Abstract
INTRODUCTION:
This post hoc analysis evaluated the efficacy of tenapanor on abdominal symptoms in patients with irritable bowel syndrome with constipation. Abdominal symptoms assessed included pain, discomfort, bloating, cramping, and fullness.
METHODS:
The abdominal symptom data were pooled from 3 randomized controlled trials (NCT01923428, T3MPO-1 [NCT02621892], and T3MPO-2 [NCT02686138]). Weekly scores were calculated for each abdominal symptom, and the Abdominal Score (AS) was derived as the average of weekly scores for abdominal pain, discomfort, and bloating. The overall change from baseline during the 12 weeks was assessed for each symptom weekly score and the AS. The AS 6/12-week and 9/12-week response rates (AS improvement of ≥2 points for ≥6/12- or ≥9/12-week) were also evaluated. The association of weekly AS response status (reduction of ≥30%) with weekly complete spontaneous bowel movement (CSBM) status (=0 and >0) was assessed.
RESULTS:
Among 1,372 patients (684 tenapanor [50 mg twice a day] and 688 placebo), the least squares mean change from baseline in AS was −2.66 for tenapanor vs −2.09 for placebo (P < 0.0001). The 6/12-week AS response rate was 44.4% for tenapanor vs 32.4% for placebo (P < 0.0001), and for 9/12-week AS, 30.6% for tenapanor vs 20.5% for placebo (P < 0.0001). A significant association between weekly CSBM status and weekly AS response status was observed each week (P < 0.0001), with a greater proportion achieving an AS reduction in patients with >0 CSBMs in a week.
DISCUSSION:
Tenapanor significantly reduced abdominal symptoms in patients with irritable bowel syndrome with constipation, particularly pain, discomfort, and bloating measured by AS, compared with placebo.
KEYWORDS: abdominal symptoms, tenapanor, Abdominal Score
INTRODUCTION
Irritable bowel syndrome (IBS) is a common disorder of gut-brain interaction characterized by abdominal pain and altered bowel movements (1). IBS is more common among women and in individuals younger than 50 years (2). IBS is classified according to the predominant bowel habit, namely, IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), or IBS with mixed bowel habits (IBS-M) (2). As defined by the Rome diagnostic criteria, the estimated prevalence of IBS ranges from 4.8% (Rome IV) to 8.8% (Rome III) in the US adult population (3), with IBS-C accounting for approximately 16% (Rome IV) to 29% (Rome III) of these cases (3). The more stringent Rome IV diagnostic criteria may underestimate the true prevalence and clinical and economic impact of IBS (4).
Individuals with IBS-C have more bothersome, frequent, and widespread abdominal pain than those with other IBS subtypes (5). They also experience other abdominal symptoms, such as bloating, discomfort, cramping, and fullness (6,7). As the pathophysiology of IBS is multifactorial, the cause of these symptoms is not completely understood. However, nonclinical studies have found that visceral hypersensitivity (8,9) and increased intestinal permeability (10–12) have been linked to abdominal pain.
Abdominal symptoms are associated with substantial morbidity, increased use of healthcare resources, decreased work productivity, and compromised health-related quality of life (13–15). Although randomized placebo-controlled studies of polyethylene glycol (16) and soluble fiber (17) demonstrated improvement in bowel movements relative to placebo, they did not significantly relieve abdominal pain or discomfort. In an online survey of patients diagnosed with IBS-C, only 37% of those taking over-the-counter treatment were satisfied with their response (18).
To assess abdominal symptoms experienced by patients with IBS-C in clinical trials, the IBS Working Group of the Critical Path Institute's Patient-Reported Outcome Consortium, with the US Food and Drug Administration (FDA) guidance on patient-reported outcomes, developed a new patient-reported outcomes instrument, the Diary for IBS Symptoms-Constipation (6,19). The Abdominal Score (AS) is a novel composite score derived from the Diary for IBS Symptoms-Constipation that measures 3 key abdominal symptoms associated with IBS: bloating, pain, and discomfort (19). AS was previously used as the primary endpoint in a phase 3b study examining the efficacy of linaclotide in reducing combined symptoms of pain, bloating, and discomfort through 12 weeks of treatment in IBS-C (20).
Tenapanor, a first-in-class, minimally absorbed, small molecule inhibitor of intestinal sodium/hydrogen exchanger 3, reduces intestinal sodium absorption, leading to retention of luminal water in the gut (21,22) that facilitates accelerated intestinal transit and softer stool consistency (21). Preclinical studies demonstrated that sodium/hydrogen exchanger 3 inhibition with tenapanor reduced intestinal permeability as measured by increased transepithelial electrical resistance (23,24). Tenapanor also inhibited transient receptor potential cation channel subfamily V member 1 signaling, resulting in reduced visceral hypersensitivity and abdominal pain (25). The degree to which these findings contribute to tenapanor's benefits for abdominal symptoms in patients with IBS-C remains to be determined.
In a phase 2 study (NCT01923428), tenapanor 50 mg twice a day (bid) significantly reduced abdominal symptoms compared with placebo (26). In 2 subsequent, phase 3 studies (T3MPO-1 [NCT02621892] and T3MPO-2 [NCT02686138]), tenapanor significantly increased the percentage of patients with IBS-C meeting the abdominal pain responder definition (a decrease of ≥30% in average weekly worst abdominal pain score from baseline) (27,28). In T3MPO-1, 6 of 12-week and 9 of 12-week responder rates were significantly higher with tenapanor than placebo (27). Similarly, in T3MPO-2, a significantly greater proportion of patients receiving tenapanor were 6 of 12-week, 9 of 12-week, and 13 of 26-week abdominal pain responders compared with patients receiving placebo (28). In addition, tenapanor had an early onset of action with robust improvements in abdominal pain observed by week 1 and maintained throughout the treatment period (27,28).
Although abdominal pain is considered a clinical hallmark of IBS, patients with IBS-C describe an array of additional bothersome abdominal symptoms, including discomfort, bloating, cramping, and fullness (6). To determine the overall effectiveness of tenapanor on abdominal symptoms, we conducted a post hoc analysis of data from the phase 2b and 3 studies to assess key IBS-associated abdominal symptoms, as well as the novel AS.
METHODS
Patients and study design
Study designs and primary results of the phase 2b, T3MPO-1, and T3MPO-2 studies have been previously reported (26–28). All 3 studies were multicenter, randomized, double-blind, placebo-controlled trials conducted in the United States that enrolled patients meeting Rome III criteria for IBS-C. The phase 2b study was conducted at 79 sites (August 2013–October 2014), T3MPO-1 at 92 sites (November 2015–March 2017), and T3MPO-2 at 92 sites (December 2015–August 2017).
In this post hoc analysis, data were pooled from the intent-to-treat populations of patients with IBS-C who received tenapanor 50 mg bid or placebo bid during the first 12 weeks of treatment of the phase 2b, T3MPO-1, and T3MPO-2 studies. Individual study analysis of the intent-to-treat populations is also reported. Patients were included in the analysis if they met the individual study eligibility criteria, were randomized, and received ≥1 dose of study drug.
In all 3 studies, IBS abdominal symptoms and constipation severity were assessed weekly through an interactive voice response system telephone diary. The interactive voice response system diary collected information on daily stool frequency, stool consistency, straining, abdominal symptoms (pain, discomfort, bloating, fullness, and cramping; each on an 11-point scale where 0 = absent and 10 = very severe), and rescue medication usage (see Supplement Information, Supplementary Digital Content 1, http://links.lww.com/AJG/D192).
Abdominal symptom scores, endpoints, and statistical analysis
Weekly scores for each abdominal symptom were calculated as the average score for all days during a week with ≥4 days of reporting of the given abdominal symptom (i.e., a valid week). In this analysis, the AS was calculated as the average of weekly scores for abdominal pain, discomfort, and bloating; this approach reflects the grouping validated by Coon et al (19).
The overall change from baseline in AS during the first 12 weeks of treatment (pooled population, phase 2b study, T3MPO-1) and during the 26-week treatment period (T3MPO-2) was assessed using a mixed-effects model with repeated measures (MMRM). The MMRM included fixed-effect factors of treatment, week, and treatment-by-week; fixed-effect covariates of baseline AS and baseline-by-week; and patient as a random effect. An unstructured (UN) covariance was used in each MMRM analysis by default. When the UN model failed to converge, a heterogeneous autoregression (ARH(1)) covariance structure was used. The cumulative distribution of the change from baseline in AS for tenapanor and placebo in week 12 (pooled population, phase 2b study, T3MPO-1) and in week 26 (T3MPO-2) was compared using the Wilcoxon rank sum test, and the P value was estimated using a Monte Carlo approach.
Weekly AS response was defined as achieving a reduction of ≥2 points in AS for a given week, which has been shown to be an appropriate threshold for clinically meaningful change in patients (19). Weekly response rates were analyzed using the Pearson χ2 test in which patients with a missing weekly AS due to discontinuation or an invalid week were included in the calculation and assumed to have no response in that week (i.e., a worst-case imputation approach).
Six of 12-week and 9 of 12-week AS responses were defined as achieving a weekly AS response for ≥6 or ≥9 weeks of the first 12 weeks. In T3MPO-2, a 13 of 26-week AS response was defined as achieving an AS response for ≥13 weeks of the 26-week treatment period. The 6 of 12-, 9 of 12-, and 13 of 26-week response rates were also analyzed using the Pearson χ2 test, with the worst-case imputation approach applied to determine a patient's response status for each week of the treatment period.
The association of the weekly AS response status with the weekly CSBM status (=0 or >0) of the pooled data was assessed using the Cochran-Mantel-Haenszel test. The CSBM status of each patient is the weekly CSBM score for the corresponding study week. The Cochran-Mantel-Haenszel P value was based on a 1 degree of freedom test for association between weekly AS response status and weekly CSBM status, stratified by the pooled investigator site.
RESULTS
Patient disposition, demographics, and baseline characteristics
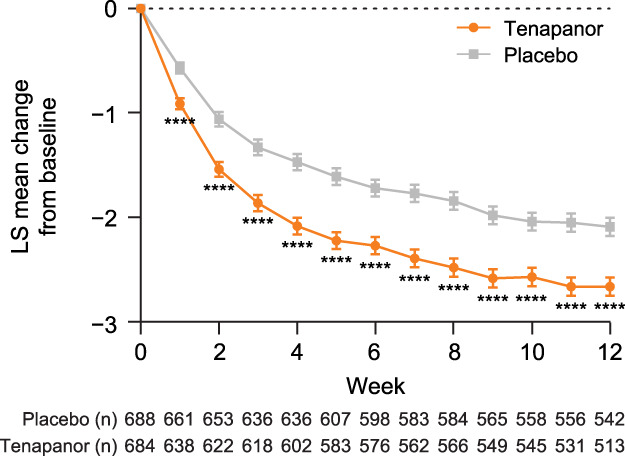
For this post hoc analysis, the pooled population of patients with IBS-C included 684 in the tenapanor group and 688 in the placebo group. Demographic and baseline characteristics of the pooled population were similar between the tenapanor and placebo groups (Table 1). Most patients were women (82.4%); the mean age was 45.3 years; and the mean weekly CSBM frequency was 0.16 at baseline. Weekly scores of baseline abdominal symptoms ranged from 5.99 to 6.69.
Table 1.
Patient demographics and baseline characteristics (pooled population)
Weekly abdominal symptoms
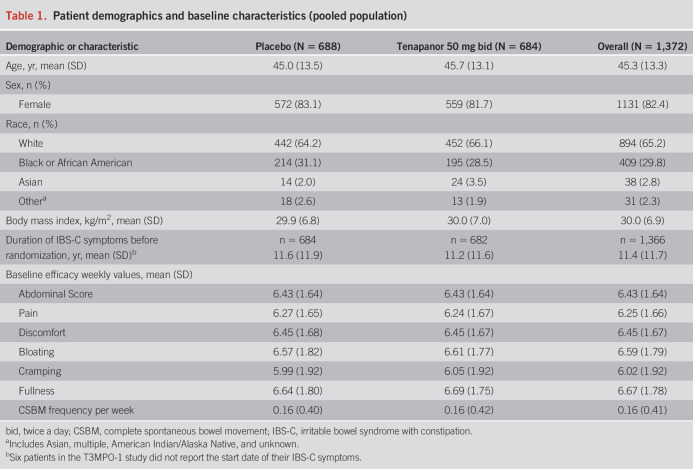
Improvements in abdominal pain, discomfort, bloating, cramping, and fullness were seen as early as the first week of treatment in the pooled population (Figure 1) and in all 3 studies separately (see Supplementary Figures S1–S3, Supplementary Digital Content 1, http://links.lww.com/AJG/D192). For patients with IBS-C who received tenapanor, significant improvements were observed in the average weekly scores from baseline to week 12 for abdominal pain, discomfort, bloating, and cramping during the phase 2b study (see Supplementary Table S1, Supplementary Digital Content 1, http://links.lww.com/AJG/D192); for abdominal pain, discomfort, bloating, cramping, and fullness during T3MPO-1 (see Supplementary Table S2, Supplementary Digital Content 1, http://links.lww.com/AJG/D192); and for the same symptoms, with the exception of abdominal cramping, from baseline to week 26 during T3MPO-2 (see Supplementary Table S3, Supplementary Digital Content 1, http://links.lww.com/AJG/D192).
Figure 1.
Raw mean change from baseline in average weekly score of each abdominal symptom over the first 12 weeks of treatment (pooled population). (a) Abdominal pain, (b) abdominal discomfort, (c) abdominal bloating, (d) abdominal cramping, and (e) abdominal fullness. Error bars represent standard error. n, the number of patients included in the summary in the corresponding study week.
Change in Abdominal Score over 12- and 26-week treatment periods
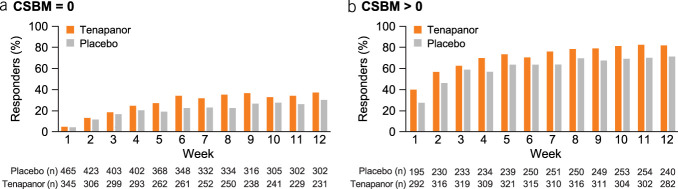
The least squares (LS) mean AS change from baseline of the pooled population was greater with tenapanor compared with placebo over the first 12 weeks of treatment (−2.66 vs −2.09, P < 0.0001) (Figure 2). Similar trends were observed in the phase 2b and T3MPO-1 studies (see Supplementary Figures S4a and S4b, Supplementary Digital Content 1, http://links.lww.com/AJG/D192), while in T3MPO-2, the significant difference between tenapanor and placebo in the LS mean AS change was sustained for the 26-week treatment period (see Supplementary Figure S4c, Supplementary Digital Content 1, http://links.lww.com/AJG/D192). At week 12, cumulative distribution of change from baseline in AS significantly favored tenapanor over placebo (estimated P < 0.0001; 99% confidence interval <0.0001–<0.0001) in the pooled population (Figure 3) and in the individual studies (see Supplementary Figure S5, Supplementary Digital Content 1, http://links.lww.com/AJG/D192).
Figure 2.
LS mean change from baseline in Abdominal Score over the first 12 weeks of treatment (pooled population). Error bars represent SE. LS mean, SE, and P values came from an unstructured MMRM with fixed-effect factors of treatment, week, and treatment-by-week; fixed-effect covariates of baseline Abdominal Score and baseline-by-week; and patient as a random effect. ****P < 0.0001. Abdominal Score was defined as the mean of weekly scores for abdominal pain, discomfort, and bloating. LS, least squares; MMRM, mixed-effects model with repeated measures; n, the number of patients included in the summary in the corresponding study week.
Figure 3.
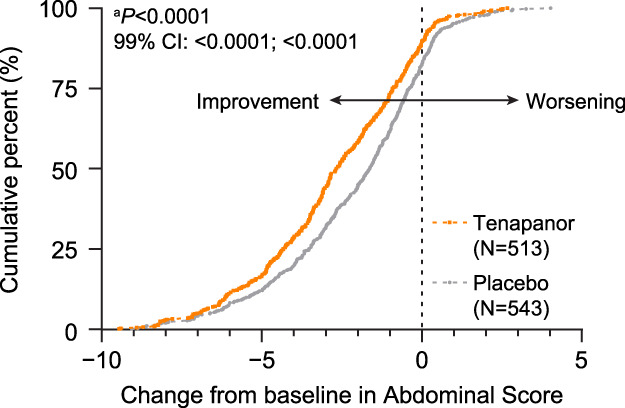
Cumulative distribution function of change from baseline to week 12 in Abdominal Score (pooled population). The cumulative distribution function plot illustrates the percentage of patients (y-axis) achieving a certain level of response as assessed by the change from baseline in Abdominal Score (x-axis). This plot shows that the tenapanor group had a consistently higher percentage of patients achieving a certain level of response compared with the placebo group, supporting the efficacy of tenapanor. Negative values of change from baseline are indicative of improvement, whereas positive values indicate worsening. aEstimated P-value comparing the cumulative distribution functions between treatment arms was obtained from a Wilcoxon 2-sample test. Abdominal Score was defined as the average of weekly scores for abdominal pain, discomfort, and bloating.
Abdominal Score response rates over 12- and 26-week periods
Weekly AS response rates were consistently higher with tenapanor compared with placebo in the pooled population over the first 12 weeks (Figure 4) and in the individual studies (see Supplementary Figure S6, Supplementary Digital Content 1, http://links.lww.com/AJG/D192). Patients receiving tenapanor had significantly higher 6 of 12-week and 9 of 12-week AS response rates compared with patients receiving placebo in the pooled population (Figure 5). Similar patterns were observed in the individual studies for the 6 of 12-week, 9 of 12-week, and 13 of 26-week response rates (see Supplementary Figure S7, Supplementary Digital Content 1, http://links.lww.com/AJG/D192).
Figure 4.
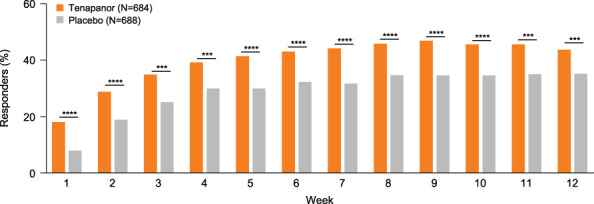
Weekly Abdominal Score response rate over the first 12 weeks of treatment (pooled population). The weekly Abdominal Score response was defined as achieving a reduction from baseline of ≥2 points in Abdominal Score for a given postbaseline week. The response rate was analyzed using the Pearson χ2 test with a worst-case imputation approach (patients with missing data included and assumed to have no response). ****P < 0.0001. The Abdominal Score was defined as the average of weekly scores for abdominal pain, discomfort, and bloating.
Figure 5.
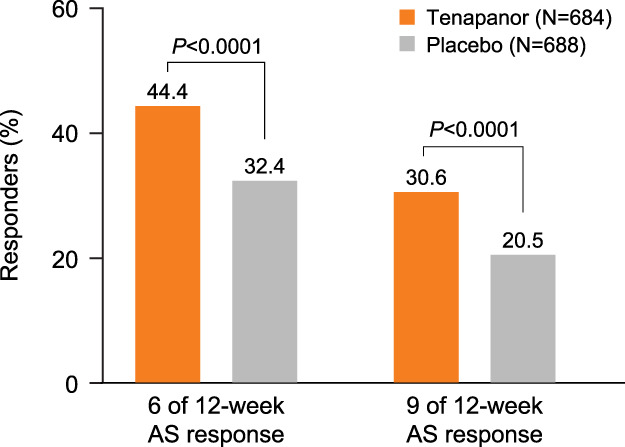
Six of 12-week and 9 of 12-week Abdominal Score response rates (pooled population). The 6 of 12- or 9 of 12-week Abdominal Score response was defined as achieving weekly Abdominal Score response with ≥2-point reduction in a week for ≥6 or 9 weeks of the first 12 weeks of treatment. Response rates were analyzed using the Pearson χ2 test with a worst-case imputation approach (patients with missing data included and assumed to have no response). P values were obtained from Pearson χ2 tests. The Abdominal Score was defined as the average of weekly scores for abdominal pain, discomfort, and bloating. AS, Abdominal Score.
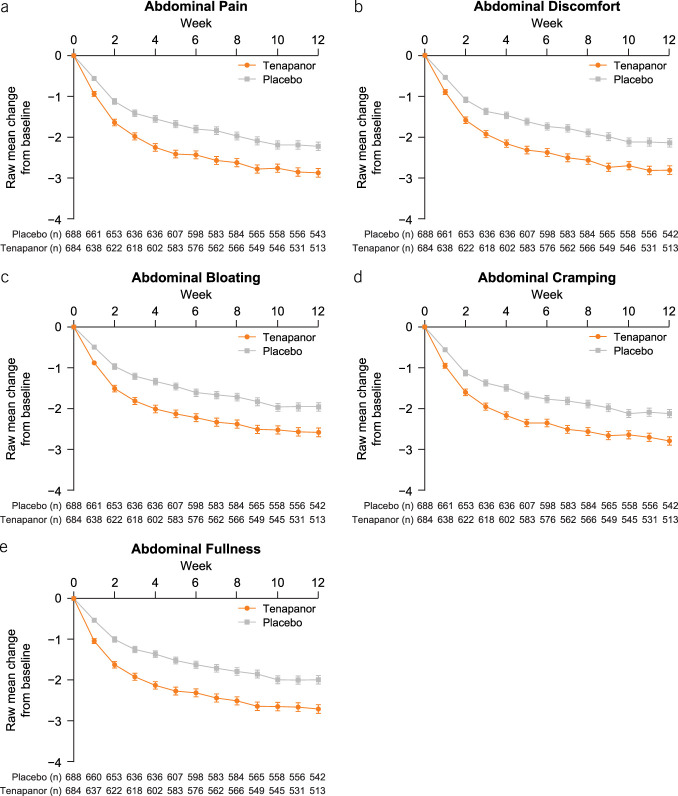
Association between weekly Abdominal Score response and weekly CSBM status
There was a statistically significant association (P < 0.0001) between weekly CSBM status and weekly AS response status in each study week (Figure 6), with a greater proportion achieving an AS reduction of ≥30% in patients with >0 CSBMs in a week compared with patients with 0 CSBMs in a week. This association was also significant when the weekly AS response was defined as achieving a reduction of ≥2 points in AS for a given postbaseline week (P < 0.0001; see Supplementary Figure S8, Supplementary Digital Content 1, http://links.lww.com/AJG/D192).
Figure 6.
Association of weekly Abdominal Score response status with weekly CSBM status. The weekly Abdominal Score response was defined as achieving a reduction of ≥30% in Abdominal Score for a given postbaseline week. Weekly CSBM status = 0 (a) or > 0 (b) for a given postbaseline week. The association of weekly Abdominal Score status with the weekly CSBM status was assessed using the Cochran-Mantel-Haenszel test. The Abdominal Score was defined as the average of weekly scores for abdominal pain, discomfort, and bloating. n, number of patients included in the summary in the corresponding study week. CSBM, complete spontaneous bowel movement.
Safety
Adverse events of the individual studies have been previously reported (26–28). Diarrhea is the most common event in the pooled population receiving tenapanor (n = 102, 14.8%). The first event of diarrhea occurred mostly within the first month of tenapanor treatment, with the median time to onset of 4.5 days (interquartile range 2–23 days; see Supplementary Figure S9, Supplementary Digital Content 1, http://links.lww.com/AJG/D192).
DISCUSSION
IBS-C is a clinically heterogeneous disorder, and available treatments may not simultaneously improve both bowel movements and the range of abdominal symptoms that patients experience (abdominal pain, discomfort, bloating, cramping, and fullness) (2,6,18,29,30). Tenapanor has previously demonstrated clinical significance in improving CSBM and abdominal pain in phase 2 and phase 3 clinical studies, leading to FDA approval for use in adult patients with IBS-C (26–28). Here, we demonstrate that tenapanor also significantly decreases average weekly scores for abdominal bloating, discomfort, cramping, and fullness from baseline to week 12 compared with placebo for patients with IBS-C. These improvements were observed as early as week 1 or 2 and sustained through 12 weeks.
The AS is a validated measure developed in conjunction with the FDA to assess global IBS-C symptoms. The AS, calculated as the average of the weekly scores of 3 key abdominal symptoms (bloating, pain, and discomfort), demonstrated a significantly greater and sustained change from baseline with tenapanor in each week over the 12-week treatment period compared with placebo (P ≤ 0.0001) in the pooled study population. In addition, the weekly AS response for the T3MPO-2 study demonstrated a sustained change from baseline with tenapanor over a 26-week treatment period compared with placebo. Significantly greater proportions of tenapanor-treated patients achieved ≥2-point improvement in AS for ≥6 and ≥9 of the first 12 weeks of treatment in the pooled population, and for ≥13 of the 26-week treatment period in the T3MPO-2 study, when compared with placebo. The weekly AS response status of tenapanor-treated patients had significant association with the weekly CSBM status.
Abdominal pain, a defining feature of IBS (31), increases IBS severity and healthcare visits, and together with bloating, decreases quality of life (13,14,18). The use of the composite AS enables assessment of clinically meaningful improvement of 3 key abdominal symptoms. In the phase 3b clinical trial of linaclotide for IBS-C that used AS as its primary endpoint, the LS mean change from baseline was −1.90 for linaclotide vs −1.18 for placebo (P < 0.0001) over 12 weeks of treatment (20), representing a difference of 0.72 points. This treatment difference was marginally greater than that observed in the pooled population, −2.66 for tenapanor vs −2.09 for placebo (P < 0.0001), with a difference of 0.57 points. This difference may be due to the nature of a post hoc analysis of data pooled from 3 studies of tenapanor vs a randomized placebo-controlled trial of linaclotide in which AS was the primary outcome. However, as with patients treated with linaclotide (20), greater proportions of patients treated with tenapanor had a consistent, significant reduction compared with placebo (P < 0.0001) in the change from baseline in 12-week AS. Furthermore, 44.4% of patients treated with tenapanor in the pooled population achieved a decrease of ≥2 points in AS in ≥6 of the first 12 weeks of treatment compared with 32.4% for placebo (P < 0.0001), representing a difference of 12.0%. This treatment difference was slightly lower than that observed in the phase 3b study of linaclotide in IBS-C, where 40.5% of patients treated with linaclotide were 6 of 12-week AS responders, compared with 23.4% of placebo, with a representative difference of 17.1%. The 2 studies had similar baseline efficacy values for the abdominal symptoms, with only marginally higher weekly CSBM frequency in the linaclotide study (0.26 vs 0.16). The age and sex of the patients in the 2 studies were also comparable with the tenapanor post hoc analysis including more Black, African American patients (29.8% vs 23.8%), but fewer Asian patients (2.8% vs 11.6%). Investigations with greater representation of these race groups are required to analyze responses to different treatments. Furthermore, responder rate differences between tenapanor and placebo are statistically significant (10%–12%) and felt to be clinically important because a ≥10% difference was considered the minimal clinically important threshold in IBS pharmacotherapy guidelines (32,33). In addition, like other IBS-C efficacy trials, real-world evidence will verify clinical significance.
Tenapanor exhibits a more gradual onset of improvement in abdominal symptoms compared with the almost immediate effect on CSBM (27,28). However, the beneficial effects of tenapanor relative to placebo for abdominal symptoms were significant from the first week of treatment. This suggests that the mechanisms of tenapanor-mediated CSBM improvement may differ with those of tenapanor-mediated abdominal symptom improvement. The mechanisms whereby tenapanor alleviates abdominal pain likely contribute to the gradual onset of response but have not been fully characterized. Nonclinical models of IBS-like colonic hypersensitivity suggest tenapanor reduces visceral hypersensitivity and normalizes colonic sensory neuronal excitability and transient receptor potential cation channel subfamily V member 1 currents (25). In addition, the study of colonic monolayer cultures from patients with IBS-C found that tenapanor modulated intestinal tight junctions and decreased permeability to macromolecules, which may enhance the etiology of abdominal symptoms (24). Further investigations to understand the antinociceptive action of tenapanor may clarify the gradual improvement in abdominal symptoms. However, the advantage of a treatment improving both abdominal symptoms and CSBM is beneficial to the totality of symptoms in patients with IBS-C.
Tenapanor has demonstrated a consistent and acceptable safety profile in phase 2b and phase 3 studies (26–28). The most commonly reported adverse event in all 3 studies was diarrhea, occurring in 9%–15% of tenapanor-treated patients. Diarrhea as an adverse event is consistent with the known mechanism of action of tenapanor: an increase in stool water content and accelerated intestinal transit. Diarrhea is generally transient, lasting for ≤1 week, and is mostly mild to moderate in severity (28).
A strength of this post hoc analysis is the pooling of data from 3 studies, resulting in a sample size larger than that in any of the individual studies. The larger sample size allows for a more precise estimate of the treatment effect, which will assist clinicians and patients in decision making regarding the use of tenapanor for IBS-C.
One limitation of this post hoc analysis is the use of Rome III criteria rather than Rome IV to diagnose and enroll patients in the individual studies because these studies started enrollment before the release of Rome IV criteria. However, Rome III criteria may be more clinically applicable because they are less stringent than Rome IV criteria (34). Like other IBS-C clinical trials (35–37), the stringent entry criteria for these studies may also reflect enrollment of a different population than that seen in real-world practice.
In conclusion, we demonstrate that tenapanor significantly improves abdominal symptoms, including pain, bloating, discomfort, cramping, and fullness, in patients with IBS-C, with an early onset of action that increases over time and is sustained throughout the treatment period. Responder rates of AS support these findings. The effects of tenapanor on the AS of the pooled population were verified by the analyses of AS of the independent studies. Cumulating evidence suggests that AS is a clinically meaningful evaluation of abdominal symptoms described by patients.
CONFLICTS OF INTEREST
Guarantor of the article: David P. Rosenbaum, PhD.
Specific author contributions: A.J.L., W.D.C., L.A.H., R.F., D.M.B., L.C., and B.E.L. were responsible for data curation and investigation. D.P.R., Y.Y., S.Z., and S.E. conceptualized the study. Y.Y. and S.Z. were responsible for the formal analysis and reviewed the manuscript critically for statistical content. A.J.L., W.D.C., L.A.H., R.F., D.M.B., L.C., B.E.L., D.P.R., and S.E. contributed to the critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript for submission.
Financial support: This study was funded by Ardelyx.
Potential competing interests: A.J.L. is a consultant for Allergan, Ardelyx, Atmo, Allakos, BioAmerica, AEON, Arena, Takeda, Evoke Pharma, Ironwood Pharmaceuticals, Aeon, Gemelli, Alkermes, Pfizer, OrthoMed, and Vibrant and has stock with Johnson & Johnson, Bristol Myer Squibb, and Allurion. W.D.C. is a consultant for Abbvie, Ardelyx, Arena, Baush, Biomerica, Gemelli, Ironwood, Isothrive, Nestle, Progenity, Salix, Takeda, Urovant, and Vibrant and has stock options with GI on Demand/Gastro Girl, Isothrive, and Modify Health. L.A.H. has received financial support from Ardelyx, AbbVie, Alnylam, Ironwood, Gemelli Biotech, Salix, and Takeda. R.F. has no conflict of interest. D.M.B. is a consultant, advisor, and/or speaker for Anji, Ardelyx, Abbive, Alnylam, Salix, Ironwood, Takeda, Bayer, Redhill, Mahana, Laborie, Owlstone, Entrinsic Bioscience, Vibrant and is a member of the Board of Directors of the International Foundation for GI Disorders (IFFGD). L.C. has served as a member of the scientific advisory boards for Ardelyx, Atmo, Immunic, Ironwood, and Vibrant. She has served as a consultant for Bausche Health, Food Marble, and Trellus Health and a speaker for Abbvie. She has received research support from Arena, AnX Robotica, and Ironwood. She has stock options with Food Marble, ModifyHealth, and Trellus Health. B.E.L. is a consultant for Allakos, Allergan, Arena, Cosmos, Ironwood, Salix, and Viver. D.P.R., Y.Y., S.Z., and S.E. are employees of Ardelyx.
Clinical trials: NCT01923428: The Efficacy of AZD1722 in Constipation Predominant Irritable Bowel Syndrome (IBS-C). NCT02621892: A 12-Week Study With a 4-Week Randomized Withdrawal Period to Evaluate the Efficacy and Safety of Tenapanor for the Treatment of IBS-C (T3MPO-1). NCT02686138: A 26-Week Study to Evaluate the Efficacy and Safety of Tenapanor in IBS-C (T3MPO-2).
Study Highlights.
WHAT IS KNOWN
✓ People with irritable bowel syndrome with constipation (IBS-C) experience bothersome abdominal symptoms including abdominal pain, bloating, discomfort, cramping, and fullness.
✓ In previously published phase 3 studies, tenapanor improved the combined response of abdominal pain and stool frequency, compared with placebo.
✓ The Abdominal Score of the Diary for IBS Symptoms-Constipation is effective in demonstrating treatment efficacy for abdominal bloating, discomfort, and pain.
WHAT IS NEW HERE
✓ Tenapanor improved the Abdominal Score through 12 weeks of treatment relative to placebo, showing a combined treatment efficacy for abdominal bloating, discomfort, and pain.
✓ Tenapanor demonstrated overall benefit for global IBS-C symptoms as compared with placebo.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients and investigators who participated in the study. Medical writing support, under the direction of the authors, was provided by Ashfield MedComms, an Inizio company, and funded by Ardelyx.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/D192
Contributor Information
William D. Chey, Email: wchey@med.umich.edu.
Lucinda A. Harris, Email: Harris.Lucinda@mayo.edu.
Rosita Frazier, Email: frazier.rosita@mayo.edu.
Darren M. Brenner, Email: Darren.Brenner@nm.org.
Lin Chang, Email: LinChang@mednet.ucla.edu.
Brian E. Lacy, Email: lacy.brian@mayo.edu.
Susan Edelstein, Email: sedelstein@ardelyx.com.
Yang Yang, Email: yyang@ardelyx.com.
Suling Zhao, Email: szhao@ardelyx.com.
David P. Rosenbaum, Email: drosenbaum@ardelyx.com.
REFERENCES
- 1.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: Management of irritable bowel syndrome. Am J Gastroenterol 2021;116(1):17–44. [DOI] [PubMed] [Google Scholar]
- 2.Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018;113(Suppl 2):1–18. [DOI] [PubMed] [Google Scholar]
- 3.Palsson OS, Whitehead W, Tornblom H, et al. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020;158(5):1262–73.e3. [DOI] [PubMed] [Google Scholar]
- 4.Black CJ, Ford AC. Assessing the impact of changes to the Rome IV criteria for clinical practice in irritable bowel syndrome. Gastroenterology 2022;162(6):1752–4.e1. [DOI] [PubMed] [Google Scholar]
- 5.Shah ED, Almario CV, Spiegel BM, et al. Presentation and characteristics of abdominal pain vary by irritable bowel syndrome subtype: Results of a nationwide population-based study. Am J Gastroenterol 2020;115(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehnel SE, Ervin CM, Carson RT, et al. Development of the diary for irritable bowel syndrome symptoms to assess treatment benefit in clinical trials: Foundational qualitative research. Value Health 2017;20(4):618–26. [DOI] [PubMed] [Google Scholar]
- 7.Heidelbaugh JJ, Stelwagon M, Miller SA, et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol 2015;110(4):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farzaei MH, Bahramsoltani R, Abdollahi M, et al. The role of visceral hypersensitivity in irritable bowel syndrome: Pharmacological targets and novel treatments. J Neurogastroenterol Motil 2016;22(4):558–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy L, Erickson A, Brierley SM. Visceral pain. Annu Rev Physiol 2019;81:261–84. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability: A new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 2016;65(1):155–68. [DOI] [PubMed] [Google Scholar]
- 13.Ballou S, McMahon C, Lee HN, et al. Effects of irritable bowel syndrome on daily activities vary among subtypes based on results from the IBS in America Survey. Clin Gastroenterol Hepatol 2019;17(12):2471–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tack J, Stanghellini V, Mearin F, et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol 2019;19(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor DCA, Abel JL, Martin C, et al. Comprehensive assessment of patients with irritable bowel syndrome with constipation and chronic idiopathic constipation using deterministically linked administrative claims and patient-reported data: The Chronic Constipation and IBS-C Treatment and Outcomes Real-World Research Platform (CONTOR). J Med Econ 2020;23(10):1072–83. [DOI] [PubMed] [Google Scholar]
- 16.Chapman RW, Stanghellini V, Geraint M, et al. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am J Gastroenterol 2013;108(9):1508–15. [DOI] [PubMed] [Google Scholar]
- 17.Bijkerk CJ, de Wit NJ, Muris JW, et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009;339:b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quigley EMM, Horn J, Kissous-Hunt M, et al. Better understanding and recognition of the disconnects, experiences, and needs of patients with irritable bowel syndrome with constipation (BURDEN IBS-C) study: Results of an online questionnaire. Adv Ther 2018;35(7):967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coon CD, Hanlon J, Abel JL, et al. Psychometric analysis of the abdominal score from the diary for irritable bowel syndrome symptoms-constipation using phase IIb clinical trial data. Value Health 2020;23(3):362–9. [DOI] [PubMed] [Google Scholar]
- 20.Chang L, Lacy BE, Moshiree B, et al. Efficacy of linaclotide in reducing abdominal symptoms of bloating, discomfort, and pain: A phase 3B trial using a novel abdominal scoring system. Am J Gastroenterol 2021;116(9):1929–37. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum DP, Yan A, Jacobs JW. Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor tenapanor: Two trials in healthy volunteers. Clin Drug Investig 2018;38(4):341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer AG, Labonte ED, Rosenbaum DP, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 2014;6(227):227ra36. [DOI] [PubMed] [Google Scholar]
- 23.King AJ, Siegel M, He Y, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med 2018;10(456):eaam6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Larauche MH, Siegel M, et al. Sa1600: Tenapanor attenuates increased macromolecule permeability in human colon monolayer cultures induced by inflammatory cytokines and human fecal supernatants. Gastroenterology 2018;154(6):S-326. [Google Scholar]
- 25.Li Q, King AJ, Liu L, et al. Tenapanor reduces IBS pain through inhibition of TRPV1-dependent neuronal hyperexcitability in vivo. Am J Gastroenterol 2017;112:S255. [Google Scholar]
- 26.Chey WD, Lembo AJ, Rosenbaum DP. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: A phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol 2017;112(5):763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chey WD, Lembo AJ, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: A 12-week, placebo-controlled phase 3 trial (T3MPO-1). Am J Gastroenterol 2020;115(2):281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chey WD, Lembo AJ, Yang Y, et al. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: A 26-week, placebo-controlled phase 3 trial (T3MPO-2). Am J Gastroenterol 2021;116(6):1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mearin F, Caballero AM, Serra J, et al. A retrospective and prospective 12-month observational study of the socioeconomic burden of moderate to severe irritable bowel syndrome with constipation in Spain. Gastroenterol Hepatol 2019;42(3):141–149. [DOI] [PubMed] [Google Scholar]
- 30.Rangan V, Ballou S, Shin A, et al. Use of treatments for irritable bowel syndrome and patient satisfaction based on the IBS in America Survey. Gastroenterology 2020;158(3):786–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016;150(6):1393–1407.e5. [DOI] [PubMed] [Google Scholar]
- 32.Chang L, Lembo A, Sultan S. American Gastroenterological Association Institute Technical Review on the pharmacological management of irritable bowel syndrome. Gastroenterology 2014;147(5):1149–72.e2. [DOI] [PubMed] [Google Scholar]
- 33.Chang L, Sultan S, Lembo A, et al. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with constipation. Gastroenterology 2022;163(1):118–36. [DOI] [PubMed] [Google Scholar]
- 34.Shin A, Chang L. The transition from Rome III to Rome IV irritable bowel syndrome: What we gain and lose. Clin Gastroenterol Hepatol 2022;20(3):508–10. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: Results of two phase 3 randomized clinical trials. Am J Gastroenterol 2018;113(5):735–45. [DOI] [PubMed] [Google Scholar]
- 36.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012;107(11):1702–12. [DOI] [PubMed] [Google Scholar]
- 37.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: Lubiprostone in patients with constipation-associated irritable bowel syndrome: Results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther 2009;29(3):329–41. [DOI] [PubMed] [Google Scholar]