Abstract
Purpose of review
To examine impact of vaginal dysbiosis (VD), including bacterial vaginosis (BV) and aerobic vaginitis (AV) on reproductive outcomes of in vitro fertilization (IVF) patients.
Recent findings
BV-bacteria (e.g. Gardnerella) and AV-bacteria (e.g. Streptococci and Enterococci) have been identified in the endometrium. However, there is inconclusive evidence whether IVF patients with VD have lower success rates.
Summary
The present systematic review and meta-analysis of PubMed/Medline, until December 2023 included 25 studies, involving 6835 IVF patients. Overall VD was defined as an approximation of community state type IV, including BV and AV-type dysbiosis based on either molecular or microscopy methods. Outcomes were live birth rate (LBR), early pregnancy loss (EPL), clinical pregnancy rate (CPR), and biochemical pregnancy rate (BPR).
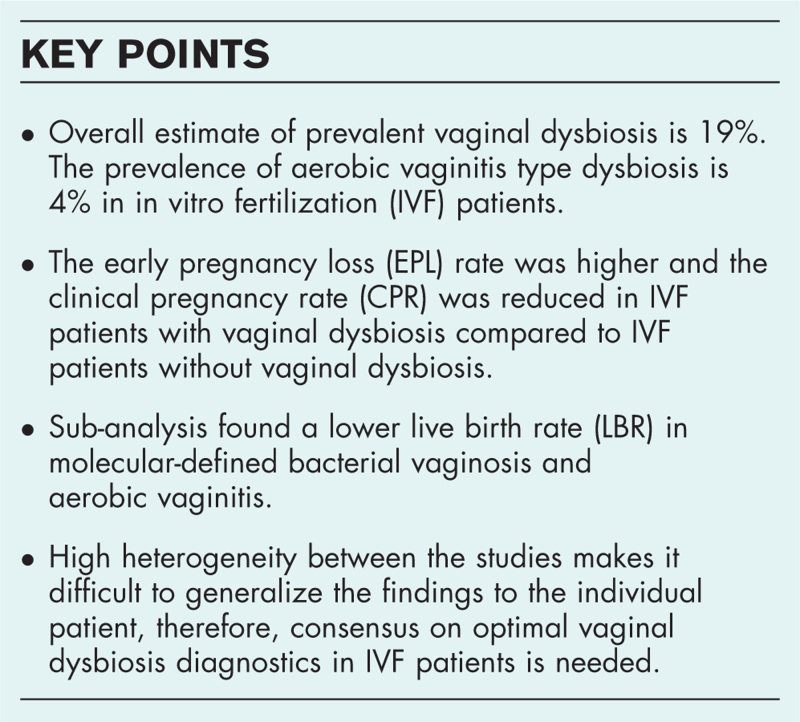
Vaginal dysbiosis prevalence was 19% [1271/6835, 95% confidence interval (CI) 18–20%]. Six studies examined AV-type dysbiosis with a prevalence of 4% (26/628, 95% CI 3–6%). Vaginal dysbiosis correlates with a higher EPL [relative risk (RR) = 1.49, 95% CI 1.15–1.94] and lower CPR (RR = 0.82, 95% CI 0.70–0.95). No statistically significant impact of VD, BV, or AV was found on LBR and BPR.
Thus, the association between VD and reproductive outcome remains puzzling as it is difficult to explain how VD impacts CPR and EPL but not LBR and BPR.
Keywords: aerobic vaginitis, bacterial vaginosis, in vitro fertilization, reproductive outcomes, vaginal dysbiosis
INTRODUCTION
Vaginal dysbiosis (VD) is an imbalance in the normal vaginal microbiota. Bacterial vaginosis (BV) is the most frequent VD and is prevalent in approximately 18% of infertile women [1]. BV is defined as a disruption of the vaginal microbiota, causing reduced abundance of Lactobacillus (L.) species (spp.), and increased presence of BV bacteria like Gardnerella (G.) spp., Fannyhessea vaginae, and Mobiluncus spp. [2]. BV may cause symptoms such as vaginal discharge and fishy odor but remains asymptomatic in up to 80% of women. Consequently, there is a risk of BV being clinically underestimated [3]. An increasing number of studies report that IVF patients with BV might be associated with poor reproductive outcomes, for example, repeated implantation failure, spontaneous abortion, and poor pregnancy rates [4–6].
Microscopic examination of vaginal fluid alongside clinical evaluation and pH measurement remains a valid method of diagnosing vaginal dysbiosis, including BV [7] using, for example, the Amsel criteria [8] or the Nugent score [9]. Research, however, has shown that the functions and diversity of the human microbiome were underestimated using microscopy methods [10]. In 2011, Ravel et al. published a study using the 16S rRNA marker gene to introduce the concept of “community state types” (CSTs). About 80% of reproductive age women can be stratified into four distinct low-diversity CSTs, each primarily dominated by a single Lactobacillus spp.: L. crispatus (CST-I), L. gasseri (CST-II), L. iners (CST-III), and L. jensenii (CST-V). The remaining 20% of women exhibited a more diverse CST-IV with multiple sub-groups covering genera like Gardnerella, Prevotella, Corynebacterium, Fannyhessea, Streptococci, and Enterococci[11].
Another common cause of VD is aerobic vaginitis (AV) type bacteria. In contrast to BV, which is considered endogenous, AV is caused by intestinal microorganisms, such as group B Streptococcus, Escherichia coli, Staphylococcus aureus, Enterococcus faecalis, and Klebsiella pneumoniae[12]. Microscopy and clinical manifestation remain the gold standard for diagnosis [13].
The impact of the vaginal microbiota on reproduction and fertility continues to attract significant scientific interest. The number of new publications considering BV and assisted reproductive technology is growing rapidly [10], questioning whether newly published data can update the current state of knowledge. Thus, the primary aim of this study was to update an earlier systematic review and meta-analysis by our group [1]. In addition to our previous methodology, we aimed to systematically summarize and differentiate between BV- and AV- types of vaginal dysbiosis to investigate their combined and individual association with reproductive outcomes in IVF patients.
Box 1.
no caption available
MATERIALS AND METHODS
The present study is a systematic review and meta-analysis of studies investigating the association between VD and predefined pregnancy outcomes in women undergoing in vitro fertilization (IVF) treatment. The analysis is an updated analysis of previously published meta-analyses [1,14] from 2019 and 2021, carrying the PROSPERO registration: CRD42016050603.
Eligibility criteria for study inclusion in this analysis were defined in our previously published study [1]. The study included infertile women undergoing IVF/intracytoplasmic sperm injection (ICSI) treatment for all causes while intrauterine insemination (IUI) patients were excluded. Sub-Saharan African studies were excluded due to a higher background prevalence of competitive co-infections with BV, such as HIV, Trichomonas vaginalis, and Chlamydia trachomatis. Additionally, case reports and reviews were excluded.
Primary outcomes were live birth rate (LBR) and early pregnancy loss (EPL). Secondary outcome measures were clinical pregnancy rate (CPR), defined as ultrasound-verified intrauterine heartbeat, and biochemical pregnancy rate (BPR) defined as human chorionic gonadotropin (hCG) serum-positive pregnancies, according to local laboratory standard [1]. The common denominator was per embryo transfer except EPL, which was biochemical pregnancies. For the calculation of prevalence, we used N = patients as the denominator. Many studies did not provide information about EPL and, thus, clinical pregnancies were subtracted from biochemical pregnancies to deduct the number of early pregnancy losses. In this updated meta-analysis, we decided to use the ongoing pregnancy rate instead of the CPR, if CPR was defined in the individual studies as an intrauterine gestational sac with or without fetal heartbeat by transvaginal ultrasound. In these cases, we decided ongoing pregnancy rate was a more precise outcome, according to our criteria.
In a study by Van den Tweel et al.[17▪▪], the authors provided the raw data upon request, so additional statistics have been performed, separating IVF/ICSI patients from IUI patients to assess the outcomes.
LITERATURE SEARCH STRATEGY
The PubMed (Medline) database was used to make an updated systematic literature search, using relevant keywords and MeSH terms (Supplementary Material 1). M.M. and A.S.H. initially screened publications based on their titles, followed by an abstract review. If an abstract contained elements related to the eligibility criteria and/or outcomes, the publication was read in full. In case of doubt, T.H. was consulted to reach a conclusive decision regarding the inclusion of the study. Further searches were carried out in Embase, Scopus, and Cochrane using the keywords ‘IVF’ and ‘microbiota’. Before conducting the meta-analysis, we repeated the literature search on December 16, 2023, to identify new publications.
QUALITY OF ARTICLES
To evaluate the quality of the individual studies, the Newcastle-Ottawa Scale (NOS) [18] was performed for each study included in our systematic review and meta-analysis [15,16▪▪,17▪▪,19–21,22▪▪,23▪], as presented in Supplementary Material 2. We did not use GRADE [24] for assessing the quality of evidence per outcome.
DATA EXTRACTION AND VAGINAL DYSBIOSIS DEFINITION
The prevalence of VD was calculated by summarizing all the included studies (Supplementary Material 3). In the studies that analyzed both molecular and microscopy methods, only the result for the molecular method was used [16▪▪,25,26].
Data extraction included the following characteristics in the individual studies: author, analysis method, outcomes, and sample size of individual studies (Supplementary Material 4–7).
Overall VD is herein defined broadly as a CST IV like microbiota with whatever method used in the individual studies, including microscopy methods of BV and AV diagnosis [11]. Moreover, this meta-analysis included subgroups of VD: (1) overall BV- or AV-type (2) microscopy or molecular defined BV-/AV-type. Microscopy comprised all studies based on, for example, Nugent and Amsel's criteria of BV as well as wet-smear criteria to diagnose AV. The molecular group constituted qPCR, IS-pro technique, and 16S rRNA gene sequencing. When possible, we sub-stratified for BV-type and AV-type dysbiosis by manually approximating to VALENCIA subgroups [11] of CST IV-A and IV-B as BV-type whereas CST IV-C was AV-type. In case sub-stratification of the CST-IV group was not possible in the individual studies they were not considered for sub analyses. Results for two studies [4,27] from the previous meta-analysis [1] are updated with AV results (Supplementary Material 3). The corresponding control group to the abovementioned groups did not have VD by any method. For example, and when possible, we subtracted BV patients from the control group in case AV-type microbiota was investigated.
STATISTICS
The relative risk (RR) with a 95% confidence interval, Forest plot, and Funnel plot was determined using a random effects model. This analysis utilized the Mantel–Haenszel method in REVIEW Manager version 5.3 (Cochrane, London, UK) [28]. Regarding VD prevalence, calculations were performed using R (version 4.1.3; RStudio).
RESULTS
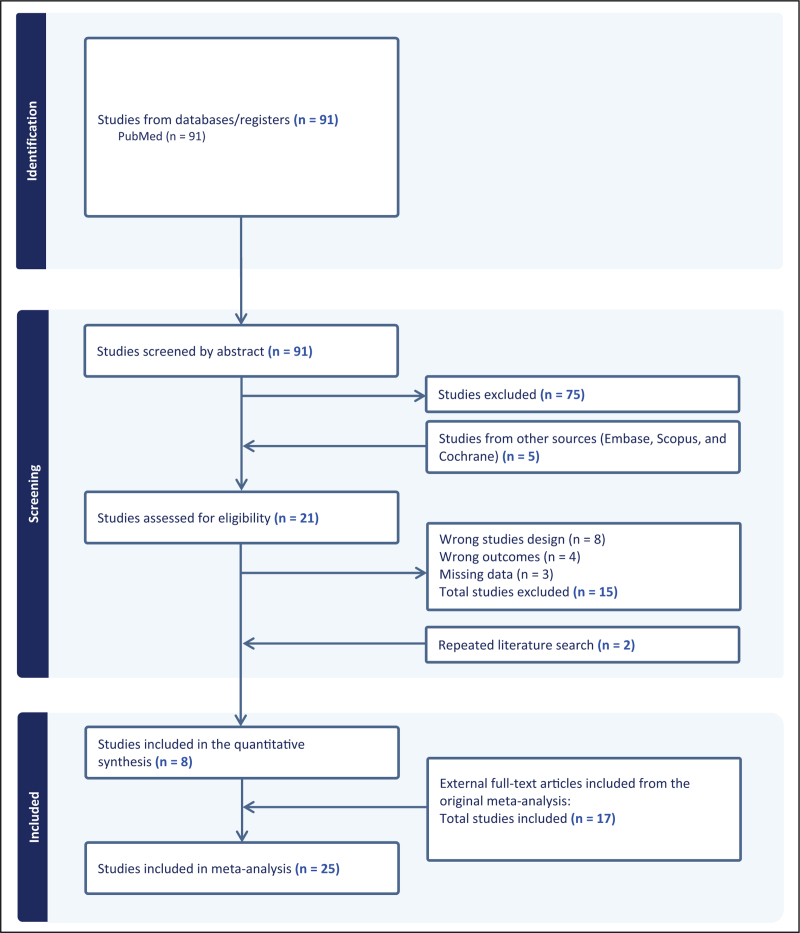
The present literature search found 91 publications as of October 7, 2023. A total of 75 citations were excluded based on title and abstract. Hence, a total of 16 citations [15,16▪▪,19–21,29,30–39] were assessed for eligibility by examination of the full text. Seven studies were removed due to study design [29,33–36,38,39] and four were removed as it was not possible to extract pregnancy outcome data for the meta-analysis [30–32,37]. Further searches in additional databases yielded five relevant papers [23▪,40–43], however, only the study by Väinämö et al.[23▪] met the eligibility criteria and was included. Additionally, we contacted the authors of four studies [40–43] to provide additional data. From three studies [40–43], we received no response, and consequently, their data could not be evaluated. The authors of one study [41], provided data, however, the study did not meet the inclusion criteria regardless. In the repeated literature search, two articles [17▪▪,22▪▪] met the eligibility criteria, as the authors of Van den Tweel et al. provided data upon request [17▪▪]. Thus, a total of eight studies were included [15,16▪▪,17▪▪,19–21,22▪▪,23▪] and added to the 17 studies [4,25–27,44–56] previously included in a systematic review and meta-analysis published by our group [1]. Thus, the present meta-analysis included 25 studies [4,15,16▪▪,17▪▪,19–21,22▪▪,23▪,25–27,44–56], comprising 6835 IVF patients. The full selection of the studies is shown in the flow diagram, Fig. 1.
FIGURE 1.
Vaginal dysbiosis – the association with reproductive outcomes in IVF-patients: a systematic PRISMA review and meta-analysis. IVF, in vitro fertilization. Created with www.covidence.org.
PREVALENCE OF VAGINAL DYSBIOSIS, AEROBIC VAGINITIS, AND INDIVIDUAL STUDY CHARACTERISTICS
The overall prevalence of VD was 18.6% (1271/6835, 95% CI 17.7–19.5%) (Supplementary Material 3). The prevalence of AV was 4.1% (26/628, 95% CI 2.7–6.0%) (Supplementary Material 3). In general, only six studies [4,16▪▪,19,21,22▪▪,27] were included in the present analysis considering AV.
The heterogeneity of VD prevalence was large, ranging from 4% (53) to 45% [20].
Seventeen publications were based on microscopy [15,16▪▪,21,23▪,25–27,44–53], and in these studies the BV prevalence was 17% (1007/6026) whereas the overall BV prevalence was 24% (323/1339) for molecular methods. The timing of sampling was different across the included studies. Nine studies performed sampling on oocyte retrieval day [16▪▪,21,25,44,47,48,51–53], whereas eight studies performed the sampling on the embryo transfer day [15,20,22▪▪,45,46,49,50,55], and four studies performed the sampling prior to IVF stimulation [23▪,26,50,56], with BV prevalence of 16% (462/2829), 32% (333/1057), and 17% (400/2290) respectively. In three studies, the sampling was performed in different phases of the menstrual cycle or IVF stimulation [17▪▪,19,55]. In one study, the time of sampling was not reported [27]. A full view of the individual study characteristics of the eight studies included can be seen in Table 1[15,16▪▪,17▪▪,19–21,22▪▪,23▪].
Table 1.
Study characteristics
| Study | Method | VD prevalence | Age (normal microbiota) | Age BV | Treatment | Timing of sampling | Country/ethnicity | IVF cycle of sampling |
| Eskew et al. | 16S rRNA | 24.0% | 18–43 (range) for all included patients | Azithromycin orally, 1 g daily once for both partners on the day of initiation of injectable gonadotropins for controlled ovarian stimulation | At 3 time points: 1. immediately before baseline ultrasound; 2. immediately before egg retrieval; and 3. immediately before embryo transfer | USA/no data | No data on cycle | |
| Ji et al. | Nugent score | 40.4% | 30.1 (mean) | 29.7 (mean) | No | On the day of endometrial transformation | China/no data | Frozen embryo transfer cycles. Some patients participated more than once |
| Karaer et al. | 16S rRNA | 44.8% | 23–39 (range) for all included patients | No | On the day of embryo transfer | Turkey/no data | No data on cycle | |
| Koort et al. | 16S rRNA and Nugent score | 26.9% | 34.1 (average) for all included patients | No | On follicle puncture day | Estonia/no data | No data on cycle | |
| Vieira-Baptista et al. | Amsel's criteria | 17.9% | 34.6 (mean) for all included patients | No | Immediately before vaginal disinfection and oocyte retrieval (IVF/ICSI) or IUI | Portugal/no data | No data on cycle | |
| Väinämö et al. | 16S rRNA | 22.6% | 32.9 (mean) for all included patients | No | At the time of fresh embryo transfer and at the eighth gestational week from those women who got pregnant | Finland/no data | No data on cycle | |
| Van den Tweel et al. | 16S rRNA | 32.3% | 37.1 (mean) | 38.5 (mean) | No | Vaginal samples taken at oocyte retrieval /at the last ultrasound before the frozen embryo transfer | Netherlands/60% Caucasian | Any cycle |
| Zeng et al. | Nugent score | 19.0% | 30.8 (mean) | 31.35 (mean) | Women with other bacterial morphologies or BV-positive (Nugent score 7–10) were empirically treated with Ornidazole via vagina for a week. No control after therapy with Ornidazole | Vaginal sample within 2 months before IVF and embryo transfer | China/no data | First cycle |
BV, bacterial vaginosis; IVF, in vitro fertilization; VD, vaginal dysbiosis.
Live birth rate
Among 4605 patients, a total of 1640 live births were reported (Supplementary Material 8, Supplementary Material 12). Compared to IVF patients without VD, the relative risk (RR) for LBR in patients with VD was 0.94 (95% CI 0.76–1.16, I2 = 46%), Table 2.
Table 2.
Vaginal dysbiosis (VD) – relative risk on reproductive outcomes
| Outcome | RR (CI 95%) | No. of participants (studies) |
| Primary outcomes | ||
| Live birth rate (CST IV) | 0.94 (0.76–1.16) | 4605 (14) |
| Live birth rate (BV) | 0.96 (0.76–1.21) | 4359 (13) |
| Microscopy | 1.02 (0.75–1.40) | 3776 (8) |
| Molecular | 0.76 (0.51–1.13) | 658 (6) |
| Early pregnancy loss (CST IV) | 1.49 (1.15–1.94) | 3153 (20) |
| Early pregnancy loss (BV) | 1.62 (1.26–2.08) | 3045 (19) |
| Microscopy | 1.51 (1.16–1.96) | 2792 (14) |
| Molecular | 2.35 (1.36–4.07) | 291 (6) |
| Secondary outcomes | ||
| Clinical pregnancy rate (CST IV) | 0.82 (0.70–0.95) | 6550 (25) |
| Clinical pregnancy rate (BV) | 0.85 (0.71–1.01) | 6092 (22) |
| Microscopy | 0.85 (0.69–1.05) | 5488 (16) |
| Molecular | 0.81 (0.61–1.07) | 781 (8) |
| Biochemical pregnancy rate (CST IV) | 0.95 (0.82–1.10) | 5455 (17) |
| Biochemical pregnancy rate (BV) | 0.95 (0.82–1.10) | 5455 (17) |
| Microscopy | 0.98 (0.82–1.17) | 4938 (13) |
| Molecular | 0.78 (0.59–1.02) | 601 (5) |
BV, bacterial vaginosis; CI, confidence interval; CST, community state type; RR, relative risk.
Considering subgroup analyses and compared to the corresponding control group, the overall BV-type RR was 0.96 (95% CI 0.76–1.21, I2 = 50%). For BV by microscopy, the RR was 1.02 (95% CI 0.75–1.40, I2 = 65%) whereas for BV-type by molecular methods the RR was 0.76 (95% CI 0.51–1.13, I2 = 15%), Table 2.
For IVF patients with AV-type microbiota and compared to IVF patients without VD, the RR was 0.76 (95% CI 0.33–1.72, I2 = 0%). Based on methodology, RR was 0.87 (95% CI 0.37–2.07, I2 = 0%) for microscopy and 0.18 (95% CI 0.01–2.73, heterogeneity not applicable) for molecular methods (Table 3, Supplementary Material 7d–f, Supplementary Material 11a–c, Supplementary Material 15a–c).
Table 3.
Aerobic vaginitis (AV) – relative risk on reproductive outcomes
| Outcome | RR (CI 95%) | No. of oarticipants (studies) |
| Primary outcomes | ||
| Live birth rate | 0.76 (0.33–1.72) | 442 (4) |
| Microscopy | 0.87 (0.37–2.07) | 355 (2) |
| Molecular | 0.18 (0.01–2.73) | 87 (2) |
| Early pregnancy loss | 0.57 (0.04–7.85) | 201 (4) |
| Microscopy | 0.57 (0.04–7.85) | 161 (2) |
| Molecular | Not estimable | 40 (2) |
| Secondary outcomes | ||
| Clinical pregnancy rate | 0.78 (0.37–1.66) | 545 (6) |
| Microscopy | 0.84 (0.35–1.98) | 360 (2) |
| Molecular | 0.60 (0.11–3.40) | 185 (4) |
| Biochemical pregnancy rate | 0.82 (0.35–1.93) | 391 (3) |
| Microscopy | 0.90 (0.39–2.09) | 401 (2) |
| Molecular | Not estimable | 31 (1) |
Early pregnancy loss
Out of 3153 patients with hCG-positive pregnancy, a total of 571 early pregnancy losses were reported (Supplementary Material 9, Supplementary Material 13).
Overall VD patients undergoing IVF had a significantly higher risk for EPL compared to patients without VD, RR 1.49 (95% CI 1.15–1.94, I2 = 38%) (Table 2).
Sub-analysis for overall BV-type revealed a RR of 1.62 (95% CI 1.26–2.08, I2 = 25%) whereas BV by microscopy had a RR of 1.51 (95% CI 1.16–1.96, I2 = 23%) and BV by molecular methods had a RR of 2.35 (95% CI 1.36–4.07, I2 = 8%) (Table 2).
For AV-type there was only one study and when compared to IVF patients without VD the RR was 0.57 (95% CI 0.04–7.85, heterogeneity not applicable), as seen in Table 3, Supplementary Material 7d–f, Supplementary Material 11d–f, Supplementary Material 15d–f.
Clinical pregnancy
Within 6550 patients, a total of 2555 clinical pregnancies were reported (Supplementary Material 10a–c, Supplementary Material 14a–c). Overall VD patients undergoing IVF had significantly lower RR 0.82 (95% CI 0.70–0.95, I2 = 49%) for CPR per embryo transfer compared to IVF patients without VD (Table 2).
Sub analysis for BV-type revealed an overall RR of 0.85 (95% CI 0.71–1.01, I2 = 54%). Considering microscopy methods for BV diagnosis the RR was 0.85 (95% CI 0.69–1.05, I2 = 63%) the RR was 0.81 (95% CI 0.61–1.07, I2 = 8%) for molecular BV methods (Table 2).
For AV, RR was 0.78 (95% CI 0.37–1.66, I2 = 14%). Based on methodology, RR was 0.84 (95% CI 0.35–1.98, I2 = 0%) for microscopy and 0.60 (95% CI 0.11–3.40, I2 = 61%) for molecular methods (Table 3, Supplementary Material 7g–i, Supplementary Material 11g–i, Supplementary Material 15g–i).
Biochemical pregnancy
In total, 2549 biochemical pregnancies per embryo transfer were reported among 5455 patients (Supplementary Material 10d–f, Supplementary Material 14d–f). Compared to IVF patients without VD, the RR for BPR for patients with VD was 0.95 (95% CI 0.82–1.10, I2 = 39%) for BPR per embryo transfer. Subgroup analyses revealed RR of 0.98 (95% CI 0.82–1.17, I2 = 54%) for microscopy BV methods and 0.78 (95% CI 0.59–1.02, I2 = 0%) for molecular BV methods (Table 2).
For AV, RR was 0.82 (95% CI 0.35–1.93, I2 = 0%). Based on methodology, RR was 0.90 (95% CI 0.39–2.09, I2 = 0%) for microscopy and not estimable for molecular methods (Table 3, Supplementary Material 7j–l, Supplementary Material 11j–l, Supplementary Material 15j–l).
DISCUSSION
Four meta-analyses were previously published, investigating the association of VD with reproductive outcomes in IVF patients, two of them by our group [1,14,57,58]. Overall, the analyses reported lower clinical pregnancy rates [14,58] and higher early pregnancy loss rates [1,14,57] in IVF patients with BV compared to IVF patients without BV. The present study contributes with eight recently published studies and reports a significant increase in EPL in IVF patients with VD (RR 1.49, 95% CI 1.15–1.94), confirming previous findings. The overall CPR was significantly decreased in women with VD (RR 0.82, 95% CI 0.70–0.95). Our previous study from 2021 [1] only detected a statistically significant decrease in CPR in the sub-group of molecular methods RR 0.55 (95% CI 0.32–0.93), which in the present study was not statically significant and closer to unity, RR 0.81 (95% CI 0.61–1.07).
In the present systematic review and meta-analysis, the overall prevalence of VD was 18.6% (95% CI 17.7–19.5), which was similar to the prevalence reported in our previous study, in which the prevalence of VD was 18% [1], and higher compared to our first study, which was 16% [14]. Although we believe a rough estimate of 19% VD-positive IVF patients is probably a good overall estimate, this may be affected by different methods to diagnose individual patients with VD. All the studies that included both microscopy and molecular methods reported a higher VD prevalence with molecular methods [16▪▪,25,26]. Prevalence of BV-type dysbiosis differed between the molecular methods: qPCR, IS-pro technique, and or 16S technique, with BV-type dysbiosis prevalence of 24%, 18%, and 34% respectively (Table 1, Supplementary Material 3). An explanation might be that molecular methods are more sensitive and reveal more patients with BV – for example, more optimal classification of patients in the Nugent intermediate group. Ultimately, the different definitions need to be subjected to treatment in order to investigate optimal classification in terms of impact on reproductive success.
Only six studies [4,16▪▪,19–22▪▪,27] reported data on AV-type dysbiosis. The present meta-analysis did not find any statistically significant association between AV-type dysbiosis and reproductive outcomes, but the number of patients was too small to be conclusive. However, RR estimates for LBR and CPR were considerably lower in the presence of AV compared to patients without VD, especially when using molecular methods to define AV, where the RR was 0.18 (95% CI 0.01–2.73) for LBR and 0.60 (95% CI 0.11–3.40) for CPR.
STRENGTHS AND LIMITATIONS
The strength of this study is the inclusion of eight new studies for a total of 25 studies, (Fig. 1). The additional data for molecular and microscopy methods enabled the sub-stratification to BV- and AV-type dysbiosis. Despite a higher number of studies included, there remains interstudy heterogeneity, which may be overcome in a future meta-analysis by individual participant meta-analysis linking the microbiome data directly to IVF outcome across studies. The most relevant explanation for high heterogeneity is the use of different diagnostic methods for VD across the studies. The present study aimed to counter some of this heterogeneity by stratifying for molecular and microscopy methods as well as sub-stratifying patients with BV- and AV-type microbiota. Differences in VD prevalence between studies can also be explained by the timing of sampling during the different phases of the menstrual cycle and different phases of IVF treatment (Table 1). Hormonal fluctuations influence the vaginal microbiota during the cycle [59]. Previous studies indicated that vaginal bacterial diversity at the time of transfer, as opposed to other time points may be associated with clinical outcomes [19,59]. However, other studies did not find any difference regarding timing of sampling [55]. Future design of systematic reviews may need to stratify according to the time of sampling.
Seven studies used antibiotics during IVF treatment, possibly resulting in biased results [19,46,48,50,51,60]. In the study by Zeng et al.[23▪], patients with symptomatic BV (Nugent score 7–10) underwent a 7-day Ornidazole treatment. Despite treatment, the results of the study suggest that BV might still adversely affect pregnancy. On the other side, we excluded a randomized control trial (RCT) study by Thanaboonyawat et al.[39], in which probiotics were systematically administered, and the results suggest their positive influence on reproductive outcomes.
CONCLUSION
The present systematic review and meta-analysis concludes that the presence of a broad definition of VD in IVF patients is significantly associated with a higher early pregnancy loss rate and lower clinical pregnancy rate compared to IVF patients without VD. The sub-analysis found a lower live birth rate in molecular-defined BV and AV, although none of these outcomes reached statistical significance. More studies reporting live birth rate might change this in the future. However, different molecular methods and different criteria were used across the studies, which make it difficult to generalize these findings to the individual patient. Consensus about optimal VD diagnostics may enable more robust evidence concerning the prevalence, reproductive outcome and risks of VD in IVF patients.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Skafte-Holm A, Humaidan P, Bernabeu A, et al. The association between vaginal dysbiosis and reproductive outcomes in sub-fertile women undergoing IVF-treatment: a systematic PRISMA review and meta-analysis. Pathogens 2021; 10:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coudray MS, Madhivanan P. Bacterial vaginosis—a brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol 2020; 245:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klebanoff MA, Schwebke JR, Zhang J, et al. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol 2004; 104:267–272. [DOI] [PubMed] [Google Scholar]
- 4.Bernabeu A, Lledo B, Diaz MC, et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet 2019; 36:2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracewell-Milnes T, Saso S, Nikolaou D, et al. Investigating the effect of an abnormal cervico-vaginal and endometrial microbiome on assisted reproductive technologies: a systematic review. Am J Reprod Immunol 2018; 80:e13037. [DOI] [PubMed] [Google Scholar]
- 6.Haahr T, Jensen JS, Humaidan P. Vaginal microbiota and IVF outcomes: poor diagnosis results in flawed conclusions. Reprod Biomed Online 2019; 39:178. [DOI] [PubMed] [Google Scholar]
- 7.Sherrard J, Wilson J, Donders G, et al. 2023 update to 2018 European (IUSTI/WHO) guideline on the management of vaginal discharge. Int J STD AIDS 2023; 34:745. [DOI] [PubMed] [Google Scholar]
- 8.Amsel R, Totten PA, Spiegel CA, et al. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 74:14–22. [DOI] [PubMed] [Google Scholar]
- 9.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Velasco JA, Menabrito M, Catalán IB. What fertility specialists should know about the vaginal microbiome: a review. Reprod Biomed Online 2017; 35:103–112. [DOI] [PubMed] [Google Scholar]
- 11.France MT, Ma B, Gajer P, et al. VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 2020; 8:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaambo E, Africa C, Chambuso R, Passmore JS. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front Public Health 2018; 6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donders GGG, Bellen G, Grinceviciene S, et al. Aerobic vaginitis: no longer a stranger. Res Microbiol 2017; 168:845–858. [DOI] [PubMed] [Google Scholar]
- 14.Haahr T, Zacho J, Brauner M, et al. Reproductive outcome of patients undergoing in vitro fertilisation treatment and diagnosed with bacterial vaginosis or abnormal vaginal microbiota: a systematic PRISMA review and meta-analysis. BJOG 2019; 126:200–207. [DOI] [PubMed] [Google Scholar]
- 15.Ji L, Peng C, Bao X. Effect of vaginal flora on clinical outcome of frozen embryo transfer. Front Cell Infect Microbiol 2022; 12:987292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪▪.Koort K, Sosa K, Turk S, et al. Lactobacillus crispatus-dominated vaginal microbiome and Acinetobacter-dominated seminal microbiome support beneficial ART outcome. Acta Obstet Gynecol Scand 2023; 102:921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined both vaginal and semen samples using 16 s rRNA gene sequencing to investigate how microbiome composition affects ART outcomes. The findings indicate that disruptions in the microbiome of both partners may result in poor reproductive outcome. It is recommended that microbial screening needs to be integrated into the standard practice for ART patients.
- 17▪▪.van den Tweel MM, van den Munckhof EHA, van der Zanden M, et al. Bacterial vaginosis in a subfertile population undergoing fertility treatments: a prospective cohort study. J Assist Reprod Genet 2023; 41:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]; An observational prospective study investigated the impact of bacterial vaginosis (BV), using qPCR and 16S rRNA gene microbiota analysis of V1-V2 region, on pregnancy rates across various fertility treatments (IUI/IVF/ICSI). The study found no significant differences in ongoing pregnancy or live birth rates based on BV status or microbiome community state type (CST). However, ongoing pregnancy rate was slightly lower in the BV qPCR positive group. The study suggests an increased risk in miscarriages in patients with positive BV qPCR status and in those categorized under CST group III and IV. Additionally, baseline qPCR positive participants had significantly higher body mass index and smoked more often.
- 18. Wells Gas B, O’Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf. [Google Scholar]
- 19.Eskew AM, Stout MJ, Bedrick BS, et al. Association of vaginal bacterial communities and reproductive outcomes with prophylactic antibiotic exposure in a subfertile population undergoing in vitro fertilization: a prospective exploratory study. F S Sci 2021; 2:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karaer A, Dogan B, Gunal S, et al. The vaginal microbiota composition of women undergoing assisted reproduction: a prospective cohort study. BJOG 2021; 128:2101–2109. [DOI] [PubMed] [Google Scholar]
- 21.Vieira-Baptista P, Silva-Soares S, Lyra J, et al. Wet mount microscopy of the vaginal milieu does not predict the outcome of fertility treatments: a cross-sectional study. J Low Genit Tract Dis 2022; 26:176–180. [DOI] [PubMed] [Google Scholar]
- 22▪▪.Väinämö S, Saqib S, Kalliala I, et al. Longitudinal analysis of vaginal microbiota during IVF fresh embryo transfer and in early pregnancy. Microbiol Spectr 2023; 11:e0165023. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study explored how the vaginal microbiota, particularly Lactobacillus crispatus, affects IVF success and early pregnancy. Results revealed a higher abundance of L. crispatus in women who achieved clinical pregnancy and live birth compared to nonpregnant women. During early pregnancy, Lactobacilli, especially L. crispatus, dominated all early pregnancy samples. Microbiota shifted from one Lactobacillus-dominated type to another, or from mixed to Lactobacillus-dominated, but never from Lactobacillus-dominated to non-Lactobacillus-dominated. The presence of L. crispatus was associated with better IVF outcomes, highlighting its significance in reproductive health.
- 23▪.Zeng H, He D, Hu L, et al. Non-Lactobacillus dominance of the vagina is associated with reduced live birth rate following IVF/ICSI: a propensity score-matched cohort study. Arch Gynecol Obstet 2022; 305:519–528. [DOI] [PubMed] [Google Scholar]; The retrospective cohort study investigated whether the dominance of Lactobacillus in the vagina is associated with IVF/ICSI outcomes. They analyzed data from 2285 women undergoing ART, who were divided into Lactobacillus-dominant and non-Lactobacillus-dominant groups. The non-Lactobacillus-dominant group had lower rates of biochemical and clinical pregnancy, as well as live birth, but higher rates of preclinical pregnancy loss and preterm birth. The miscarriage and ectopic pregnancy rates were similar between the two groups.
- 24. Holger Schünemann JB, Guyatt G, Oxman A. GRADE handbook. Available at: https://gdt.gradepro.org/app/handbook/handbook.html [Accessed 20 January 2021]. [Google Scholar]
- 25.Mangot-Bertrand J, Fenollar F, Bretelle F, et al. Molecular diagnosis of bacterial vaginosis: impact on IVF outcome. Eur J Clin Microbiol Infect Dis 2013; 32:535–541. [DOI] [PubMed] [Google Scholar]
- 26.Haahr T, Jensen JS, Thomsen L, et al. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum Reprod 2016; 31:795–803. [DOI] [PubMed] [Google Scholar]
- 27.Moragianni D, Dryllis G, Andromidas P, et al. Genital tract infection and associated factors affect the reproductive outcome in fertile females and females undergoing in vitro fertilization. Biomed Rep 2019; 10:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The Nordic Cochrane Centre. Review Manager (RevMan) [Computer Program]; Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre; 2014. [Google Scholar]
- 29.Hamdoun M, Braham M, Kacem K, et al. Does bacterial colonization during embryo transfer have an impact on pregnancy rate in ICSI?: Tunisian preliminary results. J Gynecol Obstet Hum Reprod 2021; 50:101727. [DOI] [PubMed] [Google Scholar]
- 30.Saxtorph MH, Hallager T, Persson G, et al. Assessing endometrial receptivity after recurrent implantation failure: a prospective controlled cohort study. Reprod Biomed Online 2020; 41:998–1006. [DOI] [PubMed] [Google Scholar]
- 31.Jain M, Mladova E, Dobychina A, et al. Comparison of microbial profiles and viral status along the vagina-cervix-endometrium continuum of infertile patients. Syst Biol Reprod Med 2023; 69:310–319. [DOI] [PubMed] [Google Scholar]
- 32.Lledo B, Fuentes A, Lozano FM, et al. Identification of vaginal microbiome associated with IVF pregnancy. Sci Rep 2022; 12:6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong Y, Sun Q, Shao X, Wang Z. Effect of vaginal microbiota on pregnancy outcomes of women from Northern China who conceived after IVF. Front Endocrinol (Lausanne) 2023; 14:1200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lüth T, Graspeuntner S, Neumann K, et al. Improving analysis of the vaginal microbiota of women undergoing assisted reproduction using nanopore sequencing. J Assist Reprod Genet 2022; 39:2659–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Tweel MM, van der Struijs S, van den Munckhof EHA, Boers KE. The relationship between vaginal pH and bacterial vaginosis as diagnosed using qPCR in an asymptomatic subfertile population. Arch Gynecol Obstet 2022; 306:1787–1793. [DOI] [PubMed] [Google Scholar]
- 36.Farooq Faisal S, Adnan Abdul Hameed W, Alwasiti E. The influence of vaginal dysbiosis on intracytoplasmic sperm injection outcome. Arch Razi Inst 2023; 78:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jepsen IE, Saxtorph MH, Englund ALM, et al. Probiotic treatment with specific lactobacilli does not improve an unfavorable vaginal microbiota prior to fertility treatment—a randomized, double-blinded, placebo-controlled trial. Front Endocrinol (Lausanne) 2022; 13:1057022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanha FD, Rahmani Z, Rezaei Z, et al. The effect of normalizing vaginal microbiome using Lactovag in improving pregnancy outcomes in frozen embryo transfer cycles: a randomized clinical trial. Arch Gynecol Obstet 2023; 308:1587–1592. [DOI] [PubMed] [Google Scholar]
- 39.Thanaboonyawat I, Pothisan S, Petyim S, Laokirkkiat P. Pregnancy outcomes after vaginal probiotic supplementation before frozen embryo transfer: a randomized controlled study. Sci Rep 2023; 13:11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Martínez MDC, Bernabeu A, Lledó B, et al. Impact of the vaginal and endometrial microbiome pattern on assisted reproduction outcomes. J Clin Med 2021; 10:4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans GE, Mahajan V, Wakeman S, et al. A pilot study using unique targeted testing of the urogenital microbiome has potential as a predictive test during IVF for implantation outcome. Arch Gynecol Obstet 2023; 307:1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vajpeyee M, Tiwari S, Yadav LB, Tank P. Assessment of bacterial diversity associated with assisted reproductive technologies through next-generation sequencing. Middle East Fertil Soc J 2022; 27:28. [Google Scholar]
- 43.Kong Y, Liu Z, Shang Q, et al. The disordered vaginal microbiota is a potential indicator for a higher failure of in vitro fertilization. Front Med (Lausanne) 2020; 7:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ 1999; 319:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selim SA, El Alfy SM, Aziz MH, et al. Effective of metronidazole to bacterial flora in vagina and the impact of microbes on live birth rate during intracytoplasmic sperm injection (ICSI). Arch Gynecol Obstet 2011; 284:1449–1453. [DOI] [PubMed] [Google Scholar]
- 46.Eckert LO, Moore DE, Patton DL, et al. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect Dis Obstet Gynecol 2003; 11:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liversedge NH, Turner A, Horner PJ, et al. The influence of bacterial vaginosis on in-vitro fertilization and embryo implantation during assisted reproduction treatment. Hum Reprod 1999; 14:2411–2415. [DOI] [PubMed] [Google Scholar]
- 48.Gaudoin M, Rekha P, Morris A, et al. Bacterial vaginosis and past chlamydial infection are strongly and independently associated with tubal infertility but do not affect in vitro fertilization success rates. Fertil Steril 1999; 72:730–732. [DOI] [PubMed] [Google Scholar]
- 49.Boomsma CM, Kavelaars A, Bozkurt N, et al. Is bacterial vaginosis associated with a pro-inflammatory cytokine profile in endometrial secretions of women undergoing IVF? Reprod Biomed Online 2010; 21:133–141. [DOI] [PubMed] [Google Scholar]
- 50.Eldivan O, Evliyaoglu O, Ersoy E, et al. Does screening for vaginal infection have an impact on pregnancy rates in intracytoplasmic sperm injection cycles? Turk J Obstet Gynecol 2016; 13:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moini A, Mohammadi Yeganeh L, Shiva M, et al. Bacterial vaginosis and the risk of early miscarriage in women undergoing intracytoplasmic sperm injection cycles: a prospective cohort study. Hum Fertil (Camb) 2018; 21:263–268. [DOI] [PubMed] [Google Scholar]
- 52.Moore DE, Soules MR, Klein NA, et al. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril 2000; 74:1118–1124. [DOI] [PubMed] [Google Scholar]
- 53.Spandorfer SD, Neuer A, Giraldo PC, et al. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. J Reprod Med 2001; 46:806–810. [PubMed] [Google Scholar]
- 54.Koedooder R, Singer M, Schoenmakers S, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 2019; 34:1042–1054. [DOI] [PubMed] [Google Scholar]
- 55.Kyono K, Hashimoto T, Nagai Y, Sakuraba Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: a single-center pilot study. Reprod Med Biol 2018; 17:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vergaro P, Tiscornia G, Barragan M, et al. Vaginal microbiota profile at the time of embryo transfer does not affect live birth rate in IVF cycles with donated oocytes. Reprod Biomed Online 2019; 38:883–891. [DOI] [PubMed] [Google Scholar]
- 57.van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod 2013; 28:1809–1815. [DOI] [PubMed] [Google Scholar]
- 58.Singer M, Borg M, Ouburg S, Morre SA. The relation of the vaginal microbiota to early pregnancy development during in vitro fertilization treatment—a meta-analysis. J Gynecol Obstet Hum Reprod 2019; 48:223–229. [DOI] [PubMed] [Google Scholar]
- 59.Hyman RW, Herndon CN, Jiang H, et al. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J Assist Reprod Genet 2012; 29:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.