Abstract
There is growing evidence suggesting that vascular pathologies and dysfunction play a critical role in cognitive impairment, clinical Alzheimer’s disease, and dementia. Vascular pathologies such as macroinfarcts, microinfarcts, microbleeds, small and large vessel cerebrovascular disease, and white matter disease are common especially in the brains of older persons where they contribute to cognitive impairment and lower the dementia threshold. Vascular dysfunction resulting in decreased cerebral blood flow, and abnormalities in the blood brain barrier may also contribute to the Alzheimer’s disease (AD) pathophysiologic process and AD dementia. This review provides a clinical–pathological perspective on the role of vessel disease, vascular brain injury, alterations of the neurovascular unit, and mixed pathologies in the Alzheimer’s disease pathophysiologic process and Alzheimer’s dementia. This article is part of a Special Issue entitled: Vascular Contributions to Cognitive Impairment and Dementia edited by M. Paul Murphy, Roderick A. Corriveau and Donna M. Wilcock.
Keywords: Dementia, Alzheimer’s disease, Vascular, Neuropathology, Mixed pathologies
1. Introduction
Alzheimer’s disease (AD) is the most prevalent cause of dementia and represents a growing problem of the aging population. The International Alzheimer’s Disease Report estimates that 47 million people worldwide are living with AD in 2015, and this is estimated to increase to 131 million people by 2050 [1]. Neuropathological features of AD are the deposition of hyperphosphorylated tau proteins forming paired helical filaments in neurons called neurofibrillary tangles (NFTs), and the extracellular accumulation of amyloid beta (Aβ) in plaques. Hyperphosphorylated tau filaments are also commonly present in neurites, known as neuropil threads, and in neurites-associated within neuritic plaques [2]. Although these pathologies accumulate throughout the clinical stages of AD, they can also be observed in abundance in the aging brain of persons without cognitive impairment.
A long term strategy to reducing the prevalence of dementia is to identify risk factors of the disease thus allowing intervention of the disease process and delaying disease onset. Evidence from the literature suggests that genetic, psychosocial, vascular, and life-style risk factors co-occur during lifespan to determine the risk of developing dementia and AD [3]. Unlike early onset cases of AD dementia, there has been increasing emphasis that late-onset AD (LOAD) dementia is a multifactorial disorder with persons commonly exhibiting a complex combination and manifestation of a spectrum of brain conditions. These mixed brain pathologies often include not only AD pathology but also varying degrees of cerebrovascular disease, Lewy bodies, hippocampal sclerosis, and TDP-43 pathology. The most common of the mixed pathologies is AD with vascular pathology and there is accumulating evidence identifying how vascular lesions contribute to cognitive impairment, 1) how vascular lesions may be related to the pathogenesis of AD pathology, 2) the correlation between vascular disease with dementia, and 3) vascular risk factors which predispose individuals in developing vascular dementia and onset of AD.
Overall, the literature leads us to a critical question is the pathophysiologic process of AD a neurodegenerative disorder, a vascular disorder [4], or is AD dementia merely a varying composite of both neurodegenerative and vascular pathologies [5]? In this review we discuss the role of vascular factors in AD from a clinical–pathological perspective.
2. Vascular risk factors, pathology, and dysfunction in Alzheimer’s disease
Over the last two decades the amyloid hypothesis has been the most dominant in regards to pathophysiologic process of AD. It states the sequential cleavage of APP leading to formation of Aβ aggregates is responsible for neuronal injury and cognitive decline in AD [6]. Some authors have proposed the “vascular hypothesis of AD” which states vascular brain injuries precedes and promotes the neurodegenerative process. Studies supporting this hypothesis show vascular dysfunction leads to inability of Aβ clearance from the brain, leading to the Aβ accumulation in the parenchyma and blood vessels [4,7]. Vessel disease and vascular brain injury often coexist with the pathologic and clinical diagnosis of AD, and indeed vascular disease is considered the major pathology in 10–20% of dementia cases [8–10] However, even in these cases there is often plaque and tangles pathology, but insufficient to reach a pathologic diagnosis.
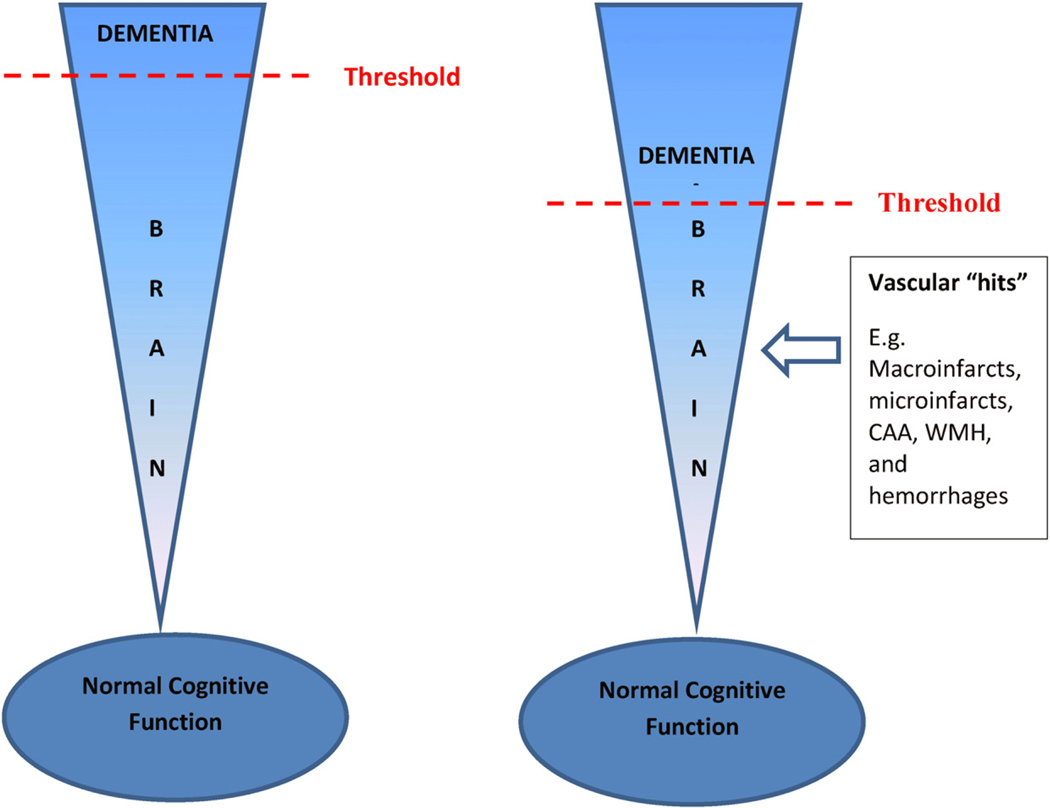
There is an increasing prevalence of AD pathology and vascular brain injury co-occurring in the aging population. Vascular pathologies include macroinfarcts, microinfarcts, atherosclerosis, arteriolosclerosis and cerebral amyloid angiopathy (CAA). Because CAA is more associated with AD pathology and not the classic cerebrovascular diseases (CVD), it is often discussed separately in pathologic studies [11]. A large body of community and population based studies document vascular pathologies in 50% of older persons, and that the overlap between vascular pathology and AD pathology is correlated with dementia (so-called mixed pathology in dementia or mixed dementia) [9,10,12]. Data from 5715 cases from the National Alzheimer’s Coordinating Center database assessed the correlation between the prevalence of CVD and vascular pathology in a variety of neurodegenerative diseases with dementia severity. The study identified a significantly higher prevalence of vascular pathology and CVD in Alzheimer’s disease than any other neurodegenerative diseases causative of dementias, such as α-synucleinopathy, fronto-temporal lobar dementias (FTLD), and prion disease [13]. Findings from the Religious Orders Study (ROS) and Rush Memory and Aging Project (MAP) suggest that vascular pathology (including macroinfarcts and microinfarcts) is very common in aging and often coexists with AD pathology, and persons diagnosed with AD dementia [10]. Indeed, AD mixed with vascular pathology was the most common mixed pathology in several studies [10,14,15]. Vascular pathology coexisting with AD pathology is thought to act as additional “hits” to the brain thereby lowering the threshold for cognitive impairment in persons with AD pathology [16] (Fig. 1). The literature also suggests that the prevalence and impact of mixed AD and vascular pathology was even greater in the oldest old, subjects over the age of 90 and the fastest growing segment of the populations [17].
Fig. 1.
A schematic representation showing the additive effect of vascular pathologies in the brain acts as “hits” and lowers the threshold for developing dementia. As aging in the brain occurs there is a threshold for developing dementia. Additional vascular “hits” lowers this threshold, thus accelerating the onset of dementia.
In adults with Down’s syndrome (DS) the risk of developing dementia has been attributed to the triplication of the APP gene. The distribution and pattern of beta-amyloid plaques and NFTs in DS adults indicate a pathological diagnosis of AD by the age of 40 [18]. Interestingly, DS adults also exhibit vascular pathologies such as CAA [19] and microhemorrhages-associated with CAA [18]. Vascular risk factors such as hypertension, obesity, and diabetes have also been reported in DS children and adults [20–22].
Epidemiological studies have shown AD and stroke share common CVD risk factors such as hypertension, diabetes, smoking, hypercholesterolemia [23], heavy alcohol consumption [24], and APOE4 isoforms. In keeping with this, CVD increases the risk of developing AD dementia or vascular dementia by three-fold [25]. It is possible the cerebrovascular changes associated with these risk factors may be related to both vascular brain injury and the propagation of AD pathology, and therefore maybe a common mechanism associated with cognitive decline. Several studies have shown hypertension is a consistent CVD risk factor for developing stroke and dementia [17,26–28]. However, most clinical–pathological studies suggest that vascular risk factors are related to infarcts rather than AD pathology. In a population-based MRC CFAS, vascular risk factors were not related to AD pathology burden, but a positive association with cerebral microinfarcts [29]. The association between diabetes and increased risk for developing dementia is well recognized. Since the risk concerns both AD and vascular dementia, multiple metabolic, inflammatory, and vascular mechanisms have been proposed but the exact pathogenesis remains unclear [30]. Community based studies from the Religious Order Study (ROS) [31] and Adult Changes in Thought (ACT) study [32] show diabetes was related to infarcts but not AD pathology. Similarly, in the ACT cohort increased blood pressure was associated with a higher prevalence of cortical microinfarcts, and not AD pathology [33].
Other studies suggest that vascular contributions in AD are much broader than the tissue injury and vessel disease seen in pathologic and imaging studies. Several data suggest that vascular contributions in AD also involve the following, 1) vascular anatomical defects such as irregularities or atrophy of the vasculature, 2) dysfunction of the blood brain barrier, and 3) insufficient cerebral blood flow. All of these vascular changes contribute to vascular pathologies observed in patients with cognitive impairment, such as focal or multifocal ischemic and hemorrhagic infarcts, white matter lesions, small vessel and large vessel disease [34]. These lesions are also associated with a plethora of other disabilities such as stroke, depression, and gait disturbances [35]. While these abnormalities are associated with vessel disease and vascular injury, contributing to mixed pathologies, their role in the pathogenesis of AD pathology remains less clear.
3. Macroscopic and microscopic infarcts
3.1. Macroscopic infarcts
Macroinfarcts encompass all of the infarcts that can be identified by the naked eye on pathologic studies. Because conventional imaging studies only diagnose infarcts when 3 mm or larger and most pathologic studies include those visualized lesions as small 1 mm, there is some distinction between macroscopic infarcts by pathology vs. imaging studies. Moreover because most imaging studies slice the brain at 5 mm intervals and pathologic studies at 1 cm intervals, both are likely imperfect at finding all macroscopic infarcts. Some imaging studies now can detect macroinfarcts smaller than 3 mm; however, these lesions are called “microinfarcts” even if they are visible without magnification [36,37].
A relatively small proportion of the larger macroscopic infarcts are recognized clinically as stroke. However, if one notes the prevalence of stroke in studies imaging infarcts in older persons [38] and neuropathological examination of macroinfarcts in older persons [38,39], these strokes are only the tip of the iceberg of macroscopic infarcts. Nonetheless these larger infarcts certainly contribute to dementia, especially in the form of poststroke dementia. Community based studies suggest that the prevalence of post-stroke dementia in people with a history of stroke is about 30% [40]. In the Rochester study, the incidence of dementia within the first year of persons who survived their first cerebral infarct was nearly 9 times greater. Compared with the community, the risks of developing Alzheimer’s disease doubled in the first year post-stroke [41].
Macroscopic infarcts are one of the most common mixed pathologies. Data from participants of the Rush MAP and ROS studies who have died with dementia has found about 50% with mixed pathologies; the most commonly observed was AD with macroscopic infarcts [12]. The pattern of cognitive impairment associated with macroscopic infarcts differed from those with AD pathology. In participants from the ROS study, the presence of cerebral infarcts was more strongly related to lower perceptual speed compared to episodic memory function in both those with and without AD pathology [42], and with motor function [43]. Though pathology data was not available, data from the Prospective Urban Rural Epidemiological (PURE) study demonstrated silent brain infarcts were also associated with slower timed gate and lower volume of white matter in the supraentorial region [44]. Cardiovascular disease burden likely plays a large role in the etiology of macroinfarcts [45].
3.2. Microscopic infarcts
Microscopic infarcts are focal regions of tissue damage often associated with gliosis and cavitation, and can be detected by light microscopy but are not seen on gross examination. They are found in many regions of the brain especially the cerebral hemispheres, and are thought to be more abundant in the vascular watershed, cortical cortex and basal ganglia [46,47]. Like macroscopic infarcts, histopathological examinations can categorize microinfarcts into three lesion subtypes, acute, subacute, and chronic. Acute lesions typically associate with eosinophilic neurons and pallor of the tissue. Sub-acute lesions, occurring 3–5 days post infarct, can be identified by the influx of activated macrophages around the microinfarct area and an increase in astrocytosis occurs at about 10 days. Astrocytes are initially gemistocytic and gradually over time become fibrillary. Chronic lesions show cavitation of the tissue with residual macrophages, some with hemosiderin, if there was associated hemorrhage, and fibrillary gliosis [36].
Several data from multiple studies show a similar prevalence of microinfarcts in older individuals. In the National Alzheimer Coordinating Center (NACC) database microinfarcts are prevalent in 19.7% in a cohort study of older adults. Similarly, data from the Religious Orders Study showed microinfarcts in about 20% [48] of older community dwelling persons, and the Adult Change in Thought (ACT) study show that 16% of brains examined had microinfarcts [39]. Microscopic infarcts are commonly associated with macroscopic infarcts, but can also identified in persons in the absence of macroscopic infarcts [12].
Most studies show microinfarcts are present in a greater percent of persons with dementia. In one study, microscopic infarcts were present in 43% of patients with AD, 62% of patients with vascular dementia, compared to 24% of subjects of normal cognitive ability [46]. Consistently, another study concludes that microinfarcts are present in one-third of subjects with dementia [42] and that multiple cortical microinfarcts are related to dementia even after controlling for macroinfarcts and AD pathology. Similarly, data from the ACT and the Honolulu Asian Aging Study both showed strong associations between microinfarcts and cognitive impairment or dementia [49]. In the Religious Orders Study, microscopic infarcts further add to the likelihood of dementia and cognitive impairment in persons with AD pathology and macroscopic infarcts. The effect of infarcts and AD pathologies was independent and there was no interaction between AD and infarct pathology.
Structural neuroimaging techniques have provided a new avenue to identify microinfarcts. A recent study assessing cerebral microinfarcts on 3 Tesla (3 T) magnetic resonance imaging (MRI) in a memory clinic study identified 32% of patients had cortical microinfarcts [50]. It is important to note the average size of most microinfarcts (approximately 0.2 to 1 mm) is typically below the lower limit of the spatial resolution for the 1.5 and 3 Tesla [36]. The higher field strength of the 7 Tesla (7 T) MRI allows for high resolution imaging with isotropic voxel sizes. Studies using this imaging method have also shown to detect cortical microinfarcts in vivo, that are confirmed by histopathological examination, in patients with known history of dementia. Upon staging criteria, lesions detected on the 7 T MRI can be characterized into cortical or non-cortical microinfarcts [51]. One limitation to be aware of these studies is the small sample size. Meanwhile, conventional neuroimaging is only able to detect infarcts 3 mm or greater in size, rendering most microinfarcts and the smaller macroscopic infarcts largely invisible to routine neuroimaging.
Overall, it is clear microinfarcts are emerging as an important contributor to cognitive impairment, but the precise pathophysiological causes of microinfarcts are not clear. Whether they are caused by those factors known to be related to other larger sized infarcts or additional molecular, biochemical or pathologic factors is uncertain.
Finally, the mechanism by which these tiny infarcts impact cognitive function is also not clear. A small number of these tiny infarcts in isolation are unlikely to be related to cognitive impairment. In an imaging pathology study, microinfarcts were related to not only infarcts but also microbleeds and white matter changes, both of which have been related to cognitive impairment. In another study, it was shown that one or several microinfarcts in the brain indicate that there are many more underlying microinfarcts present, sometimes over 1000. For instance finding 4 microscopic infarcts in a routine pathologic assessment of the brain is related to an estimated total brain load of 2000 microscopic infarcts [52]. Microinfarcts may also signal more widespread brain changes such as inflammation, hypoxia, or changes in the blood brain barrier.
3.3. Vessel disorders
Vessel disorders have been associated with vascular dementia and in some studies also related to AD dementia [53,54]. Age-related vessel disorders include atherosclerosis which affects the large arteries in the brain, and cerebral amyloid angiopathy (CAA) and arteriolosclerosis, both of which affect smaller arteries and arterioles.
3.4. Atherosclerosis
Atherosclerosis commonly occurs in the extracranial and intracranial arteries in aging. A population based prospective cohort study using 6647 participants from the Rotterdam Study suggests that atherosclerosis is associated with increased risk of dementia [25]. Some clinical–pathological studies suggest that atherosclerosis plays a role in development of not only vascular dementia but also AD dementia [55]. More than 77% participants diagnosed with AD dementia had the presence of atherosclerosis in circle of Willis observed on gross [53]. An underlying cause of large infarcts may be due to atherosclerosis. Studies show a strong correlation between atherosclerosis of the circle of Willis and large brain infarcts [56].
The relationship between atherosclerosis, AD pathology, and dementia is somewhat unclear. Although many studies suggest a direct relationship between atherosclerosis and AD pathology, some studies suggest that there is no correlation [57]. Data from the United States National Alzheimer’s Coordinating Center Database show an association between atherosclerotic disease and neuritic plaque burden [58]. Increased neuritic plaque burden and a higher Braak stages was associated with increased grades of atherosclerosis in both subjects with AD and vascular dementia [59]. In an autopsy study, the circle of Willis arteries of AD patients exhibited numerous and severe stenosis, which correlated with AD pathology [60]. More recently developed imaging techniques have been used to visualize arterial structures and atherosclerotic plaque burden. A study using positon emission tomography showed an increase in amyloid deposition in patients having both carotid artery occlusions and dementia [61]. In comparison, examining 200 brains in the Baltimore Longitudinal Study of Aging showed no correlation between atherosclerosis and AD pathology [57]. On the other hand, atherosclerosis had a direct relationship with dementia after controlling for both AD pathology and infarcts. New neuroimaging techniques used in clinical practice and research to measure cerebral blood flow in the circle of Willis will be important in future studies relating atherosclerosis to AD pathology and cognitive impairment.
3.5. Cerebral amyloid angiopathy
CAA pathology has been suggested to be a result from an imbalance between Aβ production and Aβ clearance, and is an important cause of lobar and cerebral hemorrhages resulting in profound damage. It is a result of Aβ aggregation within the basement membrane of meningeal and intracerebral arteries and arterioles, and less frequently in capillaries and veins, and leads to the replacement of smooth muscle cells and connective tissue [62]. Aβ clearance can occur via multiple routes: LRP-mediated clearance of Aβ across the BBB, APOE-mediated clearance of Aβ from the interstitial fluid, clearance of Aβ by microglia and macrophages, enzymatic degradation of Aβ, and the clearance of Aβ via the perivascular space [34].
CAA is present in over 75% of autopsy-confirmed AD brains [63]. Moderate to severe CAA in more than one cortical region show a significantly higher frequency of ischemic lesions or hemorrhages [64]. In keeping with this, examination of the temporal and parietal regions of cases from the Lund Longitudinal Dementia Study showed a strong correlation CAA with the presence of cortical microinfarcts [65]. Data from the Oxford Project to Investigate Memory and Aging (OPTIMA), and the Honolulu-Asia Aging Study (HAAS) suggest that the presence of CAA is associated with subjects having the APOE4 allele [66,67]. Imaging studies can be suggestive of CAA via the presence of cerebral microbleeds [68] or an atypical pattern of amyloid deposition.
Additionally, there are multiple studies suggesting that CAA is related to dementia. The HAAS study identified a relationship between CAA and cognitive impairment [67]. Data from the ROS study suggest that moderate to severe CAA is independently associated with lower perceptual speed and episodic memory, identifying a role of CAA in the functional decline of a specific cognitive domain [42]. More recently, data from the ROS study and Rush Memory and Aging Project, show that CAA is related to the diagnosis of probable AD even after controlling for infarcts and AD pathology. In addition, CAA was related to a steeper trajectory of cognitive decline in persons with and without dementia [11].
3.6. Arteriolosclerosis
Microscopic features of arteriolosclerosis (AS) occurring in smaller vessels (40–150 μm in diameter) include hyaline thickening, stenosis and narrowing of the lumen. Small vessels with diameter of 40–300 μm can also exhibit fibrosis associated macrophages or foam cells and leakage of plasma proteins, termed lipohyalinosis [69]. The term lipohyalinosis is sometimes used synonymously with arteriolosclerosis even without evidence of macrophage infiltration or plasma proteins preceding the fibrosis. Lipohyalinosis is well described in the neurologic literature as being related to hypertension and diabetes and having a predilection for striate vessels of subcortical gray and white matter, where they predispose to lacunar infarcts.
Examination of small vessel pathologies, showed a high prevalence arteriosclerosis (AS) and CAA in brains of patients with sporadic late-onset of AD [70]. In autopsy-AD brains, AS affected the basal ganglia first, and then progressed into the white matter, and meningeal arteries of the cortex, cerebellum, and thalamus. Lastly, progression of AS was identified in the brainstem vessels [71]. We are not aware of this pattern of AS progression being confirmed in other studies.
Older Black patients with dementia have more severe arteriolosclerosis compared to Whites [72]. APOE4 allele has been related to arteriolosclerosis in persons with pathologic AD [70]. In addition, genetic studies on 755 ROS participants identified a diabetes risk and other variants associated with AS, highlighting genetic factors in the development of AS [73].
3.7. Cerebral microbleeds
Cerebral microbleeds (CMB), also known as microhemorrhages, are small hemorrhagic lesions typically visualized on neuroimaging which reflect vascular fragility and may be an indicator for future macroscopic hemorrhages. Cerebral microbleeds may be cortical or subcortical reflecting hemorrhagic lesions adjacent to cerebral amyloid angiopathy and arteriolosclerosis, respectively [74,75]. Using amyloid imaging and MRI, a study indicates that CMB occur in regions of increased amyloid deposition [9]. Consistently, in a study assessing 371 patients with probable AD, microbleeds are associated with cerebral amyloid angiopathy [76]. Clinical–pathological studies have linked the presence of CMB with cognitive impairment. The prevalence of microbleeds is much higher in patients with mild cognitive impairment (MCI) and AD compared to cognitive normal controls [74]. A study showed patients with more than 3 CMB were associated with a decline in cognitive function [77]. In keeping with this, data from 3979 subjects from the Rotterdam study identified an association between microbleeds and lower mini-mental scores and a decline in cognitive domain functionality when testing for information processing speed and motor speed. These associations were in individuals which had more than 5 microbleeds in lobar locations [78]. Together, these studies suggest a relationship between amyloid deposition, CMB, and cognitive impairment. It is clear that numerous microbleeds in lobar locations are associated with a decline in cognitive function, but the specific number which may be harmful needs further investigation. It would seem likely that CMB at least partly mediates the relationship between CAA and cognitive decline. Other CAA related factors that may mediate cognitive decline include microinfarcts, white matter pathology, hypoperfusion and alterations in the BBB.
3.8. White matter changes
Any lesions which cause damage to the white matter can be identified as white matter hyperintensities (WMH) using structural magnetic resonance imaging (MRI) techniques. Upon scanning, the WMH areas show increased signal on T2-weighted MRI images. Various neuropathological studies have linked the severity of WMH with arteriosclerosis, demyelination, infarcts, hemorrhages, and hypoperfusion, with hypoperfusion being the most dominant [79–81]. Emerging studies have shown a relationship between WMH, AD pathology, and cerebral blood flow. Analysis of regional blood flow was significantly reduced in the frontal and mesial temporal regions in AD patients with white matter lesions (WML), than in AD patients without WML [81]. White matter lesions were significantly related to progression of amyloid deposition in sporadic AD patients [82]. In addition, WM volume has also been inversely related to AD pathology [83] and reduced cerebral autoregulation [80]. In another study, overall T2 signal prolongation was related to AD pathology and gross infarcts [84].
These studies highlight the importance of the relationship between vascular factors, the white matter and neurodegenerative pathologies and how they may be interrelated in the pathogenesis of AD dementia. The underlying pathology causing WMH maybe causally related to AD pathology itself [80].
Many studies have found relationships between WMH and cognition or incident dementia. In addition, several groups have investigated whether white matter changes are associated with SCD. Subjective cognitive decline (SCD) is a terminology introduced to identify patients who exhibit a normal neuropsychological test score but report memory-related complaints, thus may reflect an early indicator of cognitive decline. During a 2–3 year follow up, 16% of patients with subjective cognitive decline progressed clinically to MCI or dementia. A 4-fold higher risk of clinically developing MCI or dementia was identified in patients with severe white matter hyperintensities [85]. In contrast, a study investigating a cohort sample from Ireland found no association between white matter hyperintensities and SCD [86]. Few studies have been able to examine the role of WMH, after controlling for AD pathology. In one post-mortem imaging study, T2 signal prolongation in the white matter was related to episodic, semantic, and working memory after controlling for AD and gross infarct pathology [84].
Overall, current findings from the literature suggest that WMH and other white matter changes are complex abnormalities that are important markers of cerebrovascular burden, but may also be related to neurodegenerative pathologies and have independent relationships with cognition. Importantly, these abnormalities may represent an additional “hit” which in turn lowers the threshold for developing AD (Fig. 1). With newer MRI techniques patterns of white matter changes can be identified, and also may predict AD before the onset of the clinical disease [87].
3.9. The neurovascular unit
The neurovascular unit is a specialized network of cells which maintain the cerebral microenvironment. The network consists of vascular endothelial cells, pericytes, astrocytes, and neurons and plays a role in maintaining the blood brain barrier (BBB), regulate cerebral blood flow (CBF), and contribute to immune surveillance. Abnormalities of the neurovascular unit are not easily assessed using routine pathologic assessments and therefore their contribution to mixed pathologies and their independent contributions to the clinical expression of AD dementia are less clear. Meanwhile dysfunction of the BBB and cerebral blood flow changes appear to play a role in the AD pathophysiologic process and cognitive impairment.
3.10. Dysfunction of the blood brain barrier in AD
The role of the BBB is to regulate the entrance of blood-derived products, neurotoxic proteins, and pathogens into the brain. Increasing evidence from brain imaging and post-mortem tissue studies in AD patients suggests the BBB becomes “leaky”, at least partly due to accumulation of toxic Aβ molecules in cerebral blood vessels, and associated inflammation [88–91]. A body of evidence from animal models of AD also suggests the integrity of the BBB is maybe compromised before the manifestation of plaques, tangles, and cognitive impairment, suggesting that this could be the earliest sign of AD [88,92].
Various BBB defects occur in the AD pathophysiologic process such as reduced staining of endothelial markers, suggestive of accelerated degeneration of brain endothelial cells [93]. Transcriptional profiling of brain endothelial cells from AD individuals show an alteration of a homeobox gene, important in regulating vascular differentiation, and leads to aberrant angiogenesis and reduction in cerebral blood flow [94]. Together, these studies suggest that degeneration of endothelial cells in AD patients may not be due to hypoxic vascular injury but may reflect a vascular remodeling defect. Accelerated pericyte degeneration is also identified in individuals carrying the APOE4 allele, a risk factor for developing late onset of Alzheimer’s disease [95]. Post-mortem studies of brains with AD show there is a breakdown in the BBB that leads to an accumulation of blood derived molecules in the hippocampus and degeneration of pericytes [89]. Clinical neuroimaging studies using positron emission tomography with 18F-fluorodeoxyglucose, which measures cerebral glucose transport across the BBB, show a significantly reduced glucose metabolism in fronto-temporal–parietal and cingulate cortices in patients with AD [96]. Another study using the same technology identified reductions in hippocampal glucose metabolism in both patients with MCI and AD [97], thus a decreased glucose metabolism may represent an early sign in the AD pathophysiologic process and in AD dementia. One study used MRI imaging to assess WHM and BBB fragility, using intravenous Gadolinium. They found an increased signal in subjects with more WMH, and an increased signal, especially in the basal ganglia, of patients with type II diabetes. This study is one the very few studies in humans identifying dysfunction of the BBB may be associated with a specific vascular pathology [98].
A compromised BBB alters the transport across the BBB, promoting entry of blood-borne molecules, also has an impact on Aβ accumulation, and triggers an inflammatory cascade. Much evidence suggests that neuroinflammation may modify or contribute the progression of neurodegeneration in AD. Identifying inflammatory mechanisms may provide us with a better understanding of the changes occurring in brain metabolism in patients with AD. The degeneration of endothelial cells and pericytes in carriers with the APOE4 allele showed an accumulation of proinflammatory cytokines and metalloproteinases, cyclophilin A and matrix metalloproteinase 9, respectively [95]. A study performing cytokine profiling found a significant correlation between interleukin 8 (IL8) and macrophage inflammatory protein 1 beta with patients with severe VD, defined by the mini mental status test [99].
Overall, the literature suggests that the dysfunction of the BBB may represent an early sign in the AD pathophysiologic process and in AD dementia [34,89,92]. Targeting the compromise of the neurovascular unit and BBB vasculature may represent an option to prevent or delay onset of clinical AD.
3.11. Changes in cerebral blood flow in AD
The endfeet of astrocytes which line the wall of cerebral vessels and interact with neurons play a role in regulating cerebral blood flow (CBF). Specialized receptors on the endothelial cells release signaling molecules which induces mechanisms to maintain a constant CBF [100]. Data from clinical imaging studies indicate that changes in cerebral blood flow may play a role in early AD. Functional MRI (fMRI) studies using blood oxygen level dependent (BOLD) responses in patients with a test that challenged episodic memory showed a delayed cerebral blood flow response in patients with MCI. When testing patients with AD, the delay in BOLD response was further delayed, suggesting that alterations in CBF are presented in early stages of AD [101]. Examination of CBF velocity, measured by transcranial Doppler, in participants of the Rotterdam Study showed subjects with a higher CBF velocity were less likely to have dementia [102]. In keeping with this, another study showed cerebrovascular reactivity was significantly reduced in subjects with AD [103]. It has been proposed that the presence of atherosclerotic occlusion in the Circle of Willis contributes to the changes in the hemodynamics present in AD [104]. Together these studies suggest that cerebral hemodynamics may be an important factor in the pathologic process of LOAD. Interestingly, reduced CBF was associated with amyloid deposition in several cortical regions in patients with autosomal dominant AD (ADAD), subjects who present clinical symptoms at an early age. Whether the relationship between amyloid deposition and CBF is direct or whether CBF decline is a byproduct of a different AD pathophysiologic process needs further study [105].
Cerebral autoregulation is a mechanism in which the vasculature of the brain can adapt to changes in blood pressure, thus maintain a stable cerebral blood flow. Reduced cerebral autoregulation has been associated with increased amyloid deposition and increased white matter hyperintensities [80]. Changes in blood flow regulation and glucose metabolism has been identified in the medial parietal cortex (precuneus) in very early stages of AD. Analysis of the precuneus in early stage AD patients (Braak III-IV) showed nearly 50% reduction in myelinassociated glycoprotein: proteolipid protein 1 ratio levels, an indicator of oxygenation, suggesting that this may be a molecular mechanism contributing to cerebral hypoperfusion in early AD [106]. It is possible that autoregulatory dysfunction can lead to an imbalance of Aβ deposition/clearance; alternatively, amyloid pathology can lead to a dysfunction in the autoregulatory mechanism.
3.12. Mixed pathologies from community based studies
It is increasingly recognized that persons with dementia and probably AD dementia commonly have mixed pathologies. While most persons diagnosed with AD dementia are confirmed to have a pathologic diagnosis of AD, they very often have AD with additional pathologies (referred to as mixed pathologies) contributing to impairment. Cognitive and neuropathological data available from 653 autopsied participants showed persons with AD pathology alone doubled the odds of developing dementia, and persons displaying mixed pathologies such as AD with macroinfarcts and/or Lewy body (LB) pathology markedly increases the odds [107]. Data from the Memory and Aging Project (MAP) and Religious Order Study (ROS) found that about half the subjects whom were clinically-diagnosed with probable AD had mixed pathologies, the most common being AD and infarct pathology. These studies suggest that AD pathology and infarct pathology are both associated with episodic memory impairment the supposed hallmark for AD dementia. Due to the overlap of pathologies there may not be a single causative pathology for mixed dementia, and even the initiating pathology becomes unclear. Indeed, macroscopic infarcts, microscopic infarcts, and amyloid angiopathy were each found to be associated with perceptual speed and episodic memory [10,16]. A study using subjects from the Baltimore Longitudinal Study of Aging Autopsy Program documents that AD pathology accounts for 50% in this cohort, and that AD with infarct pathology accounts for 35% [108]. Neuropathological examination of brains from participants of the Medical Research Council Cognitive Function and Ageing Study (MRC-CCFAS) report 78% with vascular pathology and 70% with AD pathology in this cohort [14]. Overall, there is a striking overlap between AD and vascular pathologies contributing to probable AD and dementia in these community cohorts.
There is limited data on mixed pathologies in special populations. Data from the ROS and Rush MAP, show that mixed pathologies, particular AD and infarcts is dramatically increased in the oldest old, the fastest growing segment of our population. On the other hand, the prevalence of a pathologic diagnosis of AD or infarcts alone was not increased in the oldest old compared to the old. Clearly, prevention and treatments in the oldest old should consider both AD and vascular pathologies.
Mixed pathologies are common in both blacks and whites, but frequency patterns appear to differ. Black clinic patients are much more likely to have mixed pathologies compared to white participants. In particular, the most common mixed pathology for black participants was AD pathology with Lewy bodies. Second most common was AD pathology with Lewy bodies and infarcts. The Black cohort also had more severe arteriolosclerosis and atherosclerosis but not more infarcts. Selection bias may have contributed to the very high rates of Lewy body disease in this Black clinic cohort [72]. Nonetheless, this has important implications given that these are the Blacks that may be most likely to seek help in memory disorders clinic. Similar studies in community cohorts with Black participants will be important.
In addition to AD pathology, other common co-existing pathologies are hippocampal sclerosis and TDP-43 pathology, both documented to play a role in cognitive decline and dementia [109,110]. Interestingly, hippocampal sclerosis of aging has been linked to cerebrovascular pathology in some, but not all studies [109–111]. Data from a large autopsy study show a strong correlation between arteriolosclerosis in brain regions outside the hippocampus and hippocampal sclerosis in aging brains [111], suggesting that hippocampal sclerosis may be a result of vascular brain injury.
Data from longitudinal clinical-neuropathological studies suggest that Parkinsonian signs may be a risk factor for cognitive impairment. Data from the Arizona Study of Aging and Neurodegenerative Disorders show 38% of pathological diagnosis of Parkinson’s disease cases had a diagnosis of AD [112]. Data from 418 autopsied subjects suggest that cerebrovascular pathologies are associated with Parkinsonian signs. In particular, multiple cortical and subcortical macroscopic infarcts were related to higher global Parkinsonian scores [113].
Community-based clinical-neuropathological cohort studies are imperative in understanding the contribution of mixed pathologies to dementia. Together, the data from these studies suggest that vascular lesions’ acting in combination with other pathologies is detrimental to cognitive function. It is also possible that risk factors work through additional functional or yet unrecognized pathologies. Moreover there are a host of risk factors that have not been definitely linked to specific pathologies including such things as depression, education, and specific genetic polymorphisms. Mixed vascular pathology with AD pathology is the most prevalent ahead of dementia with lewy bodies, and hippocampal sclerosis [10,13,14]. Due to the high prevalence of the vascular pathologies in mixed dementia, prevention and treatment of the vascular component is important to reduce risk of dementia.
3.13. Biomarkers and neuropathology
To develop and implement successful treatments for AD, the ideal goal is to identify pre-symptomatic detection of reliable biomarkers. The field of neuroimaging has been widely used to improve diagnosis of AD dementia and other neurodegenerative and vascular dementias. Some studies have used MRI techniques to assess presence of hippocampal atrophy [114] and cortical microinfarcts [50,51], though these pathologies overall remain a challenge to detect in vivo. FDG-PET imaging has also been used to identify decreased glucose uptake in brain regions, and the decrease in glucose uptake correlated with AD pathology. FDG-PET however is not a specific marker for AD pathology and studies have related this more to neurodegeneration and specifically neuronal loss and gliosis [115] in the progression of AD dementia [116]. More recent studies show that FDG-PET is strongly related to measurements of regional blood flow [117]. FDG-PET has also been used for hypoxic ischemic injury [118] and in acute stroke [118,119]. We are not aware of FDG-PET being used to determine vascular contributions to dementia.
One of the most important biomarkers recently discovered, which specifically marks a central pathology of AD, is the use of PET with molecular ligand targets with beta-amyloid binding. This was first introduced using a tracer termed Pittsburgh compound B (PiB) [5,120]. More recently, beta-amyloid tracers with shorter half-life have been FDA approved for diagnostic use [121]. Greatest changes in beta-amyloid tracer uptake in the cortex are observed in pre-dementia stages with a plateau reaching around the time dementia develops [122]. Indeed, amyloid accumulation appears to be an early event possibly occurring a decade or more prior to dementia. These models may differ in late life dementia with mixed pathologies [116]. More recently tau radioligands targeting NFT have been developed [123], and are being studied as potential biomarkers for AD. Especially, since neocortical NFT are presumed by many experts to be later in the AD progression compared to amyloid [123], and is strongly related to cognition. Neither beta-amyloid nor tangle biomarkers however exclude the presence of mixed pathologies [124].
Other studies have utilized functional MRI imaging to determine impaired cerebral blood flow associated with CAA [125]. CSF biomarkers are another field being explored. Three CSF biomarkers have been well established to diagnose AD, Aβ (1–42), total Tau, and hyperphosphorylated tau. Novel neurovascular unit-based CSF biomarkers are also being identified to investigate vascular injury and potentially aid in the diagnosis of vascular dysfunction in AD [126]. The field of genomics and proteomics is emerging rapidly to further biomarker discovery. Specific genetic polymorphisms are related to cerebrovascular disease, and identification of these genes or protein metabolism associated with disease pathogenesis will lead to better strategies to optimize drug development [127].
4. Conclusion
The contribution of vascular disease to the AD pathophysiologic process, clinical expression and progression of AD dementia is a complicated and varied one. Vascular pathologies such as macroinfarcts, microinfarcts, CAA, microbleeds, and white matter changes add to the likelihood of expressing AD pathology and all contribute to cognitive impairment. Though there is evidence that atherosclerosis and impairment of the BBB and blood flow may contribute to the AD pathophysiologic process, whether these processes and pathologies initiate or accelerate the pathologic process of plaque and tangle progression remains incompletely understood. Overall, it is clear that vascular and AD pathologies rarely occur in isolation, and that the entity that we consider to be “AD dementia” in older persons is a complex composite of vascular and neurodegenerative processes. By targeting the vascular component we may be able to not only reduce the risk of developing dementia, but potentially decelerate the pathologic progression of AD pathology. Further research in elucidating molecular and cellular mechanisms underlying vascular pathologies present in AD and dementia, and facilitating the development of noninvasive vascular biomarkers will be imperative for finding preventions and treatments for AD dementia and other dementias.
Acknowledgments
National Institutes of Aging grants: R01AG17917, P30AG010161, R01AG034374, R01 AG15819, R01AG042210.
Footnotes
This article is part of a Special Issue entitled: Vascular Contributions to Cognitive Impairment and Dementia edited by M. Paul Murphy, Roderick A. Corriveau and Donna M. Wilcock.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- [1].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H, Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective, Alzheimers Dement. 11 (2015) 718–726. [DOI] [PubMed] [Google Scholar]
- [2].Spillantini MG, Goedert M, Tau pathology and neurodegeneration, Lancet Neurol. 12 (2013) 609–622. [DOI] [PubMed] [Google Scholar]
- [3].Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA, Cognitive and social lifestyle: links with neuropathology and cognition in late life, Acta Neuropathol. 127 (2014) 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Canobbio I, Abubaker AA, Visconte C, Torti M, Pula G, Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer’s disease, Front. Cell. Neurosci. 9 (2015) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC, Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease, Alzheimers Dement. 11 (2015) 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hardy J, Selkoe DJ, The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics, Science 297 (2002) 353–356. [DOI] [PubMed] [Google Scholar]
- [7].Janota C, Lemere CA, Brito MA, Dissecting the contribution of vascular alterations and aging to Alzheimer’s disease, Mol. Neurobiol. (2015). [DOI] [PubMed] [Google Scholar]
- [8].Venkat P, Chopp M, Chen J, Models and mechanisms of vascular dementia, Exp. Neurol. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Attems J, Jellinger KA, The overlap between vascular disease and Alzheimer’s disease — lessons from pathology, BMC Med. 12 (2014) (206–014–0206-2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schneider JA, Arvanitakis Z, Bang W, Bennett DA, Mixed brain pathologies account for most dementia cases in community-dwelling older persons, Neurology 69 (2007) 2197–2204. [DOI] [PubMed] [Google Scholar]
- [11].Boyle PP, Yu LP, Nag SMP, Leurgans SP, Wilson RP, Bennett DM, Schneider JMM, Cerebral Amyloid Angiopathy and Cognitive Outcomes in Community-based Older Persons, 2015. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schneider JA, High blood pressure and microinfarcts: a link between vascular risk factors, dementia, and clinical Alzheimer’s disease, J. Am. Geriatr. Soc. 57 (2009) 2146–2147. [DOI] [PubMed] [Google Scholar]
- [13].Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ, Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre, Brain 136 (2013) 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Group N, Medical Research Council Cognitive Function and Aging Study, pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS), Lancet 357 (2001) 169–175. [DOI] [PubMed] [Google Scholar]
- [15].White L, Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu–Asia aging study, J. Alzheimers Dis. 18 (2009) 713–725. [DOI] [PubMed] [Google Scholar]
- [16].Schneider JA, Bennett DA, Where vascular meets neurodegenerative disease, Stroke 41 (2010) (S144–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA, Dementia from Alzheimer disease and mixed pathologies in the oldest old, JAMA 307 (2012) 1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wilcock DM, Schmitt FA, Head E, Cerebrovascular contributions to aging and Alzheimer’s disease in Down syndrome, Biochim. Biophys. Acta (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McCarron MO, Nicoll JA, Graham DI, A quartet of Down’s syndrome, Alzheimer’s disease, cerebral amyloid angiopathy, and cerebral haemorrhage: interacting genetic risk factors, J. Neurol. Neurosurg. Psychiatry 65 (1998) 405–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sobey CG, Judkins CP, Sundararajan V, Phan TG, Drummond GR, Srikanth VK, Risk of major cardiovascular events in people with Down syndrome, PLoS One 10 (2015), e0137093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Melville CA, Cooper SA, McGrother CW, Thorp CF, Collacott R, Obesity in adults with Down syndrome: a case–control study, J. Intellect. Disabil. Res. 49 (2005) 125–133. [DOI] [PubMed] [Google Scholar]
- [22].Esbensen AJ, Health conditions associated with aging and end of life of adults with Down syndrome, Int. Rev. Res. Ment. Retard. 39 (2010) 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA, Association between atrial fibrillation and dementia in the general population, JAMA Neurol. 1–7 (2015). [DOI] [PubMed] [Google Scholar]
- [24].Langballe EM, Ask H, Holmen J, Stordal E, Saltvedt I, Selbaek G, Fikseaunet A, Bergh S, Nafstad P, Tambs K, Alcohol consumption and risk of dementia up to 27 years later in a large, population-based sample: the HUNT study, Norway, Eur. J. Epidemiol. 30 (2015) 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM, Atherosclerosis and risk for dementia, Ann. Neurol. 61 (2007) 403–410. [DOI] [PubMed] [Google Scholar]
- [26].Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ, Midlife blood pressure and dementia: the Honolulu–Asia aging study, Neurobiol. Aging 21 (2000) 49–55. [DOI] [PubMed] [Google Scholar]
- [27].Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, Nelson J, Davis DG, Hardman J, Foley DJ, Launer LJ, Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu–Asia aging study, Neurobiol. Aging 21 (2000) 57–62. [DOI] [PubMed] [Google Scholar]
- [28].Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC 3rd, Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension, J. Neurol. Sci. 131 (1995) 162–169. [DOI] [PubMed] [Google Scholar]
- [29].Richardson K, Stephan BC, Ince PG, Brayne C, Matthews FE, Esiri MM, The neuropathology of vascular disease in the Medical Research Council Cognitive Function And Ageing Study (MRC CFAS), Curr. Alzheimer Res. 9 (2012) 687–696. [DOI] [PubMed] [Google Scholar]
- [30].Jayaraman A, Pike CJ, Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions, Curr. Diab Rep. 14 (2014) (476–014–0476–2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA, Diabetes is related to cerebral infarction but not to AD pathology in older persons, Neurology 67 (2006) 1960–1965. [DOI] [PubMed] [Google Scholar]
- [32].Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, Craft S, Different patterns of cerebral injury in dementia with or without diabetes, Arch. Neurol. 66 (2009) 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang LY, Larson EB, Sonnen JA, Shofer JB, McCormick W, Bowen JD, Montine TJ, Li G, Blood pressure and brain injury in older adults: findings from a community-based autopsy study, J. Am. Geriatr. Soc. 57 (2009) 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bell RD, Zlokovic BV, Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease, Acta Neuropathol. 118 (2009) 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mok V, Kim JS, Prevention and management of cerebral small vessel disease, J. Stroke 17 (2015) 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Smith EE, Schneider JA, Wardlaw JM, Greenberg SM, Cerebral microinfarcts: the invisible lesions, Lancet Neurol. 11 (2012) 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].de Rotte AA, Koning W, den Hartog AG, Bovens SM, Zwanenburg JJ, Klomp DW, Pasterkamp G, Moll FL, Luijten PR, de Borst GJ, Hendrikse J, 7.0 T MRI detection of cerebral microinfarcts in patients with a symptomatic high-grade carotid artery stenosis, J. Cereb. Blood Flow Metab. 34 (2014) 1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Longstreth WT Jr., Brain abnormalities in the elderly: frequency and predictors in the United States (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group, J. Neural Transm. Suppl. 53 (1998) 9–16. [DOI] [PubMed] [Google Scholar]
- [39].Longstreth WT Jr., Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ, Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases, Alzheimer Dis. Assoc. Disord. 23 (2009) 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F, Poststroke dementia, Lancet Neurol. 4 (2005) 752–759. [DOI] [PubMed] [Google Scholar]
- [41].Kokmen E, Whisnant JP, O’Fallon WM, Chu CP, Beard CM, Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960–1984), Neurology 46 (1996) 154–159. [DOI] [PubMed] [Google Scholar]
- [42].Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA, Microinfarct pathology, dementia, and cognitive systems, Stroke 42 (2011) 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA, Parkinsonism in older adults and its association with adverse health outcomes and neuropathology, J. Gerontol. A Biol. Sci. Med. Seci. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Smith EE, O’Donnell M, Dagenais G, Lear SA, Wielgosz A, Sharma M, Poirier P, Stotts G, Black SE, Strother S, Noseworthy MD, Benavente O, Modi J, Goyal M, Batool S, Sanchez K, Hill V, McCreary CR, Frayne R, Islam S, DeJesus J, Rangarajan S, Teo K, Yusuf S, et al. , Early cerebral small vessel disease and brain volume, cognition, and gait, Ann. Neurol. 77 (2015) 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Qiu C, Fratiglioni L, A major role for cardiovascular burden in age-related cognitive decline, Nat. Rev. Cardiol. 12 (2015) 267–277. [DOI] [PubMed] [Google Scholar]
- [46].Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ, Cerebral microinfarcts: a systematic review of neuropathological studies, J. Cereb. Blood Flow Metab. 32 (2012) 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gold G, Giannakopoulos P, Herrmann FR, Bouras C, Kovari E, Identification of Alzheimer and vascular lesion thresholds for mixed dementia, Brain 130 (2007) 2830–2836. [DOI] [PubMed] [Google Scholar]
- [48].Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA, Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons, Ann. Neurol. 62 (2007) 59–66. [DOI] [PubMed] [Google Scholar]
- [49].Sonnen JA, Larson EB, Haneuse S, Woltjer R, Li G, Crane PK, Craft S, Montine TJ, Neuropathology in the adult changes in thought study: a review, J. Alzheimers Dis. 18 (2009) 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].van Veluw SJ, Hilal S, Kuijf HJ, Ikram MK, Xin X, Yeow TB, Venketasubramanian N, Biessels GJ, Chen C, Cortical Microinfarcts on 3 T MRI: Clinical Correlates in Memory-clinic Patients, Alzheimers Dement, 2015. [DOI] [PubMed] [Google Scholar]
- [51].van Veluw SJ, Jolink WM, Hendrikse J, Geerlings MI, Luijten PR, Biessels GJ, Klijn CJ, Cortical microinfarcts on 7 T MRI in patients with spontaneous intracerebral hemorrhage, J. Cereb. Blood Flow Metab. 35 (2015) 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM, Estimating cerebral microinfarct burden from autopsy samples, Neurology 80 (2013) 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, Van Deerlin V, Lee VM, Trojanowski JQ, Arnold SE, Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias, Brain 135 (2012) 3749–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Thal DR, von Arnim C, Griffin WS, Yamaguchi H, Mrak RE, Attems J, Upadhaya AR, Pathology of clinical and preclinical Alzheimer’s disease, Eur. Arch. Psychiatry Clin. Neurosci. 263 (Suppl. 2) (2013) S137–S145. [DOI] [PubMed] [Google Scholar]
- [55].Gupta A, Iadecola C, Impaired Abeta clearance: a potential link between atherosclerosis and Alzheimer’s disease, Front. Aging Neurosci. 7 (2015) 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR, Brain infarction and the clinical expression of Alzheimer disease. The Nun Study, JAMA 277 (1997) 813–817. [PubMed] [Google Scholar]
- [57].Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ, Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort, Ann. Neurol. 68 (2010) 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Honig LS, Kukull W, Mayeux R, Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center, Neurology 64 (2005) 494–500. [DOI] [PubMed] [Google Scholar]
- [59].Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, Walker DG, Roher AE, Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles, Acta Neuropathol. 113 (2007) 13–21. [DOI] [PubMed] [Google Scholar]
- [60].Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, Sue LI, Beach TG, Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease, Arterioscler. Thromb. Vasc. Biol. 23 (2003) 2055–2062. [DOI] [PubMed] [Google Scholar]
- [61].Huang KL, Lin KJ, Ho MY, Chang YJ, Chang CH, Wey SP, Hsieh CJ, Yen TC, Hsiao IT, Lee TH, Amyloid deposition after cerebral hypoperfusion: evidenced on [(18)F]AV-45 positron emission tomography, J. Neurol. Sci. 319 (2012) 124–129. [DOI] [PubMed] [Google Scholar]
- [62].Keable A, Fenna K, Yuen HM, Johnston DA, Smyth NR, Smith C, Salman RA, Samarasekera N, Nicoll JA, Attems J, Kalaria RN, Weller RO, Carare RO, Deposition of amyloid beta in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy, Biochim. Biophys. Acta (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA, Cerebral amyloid angiopathy pathology and cognitive domains in older persons, Ann. Neurol. 69 (2011) 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A, Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV, Neurology 46 (1996) 1592–1596. [DOI] [PubMed] [Google Scholar]
- [65].Haglund M, Passant U, Sjobeck M, Ghebremedhin E, Englund E, Cerebral amyloid angiopathy and cortical microinfarcts as putative substrates of vascular dementia, Int. J. Geriatr. Psychiatry 21 (2006) 681–687. [DOI] [PubMed] [Google Scholar]
- [66].Esiri M, Chance S, Joachim C, Warden D, Smallwood A, Sloan C, Christie S, Wilcock G, Smith AD, Cerebral amyloid angiopathy, subcortical white matter disease and dementia: literature review and study in OPTIMA, Brain Pathol. 25 (2015) 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ, Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study, Neurology 58 (2002) 1629–1634. [DOI] [PubMed] [Google Scholar]
- [68].Charidimou A, Shakeshaft C, Werring DJ, Cerebral microbleeds on magnetic resonance imaging and anticoagulant-associated intracerebral hemorrhage risk, Front. Neurol. 3 (2012) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Grinberg LT, Thal DR, Vascular pathology in the aged human brain, Acta Neuropathol. 119 (2010) 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA, APOE, vascular pathology, and the AD brain, Neurology 65 (2005) 259–265. [DOI] [PubMed] [Google Scholar]
- [71].Thal DR, Ghebremedhin E, Orantes M, Wiestler OD, Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline, J. Neuropathol. Exp. Neurol. 62 (2003) 1287–1301. [DOI] [PubMed] [Google Scholar]
- [72].Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA, Schneider JA, Mixed pathology is more likely in black than white decedents with Alzheimer dementia, Neurology 85 (2015) 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chou SH, Shulman JM, Keenan BT, Secor EA, Buchman AS, Schneider J, Bennett DA, De Jager PL, Genetic susceptibility for ischemic infarction and arteriolosclerosis based on neuropathologic evaluations, Cerebrovasc. Dis. 36 (2013) 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC, Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations, Front. Neurol. 4 (2014) 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Smith EE, Nandigam KR, Chen YW, Jeng J, Salat D, Halpin A, Frosch M, Wendell L, Fazen L, Rosand J, Viswanathan A, Greenberg SM, MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage, Stroke 41 (2010) 1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM, Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer’s disease, Neurobiol. Aging 34 (2013) 2488–2494. [DOI] [PubMed] [Google Scholar]
- [77].Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ, Utrecht Vascular Cognitive Impairment (VCI) Study Group, multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease, J. Alzheimers Dis. 38 (2014) 211–221. [DOI] [PubMed] [Google Scholar]
- [78].Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW, Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study, Neurology 78 (2012) 326–333. [DOI] [PubMed] [Google Scholar]
- [79].Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H, Pathologic correlates of incidental MRI white matter signal hyperintensities, Neurology 43 (1993) 1683–1689. [DOI] [PubMed] [Google Scholar]
- [80].Brickman AM, Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities, Curr. Neurol. Neurosci. Rep. 13 (2013) (415–013–0415-7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hanaoka T, Kimura N, Aso Y, Takemaru M, Kimura Y, Ishibashi M, Matsubara E, Relationship between white matter lesions and regional cerebral blood flow changes during longitudinal follow up in Alzheimer’s disease, Geriatr. Gerontol. Int. (2015). [DOI] [PubMed] [Google Scholar]
- [82].Grimmer T, Faust M, Auer F, Alexopoulos P, Forstl H, Henriksen G, Perneczky R, Sorg C, Yousefi BH, Drzezga A, Kurz A, White matter hyperintensities predict amyloid increase in Alzheimer’s disease, Neurobiol. Aging 33 (2012) 2766–2773. [DOI] [PubMed] [Google Scholar]
- [83].Kotrotsou A, Schneider JA, Bennett DA, Leurgans SE, Dawe RJ, Boyle PA, Golak T, Arfanakis K, Neuropathologic correlates of regional brain volumes in a community cohort of older adults, Neurobiol. Aging 36 (2015) 2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dawe RJ, Bennett DA, Schneider JA, Leurgans SE, Kotrotsou A, Boyle PA, Arfanakis K, Ex vivo T2 relaxation: associations with age-related neuropathology and cognition, Neurobiol. Aging 35 (2014) 1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Benedictus MR, van Harten AC, Leeuwis AE, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM, White matter hyperintensities relate to clinical progression in subjective cognitive decline, Stroke 46 (2015) 2661–2664. [DOI] [PubMed] [Google Scholar]
- [86].Bartley M, Bokde AL, Ewers M, Faluyi YO, Tobin WO, Snow A, Connolly J, Delaney C, Coughlan T, Collins DR, Hampel H, O’Neill D, Subjective memory complaints in community dwelling healthy older people: the influence of brain and psychopathology, Int. J. Geriatr. Psychiatry 27 (2012) 836–843. [DOI] [PubMed] [Google Scholar]
- [87].Love S, Miners JS, White matter hypoperfusion and damage in dementia: postmortem assessment, Brain Pathol. 25 (2015) 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Takeda S, Sato N, Morishita R, Systemic inflammation, blood–brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy, Front. Aging Neurosci. 6 (2014) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV, Blood–brain barrier breakdown in the aging human hippocampus, Neuron 85 (2015) 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tai LM, Holloway KA, Male DK, Loughlin AJ, Romero IA, Amyloid-beta-induced occludin down-regulation and increased permeability in human brain endothelial cells is mediated by MAPK activation, J. Cell. Mol. Med. 14 (2010) 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV, Deficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer’s disease, Brain Pathol. 23 (2013) 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ujiie M, Dickstein DL, Carlow DA, Jefferies WA, Blood–brain barrier permeability precedes senile plaque formation in an Alzheimer disease model, Microcirculation 10 (2003) 463–470. [DOI] [PubMed] [Google Scholar]
- [93].Kalaria RN, Hedera P, Differential degeneration of the cerebral microvasculature in Alzheimer’s disease, Neuroreport 6 (1995) 477–480. [DOI] [PubMed] [Google Scholar]
- [94].Wu MH, Endothelial focal adhesions J. Physiol. 569 (2005) 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Halliday MR, Rege SV, Ma Q, Zhao Z, Miller CA, Winkler EA, Zlokovic BV, Accelerated pericyte degeneration and blood–brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease, J. Cereb. Blood Flow Metab. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J, Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease, Psychiatry Res. 155 (2007) 147–154. [DOI] [PubMed] [Google Scholar]
- [97].Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, Li Y, Boppana M, de Leon MJ, Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis, Neurology 64 (2005) 1860–1867. [DOI] [PubMed] [Google Scholar]
- [98].Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I, Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging, J. Neurol. Neurosurg. Psychiatry 74 (2003) 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Schmitz M, Hermann P, Oikonomou P, Stoeck K, Ebert E, Poliakova T, Schmidt C, Llorens F, Zafar S, Zerr I, Cytokine profiles and the role of cellular prion protein in patients with vascular dementia and vascular encephalopathy, Neurobiol. Aging 36 (2015) 2597–2606. [DOI] [PubMed] [Google Scholar]
- [100].Iadecola C, Nedergaard M, Glial regulation of the cerebral microvasculature, Nat. Neurosci. 10 (2007) 1369–1376. [DOI] [PubMed] [Google Scholar]
- [101].Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P, Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study, Hum. Brain Mapp. 26 (2005) 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM, Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study, Ann. Neurol. 57 (2005) 789–794. [DOI] [PubMed] [Google Scholar]
- [103].Shim Y, Yoon B, Shim DS, Kim W, An JY, Yang DW, Cognitive correlates of cerebral vasoreactivity on transcranial Doppler in older adults, J. Stroke Cerebrovasc. Dis. 24 (2015) 1262–1269. [DOI] [PubMed] [Google Scholar]
- [104].Kalback W, Esh C, Castano EM, Rahman A, Kokjohn T, Luehrs DC, Sue L, Cisneros R, Gerber F, Richardson C, Bohrmann B, Walker DG, Beach TG, Roher AE, Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease, Neurol. Res. 26 (2004) 525–539. [DOI] [PubMed] [Google Scholar]
- [105].McDade E, Kim A, James J, Sheu LK, Kuan DC, Minhas D, Gianaros PJ, Ikonomovic S, Lopez O, Snitz B, Price J, Becker J, Mathis C, Klunk W, Cerebral perfusion alterations and cerebral amyloid in autosomal dominant Alzheimer disease, Neurology 83 (2014) 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Miners JS, Palmer JC, Love S, Pathophysiology of hypoperfusion of the precuneus in early Alzheimer’s disease, Brain Pathol. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Yu L, Boyle PA, Leurgans S, Schneider JA, Kryscio RJ, Wilson RS, Bennett DA, Effect of common neuropathologies on progression of late life cognitive impairment, Neurobiol. Aging 36 (2015) 2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ, Effect of infarcts on dementia in the Baltimore longitudinal study of aging, Ann. Neurol. 64 (2008) 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA, Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease, Ann. Neurol. 77 (2015) 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rahimi J, Kovacs GG, Prevalence of mixed pathologies in the aging brain, Alzheimers Res. Ther. 6 (2014) (82–014–0082-1. eCollection 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Neltner JH, Abner EL, Baker S, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Hammack E, Kukull WA, Brenowitz WD, Van Eldik LJ, Nelson PT, Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing, Brain 137 (2014) 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dugger BN, Adler CH, Shill HA, Caviness J, Jacobson S, Driver-Dunckley E, Beach TG, Arizona Parkinson’s Disease Consortium, concomitant pathologies among a spectrum of Parkinsonian disorders, Parkinsonism Relat. Disord. 20 (2014) 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA, Cerebrovascular disease pathology and Parkinsonian signs in old age, Stroke 42 (2011) 3183–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].de Flores R, La Joie R, Chetelat G, Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease, Neuroscience (2015). [DOI] [PubMed] [Google Scholar]
- [115].McGeer PL, Kamo H, Harrop R, McGeer EG, Martin WR, Pate BD, Li DK, Comparison of PET MRI, and CT with pathology in a proven case of Alzheimer’s disease, Neurology 36 (1986) 1569–1574. [DOI] [PubMed] [Google Scholar]
- [116].Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ, Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers, Lancet Neurol. 12 (2013) 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Albin RL, Koeppe RA, Burke JF, Giordani B, Kilbourn MR, Gilman S, Frey KA, Comparing fludeoxyglucose F18-PET assessment of regional cerebral glucose metabolism and [11C]dihydrotetrabenazine-PET in evaluation of early dementia and mild cognitive impairment, Arch. Neurol. 67 (2010) 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Blennow M, Ingvar M, Lagercrantz H, Stone-Elander S, Eriksson L, Forssberg H, Ericson K, Flodmark O, Early [18F]FDG positron emission tomography in infants with hypoxic-ischaemic encephalopathy shows hypermetabolism during the postasphyctic period, Acta Paediatr. 84 (1995) 1289–1295. [DOI] [PubMed] [Google Scholar]
- [119].Bunevicius A, Yuan H, Lin W, The potential roles of 18F-FDG-PET in management of acute stroke patients, Biomed. Res. Int. 2013 (2013) 634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B, Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B, Ann. Neurol. 55 (2004) 306–319. [DOI] [PubMed] [Google Scholar]
- [121].Yang L, Rieves D, Ganley C, Brain amyloid imaging–FDA approval of florbetapir F18 injection, N. Engl. J. Med. 367 (2012) 885–887. [DOI] [PubMed] [Google Scholar]
- [122].Jack CR Jr., H.J. Wiste, T.G. Lesnick, S.D. Weigand, D.S. Knopman, P. Vemuri, V.S. Pankratz, M.L. Senjem, J.L. Gunter, M.M. Mielke, V.J. Lowe, B.F. Boeve, R.C. Petersen, Brain beta-amyloid load approaches a plateau, Neurology 80 (2013) 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Dani M, Edison P, Brooks DJ, Imaging biomarkers in tauopathies, Parkinsonism Relat. Disord. (2015). [DOI] [PubMed] [Google Scholar]
- [124].Dugger BN, Clark CM, Serrano G, Mariner M, Bedell BJ, Coleman RE, Doraiswamy PM, Lu M, Fleisher AS, Reiman EM, Sabbagh MN, Sadowsky CH, Schneider JA, Zehntner SP, Carpenter AP, Joshi AD, Mintun MA, Pontecorvo MJ, Skovronsky DM, Sue LI, Beach TG, Neuropathologic heterogeneity does not impair florbetapir-positron emission tomography postmortem correlates, J. Neuropathol. Exp. Neurol. 73 (2014) 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Peca S, McCreary CR, Donaldson E, Kumarpillai G, Shobha N, Sanchez K, Charlton A, Steinback CD, Beaudin AE, Fluck D, Pillay N, Fick GH, Poulin MJ, Frayne R, Goodyear BG, Smith EE, Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy, Neurology 81 (2013) 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sweeney MD, Sagare AP, Zlokovic BV, Cerebrospinal fluid biomarkers of neurovascular dysfunction in mild dementia and Alzheimer’s disease, J. Cereb. Blood Flow Metab. 35 (2015) 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Brinkmalm A, Portelius E, Ohrfelt A, Brinkmalm G, Andreasson U, Gobom J, Blennow K, Zetterberg H, Explorative and targeted neuroproteomics in Alzheimer’s disease, Biochim. Biophys. Acta 1854 (2015) 769–778. [DOI] [PubMed] [Google Scholar]