in toys and childcare products in the United States.5 Nonetheless, pregnant women are still ubiquitously exposed to phthalates, such as DEHP6–8 and its replacements such as di-iso-nonyl phthalate,9, 10 even though proportion of women exposed to legacy phthalates and newer replacement phthalates varies by the compound, location, and year of assessment. For this reason, the American Academy of Pediatrics and several other scientific organizations urged a call for action on this emerging child health concern, with recommendations to expand translational research on emerging neurotoxicants.3, 11, 12
Epidemiological studies have shown that prenatal exposures to DEHP, butyl-benzyl phthalate (BBzP), di-ethyl phthalate (DEP), and di-n-butyl phthalate (DBP) are associated with less optimal cognitive function, social development, and motor skills as well as behavioral problems in children.13, 14 In the Generation R Study, we reported that maternal prenatal urinary concentrations of phthalates, i.e., monoethyl phthalate (mEP) and a metabolite of di-n-octyl phthalate (DnOP), i.e., mono(3-carboxypropyl) phthalate (mCPP), were associated with lower IQ in school-age children.15, 16 There is some evidence that prenatal exposure to mono-n-butyl phthalate (mnBP, a metabolite of DBP) is related to brain development using ultrasound measurements.17 Most of our knowledge on specific brain regions and developmental processes influenced by phthalate exposure comes from animal models. For example, developmental DEHP exposure inhibits the cerebellar granule cell proliferation in male rat/mouse offspring.18 In animal models, prenatal phthalate exposure to a mixture of phthalates (DEHP, DEP, and others) results in a reduction in neuron number, synapse number, and size of the medial prefrontal cortex.19 Developmental exposure to DEHP also reduces proliferation and neurogenesis and leads to an abnormal neuronal distribution in the neocortex.20
Several studies have examined the neural basis of cognitive development and brain structure and function.21 The relationship between brain development as identified by structural magnetic resonance imaging (MRI) and children’s and adolescents’ cognitive abilities is not limited to global development. Relations of subcortical structural and regional development in prefrontal cortex with cognitive abilities have also been demonstrated.22–25 Neuroimaging studies have also been used to identify brain influences of environmental toxicants, such as lead, air pollution or neurotoxicants, such as organophosphate pesticides.26–29 Yet, brain MRI has rarely been used to study whether specific neurobiological influences of phthalate exposure is present in humans.30 One study examined the association between prenatal phthalate exposure and white matter microstructure in 76 preschool age children and found that higher maternal urinary concentrations of high molecular weight phthalates (metabolites of DEHP and BBzP) during the second trimester were associated with a higher mean diffusivity in diffusional tensor imaging, an indicator of impairments in white matter microstructure.30 However, other structural brain differences, including global and regional volumetric measures, and child IQ were not examined in that study.
We used data from Generation R, a population-based pediatric neuroimaging study with prenatal recruitment, to examine the extent to which repeatedly measured in utero exposure to phthalates is associated with brain volumetric measures at age 10 years and whether brain structural differences mediate the association of prenatal phthalate exposure and child IQ assessed at age 14 years. We hypothesized that prenatal exposure to phthalates would be associated with global alterations in the brain, and brain alterations would be mediators in the association of prenatal phthalates and child IQ. This hypothesis is grounded on observations from epidemiological studies that showed a wide range of behavioral and cognitive outcomes affected by prenatal phthalate exposure.12–14 These studies reported sex differences in neurodevelopmental outcomes associated with prenatal phthalate exposure. Therefore, we examined sex differences in the association between prenatal phthalate exposure and brain volumetric measures and further tested sex differences in brain alterations that explained the association between prenatal phthalate exposure and child cognition. Since a recent meta-analysis reported associations of moderate effect size between prenatal phthalate exposure and neurodevelopment in girls,14 we hypothesize that sex-specific associations will be present in girls.
Materials and Methods
Study Participants
Participants were mother-child pairs from the Generation R Study, a population-based birth cohort in Rotterdam, the Netherlands. Briefly, Generation R recruited pregnant women with a delivery date between April 2002 and January 2006 in the city of Rotterdam.31–33 A sample of women provided spot urine samples three times in pregnancy, which was used for measurement of phthalates and creatinine. Children and their families have been followed across various life stages using questionnaires and in-person visits, including brain MRI scans and other objective measurement of neuropsychological functioning. In total, 2083 pregnant women provided three spot urine samples during gestation [early (<18 weeks), mid- (18-25 weeks), and late pregnancy (>25 weeks of gestational age)]. In this group, phthalate metabolites were repeatedly measured in urine sample of 1431 women with singleton pregnancy who provided postnatal consent.34 At age 10 years, participating children underwent brain MRI scans [mean age=9.8 years, standard deviation (SD) = 0.3] and at age 14 years children’s IQ was evaluated (mean age=13.48, SD=0.26). We included 775 mother-child pairs in the analysis with maternal urinary phthalate levels and good quality brain MRI. For a subgroup including 671 mother-child pairs, data on IQ at age 14 was also available. Additional mediation analyses including IQ were performed on these 671 mother-child pairs.
The Medical Ethics Committee of the Erasmus Medical Centre approved the study. Parents provided written informed consent, and confidentiality was guaranteed. We obtained assent from children older than age 12 years.
Phthalate Exposure
Urine samples of pregnant women were collected between 8 am and 8 pm in 100 mL polypropylene urine collection containers that were kept for a maximum of 20 h at 4°C before being frozen at −20°C in 20 mL portions in 25 mL polypropylene vials. Specimens were shipped on dry ice in 4 ml polypropylene vials to the Wadsworth Center, New York State Department of Health, Albany, New York for analysis of phthalate metabolites. Phthalate metabolite concentrations were determined using high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS), with details of measurements reported elsewhere.34 Briefly, urine samples were processed using enzymatic deconjugation of the glucuronidated phthalate monoesters followed by on-line solid phase extraction (SPE) coupled with reversed phase HPLC-ESI-MS/MS. This selective method allows for rapid detection of phthalate metabolites with limits of detection in the range of 0.1-0.5 ng/mL. We quantified concentrations of phthalic acid (PA), a proxy for total phthalate exposure, and 17 metabolites, included mnBP, monobenzyl phthalate (mBzP), monocyclohexyl phthalate, mono(7-carboxy-n-heptyl) phthalate, mono(2-carboxymethyl)hexyl phthalate (mCMHP), mCPP, mono(2-ethyl-5-carboxypentyl) phthalate (mECPP), mono(2-ethyl-5-hydroxyhexyl) phthalate (mEHHP), mono(2-ethyl-5oxohexyl) phthalate (mEOHP), mEP, mono(2-heptyl) phthalate, monohexyl phthalate, monoisobutyl phthalate (mIBP), mono(8-methyl-1-nonyl) phthalate, monoisononyl phthalate, monomethyl phthalate (mMP), and monooctyl phthalate. For phthalate metabolites with detection rate higher than 80%, concentrations below the limit of detection (LOD) were imputed by LOD/√2. We calculated ∑DEHP concentration as the molar sum of mECPP, mEHHP, mEOHP, and mCMHP concentrations, after imputing the levels below LOD by LOD/√2.34 All metabolite concentrations were divided by creatinine level and effect sizes were expressed per increase in ng/g of creatinine for individual phthalates and μmol/g creatinine for ∑DEHP.
Brain MRI
In Generation R, 3992 visited the center for brain MRI at age 10 years for whom brain images were acquired on a 3T General Electric scanner (MR750w, Milwaukee, WI). A high-resolution T1-weighted sequence was obtained using a 3D coronal inversion recovery fast spoiled gradient recalled sequence (TR=8.77 ms, TE=3.4 ms, TI=600 ms, flip angle=10°, field of view=220×220 mm, number of slices=230, voxel size=1.0 mm3, ARC acceleration factor=2).31 Prior to the actual MRI scanning session, the children participated in a mock scanning session. Following the mock scanner session, the child was shown two pictures of an MRI scan of the brain, one with little movement and one with considerable movement. This was done to help the child visualize that the ‘pictures of the brain become blurred with movement’. In addition, if excessive movement was seen on the MRI console following image reconstruction, the scan was repeated.
Structural MRI data were processed through the FreeSurfer analysis suite, version 6.0 recon-all tool.35 A number of automated steps were involved to segment the brain into multiple regions. These steps include the removal of nonbrain tissue, normalizing the image intensity to account for B1 inhomogeneities, whole-brain tissue segmentation, and creatubg a surface-based model of the cortex. Measures of global brain regions (e.g., total brain volume and subcortical volume), and a number of subcortical and cortical structures (amygdala, orbitofrontal cortex, etc.) were automatically labeled according to the Desikan-Killiany atlas.36 For quality assessment, T1 images underwent both visual inspection and an automated quality assessment measure for motion-related artifacts.31 Images were once rated at the time of MRI acquisitions using a six-point Likert scale and scans were repeated for images with unusable or poor rating. After FreeSurfer reconstruction, 2-D segmentations and 3-D morphometry were visually inspected using a 3-point Likert scale with the following levels: “Excellent to Very Good,” “Good to Fair,” and “Poor to Unusable”. Eventually, scans were also systematically rated using an algorithm developed in house and tested in two external neuroimaging datasets.37 To improve precision, the MRI quality score was added as a covariates to all association models (See below).
From 3992 children who visited the center for brain MRI, 806 scans were excluded, mainly due to poor/insufficient data quality after image processing (80%) as well as missing complete T1 scan (14%), a different T1 acquisition (3%), or incidental finding (3%).38 Characteristics of children who were excluded because of poor quality scans (n=806) were comparable to 3186 children with brain scans (e.g., 49% boys in both groups, mean age = 10.1 at the time of scan or sociodemographic characteristics). See full characteristics in Table S1 (Supplemental Information). From 3186 with good quality images, data on prenatal phthalates were available in included 775 mother-child pairs who were included in this analysis (See above, Study Participants).
To address our hypothesis regarding the global effect of prenatal phthalate exposure on brain development, we examined total brain, total gray matter, subcortical gray matter, cerebral white matter, and total cerebellum volumes. See Table S2 (Supplemental Information) for the list of labels included in the analysis.
IQ
During visits to the study’s research center at age 14 years, children’s IQ was assessed using the Wechsler Intelligence Scale for Children® Fifth Edition (WISC®-V, Pearson Clinical Assessment, San Antonio, Texas).39 In collaboration with Pearson, four core subtests from the WISC-V were selected to assess specific cognitive domains and to derive an estimated full scale IQ.40 All four subtest were administered by research assistants trained by a clinical neuropsychologist. The subtests included (1) Matrix reasoning, which measures fluid reasoning; (2) Coding, measuring processing speed; (3) Vocabulary, which measures verbal comprehension; and (4) Digit Span, to assess working memory.
The Matrix Reasoning and Coding subtests were administered to the child via the Q-Interactive system of Pearson41 on an iPad Air 2 and while the examiner remained in the room. In a small subset (less than 5%), the tablet was not functioning at the time of the assessment and thus a paper/pencil version of the Matrix Reasoning and the Coding subtest was used.
In the Matrix Reasoning subtest, children were provided with an incomplete matrix and asked to select the completing response option. In the Coding subtest, children were introduced to a key with numbers and corresponding symbols and were asked to match as many numbers with the corresponding symbols within 2 min. For the Vocabulary subtest, children had to provide definitions for words read out loud by the examiner. Responses by children were recorded with an audio recorder, which were then used for scoring of the subtest, additional to the responses written down by the examiner. The Digit Span subtest consisted of three separate tasks. First, children were asked to repeat a sequence of numbers in the same order that the numbers were presented. Then they were asked to repeat a sequence of numbers in reverse order. Last they were asked to repeat a sequence ordered from low to high.
Matrix Reasoning and Coding were scored automatically, whereas the Digit Span subtest was scored by trained research assistants. The three subtasks were equally weighted to compute a Digit Span summary score. The Vocabulary subtest was also scored by trained the research assistants. All examiners first scored the same subset of tests, and if scoring was adequate (reliability higher than the predefined threshold of 0.9), they could continue scoring. If examiners were in doubt about what was written down on the forms, they were instructed to listen to the recording of the task and then decide on the score. Other issues about scoring were discussed in monthly meetings with other examiners
Raw subtest scores of the Vocabulary, Matrix Reasoning, Digit Span, and Coding subtests were summed and converted to a four-subtest estimated full scale IQ (See Table S3 in Supplementary Materials for Sum of Scaled Score Range). Age-standardized T-scores were calculated using Dutch norm-scores (data from Table A1 in the Dutch Manual of the WISC V). The reliability across 6 – 16 years was tested by Pearson, which shows high reliability of the custom index and similarity with a full-scale IQ (See Supplementary Table S4 for the intraclass correlation between custom index and a Pearson full-scale IQ for different age groups).
Other Measures
Information on maternal age (year), highest educational levels achieved, maternal national origin, and marital status was obtained with questionnaires at enrollment during pregnancy. We categorized educational levels as ‘low’ (no primary school/primary school), ‘intermediate’ (secondary school or lower vocational training), and ‘high’ (higher vocational training, university). National origin was grouped as ‘the Netherlands’ and ‘other than the Netherlands’. We combined categories of married and living with partners and compared to those with no partner. Pregnant women also reported their smoking habits at enrollment, in mid-pregnancy, and in late pregnancy. We defined any smoking during pregnancy (yes/no) using this information. Pre-pregnancy body mass index (BMI) (kg/m2) was calculated using self-reported pre-pregnancy weight and height. Maternal non-verbal IQ was assessed using a computerized version of the Ravens Advanced Progressive Matrices Test, set I during age six visits of children.42 Information on parity (nulliparous, yes/no) and a child’s sex and date of birth were obtained from hospitals and midwives records.
Statistical Analysis
Because phthalates have short half-life, the average urinary concentrations of metabolites are likely a better estimate of the exposure across gestation. Therefore, we calculated an average of creatinine-adjusted concentrations throughout pregnancy. Phthalate concentration were expressed on a creatinine basis and log10 transformed.
The primary research question was the association between phthalate metabolites and brain volumetric measures. We performed linear regression models to examine the associations of phthalate metabolite concentrations with brain volumes, adjusted for confounders (see below). To correct for multiple hypothesis testing accounting for the correlation between concentrations of eight phthalate metabolites, we used False Discovery Rate (FDR).43 In a sensitivity analysis, we also examined a more conservative approach of using FDR-corrected p values for 40 tests (eight phthalate metabolites and five brain measures). We explored the non-linearity of associations between exposures and outcomes using a smoothing curve spline. To further explore if the associations between prenatal phthalate exposure and brain volumetric measures differed by sex, we added interaction terms between phthalate measures and sex to the models and also stratified the analyses of phthalates/brain measures by sex.
We used linear regression models to examine the associations of phthalate metabolite concentrations with child IQ (full scale IQ score and the four domains), adjusted for confounders. The total effect of a specific exposure on an outcome might be very small or close to null, while in fact mediation effects exist.44 Therefore, we pursued mediation analyses if associations were observed with a specific phthalate metabolite and brain volumetric measures, even when the associations between specific phthalates and child IQ were not significant in our sample. We used causal mediation analysis providing estimation of the natural direct effect, the natural indirect effect, and the total effect45 and calculated proportion mediated (ratio of natural indirect effect and total effect). Indirect effects represent the effect of phthalate exposure on IQ that is mediated via brain volumes, while the direct effect denotes the remaining part of the total effect and represents the effect of phthalate exposure on IQ not mediated through brain volumes. The natural effect models were adjusted for potential exposure-outcomes, exposure-mediator, and mediator-outcome confounders (as listed below). Standard errors were calculated using bootstrapping. If interaction terms (phthalates X sex) were significant, we further tested if the association between prenatal phthalates and child IQ mediated by brain volumetric measures was moderated by sex (moderation of the mediated effect).
We selected potential confounders based on the directed acyclic graphs and factors shown to be associated with exposure or outcomes.13, 14, 16, 34 Models were adjusted for maternal age, national origin, marital status, IQ score, BMI, parity, smoking during pregnancy, and child sex and age at the neuroimaging assessment. Models with neuroimaging outcomes were also adjusted for MRI quality score and models with cerebellar volume for intracranial volume (ICV) to ascertain relativity to head size. There were high correlations between measures of total gray matter, subcortical gray matter, and cerebral white matter volumes and ICV (r=0.81-0.90), so models with these measured were adjusted for ICV in an additional step.
Missing data for covariates were: maternal body mass index in 93 (12%), parity in 2 (0.3%), national origin in 4 (0.5%), education in 23 (3%), marital status in 33 (4%), smoking in 67 (9%), and IQ in 11 individuals (1 %); child IQ in 104 (13%) individuals. We used the Multivariate Imputation by Chained Equations (MICE) method in R to impute the missing values for covariates, creating 20 imputed datasets using 25 iterations.46–49 Mediation analysis were performed within all 20 imputed datasets, and since the results were similar across sets, we present findings from the 10th and 20th sets, randomly. For all other analyses, we present the results of combined effect estimates across imputation sets.
Within Generation R, factors predicted inclusion in this study were maternal age, education, national origin, and IQ, parity, household income, and child gestational age at birth. We used information on these factors to estimate the probability of participation in the study and applied the inverse of those probabilities as weights in all analyses to examine if the selective nonresponse influenced our findings.
Code availability
Analyses were performed in R Package version 3.4.1. Codes will be available upon request and communication with the corresponding author.
Results
Table 1 presents participants’ characteristics. Pregnant women were on average 31.0 years (SD=4.6) at enrollment and mostly had Dutch national origin (n=439, 56.9%). More than half of women had high education levels (n=420, 55.9%), and 168 women (23.7%) smoked at any point during pregnancy. The mean (SD) full scale IQ score of children at age 14 years was 103.0 (SD=13.2). Median and interquartile range of phthalate metabolites detected in at least 80% of the samples are presented in Table S5 (Supplemental Information). These included mMP, mEP, mIBP, mnBP, mBzP), mCPP, metabolites of DEHP, as well as PA (See Table S6 for the correlation between phthalates). Intraclass correlation coefficients (ICC, estimated by using a 2-way mixed-effects model with absolute agreement) for phthalate measures varied between 0.37 (∑DEHP) and 0.68 (mEP) for the average of the three measurements across pregnancy. Concentrations of mEP was the highest among all metabolites measured.
Table 1.
Participants’ characteristics (n=775). The Generation R Study.
| Maternal characteristics | |
|---|---|
| Age at enrollment, years; mean (SD) | 31.0 (4.6) |
| Pre-pregnancy BMI; mean (SD) | 23.5 (4.1) |
| Parity; n (%) | |
| 0 | 487 (63.0) |
| ≥ 1 | 286 (37.0) |
| National origin; n (%) | |
| the Netherlands | 439 (56.9) |
| Others | 332 (43.1) |
| Education; n (%) | |
| Low | 97 (12.9) |
| Intermediate | 235 (31.2) |
| High | 420 (55.9) |
| Marital status; n (%) | |
| Married/living with partner | 663 (89.4) |
| No partner | 79 (10.6) |
| IQ score; mean (SD) | 98.6 (14.4) |
| Any smoking during pregnancy; n (%) | 168 (23.7) |
| Child characteristics | |
|
| |
| Child sex, boys; n (%) | 390 (50.3) |
| Full scale IQ score at age 14 years; mean (SD) | 103.0 (13.2) |
| Total brain volume at age 10 years, cm3; mean (SD) | 1211.3 (106.1) |
| Total gray matter volume at age 10 years, cm3, means (SD) | 761.4 (63.1) |
| Cerebral white matter volume at age 10 years, cm3, means (SD) | 422.9 (46.2) |
| Subcortical gray matter volume at age 10 years, cm3, means (SD) | 60.3 (4.5) |
| Total cerebellum volume at age 10 years, cm3, means (SD) | 144.1 (13.1) |
Body mass index: BMI; Magnetic resonance imaging: MRI; Standard deviation: SD
Associations of prenatal phthalate exposure with brain volumetric measures
Table 2 summarizes the associations of maternal urinary concentration of phthalate metabolites (averaged across pregnancy) with brain global and regional volumetric measures in children aged 10 years. After correction for multiple comparisons using FDR, higher gestational concentrations of maternal mEP were associated with smaller total gray matter volumes (β per log10 increase in creatinine adjusted mEP=−10.70, 95%CI: −18.12, −3.28), but not with other brain volumetric measure. When we adjusted the models with total gray matter, cerebral white matter, and subcortical gray matter for ICV, we found a significant association between prenatal mIBP exposure and total gray matter volume (β=−8.11, 95%CI: −13.56, −2.66) that remained significant with FDR correction (Table S7 in the Supplemental Information). The associations between other phthalate metabolites and brain measures did not remain significant after correction for multiple comparisons using FDR. When we used PFDR for 40 tests, none of the associations remained significant. We found no indication of non-linearity in the association between phthalate metabolite concentrations and brain measures (data not shown).
Table 2.
Associations of prenatal exposure to phthalate metabolites (averaged across pregnancy) with brain structure at age 10 years. The Generation R Study.
| Brain volumes, cm3 (n=775) | |||||
|---|---|---|---|---|---|
| Global measures | Regional measures | ||||
|
|
|||||
| Total brain | Total gray matter | Cerebral white matter | Subcortical gray matter | Total cerebellum | |
|
| |||||
| β 95%CI | β 95%CI | β (95%CI) | β (95%CI) | β (95%CI) | |
| mMP | −3.19 (−21.67, 15.28) | −0.91 (−11.67, 9.85) | −2.22 (−10.66, 6.23) | 0.15 (−0.69, 0.99) | −1.34 (−3.37, 0.70) |
| mEP | −16.23 (−28.98, −3.48) | −10.7 (−18.12, −3.28) * | −5.22 (−11.06, 0.62) | −0.55 (−1.13, 0.03) | −0.65 (−2.06, 0.77) |
| mCPP | 2.01 (−21.51, 25.53) | 1.28 (−12.43, 14.99) | 1.10 (−9.63, 11.83) | 0.59 (−0.49, 1.66) | −2.38 (−4.95, 0.20) |
| mIBP | −16.14 (−35.41, 3.13) | −10.87 (−22.10, 0.36) | −4.78 (−13.58, 4.01) | −0.2 (−1.08, 0.67) | −2.14 (−4.25, −0.02) |
| mnBP | −6.81 (−28.15, 14.52) | −3.53 (−15.97, 8.90) | −2.94 (−12.68, 6.8) | 0.43 (−0.54, 1.41) | −1.49 (−3.83, 0.85) |
| mBzP | −10.38 (−26.56, 5.79) | −6.18 (−15.60, 3.23) | −4.00 (−11.39, 3.39) | −0.27 (−1.01, 0.47) | −0.96 (−2.73, 0.81) |
| ∑DEHP | 7.47 (−17.07, 32.01) | 3.06 (−11.24, 17.36) | 4.65 (−6.55, 15.86) | 0.53 (−0.59, 1.65) | −2.50 (−5.20, 0.19) |
| Phthalic acid | −18.47 (−41.43, 4.49) | −14.30 (−27.66, −0.94) | −3.61 (−14.11, 6.90) | −0.41 (−1.45, 0.64) | −2.77 (−5.3, −0.24) |
CI: confidence interval; DEHP: di-2-ethylhexyl phthalate; mnBP: mono-n-butyl phthalate; mBzP: monobenzyl phthalate; mCPP: mono(3-carboxypropyl) phthalate; mEP: monoethyl phthalate; mIBP: monoisobutyl phthalate; mMP: monomethyl phthalate.
Models were adjusted for maternal age, national origin, education, marital status, IQ score, pre-pregnancy body mass index, parity, smoking during pregnancy, MRI quality score, and child sex and age at the neuroimaging assessment. Models with total cerebellar volume were additionally adjusted for intracranial volume.
Bold: association with p-value <0.05.
Associations were significant after correction for multiple comparisons.
Estimates are reported per log10 increase in creatinine adjusted maternal urinary phthalate concentrations (ng/g of creatinine of individual phthalates and μmol/g creatinine for ∑DEHP).
We found sex interactions in associations of maternal mIBP concentrations with cerebral white matter volumes (significant after correction for multiple comparison, PFDR = 0.04). We examined the associations across strata of sex and found that girls with higher phthalate exposure, including mIBP and mEP, had smaller volumes in several brain features (Table S8). The associations of prenatal phthalate exposure with brain volumetric measures were not present in boys (Table S9).
Associations of prenatal phthalate exposure with child cognition and mediation by brain morphology
Table 3 shows the associations between maternal phthalate metabolite concentrations and child cognition (total IQ scores and subtests) at age 14 years. Children exposed to higher maternal mEP and mnBP concentrations had lower IQ: β for matrix reasoning per log10 increase in creatinine adjusted mEP = −0.45, 95%CI: −0.84, −0.07; and β for verbal vocabulary per log10 increase in creatinine adjusted mnBP −0.97, 95%CI: −1.65, −0.29.
Table 3.
Associations of prenatal exposure to phthalate metabolites (averaged across pregnancy) with and child IQ at age 14 years (n=671). The Generation R Study.
| Full scale IQ score | Vocabulary | Matrix Reasoning | Digit Span | Coding | |
|---|---|---|---|---|---|
|
|
|||||
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| mMP | 0.91 (−1.91, 3.73) | 0.15 (−0.45, 0.75) | 0.02 (−0.54, 0.58) | 0.24 (−0.36, 0.84) | 0.14 (−0.58, 0.86) |
| mEP | −1.68 (−3.61, 0.25) | −0.13 (−0.54, 0.28) | −0.45 (−0.84, −0.07) | −0.32 (−0.73, 0.09) | −0.08 (−0.57, 0.41) |
| mCPP | −1.42 (−5.07, 2.24) | −0.58 (−1.36, 0.19) | 0.41 (−0.32, 1.14) | 0.11 (−0.66, 0.89) | −0.76 (−1.69, 0.16) |
| mIBP | −0.53 (−3.42, 2.36) | −0.52 (−1.13, 0.09) | −0.37 (−0.94, 0.21) | 0.56 (−0.06, 1.17) | 0.01 (−0.72, 0.74) |
| mnBP | −3.15 (−6.39, 0.09) | −0.97* (−1.65, −0.29) | −0.23 (−0.88, 0.41) | −0.20 (−0.89, 0.49) | −0.49 (−1.31, 0.34) |
| mBzP | 1.08 (−1.437, 3.59) | −0.09 (−0.62, 0.44) | 0.39 (−0.11, 0.89) | 0.42 (−0.12, 0.95) | −0.06 (−0.70, 0.58) |
| ∑DEHP | −1.83 (−5.59, 1.93) | −0.44 (−1.23, 0.36) | −0.10 (−0.85, 0.65) | −0.21 (−1.01, 0.58) | −0.34 (−1.29, 0.62) |
| Phthalic acid | −1.82 (−5.35, 1.72) | −0.44 (−1.19, 0.31) | −0.23 (−0.93, 0.48) | −0.42 (−1.17, 0.33) | 0.03 (−0.87, 0.92) |
CI: confidence interval; DEHP: di-2-ethylhexyl phthalate; mnBP: mono-n-butyl phthalate; mBzP: monobenzyl phthalate; mCPP: mono(3-carboxypropyl) phthalate; mEP: monoethyl phthalate; mIBP: monoisobutyl phthalate; mMP: monomethyl phthalate.
Bold: association with p-value <0.05.
Significant with FDR correction.
Models were adjusted for maternal age, national origin, education, marital status, IQ score, pre-pregnancy body mass index, parity, smoking during pregnancy, and child sex and age at the IQ assessment.
Estimates are reported per log10 increase in creatinine adjusted maternal urinary phthalate concentrations (ng/g of creatinine of individual phthalates and μmol/g creatinine for ∑DEHP)
We next examined the extent to which brain volumetric measures that were associated with mEP and mIBP exposures, i.e., total gray and white matter volumes, mediated the association of prenatal phthalates and child general IQ. We observed that smaller total gray volumes partially mediated the association between prenatal mEP exposure and lower full scale IQ scores (natural indirect effect which shows the mediated path: β per log10 increase in creatinine adjusted mEP = −0.31, 95%CI: −0.62, 0.01; proportion mediated = 18%) (Figure 1 and Figure S1). We did not pursue mediation analysis with subcortical measure residualized for intracranial volumes because we found no associations between residualized measures and child IQ. We found that the mediated path by white matter volume in the association of mIBP exposure and child IQ was present in girls (natural indirect effect: β per log10 increase in creatinine adjusted mIBP = −1.08, 95%CI: −1.95, −0.16), but not in boys (Figure 2 and Figure S2).
Figure 1.
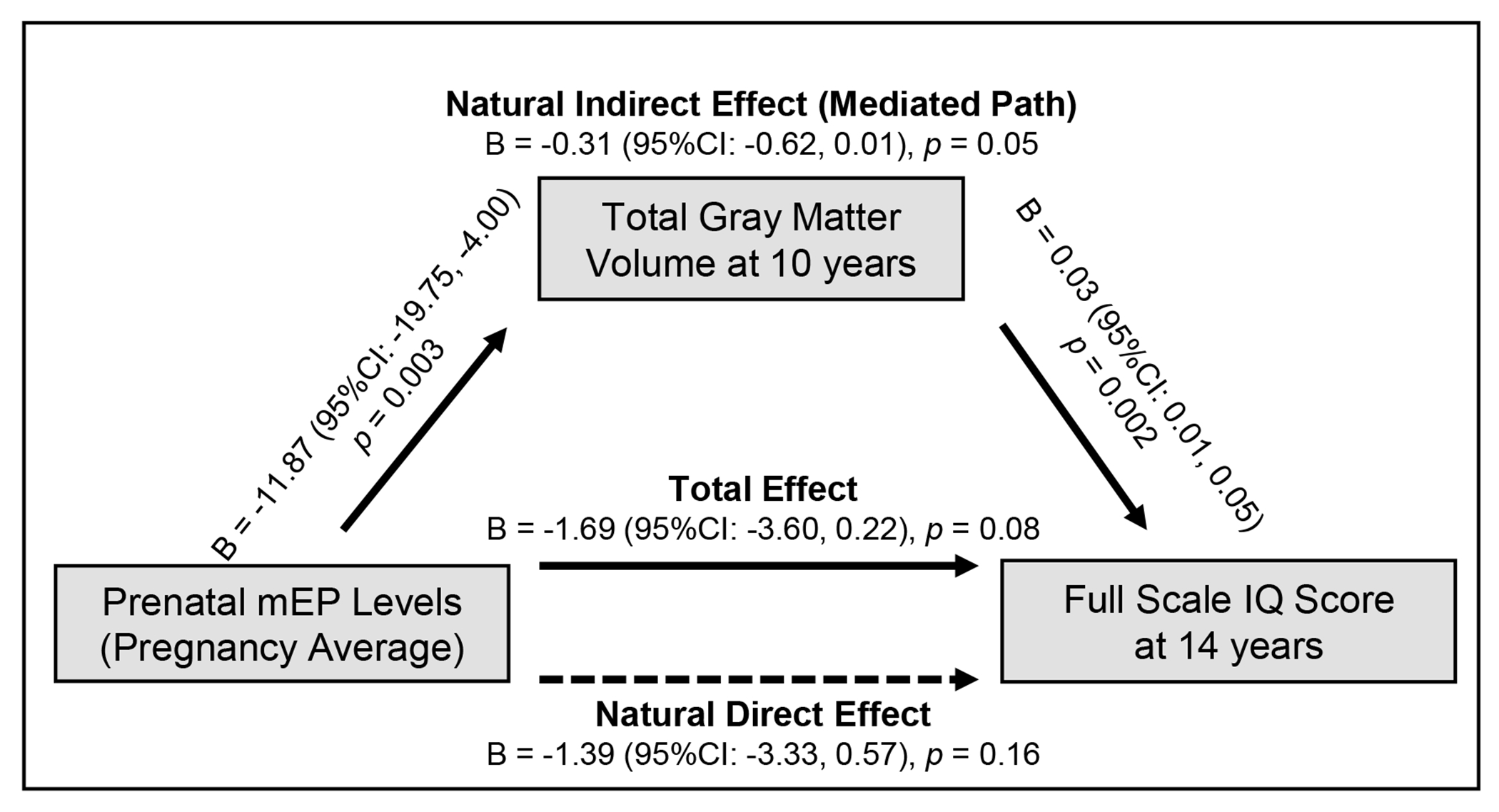
Associations between prenatal monoethyl phthalate (mEP) exposure (averaged across pregnancy) and child full scale IQ score at age 14 years, mediated by total gray matter volume at age 10 years.
Models were adjusted for maternal age, national origin, education, marital status, IQ score, pre-pregnancy body mass index, parity, smoking during pregnancy, MRI quality score, and child sex and age at the neuroimaging assessment and/or age at assessment child IQ. Data shown from the 20th imputation set.
Figure 2.
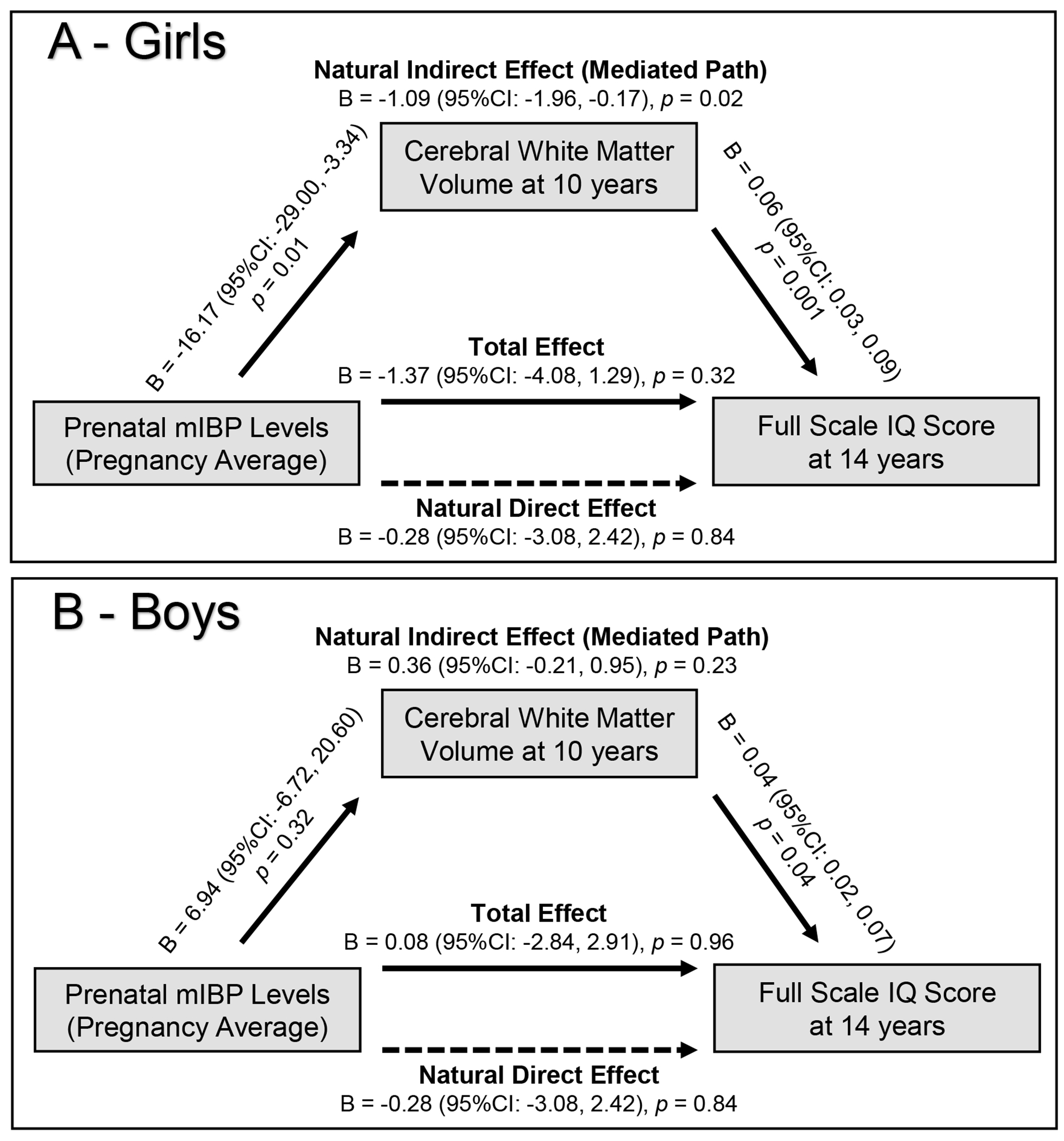
Associations between prenatal monoisobutyl phthalate (mIBP) exposure and child full scale IQ score at age 14, mediated by cerebral white matter volume at age 10 years in girls (A) and boys (B).
Models were adjusted for maternal age, national origin, education, marital status, IQ score, pre-pregnancy body mass index, parity, smoking during pregnancy, MRI quality score, and child age at the neuroimaging assessment and/or age at assessment child IQ. P value for sex interaction: 0.01. Data shown from the 20th imputation set.
Discussion
We found that higher maternal prenatal concentrations of mEP were associated with smaller total gray matter volumes in offspring at age 10 years. Smaller gray matter volumes partially mediated the association between higher maternal mEP and lower child full score IQ at age 14 years. In the association between prenatal mEP exposure and child full scale IQ scores, the proportion mediated by total gray matter volumes was 18%. The inverse association of higher mIBP and lower child IQ was mediated by smaller cerebral white matter volumes only in girls, with a proportion mediated of 76%. While effect sizes were overall small, for example, one-sixth of SD deviation decrease total gray matter volumes per 10 times increase in mEP exposure, these findings have high public health impact because of the high and widespread exposure to phthalates and poor regulations in pregnant populations. Moreover, we observed a pattern in the data that suggested an inverse relationship between phthalates with low molecular weights associated with smaller volumetric measures, even though the estimates were imprecise.
This study is the first to investigate the brain volumetric measure associated with prenatal phthalate exposure using brain MRI. Strengths include the large sample size and the availability of data on important confounders, such as maternal IQ. We used biomonitoring, with multiple urine samples during pregnancy, which is the method of choice for measuring rapidly metabolized organic compounds.50 Findings of this study should be interpreted considering the following limitations. First, phthalates are non-persistent chemicals with a short half-life and urine spot samples during pregnancy might not be the accurate reflection of the whole pregnancy exposure and long-terms effects of early life exposure should be interpreted considering this limitation. In this sample, we observed moderate ICCs between the three measures of phthalates in pregnant women and subsequently used concentrations of metabolites from three spot urine samples in the main analyses; yet, measurement error remains an issue in studies of non-persistent chemicals.51 Particularly, the short half-lives of these chemicals should be considered when interpreting trimester-specific analyses. Second, we did not examine exposure to plasticizers that are used as replacement of DEHP, while their potential neurotoxicity is a growing concern and relevant to more recent cohorts.12 Third, although we adjusted the models for several confounders, residual confounding by unmeasured factors cannot be ruled out. This is particularly important for the mediation analysis, which was performed under the assumption that no unmeasured confounding existed on any of the paths. Fourth, to minimize the participant burden, we used four subtests of WISC®-V. These subtests were selected from the 10 core subtests that are usually used to measure the four index scores to measure full IQ score, but lack information on other important constructs such as visual spatial scale. Last, Generation R sample represents the diversity of Rotterdam and its surrounding area, and as such participants reported a wide spectrum of backgrounds. Because many of these groups have a small number of participants in the Generation R Study, we operationalized this data as a two-category variable.
Developmental neurotoxicity of certain phthalates, e.g., DEHP and DBP, is widely studied in experimental studies, with possible mechanisms suggested to be thyroid disruption,52 epigenetic modifications, 53 or sex hormone disruption.54 Influences of phthalates on sex hormones are particularly important during the organizational effects of gonadal steroids on fetal brain development.55 Experimental data also suggest that the effect of phthalate exposure on brain development can be both global and early (e.g., interference in the cytoarchitecture of neocortex20) and region-specific (e.g., morphology of specific structures such as the cerebellum18, 53). Epidemiological data in humans applying neuroimaging to investigate the impact of prenatal phthalate exposure on the brain are limited. One report from the Alberta Pregnancy Outcomes and Nutrition (APrON) study showed associations between high molecular weight phthalates (metabolites of DEHP and BBzP), measured once in mid-pregnancy, and impairments in structural connectivity (white matter microstructure) in preschool age children.30 But the APrON study did not examine global or regional volumetric measures of the brain, while brain structural measures are shown to be implicated in children’s behavioral and cognitive functioning, e.g., the established relationship of cortical and subcortical gray matter volumes with IQ.22, 23 The present study is the first to examine the associations of prenatal phthalate exposure with offspring brain volumetric measure and potential implications for general cognition. We found that higher prenatal exposure to mEP, a metabolite of DEP, was associated with a global effect on the brain, as seen with smaller gray matter volume. Higher exposure to mEP was associated with lower scores in Matrix Reasoning. Fluid reasoning, important for later achievement and academic performance,56 is highly heritable but the role of environmental factors are also proposed, particularly in earlier stages of development.57 Our current analysis extends our earlier report of an association between prenatal mEP and child non-verbal IQ at age six years15, 16 by showing that the impact of prenatal phthalate exposure on the brain and child cognition continues into adolescence. DEP is less often examined in experimental studies (as opposed to DEHP and DBP); with few studies confirming that DEP exposure can interfere with brain processes,58, 59 and others report no effect.60 It is important to note that DEP is the phthalate to which individuals are most highly exposed,14 which was also found in this sample (see Table 2 for concentrations of urinary mEP concentrations, a metabolite of DEP). Thus, it is critical to identify exposure reduction strategies for DEP, possibly similar to current regulations that exist for DEHP, for example.
Our findings on sex differences in the associations of prenatal phthalates with brain measures was expected based on earlier studies of child cognition and behavior.13, 14 A recent meta-analysis of epidemiological studies on prenatal phthalate exposure and several neurodevelopmental outcomes concluded that despite some inconsistencies, girls are often more susceptible to exposure to diisobutyl phthalate (DIBP, the parent compound of mIBP) for lower motor and cognitive abilities.14 For example, two studies reported associations between higher DIBP exposure (measured by urinary mIBP) and lower child cognition only in girls.61, 62 Our study, with a large sample size that allowed examination of sex interaction and mediation analysis, provides the first evidence on the neural substrate for sex differences in cognitive outcomes associated with mIBP exposure. Phthalates interfere with sex hormone production and are associated with estrogenic and anti-androgenic reproductive effects in males and females.54 Fetal exposure to both androgens and estrogens are associated with differential growth in sexually dimorphic brain areas.55 Anti-androgenic effects of phthalates in girls, in particular mid-gestation through the end of gestation, might interfere with brain organizational development.
Findings from models adjusted for the ICV showed also associations with smaller total gray matter volumes, but in with mIBP exposure (compared to associations with mEP in models not adjusted for ICV). We speculate that these differences might be due to the mechanism of action of phthalate compounds (specific vs global effect) that should be investigated further in mechanistic studies.
In conclusion, despite increased regulations for certain phthalates, such as DEHP in the US and EU for childhood exposure, our findings suggest the global impact of prenatal exposure to phthalates, such as mEP and mIBP that are less regulated, through adolescence. Further investigations are warranted, which replicate our findings on brain global measures in larger and more diverse samples and further examine associated regional differences. Sex differences in some associations indicate potential involvement of sex steroid signaling pathways, which should be investigated in future mechanistic studies.
Supplementary Material
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaborations with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
The general design of the Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Netherlands, the Organization for Health Research and Development (ZonMw) and the Ministry of Health, Welfare and Sport. This study was supported by grant R01ES022972 and R01ES029779 from the National Institutes of Health (NIH) to LT. Neuroimaging and infrastructure was supported by the Netherlands Organization for Health Research and Development (ZonMw) TOP project number 9121102. The work of AG is supported by grant R01ES032826 from NIH. The work of MD is supported by grant 824989 from the Horizon2020 programme of the European Union. HT is supported by the ZonMW grant 016.VICI.170.200. MG is funded by a Miguel Servet fellowship (CPII18/00018) awarded by the Spanish Institute of Health Carlos III. We acknowledge support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. SME was supported by P30ES010126 and R01ES021777. VWVJ and MG received funding from the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No. 733206 LifeCycle and Grant Agreement No. 874583 ATHLETE). The research was supported in part by the Intramural Research Program of the NIMH.
Footnotes
Conflict of Interest
The spouse of H.T. is an employee of Eastman Chemical, a company that manufactures substitutes for ortho-phthalate plasticizers. Other authors have no conflict of interest.
References
- 1.GBD 2017 Child and Adolescent Health Collaborator. Diseases, Injuries, and Risk Factors in Child and Adolescent Health, 1990 to 2017: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2017 Study. JAMA pediatrics 2019; 173(6): e190337–e19033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White TJ, Shaw P, Viding E. The What, Where, and When of Childhood Psychopathology: First Steps Towards Identifying the Etiological Factors That Shape Brain Development. J Am Acad Child Adolesc Psychiatry 2017; 56(10, Supplement): S326. [Google Scholar]
- 3.Bennett D, Bellinger DC, Birnbaum LS, Bradman A, Chen A, Cory-Slechta DA et al. Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement. Environ Health Perspect 2016; 124(7): A118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annex XVII to REACH – Conditions of Restriction, Entry 51. https://echa.europa.eu/documents/10162/aaa92146-a005-1dc2-debe-93c80b57c5ee, Accessed Date December 29 2022.
- 5.Assessing and Managing Chemicals under TSCA: Phthalates. June 2022, https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/phthalates. Accessed Date December 29 2022.
- 6.Wu H, Kupsco AJ, Deierlein AL, Just AC, Calafat AM, Oken E et al. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007-2010. Environ Sci Technol 2020; 54(3): 1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Zhu BB, Tao XY, Zhu YD, Tao XG, Tao FB. Temporal Variability of Cumulative Risk Assessment on Phthalates in Chinese Pregnant Women: Repeated Measurement Analysis. Environ Sci Technol 2018; 52(11): 6585–6591. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff TJ, Zota AR, Schwartz JM. Environmental Chemicals in Pregnant Women in the United States: NHANES 2003–2004. Environ Health Perspect 2011; 119(6): 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu H, Jonsson BA, Gennings C, Svensson A, Nanberg E, Lindh CH et al. Temporal trends of phthalate exposures during 2007-2010 in Swedish pregnant women. J Expo Sci Environ Epidemiol 2018; 28(5): 437–447. [DOI] [PubMed] [Google Scholar]
- 10.Gaylord A, Kannan K, Lakuleswaran M, Zhu H, Ghassabian A, Jacobson MH et al. Variability and correlations of synthetic chemicals in urine from a New York City-based cohort of pregnant women. Environ Pollut 2022; 309: 119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trasande L, Shaffer RM, Sathyanarayana S. Food Additives and Child Health. Pediatrics. 2018; 142(2): e20181408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engel SM, Patisaul HB, Brody C, Hauser R, Zota AR, Bennet DH et al. Neurotoxicity of Ortho-Phthalates: Recommendations for Critical Policy Reforms to Protect Brain Development in Children. Am J Public Health. 2021; 111(4): 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Chen XZ, Huang X, Wang M, Wu J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology 2019; 73: 199–212. [DOI] [PubMed] [Google Scholar]
- 14.Radke EG, Braun JM, Nachman RM, Cooper GS. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ Int 2020; 137: 105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Dries MA, Guxens M, Spaan S, Ferguson KK, Philips E, Santos S et al. Phthalate and bisphenol exposure during pregnancy and offpsring non-verbal IQ. Environ Health Perspect 2020; 128(7):77009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Dries MA, Ferguson KK, Keil AP, Pronk A, Spaan S, Ghassabian A et al. Prenatal Exposure to Nonpersistent Chemical Mixtures and Offspring IQ and Emotional and Behavioral Problems. Environ Sci Technol 2021; 55(24): 16502–16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernandez MF, Garcia-Esteban R et al. Exposure to Bisphenol A and Phthalates during Pregnancy and Ultrasound Measures of Fetal Growth in the INMA-Sabadell Cohort. Environ Health Perspect 2016; 124(4): 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, Dong J, You M, Cong Z, Wei L, Fu H et al. Maternal di-(2-ethylhexyl) phthalate exposure inhibits cerebellar granule precursor cell proliferation via down-regulating the Shh signaling pathway in male offspring. Chemosphere 2019; 215: 313–322. [DOI] [PubMed] [Google Scholar]
- 19.Kougias DG, Sellinger EP, Willing J, Juraska JM. Perinatal Exposure to an Environmentally Relevant Mixture of Phthalates Results in a Lower Number of Neurons and Synapses in the Medial Prefrontal Cortex and Decreased Cognitive Flexibility in Adult Male and Female Rats. J Neurosci 2018; 38(31): 6864–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komada M, Gendai Y, Kagawa N, Nagao T. Prenatal exposure to di(2-ethylhexyl) phthalate impairs development of the mouse neocortex. Toxicol Lett. 2016; 259: 69–79. [DOI] [PubMed] [Google Scholar]
- 21.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychology 2000; 54(1): 241–257. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen NC, Flaum M, Swayze V, O’Leary DS, Alliger R, Cohen G et al. Intelligence and brain structure in normal individuals. Am J Psychiatry 1993; 150: 130–130. [DOI] [PubMed] [Google Scholar]
- 23.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: A volumetric imaging study. Brain 1996; 119(5): 1763–1774. [DOI] [PubMed] [Google Scholar]
- 24.Muetzel RL, Mous SE, van der Ende J, Blanken LM, van der Lugt A, Jaddoe VW et al. White matter integrity and cognitive performance in school-age children: A population-based neuroimaging study. Neuroimage 2015; 119: 119–128. [DOI] [PubMed] [Google Scholar]
- 25.Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 2006; 30(6): 718–729. [DOI] [PubMed] [Google Scholar]
- 26.Thomason ME, Hect JL, Rauh VA, Trentacosta C, Wheelock MD, Eggebrecht AT et al. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. NeuroImage 2019; 191: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X et al. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl. Acad Sci USA 2012; 109(20): 7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry 2015; 72(6): 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guxens M, Lubczynska MJ, Muetzel RL, Dalmau-Bueno A, Jaddoe VWV, Hoek G et al. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biol Psychiatry 2018; 84(4): 295–303. [DOI] [PubMed] [Google Scholar]
- 30.England-Mason G, Grohs MN, Reynolds JE, MacDonald A, Kinniburgh D, Liu J et al. White matter microstructure mediates the association between prenatal exposure to phthalates and behavior problems in preschool children. Environ Res 2020; 182: 109093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White T, Muetzel RL, El Marroun H, Blanken LME, Jansen P, Bolhuis K et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol 2017; 33(1), 99–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CCW et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol 2014; 29(12): 911–927. [DOI] [PubMed] [Google Scholar]
- 33.Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 2016; 31(12): 1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S et al. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ Res 2018; 161: 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004; 14(1): 11–22. [DOI] [PubMed] [Google Scholar]
- 36.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006; 31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- 37.White T, Jansen PR, Muetzel RL, Sudre G, El Marroun H, Tiemeier H et al. Automated quality assessment of structural magnetic resonance images in children: Comparison with visual inspection and surface-based reconstruction. Hum Brain Mapp 2018; 39(3): 1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muetzel RL, Mulder RH, Lamballais S, Cortes Hidalgo AP, Jansen P, Güroğlu B et al. Frequent Bullying Involvement and Brain Morphology in Children. Front Psychiatry 2019; 10(696). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaufman AS, Raiford SE, Coalson DL (2015): Intelligent Testing with the WISC-V. First Edition ed. Hoboken: John Wiley & Sons. [Google Scholar]
- 40.Blok E, Schuurmans IK, Tijburg AJ, Hillegers M, Koopman-Verhoeff ME, Muetzel RL et al. Cognitive performance in children and adolescents with psychopathology traits: A cross-sectional multicohort study in the general population. Dev Psychopathol 2023;35(2):926–940 [DOI] [PubMed] [Google Scholar]
- 41.Daniel MH, Wahlstrom D, Zhang O. Equivalence of Q-interactive™ and Paper Administrations of Cognitive Tasks: WISC®–V. Q-Interactive Technical Report 2014; 8. [Google Scholar]
- 42.Prieler J Raven’s Advanced Progressive Matrices (Vol. 24). Mödling, Austria: Schufried; 2003. [Google Scholar]
- 43.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B 1995: 289–300. [Google Scholar]
- 44.MacKinnon DP. Introduction to statistical mediation analysis. Routledge. 2008. [Google Scholar]
- 45.Steen J, Loeys T, Moerkerke B, Vansteelandt S. medflex: An R Package for Flexible Mediation Analysis using Natural Effect Models. J Stat Softw 2017 2017; 76(11): 46 [Google Scholar]
- 46.R core Team. A language and environment for statistical computing. Vienna, Austria. R Foundation for Statistical Computing. 2015 [Google Scholar]
- 47.van Buuren S, Groothuis-Oudshoorn C. MICE: Multivariate Imputation by Chained Equations in R. 2011 [Google Scholar]
- 48.Von Hippel PT. How to impute interactions, squares, and other transfored variables 2009; 39(1): 265–291. [Google Scholar]
- 49.Van Buuren S Flexible Imputation of Missing Data Second edition. 2nd edn. Chapman & Hall/CRC Boca Raton, 2018. [Google Scholar]
- 50.Nieuwenhuijsen MJ Exposure assessment in environmental epidemiology. Oxford University Press, USA. 2015 [Google Scholar]
- 51.van Smeden M, Lash TL, Groenwold RHH. Reflection on modern methods: five myths about measurement error in epidemiological research. Int J Epidemiol 2019; 49, 338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu Z, Han Y, Shen R, Huang K, Xu YY, Wang QN et al. Gestational di-(2-ethylhexyl) phthalate exposure causes fetal intrauterine growth restriction through disturbing placental thyroid hormone receptor signaling. Toxicol Lett 2018; 294: 1–10. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Jiang L, Cheng L, Chen H. Dibutyl phthalate-induced neurotoxicity in the brain of immature and mature rat offspring. Brain Dev 2014; 36(8): 653–660. [DOI] [PubMed] [Google Scholar]
- 54.Lin LC, Wang SL, Chang YC, Huang PC, Cheng JT, Su PH et al. Associations between maternal phthalate exposure and cord sex hormones in human infants. Chemosphere 2011; 83(8): 1192–1199 [DOI] [PubMed] [Google Scholar]
- 55.Miranda A, Sousa N. Maternal hormonal milieu influence on fetal brain development. Brain Behav 2018; 8(2): e00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wai J, Lubinski D, Benbow CPJJoeP. Spatial ability for STEM domains: Aligning over 50 years of cumulative psychological knowledge solidifies its importance. J Educ Psychol 2009; 101(4): 817–835. [Google Scholar]
- 57.King MJ, Katz DP, Thompson LA, Macnamara BN. Genetic and environmental influences on spatial reasoning: A meta-analysis of twin studies. Intelligence 2019; 73: 65–77. [Google Scholar]
- 58.Xu H, Shao X, Zhang Z, Zou Y, Chen Y, Han S et al. Effects of di-n-butyl phthalate and diethyl phthalate on acetylcholinesterase activity and neurotoxicity related gene expression in embryonic zebrafish. Bull Environ Contam Toxicol 2013; 91(6): 635–639. [DOI] [PubMed] [Google Scholar]
- 59.Poopal RK, Ramesh M, Maruthappan V, Babu Rajendran R. Potential effects of low molecular weight phthalate esters (C(16)H(22)O(4) and C(12)H(14)O(4)) on the freshwater fish Cyprinus carpio. Toxicol Res 2017; 6(4): 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tran CM, Do TN, Kim K-T. Comparative Analysis of Neurotoxicity of Six Phthalates in Zebrafish Embryos. Toxics. 2021; 9(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L et al. Maternal Prenatal Urinary Phthalate Metabolite Concentrations and Child Mental, Psychomotor, and Behavioral Development at 3 Years of Age. Environ Health Perspect 2012; 120 (2):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environ Res 2017; 152:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


