Abstract
Purpose of review
Postpartum anemia (PPA) is common in women after childbirth and affects about 50–80% of all women worldwide. Iron deficiency (ID) is the main cause for anemia and constitutes a potentially preventable condition with great impact on the mother's physical and mental condition after delivery. In most cases, PPA is associated with antenatal ID and peripartum blood losses. Numerous published studies confirmed the positive effect of PPA diagnosis and treatment.
Recent findings
Iron deficiency as well as iron deficiency anemia (IDA) are common in the postpartum period and represent significant health problems in women of reproductive age.
Summary
Important movements towards early detection and therapy of postpartum anemia have been observed. However, postpartum anemia management is not implemented on a large scale as many healthcare professionals are not aware of the most recent findings in the field. Diagnosis and therapy of PPA, particularly iron supplementation in ID and IDA, has proven to be highly effective with a tremendous effect on women's wellbeing and outcome.
Keywords: anemia, iron, obstetrics, postpartum
INTRODUCTION
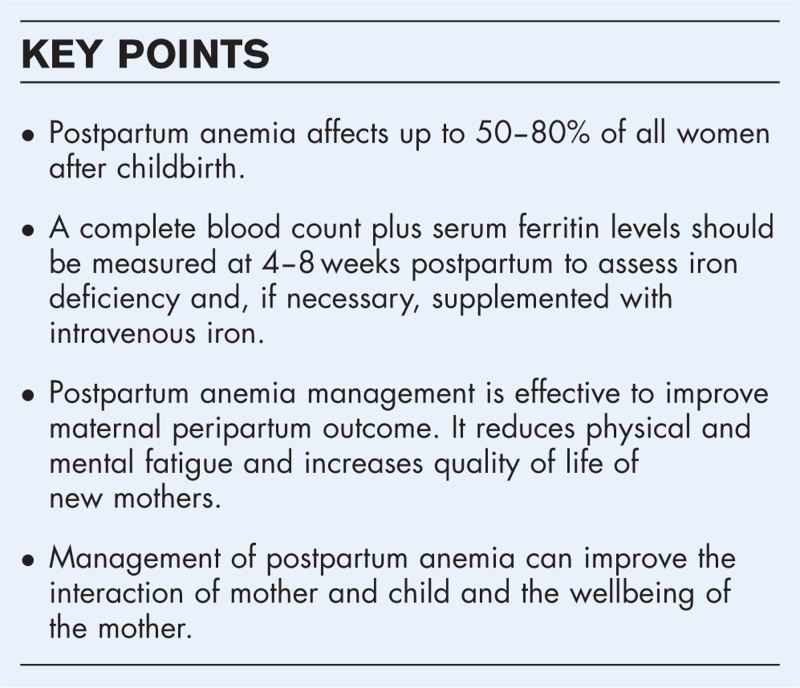
Postpartum anemia (PPA) is an increasing and severe public health issue in many parts of the world. Underlying causes are mainly untreated antenatal iron deficiency (ID) and peripartum blood loss, during or after childbirth. Although maternal iron stores are expected to replenish after delivery, the prevalence of anemia in women after childbirth remains high, in both developed (22–50%) and developing (50–80%) countries [1▪▪]. There is substantial evidence, that PPA is associated with increased morbidity in new mothers. Therefore, the management of anemia in postpartum is crucial [1▪▪,2,3]. This review illustrates the burden of PPA in women and highlights the recent state of the art of PPA management.
Box 1.
no caption available
ANEMIA IN THE PERINATAL PERIOD
According to the World Health Organization (WHO) the prevalence of anemia during pregnancy is estimated to be about 42% worldwide and still around 25% in Europe [4]. The WHO defines anemia during pregnancy as hemoglobin (Hb) values <11 g/dl, irrespective of gestational age [5]. In contrast to this definition, the Centers for Disease Control and Prevention (CDC) define anemia during pregnancy as Hb <11 g/dl in the first and third trimester, and Hb <10.5 g/dl in the second trimester [6,7].
The most common cause for anemia during pregnancy is ID, due to an increased iron demand. Increased maternal erythropoiesis is the main reason for an increased iron requirement and is estimated to be 500 mg during pregnancy. Approximately 350 mg of iron is required for fetal and placental development, and 250 mg are associated with blood loss in the peripartum period. This results in an overall maternal iron requirement of about 1 g over the entire course of pregnancy [8▪,9▪▪].
However, low iron stores during pregnancy may be carried over into the postpartum period resulting in anemia after childbirth in 5–25% [10].
The postnatal period is a critical phase in the lives of mothers and newborn babies. Most mothers recover from PPA during the weeks or sometimes months after delivery. In addition, when recovery of iron deficiency anemia (IDA) after birth takes a long time, consequences of ID and IDA may aggravate [11].
PREVALENCE OF POSTPARTUM ANEMIA
Postpartum anemia remains a persistent global health issue, affecting up to 80% of women in developing countries [12], and up to 50% of women in developed countries [13].
An analysis of 43 807 German women reveals a prevalence of PPA of 22% (defined as Hb <10.0 g/dl) 24–48 h after birth. If threshold would be a Hb value <11.0 g/dl, prevalence of anemia would be even higher, up to 50% [14]. Another retrospective study on 2990 women after vaginal delivery reveals that more than half of all women (52.1%) were anemic 24 h after birth; 45% of all women had postpartum values of Hb <11 g/dl and 7.1% had Hb values <9 g/dl [15]. Despite the correction of iron stores, their control may often be overridden by obstetric hemorrhage, a major risk during the postpartum period [16]. Together with the increase of postpartum hemorrhage (PPH), the prevalence of PPA in Germany also increased by 3.4% over the time course of five years [17].
DEFINITION OF POSTPARTUM ANEMIA
There is lack of consensus on the definition of PPA. Most guidelines define PPA as a Hb <10 g/dl after delivery, although it has also been recommended to define PPA as a Hb <11 g/dl at 1 week postpartum and <12 g/dl at 8 weeks postpartum. If Hb value is <7 g/dl, PPA should be considered severe [12].
There are different national and international evidence-based practical guidelines with different suggestions on the definition of PPA [18–23]. A recently published systematic review identified two supranational organizations [WHO and Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis (NATA)] and five guidelines developed by national professional associations covering PPA.
The WHO defines PPA as a Hb value of <11 g/dl at 1 week postdelivery and <12 g/dl in the first postpartum year [18]. In contrast, according to the “UK guidelines on the management if ID in pregnancy”, a Hb value <10.0 g/dl in the first 48 h postpartum is considered to be PPA [20]. The guideline on “Anemia and ID: Management in pregnancy and postpartum” by the Australian Health Service classifies PPA as a Hb value <10 g/dl without time frame [21]. In the French guidelines, a Hb value <11 g/dl in the first hours after delivery is considered to be anemic [22]. In Danish guidelines, Hb value should be checked within 24 h after birth and in blood loss >1000 ml [23].
WHAT ARE THE UNDERLYING CAUSES FOR POSTPARTUM ANEMIA?
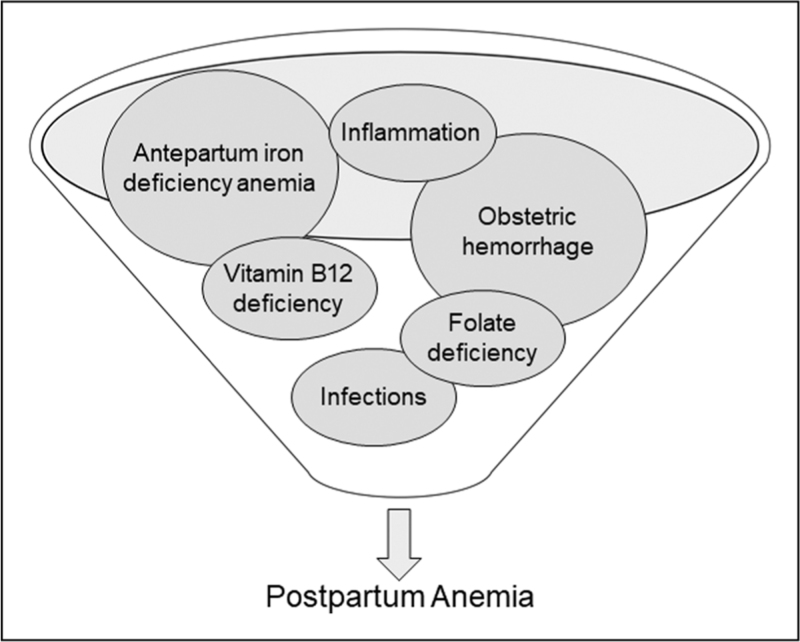
The main factors contributing to PPA are antepartum IDA or severe obstetric hemorrhage [11]. In a few women, other factors may contribute to anemia, e.g., folate and vitamin B12 deficiency and inflammatory or infectious disorders [24].
In a large observational study of 70 939 women in the United States who underwent caesarean section, the strongest risk factors for severe PPA (Hb < 8 g/dl closest to hospital discharge) were a predelivery Hb <10 g/dl [odds ratio (OR) 30.6; 95% confidence interval (CI) 27.2–34.6] and PPH (OR 8.4; 95% CI 7.8–9.2) [25]. Postpartum hemorrhage occurs in 3–8% of all deliveries [26]. Almost any complication during pregnancy, postterm deliveries, large babies, and mechanical or operative intervention during delivery are associated with increased peripartum blood losses [14]. Thus, additional risk factors that are strongly associated with severe PPA (Hb < 9 g/dl) are episiotomy (OR 3.19; 95% CI 2.10–4.84), first stage of labor >9 h (OR 2.50; 95% CI 1.58–3.94), primiparity (OR 2.50; 95% CI 1.61–3.87) and previous caesarean section (OR 2.43; 95% CI 1.51–3.90) [15]. Furthermore, assisted instrumental delivery (OR 2.72; 95% CI 1.08–6.78) and antepartum hemorrhage (OR 4.51; 95% CI 2.42–8.37) may account for immediate PPA after childbirth (Fig. 1) [27▪].
FIGURE 1.
Potential causes of postpartum anemia. Figure 1 displays different causes for the development of postpartum anemia.
IS POSTPARTUM ANEMIA HARMFUL?
Anemia is a condition in which the patient's blood has a reduced oxygen capacity to meet physiological needs due to low Hb concentration. Anemia in the postpartum period affects both, the physical and the mental health of the mother [28].
In general, anemia is associated with several adverse health consequences, such as palpitations, dizziness, weakness, impaired concentration and breathlessness [29].
Maternal morbidities linked to PPA include depression [2], fatigue [3], and impaired cognition resulting in reduced physical and mental performance [30]. These outcomes may negatively impact maternal-child bonding, the mother's ability to care for their newborn [31] and reduce the duration of breast feeding [32].
A recent meta-analysis by Moya et al.[1▪▪] in 2022 including 7 studies revealed, that women with ID or anemia in the postpartum period were 1.66 times more likely to experience symptoms of depression than nonanemic or iron-replete women [risk ratio (RR) 1.66; 95% CI 1.28–2.15]. In addition, studies showed that the administration of intravenous (i.v.) iron significantly reduces depression scores compared to women with oral iron therapy or placebo [mean difference=−1.48; 95% CI: −2.53–(−0.42)].
Anemia related depression may negatively affect the interaction of mother and child in the early postpartum period. A randomized controlled trial by Perez et al.[33] investigated the mother and child interaction in 81 women, divided into three groups: anemic women with oral iron treatment, anemic women with placebo and nonanemic women. At nine months postpartum, anemic mothers were more negative, less adept at setting appropriate goals for their infants, and less responsive compared to mothers, who were not anemic or once anemic with successful oral iron treatment (P < 0.05). To summarize, the above mentioned anemia related symptoms impair health-related quality of life and negatively affect the wellbeing of both the mother and child.
SCREENING RECOMMENDATIONS FOR POSTPARTUM ANEMIA
Limited guidelines exist on the extend of screening for anemia in postpartum women.
The NATA consensus statement on “Management of anemia and hematinic deficiencies in pregnancy and in the postpartum period” recommends the measurement of Hb concentration in all women after significant peripartum bleeding. In addition, a complete blood count plus a serum ferritin level at 4–8 weeks postpartum to assess ID should be conducted [19]. As childbirth is associated with an inflammatory response, elevated serum ferritin levels may be present [34]. Therefore, experts recommend avoidance of measuring serum ferritin levels in the first 6 weeks postpartum because of the risk of false elevation of ferritin following delivery [35].
A more precise recommendation is stated in the guidelines of the United Kingdom; after delivery, women with a blood loss >500 ml, those with uncorrected anemia detected in the antenatal period, and women with symptoms suggestive of anemia postnatally should have Hb concentration checked within 48 h of delivery [20]. The optimal postpartum time for testing for anemia is controversial. There are complex hormonal, hemodynamic and hematinic changes that occur in the postpartum period and after a normal delivery, that can take 5–7 days for the maternal extracellular and intravascular changes to reach equilibrium [11]. Therefore, the postpartum Hb value should be allowed to stabilize after delivery for at least 48 h before a reliable diagnosis of PPA can be made. The measurement of Hb concentration at one week postpartum allows a more reliable detection of PPA [13].
TREATMENT OF POSTPARTUM IRON DEFICIENCY ANEMIA
Given the fact, that antepartum ID and IDA are modifiable risk factors for anemia in the postpartum period, securing an adequate iron status during pregnancy is the first step to prevent PPA. There is substantial evidence that iron supplementation after delivery enhances the postpartum recovery of hematological and iron parameters. Postpartum anemia can be treated with either oral iron or i.v. iron supplementation. In general, guidelines recommend oral iron for mild PPA, and i.v. iron for moderate to severe anemia [16]. The NATA Network recommends 80–100 mg elemental iron daily for 3 months in women with mild to moderate PPA (Hb 9.0–11.0 g/dl) who are hemodynamically stable, asymptomatic or mildly symptomatic. In case of oral iron treatment, Hb concentration should be determined after 2–4 weeks in order to evaluate the efficacy of the treatment [19]. According to the guidelines of the United Kingdom, women with Hb <10.0 g/dl within 48 h of delivery, who are hemodynamically stable, asymptomatic, or mildly symptomatic, should be offered oral elemental iron 40–80 mg daily for at least 3 months.
The use of i.v. iron in the postpartum period should be considered in women with severe PPA, intolerance to oral iron or failed oral iron treatment [20]. More precisely, the NATA recommends the administration of i.v. iron in women with Hb value <9.0 g/dl [19].
Evidence suggests that i.v. iron should be the first-choice treatment for PPA. A recent systematic review evaluated clinical and hematological data from trials comparing oral iron with IV iron for treatment of PPA. Data shows that the maternal Hb value 6 weeks postpartum was nearly 1.0 g/dl higher among women receiving i.v. vs. oral iron [36]. Another study by Broche et al.[37] on women with postpartum Hb values <8.0 g/dl demonstrates that the administration of i.v. iron increases Hb values by 1.9 g/dl in 7 days and by 3.1 g/dl in 14 days. Furthermore, the administration of IV iron also improves medical condition of postpartum women suffering from anemia. Holm et al. supplemented i.v. iron to patients with severe fatigue after PPH and anemia. Compared to patients with oral iron supplementation, the i.v. iron group had a statistically significant and clinically relevant reduction in aggregated physical fatigue within 12 weeks after delivery [mean difference −2.3; 95% CI −3.3–(−1.3)] [38]. Another randomized trial by van Wyck et al.[39] revealed an even earlier improvement regarding the quality of life in women treated with IV iron compared to oral iron supplementation. In their study, 174 women received IV iron and 178 women oral iron with PPA (Hb ≤10 g/dl). The total fatigue score significantly improved in the IV iron group at weeks 4, 8 and 12 after delivery compared with the oral iron group.
To summarize, studies demonstrate that i.v. iron is more effective to replenish iron stores in women with PPA and should become the first choice for PPA treatment. However, it is noteworthy, that the administration of i.v. iron takes up more resources (e.g. staff, monitoring) that oral iron intake by the mother. Nonetheless, if IV iron administration is not feasible, oral iron supplementation should not be omitted.
CONCLUSION
Postpartum anemia is common and is associated with comorbidities, affecting both the physical and mental status of the mother after childbirth. The efficiency of iron supplementation in PPA has been demonstrated and therefore, postpartum management of ID and IDA in women after delivery should be mandatory. Clinicians should develop approaches and clinical care pathways to optimize anemia detection and treatment after delivery.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Germany.
Conflicts of interest
K.Z. has received honoraria for participation in advisory board meetings for Haemonetics and Vifor and received speaker fees from CSL Behring, Masimo, Pharmacosmos, Boston Scientific, Salus, iSEP, Edwards and GE Healthcare. He is the Principal Investigator of the EU-Horizon 2020 project ENVISION (Intelligent plug-and-play digital tool for real-time surveillance of COVID-19 patients and smart decision-making in Intensive Care Units) and Horizon Europe 2021 project COVend (Biomarker and AI-supported FX06 therapy to prevent progression from mild and moderate to severe stages of COVID-19). K.Z. leads as CEO the Christoph Lohfert Foundation as well as the Health, Patient Safety & PBM Foundation. P.M.'s Department received research grants from the German Research Foundation (ME 3559/1–1, ME 3559/3–1, ME 6094/3–2), BMBF (01KG1815), BMG (ZMVI1–2520DAT10E); PM received honoraria for scientific lectures from Biotest AG, CSL Behring, Haemonetics, Pharmacosmos GmbH, Vifor Pharma. V.N. received honoraria for lectures and travel expenses from Sysmex, Pharmacosmos, MCN congress organisation, and support for publication costs from the Goethe University Frankfurt.
The remaining authors have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Moya E, Phiri N, Choko AT, et al. Effect of postpartum anaemia on maternal health-related quality of life: a systematic review and meta-analysis. BMC Public Health 2022; 22:364. [DOI] [PMC free article] [PubMed] [Google Scholar]; This systematic review and meta-analysis includes 27 studies. Seven observational studies showed that iron deficient or anemic women were 1.66 times more likely to experience symptoms of depression than nonanemic or iron-replete women.
- 2.Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr 2003; 133:4139–4142. [DOI] [PubMed] [Google Scholar]
- 3.Lee KA, Zaffke ME. Longitudinal changes in fatigue and energy during pregnancy and the postpartum period. J Obstet Gynecol Neonatal Nurs 1999; 28:183–191. [DOI] [PubMed] [Google Scholar]
- 4.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009; 12:444–454. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2023; Available at: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf. [Google Scholar]
- 6.Garzon S, Cacciato PM, Certelli C, et al. Iron deficiency anemia in pregnancy: novel approaches for an old problem. Oman Med J 2020; 35:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanu FA, Hamner HC, Scanlon KS, et al. Anemia among pregnant women participating in the special supplemental nutrition program for women, infants, and children – United States, 2008–2018. MMWR Morb Mortal Wkly Rep 2022; 71:813–819. [DOI] [PubMed] [Google Scholar]
- 8▪.Wiesenack C, Meybohm P, Neef V, et al. Current concepts in preoperative anemia management in obstetrics. Cur Opin Anesthesiol 2023; 36:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides a special and holistic overview on diagnosis and treatment of prepartum anemia.
- 9▪▪.Benson AE, Shatzel JJ, Ryan KS, et al. The incidence, complications, and treatment of iron deficiency in pregnancy. Eur J Haematol 2022; 109:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides a holistic overview on management of iron deficiency in the peripartum period and during pregnancy.
- 10.Kofie P, Tarkang EE, Manu E, et al. Prevalence and associated risk factors of anaemia among women attending antenatal and postnatal clinics at a public health facility in Ghana. BMC Nutr 2019; 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butwick AJ, McDonnell N. Antepartum and postpartum anemia: a narrative review. Int J Obstet Anesth 2021; 47:102985. [DOI] [PubMed] [Google Scholar]
- 12.Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol 2011; 90:1247–1253. [DOI] [PubMed] [Google Scholar]
- 13.Milman N. Postpartum anemia II: prevention and treatment. Ann Hematol 2012; 91:143–154. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann RL, Richter R, Bergmann KE, et al. Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reprod Biol 2010; 150:126–131. [DOI] [PubMed] [Google Scholar]
- 15.Rubio-Álvarez A, Molina-Alarcón M, Hernández-Martínez A. Incidence of postpartum anaemia and risk factors associated with vaginal birth. Women Birth 2018; 31:158–165. [DOI] [PubMed] [Google Scholar]
- 16.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet 2006; 367:1066–1074. [DOI] [PubMed] [Google Scholar]
- 17.Simoes E, Kunz S, Bosing-Schwenkglenks M, et al. Mütterliche Anämie post partum - Entwicklungstendenzen und Variabilität unter Berücksichtigung unterschiedlicher Klinikkategorien. Zeitsch Geburtsh Neonatol 2004; 208:184–189. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization (WHO). Guideline: iron supplementation in postpartum women. 2016; Available at: https://apps.who.int/iris/handle/10665/249242. [PubMed] [Google Scholar]
- 19.Muñoz M, Peña-Rosas JP, Robinson S, et al. Patient blood management in obstetrics: management of anaemia and haematinic deficiencies in pregnancy and in the postpartum period: NATA consensus statement. Transfus Med 2018; 28:22–39. [DOI] [PubMed] [Google Scholar]
- 20.Pavord S, Daru J, Prasannan N, et al. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 2020; 188:819–830. [DOI] [PubMed] [Google Scholar]
- 21. Government of Western Australia. Anaemia and iron deficiency: management in pregnancy and postpartum. 2023,. Available at: https://www.kemh.health.wa.gov.au/∼/media/HSPs/NMHS/Hospitals/WNHS/Documents/Clinical-guidelines/Obs-Gyn-Guidelines/Anaemia-Management-during-Pregnancy-and-the-Postnatal-Period.pdf?thn=0. [Google Scholar]
- 22.Simon EG, Laffon M. Maternal care after vaginal delivery and management of complications in immediate postpartum – Guidelines for clinical practice. J Gynecol Obstet Biol Reprod (Paris) 2015; 44:1101–1110. [DOI] [PubMed] [Google Scholar]
- 23. Danish Society of Obstetrics and Gynecology. Anaemia and iron deficiency in pregnancy and postpartum. 2016; Available at: http://www.nfog.org/files/guidelines/NFOG_guidelines_DEN_Anaemia%20in%20pregnancy%20and%20post%20partum_2016.pdf. [Google Scholar]
- 24.Milman N. Anemia–still a major health problem in many parts of the world!. Ann Hematol 2011; 90:369–377. [DOI] [PubMed] [Google Scholar]
- 25.Butwick AJ, Walsh EM, Kuzniewicz M, et al. Patterns and predictors of severe postpartum anemia after cesarean section. Transfusion 2017; 57:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C-n, Yu F-b, Xu Y-z, et al. Prevalence and risk factors of severe postpartum hemorrhage: a retrospective cohort study. BMC Pregnancy Childbirth 2021; 21:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27▪.Agmassie GA, Alamneh GD, Ayicheh MW, et al. The magnitude and associated factors of immediate postpartum anemia among women who gave birth in east Gojjam zone hospitals, northwest- Ethiopia, 2020. PLoS One 2023; 18:e0282819. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows, that perpartum anemia and instrumental delivery are associated with immediate postpartum anemia.
- 28.Yourkavitch J, Obara H, Usmanova G, et al. A rapid landscape review of postpartum anaemia measurement: challenges and opportunities. BMC Public Health 2023; 23:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camaschella C. Iron deficiency. Blood 2019; 133:30–39. [DOI] [PubMed] [Google Scholar]
- 30.Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 2005; 135:267–272. [DOI] [PubMed] [Google Scholar]
- 31.Murray-Kolb LE, Beard JL. Iron deficiency and child and maternal health. Am J Clin Nutr 2009; 89:946s–950s. [DOI] [PubMed] [Google Scholar]
- 32.Rioux FM, Savoie N, Allard J. Is there a link between postpartum anemia and discontinuation of breastfeeding? Can J Diet Pract Res 2006; 67:72–76. [DOI] [PubMed] [Google Scholar]
- 33.Perez EM, Hendricks MK, Beard JL, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr 2005; 135:850–855. [DOI] [PubMed] [Google Scholar]
- 34.Mintsopoulos V, Tannenbaum E, Malinowski AK, et al. Identification and treatment of iron-deficiency anemia in pregnancy and postpartum: a systematic review and quality appraisal of guidelines using AGREE II. Int J Gynaecol Obstet 2023; doi: 10.1002/ijgo.14978. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Siu AL. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2015; 163:529–536. [DOI] [PubMed] [Google Scholar]
- 36.Sultan P, Bampoe S, Shah R, et al. Oral vs intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol 2019; 221:19–29. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broche DE, Gay C, Armand-Branger S, et al. Severe anaemia in the immediate postpartum period. Clinical practice and value of intravenous iron. Eur J Obstet Gyn Reprod Biol 2005; 123. [Google Scholar]
- 38.Holm C, Thomsen LL, Langhoff-Roos J. Intravenous iron isomaltoside treatment of women suffering from severe fatigue after postpartum hemorrhage. J Matern Fetal Neonatal Med 2019; 32:2797–2804. [DOI] [PubMed] [Google Scholar]
- 39.Van Wyck DB, Martens MG, Seid MH, et al. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol 2007; 110 (Pt 1):267–278. [DOI] [PubMed] [Google Scholar]