Abstract
Background:
Hypertensive arteriopathy (HA) and cerebral amyloid angiopathy (CAA) may contribute to the development of mixed cerebral microbleeds (CMBs). Recently, the total small vessel disease (SVD) scores for HA and CAA were proposed, which are determined by a combination of MRI markers to reflect overall severity of these microangiopathies.
Objective:
We investigated whether or not total HA-SVD and CAA-SVD scores could be used to predict overlap of HA and CAA in patients with mixed CMBs.
Methods:
Fifty-three subjects with mixed CMBs were retrospectively analyzed. MRI markers (CMBs, lacunes, perivascular space, white matter hyperintensity [WMH] and cortical superficial siderosis [cSS]) were assessed. The HA-SVD score and CAA-SVD score were obtained for each subject. Anterior or posterior WMH was also assessed using the age-related white matter changes scale.
Results:
The two scores were positively correlated (ρ = 0.449, p < 0.001). The prevalence of lobar dominant CMB distribution (p < 0.001) and lacunes in the centrum semiovale (p < 0.001) and the severity of WMH in the parieto-occipital lobes (p = 0.004) were significantly higher in the high CAA-SVD score group. cSS was found in four patients with high CAA-SVD score who showed lobar-dominant CMB distribution and severe posterior WMH.
Conclusion:
Mixed CMBs are mainly due to HA. Assessing both two scores may predict the overlap of HA and CAA in individuals with mixed CMBs. Patients with a high CAA-SVD score may have some degree of advanced CAA, especially when lobar predominant CMBs, severe posterior WMH, lobar lacunes, or cSS are observed.
Keywords: Cerebral amyloid angiopathy, cerebral small vessel diseases, cognitive decline, hypertension, magnetic resonance imaging
INTRODUCTION
Hypertensive arteriopathy (HA) (also known as arteriolosclerosis, deep perforator arteriopathy, or sporadic non-amyloid microangiopathy) and cerebral amyloid angiopathy (CAA) are two representative forms of cerebral small vessel disease (SVD) [1, 2]. HA is associated with deep/infratentorial (i.e., non-lobar) cerebral microbleeds (CMBs), lacunar infarction, enlargement of the perivascular space in the basal ganglia (BG-PVS), and white matter hyperintensity (WMH) with no predilection for brain region including peri-basal ganglia [3]. On the other hand, CAA is associated with multiple strictly lobar CMBs, cortical superficial siderosis (cSS), enlargement of the PVS in the centrum semiovale (CSO-PVS), and a posterior distribution of WMH [4]. Recently, the concept of a total SVD score reflecting the severity of HA has been proposed (i.e., the HA-SVD score). This score is determined from four major MRI markers of HA; lacunes, CMBs, BG-PVS, and WMH [5,6]. Subsequently, the SVD score for CAA (i.e., the CAA-SVD score) was also proposed; it is determined based on lobar CMBs, cSS, CSO-PVS, and WMH [7].
Although a pathological assessment of CAA is the gold standard for the diagnosis of CAA, probable CAA can be diagnosed noninvasively according to the modified Boston criteria in the setting of cortical hemorrhages including lobar intracerebral hemorrhage (ICH), lobar CMBs, and cSS [8]. However, these CAA markers do not strictly fulfill the modified Boston criteria for probable CAA, if there is even one hemorrhage (ICH/CMB) in the deep brain area. Therefore, mixed (deep with lobar) CMBs are thought to reflect HA [9], but the synergistic effects of HA and CAA may also contribute to the development of mixed CMBs [10,11].
Whether patients with mixed CMBs have advanced HA, CAA, or both remains controversial. Understanding the background microangiopathy has important clinical implication for medical intervention in patients with SVD. Although some studies using amyloid imaging to examine underlying SVD in patients with subcortical vascular cognitive impairment or mixed cerebral hemorrhage (ICH/CMBs) have been reported [10–12], not all institutions are able to conduct amyloid imaging. Therefore, we wondered whether or not the coexistence of HA and CAA in patients with mixed CMBs could be predicted from MRI findings alone.
We hypothesized that the total HA-SVD score and total CAA-SVD score in patients with mixed CMBs could potentially indicate the combined entity of HA and CAA. Furthermore, we asked whether each MRI marker could suggest CAA rather than HA in mixed CMBs patients with high CAA-SVD score. We therefore conducted a retrospective analysis of both the total HA-SVD score and the total CAA-SVD score in patients with mixed CMBs collectively, and characterized the significance of each neuroimaging marker for HA and CAA.
MATERIALS AND METHODS
Study subjects and data collection
We performed a retrospective analysis of our database of 426 patients who underwent cognitive impairment workup using 3-T MRI. All patients were recruited from the Department of Neurology and Memory Clinic of Mie University Hospital between January 2014 and July 2018. The topographical distribution of CMB was evaluated using the microbleed anatomical rating scale (MARS) [13]. We defined case of mixed CMBs in patients with both lobar and deep and/or infratentorial CMBs according to the CMBs distribution by the MARS. Patients with multiple (≥2) strictly lobar CMBs were classified as probable CAA (P-CAA) per the modified Boston criteria [8]. Patients with strictly deep and/or infratentorial CMBs were classified as D-CMBs. P-CAA and D-CMBs were selected as comparator cases. Patients with single lobar CMB (i.e., possible CAA) were excluded because possible CAA carries less diagnostic certainty than probable CAA [8]. Demographic and clinical information were obtained by a review of the medical records. The presence of hypertension, dyslipidemia, and diabetes was determined based on a prior medical diagnosis and treatment among all patients. Smoking was defined by a history of tobacco use.
The study was approved by the ethical review board of Mie University Hospital and the requirement for written informed consent was waived because of the retrospective study design. The present study was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its subsequent amendments.
Neuroimaging data and analyses
The MRI studies were performed with a 3T MR unit (Ingenia, Philips Medical System, Best, The Netherlands). The pulse sequence parameters were as follows. 3D-T1WI: field of view, 260 mm; matrix, 288×288; section thickness, 0.9 mm; TR (ms)/TE (ms) ratio, 7.6 (shortest); TE (shortest), 3.6 ms; flip angle, 10°; and an acquisition time of 4 min 42 s. T2WI: field of view, 220 mm; matrix, 384×345; section thickness, 3.0 mm; gap, 0.5 mm; TR (ms)/TE (ms) ratio, approximately 7,000 (shortest)/90; and acquisition time, 2 min 43 s. 3D-FLAIR: field of view, 250 mm; matrix, 256×184; section thickness, 1 mm with 0.57 mm overlap; no parallel imaging; TR/TE ratio; 6,000/378 (shortest); TI, 2,000 ms; number of signals acquired, 2; and acquisition time, 4 min 42 s. SWI: field of view, 230 mm; matrix, 320×251; section thickness, 0.8 mm over contiguous slices; minIP, 5 mm; repetition time (ms)/echo time (ms) ratio, 22/11.5 (in-phase), 37 (shifted); number of signals acquired, one; flip angle, 20°; and acquisition time, 6 min 42 s.
MRI markers
CMB, WMH, PVS, and lacunes were defined according to the Standards for Reporting Vascular changes on Neuroimaging (STRIVE) consensus [14]. Deep and periventricular WMH were both coded from 0 to 3 according to the Fazekas scale [15]. WMH was also assessed using the age-related white matter changes (ARWMC) scale [16] to investigate the distribution and severity of WMH according to the cerebral lobes because the Fazekas scale does not consider this point. PVS was rated from 0 to 4 on a previously described, validated semiquantitative scale in the basal ganglia and centrum semiovale [17]. Lacunes were classified as deep (basal ganglia, thalamus, internal capsule, and pons) and lobar (centrum semiovale) according to a previous study [18]. cSS was defined as linear residue of chronic blood products in the superficial layers of the cerebral cortex showing a characteristic gyriform pattern of a low signal on SWI [19].
Determining the total HA-SVD score and the CAA-SVD score
The total HA-SVD score was calculated according to the previously described scoring system [5,6]. The presence of one or more lacunes in the basal ganglia, internal capsule, centrum semiovale, or brainstem gave one point on the total HA-SVD score burden. The presence of one or more CMBs in the cerebellum, brainstem, basal ganglia, white matter, or cortico-subcortical junction gave one point as well. One point was awarded for the presence of moderate to severe PVS at the basal ganglia if it was grade 2 to 4. The presence of periventricular WMH extending into the deep white matter (Fazekas score 3) and/or (early) confluent deep WMH (Fazekas score 2 or 3) gave one point on the total HA-SVD score burden. Thus, the total HA-SVD score ranged from 0 to 4.
The CAA-SVD score was also calculated according to a previously described scoring system [7]. Each MRI marker (lobar CMBs, cSS, CSO-PVS, and WMH) was categorized as follows: lobar CMBs (1 point if 2–4 are present; 2 points if 5 or more are present); cSS (1 point if focal; 2 points if disseminated); CSO-PVS (1 point if 20 or more CSO-PVS are present); WMH (1 point if the score on the Fazekas scale is 3 for periventricular WMH and/or scoring 2 or 3 for deep WMH). Both HA-SVD and CAA-SVD scores were obtained from each subject.
For the purpose of the analysis, a total CAA-SVD score of 3 or more was rated as moderate to severe CAA and classified as “high CAA-SVD score”, while a total CAA-SVD score of 2 or less was classified as “low CAA-SVD score”. Similarly, a total HA-SVD score of ≥3 was rated as moderate to severe HA and classified as a “high HA-SVD score”, while a total HA-SVD score of ≤2 was classified as a “low HA-SVD score”.
Statistical analyses
All statistical analyses were performed using the SPSS software program (version 25 for Windows IBM Corp.). The χ2 test or Fisher’s exact test were used for categorical variables. Student’s t-test, the Mann-Whitney U test or the Kruskal-Wallis test with post hoc Bonferroni adjustment were used for continuous variables. Spearman’s correlation was used to analyze the relationship between the HA-SVD score and the CAA-SVD score. p values of < 0.05 were considered to indicate statistical significance in all analyses.
RESULTS
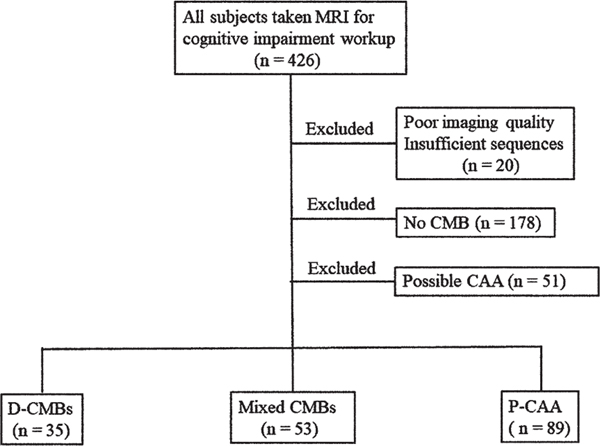
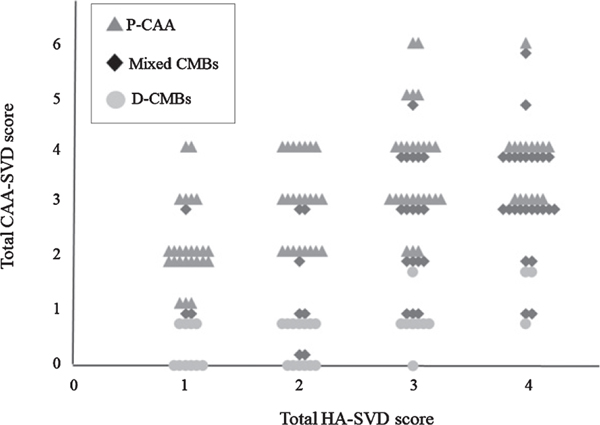
Of the 426 patients, 20 with a poor imaging quality or insufficient sequences and 178 without CMB were excluded. Fifty-one patients with possible CAA were also excluded. Of the remaining 179 patients, 53 with mixed CMBs were included in this analysis. Thirty-five patients with D-CMBs and 89 with P-CAA were selected as comparator cases. A flowchart of the patient selection is shown in Figure 1. The clinical diagnoses of the patients with mixed CMBs were as follows: mild cognitive impairment (MCI), n = 17 (amnestic type, n = 15; non-amnestic type, n = 2); Alzheimer’s disease (AD), n = 20; vascular dementia (VaD), n = 11; and other disorders, n = 5. All diagnoses were based on respective pre-established criteria [20–22]. The clinical characteristics and HA-SVD and CAA-SVD scores among the groups are shown in Table 1. In the scatterplot of the association between the total HA-SVD score and the total CAA-SVD score, the mixed CMBs group showed distribution across both the D-CMBs and P-CAA groups with a positive correlation between these two total scores (ρ = 0.449, p < 0.001) (Fig. 2).
Fig. 1.
Flowchart of patient selection. CAA, cerebral amyloid angiopathy; CMB, cerebral microbleed; D-CMBs, deep and/or infratentorial cerebral microbleeds; P-CAA, probable cerebral amyloid angiopathy.
Table 1.
Characteristics of patients with mixed CMBs, D-CMBs, and P-CAA
| Mixed CMBs (n = 53) | D-CMBs (n = 35) | p | P-CAA (n = 89) | p | |
|---|---|---|---|---|---|
| Age, y, mean±SD | 77.0±8.1 | 72.8±10.5 | 0.040∗ | 78.7±6.8 | 0.165 |
| Male, n (%) | 21 (39.6) | 23 (65.7) | 0.017∗ | 40 (44.9) | 0.538 |
| MMSE, median (IQR) | 24 (19–26) | 25 (22–27) | 0.036∗ | 23 (19–28) | 0.222 |
| Hypertension, n (%) | 36 (67.9) | 22 (62.9) | 0.624 | 51 (57.3) | 0.209 |
| Diabetes mellitus, n (%) | 9 (17.0) | 8 (22.9) | 0.494 | 20 (22.5) | 0.432 |
| Dyslipidemia, n (%) | 22 (41.5) | 11 (31.4) | 0.339 | 23 (25.8) | 0.052 |
| Smoking, n (%) | 14 (26.4) | 11 (31.4) | 0.610 | 19 (21.3) | 0.489 |
| HA-SVD-Score, median (IQR) | |||||
| Lacune | 1 (0–1) | 0 (0–1) | 0.063 | 0 (0–1) | 0.003∗ |
| CMBs | 1 (1–1) | 1 (1–1) | 1 | 1 (1–1) | 1 |
| BG-PVS | 1 (1–1) | 0 (0–1) | <0.001∗ | 0 (0–1) | <0.001∗ |
| WMH | 1 (0–1) | 0 (0–1) | <0.001∗ | 1 (0–1) | 0.185 |
| Total | 3 (3–4) | 2 (1–3) | <0.001∗ | 2 (1–3) | <0.001∗ |
| CAA-SVD-Score, median (IQR) | |||||
| Lobar CMBs | 2 (1–2) | 0 (0–0) | <0.001∗ | 1 (1–2) | 0.376 |
| cSS | 0 (0–0) | 0 (0–0) | 0.098 | 0 (0–0) | 0.303 |
| CSO-PVS | 1 (0–1) | 0 (0–1) | 0.320 | 1 (1–1) | <0.001∗ |
| WMH | 1 (0–1) | 0 (0–1) | <0.001∗ | 1 (0–1) | 0.185 |
| Total | 3 (2–4) | 0 (0–1) | <0.001∗ | 3 (2–4) | 0.396 |
BG, basal ganglia; CMB, cerebral microbleed; CSO, centrum semiovale; cSS, cortical superficial siderosis; D-CMBs, deep and/or infratentorial cerebral microbleeds; HA, hypertensive arteriopathy; IQR, interquartile range; MMSE, Mini-Mental State Examination; P-CAA, probable cerebral amyloid angiopathy; PVS, perivascular space; SD, standard deviation; SVD, small vessel disease; WMH, white matter hyperintensity.The p values reported in the column next to D-CMBs indicate comparisons between the mixed CMBs and D-CMBs groups. The p values reported in the column next to P-CAA indicate comparisons between the mixed CMBs and P-CAA groups.
p < 0.05.
Fig. 2.
Scatterplot of the association between the total HA-SVD score and the total CAA-SVD score. The mixed CMBs group shows distribution across both the D-CMBs and P-CAA groups.
Comparing the mixed CMBs and D-CMBs groups (Table 1)
Compared to the D-CMBs group, the mixed CMBs group was significantly older (p = 0.040) and had a lower male frequency (p = 0.017) and lower MMSE scores (p = 0.047). Regarding the HA-SVD score, the scores for BG-PVS and WMH in the mixed CMBs group were significantly higher than those of the D-CMBs group (BG-PVS, p < 0.001; WMH, p < 0.001). The total HA-SVD score in the mixed CMBs group was also significantly higher than that in the D-CMBs group (p < 0.001). Regarding the CAA-SVD score, the scores for lobar CMB and WMH in the mixed CMBs group were significantly higher than those in the D-CMBs group (lobar CMB, p < 0.001; WMH, p < 0.001). The total CAA-SVD score in the mixed CMBs group was also significantly higher than that in the D-CMBs group (p < 0.001).
Comparing the mixed CMBs and P-CAA groups (Table 1)
There were no significant differences in age, sex, MMSE score, or the prevalence of vascular risk factors between the mixed CMBs and P-CAA groups. Regarding the HA-SVD score, the scores for lacune and BG-PVS in the mixed CMBs group were significantly higher than those in the P-CAA group (lacune, p = 0.003; BG-PVS, p < 0.001). The total HA-SVD score in the mixed CMBs group was also significantly higher than that in the P-CAA group (p < 0.001). Regarding the CAA-SVD score, the score for CSO-PVS in the P-CAA group was significantly higher than that in the mixed CMBs group (p < 0.001). There was no significant difference in the total CAA-SVD score between these two groups (p = 0.396).
Comparing the high and low CAA-SVD score groups in the mixed CMBs group
In the mixed CMBs group, thirty-five patients (66%) showed a total CAA-SVD score of 3 or more (i.e., the high CAA-SVD score group) and 18 showed a score of 2 or less (i.e., the low CAA-SVD score group). The clinical characteristics and imaging findings between the groups are shown in Table 2. The comparison between the high CAA-SVD score and low CAA-SVD score groups revealed no significant differences in demographic characteristics, with the exception of age. The scores for lacunes and WMH in the high CAA-SVD score group were significantly higher than those in the low CAA-SVD score group (lacunes: p = 0.023; WMH: p = 0.013). The high CAA-SVD score group also showed a significantly higher total HA-SVD score than the low CAA-SVD score group (p = 0.003).
Table 2.
Comparison of the low and high CAA-SVD score groups in the mixed CMBs
| low CAA-SVD score (n = 18) | high CAA-SVD score (n = 35) | p | |
|---|---|---|---|
| Age, y, median (IQR) | 81.5 (78.75–83.25) | 75.0 (68.0–81.0) | 0.015∗ |
| Male, n (%) | 6 (33.3) | 15 (42.9) | 0.502 |
| MMSE, median (IQR) | 20 (18–26) | 24 (20–26) | 0.262 |
| Hypertension, n (%) | 12 (66.7) | 24 (68.6) | 0.888 |
| Diabetes mellitus, n (%) | 3 (16.7) | 6 (17.1) | 1 |
| Dyslipidemia, n (%) | 7 (38.9) | 15 (42.9) | 0.781 |
| Smoking, n (%) | 6 (33.3) | 8 (22.9) | 0.515 |
| HA-SVD-Score, median (IQR) | |||
| Lacune | 0 (0–1) | 1 (0–1) | 0.023∗ |
| CMBs | 1 (1–1) | 1 (1–1) | 1 |
| BG-PVS | 1 (1–1) | 1 (1–1) | 0.201 |
| WMH | 0.5 (0–1) | 1 (1–1) | 0.013∗ |
| Total | 3 (2–3.25) | 4 (3–4) | 0.003∗ |
| CAA-SVD-Score, median (IQR) | |||
| Lobar CMBs | 0.5 (0–1) | 2 (2–2) | <0.001∗ |
| cSS | 0 (0–0) | 0 (0–0) | 0.140 |
| CSO-PVS | 0 (0–1) | 1 (0–1) | 0.003∗ |
| WMH | 0.5 (0–1) | 1 (1–1) | 0.013∗ |
| Total | 1 (1–2) | 3 (3–4) | <0.001∗ |
| CMBs distribution | |||
| Deep ≥ Lobar, n (%) | 13 (72.2) | 7 (20.0) | <0.001∗ |
| Lobar > Deep, n (%) | 5 (27.8) | 28 (80.0) | <0.001∗ |
| WMH, ARWMC, median (IQR) | |||
| Frontal | 2 (1–3) | 3 (2–3) | 0.105 |
| Parieto-Occipital | 2 (1–2.25) | 3 (2–3) | 0.004∗ |
| BG | 2.5 (0–3) | 3 (1–3) | 0.648 |
| Lacune positive, n (%) | 7 (38.9) | 25 (71.4) | 0.022∗ |
| Lobar lacune-positive, n (%) | 1 (5.6) | 18 (51.4) | 0.001∗ |
| Deep lacune only, n (%) | 6 (33.3) | 7 (20.0) | 0.326 |
| BG-PVS grade, median (IQR) | 3 (2–3) | 3 (3–4) | 0.051 |
| CSO-PVS grade, median (IQR) | 2 (1–3) | 3 (2–3) | 0.009∗ |
| CSO-PVS > BG-PVS, n (%) | 3 (16.7) | 6 (17.1) | 1 |
| CSO-PVS = BG-PVS, n (%) | 4 (22.2) | 13 (37.1) | 0.270 |
| CSO-PVS < BG-PVS, n (%) | 11 (61.1) | 16 (45.7) | 0.288 |
ARWMC, age-related white matter changes; BG, basal ganglia; CAA, cerebral amyloid angiopathy; CMB, cerebral microbleed; CSO, centrum semiovale; cSS, cortical superficial siderosis; HA, hypertensive arteriopathy; IQR, interquartile range; MMSE, Mini-Mental State Examination; PVS, perivascular space; SVD, small vessel disease; WMH, white matter hyperintensity.
p < 0.05 for low CAA-SVD score versus high CAA-SVD score.
In terms of the CMB distribution, the prevalence of lobar dominant CMB distribution (i.e., more number of lobar CMBs than deep CMBs) was significantly higher in the high CAA-SVD score group (80.0% versus 27.8%, p < 0.001).
The comparison between the anterior and posterior severity of WMH revealed that the severity of the parieto-occipital lobes on the ARWMC score was significantly higher in the high CAA-SVD score group than in the low CAA-SVD score group (p = 0.004); however, the severity of the frontal lobe did not differ between the two groups to a statistically significant extent (p = 0.105) The severity of WMH in the basal ganglia also did not differ markedly between the two groups (p = 0.648).
Thirty-two patients (high CAA-SVD score group, n = 25; low CAA-SVD score group, n = 7) had at least one lacune. Among them, the prevalence of lacunes in the centrum semiovale (i.e., lobar lacunes) was significantly higher in the high CAA-SVD score group than the low CAA-SVD score group (51.4% versus 5.6%, p = 0.001), although the prevalence of exclusively deep lacunes in these two groups did not differ to a statistically significant extent (33.3% versus 20%, p = 0.326).
Although the grade of severity of BG-PVS tended to be higher in the high CAA-SVD score group than in the low CAA-SVD score group (p = 0.051), the BG-PVS scores of the high and low CAA-SVD score groups did not differ to a statistically significant extent (p = 0.201). On the other hand, the grade of severity of the CSO-PVS in the high CAA-SVD score group was significantly greater than that in the low CAA-SVD score group (p = 0.009), and the score of CSO-PVS (p = 0.003). There was no significant difference of PVS degree predominance patterns between high and low CAA-SVD score groups.
Four patients in the high CAA-SVD score group (11.4%) had cSS. In contrast, no patients in the low CAA-SVD score group had cSS. All of the four patients with cSS showed lobar-dominant CMB distribution and the grade of severity of WMH on the ARWMC scale was higher in the parieto-occipital lobes than in the frontal lobe. Three of the four patients had hypertension, and two of them had both deep and lobar lacunes.
Sub-analyses of the WMH distribution
Since the WMH scoring method is the same for both the HA-SVD and CAA-SVD scores, sub-analyses were performed to predict whether HA and CAA are dominant or additive depending on the WMH lesion. We focused on the high HA-SVD score group, in which HA was considered to be more severe than the low HA-SVD score group. In the mixed CMBs group, 43 patients (81%) showed a total HA-SVD score of ≥3 (i.e., the high HA-SVD score group). First, we investigated whether or not there were differences in the ARWMC score in the frontal lobe, parieto-occipital lobe and basal ganglia between the high and low total HA-SVD score groups. The ARWMC scores in each of the 3 regions were significantly higher in the high HA-SVD score group than in the low HA-SVD score group (frontal lobe, p = 0.001; parieto-occipital lobe, p = 0.002; basal ganglia, p = 0.032). These results suggested that the more severe the HA, the more severe WMH. Subsequently, we conducted analyses in the high HA-SVD score group, which included 32 patients with a high CAA-SVD score and 11 with a low CAA-SVD score. The clinical characteristics and imaging findings between the groups are shown in Table 3. There were no significant differences in vascular risk factors or the HA-SVD score between the two groups. However, the scores of lobar CMBs and total CAA-SVD were significantly higher in the high CAA-SVD score group than in the low CAA-SVD score group (each p < 0.001). The ARWMC score in the parieto-occipital lobe was also significantly higher in the high CAA-SVD score group than in the low CAA-SVD score group (p = 0.025), although there was no significant difference in the ARWMC score in the frontal lobe (p = 0.386) and basal ganglia (p = 0.651).
Table 3.
Comparison of the low and high CAA-SVD score groups in the mixed CMBs group with a high HA-SVD score
| low CAA-SVD score (n = 11) | high CAA-SVD score (n = 32) | p | |
|---|---|---|---|
| Age, y, mean±SD | 81.2±6.1 | 74.8±8.4 | 0.025∗ |
| Male, n (%) | 4 (36.4) | 14 (43.8) | 0.736 |
| MMSE, median (IQR) | 20 (18–23) | 24 (19–26) | 0.356 |
| Hypertension, n (%) | 5 (45.5) | 14 (43.8) | 1 |
| Diabetes mellitus, n (%) | 2 (18.2) | 5 (15.6) | 1 |
| Dyslipidemia, n (%) | 7 (38.9) | 15 (42.9) | 1 |
| Smoking, n (%) | 3 (27.3) | 7 (21.9) | 0.698 |
| HA-SVD-Score, median (IQR) | |||
| Lacune | 0 (0–1) | 1 (1–1) | 0.483 |
| CMBs | 1 (1–1) | 1 (1–1) | 1 |
| BG-PVS | 1 (1–1) | 1 (1–1) | 0.880 |
| WMH | 1 (0–1) | 1 (1–1) | 0.386 |
| Total | 3 (3–4) | 4 (3–4) | 0.154 |
| CAA-SVD-Score, median (IQR) | |||
| Lobar CMBs | 0 (0–1) | 2 (2–2) | <0.001∗ |
| cSS | 0 (0–0) | 0 (0–0) | 0.555 |
| CSO-PVS | 0 (0–1) | 1 (0–1) | 0.117 |
| WMH | 1 (0–1) | 1 (1–1) | 0.386 |
| Total | 2 (1–2) | 4 (3–4) | <0.001∗ |
BG, basal ganglia; CAA, cerebral amyloid angiopathy; CMB, cerebral microbleed; CSO, centrum semiovale; cSS, cortical superficial siderosis; HA, hypertensive arteriopathy; IQR, interquartile range; MMSE, Mini-Mental State Examination; PVS, perivascular space; SD, standard deviation; SVD, small vessel disease; WMH, white matter hyperintensity.
p < 0.05 for low CAA-SVD score versus high CAA-SVD score.
Comparing both SVD scores between the MCI, AD, and VaD patients in the mixed CMBs group
There was no significant difference in the total HA-SVD or CAA-SVD scores between MCI, AD, and VaD (total HA-SVD score, p = 0.286; total CAA-SVD score, p = 0.248).
DISCUSSION
Our study showed that the mixed CMBs group had a significantly higher total HA-SVD score than the P-CAA and D-CMBs groups. In contrast, the total CAA-SVD score of the mixed CMBs group was significantly higher than that of the D-CMBs group but was not significantly different from that of the P-CAA group. In addition, mixed CMBs patients with high CAA-SVD score showed characteristic MRI findings suggesting CAA. Unfortunately, a major limitation of this study was the lack of the neuropathological confirmation of each score; however, our results suggest that the background SVD of mixed CMBs is mainly advanced HA, but in some cases, coexistence with CAA was suspected, especially when the CAA-SVD score was high.
Histopathological studies in human brain on CAA have shown the coexistence of HA, in which evidence of HA was found in 48% to 75% of autopsy-proven CAA patients, especially in cases of moderate to severe CAA [7,23]. However, autopsy studies of dementia patients have also shown the coexistence of HA and CAA in 26% to 70%, with these two microangiopathies being found in a moderate to severe extent. [24–26]. Thus, HA and CAA are independent processes but often coexist in the brain of subjects with cognitive impairment.
Whether HA and CAA simply coexist or have a causal relationship remains unclear. However, some hypotheses concerning their relationship have been proposed. The apolipoprotein E (APOE) s4 allele is a genetic risk factor for CAA and HA [4, 27]. It is thus tempting to assume that APOE represents a pathogenic link between HA and CAA [28]. Another possible explanation involves the failure of the perivascular drainage system inducing vascular Aβ deposition [29]. A study using a transgenic mouse model of human CAA found that cerebral arterial changes similar to those seen in aging human arteries occur before the Aβ deposition and CAA formation [30]. Another animal study using spontaneously hypertensive stroke-prone rats recently showed that the triggering and progression of CAA could take place earlier in the presence of significant HA [31]. Although direct observations were made using animal models in these studies, these results suggest that the same effects may occur in humans.
Several comparative studies have been conducted on various MRI markers associated with HA and CAA. Previous studies have shown that the presence of lobar lacunes was associated with CAA, and deep lacunes with HA [18,32]. In our study, the prevalence of lobar lacune was significantly higher in the high CAA-SVD score group than in the low CAA-SVD score group. All patients with lobar lacunes also had deep lacunes together. These findings suggest that the patients with high CAA-SVD score with lobar lacunes may have some degree of CAA along with HA.
In our study, the subjects with a greater number of lobar CMBs in comparison to deep CMBs were prevalent in the high CAA-SVD score group. In accordance with this finding, a previous positron emission topography (PET) study on subcortical vascular cognitive impairment found that amyloid-positive subjects with mixed CMBs had greater number of lobar CMBs than deep CMBs, whereas amyloid-negative subjects with mixed CMBs had more numerous deep CMBs than lobar CMBs [10]. In a three-year longitudinal study by the same research group, the baseline and longitudinal changes in the tracer uptake ratio on amyloid PET were shown to be associated only with the progression of lobar CMBs, suggesting that CAA and HA have synergistic effects on the progression of lobar CMBs [11]. In contrast, a recent study using amyloid PET showed that patients with lobar and deep intracerebral hemorrhage/microbleeds (mixed ICH) have a much lower amyloid load than those with CAA-ICH, but a similar load to those with HTN-ICH [12]. These findings suggest that mixed ICH is probably caused by HA.
A recent cross-sectional study of patients with spontaneous ICH has found that a high degree and predominance of CSO-PVS was more common in CAA-ICH while a high degree and predominance of BG-PVS was more common in hypertensive ICH [33]. Another recent large memory clinic cohort study reported that CSO-PVS was mainly associated with CAA, whereas BG-PVS was mainly associated with HA, and that it is likely that there is a significant overlap between these two SVDs [34]. In the current study, the high CAA-SVD score group tended to have more severe enlarged PVS in the CSO as well as the BG in comparison to the low CAA-SVD score group. Both the high and low CAA-SVD groups similarly showed a predominance in the BG. Therefore, these findings suggest that the mixed CMBs in our study are primarily caused by HA, but that HA and CAA may overlap, especially in subjects with high CAA-SVD score.
In the present study, the ARWMC scale was also assessed because the Fazekas scale could not evaluate the anterior-posterior distribution of WMH. The severity of WMH in the parieto-occipital lobes was significantly higher in the high-CAA-SVD score group than in the low-CAA-SVD score group. Furthermore, our results for the sub-analysis of the WMH distribution suggest that more severe posterior WMH is due to the interaction of HA with CAA, especially in patients with high total HA-SVD and CAA-SVD scores. Occipital predominance of WMH has been observed in CAA patients with ICH [35,36]. A previous study has suggested that the posterior distribution of WMH is an independent predictor of pathologically confirmed CAA, even in the absence of lobar hemorrhages [37]. As these previous studies pointed out, a high CAA-SVD score with a posterior distribution of WMH may indicate a higher probability of CAA pathology.
cSS has been thought to be a potential neuroimaging marker for advanced CAA [19]. In the present study, all four patients with cSS showed characteristic MRI findings suggesting CAA, including predominantly lobar CMBs and the posterior distribution of WMH. These findings strongly indicated that patients with mixed CMBs with cSS may have advanced CAA as an underlying SVD.
In the present study, there was no significant difference in the total HA-SVD or CAA-SVD scores between MCI, AD, and VaD. In elderly patients in particular, there are pathological conditions in which AD pathology and cerebral small vessel pathology overlap, and it has been pointed out that AD and VaD fall on a continuous disease spectrum [38]. The present findings may thus be due to limited statistical power with such a small number of cases, and it may also reflect the continuous spectrum mentioned above due to the coexistence of HA and CAA in our subjects with mixed CMBs.
The present study was associated with some limitations. First, the current study was retrospective in nature. Second, the sample size was relatively small and the statistical power was therefore limited. Third, we did not examine potential biomarkers for vascular amyloid deposition, including amyloid-PET and the cerebrospinal fluid Aβ40 and Aβ42 values [39,40]. Fourth, there was no pathological verification. However, in the clinical settings, the results of our study may be helpful for predicting the underling SVDs based on MRI findings alone in cognitive decline patients who have mixed CMBs.
In conclusion, assessing both the HA-SVD and CAA-SVD scores may help predict the overlap of HA and CAA in individuals with mixed CMBs. Mixed CMBs are thought to be primarily caused by advanced HA. However, patients with mixed CMBs with high CAA-SVD score burden could have some degree of advanced CAA, especially when observed with lobar predominant numerous CMBs, WMH with posterior predominance, lobar lacunes, and cSS. Further radiological-histopathological studies are required to verify these results.
ACKNOWLEDGMENTS
We would like to thank Ryota Kogue, Shinichi Takase, and Katsuhiro Inoue in the Department of Radiology for their kind contributions to this study.
This work was funded by grant from JSPS KAK-ENHI (Grant No. JP20K08104).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0992r2).
REFERENCES
- [1].Pantoni L (2010) Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9, 689–701. [DOI] [PubMed] [Google Scholar]
- [2].Charidimou A, Pantoni L, Love S (2016) The concept of sporadic small vessel disease: A road map on key definitions and current concepts. Int J Stroke 11, 6–18. [DOI] [PubMed] [Google Scholar]
- [3].Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rosemller AM, Scheltens P, Geurts JJ (2011) Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82, 126–135. [DOI] [PubMed] [Google Scholar]
- [4].Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, Viswanathan A, Greenberg SM (2017) Emerging concepts in sporadic cerebral amyloid angiopathy. Brain 140, 1829–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Klarenbeek P, van Oostenbrugge RJ, Rouhl RP, Knottnerus IL, Staals J (2013) Ambulatory blood pressure in patients with lacunar stroke: Association with total MRI burden of cerebral small vessel disease. Stroke 44, 2995–2999. [DOI] [PubMed] [Google Scholar]
- [6].Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM (2014) Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83, 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, Frosch M, Vashkvich A, Ayres A, Rosand J, Gurol ME, Greenberg SM, Viswanathan A (2016) Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: An imaging-pathologic study of concept validation. JAMA Neurol 73, 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Greenberg SM, Charidimou A (2018) Diagnosis of cerebral amyloid angiopathy: Evolution of the Boston criteria. Stroke 49, 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, Goldstein JN, Rosand J, Viswanathan A, Pantoni L, Greenberg SM, Gurol ME (2018) Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology 90, e119–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, Kwak KC, Yoon U, Lee JM, Lee JW, Shin JS, Kim CH, Noh Y, Cho H, Kim HJ, Yoon CW, Oh SJ, Kim JS, Choe YS, Lee KH, Lee JH, Ewers M, Weiner MW, Werring DJ, Na DL (2013) Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol 73, 584–593. [DOI] [PubMed] [Google Scholar]
- [11].Kim YJ, Kim HJ, Park JH, Kim S, Woo SY, Kwak KC, Lee JM, Jung NY, Kim JS, Choe YS, Lee KH, Moon SH, Lee JH, Kim YJ, Werrinig DJ, Na DL, Seo SW (2016) Synergistic effects of longitudinal amyloid and vascular changes on lobar microbleeds. Neurology 87, 1575–1582. [DOI] [PubMed] [Google Scholar]
- [12].Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, Fotiadis P, Huang CY, Yen RF, Jeng JS, Gurol ME (2019) Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: A PiB-PET study. Neurology 92, e774–e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, Werring DJ (2009) The microbleed anatomical rating scale (MARS): Reliability of a tool to map brain microbleeds. Neurology 73, 1759–1766. [DOI] [PubMed] [Google Scholar]
- [14].Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindlei RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werrring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M; Standards for Reporting Vascular changes on nEuroimaging (STRIVE v1) (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12, 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149, 351–356. [DOI] [PubMed] [Google Scholar]
- [16].Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P; European Task Force on Age-Related White Matter Changes (2001) A new rating scale for aging-related white matter changes applicable to MRI and CT. Stroke 32, 1318–1322. [DOI] [PubMed] [Google Scholar]
- [17].Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Waldlaw JM (2010) Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41, 450–454. [DOI] [PubMed] [Google Scholar]
- [18].Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, Ayres A, Schwab KM, Goldstein JN, Rosand J, Viswanathan A, Pantoni L, Greenberg SM, Gurol ME (2017) Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology 88, 2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Charidimou A, Linn J, Vernooij MW, Opherkr HR, Akoudad S, Baron JC, Greenberg SM, Jäger HR, Werring DJ (2015) Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 138, 2126–2139. [DOI] [PubMed] [Google Scholar]
- [20].Petersen RC (2011) Clinical practice. Mild cognitive impairment. N Engl J Med 364, 2227–2234. [DOI] [PubMed] [Google Scholar]
- [21].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia (2011) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Okazaki H, Reagan TJ, Campbell RJ (1979) Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc 51, 23–31. [PubMed] [Google Scholar]
- [24].Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: The CERAD experience, Part XV. Neurology 46, 1592–1596. [DOI] [PubMed] [Google Scholar]
- [25].Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: Correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62, 1287–1301. [DOI] [PubMed] [Google Scholar]
- [26].Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, Kalaria RN (2012) Staging and natural history of cerebrovascular pathology in dementia. Neurology 78, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA (2005) APOE, vascular pathology, and the AD brain. Neurology 65, 259–265. [DOI] [PubMed] [Google Scholar]
- [28].Grinberg LT, Thal DR (2010) Vascular pathology in the aged human brain. Acta Neuropathol 19, 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weller RO, Subash M, Preston SD, Mazanti I, Carare RO (2008) Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 18, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C (2007) Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain 130, 2310–2319. [DOI] [PubMed] [Google Scholar]
- [31].Jandke S, Garz C, Schwanke D, Sendtner M, Heinze HJ, Carare RO, Schreiber S (2018) The association between hypertensive arteriopathy and cerebral amyloid angiopathy in spontaneously hypertensive stroke-prone rats. Brain Pathol 28, 844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, Fotiadis P, Huang CY, Yen RF, Gurol ME, Jeng JS (2018) Distribution of lacuner infarcts in Asians with intracranial hemorrhage: A magnetic resonance imaging and amyloid positron emission topography study. Stroke 49, 1515–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K. Ayres A, Schwab KM, Martinez-Ramirez S, Goldstein JN, Rosand J, Viswanathan A, Greenberg SM, Gurol ME (2017) MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 88, 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shams S, Martola J, Charidimou A, Larvie M, Granberg T, Shams M, Kristoffersen-Wiberg M, Wahlund LO (2017) Topography and determinants of magnetic resonance imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 6, e006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, Greenberg SM (2004) White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology 63, 1606–1612. [DOI] [PubMed] [Google Scholar]
- [36].Zhu YC, Chabriat H, Godin O, Dufouil C, Rosand J, Greenberg SM, Smith EE, Tzourio C, Viswanathan A (2012) Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. J Neurol 259, 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thanprasertsuk S, Martinez-Ramirez S, Pontes-Neto OM, Ni J, Ayres A, Reed A, Swords K, Gurol ME, Greenberg SM, Viswanathan A (2014) Posterior white matter disease distribution as a predictor of amyloid angiopathy. Neurology 83, 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Viswanathan A, Rocca WA, Tzourio C (2009) Vascular risk factors and dementia: How to move forward? Neurology 72, 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Charidimou A, Farid K, Baron JC (2017) Amyloid-PET in sporadic amyloid angiopathy: A diagnostic accuracy meta-analysis. Neurology 89, 1490–1498. [DOI] [PubMed] [Google Scholar]
- [40].Renard D, Castelnovo G, Wacongne A, Le Floch A, Thouvenot E, Mas J, Gabelle A, Labauge P, Lehmann S (2012) Interest of CSF biomarker analysis in possible cerebral amyloid angiopathy cases defined by the modified Boston criteria. J Neurol 259, 2429–2433. [DOI] [PubMed] [Google Scholar]