Abstract
Since its first identification in the 1950s as a regulator of cell division, cytokinin has been linked to many physiological processes in plants, spanning growth and development and various responses to the environment. Studies from the last two and one-half decades have revealed the pathways underlying the biosynthesis and metabolism of cytokinin and have elucidated the mechanisms of its perception and signaling, which reflects an ancient signaling system evolved from two-component elements in bacteria. Mutants in the genes encoding elements involved in these processes have helped refine our understanding of cytokinin functions in plants. Further, recent advances have provided insight into the mechanisms of intracellular and long-distance cytokinin transport and the identification of several proteins that operate downstream of cytokinin signaling. Here, we review these processes through a historical lens, providing an overview of cytokinin metabolism, transport, signaling, and functions in higher plants.
We provide an overview of cytokinin metabolism, transport, signaling, and functions in higher plants.
Introduction
In the early part of the 20th century, the Austrian botanist Gottlieb Haberlandt, the “father of plant tissue culture,” postulated the existence of “growth enzymes,” substances that are released from one set of cells to stimulate the growth and development of other cells (Haberlandt 1902, 1913). In the 1950s, cytokinin was identified as one such factor because it promoted cell division and shoot initiation in cultured cells in concert with another phytohormone, auxin (Miller et al. 1955, 1956). In recent decades, remarkable progress has been made in our understanding of the biology of cytokinin. Here, we highlight recent findings as well as the major breakthroughs that led to our understanding of how plants synthesize, degrade, and transport cytokinins, the signaling pathway underlying the perception of cytokinin, and the myriad roles that this fascinating phytohormone plays in plant growth and development and responses to biotic and abiotic stress. We also discuss how modulation of cytokinin content has been used to improve various aspects of different crops, including enhanced drought tolerance, and yield.
Cytokinin biosynthesis and metabolism
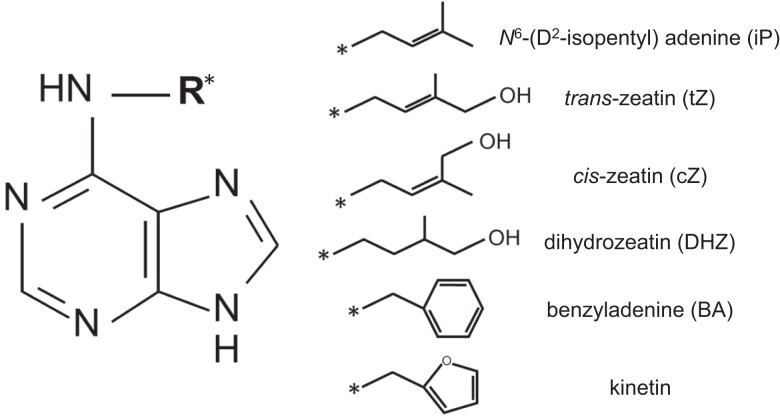
Cytokinins were first identified by Carlos Miller and others in Folke Skoog's laboratory in a search for factors that could promote the growth of plant cells in culture (for an excellent review of the discovery of cytokinin, see Amasino 2005). High activity was found in an old sample of herring sperm DNA, and subsequent work using autoclaved DNA identified the active component as an adenine derivative (6-furfural-aminopurine) that is a degradation product of DNA, which they named kinetin (Miller et al. 1955; Miller et al. 1956). Similar to other cytokinins, kinetin is an N6-substituted adenine derivative (Fig. 1). In addition to its ability to promote cell division in concert with auxin, different ratios of kinetin to auxin in media were found to promote shoot or root organogenesis, or undifferentiated growth (Skoog and Miller 1957). The first endogenous cytokinin from plants was identified from immature corn kernels and named zeatin (Letham et al. 1964; Jameson 2023). Trans-zeatin has an isoprene derivative as the N6 side chain and is the most common naturally occurring cytokinin in higher plants, which also contain N6-(Δ2-isopentenyl) adenine (iP) and dihydrozeatin (DHZ), as well as various derivatives of these (Fig. 1). Cytokinins with aromatic sides chains, including kinetin, have also been identified as minor cytokinins from some plant species (Strnad 1997). The various forms of cytokinin differ in their in planta stability, their transport characteristics throughout the plant, and their binding affinity for different cytokinin receptors (Kamada-Nobusada and Sakakibara 2009; Hluska et al. 2021).
Figure 1.
Structures of various cytokinins. Cytokinins are adenine derivatives. The general structure of the adenosine backbone of cytokinin is indicated to the left. Various sides chains, shown on the right, can be attached at the N6 position of the adenine ring (indicated by an R*), leading to cytokinin species with differing biological activity. Note that BA and kinetin are not naturally occurring cytokinins.
tRNA from most organisms, except Archea, contain cis-zeatin as a modified base (Lindner et al. 2014). It was thus postulated that breakdown of this prenylated tRNA was the primary source of cytokinin in plants. However, recent work has shown that plants lack the ability to convert cis-zeatin to trans-zeatin (Hošek et al. 2020), and in many plants, cis-zeatin is not an active cytokinin (Gajdošová et al. 2011). Thus, tRNA is most likely not a significant source of cytokinin in such plants. However, in some plants, including many crop species (e.g. rice, maize, pea, and potato), the cis form of zeatin is found at high levels (Gajdošová et al. 2011; Kudo et al. 2012; Hluska et al. 2021) and is capable of binding to and activating a subset of cytokinin receptors in some species (e.g. Yonekura-Sakakibara et al. 2004; Lomin et al. 2011). The source of this cis-zeatin in these species may be breakdown of tRNA, though an enzyme involved in the breakdown of prenylated tRNA has not yet been identified. Alternatively, there may be a distinct biosynthetic pathway for cis-zeatin.
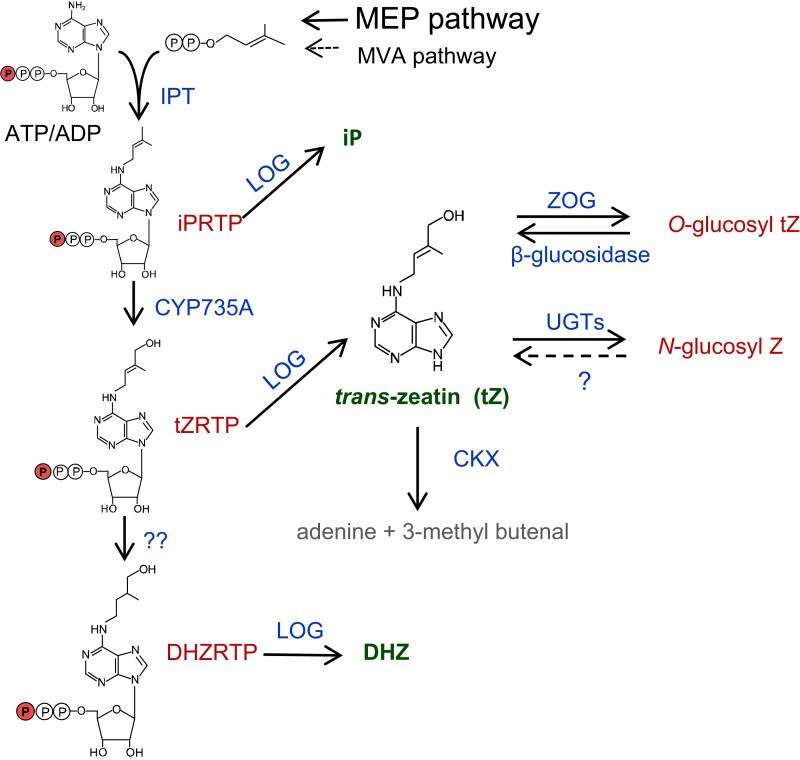
For most cytokinins, the first step in the biosynthetic pathway involves the linking of an isoprene moiety from dimethylallyl pyrophosphate (DMAPP) to the N6 position of adenosine to form N6-(Δ2-isopentenyl) adenosine (iP) (Fig. 2). The first enzyme (isopentyl transferase [IPT]) catalyzing such a reaction was found in Dictyostelium discoideum (Taya et al. 1978), and, subsequently, a gene present on the transfer-DNA from Agrobacterium tumefaciens was shown to encode an enzyme with similar activity (Akiyoshi et al. 1984). In 2001, two groups identified nine IPT genes in the Arabidopsis genome and found that in addition to the tRNA-IPTs, plants possess a unique clade of IPT genes that the research groups subsequently demonstrated were involved in cytokinin biosynthesis (Kakimoto 2001; Takei et al. 2001) (see Table 1 for a list of genes relevant to cytokinin function). An important difference between the plant and bacterial enzymes is that plant IPTs use ATP/ADP rather than AMP as the adenosine source in the reaction. A recent phylogenetic analysis suggests that the tRNA-IPTs can be traced back to the last common ancestor of eukaryotes and that the IPTs involved in cytokinin biosynthesis in plants are derived from tRNA-IPTs (Wang 2020c).
Figure 2.
Generalized pathway for cytokinin biosynthesis and catabolism. Enzymes catalyzing each step are shown in blue. Inactive cytokinin ribosides are shown in red, and the active free base forms indicated in green. The source of the dimethylallyl pyrophosphate (DMPP) side chain of cytokinin is primarily from the methylerythritol phosphate (MEP) pathway, with minor contributions from mevalonic acid (MVA) pathway. The structure of the free base form of only zeatin is indicated; for the structures of iP and DHZ, see Fig. 1. Further, the conjugation and degradation of only trans-zeatin is indicated; both iP and DHZ type cytokinin are also subject to these processes to differing extents. The formation of cis-zeatin is not shown. See text for additional details.
Table 1.
List of important genes in cytokinin metabolism, signaling, and transport
| Gene/Protein | Name | Type | Function |
|---|---|---|---|
| HK | HISTIDINE KINASE | Signaling | Cytokinin receptors |
| HP/HPT | HISTIDINE PHOSPHOTRANSFER PROTEINS | Signaling | Phosphorelay proteins from receptors to RRs |
| PHP | PSEUDO- HISTIDINE PHOSPHOTRANSFER PROTEINS | Signaling | Negative regulators; lack conserved His that is target of phosphorylation |
| Type-B RR | TYPE-B RESPONSE REGULATOR | Signaling | Primary transcription factors; positive elements in the cytokinin response |
| Type-A RR | TYPE-A RESPONSE REGULATOR | Signaling | Rapidly induced by cytokinin; act as negative regulators of the cytokinin response |
| IPT | ISOPENTENYL TRANSFERASE | Biosynthesis | Biosynthetic enzyme; links isoprene moiety to N6 position of adenosine |
| CYP735A | CYTOCHROME P450 mONOOXYGENASES | Biosynthesis | Conversion of iP ribosides to trans-zeatin |
| LOG | LONELY GUY | Biosynthesis | Cytokinin riboside 5′-monophosphate phosphoribohydrolase; activity generates active free-base forms of cytokinins |
| CKX | CYTOKININ OXIDASE | Degradation | FAD-dependent amine oxidases, cleavage of N6-side chains; degrades cytokinins |
| UGT76C1 and 2 | UDP-GLUCOSYL TRANSFERASE 76C1 and 2 | Inactivation | N-glycosyltransferases |
| OsZOG1 | ZEATIN O-GLUCOSYLTRANSFERASE 1 | Inactivation | O-glycosyltransferases |
| ENT | EQUILIBRATIVE NUCLEOSIDE TRANSPORTERS | Transport | Cytokinin transporter |
| ABCGs | ATP-BINDING CASSETE TRANSPORTER G | Transport | Cytokinin transporter |
| PUPs | PURINE PERMEASES | Transport | Cytokinin transporter |
| HvSWEET11 | SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTERS | Transport | Cytokinin and sugar transporter |
| AZG | AZAGUANINE PURINE TRANSPORTER | Transport | Purine and cytokinin transporter |
Isotope-labeling experiments in Arabidopsis demonstrated that the primary source of the DMAPP used as the prenyl donor for the IPT reaction comes from the methylerythritol phosphate pathway (Kasahara et al. 2004), which is localized to plastids. Consistent with this, four of the Arabidopsis IPTs involved in cytokinin biosynthesis are localized to plastids, though two others are localized to the cytosol (IPT4) and mitochondria (IPT7) (Miyawaki et al. 2004; Takei et al. 2004a). A minor portion of DMAPP for cytokinin biosynthesis likely also comes from mevalonic acid because lovastatin, an inhibitor of mevalonic acid biosynthesis, can reduce cytokinin content (Laureys et al. 1999). The crystal structures for both a plant and an A. tumefaciens IPT have been solved, and this analysis revealed that the prenylation reaction proceeds via an SN2-reaction mechanism (Sugawara et al. 2008; Chu et al. 2010). Interestingly, the plant enzyme has two basic residues (Lys275 and Lys220) that interact with ATP to neutralize the charge of the β- and γ-phosphate, but these residues are replaced by acidic amino acids (Asp221 and Asp171) in bacterial IPTs, which likely explains the difference in the adenosine utilized in bacterial vs plant IPTs.
The products of the IPT reaction are iP ribosides, which are converted to trans-zeatin ribosides by hydroxylation of the prenyl side chain, a reaction catalyzed by the CYP735A cytochrome P450 monooxygenases (Takei et al. 2004b). Disruption of the genes encoding these CYP735As in Arabidopsis and rice eliminates trans-zeatin biosynthesis, allowing one to define specific roles of trans-zeatin type cytokinins in regulating growth and development. In the case of Arabidopsis, the cyp735a1/2 double mutant shows reduced shoot growth but little effect on root growth (Kiba et al. 2013). This may reflect the observation that ARABIDOPSIS HISTIDINE KINASE3 (AHK3) is the cytokinin receptor most highly expressed in shoots (Higuchi et al. 2004; Nishimura et al. 2004), and it has a higher affinity for trans-zeatin than iP (Romanov et al. 2006; Stolz et al. 2011; Lomin et al. 2012). Disruption of the CYP735As in rice resulted in decreased growth of both roots and shoots, with substantial reductions in leaf size and rate of leaf formation, fewer tillers, delayed flowering, and highly reduced inflorescences (Kiba et al. 2023). This suggests that in rice, trans-zeatin is a positive regulator of shoot and inflorescence meristem function, consistent with analyses of cytokinin receptor mutants in rice (Burr et al. 2020). The enzyme responsible for the conversion of trans-zeatin to dihydrozeatin has not yet been identified in plants.
Biologically active cytokinins are produced by the removal of the riboside groups from iP riboside 5′-monophosphate, trans-zeatin riboside 5′-monophosphate, or dihydrozeatin riboside 5′-monophosphate to form the active, free-base cytokinins, a reaction catalyzed by the LONELYGUY (LOG) family of enzymes (Chen et al. 2022). LOG was first identified in a screen for rice mutants that failed to maintain shoot meristem function (Kurakawa et al. 2007). The LOG name comes from the phenotype of the spikelets in the mutants, which often contain only a single stamen and no pistil (Kurakawa et al. 2007). LOG is present as a small gene family in most angiosperms, as well as in moss (Physcomitrium patens) and liverworts (Selagenella moellendorffii), and are also found as single copy genes in green algae (Chen et al. 2022). Disruption of multiple LOG genes in Arabidopsis leads to severe retardation of shoot and root growth and defects in the maintenance of the apical meristems, with LOG4 playing a major role in the shoot apical meristem (SAM) and LOG3 and LOG4 in the root (Tokunaga et al. 2012). LOG4 expression is restricted to the L1 layer of the SAM and may supply apically derived cytokinin to underlying cell layers of the SAM to help pattern the expression of WUSCHEL (WUS), a key regulator of stem cell function within the stem cell niche (Chickarmane et al. 2012). Overexpression of OsLOGL5 in rice reduced primary root growth, tiller number, and yield (Wang et al. 2020a). In contrast, mutations that alter the C-terminal domain of OsLOGL5 did not significantly affect the growth or morphology of the plants but led to significantly increased yield in the field (Wang et al. 2020a), though the effects of these C-terminal mutations on OsLOGL5 function have not been examined.
Cytokinin levels can be decreased through either conjugation to sugars, especially glucose, or irreversible cleavage by cytokinin oxidases. In terms of conjugation, glucose can be linked to the nitrogen at primarily the N7 or N9 position of the purine ring or to the oxygen on the side chains of iP, DHZ, and zeatin (both cis and trans) (Chen et al. 2021). Glucosyl conjugates are generally inactive in bioassays and fail to bind to cytokinin receptors (Spichal et al. 2004). Conjugations at the N positions were thought to be irreversible, but recent findings are changing that notion as metabolic studies on Arabidopsis seedlings have shown that trans-zeatin N-glucosides (but not those of iP) are rapidly converted to trans-zeatin (Hošek et al. 2020). The O-glycosylated forms can also be converted into active cytokinins by β-glucosidases (Brzobohaty et al. 1993). Genes encoding enzymes catalyzing N or O (or both) glycosylation of cytokinin have been identified (Chen et al. 2021). In Arabidopsis, the glycosyltransferases UGT76C1 and UGT76C2 can glucosylate multiple different cytokinins at the N7 or N9 positions, and UGT85A1 glycosylates cytokinin at the oxygen on the side chain. As expected, disruption of UGT76C2 led to a significant decrease in cytokinin N-glucoside levels, and overexpression had the opposite effect (Wang et al. 2011). The lines with altered UGT76C2 function did not result in any obvious morphological alterations in plants, though there were some modest changes in the expression of cytokinin-regulated genes (Wang et al. 2011). The lack of effect on growth and development may be due to compensatory changes in cytokinin metabolism in these lines that keep the active, free-base forms within a physiologically suitable range. In contrast, knockdown of a rice cytokinin O-glucosyltransferase (OscZOG1) had substantial effects on growth and development, including increased lateral roots, tillering, panicle branching, grain number per panicle, and seed size; overexpression of OscZOG1 had the opposite effects, concomitant with an increased level of cis-zeatin O-glucoside (Shang et al. 2016). These results are consistent with the idea that conjugation of cytokinin plays an important role in regulating active cytokinin levels in vivo.
A major regulator of cytokinin content in plant tissues is its irreversible degradation by cytokinin oxidases (CKX), which are FAD-dependent amine oxidases that cleave the N6-side chains from a subset of cytokinins (Werner et al. 2006). Cytokinin oxidase was first cloned from Zea mays kernels (Houba-Hérin et al. 1999; Morris et al. 1999) and has since been identified in all land plants. Phylogenetic analysis suggests that the CKX genes from land plants are derived from a single chlamydial ancestral gene (Wang et al. 2020b). The substrate preferences vary among CKX isoforms, but in general the free bases and their ribosides are the preferred substrates; dihydrozeatin and aromatic cytokinins such as 6-benzylaminopurine (BA) are generally resistant to cleavage (Galuszka et al. 2007). The expression of multiple CKX genes is induced in response to elevated cytokinin, acting as a negative feedback mechanism to reduce cytokinin levels (Bhargava et al. 2013; Polko et al. 2021).
Alteration of CKX function has substantial potential to improve various agronomic traits (reviewed in Jameson and Song 2016; Jameson and Song 2020) and, indeed, various CKX genes have been disrupted in multiple crop species using overexpression, RNAi, TILLING, or, more recently, CRISPR/Cas9 (Mandal et al. 2022). Species targeted include rice, wheat, barley, rapeseed, and potato (Jameson and Song 2016). In general, CKX loss-of-function lines tend to show increased yields, while lines overexpressing CKX often have larger root systems and are more drought tolerant. For example, in a recent study in rapeseed (an allotetraploid), disruption of six of the eight copies of two CKX genes by TILLING resulted in increased seed yield from both greenhouse and field-grown plants, likely as a result of a more active inflorescence meristem leading to more flowers (Schwarz et al. 2020). Disruption of Gn1a, which encodes a CKX enzyme, in the indica subspecies of rice results in a substantial increase in grain yield (Ashikari et al. 2005; Li et al. 2016). Interestingly, overexpression of CKX in multiple crops leads to a higher content of various micro- and macro-elements in the shoots, likely due at least in part to increased size of the root systems. For example, the concentration of zinc, which is deficient in the diet of nearly 25% of the world's population, was significantly increased in the seeds of transgenic barley plants overexpressing CKX (Ramireddy et al. 2018). Likewise, in rapeseed, overexpression of a CKX gene resulted in plants that displayed an increase in the content of multiple macro- and microelements in shoots (Nehnevajova et al. 2019). In maize, overexpression of a CKX gene specifically in roots resulted in a larger root system without affecting the size of the shoot, and an increase in the content of various minerals, including K, P, and Zn in the shoot and in some lines, resulted in an increase in Cu, Zn, and Mn in seeds (Ramireddy et al. 2021). Similar results were also obtained with overexpression of a CKX gene in barley (Ramireddy et al. 2018).
Transport of cytokinins
Cytokinins are synthesized in both roots and shoots and then can be transported short distances to neighboring cells or long distances to other tissues (Sakakibara 2006; Zhang et al. 2023). Trans-zeatin and trans-zeatin riboside cytokinins are generally synthesized in the roots and transported to shoots through the xylem, whereas iP and cis-zeatin type cytokinins are mostly synthesized in the shoots and transported to the roots through the phloem (Sakakibara 2006). Although the systemic, mobile nature of cytokinins was known for many years, the identity of the transporters involved in their translocation have only recently come to light.
In the early 2000s, a series of genetic and biochemical studies identified members of the EQUILIBRATIVE NUCLEOSIDE TRANSPORTERS (ENT) family as nucleoside transporters with broad substrate specificity, with some members being able to transport cytokinins (Möhlmann et al. 2001; Hirose et al. 2005). ENT proteins typically localize to the plasma membrane and can transport nucleosides down their concentration gradients (Hyde et al. 2001). A mutation in ENT8 was identified as a suppressor of an Arabidopsis IPT overexpression line, and disruption of this gene resulted in hyposensitivity to exogenous isopentenyladenine and trans-zeatin ribosides, as well as a reduced uptake of isopentenyladenine riboside (Sun et al. 2005). In rice, OsENT2 was shown to transport cytokinin ribosides when expressed in yeast, suggesting it may also participate in cytokinin riboside uptake, though no in planta transport assays have been done for this ENT (Hirose et al. 2008). These studies suggest that a subset of ENTs is likely involved in the long-distance translocation from roots to shoots of the riboside forms of isopentenyladenine and trans-zeatin.
Another class of transporters acting in the long-distance movement of cytokinin belongs to the ATP-BINDING CASSETTE TRANSPORTER G (ABCG) family. Disruption of ABCG14 resulted in phenotypes consistent with reduced cytokinin function, suggesting that it might act as a cytokinin transporter (Zhang et al. 2014). ABCG proteins transport a variety of substrates, including auxin. Through genetic and biochemical analyses, ABCG14 was shown to be a PM-localized protein that functions in the uptake of trans-zeatin and DHZ-type cytokinins (Ko et al. 2014; Zhang et al. 2014). ABCG14 is highly expressed in roots, and quantification of cytokinins in both shoot and roots of the abcg14 mutant showed a hyperaccumulation of cytokinins in roots compared with wild-type plants, suggesting a function in the root to shoot transport of cytokinin (Ko et al. 2014; Zhang et al. 2014). Grafting experiments confirmed that ABCG14 functions as a cytokinin transporter between roots and shoots (Ko et al. 2014). ABCG14 plays a role in both the loading of cytokinin into the xylem in the roots (Ko et al. 2014; Zhang et al. 2014) and the unloading of cytokinin from the phloem in shoots via an apoplastic pathway (Zhao et al. 2021). In rice, OsABCG18 plays a similar role in root to shoot transport of cytokinin (Kim et al. 2020). Another ABCG transporter, ABCG11, has been proposed as a cytokinin transporter due to cytokinin-associated phenotypes displayed by an abcg11 loss-of-function mutant (Yang et al. 2022); however, biochemical evidence of transporter activity is lacking. Likewise, disruption of the genes encoding 3 non-intrinsic ABC transporters (ABCI19, ABCI20, and ABCI21) resulted in cytokinin hypersensitivity in Arabidopsis (Kim et al. 2020). These ABCI proteins were localized to the ER and found to be induced by light, though, like ABCG11, these have not yet been shown to transport cytokinin.
Another class of cytokinin transporters belongs to the PURINE PERMEASES (PUPs) family, which likely reflects the fact that cytokinins are purine derivatives. There are 21 PUP genes in Arabidopsis (Jelesko 2012), of which AtPUP1 was the first shown to encode a transporter of cytokinin (Gillissen et al. 2000). A survey of the other PUP genes led to the identification PUP14 as the member with highest expression in a variety of plant tissues and strongest genetic effect on cytokinin responses (Zürcher et al. 2016). PUP14 was localized to the PM, and its activity was inversely correlated with cytokinin content in the apoplast, thus leading to the hypothesis that its physiological function was to attenuate cytokinin signaling by transporting cytokinins from the apoplast into cells, reducing the signal available for PM localized cytokinin receptors (Zürcher et al. 2016). Given the observations that the affinity of this transporter is significantly lower than that of the receptors for cytokinin, that the bulk of the cytokinin receptors are found in the ER, and that a rice PUP cytokinin transporter (OsPUP1) has been localized to the ER (Xiao et al. 2019), a counter-hypothesis is that the PUPs function by transporting cytokinins intracellularly between ER, the cytosol, and the apoplast, thus regulating cytokinin signaling by depleting cytokinin from ER-localized receptors (Romanov et al. 2018).
Recently, a member of the SWEET (SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTERS) family of sugar transporters in barley (HvSWEET11b) was shown to function as a dual transporter of both sugars and cytokinins in developing barley grains (Radchuk et al. 2023). Given that cytokinin regulates sink-source relationships, which involves sugar transport, this directly links the transport of cytokinins to one of the physiological processes they control (Radchuk et al. 2023).
Finally, two transporters in the azaguanine purine transporter family (AZG) family have been linked to cytokinin transport. AZG2 transported purines and cytokinin with high affinity (Tessi et al. 2021) and localized to both the ER and the plasma membrane. AZG2 plays a role in lateral root development. Interestingly, AZG1, which was also shown to transport cytokinin, was identified as a protein that co-purified with the PIN1 auxin transporter in native gels, indicating that it may play a role in the interaction between auxin and cytokinin (Tessi et al. 2023).
Deciphering the signaling pathway
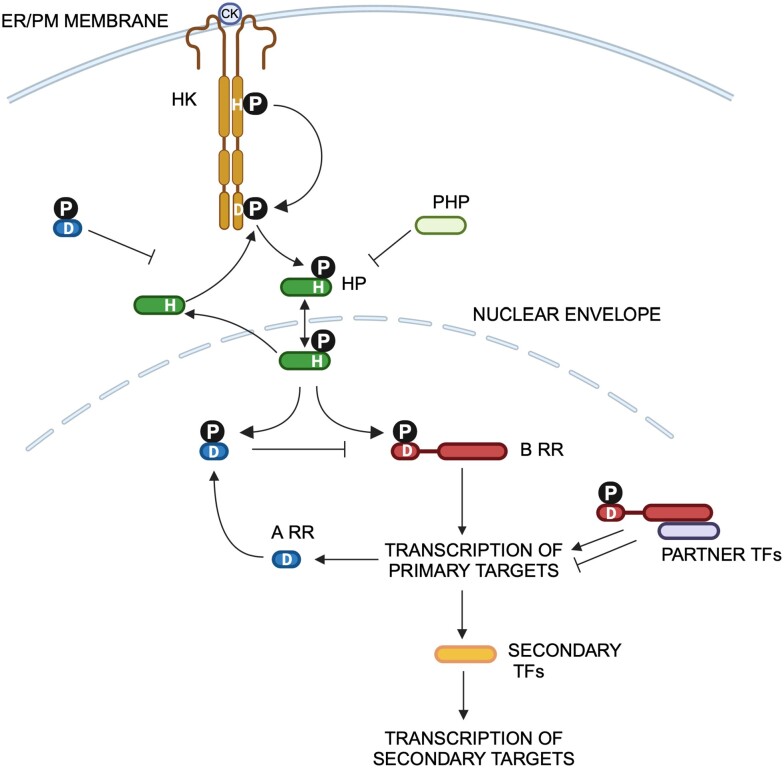
Starting in the mid-1970s, multiple groups reported the biochemical purification of cytokinin binding proteins (Takegami and Yoshida 1975; Polya and Davis 1978; Sussman and Kende 1978; Moore 1979; Keim and Fox 1980), including binding to isolated plant ribosomes (Fox and Erion 1975), but these approaches failed to identify the molecular nature of the cytokinin receptor. Recent decades have seen remarkable progress in our understanding of the molecular mechanisms underlying cytokinin perception and signaling. In the mid-1990s, 2 independent lines of investigation converged on the same signaling pathway. The CKI1 histidine kinase was identified in a screen for genes that when overexpressed in cultured cells conferred the ability to form shoots in the absence of exogenous cytokinin (Kakimoto 1996). In parallel, two genes encoding response regulators (ARR5 and ARR7) were identified in a screen for cytokinin primary response genes (Brandstatter and Kieber 1998). Histidine Kinases (HKs) and Response Regulators (RRs) comprise the elements of two-component signaling pathways in prokaryotic organisms (Stock Stock and Mottonen 1990; Stock Robinson and Goudreau 2000). In their simplest form, two-component systems are comprised of an often membrane-localized sensor HK that perceives some environmental stimuli (e.g. phosphate levels). Upon perception of the signal, the HK autophosphorylates on a His residue and then in turn transfers the phosphate to an Asp residue within the receiver domain of an RR protein. Phosphorylation of the RR regulates its activity, which most often involves direct regulation of transcription of target genes. Connecting both a HK (CKI1) and two RRs (ARR5 and ARR7) to the response to cytokinin strongly suggested that cytokinin perception involves a two-component–like pathway, which subsequent studies confirmed. However, in the case of cytokinin (and other eukaryotic two-component pathways), the response pathway is actually similar to phosphorelays, which involve an extra pair of phosphotransfers between His and Asp residues (Swanson et al. 1994; Appleby et al. 1996; Schaller et al. 2011). In these systems, there is a receiver domain fused to the HK receptor protein, and when the HK is activated, an Asp residue within this receiver domain accepts the transferred phosphate in an intramolecular reaction. A small histidine-containing phosphotransfer protein then shuttles the phosphate from the HK receiver domain to an Asp residue on the downstream RR proteins (Fig. 3).
Figure 3.
Canonical cytokinin signaling pathway. Cytokinin binds to the CHASE domain in the HK receptors and initiates a phosphorelay as indicated by the P. See text for additional details.
While CKI1 was the first HK to be linked to cytokinin signaling, it lacks a cytokinin binding domain and does not act as a cytokinin receptor, though it may feed into the downstream signaling elements (Dobisova et al. 2017). Authentic cytokinin HK receptors were identified in genetic screens for loss-of-function mutants impaired in cytokinin responses in cultured cells [e.g. shoot formation in tissue culture (Inoue et al. 2001)], by reverse genetic analysis of HK homologs in Arabidopsis (Ueguchi et al. 2001), by transient expression in Arabidopsis protoplasts (Hwang and Sheen 2001), by using bioassays in Escherichia coli or yeast, and/or by direct binding assays (Inoue et al. 2001; Suzuki et al. 2001; Ueguchi et al. 2001; Yamada et al. 2001). Plant cytokinin HK receptors are generally encoded by small gene families (e.g. three in Arabidopsis and four in rice) and share a similar structure, with two or three transmembrane domains flanking the cytokinin binding domain, a cytosolic HK domain fused to both a functional receiver domain as well as a degenerate receiver domain. Genetic analysis has demonstrated that the His kinase activity of AHK4 in Arabidopsis is required for cytokinin signaling (Mähönen et al. 2006a), as is the Asp residue that receives the phosphate within the functional receiver domain (Hodgens et al. 2020). Interestingly, multiple missense mutations that alter residues within or near the first transmembrane domain of AHK4 also eliminate its function, which may reflect disruption of its membrane insertion (Hodgens et al. 2020).
Cytokinin binds to a CHASE (cyclases/histidine kinases associated sensor extracellular) domain in these HK receptors, an evolutionarily ancient domain that binds a wide variety of ligands, including other adenine derivatives and peptides (Anantharaman and Aravind 2001). Cytokinin-binding, CHASE domain–containing HK cytokinin receptors have been identified in a bryophyte (P. patens), a lycophyte (S. moellendorffii), a gymnosperm (Picea abies), and all angiosperms examined (Lomin et al. 2021), which suggests that the ability to perceive cytokinin arose early in the evolution of land plants. In the case of P. patens, the CHASE domain HKs are essential for the response to exogenous cytokinin (von Schwartzenberg et al. 2016), even though P. patens contains only a tRNA-IPT and thus these receptors may reflect a response to endogenous cytokinin derived solely from breakdown of prenylated tRNA. The various cytokinin HK receptors in higher plants have different spatial patterns of expression and bind to different cytokinin species with distinct affinities (Spichal et al. 2004; Romanov et al. 2006; Stolz et al. 2011; Lomin et al. 2012, 2021). Thus, although the cytokinin receptors display varying degrees of genetic redundancy in the species that have been examined, they also likely have distinct functionalities.
An unresolved question in the field is the subcellular localization of these receptors. Early studies using analysis of AHK fusions to fluorescent proteins suggested a plasma membrane localization (Kim et al. 2006). However, subsequent biochemical analysis indicated that all three AHK cytokinin receptors localize to the endoplasmic reticulum (ER) (Wulfetange et al. 2011). This was based on analysis of high-affinity cytokinin binding to membrane fractions purified by two-phase partitioning in Arabidopsis lines harboring a single functional AHK receptor, as well as localization of fluorescent fusion proteins in transiently transformed tobacco leaves. Furthermore, aqueous two-phase partitioning of microsomes revealed that epitope-tagged AHK2 and AHK3 colocalized with ER membranes (Wulfetange et al. 2011). Likewise, a second study confirmed that fluorescently labeled AHK3 and AHK4 localized to the ER in both tobacco leaves and stably transformed Arabidopsis, and the authors showed that these fusion proteins complemented an ahk2 ahk3 loss-of-function mutant (Caesar et al. 2011). Likewise, the maize ZmHK1 protein was also localized to the ER (Lomin et al. 2011). Finally, the pH optima of cytokinin binding to the Arabidopsis receptors is more consistent with binding in the ER lumen than in the apoplast. This issue was extensively discussed in an excellent review (Romanov et al. 2018). However, recent results indicate that a subset of the cytokinin receptors may actually reside in the plasma membrane (Antoniadi et al. 2020). These authors found that immobilized cytokinin (i.e. covalently linked to beads), which cannot enter cells, was capable of activating all three AHK receptors in Arabidopsis protoplasts as measured by both activation of a cytokinin-responsive reporter (Liu and Müller 2017) and induction of Cytokinin Response Factors (CRF) transcript levels (Rashotte et al. 2006). Further, super-resolution microscopy of AHK4-GFP and AHK3-GFP fusion proteins indicated that a fraction of the signal is present at the cell surface (Antoniadi et al. 2020). Finally, more recent analysis of cytokinin receptors across the plant kingdom has revealed a wide range of pH optima for cytokinin binding, consistent with different subcellular localizations (Lomin et al. 2021). Thus, it seems that cytokinin receptors in higher plants are primarily localized to the ER with cytokinin binding occurring in the ER lumen, but a fraction of the functional receptors may be at the plasma membrane, binding cytokinin in the apoplast.
Histidine-containing phosphotransfer proteins
The Authentic Histidine-containing Phosphotransfer proteins (AHPs) act downstream of the cytokinin receptors. AHPs are found in unicellular algae, moss, lycophytes, gymnosperms, and angiosperms, with the AHPs from land plants forming a distinct phylogenic clade from those in algae (Pils and Heyl 2009; Rashotte 2021). The AHPs are often found as small gene families in higher plants (e.g. five in Arabidopsis and two in rice), and at least in Arabidopsis, these AHPs have overlapping functions as positive elements in cytokinin signaling (Hutchison et al. 2006). These proteins shuttle between the cytosol and the nucleus to mediate the transfer of a phosphoryl group from the receiver domain of the activated HK receptors to the receiver domain of an RR protein (Punwani et al. 2010). Their phosphotransferase activity is negatively regulated by nitric oxide–induced S-nitrosylation, suggesting a link between redox signaling and cytokinin function (Feng et al. 2013). In addition to their role in cytokinin signaling, the AHPs also act downstream of other HKs, including CKI1 and likely AHK1 in Arabidopsis. For example, disruption of multiple AHPs results in loss of central cell and antipodal cell fates coupled with a gain of egg cell or synergid cell attributes during female gametophyte development in Arabidopsis (Liu et al. 2017), similar to the phenotypes of cki1 loss-of-function mutations (Yuan et al. 2016).
The AHPs contain an essential His that is the target of phosphotransfer from the upstream AHKs. In addition to the AHPs, plant genomes also encode proteins similar to AHPs that lack the His residue required for phosphorelay. These so-called Pseudo Histidine Phosphotransfer proteins (PHPs) are negative regulators of cytokinin signaling, likely acting in a dominant negative manner to block AHP phosphotransfer activity (Mähönen et al. 2006b). AHP6 (an Arabidopsis PHP) mediates cross talk between auxin and cytokinin in multiple developmental events, including root vascular patterning, the formation of passage cells, lateral root organogenesis, proliferation of the inflorescence meristem, shoot phyllotaxy, and gynoecium development (Mähönen et al. 2006b; Moreira et al. 2013; Besnard et al. 2014; Müller et al. 2017; Andersen et al. 2018). For example, in vascular development, bisymmetric domains of auxin and cytokinin ultimately lead to the formation of phloem and xylem (Mähönen et al. 2006b) (see below). Similar to this, in the shoot apical meristem, leaves initiate at positions of auxin maxima, and the robustness of this phyllotaxy is enhanced by a spatio-temporal pattern of AHP6 that regulates fields of cytokinin signaling (Besnard et al. 2014).
Phylogenic analysis indicates that the PHPs are present in gymnosperms and early diverging angiosperms but not in bryophytes (Vaughan-Hirsch et al. 2021). The monocot and dicot PHPs fall into two distinct clades, with the monocots having a Gln at the position of the conserved His residue and dicots having an Asn at this position. This suggests that PHPs evolved twice independently from AHPs: one event in monocots event giving rise to a to a PHP(Gln) class and a second in eudicots giving rise to the PHP(Asn) class. Consistent with their independent evolutionary origin, rice PHPs appear to have functions distinct from their Arabidopsis counterparts (Vaughan-Hirsch et al. 2021).
Response regulators
The final step of the cytokinin-regulated phosphorelay is the transfer of the phosphate from the AHPs to an Asp residue on the receiver domain of response regulators (RRs). There are two main types of RRs in plants. The type-B RRs are characterized by the presence of a receiver domain and a large C-terminal extension harboring a Myb-like DNA binding domain. Type-B RRs are generally not transcriptionally regulated by cytokinin and act as positive regulators of cytokinin signaling. In contrast, type-A RRs, which form a distinct phylogenetic clade from the type-B RRs, lack a DNA binding domain, are generally transcriptionally induced by cytokinin and act as negative regulators of cytokinin signaling. Most phylogenetic analyses suggest that type-A and type-B RRs originated in the Charophyte algae and are found throughout the land plant lineage, from the liverwort Marchantia polymorpha and the moss Physcomitrella patens, through Gymnosperms and Angiosperms (Pils and Heyl 2009; Rashotte 2021).
Type-B RRs are DNA-binding transcription factors that mediate the initial transcriptional response to cytokinin (Sakai et al. 2000; Hwang and Sheen 2001; Sakai et al. 2001; Imamura et al. 2003; Mason et al. 2005; Argyros et al. 2008; Hill et al. 2013). As with other elements in the cytokinin signaling pathway, type-Bs are generally encoded by a small gene family in higher plants (e.g. 11 in Arabidopsis and 13 in rice, though not all have been shown to be involved in cytokinin signaling). There is functional overlap in the gene family in Arabidopsis; single mutants have at most modest effects, but disruption of multiple type-B RRs leads to cytokinin insensitivity, effects on growth and development similar to disruption of the cytokinin HK receptors, and, in the highest combination mutants, the near absence of a transcriptional response to cytokinin (Mason et al. 2005; Argyros et al. 2008). The receiver domain of the type-B RRs negatively regulates their transcriptional activity (Sakai et al. 2001; Liang et al. 2012), and phosphorylation of this domain at the conserved Asp residue results in increased binding of type-B RRs to their genomic targets (Zubo et al. 2017; Xie et al. 2018). Type-B RRs were found to bind upstream of genes that were both activated and repressed by cytokinin, and disruption of type-B RRs block the cytokinin regulation of both classes of genes (Mason et al. 2005; Argyros et al. 2008), suggesting that type-B RRs are necessary for both gene activation and repression in response to cytokinin. Type-B RR protein stability is regulated via the KISS ME DEADLY F-box proteins (Kim et al. 2013).
A large number of transcription factors are regulated in response to cytokinin (Argueso et al. 2010; Brenner et al. 2012; Bhargava et al. 2013), suggesting that type-B RRs operate at the top of a transcriptional cascade, with the succeeding waves of transcription being regulated by these inducible transcription factors (Fig. 3). Various other transcription factors act in concert with type-B RRs and contribute to differences in transcriptomic responses to cytokinin (Leuendorf and Schmülling 2021). These partner transcription factors likely modulate the target genes to which activated type-B RRs bind, either through direct or indirect interaction. Many such type-B RR interacting partners have been identified, including CRFs, DELLA, BPCs, NPR1, TCPs, HY5, TGA3, HD-ZIP IIIs, and EIN3 (Rashotte et al. 2006; Choi et al. 2010; Efroni et al. 2013; Marín-de la Rosa et al. 2015; Raines et al. 2016b; Shanks et al. 2016; Yan et al. 2017; Zhang et al. 2017; Hodgens et al. 2020).
The type-A RRs contain a receiver domain but, unlike the type-B RRs, lack a DNA binding domain and most are transcriptionally induced in response to cytokinin (D’Agostino et al. 2000; Tsai et al. 2012). Genetic analyses indicate that ten type-A RRs in Arabidopsis function as negative regulators of cytokinin signaling, thus acting as a negative feedback loop to dampen cytokinin signaling (Kiba et al. 2003; To et al. 2004; Leibfried et al. 2005; Lee et al. 2007; To et al. 2007). The mechanism by which type-A RRs negatively regulate cytokinin signaling is not fully understood but likely involves both competition with the type-B RRs for phosphotransfer from the upstream AHPs and phospho-specific interactions with regulatory proteins (To et al. 2007; Shanks et al. 2018).
The stability of type-A RR proteins also plays an important role in their regulation. In response to elevated cytokinin, type-A RRs are phosphorylated, and this inhibits their degradation by the 26S proteosome (To et al. 2007). This stabilization acts synergistically with the transcriptional upregulation of type-A RRs to highly increase RR protein levels in response to elevated cytokinin. Intriguingly, in contrast to unphosphorylated type-A RRs, the phosphorylated forms are targeted for degradation by autophagy in an EXO70D-dependent manner (Acheampong et al. 2020). The relative contributions of 26S proteome vs autophagic protein turnover is likely influenced by multiple regulatory inputs and differs across the type-A RR family members. These processes likely complement each other to optimize type-A RR protein levels, and hence cytokinin responsiveness, in response to various developmental and environmental cues.
Another negative feedback loop in cytokinin signaling in addition to the type-A RRs is provided by the cytokinin induction of the EAR motif-containing transcriptional repressor TIE1/TIE2 (He et al. 2022). ARR1 induces transcription of TIE1 and TIE2 by directly binding to their regulatory regions in the genome. TIE1/2 also interact directly with type-B RRs to represses transcription of their target genes in Arabidopsis roots, thus dampening the response to elevated cytokinin.
A number of cytokinin-responsive reporter transgenes have been constructed based on the transcriptional induction of the type-A RRs. The first were fusions of the promoter of the Arabidopsis ARR5 gene to either a GUS or GFP reporter (D'Agostino et al. 2000; Lohar et al. 2004). Although these reporters are useful, they suffer from lack of specificity and universality because ARR5 is likely regulated by other regulatory inputs and is not induced in all cell types. A synthetic reporter was developed comprised of concatemerized type-B binding sites fused to a GFP reporter called TCS::GFP (Müller and Sheen 2008). Although useful for reporting cytokinin signaling, TCS::GFP showed little or no expression in developmental context known to involve cytokinin (e.g. the shoot apical meristem [SAM]) and was found to rapidly silence in transgenic plants. An improved version, called TCSn::GFP, was developed with more robust expression that is more consistent with known cytokinin functions in Arabidopsis and which is stable over several generations (Zürcher et al. 2013). TCSn::GFP is a valuable tool to querying the endogenous state of cytokinin signaling, and it or derivatives have been used in multiple plant species, including Arabidopsis (Zürcher et al. 2013), rice (Tao et al. 2017), tomato (Steiner et al. 2020), and Medicago truncatula (Fonouni-Farde et al. 2017).
Role of cytokinin in plant development and responses to stress
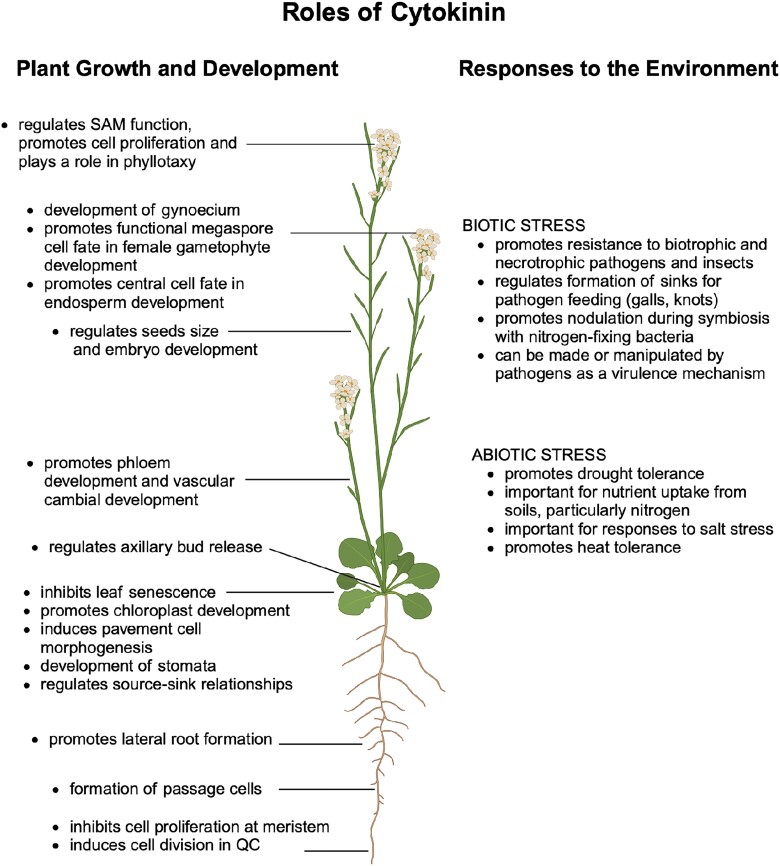
Cytokinin exerts a profound influence on nearly all aspects of plant development, orchestrating intricate processes that determine growth, differentiation, and overall morphogenesis (Fig. 4). In addition, cytokinin also plays a pivotal role in regulating several physiological processes that help maintain optimal plant development in response to environmental cues. These processes were extensively reviewed previously (Argueso et al. 2009; Hwang et al. 2012; Kieber and Schaller 2014; Schaller et al. 2015; Kieber and Schaller 2018; Wybouw and De Rybel 2019; Liu et al. 2020), and so here we simply highlight a subset of the various responses regulated by cytokinin, focusing on seminal work that elucidated these responses, the more recent findings, and the molecular mechanisms at play.
Figure 4.
Roles of cytokinins in plant growth and responses to the environment. See text for additional details.
Cell division
Cytokinin plays a crucial role in cell division and cell cycle control, orchestrating the growth and development of plants (Schaller et al. 2014). After this hormone was discovered by its ability to promote cell division, studies showed that oscillations in cytokinin levels accompanied the progression of the cell cycle, peaking at phase transitions (Hartig and Beck 2005), suggesting a link between cytokinin and cell cycle control. Further work demonstrated that cytokinin regulates the expression of CYCD3 D-type cyclins (Riou-Khamlichi et al. 1999), which in turn regulate the transition from the G1 to the S phase of the cell cycle. Cytokinin-induced shoot regeneration in vitro was also found to be dependent on cytokinin-regulated CYCD3s (Dewitte et al. 2007). The bHLH transcription factor CYTOKININ-RESPONSIVE GROWTH REGULATOR (CKG) was recently identified as a mediator of the G1/S transition in Arabidopsis (Park et al. 2021). CKG is transcriptionally induced by cytokinin, and ckg mutants show altered expression of cell cycle regulators and display a delay in the G1/S transition. Although the studies described above indicated a role for cytokinin in the G1/S transition via the action of CYCD3s and CKG, evidence for a role for cytokinin in the G2/M transition also exists. Treatment of synchronized BY-2 tobacco cell cultures with the cytokinin biosynthesis inhibitor lovastatin blocks the G2/M transition (Hartig and Beck 2005), and ahk2, ahk3, ahk4 triple mutants show a delayed G2/M transition in roots (Higuchi et al. 2004).
Cell cycle control by cytokinin regulates the activity and maintenance of meristems, ensuring sustained growth and proper development. SAM size is directly correlated to cytokinin content (Kurakawa et al. 2007; Bartrina et al. 2011), and the spatial distribution of cytokinin is highly regulated within the SAM (Gordon et al. 2009; Chickarmane et al. 2012). Cytokinin also directly regulates the activity of the homeodomain transcription factor WUS, which controls stem cell fate and maintenance in the SAM (Lopes et al. 2021). WUS expression is induced by cytokinin, and WUS is a direct transcription target of type-B ARR1, ARR10, and ARR12 (Meng et al. 2017; Zhang et al. 2017; Zubo et al. 2017; Xie et al. 2018). In turn, WUS represses type-A RR transcription (Leibfried et al. 2005) and cytokinin biosynthesis by downregulating LOG4 (Chickarmane et al. 2012). This feedback loop between WUS and cytokinin signaling has been hypothesized to control cytokinin content and signaling in the SAM and therefore SAM activity. The mechanisms by which cytokinin regulates cell division in the SAM involve CYCD3, which is a direct transcriptional target of the cytokinin-regulated SAM transcription factor SHOOT MERISTEMLESS (Scofield et al. 2013), and MYB3R4 transcription factor, which promotes the G2/M transition in dividing cells of the SAM (Yang et al. 2021a).
The role of cytokinin in the root apical meristem (RAM) is opposite of that in the SAM. Increased cytokinin content leads to a smaller RAM, and mutants with reduced cytokinin content (e.g. ipt3, ipt5, ipt7 triple mutant) have an enlarged RAM (Dello Ioio et al. 2007). Cytokinin reduces RAM size by promoting the exit of cells from the meristem, acting via an incoherent regulatory loop between IPT7 and the HD-ZIPIII transcription factor PHABULOSA (Dello Ioio et al. 2012). This negative effect of cytokinin on the RAM is in part mediated by its antagonistic interaction with auxin, via transcriptional upregulation of auxin signaling repressors (Dello Ioio et al. 2008; Moubayidin et al. 2010; Perilli et al. 2013) and altered regulation of auxin efflux and influx carriers (Marhavý et al. 2011; Zhang et al. 2013), preventing auxin-induced cell division. Cytokinin can directly regulate cell division in the RAM independently of auxin. This is mediated by the CCS52A1 E3 ligase, whose gene expression is directly regulated by ARR2 and whose activity induces endoreplication by degradation of mitotic cyclins (Takahashi et al. 2013).
After cells exit the RAM in the Arabidopsis root, they stop dividing and rapidly elongate in a region called the elongation zone and then finally stop growing and mature into their final cell forms in the differentiation zone (Petricka et al. 2012). In addition to its role in inducing the exit of cells from the root meristem, cytokinin also promotes cessation of cell growth in the distal elongation zone at least in part by increasing the rigidity of the cell wall (Liu et al. 2022).
In rice, disruption of cytokinin function by disruption of cytokinin biosynthesis (LOG genes) or perception (HK cytokinin receptors) results in a drastic effect on both the inflorescence and the spikelet meristems (Kurakawa et al. 2007; Burr et al. 2020). In Arabidopsis, disruption of all three HK cytokinin receptors or of all seven LOG genes results in a substantial reduction in the size of the SAM (Higuchi et al. 2004; Nishimura et al. 2004; Riefler et al. 2006; Tokunaga et al. 2012), though this may be at least partially a secondary consequence of defects in the vasculature in these mutants. Further, unlike in rice, there are few, if any, effects on the floral meristem in cytokinin signaling mutants in Arabidopsis. In terms of type-A RRs, disruption of a single type-A RR gene (ABPHYL1) results in an enlarged meristem in maize (Jackson and Hake 1999), but no such effect is observed even in multiple type-A RR mutants in Arabidopsis (To et al. 2004). Overall, the results suggest that cytokinin may play a more pronounced role in regulating SAM function in monocots compared with dicots.
Vascular and cambium development
The plant vascular system is essential for the survival of plants on land. The main vascular tissues, namely xylem and phloem, originate from procambial stem cells that differentiate into mature vascular tissue. A role for cytokinin in vascular morphogenesis was first revealed by the identification of the wooden leg allele of AHK4 (Scheres et al. 1995), which displays a pronounced reduction in the number of cell files in the vascular bundles, a phenotype that is even more pronounced in ahk2,3,4 triple mutants (Riefler et al. 2006). Vascular morphogenesis and differentiation require precise control of cytokinin function in concert with auxin. A feedback loop that helps define the cellular zones of auxin/cytokinin function in the developing vasculature involves the auxin transcription factor MONOPTEROS/AUXIN RESPONSE FACTOR 5, which increases the expression of AHP6, a negative regulator of cytokinin signaling. This leads to the inhibition of cytokinin signaling in specific domains of the vascular system, regulating meristematic activity of the procambium and the formation of xylem and phloem (Mähönen et al. 2006a, 2006b). Cytokinins also induce a bisymmetric distribution of the PIN-FORMED (PIN) auxin efflux proteins, resulting in accumulation of auxin in the central domain of protoxylem (Bishopp et al. 2011). In addition to its effects on AHP6, MP can also directly activate the expression of the transcription factor TARGET OF MONOPTEROS 5 (TOM5), which together with the transcription factor LONESOME HIGHWAY (LHW), regulates the expression of LOG3 and LOG4, locally increasing cytokinin levels in the protoxylem, and regulating vascular morphogenesis and growth (De Rybel et al. 2013). TOM5 and LHW also regulate the cell mobile transcription factor SHORTROOT, which can induce CKX3 expression and further regulate vascular development through localized cytokinin signaling domains (Yang et al. 2021b).
As plants grow, their stems and roots thicken, a process known as secondary growth, which results from cambial meristem activity and cell proliferation. Studies in Arabidopsis and poplar demonstrated that ipt mutants have decreased root and stem thickening, as well as reduced cambial activity, establishing a role for cytokinin in promoting cambium development (Matsumoto-Kitano et al. 2008; Nieminen et al. 2008). The genes encoding the transcription factor AINTEGUMENTA and the cyclin CYCD3;1 are both transcriptionally upregulated by cytokinin in the cambium (Randall et al. 2015). Both genes are required for proper root and stem thickening, implicating them in a conserved regulatory module controlling vascular development (Randall et al. 2015). Recently, a study in Arabidopsis identified members of the transcription factor family LATERAL ORGAN BOUNDARIES DOMAIN (LBD) as regulators of secondary growth initiation (Ye et al. 2021). LBD3 and LBD4 are transcriptionally induced in response to cytokinin and initiate activation of the cambium meristematic cells. Other members, LBD1 and LBD11, are transcriptionally induced by cytokinin later and together contribute to further radial growth and cambial stem cell maintenance. These LBD transcription factors were also determined genetically to inhibit cytokinin signaling, forming a feedback loop that regulates secondary growth (Ye et al. 2021). THE AT-HOOK MOTIF CONTAINING NUCLEAR LOCALIZED 15 transcription factor can also induce cytokinin biosynthesis locally and stimulate cambial activity (Rahimi et al. 2022).
Female gametophyte
During female gametogenesis in plants, the haploid female gametophyte (FG) develops within the ovule, surrounded by the sporophyte, to eventually generate megaspore cells. The development of the FG follows a spatial orientation along the chalazal/micropylar axis of the sporophyte. Cytokinin signaling has been shown to be essential in both the sporophyte (Kinoshita-Tsujimura and Kakimoto 2011) and in the gametophyte (Cheng et al. 2013) for female megaspore formation. In strong alleles of ahk receptor triple mutants, FG development is impaired, and the functional megaspore fails to form (Cheng et al. 2013). The effect of cytokinin on FG development was demonstrated to be associated with altered cell cycle regulation (Zhang et al. 2022). Interestingly, even though the requirement for cytokinin signaling through HK receptors for FG development has been demonstrated, cytokinin-independent signaling through two-component elements mediated by CKI1 and multiple AHPs is also required for FG development as cki1 mutant plants display specific defects in FG development (Hejátko et al. 2009) that can be phenocopied by an ahp2, ahp3, ahp5 triple mutant (Liu et al. 2017). CKI is also involved in central cell specification that generates the endosperm (Yuan et al. 2016) through a mechanism that appears to involve activation of the cytokinin signaling pathway via the AHPs (Liu et al. 2017), ultimately activating multiple type-B RRs (Zhu et al. 2022).
Leaf senescence
One of the most well-known effects of cytokinin is its anti-senescence activity. During developmental leaf senescence, which is age dependent and genetically programmed, as well as stress-induced senescence, nutrients are remobilized from leaves to other parts of the plant, where they can be reutilized for growth or storage. Levels of senescence are antagonistically correlated with levels of cytokinin content and signaling (Zwack and Rashotte 2013).
Application of cytokinin to plants delays leaf senescence (Gan and Amasino 1996). In addition, increasing endogenous cytokinin levels through transgenic approaches can also reduce senescence when properly regulated (Kant et al. 2015; Glanz-Idan et al. 2022). This was elegantly demonstrated using the promoter of a senescence-associated gene, SAG12, to drive the expression of an IPT gene in tobacco plants (Gan and Amasino 1995). Using this transgenic approach, cytokinin levels were increased specifically in cells undergoing onset of senescence, and transgenic plants expressing SAG12::IPT7 showed decreased developmental as well as stress-induced senescence (Gan and Amasino 1995).
At the level of signaling, a genetic screen for mutants with delayed senescence identified a gain-of-function allele of AHK3 as an important regulator of senescence in Arabidopsis (Kim et al. 2006). AHK3 functions in senescence by the activation of the type-B ARR2, whose transcriptional function is required for the anti-senescence activity (Kim et al. 2006). Downstream of type-B RR function, the CRF transcription factors were also found to have a role in senescence regulation. Higher-order mutant crf1,3,5,6 plants display faster developmental senescence (Raines et al. 2016a), while overexpression of CRF6 delays stress-related senescence (Zwack and Rashotte 2013).
The mechanisms underlying the reduction of leaf senescence by cytokinin are relatively unknown. The strongest association has been with cell wall-invertases (CWINV). CWINVs function by cleaving sucrose molecules into glucose and fructose, which then become available for metabolic processes associated with plant growth. A delay in developmental or stress-induced senescence is associated with increased CWINV enzymatic activity and gene expression (Gan and Amasino 1995). Moreover, expression of a CWINV driven by a cytokinin inducible promoter also correlates with decreased senescence, and that can be reverted by the use of a chemical inhibitor of CWINV activity (Balibrea Lara et al. 2004).
Biotic stress
A role for cytokinin in plant-pathogen interaction was long proposed, especially in the relationship between plants and biotrophic pathogens in which exchange of nutrients is required (Walters and McRoberts 2006). This is the case in the formation of the so-called “green islands,” which are formed around sites of biotrophic pathogen colonization, an area of reduced senescence that supports pathogen growth. Further studies in Arabidopsis showed that application of high concentrations of cytokinin, as well as genetic approaches increasing cytokinin content and signaling (Choi et al. 2010; Argueso et al. 2012), do not activate defense directly but rather prime the plants for stronger defense in a variety of plant-pathogen systems (Albrecht and Argueso 2017). This priming effect by cytokinin is also observed in biocontrol agents such as rhizobacteria, which produce cytokinin to activate plant defense and reduce disease (Großkinsky et al. 2016). Molecular mechanisms of immunity activation against biotrophs by cytokinin include formation of a transcriptional complex between the type-B ARR2, the salicylic acid co-receptor NPR1, and the TGA3 transcription factor (Choi et al. 2010). Type-A RRs also participate by negatively regulating immunity in a phosphorylation-dependent manner (Argueso et al. 2012). Stomatal immunity is also partially controlled by cytokinin via regulation of peroxidases (Arnaud et al. 2017).
Apart from a role in immunity, cytokinin is also important in interactions of susceptibility between plants and pathogens. In such cases, increased cytokinin production or signaling initiated by pathogens leads to localized regions of cell proliferation, which become the galls, tumors, and knots that are often symptoms of plant diseases (McIntyre et al. 2021). The crown gall disease caused by A. tumefaciens is a classic example of this type of interaction (Akiyoshi et al. 1984), but many other pathogens such as fungi (Malinowski et al. 2016), nematodes (Siddique et al. 2015), and even parasitic plants (Spallek et al. 2017) can also manipulate cytokinin biosynthesis or signaling to create regions of cell division at sites of infection. Such regions of cell proliferation function as sinks, to which metabolites are rerouted to, away from source leaves, aiding in pathogen growth and multiplication in a process involving sugars and amino acid transporters that are also regulated by cytokinin (McIntyre et al. 2021).
Recently, a function for cytokinin in immunity against necrotrophic pathogens was also revealed (Gupta et al. 2020; Li et al. 2021; Liu et al. 2023). In tomato, application of micromolar amounts of cytokinin had a protective effect against the necrotrophic fungal pathogen Botrytis cinerea, through mechanisms that include the expression of defense marker genes as well as production of ethylene (Gupta et al. 2020). Interestingly, cytokinin can also be produced and sensed by Botrytis, having a role in fungal development and energy utilization, which underscores the complex relationship of this hormone in plant-pathogen interactions (Gupta et al. 2021).
Finally, cytokinin also plays a role in symbiotic interactions, particularly in the interactions of legume plants and rhizobia bacteria during nodule formation for nitrogen fixation. Evidence supporting a major role of cytokinin in nodulation comes from the fact that Medicago truncatula and Lotus japonicus mutants impaired in cytokinin signaling display reduced nodule formation in response to rhizobia infection (Plet et al. 2011; Held et al. 2014), while gain-of-function mutations in these genes leads to nodulation in the absence of rhizobia. Nodule formation is initiated by perception of bacteria-secreted nodulation (Nod) factors by plant cell surface LysM-type receptor kinases. After Nod factor perception, cytokinin biosynthesis and signaling are increased locally in the root, mostly in the root cortex (Lohar et al. 2004). This localized cytokinin accumulation induces the expression of key nodulation transcription factors, including NODULATION SIGNALING PATHWAY (NSP) 1 and 2, by binding of type-B RRs to their promoters to induce their transcription (Ariel et al. 2012). NSP1 and NSP2 then regulate genes that lead to cortical cell dedifferentiation and proliferation for nodule formation, such as NODULE INCEPTION 1. Interestingly, legumes also inhibit nodulation in order to balance the carbon costs of nodule formation with their needs for nitrogen fixation, and this suppression of nodulation is also mediated by type-B RRs (Chen et al. 2022). In soybeans the type-B RR GmRRB1d can physically interact with NSP2, interfering with its transcriptional activity, resulting in reduced nodulation (Chen et al. 2022).
Drought and salt tolerance
Cytokinin regulates a myriad of responses to abiotic stress (Argueso et al. 2009; Zwack and Rashotte 2015; Mandal et al. 2022), including the responses to drought and elevated salinity. Pioneering studies from the Blumwald laboratory demonstrated that increased levels of endogenous cytokinin via a transgene harboring an IPT gene under the control of a senescence/stress-inducible promoter could confer drought tolerance in tobacco (Rivero et al. 2007) and other plant species (Peleg et al. 2011; Reguera et al. 2013, Décima Oneto et al. 2016; Beznec et al. 2021). The drought tolerance in these lines may be mediated by the increased expression and activity of antioxidant enzymes, which may act to suppress the accumulation of reactive oxygen species during drought stress (Xu et al. 2016). In contrast to these findings, Arabidopsis ipt mutants show increased drought tolerance (Tran et al. 2007), and similar results are observed in plants overexpressing CKX genes (Lubovská et al. 2014). These seemingly opposing roles for cytokinin in drought tolerance maybe be due to different mechanisms of action and specific cellular contexts. In Arabidopsis, a negative role for cytokinin in drought tolerance occurs through a crosstalk with abscisic acid signaling (Huang et al. 2018). SnRK2 kinases, which participate in abscisic acid signaling, phosphorylate type-A RRs, stabilizing their protein levels and thus inhibiting cytokinin signaling. Conversely, type-B RRs physically interact with SnRK2 kinases to inhibit their function (Huang et al. 2018). Through this mutual interaction, these hormones act to fine tune growth and drought responses.
Cytokinin also appears to affect tolerance to salinity, though this also appears to be a complex interaction. In Arabidopsis, ahk2 and ahk3 mutants were found to be more salt tolerant than the wild type (Tran et al. 2007). Consistent with this, Arabidopsis lines with reduced cytokinin content (CKX overexpression or ipt loss-of-function) displayed enhanced salt tolerance (Nishiyama et al. 2011), and elevation of endogenous cytokinin via inducible expression of an IPT gene resulted in hypersensitivity to salt (Wang et al. 2015). In tomato, elevation of cytokinin biosynthesis in roots resulted in enhanced salt tolerance (Ghanem et al. 2011). These results suggest that cytokinin negatively regulates salt tolerance. However, a different study reported that ahk2 mutants, but not mutations in the other 2 AHK receptors, displayed hypersensitivity to salt stress (Kumar and Verslues 2015). Likewise, in rice, reduced expression of AHPs via RNAi resulted in hypersensitivity to elevated salinity (Sun et al. 2014), and disruption of 2 type-A RR genes in rice displayed increased salinity tolerance (Wang et al. 2019), suggesting that cytokinin positively regulated salinity tolerance in rice. Consistent with this, reduced expression of OsCKX2 (via RNAi) resulted in increased endogenous cytokinin and strong salt tolerance in vegetative shoots, which lead to a significant increase in yield in the presence of high salinity (Joshi et al. 2018). In contrast, overexpression of a cytokinin glucosyltransferase in rice, which would decrease active cytokinin levels, also enhanced salt tolerance in adult plants, though it led to increased salt sensitivity in young seedlings (Li et al. 2020). Modulation of cytokinin redistribution in rice such that there were elevated levels of cytokinin specifically in roots resulted in increased salt tolerance (Yin et al. 2020). Overall, these and other studies suggest a complex relationship between cytokinin function and salinity tolerance, with perhaps differing effects depending on the tissues in which cytokinin function is altered and on the particular plant species examined.
A recent study revealed that salt stress induced degradation of multiple type-B RRs in Arabidopsis via phosphorylation by 2 MAP kinases (MPK3/6) (Yan et al. 2021), suggesting that these MAPKs modulate salt sensitivity by reducing cytokinin sensitivity by targeted degradation of type-B RRs.
Nutrient uptake
Cytokinin influences the uptake and distribution of essential nutrients, such as nitrogen, phosphorus, and potassium (Pavlů et al. 2018). This can enhance nutrient acquisition efficiency, critical for sustaining growth during environmentally stressful conditions. Of these essential nutrients, nitrogen, in the form of nitrate, is one of the most growth limiting nutrients and is tightly linked to cytokinin levels and signaling. In response to nitrate supply, the expression of IPT genes is increased (Miyawaki et al. 2004), leading to the accumulation of cytokinin in the roots via the action of IPT3 (Takei et al. 2004a). The increased cytokinin levels in the roots results in changes in root architecture (Ruffel et al. 2011) and also changes in expression of nitrate transporters (Lezhneva et al. 2014), fine-tunning root growth and nitrate uptake to the environmental conditions present. Cytokinins produced in the roots are then translocated to shoots via the xylem, mainly via the ABCG14 cytokinin transporter (Poitout et al. 2018). In the shoot, cytokinin influences multiple aspects of plant growth, including glutamine/glutamate metabolism (Poitout et al. 2018) and SAM activity (Landrein et al. 2018). Thus, levels of cytokinin within the plant function as a long-distance systemic signal of the nitrogen status, modulating plant growth. A possible mechanism mediating this long-distance signal has been recently elucidated: NPL7, a master transcriptional regulator of nitrate signaling, binds to the promoters of the cytokinin transporters ABCG14, PUP14 and PUP18 (Alvarez et al. 2020), inducing cytokinin transport to shoots. NLP7 is also required for the cytokinin-dependent expression of CRFs in the shoot, which in turn regulate expression of PIN auxin efflux transporters that mediate shoot growth in response to nitrate (Abualia et al. 2022).
Conclusions
Cytokinin plays pleiotropic roles throughout plant development, with often very distinct roles and effects in different tissues, at different times in development, and in response to various biotic and abiotic factors. An unresolved question remains: What is the molecular basis for the specificity of cytokinin? That is, how does this particular phytohormone have such distinct effects on different cells and tissues throughout the plant and across development? Differences in the cellular context in which the cytokinin signal is perceived likely account for the specificity. The cellular context is defined by the suite of cytokinin signaling elements expressed in a target cell (multiple paralogs of each signaling element in most plants), the activity of various partner transcription factors (multiple such factors identified as described above), the epigenetic state of the genome, and other interacting signaling pathways impinging on the cell. Differences in chromatin accessibility have been linked to differences in the effect of cytokinin on particular target genes in roots and shoots, and cytokinin also alters the accessibility of chromatin throughout the Arabidopsis genome in a type-B RR-dependent manner (Potter et al. 2018). Single cell approaches may shed light on the specifics of various interactions and specificity involved in cytokinin signaling.
Much of this review has focused on results in the model plant Arabidopsis, with some brief highlights from other species. Cytokinin function is being analyzed in many other plant species, including monocots such as maize and rice, as well as the lower plant Marchantia polymorpha (Aki et al. 2019). The future will likely reveal new aspects of cytokinin function beyond the Arabidopsis paradigm.
There are undoubtably additional inputs into cytokinin signaling yet to be identified, as well as missing components involved in cytokinin metabolism (e.g. what is the enzyme involved in the biosynthesis of dihydrozeatin? What is the source of cis-zeatin?). Further, we are only just beginning to understand cytokinin transport throughout the plant and within the cell. Finally, the modulation of cytokinin function to improve various aspects of agriculture is just starting. Although the past decades have seen remarkable progress in our understanding of cytokinin biology, the future should continue to reveal the secrets of this remarkable signaling molecule.
Acknowledgments
We apologize to our colleagues whose work we could not discuss due to length considerations. We thank members of the Kieber laboratory for helpful comments on the text.
Contributor Information
Cristiana T Argueso, Department of Agricultural Biology, Colorado State University, Fort Collins, CO 80523, USA.
Joseph J Kieber, Department of Biology, University of North Carolina, Chapel Hill, NC 27599, USA.
Author contributions
Both JJK and CTA contributed to the writing of this manuscript.
Funding
Work in the authors laboratory is generously supported by grants for the National Science Foundation (IOS IOS-2126144 to JJK and MCB-1818211 to CA), the USDA (2023-67013-39413 to JJK), and the USDA National Institute of Food and Agriculture HATCH Project COL00781 (COL0781A) to CA.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Abualia R, Ötvös K, Novák O, Bouguyon E, Domanegg K, Krapp A, Nacry P, Gojon A, Lacombe B, Benková E. Molecular framework integrating nitrate sensing in root and auxin-guided shoot adaptive responses. Proc Natl Acad Sci USA. 2022:119(31):e2122460119. 10.1073/pnas.2122460119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheampong AK, Shanks C, Cheng CY, Schaller GE, Dagdas Y, Kieber JJ. EXO70D isoforms mediate selective autophagic degradation of type-A ARR proteins to regulate cytokinin sensitivity. Proc Natl Acad Sci USA. 2020:117(43):27034–27043. 10.1073/pnas.2013161117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aki SS, Mikami T, Naramoto S, Nishihama R, Ishizaki K, Kojima M, Takebayashi Y, Sakakibara H, Kyozuka J, Kohchi T, et al. Cytokinin signaling is essential for organ formation in Marchantia polymorpha. Plant Cell Physiol. 2019:60(8):1842–1854. 10.1093/pcp/pcz100 [DOI] [PubMed] [Google Scholar]
- Akiyoshi D, Klee H, Amasino RM, Nester EW, Gordon MP. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA. 1984:81(19):5994–5998. 10.1073/pnas.81.19.5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht T, Argueso CT. Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann Bot. 2017:119(5):725–735. 10.1093/aob/mcw211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JM, Schinke AL, Brooks MD, Pasquino A, Leonelli L, Varala K, Safi A, Krouk G, Krapp A, Coruzzi GM. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat Commun. 2020:11(1):1157. 10.1038/s41467-020-14979-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. 1955: kinetin arrives: the 50th anniversary of a new plant hormone. Plant Physiol. 2005:138(3):1177–1184. 10.1104/pp.104.900160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem Sci. 2001:26(10):579–582. 10.1016/S0968-0004(01)01968-5 [DOI] [PubMed] [Google Scholar]
- Andersen TG, Naseer S, Ursache R, Wybouw B, Smet W, De Rybel B, Vermeer JEM, Geldner N. Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells. Nature. 2018:555(7697):529–533. 10.1038/nature25976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadi I, Novák O, Gelová Z, Johnson A, Plíhal O, Simerský R, Mik V, Vain T, Mateo-Bonmatí E, Karady M, et al. Cell-surface receptors enable perception of extracellular cytokinins. Nat Commun. 2020:11(1):4284. 10.1038/s41467-020-17700-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996:86(6):845–848. 10.1016/S0092-8674(00)80158-0 [DOI] [PubMed] [Google Scholar]
- Argueso CT, Ferreira FJ, Epple P, To JPC, Hutchison CE, Mathews DE, Schaller GE, Dangl JL, Kieber JJ. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012:8(1):e1002448. 10.1371/journal.pgen.1002448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso CT, Ferreira FJ, Kieber JJ. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell Environ. 2009:32(9):1147–1160. 10.1111/j.1365-3040.2009.01940.x [DOI] [PubMed] [Google Scholar]
- Argueso CT, Raines T, Kieber JJ. Cytokinin signaling and transcriptional networks. Curr Opin Plant Biol. 2010:13(5):533–559. 10.1016/j.pbi.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Argyros RD, Mathews DE, Chiang YH, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell. 2008:20(8):2102–2116. 10.1105/tpc.108.059584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel F, Brault-Hernandez M, Laffont C, Huault E, Brault M, Plet J, Moison M, Blanchet S, Ichanté JL, Chabaud M, et al. Two direct targets of cytokinin signaling regulate symbiotic nodulation in Medicago truncatula. Plant Cell. 2012:24(9):3838–3852. 10.1105/tpc.112.103267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud D, Lee S, Takebayashi Y, Choi D, Choi J, Sakakibara H, Hwang I. Cytokinin-mediated regulation of reactive oxygen species homeostasis modulates stomatal immunity in Arabidopsis. Plant Cell. 2017:29(3):543–559. 10.1105/tpc.16.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. Cytokinin oxidase regulates rice grain production. Science. 2005:309(5735):741–745. 10.1126/science.1113373 [DOI] [PubMed] [Google Scholar]
- Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehneß R, Lee TK, Proels R, Tanner W, Roitsch T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004:16(5):1276–1287. 10.1105/tpc.018929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell. 2011:23(1):69–80. 10.1105/tpc.110.079079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard F, Refahi Y, Morin V, Marteaux B, Brunoud G, Chambrier P, Rozier F, Mirabet V, Legrand J, Lainé S, et al. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature. 2014:505(7483):417–421. 10.1038/nature12791 [DOI] [PubMed] [Google Scholar]
- Beznec A, Faccio P, Miralles DJ, Abeledo LG, Oneto CD, Garibotto MB, Gonzalez G, Moreyra F, Elizondo M, Ruíz M, et al. Stress-induced expression of IPT gene in transgenic wheat reduces grain yield penalty under drought. J Genet Eng Biotechnol. 2021:19(1):67. 10.1186/s43141-021-00171-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A, Clabaugh I, To JP, Maxwell BB, Chiang YH, Schaller GE, Loraine A, Kieber JJ. Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-Seq in Arabidopsis. Plant Physiol. 2013:162(1):272–294. 10.1104/pp.113.217026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen A, Helariutta Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol. 2011:21(11):927–932. 10.1016/j.cub.2011.04.049 [DOI] [PubMed] [Google Scholar]
- Brandstatter I, Kieber JJ. Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell. 1998:10(6):1009–1020. 10.1105/tpc.10.6.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner WG, Ramireddy E, Heyl A, Schmülling T. Gene regulation by cytokinin in Arabidopsis. Front Plant Sci. 2012:3:8. 10.3389/fpls.2012.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzobohaty B, Moore I, Kristoffersen P, Bako L, Campos N, Schell J, Palme K. Release of active cytokinin by a b-glucosidase localized to the maize root meristem. Science. 1993:262(5136):1051–1054. 10.1126/science.8235622 [DOI] [PubMed] [Google Scholar]
- Burr CA, Sun J, Yamburenko MV, Willoughby A, Hodgens C, Boeshore SL, Elmore A, Atkinson J, Nimchuk ZL, Bishopp A, et al. The HK5 and HK6 cytokinin receptors mediate diverse developmental pathways in rice. Development. 2020:147(20):dev191734. 10.1242/dev.191734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Thamm AMK, Witthöft J, Elgass K, Huppenberger P, Grefen C, Horak J, Harter K. Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. J Exp Bot. 2011:62(15):5571–5580. 10.1093/jxb/err238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jameson GB, Guo Y, Song J, Jameson PE. The LONELY GUY gene family: from mosses to wheat, the key to the formation of active cytokinins in plants. Plant Biotechnol J. 2022:20(4):625–645. 10.1111/pbi.13783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhao J, Song J, Jameson PE. Cytokinin glucosyl transferases, key regulators of cytokinin homeostasis, have potential value for wheat improvement. Plant Biotechnol J. 2021:19(5):878–896. 10.1111/pbi.13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-Y, Mathews DE, Eric Schaller G, Kieber JJ. Cytokinin-dependent specification of the functional megaspore in the Arabidopsis female gametophyte. Plant J. 2013:73(6):929–940. 10.1111/tpj.12084 [DOI] [PubMed] [Google Scholar]
- Chickarmane VS, Gordon SP, Tarr PT, Heisler MG, Meyerowitz EM. Cytokinin signaling as a positional cue for patterning the apical–basal axis of the growing Arabidopsis shoot meristem. Proc Natl Acad Sci U S A. 2012:109(10):4002–4007. 10.1073/pnas.1200636109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Huh SU, Kojima M, Sakakibara H, Paek KH, Hwang I. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev Cell. 2010:19(2):284–295. 10.1016/j.devcel.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Chu HM, Ko TP, Wang AH. Crystal structure and substrate specificity of plant adenylate isopentenyltransferase from Humulus lupulus: distinctive binding affinity for purine and pyrimidine nucleotides. Nucl Acids Res. 2010:38(5):1738–1748. 10.1093/nar/gkp1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino I, Deruère J, Kieber JJ. Characterization of the response of the Arabidopsis ARR gene family to cytokinin. Plant Physiol. 2000:124(4):1706–1717. 10.1104/pp.124.4.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décima Oneto C, Otegui ME, Baroli I, Beznec A, Faccio P, Bossio E, Blumwald E, Lewi D. Water deficit stress tolerance in maize conferred by expression of an isopentenyltransferase (IPT) gene driven by a stress- and maturation-induced promoter. J Biotechnol. 2016:220:66–77. 10.1016/j.jbiotec.2016.01.014 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Galinha C, Fletcher AG, Grigg SP, Molnar A, Willemsen V, Scheres B, Sabatini S, Baulcombe D, Maini PK, et al. A PHABULOSA/cytokinin feedback loop controls root growth in Arabidopsis. Curr Biol. 2012:22(18):1699–1704. 10.1016/j.cub.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol. 2007:17(8):678–682. 10.1016/j.cub.2007.02.047 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. A genetic framework for the control of cell division and differentiation in the root meristem. Science. 2008:322(5906):1380–1384. 10.1126/science.1164147 [DOI] [PubMed] [Google Scholar]
- De Rybel B, Möller B, Yoshida S, Grabowicz I, Barbier de Reuille P, Boeren S, Smith RS, Borst JW, Weijers D. A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in Arabidopsis. Dev Cell. 2013:24(4):426–437. 10.1016/j.devcel.2012.12.013 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V, et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci U S A. 2007:104(36):14537–14542. 10.1073/pnas.0704166104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobisova T, Hrdinova V, Cuesta C, Michlickova S, Urbankova I, Hejatkova R, Zadnikova P, Pernisova M, Benkova E, Hejatko J. Light controls cytokinin signaling via transcriptional regulation of constitutively active sensor histidine kinase CKI1. Plant Physiol. 2017:174(1):387–404. 10.1104/pp.16.01964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efroni I, Han S-K, Kim HJ, Wu M-F, Steiner E, Birnbaum KD, Hong JC, Eshed Y, Wagner D. Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev Cell. 2013:24(4):438–445. 10.1016/j.devcel.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Wang C, Chen Q, Chen H, Ren B, Li X, Zuo J. S-nitrosylation of phosphotransfer proteins represses cytokinin signaling. Nat Commun. 2013:4(1):1529. 10.1038/ncomms2541 [DOI] [PubMed] [Google Scholar]
- Fonouni-Farde C, Kisiala A, Brault M, Emery RJN, Diet A, Frugier F. DELLA1-Mediated gibberellin signaling regulates cytokinin-dependent symbiotic nodulation. Plant Physiol. 2017:175(4):1795–1806. 10.1104/pp.17.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JE, Erion JL. A cytokinin binding protein from higher plant ribosomes. Biochem Biophys Res Commun. 1975:64(2):694–700. 10.1016/0006-291X(75)90376-9 [DOI] [PubMed] [Google Scholar]
- Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Žižková E, et al. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot. 2011:62(8):2827–2840. 10.1093/jxb/erq457 [DOI] [PubMed] [Google Scholar]
- Galuszka P, Popelková H, Werner T, Frébortová J, Pospíšilová H, Mik V, Köllmer I, Schmülling T, Frébort I. Biochemical characterization of cytokinin oxidases/dehydrogenases from Arabidopsis thaliana expressed in Nicotiana tabacum L. J Plant Growth Regul. 2007:26(3):255–267. 10.1007/s00344-007-9008-5 [DOI] [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995:270(5244):1986–1988. 10.1126/science.270.5244.1986 [DOI] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Cytokinins in plant senescence: from spray and pray to clone and play. Bioessays. 1996:18(7):557–565. 10.1002/bies.950180707 [DOI] [Google Scholar]
- Ghanem ME, Albacete A, Smigocki AC, Frébort I, Pospísilová H, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Lutts S, Dodd IC, et al. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot. 2011:62(1):125–140. 10.1093/jxb/erq266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen B, Bürkle L, André B, Kühn C, Rentsch D, Brandl B, Frommer WB. A new family of high-affinity transporters for adenine, cytosine, and purine derivatives in Arabidopsis. Plant Cell. 2000:12(2):291–300. 10.1105/tpc.12.2.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz-Idan N, Lach M, Tarkowski P, Vrobel O, Wolf S. Delayed leaf senescence by upregulation of cytokinin biosynthesis specifically in tomato roots. Front Plant Sci. 2022:13:922106. 10.3389/fpls.2022.922106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci U S A. 2009:106(38):16529–16534. 10.1073/pnas.0908122106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Großkinsky DK, Tafner R, Moreno MV, Stenglein SA, García de Salamone IE, Nelson LM, Novák O, Strnad M, van der Graaff E, Roitsch T. Cytokinin production by Pseudomonas fluorescens G20-18 determines biocontrol activity against Pseudomonas syringae in Arabidopsis. Sci Rep. 2016:6(1):23310. 10.1038/srep23310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Leibman-Markus M, Pizarro L, Bar M. Cytokinin induces bacterial pathogen resistance in tomato. Plant Path. 2021:70(2):318–325. 10.1111/ppa.13279 [DOI] [Google Scholar]
- Gupta R, Pizarro L, Leibman-Markus M, Marash I, Bar M. Cytokinin response induces immunity and fungal pathogen resistance, and modulates trafficking of the PRR LeEIX2 in tomato. Mol Plant Pathol. 2020:21(10):1287–1306. 10.1111/mpp.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt G. Culturversuche mit isolierten pflanzenzellen. Sitz-Ber Mat Nat Kl Kais Akad Wiss Wien. 1902:111:69–91. https://biostor.org/reference/221963 [Google Scholar]
- Haberlandt G. Zur physiologie der zellteilung. Sitzungsber. Voll XX. Wien: Sitzungsberichte der Preussischen Akademie der Wissenschaften. 1913; p318–345. [Google Scholar]
- Hartig K, Beck E. Endogenous cytokinin oscillations control cell cycle progression of tobacco BY-2 cells. Plant Biol (Stuttg). 2005:7(1):33–40. 10.1055/s-2004-830474 [DOI] [PubMed] [Google Scholar]
- He Q, Yuan R, Zhang T, An F, Wang N, Lan J, Wang X, Zhang Z, Pan Y, Wang X, et al. Arabidopsis TIE1 and TIE2 transcriptional repressors dampen cytokinin response during root development. Sci Adv. 2022:8(36):eabn5057. 10.1126/sciadv.abn5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejátko J, Ryu H, Kim GT, Dobešová R, Choi S, Choi SM, Souček P, Horák J, Pekárová B, Palme K, et al. The histidine kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots. Plant Cell. 2009:21(7):2008–2021. 10.1105/tpc.109.066696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held M, Hou H, Miri M, Huynh C, Ross L, Hossain MS, Sato S, Tabata S, Perry J, Wang TL, et al. Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. Plant Cell. 2014:26(2):678–694. 10.1105/tpc.113.119362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci U S A. 2004:101(23):8821–8826. 10.1073/pnas.0402887101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Mathews DE, Kim HJ, Street IH, Wildes SL, Chiang YH, Mason MG, Alonso JM, Ecker JR, Kieber JJ, et al. Functional characterization of type-B response regulators in the Arabidopsis cytokinin response. Plant Physiol. 2013:162(1):212–224. 10.1104/pp.112.208736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Makita N, Yamaya T, Sakakibara H. Functional characterization and expression analysis of a gene, OsENT2, encoding an equilibrative nucleoside transporter in rice suggest a function in cytokinin transport. Plant Physiol. 2005:138(1):196–206. 10.1104/pp.105.060137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot. 2008:59(1):75–83. 10.1093/jxb/erm157 [DOI] [PubMed] [Google Scholar]
- Hluska T, Hlusková L, Emery RJN. The hulks and the deadpools of the cytokinin universe: a dual strategy for cytokinin production, translocation, and signal transduction. Biomolecules. 2021:11(2):209. 10.3390/biom11020209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgens C, Chang N, Schaller GE, Kieber JJ. Mutagenomics: a rapid, high-throughput method to identify causative mutations from a genetic screen. Plant Physiol. 2020:184(4):1658–1673. 10.1104/pp.20.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hošek P, Hoyerová K, Kiran NS, Dobrev PI, Zahajská L, Filepová R, Motyka V, Müller K, Kamínek M. Distinct metabolism of N-glucosides of isopentenyladenine and trans-zeatin determines cytokinin metabolic spectrum in Arabidopsis. New Phytol. 2020:225(6):2423–2438. 10.1111/nph.16310 [DOI] [PubMed] [Google Scholar]
- Houba-Hérin N, Pethe C, d’Alayer J, Laloue M. Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. Plant J. 1999:17(6):615–626. 10.1046/j.1365-313X.1999.00408.x [DOI] [PubMed] [Google Scholar]
- Huang X, Hou L, Meng J, You H, Li Z, Gong Z, Yang S, Shi Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol Plant. 2018:11(7):970–982. 10.1016/j.molp.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell. 2006:18(11):3073–3087. 10.1105/tpc.106.045674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J. Two-component circuitry in Arabidopsis signal transduction. Nature. 2001:413(6854):383–389. 10.1038/35096500 [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J, Müller B. Cytokinin signaling networks. Annu Rev Plant Biol. 2012:63(1):353–380. 10.1146/annurev-arplant-042811-105503 [DOI] [PubMed] [Google Scholar]
- Hyde RJ, Cass CE, Young JD, Baldwin SA. The ENT family of eukaryote nucleoside and nucleobase transporters: recent advances in the investigation of structure/function relationships and the identification of novel isoforms. Mol Membr Biol. 2001:18(1):53–63. 10.1080/09687680118799 [DOI] [PubMed] [Google Scholar]
- Imamura A, Kiba T, Tajima Y, Yamashino T, Mizuno T. In vivo and in vitro characterization of the ARR11 response regulator implicated in the his-to-asp phosphorelay signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2003:44(2):122–131. 10.1093/pcp/pcg014 [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001:409(6823):1060–1063. 10.1038/35059117 [DOI] [PubMed] [Google Scholar]
- Jackson D, Hake S. Control of phyllotaxy in maize by the abphyl1 gene. Development. 1999:126(2):315–323. 10.1242/dev.126.2.315 [DOI] [PubMed] [Google Scholar]
- Jameson PE. Zeatin: the 60th anniversary of its identification. Plant Physiol. 2023:192(1):34–55. 10.1093/plphys/kiad094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson PE, Song J. Cytokinin: a key driver of seed yield. J Exp Bot. 2016:67(3):593–606. 10.1093/jxb/erv461 [DOI] [PubMed] [Google Scholar]
- Jameson PE, Song J. Will cytokinins underpin the second ‘green revolution'? J Exp Bot. 2020:71(22):6872–6875. 10.1093/jxb/eraa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelesko JG. An expanding role for purine uptake permease-like transporters in plant secondary metabolism. Front Plant Sci. 2012:3:78. 10.3389/fpls.2012.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R, Sahoo KK, Tripathi AK, Kumar R, Gupta BK, Pareek A, Singla-Pareek SL. Knockdown of an inflorescence meristem-specific cytokinin oxidase—osCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ. 2018:41(5):936–946. 10.1111/pce.12947 [DOI] [PubMed] [Google Scholar]
- Kakimoto T. CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science. 1996:274(5289):982–985. 10.1126/science.274.5289.982 [DOI] [PubMed] [Google Scholar]
- Kakimoto T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: aTP/ADP isopentenyltransferases. Plant Cell Physiol. 2001:42(7):677–685. 10.1093/pcp/pce112 [DOI] [PubMed] [Google Scholar]
- Kamada-Nobusada T, Sakakibara H. Molecular basis for cytokinin biosynthesis. Phytochemistry. 2009:70(4):444–449. 10.1016/j.phytochem.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Kant S, Burch D, Badenhorst P, Palanisamy R, Mason J, Spangenberg G. Regulated expression of a cytokinin biosynthesis gene IPT delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS One. 2015:10(1):e0116349. 10.1371/journal.pone.0116349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H. Distinct isoprenoid origins of cis- and trans-zeatin biosyntheses in Arabidopsis. J Biol Chem. 2004:279(14):14049–14054. 10.1074/jbc.M314195200 [DOI] [PubMed] [Google Scholar]
- Keim P, Fox JE. Interaction of a radiolabeled cytokinin photoaffinity probe with a receptor protein. Biochem Biophys Res Commun. 1980:96(3):1325–1334. 10.1016/0006-291X(80)90096-0 [DOI] [PubMed] [Google Scholar]
- Kiba T, Mizutani K, Nakahara A, Takebayashi Y, Kojima M, Hobo T, Osakabe Y, Osakabe K, Sakakibara H. The trans-zeatin-type side-chain modification of cytokinins controls rice growth. Plant Physiol. 2023:192(3):2457–2474. 10.1093/plphys/kiad197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Takei K, Kojima M, Sakakibara H. Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Dev Cell. 2013:27(4):452–461. 10.1016/j.devcel.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Kiba T, Yamada H, Sato S, Kato T, Tabata S, Yamashino T, Mizuno T. The type-A response regulator, ARR15, acts as a negative regulator in the cytokinin-mediated signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2003:44(8):868–874. 10.1093/pcp/pcg108 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE. Cytokinins. Arabidopsis Book. 2014:12:e0168. 10.1199/tab.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE. Cytokinin signaling in plant development. Development. 2018:145(4):dev149344. 10.1242/dev.149344 [DOI] [PubMed] [Google Scholar]
- Kim A, Chen J, Khare D, Jin JY, Yamaoka Y, Maeshima M, Zhao Y, Martinoia E, Hwang JU, Lee Y. Non-intrinsic ATP-binding cassette proteins ABCI19, ABCI20 and ABCI21 modulate cytokinin response at the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell Rep. 2020:39(4):473–487. 10.1007/s00299-019-02503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Chiang Y-H, Kieber JJ, Schaller GE. SCF(KMD) controls cytokinin signaling by regulating the degradation of type-B response regulators. Proc Natl Acad Sci U S A. 2013:110(24):10028–10033. 10.1073/pnas.1300403110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ryu H, Hong SH, Woo HR, Lim PO, Lee IC, Sheen J, Nam HG, Hwang I. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proc Natl Acad Sci U S A. 2006:103(3):814–819. 10.1073/pnas.0505150103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita-Tsujimura K, Kakimoto T. Cytokinin receptors in sporophytes are essential for male and female functions in Arabidopsis thaliana. Plant Signal Behav. 2011:6(1):66–71. 10.4161/psb.6.1.13999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D, Kang J, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song W-Y, et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci USA. 2014:111(19):7150–7155. 10.1073/pnas.1321519111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012:160(1):319–331. 10.1104/pp.112.196733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MN, Verslues PE. Stress physiology functions of the Arabidopsis histidine kinase cytokinin receptors. Physiol Plant. 2015:154(3):369–380. 10.1111/ppl.12290 [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007:445(7128):652–655. 10.1038/nature05504 [DOI] [PubMed] [Google Scholar]
- Landrein B, Formosa-Jordan P, Malivert A, Schuster C, Melnyk CW, Yang W, Turnbull C, Meyerowitz EM, Locke JCW, Jönsson H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc Natl Acad Sci U S A. 2018:115(6):1382–1387. 10.1073/pnas.1718670115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys F, Smetsa R, Lenjoub M, Van Bockstaeleb D, Inzéc D, Van Onckelena H. A low content in zeatin type cytokinins is not restrictive for the occurrence of G1/S transition in tobacco BY-2 cells. FEBS Lett. 1999:460(1):123–128. 10.1016/S0014-5793(99)01264-8 [DOI] [PubMed] [Google Scholar]
- Lee DJ, Park JY, Ku SJ, Ha YM, Kim S, Kim MD, Oh MH, Kim J. Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7(ARR7) overexpression in cytokinin response. Mol Genet Genomics. 2007:277(2):115–137. 10.1007/s00438-006-0177-x [DOI] [PubMed] [Google Scholar]
- Leibfried A, To JPC, Stehling SK, Busch W, Demar M, Kieber JJ, Lohmann JU. WUSCHEL controls meristem size by direct transcriptional regulation of cytokinin inducible response regulators. Nature. 2005:438(7071):1172–1175. 10.1038/nature04270 [DOI] [PubMed] [Google Scholar]
- Letham DS, Shannon JS, McDonald IR. The structure of zeatin, a factor inducing cell division. Proc Chem Soc. 1964:8:230–231. [Google Scholar]
- Leuendorf JE, Schmülling T. Meeting at the DNA: specifying cytokinin responses through transcription factor Complex formation. Plants (Basel). 2021:10(7):1458. 10.3390/plants10071458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezhneva L, Kiba T, Feria-Bourrellier AB, Lafouge F, Boutet-Mercey S, Zoufan P, Sakakibara H, Daniel-Vedele F, Krapp A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014:80(2):230–241. 10.1111/tpj.12626 [DOI] [PubMed] [Google Scholar]
- Li M, Li X, Zhou Z, Wu P, Fang M, Pan X, Lin Q, Luo W, Wu G, Li H. Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/cas9 system. Front Plant Sci. 2016:7:377. 10.3389/fpls.2016.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu F, Li P, Wang T, Zheng C, Hou B. An Arabidopsis cytokinin-modifying glycosyltransferase UGT76C2 improves drought and salt tolerance in rice. Front Plant Sci. 2020:11:560696. 10.3389/fpls.2020.560696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang R, Wang S, Zhang J, Chang L. Diversified regulation of cytokinin levels and signaling during Botrytis cinerea infection in Arabidopsis. Front Plant Sci. 2021:12:584042. 10.3389/fpls.2021.584042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Wang X, Hong S, Li Y, Zuo J. Deletion of the initial 45 residues of ARR18 induces cytokinin response in Arabidopsis. J Genet Genomics. 2012:39(1):37–46. 10.1016/j.jgg.2011.12.004 [DOI] [PubMed] [Google Scholar]
- Lindner AC, Lang D, Seifert M, Podlešáková K, Novák O, Strnad M, Reski R, von Schwartzenberg K. Isopentenyltransferase-1 (IPT1) knockout in Physcomitrella together with phylogenetic analyses of IPTs provide insights into evolution of plant cytokinin biosynthesis. J Exp Bot. 2014:65(9):2533–2543. 10.1093/jxb/eru142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Müller B. Imaging TCSn::GFP, a synthetic cytokinin reporter, in Arabidopsis thaliana. Methods Mol Biol. 2017:1497:81–90. 10.1007/978-1-4939-6469-7_9 [DOI] [PubMed] [Google Scholar]
- Liu S, Strauss S, Adibi M, Mosca G, Yoshida S, Dello Ioio R, Runions A, Andersen TG, Grossmann G, Huijser P, et al. Cytokinin promotes growth cessation in the Arabidopsis root. Curr Biol. 2022:32(9):1974–1985.e1973. 10.1016/j.cub.2022.03.019 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yuan L, Song X, Yu X, Sundaresan V. AHP2, AHP3, and AHP5 act downstream of CKI1 in Arabidopsis female gametophyte development. J Exp Bot. 2017:68(13):3365–3373. 10.1093/jxb/erx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang M, Meng Z, Wang B, Chen M. Research progress on the roles of cytokinin in plant response to stress. Int J Mol Sci. 2020:21:6574. 10.3390/ijms22010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhou X, Li D, Hong B, Gao J, Zhang Z. Rose WRKY13 promotes disease protection to Botrytis by enhancing cytokinin content and reducing abscisic acid signaling. Plant Physiol. 2023:191(1):679–693. 10.1093/plphys/kiac495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM. Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J. 2004:38:203–214. 10.1111/j.1365-313X.2004.02038.x [DOI] [PubMed] [Google Scholar]
- Lomin SN, Krivosheev DM, Steklov MY, Osolodkin DI, Romanov GA. Receptor properties and features of cytokinin signaling. Acta Naturae. 2012:4(3):31–45. 10.32607/20758251-2012-4-3-31-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomin SN, Savelieva EM, Arkhipov DV, Pashkovskiy PP, Myakushina YA, Heyl A, Romanov GA. Cytokinin perception in ancient plants beyond angiospermae. Int J Mol Sci. 2021:22(23):13077. 10.3390/ijms222313077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomin SN, Yonekura-Sakakibara K, Romanov GA, Sakakibara H. Ligand-binding properties and subcellular localization of maize cytokinin receptors. J Exp Bot. 2011:62(14):5149–5159. 10.1093/jxb/err220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FL, Galvan-Ampudia C, Landrein B. WUSCHEL in the shoot apical meristem: old player, new tricks. J Exp Bot. 2021:72(5):1527–1535. 10.1093/jxb/eraa572 [DOI] [PubMed] [Google Scholar]
- Lubovská Z, Dobrá J, Štorchová H, Wilhelmová N, Vanková R. Cytokinin oxidase/dehydrogenase overexpression modifies antioxidant defense against heat, drought and their combination in Nicotiana tabacum plants. J Plant Physiol. 2014:171(17):1625–1633. 10.1016/j.jplph.2014.06.021 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006b:311(5757):94–98. 10.1126/science.1118875 [DOI] [PubMed] [Google Scholar]
- Mähönen AP, Higuchi M, Törmäkangas K, Miyawaki K, Pischke MS, Sussman MR, Helariutta Y, Kakimoto T. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr Biol. 2006a:16(11):1116–1122. 10.1016/j.cub.2006.04.030 [DOI] [PubMed] [Google Scholar]
- Malinowski R, Novák O, Borhan MH, Spichal L, Strnad M, Rolfe SA. The role of cytokinins in clubroot disease. Eur J Plant Pathol. 2016:145(3):543–557. 10.1007/s10658-015-0845-y [DOI] [Google Scholar]
- Mandal S, Ghorai M, Anand U, Samanta D, Kant N, Mishra T, Rahman MH, Jha NK, Jha SK, Lal MK, et al. Cytokinin and abiotic stress tolerance -what has been accomplished and the way forward? Front Genet. 2022:13:943025. 10.3389/fgene.2022.943025 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marhavý P, Bielach A, Abas L, Abuzeineh A, Duclercq J, Tanaka H, Pařezová M, Petrášek J, Friml J, Kleine-Vehn J, et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell. 2011:21(4):796–804. 10.1016/j.devcel.2011.08.014 [DOI] [PubMed] [Google Scholar]
- Marín-de la Rosa N, Pfeiffer A, Hill K, Locascio A, Bhalerao RP, Miskolczi P, Grønlund AL, Wanchoo-Kohli A, Thomas SG, Bennett MJ, et al. Genome wide binding site analysis reveals transcriptional coactivation of cytokinin-responsive genes by DELLA proteins. PLoS Genet. 2015:11(7):e1005337. 10.1371/journal.pgen.1005337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JRS, Schaller GE. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell. 2005:17(11):3007–3018. 10.1105/tpc.105.035451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T. Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA. 2008:105(50):20027–20031. 10.1073/pnas.0805619105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre KE, Bush DR, Argueso CT. Cytokinin regulation of source-sink relationships in plant-pathogen interactions. Front Plant Sci. 2021:12:677585. 10.3389/fpls.2021.677585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng WJ, Cheng ZJ, Sang YL, Zhang MM, Rong XF, Wang ZW, Tang YY, Zhang XS. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell. 2017:29(6):1357–1372. 10.1105/tpc.16.00640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CO, Skoog F, Okomura FS, von Saltza MH, Strong FM. Isolation, structure and synthesis of kinetin, a substance promoting cell division. J Am Chem Soc. 1956:78(7):1345–1350. 10.1021/ja01588a032 [DOI] [Google Scholar]
- Miller CO, Skoog F, Von Saltza MH, Strong F. Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc. 1955:77(5):1392. 10.1021/ja01610a105 [DOI] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. Expression of cytokinin biosynthetic isopentyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 2004:37(1):128–138. 10.1046/j.1365-313X.2003.01945.x [DOI] [PubMed] [Google Scholar]
- Möhlmann T, Mezher Z, Schwerdtfeger G, Neuhaus HE. Characterisation of a concentrative type of adenosine transporter from Arabidopsis thaliana (ENT1. At). FEBS Lett. 2001:509(3):370–374. 10.1016/S0014-5793(01)03195-7 [DOI] [PubMed] [Google Scholar]
- Moore FH. A cytokinin-binding protein from wheat germ: isolation by affinity chromatography and properties. Plant Physiol. 1979:64(4):594–599. 10.1104/pp.64.4.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S, Bishopp A, Carvalho H, Campilho A. AHP6 inhibits cytokinin signaling to regulate the orientation of pericycle cell division during lateral root initiation. PLoS One. 2013:8(2):e56370. 10.1371/journal.pone.0056370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RO, Bilyeu KD, Laskey JG, Cheikh NN. Isolation of a gene encoding a glycosylated cytokinin oxidase from maize. Biochem Biophys Res Comm. 1999:255(2):328–333. 10.1006/bbrc.1999.0199 [DOI] [PubMed] [Google Scholar]
- Moubayidin L, Perilli S, Dello Ioio R, Di Mambro R, Costantino P, Sabatini S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr Biol. 2010:20(12):1138–1143. 10.1016/j.cub.2010.05.035 [DOI] [PubMed] [Google Scholar]
- Müller CJ, Larsson E, Spíchal L, Sundberg E. Cytokinin-auxin crosstalk in the gynoecial primordium ensures correct domain patterning. Plant Physiol. 2017:175:1144–1157. 10.1104/pp.17.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008:453(7198):1094–1097. 10.1038/nature06943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehnevajova E, Ramireddy E, Stolz A, Gerdemann-Knörck M, Novák O, Strnad M, Schmülling T. Root enhancement in cytokinin-deficient oilseed rape causes leaf mineral enrichment, increases the chlorophyll concentration under nutrient limitation and enhances the phytoremediation capacity. BMC Plant Biol. 2019:19(1):83. 10.1186/s12870-019-1657-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen K, Immanen J, Laxell M, Kauppinen L, Tarkowski P, Dolezal K, Tähtiharju S, Elo A, Decourteix M, Ljung K, et al. Cytokinin signaling regulates cambial development in poplar. Proc Natl Acad Sci U S A. 2008:105(50):20032–20037. 10.1073/pnas.0805617106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004:16(6):1365–1377. 10.1105/tpc.021477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 2011:23(6):2169–2183. 10.1105/tpc.111.087395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee S, Park G, Cho H, Choi D, Umeda M, Choi Y, Hwang D, Hwang I. CYTOKININ-RESPONSIVE GROWTH REGULATOR regulates cell expansion and cytokinin-mediated cell cycle progression. Plant Physiol. 2021:186(3):1734–1746. 10.1093/plphys/kiab180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlů J, Novák J, Koukalová V, Luklová M, Brzobohatý B, Černý M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int J Mol Sci. 2018:19(8):2450. 10.3390/ijms19082450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E. Cytokinin-mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J. 2011:9(7):747–758. 10.1111/j.1467-7652.2010.00584.x [DOI] [PubMed] [Google Scholar]
- Perilli S, Perez-Perez JM, Di Mambro R, Peris CL, Díaz-Triviño S, Del Bianco M, Pierdonati E, Moubayidin L, Cruz-Ramírez A, Costantino P, et al. RETINOBLASTOMA-RELATED protein stimulates cell differentiation in the Arabidopsis root meristem by interacting with cytokinin signaling. Plant Cell. 2013:25(11):4469–4478. 10.1105/tpc.113.116632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricka JJ, Winter CM, Benfey PN. Control of Arabidopsis root development. Annu Rev Plant Biol. 2012:63(1):563–590. 10.1146/annurev-arplant-042811-105501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pils B, Heyl A. Unraveling the evolution of cytokinin signaling. Plant Physiol. 2009:151(2):782–791. 10.1104/pp.109.139188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plet J, Wasson A, Ariel F, Le Signor C, Baker D, Mathesius U, Crespi M, Frugier F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011:65(4):622–633. 10.1111/j.1365-313X.2010.04447.x [DOI] [PubMed] [Google Scholar]
- Poitout A, Crabos A, Petřík I, Novák O, Krouk G, Lacombe B, Ruffel S. Responses to systemic nitrogen signaling in Arabidopsis roots involve trans-zeatin in shoots. Plant Cell. 2018:30(6):1243–1257. 10.1105/tpc.18.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polko JK, Potter KC, Burr CA, Schaller GE, Kieber JJ. Meta-analysis of transcriptomic studies of cytokinin-treated rice roots defines a core set of cytokinin response genes. Plant J. 2021:107(5):1387–1402. 10.1111/tpj.15386 [DOI] [PubMed] [Google Scholar]
- Polya GM, Davis AW. Properties of a high-affinity cytokinin-binding protein from wheat germ. Planta. 1978:139(2):139–147. 10.1007/BF00387139 [DOI] [PubMed] [Google Scholar]
- Potter KC, Wang J, Schaller GE, Kieber JJ. Cytokinin modulates context-dependent chromatin accessibility through the type-B response regulators. Nat Plants. 2018:4(12):1102–1111. 10.1038/s41477-018-0290-y [DOI] [PubMed] [Google Scholar]
- Punwani JA, Hutchison CE, Schaller GE, Kieber JJ. The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. Plant J. 2010:62(3):473–482. 10.1111/j.1365-313X.2010.04165.x [DOI] [PubMed] [Google Scholar]
- Radchuk V, Belew ZM, Gündel A, Mayer S, Hilo A, Hensel G, Sharma R, Neumann K, Ortleb S, Wagner S, et al. SWEET11b transports both sugar and cytokinin in developing barley grains. Plant Cell. 2023:35(6):2186–2207. 10.1093/plcell/koad055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi A, Karami O, Lestari AD, de Werk T, Amakorová P, Shi D, Novák O, Greb T, Offringa R. Control of cambium initiation and activity in Arabidopsis by the transcriptional regulator AHL15. Curr Biol. 2022:32(8):1764–1775.e3. 10.1016/j.cub.2022.02.060 [DOI] [PubMed] [Google Scholar]
- Raines T, Blakley IC, Tsai Y-C, Worthen JM, Franco-Zorrilla JM, Solano R, Schaller GE, Loraine AE, Kieber JJ. Characterization of the cytokinin-responsive transcriptome in rice. BMC Plant Biol. 2016a:16(1):260. 10.1186/s12870-016-0932-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines T, Shanks C, Cheng C-Y, McPherson D, Argueso CT, Kim HJ, Franco-Zorrilla JM, López-Vidriero I, Solano R, Vaňková R, et al. The cytokinin response factors modulate root and shoot growth and promote leaf senescence in Arabidopsis. Plant J. 2016b:85(1):134–147. 10.1111/tpj.13097 [DOI] [PubMed] [Google Scholar]
- Ramireddy E, Hosseini SA, Eggert K, Gillandt S, Gnad H, von Wirén N, Schmülling T. Root engineering in barley: increasing cytokinin degradation produces a larger root system, mineral enrichment in the shoot and improved drought tolerance. Plant Physiol. 2018:177(3):1078–1095. 10.1104/pp.18.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramireddy E, Nelissen H, Leuendorf JE, Van Lijsebettens M, Inzé D, Schmülling T. Root engineering in maize by increasing cytokinin degradation causes enhanced root growth and leaf mineral enrichment. Plant Mol Biol. 2021:106(6):555–567. 10.1007/s11103-021-01173-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RS, Miyashima S, Blomster T, Zhang J, Elo A, Karlberg A, Immanen J, Nieminen K, Lee JY, Kakimoto T, et al. AINTEGUMENTA and the D-type cyclin CYCD3; 1 regulate root secondary growth and respond to cytokinins. Biol Open. 2015:4(10):1229–1236. 10.1242/bio.013128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM. The evolution of cytokinin signaling and its role in development before angiosperms. Semin Cell Dev Biol. 2021:109:31–38. 10.1016/j.semcdb.2020.06.010 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Mason MG, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ. A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proc Natl Acad Sci. U S A. 2006:103(29):11081–11085. 10.1073/pnas.0602038103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013:163(4):1609–1622. 10.1104/pp.113.227702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006:18(1):40–54. 10.1105/tpc.105.037796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999:283(5407):1541–1544. 10.1126/science.283.5407.1541 [DOI] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci U S A. 2007:104(49):19631–19636. 10.1073/pnas.0709453104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov GA, Lomin SN, Schmülling T. Biochemical characteristics and ligand-binding properties of Arabidopsis cytokinin receptor AHK3 compared to CRE1/AHK4 as revealed by a direct binding assay. J Exp Bot. 2006:57(15):4051–4058. 10.1093/jxb/erl179 [DOI] [PubMed] [Google Scholar]
- Romanov GA, Lomin SN, Schmülling T. Cytokinin signaling: from the ER or from the PM? That is the question! New Phytol. 2018:218(1):41–53. 10.1111/nph.14991 [DOI] [PubMed] [Google Scholar]
- Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM. Nitrogen economics of root foraging: transitive closure of the nitrate–cytokinin relay and distinct systemic signaling for N supply vs. Demand. Proc Natl Acad Sci U S A. 2011:108(45):18524–18529. 10.1073/pnas.1108684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Aoyama T, Oka A. Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J. 2000:24(6):703–711. 10.1111/j.1365-313X.2000.00909.x [DOI] [PubMed] [Google Scholar]
- Sakai H, Honma T, Aoyama T, Sato S, Kato T, Tabata S, Oka A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science. 2001:294(5546):1519–1521. 10.1126/science.1065201 [DOI] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol. 2006:57(1):431–449. 10.1146/annurev.arplant.57.032905.105231 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Bishopp A, Kieber JJ. The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell. 2015:27(1):44–63. 10.1105/tpc.114.133595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Shiu S-H, Armitage JP. Two-component systems and their co-option for eukaryotic signal transduction. Curr Biol. 2011:21(9):R320–R330. 10.1016/j.cub.2011.02.045 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Street IH, Kieber JJ. Cytokinin and the cell cycle. Curr Opin Plant Biol. 2014:21C:7–15. 10.1016/j.pbi.2014.05.015 [DOI] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzo L, Willemsen V, Hauser M-T, Janmaat K, Weisbeek P, Benfey PN. Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995:121(1):53–62. 10.1242/dev.121.1.53 [DOI] [Google Scholar]
- Schwarz I, Scheirlinck MT, Otto E, Bartrina I, Schmidt RC, Schmülling T. Cytokinin regulates the activity of the inflorescence meristem and components of seed yield in oilseed rape. J Exp Bot. 2020:71(22):7146–7159. 10.1093/jxb/eraa419 [DOI] [PubMed] [Google Scholar]
- Scofield S, Dewitte W, Nieuwland J, Murray JAH. The Arabidopsis homeobox gene SHOOT MERISTEMLESS has cellular and meristem-organisational roles with differential requirements for cytokinin and CYCD3 activity. Plant J. 2013:75(1):53–66. 10.1111/tpj.12198 [DOI] [PubMed] [Google Scholar]
- Shang X-L, Xie R-R, Tian H, Wang Q-L, Guo F-Q. Putative zeatin O-glucosyltransferase OscZOG1 regulates root and shoot development and formation of agronomic traits in rice. J Integr Plant Biol. 2016:58(7):627–641. 10.1111/jipb.12444 [DOI] [PubMed] [Google Scholar]
- Shanks CM, Hecker A, Cheng C-Y, Brand L, Collani S, Schmid M, Schaller GE, Wanke D, Harter K, Kieber JJ. Role of BASIC PENTACYSTEINE transcription factors in a subset of cytokinin signaling responses. Plant J. 2018:95(3):458–473. 10.1111/tpj.13962 [DOI] [PubMed] [Google Scholar]
- Shanks CM, Rice H, Hubo Y, Schaller E, Hewezi T, Kieber JJ. The role of cytokinin during infection of Arabidopsis thaliana by Heterodera schachtii. Mol Plant Microbe Interact. 2016:29(1):57–68. 10.1094/MPMI-07-15-0156-R [DOI] [PubMed] [Google Scholar]
- Siddique S, Radakovic ZS, De La Torre CM, Chronis D, Novák O, Ramireddy E, Holbein J, Matera C, Hutten M, Gutbrod P, et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc Natl Acad Sci USA. 2015:112(41):12669–12674. 10.1073/pnas.1503657112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Miller C. Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp Soc Exp Biol. 1957:11:118–130. [PubMed] [Google Scholar]
- Spallek T, Melnyk CW, Wakatake T, Zhang J, Sakamoto Y, Kiba T, Yoshida S, Matsunaga S, Sakakibara H, Shirasu K. Interspecies hormonal control of host root morphology by parasitic plants. Proc Natl Acad Sci U S A. 2017:114(20):5283–5288. 10.1073/pnas.1619078114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spichal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T. Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol. 2004:45(9):1299–1305. 10.1093/pcp/pch132 [DOI] [PubMed] [Google Scholar]
- Steiner E, Israeli A, Gupta R, Shwartz I, Nir I, Leibman-Markus M, Tal L, Farber M, Amsalem Z, Ori N, et al. Characterization of the cytokinin sensor TCSv2 in Arabidopsis and tomato. Plant Methods. 2020:16(1):152. 10.1186/s13007-020-00694-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000:69(1):183–215. 10.1146/annurev.biochem.69.1.183 [DOI] [PubMed] [Google Scholar]
- Stock JF, Stock AN, Mottonen JM. Signal transduction in bacteria. Nature. 1990:344(6265):395–400. 10.1038/344395a0 [DOI] [PubMed] [Google Scholar]
- Stolz A, Riefler M, Lomin SN, Achazi K, Romanov GA, Schmülling T. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J. 2011:67(1):157–168. 10.1111/j.1365-313X.2011.04584.x [DOI] [PubMed] [Google Scholar]
- Strnad M. The aromatic cytokinins. Physiol Plant. 1997:101(4):674–688. 10.1111/j.1399-3054.1997.tb01052.x [DOI] [Google Scholar]
- Sugawara H, Ueda N, Kojima M, Makita N, Yamaya T, Sakakibara H. Structural insight into the reaction mechanism and evolution of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 2008:105(7):2734–2739. 10.1073/pnas.0707374105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hirose N, Wang X, Wen P, Xue L, Sakakibara H, Zuo J. Arabidopsis SOI33/AtENT8 gene encodes a putative equilibrative nucleoside transporter that is involved in cytokinin transport in planta. J Integr Plant Biol. 2005:47(5):588–603. 10.1111/j.1744-7909.2005.00104.x [DOI] [Google Scholar]
- Sun L, Zhang Q, Wu J, Zhang L, Jiao X, Zhang S, Zhang Z, Sun D, Lu T, Sun Y. Two rice authentic histidine phosphotransfer proteins, OsAHP1 and OsAHP2, mediate cytokinin signaling and stress responses in rice. Plant Physiol. 2014:165(1):335–345. 10.1104/pp.113.232629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman MR, Kende H. In vitro cytokinin binding to a particulate fraction of tobacco cells. Planta. 1978:140(3):251–259. 10.1007/BF00390256 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Miwa K, Ishikawa K, Yamada H, Aiba H, Mizuno T. The Arabidopsis sensor his-kinase, AHK4, can respond to cytokinins. Plant Cell Physiol. 2001:42(2):107–113. 10.1093/pcp/pce037 [DOI] [PubMed] [Google Scholar]
- Swanson RV, Alex LA, Simon MI. Histidine and aspartate phosphorylation: two-component systems and the limits of homology. Trends Biochem Sci. 1994:19(11):485–490. 10.1016/0968-0004(94)90135-X [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kajihara T, Okamura C, Kim Y, Katagiri Y, Okushima Y, Matsunaga S, Hwang I, Umeda M. Cytokinins control endocycle onset by promoting the expression of an APC/C activator in Arabidopsis roots. Curr Biol. 2013:23(18):1812–1817. 10.1016/j.cub.2013.07.051 [DOI] [PubMed] [Google Scholar]
- Takegami T, Yoshida K. Isolation and purification of cytokinin binding protein from tobacco leaves by affinity column chromatography. Biochem Biophys Res Commun. 1975:67(2):782–789. 10.1016/0006-291X(75)90881-5 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Sugiyama T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem. 2001:276(28):26405–26410. 10.1074/jbc.M102130200 [DOI] [PubMed] [Google Scholar]
- Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H. AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol. 2004a:45(8):1053–1062. 10.1093/pcp/pch119 [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem. 2004b:279(40):41866–41872. 10.1074/jbc.M406337200 [DOI] [PubMed] [Google Scholar]
- Tao J, Sun H, Gu P, Liang Z, Chen X, Lou J, Xu G, Zhang Y. A sensitive synthetic reporter for visualizing cytokinin signaling output in rice. Plant Methods. 2017:13(1):89. 10.1186/s13007-017-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y, Tanaka Y, Nishimura S. 5′-AMP is a direct precursor of cytokinin in Dictyostelium discoidum. Nature. 1978:271(5645):545–547. 10.1038/271545a0 [DOI] [PubMed] [Google Scholar]
- Tessi TM, Brumm S, Winklbauer E, Schumacher B, Pettinari G, Lescano I, González CA, Wanke D, Maurino VG, Harter K, et al. Arabidopsis AZG2 transports cytokinins in vivo and regulates lateral root emergence. New Phytol. 2021:229(2):979–993. 10.1111/nph.16943 [DOI] [PubMed] [Google Scholar]
- Tessi TM, Maurino VG, Shahriari M, Meissner E, Novak O, Pasternak T, Schumacher BS, Ditengou F, Li Z, Duerr J, et al. AZG1 is a cytokinin transporter that interacts with auxin transporter PIN1 and regulates the root stress response. New Phytol. 2023:238(5):1924–1941. 10.1111/nph.18879 [DOI] [PubMed] [Google Scholar]
- To JP, Deruère J, Maxwell BB, Morris VF, Hutchison CE, Schaller GE, Kieber JJ. Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell. 2007:19(12):3901–3914. 10.1105/tpc.107.052662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To JPC, Haberer G, Ferreira FJ, Deruère J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ. Type-A ARRs are partially redundant negative regulators of cytokinin signaling in Arabidopsis. Plant Cell. 2004:16(3):658–671. 10.1105/tpc.018978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H, Kojima M, Kuroha T, Ishida T, Sugimoto K, Kiba T, Sakakibara H. Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in cytokinin activation. Plant J. 2012:69(2):355–365. 10.1111/j.1365-313X.2011.04795.x [DOI] [PubMed] [Google Scholar]
- Tran LSP, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc Natl Acad Sci U S A. 2007:104(51):20623–20628. 10.1073/pnas.0706547105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Weir NR, Hill K, Zhang W, Kim HJ, Shiu SH, Schaller E, Kieber JJ. Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiol. 2012:158(4):1666–1684. 10.1104/pp.111.192765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi C, Sato S, Kato T, Tabata S. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol. 2001:42(7):751–755. 10.1093/pcp/pce094 [DOI] [PubMed] [Google Scholar]
- Vaughan-Hirsch J, Tallerday EJ, Burr CA, Hodgens C, Boeshore SL, Beaver K, Melling A, Sari K, Kerr ID, Šimura J, et al. Function of the pseudo phosphotransfer proteins has diverged between rice and Arabidopsis. Plant J. 2021:106(1):159–173. 10.1111/tpj.15156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwartzenberg K, Lindner AC, Gruhn N, Šimura J, Novák O, Strnad M, Gonneau M, Nogué F, Heyl A. CHASE domain-containing receptors play an essential role in the cytokinin response of the moss Physcomitrella patens. J Exp Bot. 2016:67(3):667–679. 10.1093/jxb/erv479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DR, McRoberts N. Plants and biotrophs: a pivotal role for cytokinins? Trends Plant Sci. 2006:11(12):581–586. 10.1016/j.tplants.2006.10.003 [DOI] [PubMed] [Google Scholar]
- Wang X, Ding J, Lin S, Liu D, Gu T, Wu H, Trigiano RN, McAvoy R, Huang J, Li Y. Evolution and roles of cytokinin genes in angiosperms 2: do ancient CKXs play housekeeping roles while non-ancient CKXs play regulatory roles? Hortic Res. 2020b:7(1):29. 10.1038/s41438-020-0246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W-C, Lin T-C, Kieber J, Tsai Y-C. Response regulators 9 and 10 negatively regulate salinity tolerance in rice. Plant Cell Physiol. 2019:60(11):2549–2563. 10.1093/pcp/pcz149 [DOI] [PubMed] [Google Scholar]
- Wang X, Lin S, Liu D, Gan L, McAvoy R, Ding J, Li Y. Evolution and roles of cytokinin genes in angiosperms 1: do ancient IPTs play housekeeping while non-ancient IPTs play regulatory roles? Hort Res. 2020c:7(1):28. 10.1038/s41438-019-0211-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma XM, Kojima M, Sakakibara H, Hou BK. N-glucosyltransferase UGT76C2 is involved in cytokinin homeostasis and cytokinin response in Arabidopsis thaliana. Plant Cell Physiol. 2011:52(12):2200–2201. 10.1093/pcp/pcr152 [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen W, Chan Z, Wu Y. Endogenous cytokinin overproduction modulates ROS homeostasis and decreases salt stress resistance in Arabidopsis thaliana. Front Plant Sci. 2015:6:1004. 10.3389/fpls.2015.01004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang G, Gao Y, Lu G, Habben JE, Mao G, Chen G, Wang J, Yang F, Zhao X, et al. A cytokinin-activation enzyme-like gene improves grain yield under various field conditions in rice. Plant Mol Biol. 2020a:102(4–5):373–388. 10.1007/s11103-019-00952-5 [DOI] [PubMed] [Google Scholar]
- Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T. New insights into the biology of cytokinin degradation. Plant Biol (Stuttg). 2006:8(3):371–381. 10.1055/s-2006-923928 [DOI] [PubMed] [Google Scholar]
- Wulfetange K, Lomin SN, Romanov GA, Stolz A, Heyl A, Schmülling T. The cytokinin receptors of Arabidopsis are located mainly to the endoplasmic reticulum. Plant Physiol. 2011:156(4):1808–1818. 10.1104/pp.111.180539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybouw B, De Rybel B. Cytokinin—a developing story. Trends Plant Sci. 2019:24(2):177–185. 10.1016/j.tplants.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Liu D, Zhang G, Gao S, Liu L, Xu F, Che R, Wang Y, Tong H, Chu C. Big grain3, encoding a purine permease, regulates grain size via modulating cytokinin transport in rice. J Integr Plant Biol. 2019:61(5):581–597. 10.1111/jipb.12727 [DOI] [PubMed] [Google Scholar]
- Xie M, Chen H, Huang L, O'Neil RC, Shokhirev MN, Ecker JR. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat Commun. 2018:9(1):1604. 10.1038/s41467-018-03921-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Burgess P, Huang B. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J Exp Bot. 2016:67(6):1979–1992 10.1093/jxb/erw019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T. The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol. 2001:41(9):1017–1023. 10.1093/pcp/pce127 [DOI] [PubMed] [Google Scholar]
- Yan Z, Liu X, Ljung K, Li S, Zhao W, Yang F, Wang M, Tao Y. Type B response regulators act as central integrators in transcriptional control of the auxin biosynthesis enzyme TAA1. Plant Physiol. 2017:175(3):1438–1454. 10.1104/pp.17.00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Wang J, Wang F, Xie C, Lv B, Yu Z, Dai S, Liu X, Xia G, Tian H, et al. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis. EMBO Rep. 2021:22(10):e52457. 10.15252/embr.202152457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Cortijo S, Korsbo N, Roszak P, Schiessl K, Gurzadyan A, Wightman R, Jönsson H, Meyerowitz E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science. 2021a:371(6536):1350–1355. 10.1126/science.abe2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Minne M, Brunoni F, Plačková L, Petřík I, Sun Y, Nolf J, Smet W, Verstaen K, Wendrich JR, et al. Non-cell autonomous and spatiotemporal signalling from a tissue organizer orchestrates root vascular development. Nat Plants. 2021b:7(11):1485–1494. 10.1038/s41477-021-01017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhang J, Kojima M, Takebayashi Y, Uragami T, Kiba T, Sakakibara H, Lee Y. ABCG11 modulates cytokinin responses in Arabidopsis thaliana. Front Plant Sci. 2022:13:976267. 10.3389/fpls.2022.976267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wang X, Lyu M, Siligato R, Eswaran G, Vainio L, Blomster T, Zhang J, Mähönen AP. Cytokinins initiate secondary growth in the Arabidopsis root through a set of LBD genes. Curr Biol. 2021:31(15):3365–3373. 10.1016/j.cub.2021.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Xiao Y, Niu M, Meng W, Li L, Zhang X, Liu D, Zhang G, Qian Y, Sun Z, et al. ARGONAUTE2 enhances grain length and salt tolerance by activating BIG GRAIN3 to modulate cytokinin distribution in rice. Plant Cell. 2020:32(7):2292–2306. 10.1105/tpc.19.00542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H. Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol. 2004:134(4):1654–1661. 10.1104/pp.103.037176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu Z, Song X, Johnson C, Yu X, Sundaresan V. The CKI1 histidine kinase specifies the female gametic precursor of the endosperm. Dev Cell. 2016:37(1):34–46. 10.1016/j.devcel.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Berman A, Shani E. Plant hormone transport and localization: signaling molecules on the move. Annu Rev Plant Biol. 2023:74(1):453–479. 10.1146/annurev-arplant-070722-015329 [DOI] [PubMed] [Google Scholar]
- Zhang T-Q, Lian H, Zhou C-M, Xu L, Jiao Y, Wang J-W. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell. 2017:29(5):1073–1087. 10.1105/tpc.16.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Novak O, Wei Z, Gou M, Zhang X, Yu Y, Yang H, Cai Y, Strnad M, Liu CJ. Arabidopsis ABCG14 protein controls the acropetal translocation of root-synthesized cytokinins. Nat Commun. 2014:5(1):3274. 10.1038/ncomms4274 [DOI] [PubMed] [Google Scholar]
- Zhang J, Pai Q, Yue L, Wu X, Liu H, Wang W. Cytokinin regulates female gametophyte development by cell cycle modulation in Arabidopsis thaliana. Plant Sci. 2022:324:111419. 10.1016/j.plantsci.2022.111419 [DOI] [PubMed] [Google Scholar]
- Zhang W, Ranjan Swarup R, Bennett M, Schaller GE, Kieber JJ. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol. 2013:23(20):1979–1989. 10.1016/j.cub.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Zhao J, Ding B, Zhu E, Deng X, Zhang M, Zhang P, Wang L, Dai Y, Xiao S, Zhang C, et al. Phloem unloading via the apoplastic pathway is essential for shoot distribution of root-synthesized cytokinins. Plant Physiol. 2021:186(4):2111–2123. 10.1093/plphys/kiab188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tao L, Zhang J, Liu R, Tian H, Hu C, Zhu Y, Li M, Wei Z, Yi J, et al. The type-B response regulators ARR10, ARR12, and ARR18 specify the central cell in Arabidopsis. Plant Cell. 2022:34(12):4714–4737. 10.1093/plcell/koac285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubo YO, Blakley IC, Yamburenko MV, Worthen JM, Street IH, Franco-Zorrilla JM, Zhang W, Hill K, Raines T, Solano R, et al. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proc Natl Acad Sci U S A. 2017:114(29):E5995–E6004. 10.1073/pnas.1620749114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher E, Liu J, di Donato M, Geisler M, Müller B. Plant development regulated by cytokinin sinks. Science. 2016:353(6303):1027–1030. 10.1126/science.aaf7254 [DOI] [PubMed] [Google Scholar]
- Zürcher E, Tavor-Deslex D, Lituiev D, Enkerli K, Tarr PT, Müller B. A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiol. 2013:161(3):1066–1075. 10.1104/pp.112.211763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwack PJ, Rashotte AM. Cytokinin inhibition of leaf senescence. Plant Signal Behav. 2013:8(7):e24737. 10.4161/psb.24737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwack PJ, Rashotte AM. Interactions between cytokinin signalling and abiotic stress responses. J Exp Bot. 2015:66(16):4863–4871. 10.1093/jxb/erv172 [DOI] [PubMed] [Google Scholar]