Abstract
Background:
Air pollution is a health risk in pregnant women and children. Despite the importance of refined exposure assessment, the characterisation of personalised air pollution exposure remains a challenge in paediatric and perinatal epidemiology.
Objective:
We used portable personal air monitors to characterise personalised exposure to air pollutants in pregnant women.
Methods:
Between November 2019 and May 2022, we offered personal air monitors to pregnant women participating in a birth cohort in New York City. During pregnancy, women used air monitors, which measured particulate matter (PM), nitrogen dioxide (NO2), and volatile organic compounds (average use = 14 days). Data were stored in real-time on a secure database via synchronisation with a smartphone application. Of 497 women who agreed to use air monitors, 273 women (55%) were successful in using air monitors for longer than a day. For these participants, we identified daily patterns of exposure to air pollutants using functional principal component analysis (3827 days of air monitoring).
Results:
Compared to women with no pollution data (n = 224), women who successfully used monitors were more likely to be non-Hispanic White and Asian (vs. Hispanic), nulliparous, unemployed, married/partnered, and received the device in-person (vs. mailed). We identified different daily patterns of exposure to air pollutants. The most dominant pattern for all pollutants was low exposure levels with little variations within 24 h, followed by a pattern that showed differences between day and night levels. NO2 had higher daily variations compared to PM.
Conclusions:
Small wearables are useful for the measurement of personalised air pollution exposure in birth cohorts and identify daily patterns that cannot be captured otherwise. Successful participation, however, depends on certain individual characteristics. Future studies should consider strategies in design and analysis to account for selective participation.
Keywords: air pollution, birth cohort, personal monitor, pregnancy
1 |. BACKGROUND
Air pollution is a health risk in pregnant women and young children. During pregnancy, air pollution exposure is associated with poor maternal health and adverse pregnancy outcomes.1–3 Developmental exposure to air pollutants such as particulate matter (PM) and nitrogen oxides (NOx) contributes to poor health outcomes in children.4–6 While a marked improvement in air quality has happened over the past decades,7 recent national data confirm that the trend towards better air quality is reversing,8 adding to growing concerns on potential impacts of low-level exposure, particularly in vulnerable groups.9,10
Despite the importance of refined exposure assessment, particularly at low exposure levels, the characterisation of personalised air pollution exposure remains a challenge in paediatric and perinatal epidemiology. Epidemiological studies of air pollution exposure and child health apply various methods for exposure assessment, that is geographical information system (GIS) methods, personal and/or residential air-monitoring methods, and biomarker assessments.6,11–13 GIS-based methods are commonly used in large studies and vary in precision, for example, distance from major roads or leveraging data from air quality monitoring stations and combining it with statistical modelling, for example, land-use regression models (LUR).14,15 GIS-based methods are often bound to the spatial and temporal resolution of data provided by air monitors or other geographical indicators. Integration of satellite-derived remote sensing into LUR improves both spatial and temporal predictions. Yet, ambient air pollution is a proxy of personal exposure, with correlations between the two varying considerably.16,17 While previous studies applied personal air monitoring in epidemiological settings,18 the use of personal exposure monitoring has remained restricted in epidemiological settings due to practical issues (e.g. large size of monitors), high cost and a limited number of pollutants assessed by one monitor.19–22 Portability of personal air monitors would allow granular measurement of changing exposure when study participants travel to different locations during the day (indoor and/or outdoor). With advancements in sensor technology, small and portable personal air monitors are available with acceptable durability, which favours their use for an extended period. Importantly, these devices can measure multiple pollutants simultaneously and from various indoor and outdoor emission sources. Yet, compliance and cost limit the use of these monitors in large epidemiological studies.22,23
We used small wearable air monitors to characterise exposure to fine PM, nitrogen dioxide (NO2), and volatile organic compounds (VOC) in pregnant women participating in a birth cohort. We identified characteristics that were associated with the successful use of personal air monitors. In those women who successfully used air monitor, we characterised patterns in daily exposure to air pollutants.
2 |. METHODS
2.1 |. Cohort selection
This study was nested in the New York University Children’s Health and Environment Study (NYU CHES), an ongoing birth cohort in New York City (NYC, 2016–present).24 NYU CHES is one of the participating paediatric cohorts in the National Institutes of Health Environmental influences on Child Health Outcome (ECHO) program and was designed to examine the role of the environment in children’s growth and development. Briefly, pregnant women who are 18 years or older, less than 18 weeks pregnant, and planning to deliver in one of the NYU-affiliated hospitals are invited to participate.
From November 2019 to May 2022, we invited all pregnant participants of NYU CHES to use a personal air monitor over the course of pregnancy. Study recruitment happened at two sites, NYU–Manhattan and NYU–Brooklyn.24 Trained research assistants approached participants during their routine prenatal care, provided a personal air monitor, and initiated the operation and pairing of the device with participants’ smartphones. From 1097 pregnant women who were recruited in NYU CHES within this period, 864 had live birth within the study period (excluding miscarriages, terminations, stillbirths, and loss to follow-up). Among 864 women, 497 women (57.5%) agreed to use air monitors and 367 women (42.5%) did not. We found differences between these two groups. For example, women who agreed to use air monitors were older, and likely to be of non-Hispanic origin and highly educated, to have private insurance, and to be from households with higher annual income than those who did not (Table S1).
2.2 |. Exposure
We used the Flow by Plume Labs (Paris, France), which measures real-time exposure to fine particular matter (with aerodynamic diameters <1 μm (PM1) and <2.5 μm (PM2.5), NO2 and VOC). Flow is a small (dimensions: 12.5 × 4.0 × 2.5 cm; weight ~70 g) portable air monitor with durable battery life (~24 h), which is designed to track the quality of personal air breathed by individuals (indoor and outdoor). Flow contains a light-scattering laser to count airborne particles and two reactive metal oxide membranes for the detection of NO2 and VOC. The Flow device provides an estimate of the air pollutants each minute and is designed to self-calibrate using machine learning contained in Flow’s firmware. As reported by the manufacturer, the detection range for PM is 0–200 μg/m3, 0–300 ppb for NO2 and 0–10 ppm for VOC. Air pollution and geolocation data are stored in real-time in a secure database via HTTPS synchronisation with a smartphone application available on Android or iOS. In addition to that, participants had access to their air pollution data in real-time via a live map and could download the data on the smartphone application, if interested. For individual users, the application also shows the Air Quality Index for each pollutant and applies a colour-coded messaging system (LED light) to inform users about air quality. Air pollutant concentrations estimated by flow were highly correlated with estimates obtained by the reference device in the lab setting with coefficients of correlation varying between 0.69 (for VOC) and 0.96 (for NO2).25 We used geolocation data as a proxy for mobility and inferred adherence to the recommendation of carrying the device based on the average distance travelled per day. See Supplementary Material for more information about the device and instructions for use.
Per convenience for participants, about half of the participants (n = 260, 53.1%) received the device by mail and others during their in-person visits. Participants who received the devices by mail were provided with an educational video by email/text. Study staff monitored air quality data in real-time and performed follow-up phone calls with instruction/assistance on device utility if participants had the device but no data were stored in the secure database.
2.3 |. Other measures
At enrolment during early pregnancy, NYU CHES participants filled in a questionnaire (online or via interviews), which was used to obtain information on race and ethnicity, highest education achieved, marital status, employment status, and household annual income. We used electronic health records to obtain data for the date of birth, parity, pregnancy outcome, and pregnancy start/end date as well as data on race and ethnicity, marital status, and employment status, if missing from the questionnaires.
2.4 |. Statistical analysis
We compared individual characteristics of women who successfully used air monitors and had air pollution data with those women who failed to pair the device with their smartphone (mean for continuous variables and percentages for categorical variables). To examine the association between participants’ characteristics and the successful use of air monitors, we estimated risk ratio (RR) using log-linear models (all variables included in one model).
Minute-by-minute concentrations of air pollutants for each woman were evaluated for outliers, and data were truncated at the 99th percentile. Raw data had a skewed distribution. Therefore, we calculated hourly averaged concentrations of air pollutants per participant using the geometric mean of minutes in an hour. For each participant, the daily average exposure was calculated using the arithmetic mean of hourly-level data. We report the mean [standard deviation (SD)] and median [interquartile range (25th and 75th percentiles)] of daily exposure for each pollutant.
Individual averaged hourly levels were used to identify patterns of daily exposure in participating women using various approaches. First, we calculated the Spearman correlation coefficient between concentrations of pollutants within 24 h of each day. Higher coefficient correlations between consecutive hours suggested less variation in concentrations around that time of the day. Second, we plotted hourly exposure levels within a day for each participant and then calculated the area under the average curve (AUC) of these connected values. AUCs were calculated for each day with higher AUCs reflecting higher cumulative daily exposure.
Variations in daily exposure may not entirely be captured by using standard data analysis techniques such as mean and measures of dispersion. Moreover, an average curve could not closely resemble any of the individual daily exposure curves. Because data generated by the air monitors are continuous functions of concentrations for hours of a day, we performed functional principal component analysis (fPCA) on these functions at 24 time points to depict the major patterns, which explained the variations underlying air pollution exposure during a day. fPCA is an extension of the traditional PCA where the principal components are represented by functions rather than vectors.26,27 The top functional principal components (PCs) explain major sources of variation in the data and the corresponding functional PC scores can reduce the dimensionality from functional data to lower dimensions. We identified the major functional PC explaining over 80% of variations in the data and visualised and interpreted each functional PC. We further explored the loading of these components for each day of exposures to identify which hourly exposures are strongly correlated with the top fPCs. We performed fPCA once with all 3827 days and once with one observation per study participant (average of hourly level data) to examine if daily patterns were independent of clustering of daily data around one participant.
We reran the analyses excluding the first 7 days of air pollution data as independent field evaluation with a stationary ambient monitoring site suggested that the device requires a 7-day calibration period, particularly for NO2.28 We also reran the analyses stratifying data by summer and winter months to identify seasonal variation.
Statistical analysis was performed using R Package Version 4.0.2.
2.5 |. Missing data
Missing values for covariates were 2.2% for race/ethnicity, 11.5% for education, 2.2% for marital status, 13.5% for income, 17.3% for insurance type, 5.0% for employment status and 2.4% for parity. We used the Multivariate Imputation by Chained Equations (MICE) method in R with 50 data sets to impute missing values in covariates.
3 |. RESULTS
In total, 273 women activated the device and paired it with the smartphone, which resulted in a successful collection of air pollution data for an average period of 14 days [median number of days = 10; 25th and 75th percentile: 5 and 17 days; 3827 person-day data]. Data were collected with no interruption during the period of use for 237 women. Characteristics of study participants, whether using the device successfully or not and if they provided geolocation data, are shown in Table 1. Women with Asian, non-Hispanic White or mixed racial/ethnic background were more likely to use the device successfully than women who were Hispanic (Table S2). Lower education was inversely associated with the successful use of the device. Women who were separated, divorced or single had lower odds of using the device successfully compared to those who were married/partnered. Women who received air monitors by mail were also less likely to use them successfully compared to those women who received the device in-person. In contrast, unemployment and nulliparity were associated with a better response.
TABLE 1.
Participan’s characteristics, New York University Children's Health and Environment Study (NYU CHES).
| All participantsa (n = 497) | Fail to use air monitors (n = 224) | Successfully used air monitors (n = 273) | Successfully used air monitors, with geolocation data (n = 115) | |
|---|---|---|---|---|
| Age, year; mean (SD) | 32.2 (5.6) | 31.7 (5.8) | 33.4 (5.2) | 33.8 (4.9) |
| Race/Ethnicity; n (%) | ||||
| Hispanic | 198 (40.7) | 115 (52.8) | 83 (31.0) | 35 (31.0) |
| Non-hispanic white | 181 (37.2) | 62 (28.4) | 119 (44.4) | 46 (40.7) |
| Non-hispanic black | 34 (7.0) | 19 (8.7) | 15 (5.6) | 8 (7.1) |
| Asian | 57 (11.7) | 18 (8.3) | 39 (14.6) | 19 (16.8) |
| Other | 6 (1.2) | 4 (1.8) | 2 (0.7) | 0 (0) |
| Mixed | 7 (1.4) | 0 (0) | 7 (2.6) | 3 (2.7) |
| Refused to disclose | 3 (0.6) | 0 (0) | 3 (1.1) | 2 (1.8) |
| Education; n (%) | ||||
| High school or less | 105 (24.1) | 61 (33.5) | 44 (17.0) | 21 (19.3) |
| Some college | 41 (9.4) | 23 (12.6) | 18 (7.0) | 7 (6.4) |
| Associate or bachelor's degree | 129 (29.6) | 46 (25.3) | 83 (32.2) | 33 (30.3) |
| Postgraduate degree | 165 (37.8) | 52 (28.6) | 113 (43.8) | 48 (44.0) |
| Marital status; n (%) | ||||
| Married/Partnered | 436 (89.7) | 178 (82.4) | 258 (91.8) | 108 (94.7) |
| Divorced/Separated/Single | 50 (10.3) | 38 (17.6) | 23 (8.2) | 6 (5.3) |
| Household income; n (%) | ||||
| Less than $30,000 | 53 (12.3) | 25 (14.3) | 28 (11.0) | 12 (10.9) |
| $30,000 to $49,999 | 29 (6.7) | 18 (10.3) | 11 (4.3) | 5 (4.5) |
| $50,000 to $99,999 | 51 (11.9) | 19 (10.9) | 32 (12.5) | 16 (14.5) |
| $100,000 or more | 231 (53.7) | 75 (42.9) | 156 (61.2) | 62 (56.5) |
| Do not know | 66 (15.3) | 38 (21.7) | 28 (11.0) | 15 (13.6) |
| Study site; n (%) | ||||
| Brooklyn | 176 (35.4) | 99 (44.2) | 77 (28.2) | 33 (28.7) |
| Manhattan | 321 (64.6) | 125 (55.8) | 196 (71.8) | 82 (71.3) |
| Insurance type; n (%) | ||||
| Private | 264 (64.2) | 89 (50.0) | 175 (75.1) | 71 (73.2) |
| Public | 147 (35.8) | 89 (50.0) | 58 (24.9) | 26 (26.8) |
| Employment status; n (%) | ||||
| Employed | 356 (75.4) | 149 (72.0) | 207 (78.1) | 84 (75.7) |
| Unemployed | 116 (24.6) | 58 (28.0) | 58 (21.9) | 27 (24.3) |
| Nulliparous, n (%) | 232 (47.8) | 89 (40.5) | 146 (54.5) | 63 (55.8) |
| Trimester air monitor given; n (%) | ||||
| One | N/A | 161 (71.9) | 221 (80.9) | 108 (93.9) |
| Two | N/A | 16 (7.1) | 18 (6.6) | 3 (2.6) |
| Three | N/A | 47 (21.0) | 34 (12.5) | 4 (3.5) |
| Methods air monitor given; n (%) | ||||
| In-person | N/A | 86 (38.4) | 144 (52.7) | 45 (39.1) |
| Mailed | N/A | 138 (61.6) | 129 (47.3) | 70 (60.9) |
Note: For each categorical variable, percentages are calculated among successful users and those who failed to use the device (columns). Missing values: 2.2% for race/ethnicity, 11.5% for education, 2.2% for marital status, 13.5% for income, 17.3% for insurance type, 5.0% for employment status and 2.4% for parity.
Abbreviations: NA, not applicable; SD, standard deviation.
All recruitment occurred between November 2019 and May 2022 in New York City.
The majority of participants (225, 82.4%) started using an air monitor in their first trimester of pregnancy, driven by the study design, which aimed to recruit women in early pregnancy. Geolocation data showed mobility during the period of use; for example median distance travelled per day of use was 10.6 km (IQR = 17.5). Figure S2 presents examples of daily exposure concentrations for pollutants in one participant over eight consecutive days. Table 2 summarises descriptive statistics of average hourly and daily concentrations for pollutants. Descriptive statistics of AUCs, estimating the cumulative exposure in a day for each participant, are shown in Table S3. Spearman correlation coefficients between concentrations of pollutants in 24 h per day showed strong positive correlations between concentrations in consecutive hours (r = 0.73–0.96 for all pollutants), which were the highest from 7 to 11 in the morning (r > 0.9). Correlations were the lowest between consecutive hours in the evening, suggesting more variations during the nighttime (Figure 1).
TABLE 2.
Descriptive statistics of hourly and daily exposure to air pollutants as measured by Flow personal air monitor in pregnant women.
| Hourly average |
Daily average |
|||
|---|---|---|---|---|
| Mean (SD) | Median (25th, 75th) | Mean (SD) | Median (25th, 75th) | |
| NO2, ppb | 13.5 (13.3) | 9.6 (3.6, 19.2) | 9.8 (8.4) | 7.6 (4.0, 13.3) |
| PM1, μ/m3 | 3.1 (6.5) | 1.1 (1.0, 1.95) | 2.7 (5.1) | 1.2 (1.0, 2.0) |
| PM2.5, μ/m3 | 5.7 (8.6) | 2.4 (2.0, 5.0) | 5.0 (6.8) | 2.6 (2.2, 4.7) |
| VOC, ppb | 185.4 (117.1) | 162.3 (139.1, 193.1) | 173.4 (73.2) | 163.9 (147.3, 184.0) |
Note: Data were from 3827 days of air monitoring in 273 participants between November 2019 and May 2022 in New York City.
Hourly averages were calculated using the geometric mean of minute-by-minute data, after data truncation at 99th percentile. Daily averages were calculated using the arithmetic mean of hourly averages.
Abbreviations: NO2, nitrogen dioxide; PM1, particulate matter with aerodynamic diameter <1 μm; PM2.5, particulate matter with aerodynamic diameter <2.5 μm; SD, standard deviation; VOC, volatile organic compounds.
FIGURE 1.
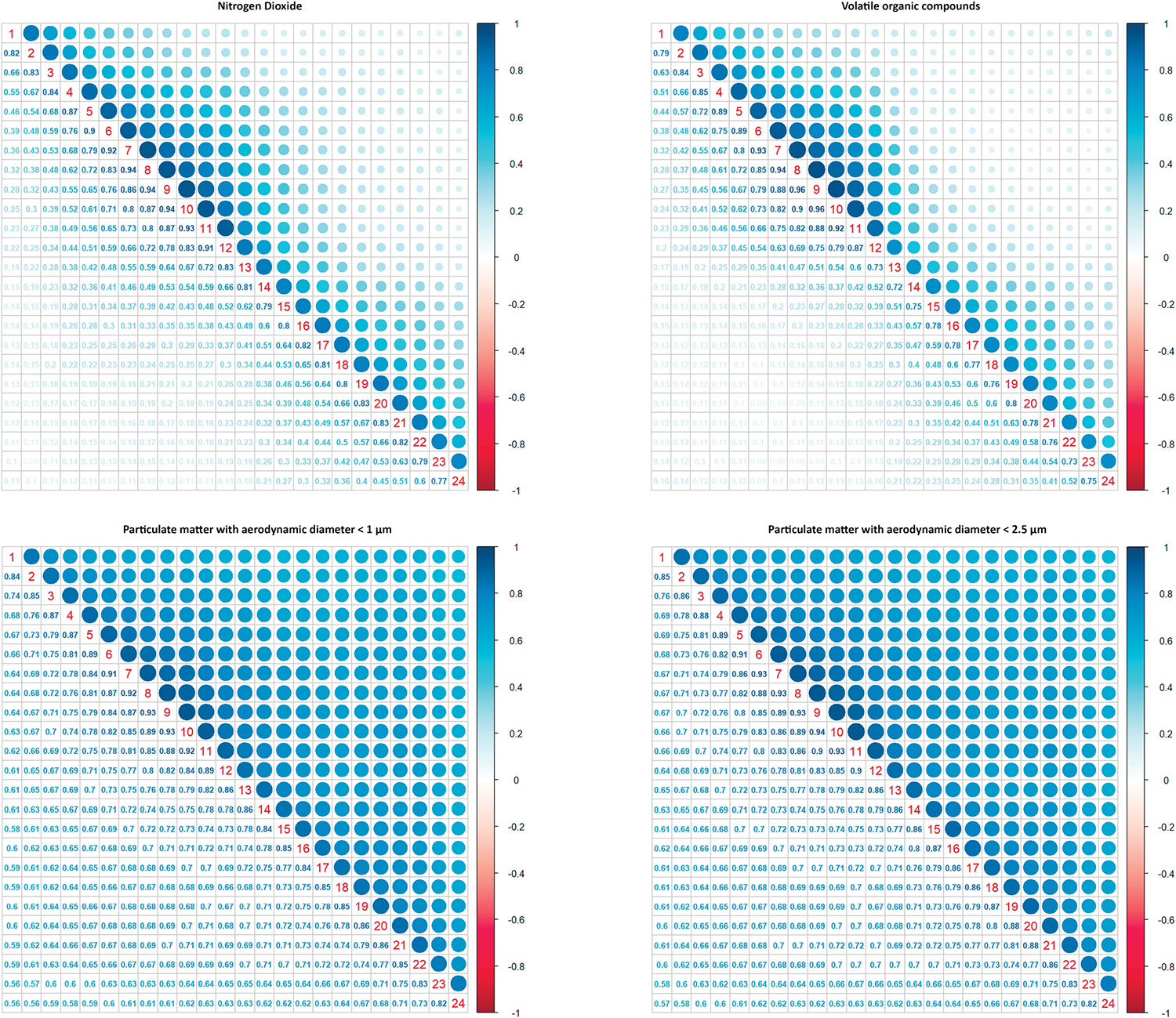
Spearman correlation coefficients between hourly concentrations of air pollutants in 24 h. Diagonal numbers represent 24 h. Data were from 3827 days of air monitoring in 273 participants between November 2019 and May 2022 in New York City.
Results of the fPCA showed that three major components explained 80–90% of the variation in the data for all pollutants. For NO2, the first principal component (PC1, solid black line, Figure S3) presented the overall daily exposure and accounted for about 55% of the variation in the data. PC2 (dashed red line), a component in data with the lowest eigenfunction around late morning (10–12), explained 15% of the variation in the data. PC3 (dotted green line) accounted for about 10% of the variation in data and presented a component with highest levels of exposure around noon and substantially lower exposure levels in other hours of the day. We plotted the loading of data for the first two PCs for PM2.5 and NO2 in 273 women (Figure 2), which showed days were equally distributed around zero for both factors, suggesting most days had high variability in NO2 levels. For PM2.5, as shown in Figure 2, the majority of data points were around 0 for PC2 but negative loading for PC1, suggesting most days had low exposure levels with low variability. Some days had loading around zero for PC2 but positive loading for PC1 suggesting low variability around high exposure levels. Occasionally, there were days with high exposure levels and high variability around 24 h. The patterns were similar for PM1. For VOC, most days had low exposure of VOC with some outliers with high variability and high exposure levels.
FIGURE 2.
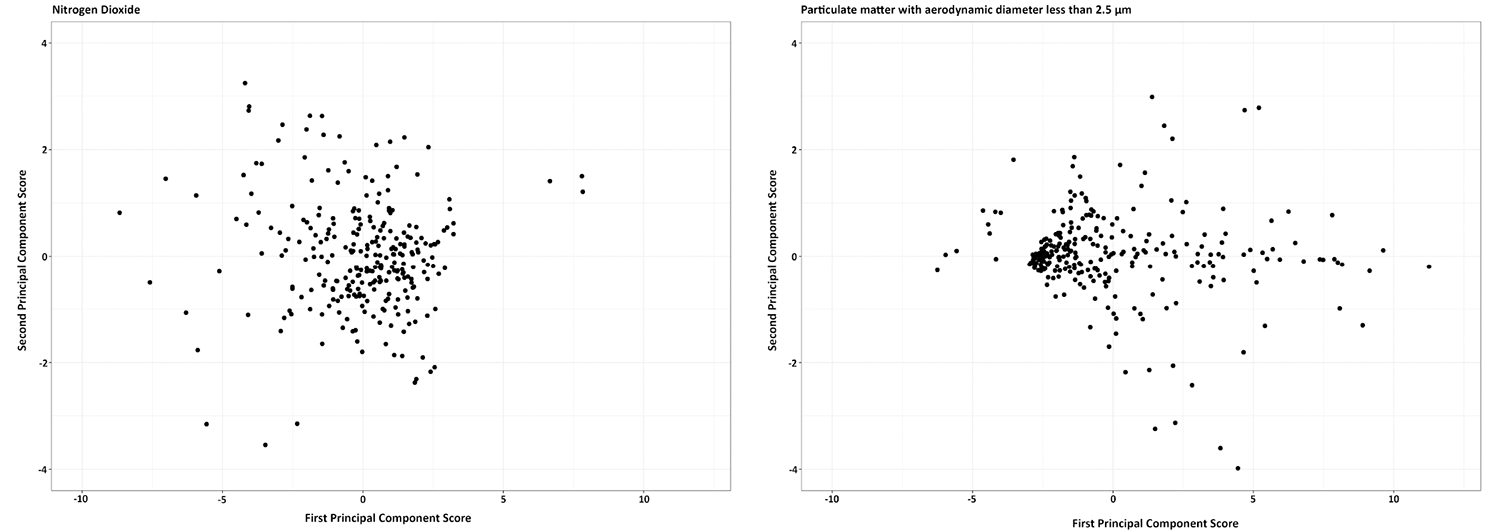
Daily patterns of exposure to air pollutants by functional principal component analysis. Each dot represents an average day for a participant during the study period. First principal component score (Y axis) represents the overall low daily exposure (with little variation) to pollutants within 24 h and the second principal component score (X axis) presents the difference between daily and nighttime exposure. Data were from 3827 days of air monitoring in 273 participants between November 2019 and May 2022 in New York City metropolitan area.
Excluding the first 7 days of air pollution data from each monitor resulted in similar exposure levels (3176 days of observations from 223 participants, Table S4). There were seasonal variations in exposure levels, particularly for PM and VOC, with higher daily averages for VOC in winter months and higher averages for PM in summer months (Table S5).
4 |. COMMENT
4.1 |. Principal findings
In a large and socio-economically diverse sample of pregnant women in NYC, we used personal air monitors to characterise exposure to air pollutants during pregnancy. We found that women who agreed to use air monitors differed in their socio-d emographic characteristics compared to women who did not agree to use the device. Among users of air monitors, a successful use was particularly more likely among women who were non-Hispanic White or Asian, married/partnered, unemployed and nulliparous and received the device in-person (vs in mail). Because of a large sample size (3827 person-day data), we were able to characterise patterns of intra-day exposure to air pollutants, which was otherwise not detectible looking at daily averages. Using fPCA, we identified different daily patterns of exposure for NO2, PM and VOC. The most dominant pattern for all pollutants was low exposure levels with little variation within 24 h, followed by a pattern that showed differences between day and night levels. NO2 exposure had higher variation across days of assessment compared to PM.
4.2 |. Strengths of the study
Strengths of this study included a large sample and a relatively long period of monitor use by participants. Therefore, we were able to examine daily patterns of exposure. The study was embedded in a well-characterised birth cohort, which gave us access to a wide range of characteristics to study predictors of participation and successful use of the device.
4.3 |. Limitations of the study
First, the Flow air monitor measures PM, NO2 and VOC, but not ozone. Moreover, the Flow monitor does not provide any information on the components of PM or VOC. Second, the Flow air monitor is a low-cost air monitor with validation data only available in the limited laboratory setting. Also, because of the large number of devices used in the study, we were not able to perform calibration against gravimetric concentrations measured by filter samples23 and had to rely on self-calibration by machine-learning algorithm in Flow’s firmware. Third, we did not collect information on women’s activity nor did we directly measured compliance.29 However, we had access to geolocation, a proxy that participants followed the recommendations and carried the device during the period of use. Lastly, we did not particularly investigate motives, barriers or individual behaviour associated with the use of air monitors.
4.4 |. Interpretation
Several studies have reported the protocol and design of using personal air monitors in prospective studies.22,23,30 Two of these are particularly important for paediatric and perinatal epidemiology as they recruited pregnant women or individuals trying to become pregnant: The Air Pollution, In Vitro Fertilisation (IVF) and Reproductive Outcomes (AIR) Study and the Breathe—Well-Being, Environment, Lifestyle and Lung Function Study (B-WELL-MOM).22,23 Gaskins and Hart reported the use of personal air monitors in six participants of the AIR study over 72 h and outlined difficulties with battery life, connectivity and usability of air monitors.22 Compliance was self-report, and the small sample size did not allow examination of individual characteristics associated with compliance. In B-WELL-MOM, Ha et al. monitored personal air pollution exposure in 40 pregnant women for 2–4 days. Compliance was defined as the proportion of time the monitor was worn during waking hours and calculated using activity level recorded by the accelerometer and self-reported sleeping hours. They then examined characteristics, such as asthma status, age, cigarette smoking and activity levels, and found that smoking and activity were associated with compliance.23 They did not look at socio-demographic characteristics such as race/ethnicity, education, etc. None of the studies examined daily patterns quantitatively although Gaskins and Hart qualitatively examined the patterns and reported a peak in exposure to PM2.5 in mid-day or evening. In both studies, participants spent the majority of their time indoors.
We could only recruit women who had a smartphone, which might explain some of the differences in characteristics between those who agreed to use air monitors and those who did not. Among women who agreed to use the air monitors, 55% used the device for longer than a day. An uptake of 55% is acceptable particularly because of low cost and burden (e.g. introducing a wearable during an existing study visit does not require additional staff time). This percentage can be improved by, for example introducing the wearable to participants during in-person visits. It is likely that participants who received the device in the mail could not find and download the smartphone application or failed to pair the device with the application. We did not find any association between characteristics, such as age and educational levels, and the successful use of air monitors in the fully adjusted model. In contrast, women who were married or partnered and were nulliparous were more likely to use the device successfully. Few pregnant women in NYU CHES smoked during pregnancy (1%)24 and as such there was not enough variation in the data to replicate the finding from Ha et al. regarding smoking.23 Using geolocation data, we observed substantial movement/change in location each day during the period of use that suggested good adherence to recommendations of carrying the device by women. We speculate that the portability of the device, lightweight, good battery life and reliable connectivity to the smartphone are important factors for participants to use the device as recommended.
Importantly, examination of patterns showed variations in daily exposure levels, which were different for various pollutants. We observed the highest daily variation for NO2, followed by PM, and VOC. Our quantitative measure of PM daily patterns is in line with a qualitative publication with a limited number of participants, which showed higher levels of PM2.5 during mid-day and evening, which was posited by the authors to be due to cooking and meal preparation in indoor settings.22 Since portable monitors can be used both indoors and outdoors, we cannot entirely rule out other sources of exposure, for example in the underground subway system during mid-day or early evening commutes.31 Studies on daily variation of NO2 were limited to those based on ambient levels, highly influenced by traffic.32 But outdoor ambient variation in NO2 might not directly translate into personal exposure. Indoor sources of NO2, such as gas-burning cooking appliances, can be a major source, especially in pregnant women. Daily variations are of particular interest in studies of health outcomes for which acute exposure in a short period might have a substantial effect on the health outcomes. Examples are prospective studies that aimed to identify sensitive windows of exposure during a reproductive cycle for a potential impact of air pollution exposure on odds of conception or studies on air pollution exposure and asthma attack.
5 |. CONCLUSIONS
Our study shows that small wearables can be used to measure personal air pollution exposure and identify daily patterns that cannot be captured otherwise. Another important finding is that the successful use of personal air monitors depended on certain individual characteristics. Additional attention should be directed towards recruitment strategies during design and address selective recruitment in the analyses.
Supplementary Material
Synopsis.
Study question
Can we use portable personal air monitors to characterise exposure to air pollution in pregnant women participating in a birth cohort?
What’s already known
Epidemiological studies of air pollution exposure and child health apply various methods for exposure assessment, that is geographical information system methods, personal and/or residential air monitoring methods and biomarker assessments. The use of personal monitoring has remained restricted in epidemiological settings due to practical issues, high cost and a limited number of pollutants assessed by one monitor.
What this study adds
Small wearables can be used for measurement of personalised air pollution exposure in birth cohorts and identify daily patterns that cannot be captured otherwise. Successful participation, however, depends on certain individual characteristics.
ACKNOWLEDGEMENTS
We thank all of the NYU CHES participants and staff for their important contributions. We also wish to thank our colleagues in the Environmental influences on Child Health Outcome (ECHO) program, the medical, nursing and program staff, as well as the children and families participating in the ECHO cohorts. We acknowledge the contribution of the ECHO program Coordinating Center, Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL, Benjamin DK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
FUNDING INFORMATION
Research reported in this publication was supported by the institutional funds of NYU Grossman School of Medicine as well as the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core) and UG3/UH3OD023305.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Mendola P, Nobles C, Williams A, et al. Air pollution and preterm birth: do air pollution changes over time influence risk in consecutive pregnancies among low-risk women? Int J Environ Res Public Health. 2019;16:3365. doi: 10.3390/ijerph16183365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghassabian A, Pierotti L, Basterrechea M, et al. Association of Exposure to ambient air pollution with thyroid function during pregnancy. JAMA Netw Open. 2019;2:e1912902. doi: 10.1001/jamanetworkopen.2019.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leiser CL, Hanson HA, Sawyer K, et al. Acute effects of air pollutants on spontaneous pregnancy loss: a case-crossover study. Fertil Steril. 2019;111:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeni I, Ha S, Nobles C, Liu D, Sherman S, Mendola P. Air pollution exposure during pregnancy: maternal asthma and neonatal respiratory outcomes. Ann Epidemiol. 2018;28:612–618.e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volk HE, Perera F, Braun JM, et al. Prenatal air pollution exposure and neurodevelopment: a review and blueprint for a harmonized approach within ECHO. Environ Res. 2021;196:110320. doi: 10.1016/j.envres.2020.110320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guxens M, Garcia-Esteban R, Giorgis-Allemand L, et al. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology. 2014;25:636–647. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan TJ, Driscoll CT, Beier CM, et al. Air pollution success stories in the United States: the value of long-term observations. Environ Sci Pol. 2018;84:69–73. [Google Scholar]
- 8.Clay K, Muller NZ. Recent Increases in Air Pollution: Evidence and Implications for Mortality. National Bureau of Economic Research; 2019. [Google Scholar]
- 9.Papadogeorgou G, Kioumourtzoglou MA, Braun D, Zanobetti A. Low levels of air pollution and health: effect estimates, methodological challenges, and future directions. Current Environmental Health Report. 2019;6:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. World Health Organization; 2021. [PubMed] [Google Scholar]
- 11.Guxens M, Ghassabian A, Gong T, et al. Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: the ESCAPE project. Environ Health Perspect. 2016;124:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vishnevetsky J, Tang D, Chang HW, et al. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol Teratol. 2015;49:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson BS, Rauh VA, Bansal R, et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiat. 2015;72:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieuwenhuijsen MJ. Exposure assessment in environmental epidemiology. Oxford University Press; 2015. [Google Scholar]
- 15.Jerrett M, Arain A, Kanaroglou P, et al. A review and evaluation of intraurban air pollution exposure models. J Expo Anal Environ Epidemiol. 2005;15:185–204. [DOI] [PubMed] [Google Scholar]
- 16.Avery CL, Mills KT, Williams R, et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures. Epidemiology. 2010;21:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng QY, Svendsgaard D, Kotchmar DJ, Pinto JP. Associations between personal exposures and ambient concentrations of nitrogen dioxide: a quantitative research synthesis. Atmos Environ. 2012;57:322–329. [Google Scholar]
- 18.Kehm RD, Walter EJ, Oskar S, et al. Exposure to polycyclic aromatic hydrocarbons during pregnancy and breast tissue composition in adolescent daughters and their mothers: a prospective cohort study. Breast Cancer Res. 2022;24:47. doi: 10.1186/s13058-022-01546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKercher GR, Salmond JA, Vanos JK. Characteristics and applications of small, portable gaseous air pollution monitors. Environ Pollut. 2017;223:102–110. [DOI] [PubMed] [Google Scholar]
- 20.Jovašević-Stojanović M, Bartonova A, Topalović D, Lazović I, Pokrić B, Ristovski Z. On the use of small and cheaper sensors and devices for indicative citizen-based monitoring of respirable particulate matter. Environ Pollut. 2015;206:696–704. [DOI] [PubMed] [Google Scholar]
- 21.Gozzi F, Della Ventura G, Marcelli A. Mobile monitoring of particulate matter: state of art and perspectives. Atmos Pollut Res. 2016;7:228–234. [Google Scholar]
- 22.Gaskins AJ, Hart JE. The use of personal and indoor air pollution monitors in reproductive epidemiology studies. Pediatric and Perinatal Epidemiology. 2020;34:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha S, Nobles C, Kanner J, et al. Air pollution exposure monitoring among pregnant women with and without asthma. Int J Environ Res Public Health. 2020;17:4888. doi: 10.3390/ijerph17134888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trasande L, Ghassabian A, Kahn LG, et al. The NYU Children’s health and environment study. Eur J Epidemiol. 2020;35:305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PlumeLabs. Evaluation of Flow, a personal air quality sensor; 2019. [cited December 7, 2022]. https://plumelabs.zendesk.com/hc/en-us/articles/360025092554-How-Accurate-is-Flow-. [Google Scholar]
- 26.Ramsay IO, Silverman BW. Applied Funcational Data Analysis. Springer-Verlag; 2002. [Google Scholar]
- 27.Ryan W, Harrison A, Hayes KJSB. Functional data analysis of knee joint kinematics in the vertical jump. Sports Biomech. 2006;5:121–138. [DOI] [PubMed] [Google Scholar]
- 28.AQMD South Coast. Plume Labs Flow 2 –Field Evaluation; 2020. [cited December 7, 2020]. http://www.aqmd.gov/docs/default-source/aq-spec/field-evaluations/plume-labs-flow-2---field-evaluation.pdf?sfvrsn=8.
- 29.Lawless P, Thornburg J, Rodes C, Williams R. Personal exposure monitoring wearing protocol compliance: an initial assessment of quantitative measurement. J Expo Sci Environ Epidemiol. 2012;22:274–280. [DOI] [PubMed] [Google Scholar]
- 30.Chartier R, Phillips M, Mosquin P, et al. A comparative study of human exposures to household air pollution from commonly used cookstoves in Sri Lanka. Indoor Air. 2017;27:147–159. [DOI] [PubMed] [Google Scholar]
- 31.Luglio DG, Katsigeorgis M, Hess J, et al. Concentration and composition in Subway Systems in the Northeastern United States. Enviornmental Health Perspective. 2021;129:027001. doi: 10.1289/EHP7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SU, Kim KY. Physical and chemical mechanisms of the daily-to-seasonal variation of PM(10) in Korea. Sci Total Environ. 2020;712:136429. doi: 10.1016/j.scitotenv.2019.136429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


