Abstract
The U.S. Centers for Disease Control and Prevention (CDC) developed and implemented the CDC COVID-19 Vaccine Pregnancy Registry (C19VPR) to monitor vaccine safety. Potential participants who received a COVID-19 vaccine in pregnancy or up to 30 days prior to their pregnancy-associated last menstrual period were eligible to participate in the registry, which monitored health outcomes of participants and their infants through phone interviews and review of available medical records. Data for select outcomes, including birth defects, were reviewed by clinicians. In certain cases, medical records through phone interviews and review of available medical records. Data for select outcomes, including birth defects, were reviewed by clinicians. In certain cases, medical records were used to confirm and add detail to participant-reported health conditions.
This paper serves as a description of CDC C19VPR protocol. We describe the development and implementation for each data collection aspect of the registry (i.e., participant phone interviews, clinical review, and medical record abstraction), data management, and strengths and limitations. We also describe the demographics and vaccinations received among eligible and enrolled participants. There were 123,609 potential participants 18–54 years of age identified from January 2021 through mid-June 2021; 23,339 were eligible and enrolled into the registry. Among these, 85.3 % consented to medical record review for themselves and/or their infants. Participants were majority non-Hispanic White (79.1 %), residents of urban areas (93.3 %), and 48.3 % were between 30 and 34 years of age. Most participants completed the primary series of vaccination by the end of pregnancy (89.7 %). Many participants were healthcare personnel (44.8 %), possibly due to the phased roll-out of the vaccination program.
The registry continues to provide important information about the safety of COVID-19 vaccination among pregnant people, a population with higher risk of poor outcomes from COVID-19 who were not included in preauthorization clinical trials. Lessons learned from the registry may guide development and implementation of future vaccine safety monitoring efforts for pregnant people and their infants.
Keywords: COVID-19, COVID-19 vaccine, Vaccine safety, Vaccination, Immunization, Pregnancy surveillance, Pregnancy
1. Background
Vaccination is a key public health tool to mitigate morbidity and mortality from COVID-19 disease caused by infection with the SARS-CoV-2 virus [1] COVID-19 vaccines were initially administered after the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorizations (EUA) in December 2020 following expedited vaccine development and clinical trial processes [2]. The U.S. Centers for Disease Control and Prevention (CDC) and the FDA planned for robust and extensive vaccine safety monitoring by utilizing existing monitoring systems (e.g., Vaccine Adverse Event Reporting System and Vaccine Safety Datalink) and implementing new safety monitoring systems, (e.g., v-safe after vaccination health checkerSM (v-safe) [3] and the CDC COVID-19 Vaccine Pregnancy Registry (C19VPR) [4]) to rapidly identify and assess potential safety issues. Though developmental and reproductive toxicity (DART) studies using animal models were found to be reassuring, pregnant people were excluded from pre-authorization clinical trials, resulting in initially limited data about COVID-19 vaccine safety in human pregnancy [5]. The FDA required postmarketing safety monitoring in pregnant people, including both passive surveillance and sponsor conducted active surveillance (e.g., the COVID-19 Vaccines International Pregnancy Exposure Registry (C-VIPER), Organization of Teratology Information Specialists (OTIS)/MotherToBaby Pregnancy Registry) and database studies [6–8]. However, initial lack of data about the safety of COVID-19 vaccination during pregnancy may have contributed to relatively low uptake of COVID-19 vaccine among pregnant people and people who could become pregnant, as safety concerns were a leading reason for vaccine hesitancy among pregnant people [9–11].
People who experience SARS-CoV-2 infection during pregnancy have an increased risk of adverse outcomes, including stillbirth, preterm delivery, intensive care unit admission, mechanical ventilation, and death, and their infants have an increased risk of neonatal intensive care unit admission and neonatal death [12,13]. The exclusion of pregnant people from COVID-19 vaccine clinical trials heightened the importance of monitoring vaccine safety among pregnant people in the post-authorization period and led to the rapid development and implementation of the CDC C19VPR. Throughout data acquisition, analyses of preliminary data were conducted to inform CDC’s COVID-19 Vaccine Safety Technical Work Group (VaST) and Advisory Committee on Immunization Practices (ACIP) [14,15]. As of November 2023, data collection and analyses remain ongoing. This review describes (1) methodology of the CDC C19VPR including participant identification, eligibility criteria, interview methodology, clinical review, and medical record acquisition and abstraction and (2) response rates and characteristics of enrolled participants.
2. Registry overview
The CDC C19VPR is a voluntary registry evaluating participant, pregnancy, and infant outcomes related to pregnancies during which a primary series COVID-19 vaccine was received. Participants were enrolled beginning in January 2021 and initial data collection (Phase 1) ended in August 2022. Extended follow-up (Phase 2) among participants who expressed interest in continued participation began in November 2022. This paper focuses on the methods for Phase 1, which are largely applicable to Phase 2. The registry aimed to monitor a broad range of pregnancy outcomes (e.g., spontaneous abortion, stillbirth, preterm birth), infant outcomes (e.g., infant death and infant medical conditions, including birth defects) and pregnancy-related conditions (e.g., hypertensive disorders of pregnancy). Fig. 1 illustrates the different components of the registry. In brief, interview data were collected through standardized, structured phone interviews by trained interviewers. Medical records, selectively requested, were abstracted, and reviewed by clinicians to classify and/or validate participant-reported outcomes (e.g., stillbirths, hypertensive disorders of pregnancy, birth defects). Due to the complexity of birth defect classification criteria that often require consideration of nuanced details (e.g., anatomic location, timing of diagnosis, treatment), fetal and infant health data (both participant-reported and medical record) underwent a multi-step clinical review process to systematically identify and classify birth defects. Data were protected through strict safety measures.1 Registry operating procedures were refined over time as experience was gained in the emergency response setting; methods described reflect those used for the majority of the registry cohort unless otherwise specified. This activity was reviewed by the CDC, conducted consistent with applicable federal law and CDC policy, and met requirements of public health surveillance as defined in 45 CFR 46.102(I)(2). An Assurance of Confidentiality was obtained to protect sensitive and potentially identifiable participant information [16].
Fig. 1.
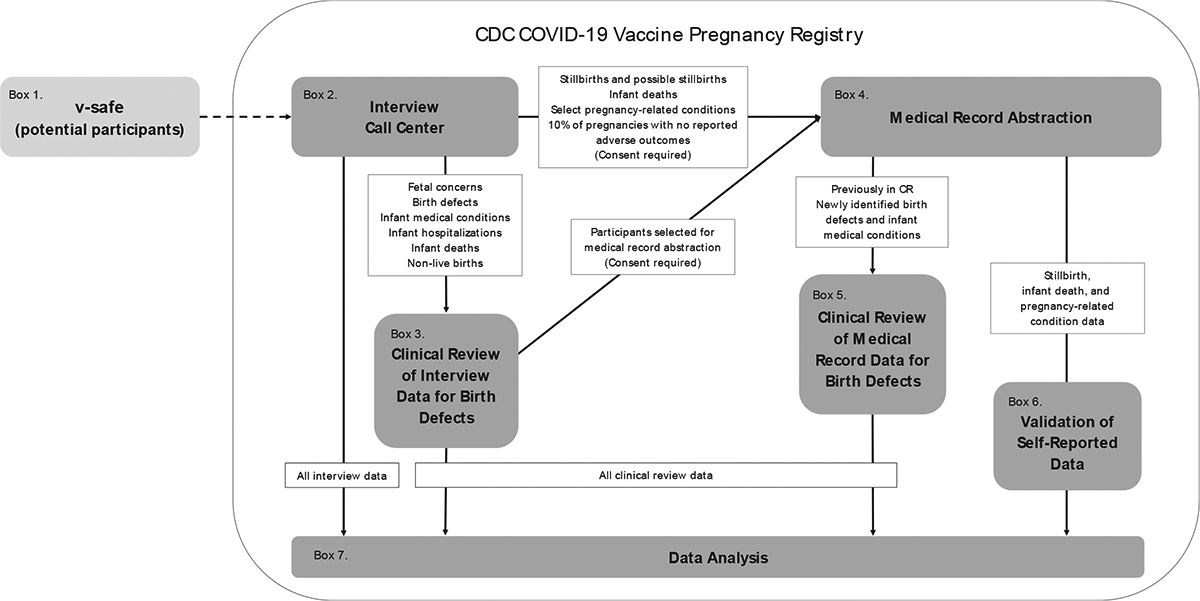
CDC COVID-19 Vaccine Pregnancy Registry Data System Components and Data Flow.
3. Methods
3.1. Identifying potential participants (Fig. 1, Box 1)
V-safe is a voluntary, smart-phone based active surveillance system developed by CDC during the COVID-19 pandemic to monitor for adverse events after receipt of COVID-19 vaccines [3]. V-safe sends enrolled participants surveys at scheduled intervals following vaccination to screen for adverse health effects. From December 15, 2020 through June 20, 2021, 123,609 v-safe participants, ages 18–54 years, who indicated that they were “female,” “other,” or preferred not to say were asked about their pregnancy status after each reported COVID-19 vaccination; those reporting a positive pregnancy test were identified as potential registry participants and were subsequently contacted, to screen for eligibility.
Enrollment in the registry began January 11, 2021. A total of 123,609 potential participants were contacted. Table 1 details initial contact disposition of potential participants. Initially, potential participants were called by CDC emergency response staff. In May 2021, a Pregnancy Follow-up Survey (PFUS) was sent through v-safe to confirm pregnancy and to better identify likely participants among potential participants who had not yet been reached; potential participants who indicated that they were not pregnant around the time of vaccination or were not interested in participating in the registry were removed from the list of potential participants. Beginning in July 2021, a contractor conducted the remaining participant interviews. In total, 65,076 potential participants were contacted via phone to assess eligibility.
Table 1.
Initial contact disposition among potential CDC COVID-19 Vaccine Pregnancy Registry participants, by manufacturer of first COVID-19 vaccine dose received, January 2021–August 2022 (N = 123,609).
| Pfizer-BioNTech | Moderna | Janssen | Unknown* | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Pregnancies reported to v-safe** | 66,509 | (53.8) | 47,192 | (38.2) | 4,180 | (3.4) | 5,728 | (4.6) | 123,609 | (100.0) |
| Originally contacted by CDC | 3,816 | (49.6) | 3,444 | (44.7) | 441 | (5.7) | 0 | (0.0) | 7,701 | (6.2) |
| Sent pregnancy follow-up survey (PFUS) | 62,693 | (54.1) | 43,748 | (37.7) | 3,739 | (3.2) | 5,728 | (4.9) | 115,908 | (93.8) |
| No response | 19,957 | (53.7) | 15,176 | (40.8) | 1,398 | (3.8) | 635 | (1.7) | 37,166 | (32.1) |
| Responded “Never pregnant” | 4,126 | (31.1) | 4,188 | (31.6) | 277 | (2.1) | 4,668 | (35.2) | 13,259 | (11.4) |
| Responded “Pregnant” | 38,610 | (59.0) | 24,384 | (37.2) | 2,064 | (3.2) | 425 | (0.6) | 65,483 | (56.5) |
| No permission to contact | 4,247 | (52.4) | 3,167 | (39.1) | 275 | (3.4) | 418 | (5.2) | 8,107 | (12.4) |
| Permission to contact | 34,363 | (59.9) | 21,217 | (37.0) | 1,789 | (3.1) | 7 | (0.0) | 57,376 | (87.6) |
| Total participants called*** | 38,179 | (58.7) | 24,660 | (37.9) | 2,230 | (3.4) | 7 | (0.0) | 65,076 | (52.6) |
5,728 potential participants did not report vaccine manufacturer to v-safe. Though this information could have been collected through CDC C19VPR, these potential participants were sent the PFUS but (a) did not respond (n = 635), (b) responded that they were never pregnant (n = 4,668), (c) responded that they were pregnant but did not give permission to be contacted (n = 418), or (d) gave permission to be contacted but were unreachable (n = 7).
Row percents are presented for manufacturer of first dose among “pregnancies reported to v-safe” and “total participants called.” All other percents represent the column percent describing each subgroup.
Contacted by either CDC or Abt after the PFUS survey.
3.2. Eligibility (Fig. 1, Box 2)
Potential participants were asked to confirm their identity and provide verbal consent for participation in the registry. Those who did not confirm date of birth or the vaccination dates they provided to v-safe were excluded due to inability to verify their identity (Table 2). Interviewers determined eligibility by assessing pregnancy timing relative to vaccine timing (Table 2). Potential participants who did not speak English or Spanish were excluded due to resource limitations.
Table 2.
Eligibility of called potential CDC COVID-19 Vaccine Pregnancy Registry participants by manufacturer of first COVID-19 vaccine dose received, January 2021 – August 2022 (N = 65,076)*.
| Pfizer-BioNTech | Moderna | Janssen | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| (N = 38,179) | (N = 24,660) | (N = 2,230) | (N = 65,076) | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Unreachable | 23,093 | (35.5) | 14,409 | (22.1) | 1,446 | (2.2) | 38,955 | (59.9) |
| Reached | 15,086 | (23.5) | 10,251 | (15.7) | 784 | (1.2) | 26,121 | (40.1) |
| Refused participation | 761 | (5.0) | 467 | (4.6) | 29 | (3.7) | 1,257 | (4.8) |
| Not eligible** | 725 | (4.8) | 654 | (6.4) | 24 | (3.1) | 1,403 | (5.4) |
| Eligible | 13,600 | (90.1) | 9,130 | (89.1) | 731 | (93.2) | 23,461 | (89.8) |
| Declined enrollment | 63 | (0.5) | 45 | (0.5) | 7 | (1.0) | 115 | (0.5) |
| Eligible and enrolled | 13,537 | (99.5) | 9,085 | (99.5) | 724 | (99.0) | 23,339 | (99.5) |
| Withdrew | 28 | (0.4) | 26 | (0.3) | 5 | (0.7) | 59 | (0.3) |
| Lost to follow-up | 342 | (2.5) | 299 | (3.3) | 75 | (10.4) | 716 | (3.1) |
| Analytically ineligible*** | 44 | (0.3) | 45 | (0.5) | 1 | (0.1) | 90 | (0.4) |
| Total analytically eligible† | 13,491 | (99.7) | 9,035 | (99.5) | 723 | (99.9) | 23,249†† | (99.6) |
Potential participants called included those who were initially called by CDC or provided consent for contact in response to the Pregnancy Follow-up Survey (PFUS) to assess willingness to participate. Potential participants with unknown manufacturer of first dose (n = 7) were all unreachable and are included in the overall column, though there is no distinct column for participants with unknown manufacturer of first dose.
Includes reached potential participants who were ineligible based on timing of vaccination, did not provide enough information to determine eligibility, or spoke a language other than English or Spanish.
Deemed analytically ineligible based on gestational age at pregnancy outcome and date of outcome relative to timing of vaccination.
Analytically eligible is not mutally exclusive from participants who withdrew or were lost to follow-up, but excludes those who withdrew and requested data exclusion (n = 7) and those who were deemed analytically ineligible.
23,249 participants reported and contributed data on a total of 23,265 eligible pregnancies to the registry.
Prior to eligibility screening, gestational age at time of registry-eligible vaccination was unknown, as this information was not collected in v-safe. Potential participants who received at least one primary series COVID-19 vaccination available in the United States under EUA (i.e., Pfizer-BioNTech, Moderna, or Janssen) between the 30th day prior to their last menstrual period (LMP) and the end of their pregnancy were eligible to participate in the registry. A primary series dose was defined as the first or second dose of an original monovalent mRNA vaccine (i.e., Moderna or Pfizer-BioNTech) or the first dose of the Janssen vaccine. Menstrual cycles are variable and date of LMP is often unknown or estimated [17]. Thus, eligibility was based on timing of vaccination relative to participant-reported LMP or LMP calculated from participant-reported estimated due date (EDD).
Participants could report more than one pregnancy around the time of vaccination (e.g., a pregnancy at the time of first vaccination ending in spontaneous abortion followed by a subsequent pregnancy at the time of second vaccination). Eligibility was determined independently for each pregnancy and a separate record was created for each eligible pregnancy. Potential participants were called six times at least 3–4 days apart at varying times before they were considered unreachable. After initial contact, potential participants could have one of the following mutually exclusive dispositions: (a) eligible and enrolled, (b) eligible but declined enrollment, (c) not eligible, or (d) refused participation before eligibility was determined.
3.3. Interview (Fig. 1, Box 2)
Interview forms were developed to be administered by phone at multiple timepoints: once during each trimester following enrollment (1 to 3 interviews), approximately 4–8 weeks after end of pregnancy (1 interview), and, for livebirths, when the infant was at least three months old (1 interview) (Fig. 2). At each interview before the EDD, participants were asked whether they were still pregnant and, based on their response, applicable interview forms were completed.
Fig. 2.
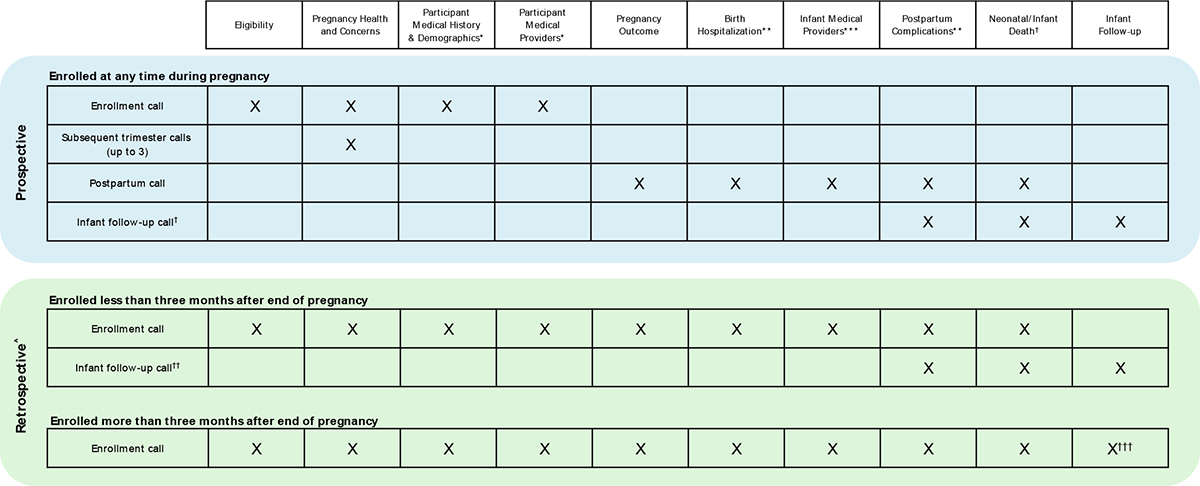
Data collection forms completed at each interview for Phase 1 of the CDC COVID-19 Vaccine Pregnancy Registry, by timing of enrollment.
* Participant refers to the pregnant person enrolled into the COVID-19 Vaccine Pregnancy Registry through v-safe.
** Completed only for stillbirths and live births.
*** Completed only for live births.
† Completed only for live births where participant reported that the infant is no longer living.
†† Call completed only for live births with infant still living at last contact.
††† Form completed only for live births with infant still living.
^ Only one interview was possible for retrospectively enrolled participants if at the time of the initial call the pregnancy did not result in a live birth, or the live-born infant had died, or the infant was older than three months of age. A maximum of two interviews were possible if the part icipant had a live birth and their infant was younger than three months old at the time of the initial call
When the registry began contacting potential participants, enrollment was predominantly prior to pregnancy outcome; these participants were considered “prospective participants.” As the volume of potentially eligible participants identified by v-safe increased, many participants’ pregnancies had already ended by the time they were contacted for enrollment, making data collection retrospective relative to pregnancy outcome (“retrospective participants”). Among retrospective participants, median weeks since end of pregnancy was 26.4 (IQR = 20.9 weeks), regardless of outcome (data not shown). Duration of the enrollment call was considerably longer (20–40 min) for retrospective participants compared with prospective participants (15–20 min), as retrospective data collection required completion of more forms during each interview (Fig. 2).
Research Electronic Data Capture (REDCap) (Version 13.1.14, Vanderbilt University 2023) was used to create a series of forms and serve as an interactive structured script and data entry platform for interviewers. Branching logic was used to ensure questions were appropriate based on participants’ previous responses. Prior to beginning each interview, interviewers verified the participant’s identity, and participants were reminded that participation was voluntary. Question domains were separated into the following eight REDCap forms:
Eligibility
Participant medical history and demographics
Pregnancy health and concerns
Pregnancy outcome2
Birth hospitalization
Participant postpartum health
Infant follow-up2
Neonatal/infant death2
For each interview following the enrollment call, up to four call attempts were made. If contact was made with the participant but they could not complete the interview at that time, the number of call attempts was reset to maximize participation. Text messaging was used to notify participants the day before first call attempts, scheduled callbacks, and final call attempts. Prospective participants were considered lost to follow-up (LTFU) if the participant was unreachable at two consecutive interview points (e.g., 2nd trimester and 3rd trimester interviews); no additional calls were made. Eligible retrospective participants were considered LTFU if they did not complete at least two forms and were then unable to be reached again.
Interviewers requested participants’ verbal consent3 to obtain medical records for the eligible pregnancy (prenatal through six weeks post-pregnancy) and, among live births, for the infant (birth through three months of life). If consent was given, contact information was collected for participants’ prenatal care providers and delivery facilities, and for infants’ primary care providers.
Participants who requested to be withdrawn from the registry after enrollment were given the option to have their data excluded from future analyses; no additional call attempts were made. Participants who were LTFU or withdrew from the registry and did not request data exclusion contributed partial data. All Phase 1 interviews were completed by July 26, 2022.
There were limitations to the registry’s interview methodology. Given the initial paucity of safety data about COVID-19 vaccines among pregnant people, the registry sought to gather information about a broad range of participant, pregnancy, and infant outcomes by asking general questions about specific conditions (e.g., “Was your baby diagnosed with any medical conditions for which he or she sees a healthcare provider?” rather than “Was your baby diagnosed with [a specific condition]?”). Thus, conditions may have gone unreported, possibly because the condition had resolved, or the participant did not recall it or believe it to be serious enough to warrant reporting. Some participants reported complicated health information, which may not have been comprehensively described by the participant, recorded by the interviewer, or entirely captured in a standardized, structured interview form. Weekly data cleaning and quality assessments were used to identify challenges and improve the interview process through additional training and interview updates.
3.4. Clinical review of interview data for birth defects (Fig. 1, Box 3)
Given the complexity of birth defect identification, interview data were reviewed by clinicians in a multi-step process (“clinical review”) to determine presence of birth defects among fetuses or infants. Participants reporting any of the following were selected for clinical review of interview data for birth defects:
Concern for a birth defect at any gestational age
Pregnancy loss ≥ 14 weeks’ gestation
Induced abortion
Infant medical condition
Infant referral to a clinical specialist
Infant hospital admission, not including birth hospitalization
Infant death
Interview data were exported from REDCap into a Microsoft Access database, referred to as V-POINT (Vaccination in Pregnancy of Interest Navigation Tool), designed to facilitate clinical review for the registry. Each fetal or infant condition reported was classified as (a) a major birth defect, (b) a possible/probable birth defect, (c) a minor birth defect, or (d) not a birth defect the criteria established in the 2021 Metropolitan Atlanta Congenital Defects Program (MACDP) Code Defect List as a guide [18]. MACDP is a population-based active surveillance system established by the CDC, Emory University, and the Georgia Mental Health Institute that ascertains structural and chromosomal anomalies among live births, stillbirths, and induced abortions. MACDP was developed to be applied to medical record data [18,19]; therefore, CDC birth defect experts established additional considerations for several birth defects for classification of registry participant-reported data. All conditions classified as a major, possible/probable, or minor birth defect were assigned an MACDP code. Classifications were based on certainty of the description provided by the participant, treatment, specialist referrals, and timing of diagnosis (e.g., prenatal versus postnatal, age of sign/symptom onset).
Data for each participant were reviewed by two clinicians. Discrepancies between clinicians’ classifications were discussed by the reviewing clinicians and resolved. Unresolved discrepancies and nonspecific or unclear descriptions were reviewed by birth defect experts; classifications were modified as necessary. Medical records were requested when additional information was required to determine more accurate classification or specific coding of a condition.
Limitations of clinical review included reliance on participant-reported information and interviewer documentation; interview data was often missing details pertinent to birth defect classification. Participant-report of birth defects is not standard methodology used by birth defect surveillance programs [18,20]. While the process to develop and refine criteria for registry purposes was iterative, protocols and procedures were implemented to ensure consistent coding and classification. Nuanced details, such as whether a prenatally detected condition resolved during pregnancy or was still present after birth, were often not reported, resulting in possible/probable classifications that required medical records or additional participant follow-up for adjudication.
3.5. Medical record abstraction (Fig. 1, Box 4)
Medical records were requested and abstracted for select participants and infants to validate and classify priority participant-reported conditions and outcomes. Among participants who consented to medical record release for themselves and/or their infants, medical records were requested and abstracted for participants who reported any of the following:
Pregnancy loss ≥ 14 weeks’ gestation
Neonatal or infant death
Hypertensive disorder during pregnancy, delivery, or postpartum
Participant admission into the intensive care unit during birth hospitalization
Fetal/infant conditions, including birth defects Participant records were requested for the pregnancy of interest through six weeks after the pregnancy ended, and infant records were requested for birth through three months of age. Requests for medical records were sent to each facility/provider once a week up to four times before records were considered unobtainable. Requests for medical records began in August 2021. As of November 2023, records were requested for 4,351 participants and 3,816 infants; of these, medical records were obtained for 84.8 % and 73.4 %, respectively. Requested records were unobtainable for various reasons: incorrect clinical facility/provider information, inability of facility to verify participant as their patient, or lack of response from the facility. For participants whose medical records could not be obtained and who reported a complex outcome (e.g., pregnancy loss ≥ 17 weeks, infant death), CDC clinicians called the provider to verbally verify the reported outcome.
All medical record abstractors had a clinical background (e.g., physicians and nurses) and received standardized training on review and abstraction of data from medical records. Abstracted conditions were identified through provider diagnoses noted in the records and not solely ICD-10 codes, as ICD-10 codes are not always reflective of a confirmed diagnosis or specific condition. Abstractors followed a comprehensive protocol that contained explicit abstraction instructions for each variable. The following information was abstracted and entered into REDCap:
Types of records received
Participant medical history
Family history of birth defects
Medication use during pregnancy
Substance use during pregnancy
Obstetrical information
Gestational conditions
Fetal imaging
Genetic testing
Pregnancy outcome
Participant hospitalization or ICU admission
Delivery and postpartum complications
NICU admission
Placental pathology
Anthropometric measurements
Infant medical conditions and infections
Infant referrals and consultations
Infant hospitalization
Infant radiology reports
Autopsy reports
REDCap was configured to notify abstractors of potential data entry errors, and quality control assessments were performed weekly. To evaluate accuracy and completeness of abstractions, up to 25 % of medical recordswere randomly selected for re-abstraction. Discrepancies were reconciled and used to inform clarifications to abstraction guidance. Discrepancy rates will be published within specific analyses to reflect how accurate medical record abstraction was by topic due to the breadth of data abstracted.
Medical record data are considered the gold standard for classifying and confirming pregnancy outcomes and medical conditions [21,22]. For the registry, abstraction was performed manually, making it time-consuming and resource intensive. There are variations in the structure and format of medical records received from thousands of clinics and hospitals nationally, with many facilities using different versions of electronic health records; some records were handwritten, making them difficult to read, and some records contained inconsistent information within the record related to the outcome of interest. Reliance on facilities and providers to send medical records also resulted in challenges to timely abstraction. Another limitation was the incompleteness of obtained medical records for an entire pregnancydue to the multiple clinical facilities involved in prenatal, delivery, and infant care, potentially limiting understanding of the full clinical context of conditions that required classification. Abstractors were instructed to avoid using clinical judgement in abstraction of diagnoses; however, a degree of subjectivity was sometimes unavoidable, especially in cases where there was inconsistent information within medical records. Medical record acquisition and abstraction are still in progress at the time of publication, with completion expected in 2024.
3.6. Clinical review of medical record data for birth defects (Fig. 1, Box 5)
Additional clinical review for birth defects was conducted using medical record data. Clinicians reviewed medical record data to confirm or update the interview-based classification of potential birth defects for the following: (1) conditions identified through clinical review of interview data for which medical records were abstracted (2) conditions newly identified during medical record abstraction. Conditions were not re-reviewed if medical records could not be obtained or if interview data alone were sufficient for birth defect classification and medical records were not requested. The same protocol for classifying birth defects, as described above, was utilized during clinical reviews based on medical records.
If a condition was reported by the participant, and medical records could not confirm or rule out whether the condition was a birth defect, classification and coding based on interview data alone were retained. Similarly, if medical records were not available, final classification was based on interview data alone. Clinical review based on medical record data was limited by availability, completeness, and quality of medical records.
3.7. Validation of participant-reported data (Fig. 1, Box 6)
For select outcomes of interest that have very specific clinical diagnostic criteria and, therefore, may be less accurately reported by patients (e.g., stillbirths and hypertensive disorders of pregnancy), participant-reported outcomes are being compared to medical record data, if available. Validation efforts remain in process as of November 2023, as medical records are still being acquired and abstracted. For example, to characterize stillbirths accurately based on clinical definitions, medical records were requested for participants who reported their pregnancy outcome as any of the following: (a) a “stillbirth” at any gestational age, (b) a “miscarriage” at or after 17 weeks’ gestation, or (c) a “live birth” with an infant death within 1 day of delivery. Clinicians used the National Center for Health Statistics and American College of Obstetrics and Gynecology’s definition of stillbirth [23] (fetal loss at ≥20 weeks’ gestation) and data found in medical records (e.g., APGAR scores, presence of heartbeat) to verify whether the participant-reported outcome aligned with the outcome documented in medical records and correctly classify the outcome. For other outcomes, such as hypertensive disorders of pregnancy, medical record data will be used to validate and understand the quality of participant-reported data. If a medical condition or pregnancy outcome reported by the participant could not be confirmed or ruled out through available medical records, or in select cases from healthcare providers’ verbal confirmation, classification and coding based on interview data alone was retained.
3.8. Data analysis (Fig. 1, Box 7)
Data analysis is ongoing. During data collection, the registry regularly monitored outcomes of interest (e.g., spontaneous abortion, stillbirth, birth defects, neonatal death) by comparing data from the enrolled cohort to published background rates [4,14,24]. Background rates were used to assess whether reported incidences of outcomes in this vaccinated cohort exceeded reported incidences in unvaccinated cohorts, both before and during the COVID-19 pandemic [25–28].
4. Cohort description and response rates
Among the 65,076 potential participants contacted via phone to assess eligibility, 38,955 (59.9 %) were unreachable, resulting in a response rate of 40.1 % (Table 2). Among the 26,121 potential participants reached by phone, 23,461 (89.8 %) were eligible and enrolled, 1,403 (5.4 %) were not eligible, and 1,257 (4.8 %) refused participation (Table 2). The participation rate among those who were reached was 95.2 % (24,867/26,121), including potential participants who were eligible and enrolled and potential participants who were not eligible but participated to the maximum extent possible. Among eligible and enrolled participants, 59 (0.3 %) withdrew from participation and 717 (3.1 %) were lost to follow-up. Although participants were deemed eligible during the initial call based on LMP (participant-reported or estimated from participant-reported EDD), these dates may not always be a reliable indicator of the beginning of a pregnancy. For example, fetal measurements during early ultrasounds sometimes indicate a gestational age that is inconsistent with LMP, resulting in updated pregnancy dating. Therefore, we compared an updated calculated LMP4 and LMP from the initial call and found that 90 (0.4 %) participants received their registry-eligible vaccination more than 30 days prior to the updated, calculated LMP. Although these participants were interviewed for the registry, they were later deemed ineligible for analysis (Table 2).
Though potential participants were identified through v-safe, the CDC C19VPR participants were not a representative sample of v-safe participants overall, as almost half of v-safe participants were 50 years of age or older [3]) CDC C19VPR participants were largely non-Hispanic White (79.1 %), aged 30 through 34 years (48.3 %), and resided in an urban area (93.3 %) (Fig. 3). Participant residences included all 50 states, Washington D.C., and Puerto Rico (data not shown). When asked if they were part of a vaccination priority group, a large proportion of the cohort (44.8 %) identified as healthcare personnel (data not shown), likely due to the coincidence of the registry enrollment period and the phased roll-out of the vaccination program. Among 23,249 analytically eligible participants, 16 contributed two eligible pregnancies, resulting in 23,265 pregnancies available for analysis. For the majority of pregnancies, participants received an mRNA vaccine as their first dose (i.e., Moderna or Pfizer-BioNTech) (96.9 %), completed the primary series of vaccination by the end of pregnancy (90.1 %), and received two doses of a COVID-19 vaccine in pregnancy (78.5 %) (Table 3. Overall, 85.3 % of participants consented to medical record release for themselves, their infants, or both; consent was obtained for 84.4 % of participants and 79.3 % of live born infants. All analyses were conducted using SAS (version 9.4; SAS Institute).
Fig. 3.
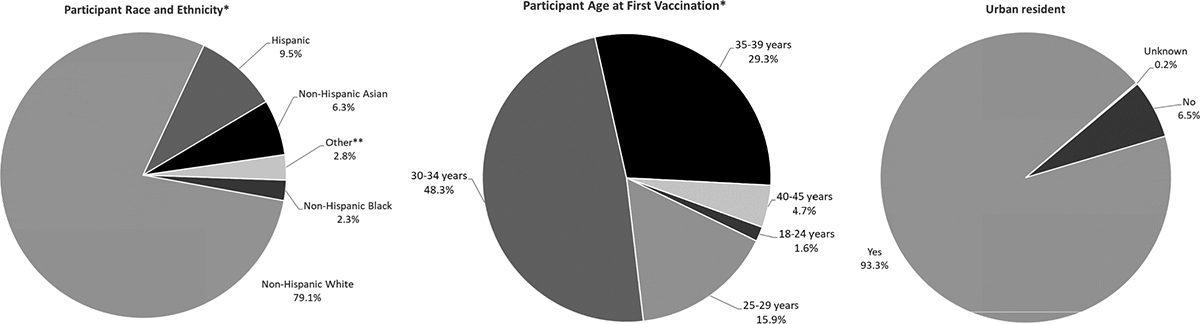
Demographic distribution among participants eligible and enrolled in the CDC COVID-19 Vaccine Pregnancy Registry, January 2021 – August 2022.
* Participant refers to the pregnant person enrolled into the COVID-19 Vaccine Pregnancy Registry through v-safe.
** Other includes non-Hispanic (NH) American Indian or Alaskan Native (0.2%), NH Native Hawaiian or Pacific Islander (0.1%), NH Multi-racial (2.4%), and Unknown (0.2%)
Table 3.
Vaccine manufacturer and number of COVID-19 vaccine doses received between last menstrual period (LMP) and pregnancy end for each pregnancy (N = 23,265) reported by CDC COVID-19 Vaccine Pregnancy Registry participants, January 2021 – August 2022 (N = 23,249)*.
| Manufacturer of first COVID-19 vaccine dose received** | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pfizer-BioNTech N = 13,501 (58.0 %) | Moderna N = 9,040 (38.9 %) | Janssen N = 724 (3.1 %) | Overall N = 23,265 | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| Completed primary series by end of pregnancy*** | ||||||||
| Yes | 12,318 | (91.3) | 7,910 | (87.5) | 724 | (100.0) | 20,952 | (90.1) |
| No | 1,183 | (8.7) | 1,130 | (12.5) | 0 | (0.0) | 2,313 | (9.9) |
| Number of doses reported between LMP and pregnancy end including primary series and booster doses | ||||||||
| One | 1,488 | (11.0) | 1,385 | (15.3) | 687 | (95.0) | 3,560 | (15.3) |
| Two | 11,111 | (82.3) | 7,142 | (79.0) | 13 | (1.8) | 18,266 | (78.5) |
| Three | 613 | (4.5) | 256 | (2.8) | 0 | (0.0) | 869 | (3.7) |
| None† | 289 | (2.1) | 257 | (2.8) | 24 | (3.2) | 570 | (2.5) |
Vaccination through the end of pregnancy or, for participants lost to follow-up, through last report. 16 participants contributed more than one pregnancy; participants may have been fully vaccinated by the end of one pregnancy but not another and may have received a different number of vaccinations in each pregnancy.
Among participants who received a Pfizer-BioNTech or Moderna vaccination for their first dose, 10 participants received a vaccine from a different manufacturer for their second dose.
Primary series doses included doses 1 and 2 for mRNA vaccinations (Pfizer-BioNTech and Moderna) and dose 1 for Janssen vaccinations.
Participants who reported receiving at least one dose in the 30 days prior to their LMP but no doses between LMP and pregnancy end.
5. Discussion
The identification of potential participants through v-safe, a new active surveillance system developed during the pandemic resulted in a large, national pregnancy registry implemented for a novel vaccine. A key strength of the C19VPR was the ability, through v-safe, to directly engage a large population of pregnant people distributed nationwide shortly after COVID-19 vaccines became available and nearly immediately after people chose to be vaccinated. The registry provided some of the earliest, real-time data used to inform public health and clinical recommendations, which was essential since pregnant people were excluded from pre-authorization clinical trials. As data accrued, the registry routinely evaluated data for potential safety signals and reported the findings [14,15]. The registry continues to provide data used for updates at public ACIP meetings as additional data are obtained. While some vaccine safety surveillance programs, such as VAERS, rely only on reports of adverse events, the registry collects comprehensive data about participant and infant health outcomes among all participants in the cohort. This methodology allows for both identification of potential safety concerns and confirmation of a lack of safety concerns over the course of and after pregnancy. High participation and medical record consent rates as well as the low rate of loss to follow-up indicate that the registry was able to effectively enroll and obtain full participation from the majority of reachable potential participants.
Though participation was high among reachable potential participants, a limitation of the registry was its large number of unreachable potential participants (N = 38,948; 59.9 %). Attempts were made to encourage potential participants to respond, including programming interviewers’ caller IDs and using text message alerts. The low response rate may be due in part to the sensitive nature of pregnancy-related conversations or to the inability of the registry to disclose its full purpose in text notifications. Due to privacy concerns, text communication about the registry did not include mention of pregnancy. The identification of potential C19VPR participants through v-safe resulted in a convenience sample of early vaccine recipients. These characteristics may limit the generalizability of our findings. For example, our cohort is not representative of the U.S. population’s racial and ethnic profile. The voluntary nature of both v-safe and the registry may contribute to biases that are difficult to predict. For example, healthcare workers vaccinated early may represent a healthier population, have greater healthcare access, and may be less likely to experience negative pregnancy-related conditions/outcomes or infant health outcomes and more likely to participate in the registry (healthy vaccinee bias) [29]. Conversely, participants may have been vaccinated early due to underlying medical conditions (e.g., diabetes, heart disease) that may increase the likelihood of negative pregnancy and infant health outcomes. Furthermore, due to the voluntary nature of the registry, pregnant people who experienced adverse outcomes themselves or in their infants may have been more inclined to participate (i.e., selection bias) [30–32]. It is also possible that some potential participants who experienced adverse outcomes (e.g., stillbirth, infant death) chose not to participate due to the difficult nature of the conversation. In addition, participant-reported data may introduce recall and misclassification biases regarding health outcomes (e.g., infant medical condition details, gestational age at key outcomes).
Importantly, the registry does not include a control group of unvaccinated pregnant people as enrollment was based on receipt of COVID-19 vaccine during or shortly before pregnancy. Thus, contextualization of registry results relies on available background rates of outcomes. Most published background rates reflect the pre-pandemic period; however, rates of some perinatal outcomes (e.g., preterm birth, low birthweight, stillbirth) may have changed during the pandemic [25–28].
6. Summary
As COVID-19 vaccines became publicly available, the C19VPR was rapidly developed and implemented in January 2021 to monitor vaccine safety among pregnant people as a part of CDC’s COVID-19 emergency response efforts. The registry continues to monitor the safety of COVID-19 vaccination during pregnancy. Initial information collection for the registry aimed to gather data through three months post-pregnancy; however, some participant and infant health conditions (e.g., cardiomyopathy, birth defects) may be diagnosed beyond that 3-month postpartum period. As of November 2023, Phase 2 interviews were completed; data collection included participant health through 15 months after end of pregnancy, and infant health and COVID-19 vaccination through 15 months of age. Phase 2 clinical review and medical record abstraction are in progress. The unique combination of participant-report, medical record, and clinical review data allows for a comprehensive understanding of the health of participants and their infants. While preliminary results have been reported, analyses of the complete CDC C19VPR cohort are forthcoming.
Experience gained from the CDC C19VPR may inform the development of similar large-scale, post-authorization vaccine safety surveillance systems in the future. Launching v-safe at the same time COVID-19 vaccines became available was paramount to the early identification of pregnant people receiving a COVID-19 vaccine for inclusion in a pregnancy registry. The ability to begin enrolling participants in the registry just two weeks after FDA’s EUA of the initial COVID-19 vaccines and to scale up interview capacity led to rapid acquisition of pregnancy-related data. The registry contributed essential data to fill the initial information void created by the exclusion of pregnant people from pre-authorization clinical trials and informed vaccine guidance for this high-risk population during an unprecedented pandemic and global new vaccine implementation.
Acknowledgements
We would like to thank all the registry participants for their time and willingness to participate in the registry as well as the numerous individuals across CDC and the contracting agencies, Abt Associates, Inc., Chickasaw Nation Industries, Inc., and Lukos, LLC, who contributed substantially to the C19VPR.
Funding sources
This activity did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Interview and medical record data are housed in separate Research Electronic Data Capture (REDCap) projects (Version 10.0.8, Vanderbilt University 2023) in CDC’s secure access management services (SAMS) environment and transferred to the secure server at regularly scheduled intervals to a secure server. REDCap user rights ensured that only approved team members have access to the data, including restricted ability to download Personal Identifiable Information (PII). All medical records were maintained on an encrypted network server with limited, controlled user access within the CDC secure network.
Separate pregnancy outcome, infant follow-up, and neonatal/infant death forms were completed for each fetus or infant in multiple gestation pregnancies.
Verbal consent was obtained, as CDC is a public health authority defined by the Health Insurance Portability and Accountability Act (HIPAA), Standards for Privacy of Individually Identifiable Health Information; Final Rule (Privacy Rule) [45 CFR §164.501]. Pursuant to 45 CFR §164.512(b) of the Privacy Rule, healthcare providers may disclose, without individual authorization, protected health information to public health authorities for the purpose of conducting public health surveillance, investigations, and interventions.
Updated calculated LMP was based on participant-reported gestational age and date of pregnancy outcome where available. If both pregnancy outcome date and gestational age were not available, the most recently reported EDD was used to calculate LMP.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention (CDC).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007;298(18):2155–63. 10.1001/jama.298.18.2155. [DOI] [PubMed] [Google Scholar]
- [2].Tran A, Witek TJ Jr. The emergency use authorization of pharmaceuticals: history and utility during the COVID-19 pandemic. Pharmaceut Med 2021;35(4):203–13. 10.1007/s40290-021-00397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Myers TR, Marquez PL, Gee JM, et al. The v-safe after vaccination health checker: active vaccine safety monitoring during CDC’s COVID-19 pandemic response. Vaccine 2023;41(7):1310–8. 10.1016/j.vaccine.2022.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 2021;384(24):2273–82. 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shook LL, Fallah PN, Silberman JN, Edlow AG. COVID-19 vaccination in pregnancy and lactation: current research and gaps in understanding. Front Cell Infect Microbiol 2021;11:735394. 10.3389/fcimb.2021.735394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].US Food and Drug Administration. Approval Letter - Comirnaty 2021. https://www.fda.gov/media/151710/download.
- [7].US Food and Drug Administration. Approval Letter - SPIKEVAX 2022. https://www.fda.gov/media/155815/download.
- [8].Wyszynski DF, Bhattacharya M, Martinez-Perez O, et al. The COVID-19 vaccines international pregnancy exposure registry (C-VIPER): protocol and methodological considerations. Drug Saf 2023;46(3):297–308. 10.1007/s40264-022-01271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Battarbee AN, Stockwell MS, Varner M, et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020. Am J Perinatol 2022;39(1):75–83. 10.1055/s-0041-1735878. [DOI] [PubMed] [Google Scholar]
- [10].Razzaghi H, Yankey D, Vashist K, et al. COVID-19 vaccination coverage and intent among women aged 18–49 years by pregnancy status, United States, April-November 2021. Vaccine 2022. 10.1016/j.vaccine.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;09(01):370. 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69(44):1641–7. 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP). COVID-19 vaccine safety technical (VaST) work group reports. CDC. Accessed 17 May 2023, https://www.cdc.gov/vaccines/acip/work-groups-vast/index.html. [Google Scholar]
- [15].Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP). CDC. Accessed 17 May 2023, https://www.cdc.gov/vaccines/acip/index.html. [Google Scholar]
- [16].Centers for Disease Control and Prevention. Assurances of confidentiality; 2023. https://www.cdc.gov/os/integrity/confidentiality/index.htm.
- [17].Wegienka G, Baird DD. A comparison of recalled date of last menstrual period with prospectively recorded dates. J Womens Health (Larchmt) 2005;14(3):248–52. 10.1089/jwh.2005.14.248. [DOI] [PubMed] [Google Scholar]
- [18].Centers for Disease Control and Prevention. Metropolitan Atlanta congenital defects program (MACDP). CDC. Accessed 17 May 2023, https://www.cdc.gov/ncbddd/birthdefects/macdp.html. [Google Scholar]
- [19].Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res 2007;79(2):66–93. 10.1002/bdra.20351. [DOI] [PubMed] [Google Scholar]
- [20].National Birth Defects Prevention Network. Birth defects surveillance guidelines. Accessed May 31, 2023; 2023. https://www.nbdpn.org/guidelines.php#NSBDS.
- [21].Anderka M, Mai CT, Romitti PA, et al. Development and implementation of the first national data quality standards for population-based birth defects surveillance programs in the United States. BMC Public Health 2015;15:925. 10.1186/s12889-015-2223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mai CT, Correa A, Kirby RS, Rosenberg D, Petros M, Fagen MC. Assessing the practices of population-based birth defects surveillance programs using the CDC strategic framework, 2012. Public Health Rep 2015;130(6):722–30. 10.1177/003335491513000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Metz TD, Berry RS, Fretts RC, Reddy UM, Turrentine MA. Obstetric care consensus #10: management of stillbirth: (Replaces Practice Bulletin Number 102, March 2009). Am J Obstet Gynecol 2020;222(3):B2–20. 10.1016/j.ajog.2020.01.017. [DOI] [PubMed] [Google Scholar]
- [24].Zauche LH, Wallace B, Smoots AN, et al. Receipt of mRNA COVID-19 vaccines preconception and during pregnancy and risk of self-reported spontaneous abortions, CDC v-safe COVID-19 Vaccine Pregnancy Registry 2020–21. Res Sq 2021. 10.21203/rs.3.rs-798175/v1. [DOI] [Google Scholar]
- [25].Jeong Y, Kim MA. SARS-CoV-2 infection in pregnancy and adverse pregnancy outcomes: a systematic review and meta-analysis. Obstet Gynecol Sci 2023. 10.5468/ogs.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vaccaro C, Mahmoud F, Aboulatta L, Aloud B, Eltonsy S. The impact of COVID-19 first wave national lockdowns on perinatal outcomes: a rapid review and meta-analysis. BMC Pregnancy Childbirth 2021;21(1):676. 10.1186/s12884-021-04156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Caniglia EC, Magosi LE, Zash R, et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol 2021;224(6):615.e1–615. e12. 10.1016/j.ajog.2020.12.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shah PS, Ye XY, Yang J, Campitelli MA. Preterm birth and stillbirth rates during the COVID-19 pandemic: a population-based cohort study. CMAJ 2021;193(30): E1164–72. 10.1503/cmaj.210081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Remschmidt C, Wichmann O, Harder T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC Infect Dis 2015;15:429. 10.1186/s12879-015-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moro PL, Broder K, Zheteyeva Y, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol 2011;205(5):e1–9. 10.1016/j.ajog.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McNeil MM, Li R, Pickering S, Real TM, Smith PJ, Pemberton MR. Who is unlikely to report adverse events after vaccinations to the Vaccine Adverse Event Reporting System (VAERS)? Vaccine 2013;31(24):2673–9. 10.1016/j.vaccine.2013.04.009. [DOI] [PubMed] [Google Scholar]
- [32].Santi Laurini G, Montanaro N, Motola D. Safety of COVID-19 vaccines in pregnancy: a VAERS based analysis. Eur J Clin Pharmacol 2023. 10.1007/s00228-023-03482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


