Abstract
Rationale:
Electronic cigarette (E-cigarette) aerosol contains volatile aldehydes, including flavorings and oxidant metals with known pulmonary toxicity.
Objectives:
To evaluate the associations of e-cigarette use with symptoms of wheeze, bronchitic symptoms and shortness of breath across four years of prospective data.
Methods:
Participants completed questionnaires on respiratory symptoms and past 30-day e-cigarettes, cigarettes, and cannabis use in 2014 (wave 1; N = 2094; mean age 17.3 years, standard deviation [SD] = 0.6 years. Follow-up information was collected in 2015 (wave 2; N = 1609), 2017 (wave 3; N = 1502), and 2018 (wave 4; N = 1637) using online surveys. Mixed effects logistic regression models evaluated associations of e-cigarette use with respiratory symptoms.
Measurements and Main Results:
Participants were mostly Hispanic White (51.8%) and evenly representative by sex (49.6% female; 50.4% male). Compared to never e-cigarette users, past 30-day e-cigarette users reported increased odds of wheeze (odds ratio [OR]: 1.95; 95% CI 1.39, 2.72), bronchitic symptoms (OR: 2.10; 95% CI (1.61, 2.73), and shortness of breath (SOB - OR: 1.81; 95% CI 1.25, 2.60), adjusting for study wave, age, sex, race, lifetime asthma diagnosis, and parental education. Effect estimates were attenuated [wheeze (OR: 1.48; 95% CI 1.05, 2.09), bronchitic symptoms (OR: 1.58; 95% CI 1.20, 2.07), shortness of breath (OR: 1.52; 95% CI 1.04, 2.22)], after adjusting additionally for current cigarette use, cannabis use, and secondhand exposure to e-cigarettes/cigarettes/cannabis.
Conclusions:
E-cigarette use in young adults was associated with respiratory symptoms, independent of combustible cannabis and cigarette exposures.
Keywords: electronic cigarette use, respiratory health, adolescents and young adults
Introduction
Use of electronic cigarettes (e-cigarettes) has increased in recent years among adolescents and young adults. In the United States, prevalence of past 30-day e-cigarette use among high school students 9th to 12th grade in the U.S. National Youth Tobacco Survey increased from 1.5% in 2011[1] to a high of 27.5% in 2019[2], later decreased to 19.6% in 2020,[2] and in 2022 estimates suggest 14.1% of youth currently used e-cigarettes.[3] Components of the e-cigarette aerosol, including volatile aldehydes in flavorings, and oxidant metals, have known lung toxicity.[4, 5] For example, diacetyl and related diketone flavorings that cause bronchiolitis obliterans in occupationally exposed populations have also been found in e-cigarette liquids.[4] E-cigarette aerosol has high concentrations of fine and ultrafine particles that can deliver these toxicants to the distal airways and alveoli,[6] making the lung a target organ for injury and increasing risk for adverse respiratory health effects in e-cigarette users. To date, several cross-sectional and longitudinal studies have reported respiratory symptoms (e.g., chronic cough, wheeze, asthma, shortness of breath) among both adolescent[7–14] and young adult[10, 15] e-cigarette users. Most studies to date have focused on e-cigarette use without examining how multiple product use (e.g., cigarette and/or cannabis prior or co-use) may impact respiratory health.[16, 17]
A 2018 report from the National Academies of Sciences, Engineering, and Medicine concluded that there was not enough evidence to determine whether e-cigarettes cause human respiratory diseases, although the report found moderate evidence that e-cigarette use increases cough, wheeze, and asthma exacerbations in adolescents.[18] These conclusions were based on a few cross-sectional studies.[7, 11, 14] In a study of adolescents, we found that a history of e-cigarette use was associated with increased prevalence of bronchitic symptoms, including among study participants with no history of cigarette use,[7] consistent with findings in another cross-sectional study.[14] Other studies found associations of e-cigarette use with asthma prevalence,[9, 11, 13] and two recent prospective studies[19, 20] found associations of e-cigarette use with respiratory symptoms in the US, controlling for combustible tobacco use. One cross-sectional study[10] found that using e-cigarettes at least 5 days in the past 30-days was associated with bronchitis and shortness of breath after controlling for both combustible cigarette and cannabis use. However, to date, there has been little study of associations of e-cigarette use with respiratory symptoms in prospective investigations while also controlling for exposure to cannabis and combustible cigarette use.
In this study prospective data spanning four years of follow-up (2014 – 2018) were collected on e-cigarette use and respiratory symptoms in the Southern California Children’s Health Study (CHS).[21] Analyses used prospectively collected data to evaluate the associations of e-cigarette use with subsequent symptoms of chronic bronchitis, shortness of breath and wheeze, controlling for co-use of combustible tobacco and cannabis and secondhand exposure to combustibles and e-cigarettes in these young adults. The primary hypothesis was that current e-cigarette use compared to never use of e-cigarettes would be associated with subsequent wheeze, bronchitic symptoms, and shortness of breath at each wave of data collection, independent of use of or secondhand exposure to combustible cannabis and other tobacco products.
Methods
Study design:
There were 2097 CHS participants who completed a school-based survey on tobacco products and respiratory symptoms in 2014 (wave 1) when study participants were in 11th and 12th grade (mean age 17.3 years, standard deviation 0.6). Follow-up information was also collected from 1609 participants in 2015 (wave 2), from 1502 in 2017 (wave 3), and 1637 in wave 4 (2018) using online surveys.[22] At each survey wave, data were collected on ever and past 30-day use of e-cigarettes and cigarettes. At wave 3, cannabis use was added to the survey. There were 2094 participants who contributed to the analytic sample (see supplemental figure 1), based on reporting of e-cigarette use and respiratory outcomes at each wave; at wave 4 there were 1438 participants who provided information on wheeze, 1383 who provided information on bronchitic symptoms, and 1445 who provided information on shortness of breath. Additional description of the cohort, including initial study recruitment, has been reported previously.[7]
Ethics Statement.
The study was approved by the University of Southern California Institutional Review Board [IRB # HS-18-00706]. Written informed consent was obtained from parents of the participants and assent from participants prior to initial data collection. Informed consent was obtained from study participants as they reached 18 years of age.
Measures
Respiratory Symptoms:
Participants were considered to have had symptoms of chronic bronchitis during the previous wave based on the report of a daily cough for 3 months in a row or bronchitis in the previous 12 months, or congestion or phlegm other than when accompanied by a cold, as previously described.[7] Wheeze was assessed based on a report of wheezing or whistling in the chest during the previous 12 months.[23] Shortness of breath was evaluated by a question asking if participants were troubled by shortness of breath when hurrying on level ground or walking up a slight hill. This question was added in wave 2.
Cigarette, E-Cigarette, and Cannabis Exposure:
At each survey, participants reported never, ever, and past 30-day use of cigarettes, e-cigarettes, and cannabis. Specifically, participants were asked if they had ever used the products, and if participants indicated yes, they were asked a follow up question about the number of days they had used the product(s) in the past 30 days. Participants who had never tried a product were classified as never-users. Participants who had used a product on at least 1 of the past 30-days were classified as “past 30-day users” of that product(s), a classification consistent with prior study of e-cigarette use and respiratory symptoms.[7] Use of cannabis was first reported at wave 3 (coded as a three level variables: missing, past 30-day use, no use).
Covariate Information:
Information was available on participants’ sex, ethnicity (Hispanic, Non-Hispanic White, other), and parental education (highest level of education of either parent, categorized as high school diploma/GED or less, some college, college degree, or more), as previously described.[7] Report of any lifetime asthma was assessed at wave 1.[24] At each wave, secondhand exposure to e-cigarette vapor and to cigarette (and cannabis smoke starting at wave 3) at home was assessed by whether anyone in the household (other than the participant) used these products in their presence.
Statistical Analysis
Separate mixed effects logistic regression models were used to relate each respiratory symptom (wheeze, bronchitic symptoms, and shortness of breath) to past 30-day e-cigarette use assessed at the same wave (lag 0) or at the prior wave (lag 1). All models included a participant-level random intercept (random effect) to account for repeated observations over time within participant, and adjusted (fixed effects) for sex, race/ethnicity, age, lifetime asthma diagnosis, parental education, wave; additional models also adjusted for concurrent (lag 0), past 30-day use of cigarettes and/or cannabis, and secondhand exposure to e-cigarettes/cigarettes/cannabis. Although the sample size varied from wave to wave, all available data for each participant in each wave was included in the initial analysis. In sensitivity analyses conducted on associations of concurrent (lag 0) past 30-day e-cigarette use, we additionally restricted the sample to participants (1) without concurrent past 30-day (lag 0) cigarette or cannabis use, in order to remove potential confounding effects of co-exposure, (2) who participated both in wave 1 and 2, in order to assess whether loss to follow-up resulted in biased estimates, and (3) with no history of physician diagnosed asthma at wave 1, in order to assess the influence of asthma on the patterns of observed associations. Further, multiplicative interactions between concurrent and 1 year lagged past-30-day e-cigarettes use were tested. To assess associations with the joint distribution of 1-wave lagged and current past 30-day e-cigarette use we also estimated effects of a four-level e-cigarette use variable: no past 30-day e-cigarette use in the previous and current wave (reference group), past 30-day e-cigarette use in previous wave only, past 30-day e-cigarette use in current wave only and past 30-day e-cigarette use in both previous and current wave. Statistical analyses were conducted using SAS V.9.4 (SAS Institute).
RESULTS
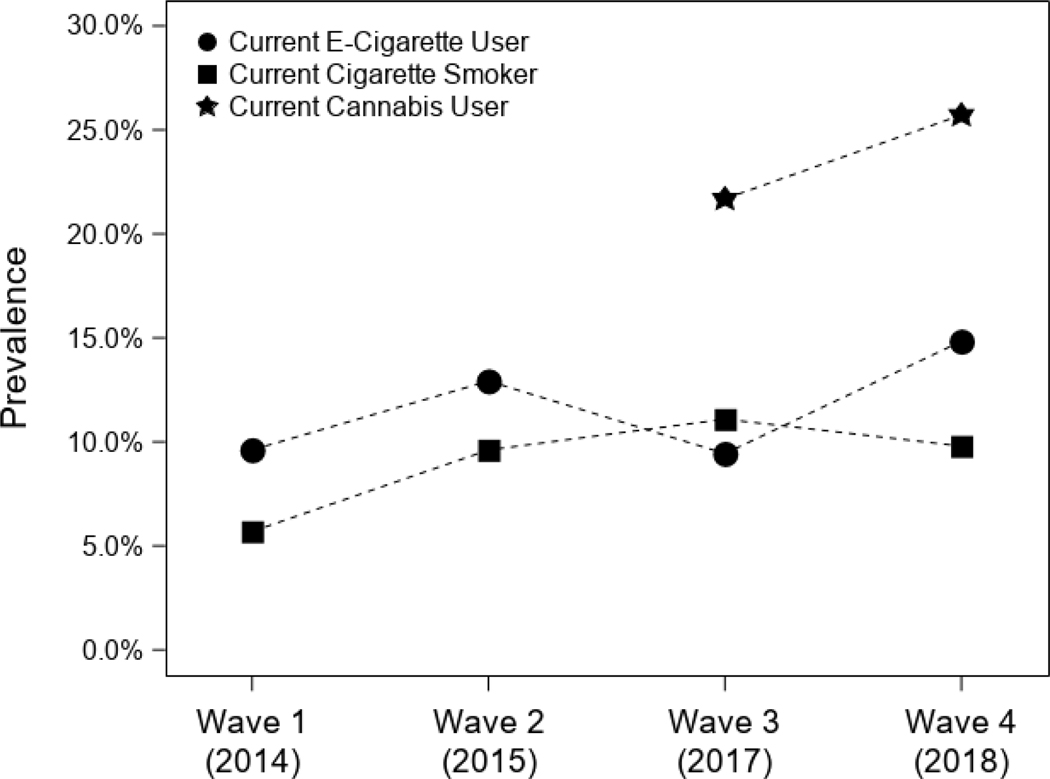
Among the analytic sample of 2094 participants, 49.6% were female, and Hispanic Whites were the largest ethnic group (51.8%), as shown in Table 1. At wave 1 (the only time this question was asked), 22.7% (n = 476) participants reported a lifetime history of physician diagnosed asthma. Current wheeze (12.2%, 13.9%, 14.2%, and 14.8%, waves 1– 4, respectively) and shortness of breath (16.5%, 18.1%, and 17.0%, waves 2– 4, respectively) symptoms varied by wave, with bronchitic symptoms remaining the most commonly reported symptom across each wave (19.4%, 22.5%, 23.5%, and 26% waves 1 – 4, respectively), as shown in Figure 1. Prevalence of respiratory symptoms by sociodemographic characteristics are reported in Table 1. Prevalence of past 30-day e-cigarette use was11.7% (wave 1), 11.8% (wave 2), 11.0% (wave 3), and increased to 15.6% at waves 4 (Figure 2; Table 2). Past 30-day combustible use was reported by 25.6% (wave 1), 25.9% (wave 2), 21.1% (wave 3), and 20.6% (wave 4) of participants. Finally, cannabis use was reported by 21.7% of participants at wave 3 (when participants were first asked) and increased to 25.7% at wave 4 (see Table 2).
Table 1.
Demographic characteristics of 2094 participants and by report of wheeze, bronchitic symptoms, shortness of breath or past 30-day e-cigarette use during the study period (2014 −2018).
| Total Sample N = 2094 | Respiratory Symptoms | Past 30-Day E-Cigarette Use | |||
|---|---|---|---|---|---|
| Wheeze | Bronchitic Symptoms | Shortness of Breath | |||
| Race | Frequency (Percent) | ||||
| Asian | 72 (3.4) | 16 (3.1) | 27 (3.3) | 19 (4.0) | 17 (3.5) |
| Black | 31 (1.5) | 9 (1.8) | 12 (1.5) | 2 (0.4) | 4 (0.8) |
| Hispanic White | 1083 (51.8) | 214 (42.0) | 379 (46.7) | 254 (53.0) | 228 (46.9) |
| Non-Hispanic White | 734 (35.1) | 222 (43.5) | 321 (39.5) | 168 (35.1) | 208 (42.8) |
| Another Race | 172 (8.2) | 49 (9.6) | 73 (9.0) | 36 (7.5) | 29 (6.0) |
| Parental Education at Baseline | |||||
| Completed Grade 12 or Less | 575 (27.5) | 113 (22.2) | 185 (22.8) | 142 (29.6) | 108 (22.2) |
| Some College | 649 (31.0) | 164 (32.2) | 268 (33.0) | 154 (32.1) | 168 (34.6) |
| Completed College or More | 623 (29.8) | 183 (35.9) | 280 (34.4) | 136 (28.3) | 153 (31.5) |
| Missing | 247 (11.8) | 50 (9.8) | 80 (9.8) | 48 (10.0) | 57 (11.7) |
| Sex | |||||
| Female | 1038 (49.6) | 258 (50.6) | 419 (51.5) | 337 (70.2) | 210 (43.2) |
| Male | 1056 (50.4) | 252 (49.4) | 394 (48.5) | 143 (29.8) | 276 (56.8) |
Note. Another Race = Hispanic black, Hispanic Asian/Pacific Islander, Missing, Two or more racial/ethnic identities, Native American/American Indian. Respiratory symptoms are an average of reported symptoms across the study period.
Figure 1.
Prevalence of current respiratory symptoms (wheeze, shortness of breath, and bronchitic symptoms) among study participants across each wave.
Figure 2.
Prevalence of current e-cigarette, cigarette, and cannabis use among study participants across each wave.
Table 2.
Participant age, frequency of nicotine/tobacco and cannabis use by study wave (2014 −2018).
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | |
|---|---|---|---|---|
| (N = 2094) | (N = 1609) | (N = 1502) | (N = 1637) | |
| Participant Age (M, SD) | 17.3 (0.6) | 18.9 (0.6) | 20.2 (0.6) | 21.9 (0.7) |
| Secondhand Exposure | ||||
| E-Cigarettes | 242 (11.7) | 184 (11.8) | 161 (11.0) | 223 (15.6) |
| Cigarettes | 552 (26.6) | 402 (25.9) | 309 (21.1) | 293 (20.6) |
| Cannabis Use | --- | --- | 301 (20.5) | 375 (26.3) |
| Past 30-day Product Use (number of days in past 30-days; M, SD) | ||||
| E-Cigarettes | 5.5 (7.3) | 8.5 (10.0) | 10.6 (11.1) | 11.9 (11.2) |
| Cigarettes | 9.7 (10.8) | 8.6 (10.1) | 8.2 (9.0) | 7.7 (9.9) |
| Cannabis | --- | --- | 12.3 (10.7) | 11.9 (10.7) |
Participants who currently (lag 0) reported past 30-day e-cigarette use (Table 2, Model 1) had increased odds of wheeze (OR: 1.81, 95% CI: 1.28, 2.56), bronchitic symptoms (OR: 2.06, 95% CI: 1.58, 2.69), and shortness of breath (OR: 1.78, 95% CI: 1.23, 2.57), controlling for wave, age, sex, race, and parental education. After further adjustment for concurrent (lag 0) use of cigarettes, cannabis, and secondhand exposure to e-cigarettes, cigarettes, and/or cannabis (Table 3, Model 2), all associations with lag 0 past 30-day e-cigarette use were attenuated but all except wheeze (p =0.06) remained significant (wheeze, OR: 1.41, 95% CI: 0.99, 2.01; bronchitic symptoms, OR: 1.55, 95% CI: 1.18, 2.05; shortness of breath, OR: 1.48, 95% CI: 1.01, 2.18). In models (Table 3) using past 30-day e-cigarette use from the previous wave (lag 1), associations were stronger for wheeze (OR: 2.17, 95% CI: 1.41, 3.35; and OR: 1.77, 95% CI: 1.14, 2.74 with additional adjustment for lag 0 use of cigarette/cannabis and secondhand exposures) than in lag 0 models; associations of lag 1 e-cigarette use with bronchitic symptoms and wheeze were weaker than were lag 0 exposures, and after adjustment (for lag 0 co-exposures) lag 1 associations with bronchitic symptoms and wheeze were no longer significant.
Table 3.
Adjusted odds ratios (OR) relating past-30-day e-cigarette use to respiratory symptoms, from mixed-effect logistic regression models.
| Model | Wheeze | Bronchitic Symptoms | Shortness of Breath |
|---|---|---|---|
| Concurrent wave e-cigarette use (lag 0) | OR (95%CI) | OR (95%CI) | OR (95%CI) |
| Model 1a | 1.81 (1.28, 2.56) | 2.06 (1.58, 2.69) | 1.78 (1.23, 2.57) |
| Model 2b | 1.41 (0.99, 2.01) | 1.55 (1.18, 2.05) | 1.48 (1.01, 2.18) |
| Prior wave e-cigarette use (lag 1) | |||
| Model 1a | 2.17 (1.41, 3.35) | 1.51 (1.07, 2.14) | 1.59 (1.08, 2.34) |
| Model 2b | 1.77 (1.14, 2.74) | 1.23 (0.86, 1.74) | 1.41 (0.96, 2.09) |
Note.
= Model included participant-level random intercept and adjustment for wave, age, sex, race, and parental education.
= additional adjustment for concurrent (lag 0) use of cigarettes, cannabis, and secondhand exposure to e-cigarettes, cigarettes, and cannabis.
Sensitivity analyses were conducted on lag 0 past 30-day e-cigarette use. Estimated effects in the subset of participants reporting no concurrent (lag 0) cigarette or cannabis use (sensitivity analysis 1 in Table 4) were substantially larger than effects co-adjusted for combustibles use in the full sample (from Table 3). For example, the OR for bronchitic symptoms in the participants without concurrent combustible use was 1.86 (95% CI 0.85,10.1) compared with estimates (reported above) adjusted for combustibles (OR: 1.55, 95% CI: 1.18, 2.05). In models restricted to participants who were not lost to follow-up in wave 2 (n=1609; Table 4, sensitivity analysis 2), estimated effects of concurrent past 30-day e-cigarette use (with adjustment for concurrent use of combustibles and secondhand exposures) were almost identical to effects in the full sample. In the subset of participants with no lifetime history of physician diagnosed asthma at wave 1 (n=1618),[24] the effects of e-cigarette use were generally similar in magnitude to associations in the full sample.
Table 4.
Sensitivity analyses showing adjusted odds ratios (OR) relating past-30-day e-cigarette use (lag 0) to respiratory symptoms, from mixed-effect logistic regression models.a
| Sensitivity Analysis Subgroup | Wheezea | Bronchitic Symptomsa | Shortness of Breatha |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| No past 30-day cigarette or cannabis use, n=1235b | 2.92 (0.85, 10.10) | 1.86 (0.83, 4.19) | 1.53 (0.65, 3.63) |
| Participated in both waves 1–2, n=1609c | 1.43 (0.99, 2.05) | 1.52 (1.14, 2.02) | 1.52 (1.03, 2.24) |
| No asthma, n=1618d | 1.66 (1.11, 2.48) | 1.53 (1.10, 2.12) | 1.61 (1.04, 2.48) |
Note.
= OR and 95% CI are based on mixed-effect logistic regression model with person-specific random intercept and adjustment (fixed effect) for wave, age, sex, race, parental education, secondhand exposure to cigarettes, e-cigarettes or cannabis.
= Sensitivity Analysis 1: Restricted to participants without any concurrent cigarette and/or cannabis use in the past 30 days.
= Sensitivity Analysis 2: Participants were included if they participated in both wave 1 and wave 2, adjusted additionally for concurrent cigarette and/or cannabis use.
= Sensitivity Analysis 3: Participants who reported no history of physician diagnosed asthma at wave 1, adjusted additionally for concurrent cigarette and/or cannabis use.
Between wave 1 and wave 2, there were 485 participants who were lost to follow up (supplement table 1). Participants who were not included in wave 2 differed little from those included in rates of each outcome and in rates of exposure to current e-cigarette use at wave 1. Those lost to follow-up were more likely to be male, Hispanic White, and to have a parent completing twelfth grade or less. We adjusted for these sociodemographic characteristics that varied between those included and lost to the analysis, and the effect estimates (shown in Table 2) were quite similar in size to the unadjusted estimates (results not shown). In addition, in a sensitivity analysis excluding these 485 participants, the effect estimates were similar in magnitude in the subset of participants with the same data at both waves 1–2 (Table 4).
Finally, when considering the history of past 30-day e-cigarette use in the current wave (lag 0) only, in the prior wave (lag 1) only, and in both waves, participants who reported past-30 day use at both waves had the largest OR (reference: no past 30-day e-cigarette use in both waves), particularly for wheeze and shortness of breath (supplementary table 2, although the interaction of lag 0 x lag 1 exposure was not statistically significant).
Discussion
This study used four years of prospective data to examine the association of e-cigarette use and respiratory symptoms in late adolescence and early young adulthood. Past 30-day e-cigarette use at the time of the assessment of outcome was associated with increased risk of wheeze, bronchitic symptoms, and shortness of breath, after adjusting for use of and secondhand exposure to combustibles. Associations persisted in a sensitivity analysis excluding participants with a baseline lifetime history of asthma, indicating that associations were present in all participants, not just those with asthma. Past 30-day use of e-cigarettes the year prior to symptom assessment had a stronger association with the current year wheeze, but weaker (and not significant) associations with bronchitic symptoms and shortness of breath. The estimated effect of persistent past 30-day e-cigarette use in the previous (lag 1) wave and the current (lag 0) wave was larger for all three outcomes than for either previous or current use alone. Results were consistent with another recent prospective study in adults showing e-cigarette use was an independent risk factor for poor respiratory outcomes.[20]
The associations between past 30-day e-cigarette use and symptoms were moderately attenuated after controlling for concurrent use of or exposure to secondhand cigarettes and/or cannabis. Increasing frequency of co-occurrence of adult use of e-cigarettes with cannabis[17, 25, 26] and cigarettes[20] makes it difficult to establish that respiratory associations with e-cigarettes are independent of co-exposures. Recent analyses of the Population Assessment of Tobacco and Health (PATH) Study data found that in adults e-cigarette use was associated with development of respiratory disease, independent of cigarette smoking,[20] but that past 30-day e-cigarette use among adolescents was not statistically significantly associated with self-reported symptoms of wheeze after controlling for cannabis vaping.[26] In our study, a causal interpretation was strengthened by sensitivity analysis showing that estimates of e-cigarette effects were stronger in participants with no (potentially confounding) past 30-day use of cigarettes or cannabis. Our observational results are supported by experimental studies. In cultured human airway epithelial cells, ex vivo immune cells, and mice, e-cigarette vapor increased markers of oxidative stress and inflammatory effects, and decreased cell viability.[27, 28] Further, recent human laboratory studies conducted among adult users of e-cigarettes showed that e-cigarette exposure causes short-term adverse respiratory effects[29] and immune suppression.[30–32]
This study has some limitations. First, these data were collected via self-reported questionnaires. Second, questionnaire items for shortness of breath and cannabis were not available until waves 2 and 3, respectively, so analyses of these variables made use of more limited data than other analyses. Unlike cigarettes, which can be quantified by number of cigarettes smoked per day, validated questionnaire-based methods for assessing e-cigarette use frequency, for example by reporting the number of e-cigarette inhalations/vaping sessions or duration of inhalation, were not available to us.[33, 34] Even when self-reported e-cigarette puffs are available, these estimates under-reported objectively collected user puffs from an e-cigarette device by approximately 2-fold.[35] It is likely that such misclassified exposure estimates from our self-report of any past 30-day use would result in under-estimation of a true causal effect. In addition, e-cigarettes come in a variety of device types (e.g., pod-mods, tank systems, disposables), e-liquid flavors (fruit, mint, fruit-ice, menthol), and various nicotine types (e.g., synthetic nicotine, free-base nicotine, nicotine-salt, various nicotine strength), which impact the user experience,[36] frequency of use,[37] and may cause heterogeneous respiratory effects.[10, 19] [4] For example, devices that offer greater power to heat e-liquids can generate substantially greater concentrations of aldehydes and other lung toxicants compared to lower-powered devices.[38, 39] Recent cross-sectional data from four study populations across the United States found that e-cigarette use was associated with bronchitic symptoms and shortness of breath, and device types did not substantially affect the strength of these associations.[10] Finally, the direction of possible selection bias due to loss to follow-up is not clear. Those lost to follow-up differed little either in rates of outcome or exposure at wave, suggesting that the pattern of e-cigarette effects would have been similar had the entire cohort been included in the prospective analysis. Some sociodemographic covariates varied by symptoms outcomes between those retained and lost to wave 2 follow-up, but sensitivity analyses found that the effect estimates of e-cigarette effects varied very little between models with and without these covariates. Estimates excluding in wave 1 those subjects that were lost to follow-up were also very similar to the analyses including all available information.
Conclusions
Data collected from 2014 – 2018 among a diverse sample of young adults in southern California showed that e-cigarette use was associated with respiratory symptoms after accounting for concurrent cigarette and cannabis use as well as secondhand exposure to each of these products and secondhand exposure to e-cigarettes. The results strengthen epidemiologic evidence of adverse respiratory effects of e-cigarettes that is consistent with known effects of e-cigarette ingredients.[7, 10]
Supplementary Material
Key Message:
What is already known on this topic?
E-cigarette use has been associated with increased respiratory symptoms like wheeze, bronchitic symptoms and shortness of breath, but limited studies have examined these associations in prospective, longitudinal population cohorts.
What this study adds?
This study identified prospective associations between e-cigarette use and wheeze, bronchitic symptoms and shortness of breath, even when controlling for use of combustible cannabis and cigarette use and secondhand exposure to e-cigarettes/cigarettes/cannabis.
How might this study affect research, practice, or policy?
This study contributes to emerging evidence from human and toxicological studies that e-cigarettes cause respiratory symptoms that warrant consideration in regulation of e-cigarettes. It suggests that regulatory assessments of the population health cost under-estimate the effects of late adolescent and young adult e-cigarette, cannabis, and tobacco product use.
Funding/Support
This Research reported was supported by NIH grants # P50CA180905, R21HD084812, K01DA042950, K01HL148907, P30ES007048, P2CES033433 and the Hastings Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Competing of Interests
None
Ethics Approval
This study involves human participants and was approved by an Ethics Committee(s) or Institutional Board(s): University of Southern California Institutional Review Board (HS-18–00706).
Data Sharing
Data are available upon reasonable request.
References
- 1.Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. Tobacco product use among middle and high school students—United States, 2011–2017. Morbidity and Mortality Weekly Report. 2018;67(22):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi BM, Abraham I. The Decline in e-Cigarette Use Among Youth in the United States—An Encouraging Trend but an Ongoing Public Health Challenge. JAMA Network Open. 2021;4(6):e2112464-e. [DOI] [PubMed] [Google Scholar]
- 3.Cooper M Notes from the field: E-cigarette use among middle and high school students—United States, 2022. MMWR Morbidity and Mortality Weekly Report. 2022;71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2, 3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environmental health perspectives. 2016;124(6):733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health. 2017;16(1):42. Epub 2017/04/30. doi: 10.1186/s12940-017-0249-x. PubMed PMID: 28449666; PubMed Central PMCID: PMCPMC5406907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicology and applied pharmacology. 2014;278(1):9–15. [DOI] [PubMed] [Google Scholar]
- 7.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, et al. Electronic cigarette use and respiratory symptoms in adolescents. American journal of respiratory and critical care medicine. 2017;195(8):1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tackett AP, Keller-Hamilton B, Smith CE, Hébert ET, Metcalf JP, Queimado L, et al. Evaluation of Respiratory Symptoms Among Youth e-Cigarette Users. JAMA Network Open. 2020;3(10):e2020671-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alnajem A, Redha A, Alroumi D, Alshammasi A, Ali M, Alhussaini M, et al. Use of electronic cigarettes and secondhand exposure to their aerosols are associated with asthma symptoms among adolescents: a cross-sectional study. Respiratory Research. 2020;21(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaffee BW, Barrington-Trimis JL, Liu F, Wu R, McConnell R, Krishnan-Sarin S, Leventhal AM, Kong G E-Cigarette Use and Adverse Respiratory Symptoms Among Adolescents and Young Adults in the United States. Preventive Medicine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schweitzer RJ, Wills TA, Tam E, Pagano I, Choi K. E-cigarette use and asthma in a multiethnic sample of adolescents. Preventive medicine. 2017;105:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi K, Bernat D. E-cigarette use among Florida youth with and without asthma. American journal of preventive medicine. 2016;51(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SI, Dardari ZA, et al. The association between e-cigarette use and asthma among never combustible cigarette smokers: behavioral risk factor surveillance system (BRFSS) 2016 & 2017. BMC pulmonary medicine. 2019;19(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang MP, Ho SY, Leung LT, Lam TH. Electronic cigarette use and respiratory symptoms in Chinese adolescents in Hong Kong. JAMA pediatrics. 2016;170(1):89–91. [DOI] [PubMed] [Google Scholar]
- 15.Gotts JE, Jordt S-E, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? bmj. 2019;366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadi N, Schroeder R, Jensen JW, Levy S. Association between electronic cigarette use and marijuana use among adolescents and young adults: a systematic review and meta-analysis. JAMA pediatrics. 2019;173(10):e192574-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braymiller JL, Barrington-Trimis JL, Leventhal AM, Islam T, Kechter A, Krueger EA, et al. Assessment of nicotine and cannabis vaping and respiratory symptoms in young adults. JAMA network open. 2020;3(12):e2030189-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Academies of Sciences, Engineering, and Medicine. Public health consequences of e-cigarettes. Committee on the review of the health effects of electronic cigarettes of electronic nicotine delivery systems, National Academies Press, Washington, DC: 2018, Paperback, 680 Pages, ISBN 9780309468312 0309468310. 2018. doi: 10.17226/24952. [DOI] [Google Scholar]
- 19.Xie W, Tackett AP, Berlowitz JB, Harlow AF, Kathuria H, Galiatsatos P, et al. Association of electronic cigarette use with respiratory symptom development among US young adults. American Journal of Respiratory and Critical Care Medicine. 2022;(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatta DN, Glantz SA. Association of e-cigarette use with respiratory disease among adults: a longitudinal analysis. American journal of preventive medicine. 2020;58(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, et al. Traffic, susceptibility, and childhood asthma. Environmental health perspectives. 2006;114(5):766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrington-Trimis JL, Berhane K, Unger JB, Cruz TB, Huh J, Leventhal AM, et al. Psychosocial factors associated with adolescent electronic cigarette and cigarette use. Pediatrics. 2015;136(2):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISAAC Steering Committee. International study of asthma and allergies in children: Phase II modules. Muenster, Germany: Institute of Epidemiology and Social Medicine, University of Muenster. 1998.. [Google Scholar]
- 24.Islam T, Braymiller J, Eckel SP, Liu F, Tackett AP, Rebuli ME, et al. Secondhand nicotine vaping at home and respiratory symptoms in young adults. Thorax. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts ME, Tackett AP, Singer JM, Wagner DD, Lu B, Wagener TL, et al. Dual use of e-cigarettes and cannabis among young people in America: A new public health hurdle? Journal of Studies on Alcohol and Drugs. 2022:jsad. 22–00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd CJ, McCabe SE, Evans-Polce RJ, Veliz PT. Cannabis, Vaping, and Respiratory Symptoms in a Probability Sample of U.S. Youth. Journal of Adolescent Health. 2021;69(1):149–52. doi: 10.1016/j.jadohealth.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowell TR, Reeber SL, Lee SL, Harris RA, Nethery RC, Herring AH, et al. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017;313(1):L52–L66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017;313(2):L278–L92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wills TA, Pagano I, Williams RJ, Tam EK. E-cigarette use and respiratory disorder in an adult sample. Drug and alcohol dependence. 2019;194:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141(6):1400–6. [DOI] [PubMed] [Google Scholar]
- 31.Dicpinigaitis PV, Chang AL, Dicpinigaitis AJ, Negassa A. Effect of e-cigarette use on cough reflex sensitivity. Chest. 2016;149(1):161–5. [DOI] [PubMed] [Google Scholar]
- 32.Rebuli ME, Glista-Baker E, Hoffman JR, Duffney PF, Robinette C, Speen AM, et al. Electronic-cigarette use alters nasal mucosal immune response to live-attenuated influenza virus. A clinical trial. American journal of respiratory cell and molecular biology. 2021;64(1):126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weaver SR, Kim H, Glasser AM, Sutfin EL, Barrington-Trimis J, Payne TJ, et al. Establishing consensus on survey measures for electronic nicotine and non-nicotine delivery system use: current challenges and considerations for researchers. Addictive behaviors. 2018;79:203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halpern-Felsher B, Kim H. Measuring E-cigarette use, dependence, and perceptions: Important principles and considerations to advance tobacco regulatory science. Addictive behaviors. 2018;79:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Benowitz-Fredericks C, Ling PM, Cohen JE, Thrul J. Assessing Young Adults’ ENDS Use via Ecological Momentary Assessment and a Smart Bluetooth Enabled ENDS Device. Nicotine & Tobacco Research. 2020;23(5):842–8. doi: 10.1093/ntr/ntaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park-Lee E, Ren C, Sawdey MD, Gentzke AS, Cornelius M, Jamal A, et al. Notes from the field: e-cigarette use among middle and high school students—National Youth Tobacco Survey, United States, 2021. Morbidity and Mortality Weekly Report. 2021;70(39):1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel EA, Ramo DE, Rubinstein ML. Prevalence and correlates of adolescents’e-cigarette use frequency and dependence. Drug and Alcohol Dependence. 2018;188:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. International journal of hygiene and environmental health. 2016;219(3):268–77. [DOI] [PubMed] [Google Scholar]
- 39.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, et al. Aldehyde detection in electronic cigarette aerosols. ACS omega. 2017;2(3):1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.