Abstract
Genetic evidence suggests that regulation of β-catenin and regulation of Tcf/Lef family transcription factors are downstream events of the Wnt signal transduction pathway. However, a direct link between Wnt activity and Tcf/Lef transcriptional activation has yet to be established. In this study, we show that Wnt-1 induces a growth response in a cultured mammalian cell line, Rat-1 fibroblasts. Wnt-1 induces serum-independent cellular proliferation of Rat-1 fibroblasts and changes in morphology. Rat-1 cells stably expressing Wnt-1 (Rat-1/Wnt-1) show a constitutive up-regulation of cytosolic β-catenin, while membrane-associated β-catenin remains unaffected. Induction of cytosolic β-catenin in Rat-1/Wnt-1 cells is correlated with activation of a Tcf-responsive transcriptional element. We thus provide evidence that Wnt-1 induces Tcf/Lef transcriptional activation in a mammalian system. Expression of a mutant β-catenin (β-CatS37A) in Rat-1 cells does not result in a proliferative response or a detectable change in the cytosolic β-catenin protein level. However, β-CatS37A expression in Rat-1 cells results in strong Tcf/Lef transcriptional activation, comparable to that seen in Wnt-1-expressing cells. These results suggest that Wnt-1 induction of cytosolic β-catenin may have functions in addition to Tcf/Lef transcriptional activation.
The Wnt gene family encodes secreted factors involved in cell growth, differentiation, organogenesis, and oncogenesis (31). Wnt proteins have been demonstrated to possess mitogenic (31), inductive (19), and morphogenetic (47a) activities. A mitogenic function of Wnt proteins is supported by the finding that misexpression of Wnt-1 promotes mammary tumorigenesis (11, 47) or neural tube hyperplasia (9, 10). Wnt-1 expression in mammalian cells results in morphological changes and growth postconfluence in C57MG (7) and RAC311C (39) mammary epithelial cell lines and C3H10T1/2 fibroblasts (5), indicating that Wnt proteins can induce cell growth and loss of contact inhibition. In humans, components of the Wnt signaling cascade have been implicated in the development of both colon cancer (28) and melanomas (42).
Understanding of the Wnt signal transduction pathway has come mostly from genetic analyses of the Wnt-1 Drosophila ortholog, wingless (wg) (37, 40). Wnt/wg signaling events are initiated by receptor activation involving the frizzled cell surface protein (4). This signal suppresses the activity of the glycogen synthase kinase 3 (GSK-3) homolog, zeste-white 3 (zw-3) kinase, leading to changes in phosphorylation and increased stability of the armadillo protein (36). Armadillo protein is then thought to form complexes with members of Drosophila Tcf (dTcf) family transcription factors, regulating the expression of wg target genes (48).
β-Catenin, the mammalian homolog of Drosophila armadillo protein, was first identified as a cadherin-associated protein at cell-cell junctions (24). β-Catenin links membrane-bound cadherin and actin-bound α-catenin, coordinating processes essential to cell-cell adhesion and possibly cell migration (16). In mammalian cells, Wnt-1 expression results in an increase in the steady-state levels of β-catenin protein, leading to formation of β-catenin–cadherin heterocomplexes (20, 29).
β-Catenin also associates with the tumor suppressor protein adenomatous polyposis coli (APC) (43, 45). Wnt-1 expression induces the accumulation of β-catenin–APC complexes (33), uncomplexed monomeric β-catenin (33), or cytosolic β-catenin (23). β-Catenin–APC complexes have been identified in human cancer cell lines (42) and are implicated in the regulation of steady-state levels of β-catenin (18, 29), while monomeric or cytosolic β-catenin is proposed to be critical to Wnt signaling (44).
β-Catenin is thought to be essential for activating mammalian target genes in response to Wnt signaling (21, 25). Recent studies have provided evidence for interactions between armadillo, or β-catenin, and Tcf/Lef HMG transcription factors in Drosophila and Xenopus (2, 8, 26, 38, 48). β-Catenin/Tcf transcriptional complexes have been identified in human cancer cell lines and are implicated in deregulation of cell growth (22, 28). In addition, a mutant β-catenin displays transforming activity in NIH 3T3 cells (51) and transformation of mammary epithelial C57MG cells by Wnts correlates with regulation of β-catenin (44), further implicating the β-catenin gene as a potential oncogene in mammalian cells (35). These findings point to a possible role for β-catenin in transduction of Wnt-1 proliferative signals in mammalian cells. However, although Wnt-1 regulates β-catenin protein levels, it is not clear if this induction is required or sufficient for Wnt signaling. In addition, it is not known if Wnt-1 regulates Tcf/Lef transcriptional activity and if this activation is required or sufficient to transduce Wnt-1 proliferative signals in mammalian cells.
We report that Rat-1 fibroblasts show proliferative responses and morphological alterations in response to Wnt-1. Wnt-1 expression results in constitutive up-regulation of cytosolic β-catenin and activation of Tcf/Lef-dependent transcription in Rat-1 cells. However, our data suggest that Tcf/Lef transcriptional activation alone is not sufficient to elicit Wnt-1 proliferative responses in Rat-1 fibroblasts.
MATERIALS AND METHODS
Adenovirus expression vectors.
Recombinant adenovirus vectors expressing either Wnt-1 or Wnt-5A from the cytomegalovirus immediate-early promoter were constructed by cre/lox recombination (17). To accomplish this, Wnt-1HA and Wnt-5AHA cDNAs (44) were subcloned into the pAdlox shuttle vector, creating pAdlox Wnt-1HA and pAdlox Wnt-5A, which were then recombined with donor virus to create Ad/Wnt-1 and Ad/Wnt-5A. These adenovirus vectors contain the Wnt-HA expression cassette replacing the E1-region genes and a 2.6-kb deletion in the E3 region (3).
Retrovirus expression vectors.
Mammalian retrovirus expression vectors, pZNCX and pQNCX, were used to construct and express full-length murine Wnt-1, murine Wnt-5A, and human β-catenin (S37A). cDNA expression in pZNCX and pQNCX is driven by an internal cytomegalovirus enhancer/promoter (unpublished observations). Wnt-5A cDNA was generously provided by Andrew McMahon (Harvard University). Mutant β-catenin (S37A) cDNA was generously provided by Steven Byers (Georgetown University). All cDNAs are modified to encode a hemagglutinin (HA) epitope tag on the 3′ end.
Cell culture.
Rat-1 fibroblasts (13) and BOSC 23 packaging cells (34) were maintained in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum and penicillin-streptomycin, at 37°C under 8% CO2 and at 90% humidity. To measure growth beyond confluence, Rat-1 cells were seeded at approximately 75% confluence and allowed to achieve confluence in the presence of serum overnight. The following day, the serum was removed by rinsing the cell monolayers once with phosphate-buffered saline (PBS) and once with DMEM. The cells were then cultured in DMEM. At several time points after serum removal, the cells were dissociated by trypsinization and viable cells were counted after trypan blue staining. To visualize cellular growth postconfluence, cell monolayers were fixed with cold methanol-acetone (1:1), stained with Giemsa nuclear stain, and photographed. To measure serum-independent cell proliferation, Rat-1 cells were seeded at approximately 15% confluence in the presence of serum. The following day, serum was removed and viable-cell numbers were obtained at several time points after serum removal, as described above.
Adenovirus infection.
Rat-1 fibroblasts were seeded at 80% confluence and were subsequently mock infected or infected with recombinant adenoviruses at various multiplicities of infection (MOIs). Before infection, the cells were rinsed with DMEM. Infections were carried out with virus-containing inoculum in DMEM–2% fetal bovine serum that was added to the cells for 1 h at 37°C. The cells were then maintained in DMEM containing 2% fetal bovine serum.
Transient transfection and retrovirus infection.
Recombinant retroviruses were generated by calcium phosphate transfection of the retroviral constructs into BOSC packaging cells (34). One day later, the BOSC cells were treated with mitomycin C (10 μg/ml; Sigma) for 4 h. At 24 h after treatment, transfected BOSC cells were trypsinized and seeded with target Rat-1 fibroblasts for coculture. After 48 h, the cells were cultured with medium containing 500 μg of Geneticin (G418; Gibco) per ml. Resistant colonies were pooled as polyclonal, stable cell lines maintained in medium containing 250 μg of Geneticin per ml.
Western blot analysis.
Wnt-1, Wnt-5A, and β-catenin (S37A) protein expression was assessed by Western blot analysis of cell lysates. Briefly, cells were washed twice with cold PBS, pelleted, and resuspended in lysis buffer (50 mM Tris [pH 8], 150 mM NaCl, 1% Triton X-100, 10 μg of aprotinin per ml, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg of leupeptin per ml, 2 μg of pepstatin per ml) for 30 min on ice. Cell lysates were then clarified by microcentrifugation at 10,000 rpm (8,000 × g) for 10 min at 4°C. The protein concentrations were determined with Bio-Rad protein determination dye. Cellular proteins (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide) and transferred onto nitrocellulose filters.
The filters were blocked with TBST (10 mM Tris [pH 8], 150 mM NaCl, 0.2% Tween 20) containing 1% bovine serum albumin (BSA) (fraction V) and immunoblotted for protein expression by incubation with anti-HA monoclonal antibody 12CA5 (Berkeley Antibody Co.) at a 1:50 dilution in TBST–1% BSA for 2 h at room temperature. The blots were washed for 25 min with TBST at room temperature, exposed to the secondary antibody (horseradish peroxidase-conjugated sheep anti-mouse immunoglobulin G [Amersham]) at a 1:5,000 dilution for 45 min at room temperature, and washed with TBST at room temperature. Protein expression was visualized with enhanced chemiluminescence detection reagents (Amersham) and by exposure to X-ray film.
Cell fractionation and β-catenin analysis.
β-Catenin protein levels were assessed in cytosolic and membranous pools prepared by fractionation of cellular lysates as described previously (14a, 44). Briefly, cells were washed twice with cold PBS and collected in physiological buffer (PB) (10 mM Tris [pH 7.4], 140 mM NaCl, 5 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, 2 μg of leupeptin per ml, 2 μg of pepstatin per ml) (500 μl per 100-mm confluent dish). The cells were homogenized in PB by 30 strokes of a Dounce homogenizer, and the lysate was centrifuged with a microcentrifuge for 10 min at 3,000 rpm (500 × g) at 4°C. Membranous and cytosolic material was obtained by ultracentrifugation at 30,000 rpm (100,000 × g) for 90 min at 4°C. The supernatant was designated the cytosolic fraction, and pellets were resuspended in PB containing 0.1% SDS (150 μl per 100-mm confluent dish) and designated the membranous fraction. The protein concentrations in the respective fractions were determined with Bio-Rad protein determination dye. β-Catenin was analyzed by separation of 30 μg of cytosolic proteins or 15 μg of membranous proteins on SDS–10%-polyacrylamide gels and by Western blot analyses with an anti-β-catenin monoclonal antibody (Transduction Laboratories) at a 1:5,000 dilution in TBST–1% BSA for 2 h at room temperature. Protein expression was visualized with enhanced chemiluminescence detection reagents (Amersham) and by exposure to X-ray film.
Tcf luciferase assay.
pTOPFLASH and pFOPFLASH Tcf luciferase reporter constructs were generously provided by Hans Clevers (University Hospital, Utrecht, The Netherlands) (22). A pSV-Luc luciferase reporter construct in which the simian virus 40 early promoter was used to constitutively drive the expression of the luciferase gene was generously provided by Andrew Henderson, Pennsylvania State University. A 4-μg sample of reporter construct was introduced into cells by the calcium phosphate transfection method (34). Two days after transfection, the cells were collected and resuspended in lysis buffer (5% Triton, 125 mM Gly-Gly [pH 7.8], 75 mM MgSO4, 20 mM EGTA [pH 7.8], 10 mM dithiothreitol). Crude lysates were clarified by pulse microcentrifugation. Supernatants were evaluated for luciferase activities by using luciferin substrates (Sigma). Measurements were obtained from transfections performed in triplicate and averaged.
RESULTS
Wnt-1 overexpression alters Rat-1 fibroblast morphology and growth.
We set out to identify Wnt-responsive cultured mammalian cell lines. We expressed Wnt-1 in several established human and rat cell lines by using a recombinant adenovirus expression vector (Ad) containing an HA-epitope tagged murine Wnt-1 cDNA (Ad/Wnt-1) (data not shown). Rat-1 fibroblasts (13) responded to Wnt-1 by undergoing changes in morphology and growth. We subsequently characterized the biological and biochemical responses to Wnt-1 in these cells.
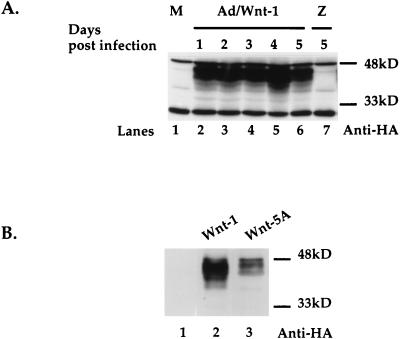
Ectopic expression of Wnt genes was accomplished with two different gene expression systems: adenovirus and retrovirus expression vectors. The adenovirus vector Ad/Wnt-1 was used to infect Rat-1 cells. Wnt-1 proteins were detected by Western blot analysis of cell lysates with anti-HA antibody (12CA5). At 24 h after Ad infection, Wnt-1 proteins were detected in cells infected by Ad/Wnt-1 (Fig. 1A, lane 2) but were not found in mock-infected cells (lane 1) or cells infected by a virus expressing the lacZ gene (Ad/LacZ) (lane 7). Wnt-1 expression persisted for up to 5 days after Ad infection (lanes 2 to 6).
FIG. 1.
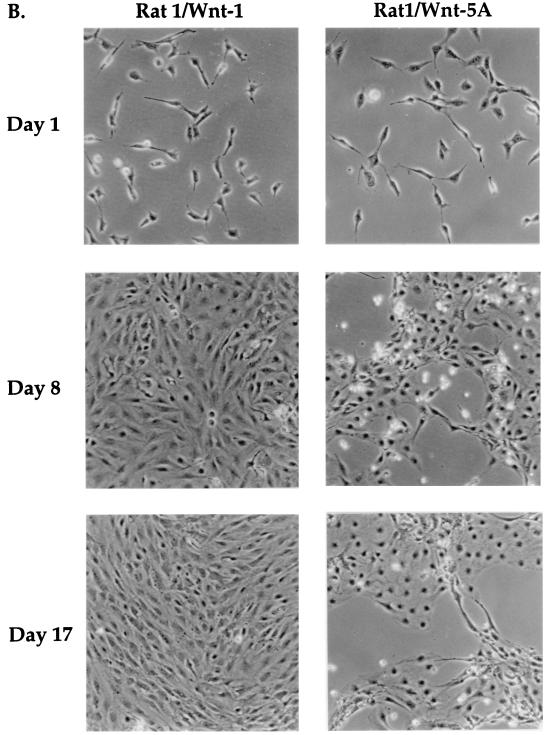
Wnt-1 expression alters Rat-1 fibroblast morphology and growth. (A) Transient expression of Wnt-1 protein by recombinant adenovirus expression vector in Rat-1 fibroblasts. Western blot analysis of cell lysates shows Wnt-1 protein expressed in cells at multiple days (days 1 to 5) after infection with Ad/Wnt-1 (lanes 2 to 6) (MOI = 5) but not with the control, Ad/LacZ (Z) (MOI = 5) (lane 7), or after mock infection (M) (lane 1). Wnt-1 was detected with anti-HA antibody (Anti-HA). (B) Stable expression of Wnt-1 and Wnt-5A proteins in Rat-1 stable cell lines, Rat-1/Wnt-1 and Rat-1/Wnt-5A. Western blot analysis of cell lysates shows Wnt-1 (lane 2) and Wnt-5A (lane 3) proteins expressed, as compared to control cells (lane 1). Wnt-1 and Wnt-5A proteins were detected with anti-HA antibody. (C) Wnt-1 alters Rat-1 fibroblast morphology and growth. Phase-contrast microscopy of polyclonal Rat-1/Wnt-1 and Rat-1/Wnt-5A stable cell lines shows that Rat-1/Wnt-1 cells attain an elongated, refractile morphology and densely pack in cord-like bundles, in contrast to Rat-1/Wnt-5A cells.
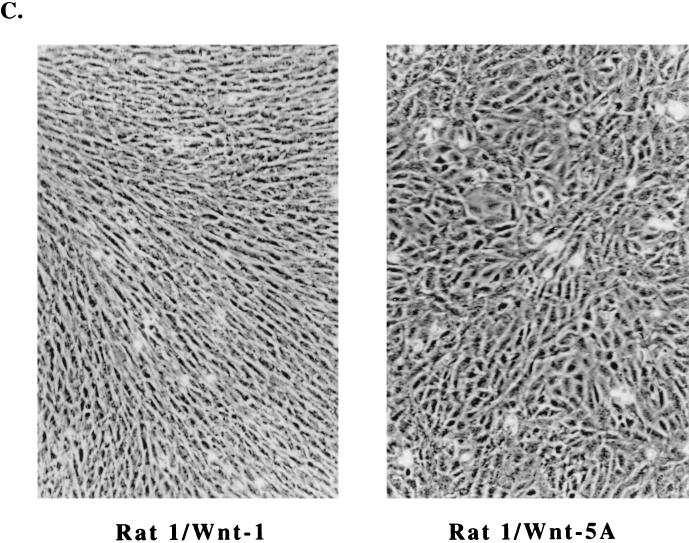
Stable Rat-1 cell lines expressing Wnt genes were established with retrovirus vectors driving the expression of HA epitope-tagged Wnt cDNAs. We generated Wnt-1-expressing cell lines and cell lines expressing vector alone (control) or murine Wnt-5A (Rat-1/Wnt-5A). Previous studies of Wnt functions in Xenopus and mammary epithelial C57MG cells suggest that Wnt-1 and Wnt-5A belong to different functional classes among the Wnt protein family members (44, 46, 52). We compared the activities of Wnt-1 and Wnt-5A in Rat-1 cells. Western blot analyses of cell lysates revealed that stable Rat-1/Wnt-1 and Rat-1/Wnt-5A cells express glycosylated isoforms of the exogenously expressed Wnt proteins, ranging in molecular mass from 36 to 45 kDa (Fig. 1B). Rat-1/Wnt-1 (Fig. 1B, lane 2) and Rat-1/Wnt-5A (lane 3) cells both showed expression of their respective exogenous Wnt proteins, while control cells showed no exogenous protein expression (lane 1).
Rat-1/Wnt-1 stable cell lines exhibit morphological changes. A similar response to Wnt-1 expression was observed in Rat-1 fibroblasts infected by Ad/Wnt-1 (data not shown). Figure 1C shows that Rat-1/Wnt-1 cells adopt an elongated and refractile appearance, unlike control Rat-1/Wnt-5A cells. Wnt-1 cells grew more densely as a monolayer, forming cord-like bundles lined up in a uniform direction. Rat-1/Wnt-5A exhibited morphologies and growth characteristics identical to those of uninfected Rat-1 cells, retaining a cuboidal morphology. In subsequent analyses, we will refer to Rat-1/Wnt-5A as the control cell line, since we observed no detectable biological change in response to Wnt-5A expression.
Wnt-1 induces cell growth postconfluence.
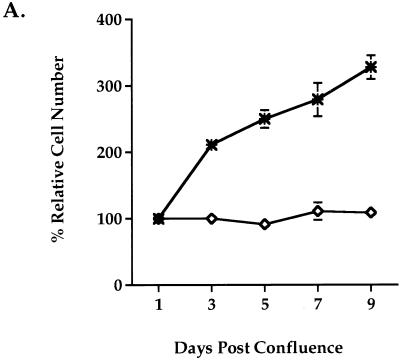
One of the growth properties observed in Rat-1 fibroblasts is that they require both contact inhibition and depletion of serum to become growth arrested. To examine whether Wnt-1 can overcome these growth-inhibitory signals in Rat-1 cells, we analyzed the ability of Wnt-1 to induce postconfluent growth. Growth postconfluence was analyzed under serum-free conditions and measured by counting viable cells. Rat-1/Wnt-1 cells continued to proliferate postconfluence in the absence of serum, while control Rat-1/Wnt-5A cells remained growth arrested (Fig. 2A). At 9 days postconfluence, Rat-1/Wnt-1 cells continued to proliferate under serum-free conditions. This response to Wnt-1 expression was reflected by a threefold increase in the relative cell number over that achieved by control Rat-1/Wnt-5A cells (Fig. 2A).
FIG. 2.
Wnt-1 induces proliferation in quiescent Rat-1 fibroblasts. Rat-1/Wnt-1 (∗) and control Rat-1/Wnt-5A (◊) cells were cultured to confluence, deprived of serum, and monitored for growth postconfluence. (A) At multiple days postconfluence, viable cells were trypsinized and counted by the trypan blue exclusion method. The percent relative cell number is calculated as the percentage relative to the cell number recorded 1 day after serum removal. Triplicate samples were counted and averaged. (B) Six days postconfluence, cell monolayers were fixed and stained with Giemsa nuclear stain and photographed. Bar, 4.75 mm.
Proliferating Rat-1/Wnt-1 cells packed together tightly, forming dense clusters of cells in a monolayer. These clusters were evident after Giemsa staining of the cell monolayer (Fig. 2B). Under similar conditions, Rat-1/Wnt-5A remained growth arrested at confluence, as shown by uniform Giemsa staining of the cell monolayer (Fig. 2B).
Wnt-1 induces serum-independent cell proliferation.
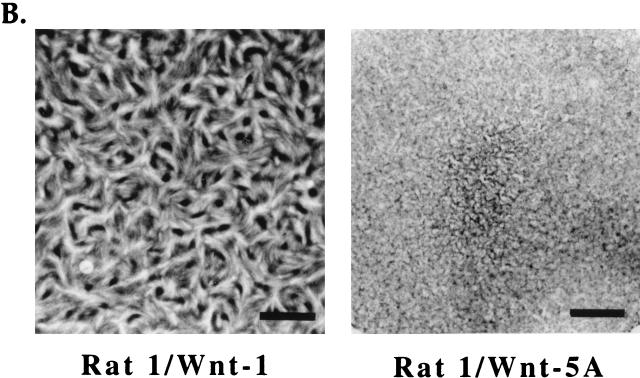
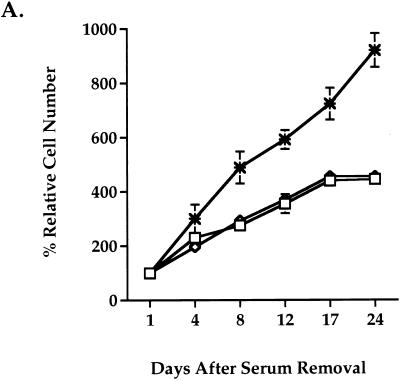
To test whether Wnt-1 is sufficient to support cell growth in place of mitogenic serum components, we analyzed the growth of Rat-1 stable cell lines under serum-free conditions. Wild-type Rat-1, Rat-1/Wnt-5A, or Rat-1/Wnt-1 fibroblasts were seeded sparsely (<15% confluence) in serum-free medium, and cell proliferation was measured by counting viable cells at multiple days after serum removal. Wild-type Rat-1 cells remained viable and grew very slowly, with growth tapering off after 17 days in serum-free culture (Fig. 3A). Overexpression of Wnt-1 dramatically increased the growth of Rat-1 fibroblasts in serum-free medium (Fig. 3A). Rat-1/Wnt-1 cells showed a 2.5-fold increase in cell number compared to control cells. Control Wnt-5A-expressing cells did not achieve confluence after 3 weeks in culture (Fig. 3B) whereas Rat-1/Wnt-1 cells achieved confluence after 1 week in culture and continued to proliferate postconfluence (Fig. 3B). Rat-1/Wnt-1 cells showed a ninefold total increase in cell number after 24 days in culture.
FIG. 3.
Wnt-1 induces serum-independent growth in Rat-1 fibroblasts. Wild-type Rat-1 (□), Rat-1/Wnt-1 (∗), and control Rat-1/Wnt-5A (◊) cells were seeded at low density (<15% confluence), deprived of serum, and monitored for serum-independent growth. (A) At multiple days after removal of serum, the cells were trypsinized and viable cells were counted. The percent relative cell number is calculated relative to the cell number recorded 1 day after serum removal. Triplicate samples were counted and averaged. (B) At multiple days (days 1, 8, and 17) after removal of serum, Rat-1/Wnt-1 and Rat-1/Wnt-5A cells were photographed and monitored for serum-independent growth.
Wnt-1 induces cytosolic accumulation of β-catenin protein in Rat-1 fibroblasts.
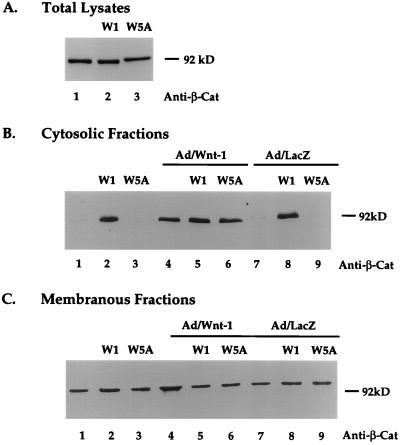
It has previously been reported that Wnt-1 expression results in increased steady-state levels of β-catenin (20, 33, 44). It has also been hypothesized that the accumulation of β-catenin in the cytosol is responsible for Wnt-1 downstream signaling (35). Having established that Wnt-1 affects the growth and morphological properties of Rat-1 fibroblasts, we investigated the effects of Wnt-1 on β-catenin protein levels in Rat-1 cells. To study changes in β-catenin in response to Wnt-1, we evaluated β-catenin protein levels in total lysates and in cytosolic and membranous fractions of Rat-1 cell lysates. Western blot analyses of protein fractions revealed that Wnt-1-expressing cells showed a dramatic increase in cytosolic β-catenin levels compared to control cells (Fig. 4B, lane 2). Wild-type Rat-1 and control Rat-1/Wnt-5A cells showed almost undetectable levels of cytosolic β-catenin protein (Fig. 4B, lanes 1 and 3). β-Catenin levels remained constant in total lysates (Fig. 4A) and membranous fractions (Fig. 4C, lanes 1 to 3) in all cell lines. Immunofluorescence analyses of Rat-1 cell lines showed a predominant membranous signal and did not show alterations in the cellular localization of β-catenin in response to Wnt-1 expression (data not shown).
FIG. 4.
Wnt-1 induces cytosolic accumulation of β-catenin protein in Rat-1 fibroblasts. Western blot analyses of steady-state β-catenin protein levels in unfractionated (A), cytosolic (B), and membranous (C) protein fractions of wild-type Rat-1 (blank), Rat-1/Wnt-1 (W1), and Rat-1/Wnt-5A (W5A) cells are shown. Cells were uninfected or infected with Ad/Wnt-1 or with the control, Ad/LacZ (MOI = 5). Cell extracts were prepared and fractionated, as described in Materials and Methods. Protein fractions were separated by SDS-PAGE (10% polyacrylamide), transferred onto nitrocellulose, and analyzed by Western blot analysis with anti-β-catenin antibody (Anti-β-Cat).
Studies with Xenopus suggest that Wnt-5A can interfere with Wnt-1 signaling (46). To study this interaction in Rat-1 cells, we introduced Wnt-1 into Rat-1/Wnt-5A cells by Ad/Wnt-1 infection and measured changes in cytosolic β-catenin levels. Both wild-type Rat-1 cells and Rat-1/Wnt-5A cells showed equivalent responsiveness to Wnt-1, resulting in an acute induction of β-catenin protein in the cytosol 2 days after Ad/Wnt-1 infection (Fig. 4B, lanes 4 and 6). The levels of β-catenin induction by Ad/Wnt-1 in either control (lane 4) or Rat-1/Wnt-5A (lane 6) cells were comparable to those observed in Rat-1/Wnt-1 stable cell lines (lane 2). These results indicate that Rat-1/Wnt-5A cell lines have not acquired altered responsiveness to Wnt-1 subsequent to selection and that Wnt-5A expression does not interfere with Wnt-1 activity. Ad/Wnt-1 infection of Rat-1/Wnt-1 cells did not further increase the cytosolic β-catenin levels (lane 5). Control Ad/LacZ infection had no effects on β-catenin levels in any of the three cell lines (Fig. 4B, lanes 7 to 9).
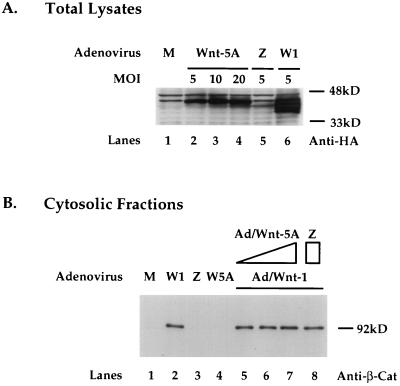
To further examine the potential interaction between Wnt-5A and Wnt-1, we generated an adenovirus expression construct expressing Wnt-5AHA, Ad/Wnt-5A. Infection of Rat-1 cells with Ad/Wnt-5A did not lead to detectable morphological or proliferative changes to the cells (data not shown). Western blot analysis of cell lysates 2 days after infection showed that Wnt-5A proteins were present in cells infected with Ad/Wnt-5A (Fig. 5A, lanes 2 to 4). Wnt-1 proteins were detected in cells infected with Ad/Wnt-1 (lane 6). No Wnt proteins were detected in mock-infected cells (lane 1) or cells infected with control adenovirus Ad/LacZ (lane 5). By increasing the MOI of Ad/Wnt-5A, a stepwise modest increase in expression of Wnt-5A proteins was detected in Rat-1 cells.
FIG. 5.
Coexpression of Wnt-5A does not interfere Wnt-1 induction of cytosolic β-catenin in Rat-1 fibroblasts. (A) Expression of Wnt-5A protein in Rat-1 cells at increasing MOIs with Ad/Wnt-5A. Western blot analysis of cell lysates 2 days after infection with Ad/Wnt-5A at an MOI of 5, 10, or 20 or with Ad/Wnt-1 (W1) (MOI = 5) or Ad/LacZ (Z) (MOI = 5) or after mock infection (M) is shown. Wnt-1 and Wnt-5A proteins are detected with anti-HA antibody. (B) Coexpression of Wnt-5A does not interfere with Wnt-1 induction of cytosolic β-catenin in Rat-1 fibroblasts. Western blot analyses of steady-state cytosolic β-catenin protein levels in Rat-1 cells following mock infection (M), infection with Ad/Wnt-1 (W1), Ad/LacZ (Z), or Ad/Wnt-5A (5A), coinfection with Ad/Wnt-1 (MOI = 5) and Ad/Wnt-5A (MOI = 5, 10, or 20), or coinfection with Ad/Wnt-1 (W1) (MOI = 5) and Ad/LacZ (Z) (MOI = 20) are shown. Cell extracts were prepared, fractionated, and analyzed by Western blot analysis with anti-β-catenin antibody (Anti-β-Cat).
To study the potential modulating effects of Wnt-5A on Wnt-1 activity, we introduced Wnt-5A and Wnt-1 into Rat-1 cells by coinfection with Ad/Wnt-5A and Ad/Wnt-1 and examined the β-catenin levels in cytosolic fractions of cell lysates 2 days following coinfection. Rat-1 cells showed a substantially increased level of cytosolic β-catenin following infection with Ad/Wnt-1 (Fig. 5B, lane 2; refer to Fig. 4B). Cells infected with Ad/Wnt-5A showed an almost undetectable level of cytosolic β-catenin (Fig. 5B, lane 4), as did mock-infected cells and cells infected with the control, Ad/LacZ (lanes 1 and 3). Coinfection of Rat-1 cells with Ad/Wnt-1 and with Ad/Wnt-5A at increasing MOIs of 5, 10, and 20 resulted in no change in the Wnt-1 induction of cytosolic β-catenin level (lanes 5 to 7). Coinfection with Ad/Wnt-1 and Ad/LacZ also had no effect on Wnt-1 induction of β-catenin. These results suggest that coexpression of Wnt-5A with adenovirus vectors does not interfere with Wnt-1 induction of β-catenin in Rat-1 fibroblasts.
Wnt-1 induces Tcf/Lef transcriptional activation in Rat-1 fibroblasts.
Current models suggest that cytosolic β-catenin interacts with downstream effectors, translocates into the nucleus, and activates target genes. One class of potential effectors are the Tcf/Lef HMG transcription factors (30, 35). We postulate that Wnt-1 may directly affect the activities of Tcf/Lef transcription factors by activation of the Wnt signal transduction pathway in Rat-1 fibroblasts. To address this hypothesis, we analyzed the effects of Wnt-1 on Tcf/Lef transcriptional activities in Rat-1 fibroblasts in a Tcf luciferase reporter assay (22).
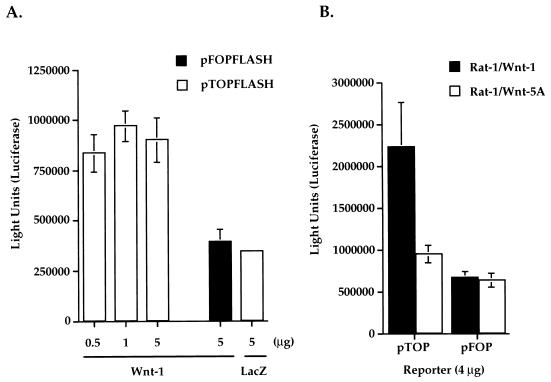
The Tcf luciferase reporter construct used in these experiments, pTOPFLASH, contains three optimal Tcf binding elements placed in tandem, upstream of a minimal c-fos promoter that drives the expression of the luciferase gene (22). The control Tcf reporter, pFOPFLASH, contains critical nucleotide replacements within the binding elements, which disrupt Tcf binding. We observed that cotransfection of the Wnt-1 expression vector and pTOPFLASH Tcf reporter into Rat-1 fibroblasts resulted in strong luciferase activities 2 days posttransfection (Fig. 6A). Background luciferase activities were measured in cells cotransfected with pTOPFLASH and a lacZ-containing plasmid (Fig. 6A). Wnt-1 induced threefold more activation of pTOPFLASH reporter than of control pFOPFLASH reporter. Transfections with increasing amounts of Wnt-1 plasmids (0.5, 1, or 5 μg) did not result in increasing activation of the pTOPFLASH reporter (Fig. 6A).
FIG. 6.
Wnt-1 induces Tcf/Lef-dependent transcription in Rat-1 fibroblasts. (A) Transient expression of Wnt-1 induces Tcf/Lef-dependent transcription. Wild-type (pTOPFLASH) or mutant (pFOPFLASH) Tcf reporter constructs (4 μg) were cotransfected into Rat-1 fibroblasts with increasing amounts of Wnt-1 or lacZ cDNA. (B) Stable expression of Wnt-1 in Rat-1/Wnt-1 cells induces constitutive Tcf/Lef transcriptional activation. Wild-type Tcf (pTOP) or mutant Tcf (pFOP) reporter constructs (4 μg) were transfected into Rat-1/Wnt-1 or Rat-1/Wnt-5A stable cell lines. Luciferase activities were measured 2 days after transfection. Triplicate samples were counted and averaged.
Next, we investigated if there are constitutive Tcf/Lef transcriptional activities in the stable Rat-1 cell lines. Rat-1/Wnt-1 and Rat-1/Wnt-5A cells were transfected with either pTOPFLASH or control pFOPFLASH reporters, and luciferase activities were measured 2 days later. Figure 6B illustrates that Rat-1/Wnt-1 cells showed threefold-greater constitutive Tcf-dependent luciferase activities than did control Rat-1/Wnt-5A cells. Rat-1/Wnt-1 and Rat-1/Wnt-5A cells showed comparable background luciferase activities when transfected with the mutant Tcf reporter, pFOPFLASH (Fig. 6B). Control transfections with a constitutive reporter (pSV2-Luc) that uses the simian virus 40 early promoter to express luciferase demonstrated that transfection efficiencies were comparable in all stable cell lines (data not shown).
Mutant β-catenin (S37A) does not accumulate in the cytosol.
It has been postulated that cytosolic accumulation of β-catenin is the key event that drives the formation of active β-catenin/Tcf transcriptional complexes. Cytosolic β-catenin accumulation would thus be the rate-limiting step in Wnt-1 signaling. Wild-type cytosolic β-catenin is generally unstable and degrades rapidly in the absence of Wnt-1 signal (1). A mutant β-catenin with a serine-to-alanine mutation at residue 37 (S37A) is thought to be more stable and thus more active than wild-type β-catenin (42).
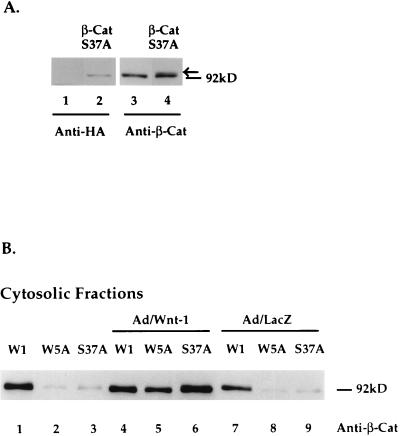
To examine the activities of β-catenin, we expressed β-catenin (S37A) in Rat-1 fibroblasts. Using a recombinant retrovirus expressing HA-epitope tagged β-catenin (S37A), we generated a polyclonal Rat-1 stable cell line, Rat-1/β-CatS37A. Expression of β-catenin (S37A) protein in Rat-1/β-CatS37A cells was shown by Western blot analyses of total-cell lysates with anti-HA antibodies. Figure 7A (lane 2) shows the HA-tagged β-catenin (S37A) protein in total-cell lysates of Rat-1/β-CatS37A cells, migrating at approximately 92 kDa. No exogenous protein was detected in mock-infected cells (Fig. 7A, lane 1). In parallel, Western blot analysis with anti-β-catenin antibodies showed exogenous β-catenin (S37A) proteins (lane 4) migrating slightly higher than endogenous β-catenin proteins (lane 3). The observed alteration in electrophoretic mobility of β-catenin (S37A) is probably due to the HA epitope tag.
FIG. 7.
Mutant β-catenin (S37A) does not accumulate in the cytosol. (A) Western blot analyses of β-catenin (S37A) in Rat-1/β-CatS37A stable cell lines. Cell lysates were separated by SDS-PAGE (10% polyacrylamide for lanes 1 and 2 and 7.5% polyacrylamide for lanes 3 and 4) and transferred to nitrocellulose, and β-catenin (S37A) was detected by Western blot analysis with anti-HA antibody (lane 2) or anti-β-catenin (lane 4). Lane 1 shows that no exogenous protein is detected in control cells. Lane 3 shows endogenous expression of β-catenin in Rat-1 cells. Exogenous β-catenin (S37A) protein is indicated by the arrow in lane 4. (B) Western blot analysis of cytosolic β-catenin protein levels in Rat-1/Wnt-1 (W1), Rat-1/Wnt-5A (W5A), and Rat-1/β-CatS37A (S37A) stable cell lines. Cells were uninfected or infected with Ad/Wnt-1 (MOI = 5) or with the control, Ad/LacZ (MOI = 5) Cytosolic protein fractions were separated by SDS-PAGE (10% polyacrylamide), transferred to nitrocellulose, and analyzed by Western blot analysis with anti-β-catenin antibody (Anti-β-Cat).
We next analyzed the levels of β-catenin (S37A) proteins in the cytosolic fraction of Rat-1/β-CatS37A cells. By cell fractionation and Western blot analysis with anti-β-catenin antibodies, we observed little accumulation of β-catenin (S37A) protein in the cytosolic fraction (Fig. 7B, lane 3) compared to that in Wnt-1-expressing cells (lane 1). Like Rat-1/Wnt-5A cells, Rat-1/β-CatS37A cells showed low levels of cytosolic β-catenin (lanes 2 and 3). However, both cell lines showed acute responsiveness to Wnt-1, demonstrated by the dramatic accumulation of β-catenin protein in the cytosol 2 days after Ad/Wnt-1 infection (lanes 5 and 6). Control infection with Ad/LacZ had no effects on β-catenin levels in any of the cell lines (lanes 7 to 9). The cytosolic pool of β-catenin in Rat-1/β-CatS37A cells following Ad/Wnt-1 infection is composed primarily of endogenous β-catenin. Ectopically expressed β-catenin (S37A), which was detected as a slower-migrating form, shows little or no responsiveness to the Wnt-1 signal and does not accumulate in the cytosol following Ad/Wnt-1 infection (data not shown) (see Discussion).
β-Catenin S37A does not affect the growth of Rat-1 fibroblasts.
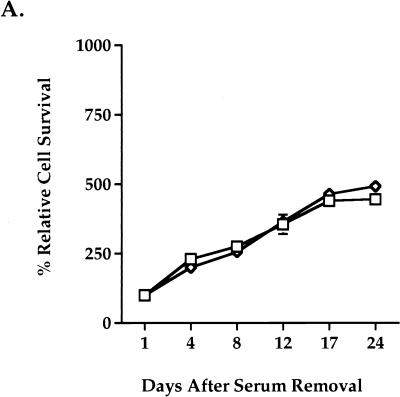
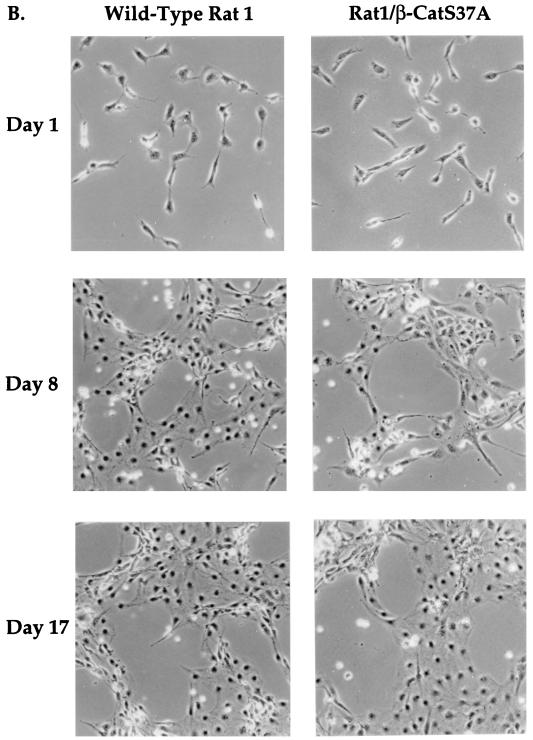
We next characterized the growth of Rat-1/β-CatS37A cells under serum-free conditions. When seeded sparsely, Rat-1/β-CatS37A cells proliferated at a rate similar to that of wild-type Rat-1 cells (Fig. 8A) and did not achieve confluence in the absence of serum (Fig. 8B). Rat-1/β-CatS37A cells showed a rate of proliferation significantly lower than that of Rat-1/Wnt-1 cells (compare Fig. 3A and Fig. 8A). Rat-1/β-CatS37A cells retained the morphological and growth properties of wild-type Rat-1 fibroblasts (Fig. 8B). In addition, Rat-1/β-CatS37A cells failed to grow postconfluence in serum-free medium (data not shown), as seen for Rat-1/Wnt-1 cells. We have also generated and analyzed eight clonal lines derived from the Rat-1/β-CatS37A cell line. Although there are clonal differences in the expression levels of β-catenin (S37A), as determined by Western blotting, there are few observable differences in growth and morphology between the polyclonal and clonal lines of Rat-1/β-CatS37A (data not shown).
FIG. 8.
Mutant β-catenin (S37A) expression does not alter Rat-1 cell morphology or growth properties. Rat-1/β-CatS37A (◊) and wild-type Rat-1 (□) cells were seeded at low density (<15% confluence), deprived of serum, and monitored for serum-independent growth. (A) At multiple days after removal of serum, the cells were trypsinized and viable cells were counted. The percent relative cell number is calculated as the percentage relative to the cell number recorded 1 day after serum removal. Samples were counted in triplicate. (B) After removal of serum, wild-type Rat-1 and Rat-1/β-CatS37A cells were photographed on the days indicated in the figure.
β-Catenin (S37A) induces Tcf/Lef transcriptional activation.
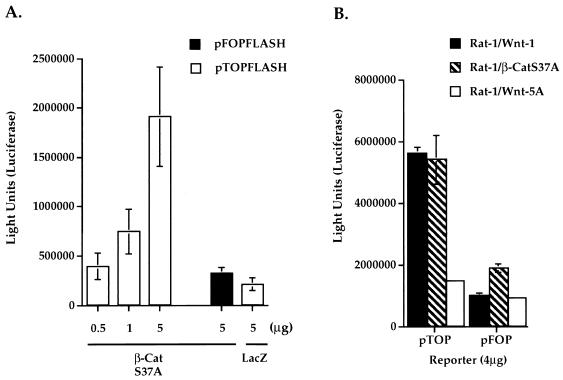
β-Catenin mutations have been implicated in constitutive Tcf transcriptional activation in cancer cell lines (28). We examined if ectopic expression of β-catenin (S37A) leads to activation of Tcf/Lef-dependent transcription in Rat-1 fibroblasts. Rat-1 fibroblasts were cotransfected with Tcf luciferase reporter constructs and β-catenin (S37A) expression plasmids. Luciferase activities were measured 2 days after transfection. A dose-dependent increase in luciferase activities was detected by cotransfecting increasing amounts of β-catenin (S37A) cDNA with pTOPFLASH reporter, with the activities reaching up to fivefold over those of the pFOPFLASH control reporter (Fig. 9A). Control transfections of pTOPFLASH reporter with lacZ plasmids showed background luciferase activities (Fig. 9A).
FIG. 9.
Mutant β-catenin (S37A) activates Tcf/Lef-dependent transcription. (A) Transient expression of β-catenin (S37A) (β-CatS37A) induces Tcf/Lef-dependent transcription. Wild-type (pTOPFLASH) or mutant (pFOPFLASH) Tcf reporter constructs (4 μg) were cotransfected into Rat-1 fibroblasts with increasing amounts of β-CatS37A or control lacZ cDNAs. (B) Stable expression of β-CatS37A in Rat-1/β-CatS37A cells induces constitutive Tcf/Lef transcriptional activation. Wild-type Tcf (pTOP) or mutant Tcf (pFOP) reporter constructs (4 μg) were transfected into Rat-1/β-CatS37A, Rat-1/Wnt-1, or Rat-1/Wnt-5A stable cell lines. Luciferase activities were measured 2 days after transfection. Triplicate samples were counted and averaged.
We next investigated whether Rat-1/β-CatS37A stable cell lines exhibited constitutive Tcf/Lef transcriptional activities. We introduced pTOPFLASH or pFOPFLASH reporters into Rat-1 stable cell lines by transient transfection and measured luciferase activities 2 days later. Rat-1/β-CatS37A cells showed strong constitutive Tcf/Lef transcriptional activities. Figure 9B illustrates that Rat-1/β-CatS37A and Rat-1/Wnt-1 cells had comparable levels of luciferase activities when transfected with pTOPFLASH Tcf reporter. For both cell lines, no significant luciferase activities were detected in cells transfected with the control pFOPFLASH reporter. Control Wnt-5A cells showed no significant luciferase activity when transfected with either Tcf reporter construct (Fig. 9B). We conclude that expression of the mutant form β-catenin (S37A) activates Tcf/Lef-dependent transcription in Rat-1 cells. Stable expression of either Wnt-1 or β-catenin (S37A) in Rat-1 fibroblasts results in constitutive Tcf/Lef transcriptional activation.
DISCUSSION
In this study, we have identified Rat-1 fibroblasts as a Wnt-1-responsive cell line. Wnt-1 supports the growth of Rat-1 cells in the absence of serum and stimulates growth beyond confluence. We have used Rat-1 cells to characterize Wnt signaling events and to relate these events to biological responses of Rat-1 cells to Wnt-1. Our data show that activation of the Wnt signal transduction pathway results in induction of β-catenin in the cytosol and Tcf/Lef transcriptional activation in Rat-1 fibroblasts.
We proposed that the biological phenotype of Rat-1 fibroblasts in response to Wnt-1 is a result of increased levels and/or activities of β-catenin, a key Wnt signaling intermediate. Studies with Xenopus have provided evidence that overexpression of β-catenin can mimic Wnt activities (14, 15). Examination of β-catenin protein levels in Rat-1 fibroblasts in response to Wnt-1 revealed an acute accumulation of β-catenin in the cytosolic cellular fraction. In contrast, little or no change in β-catenin levels was observed in the membranous fractions of these cells, indicating a selective modulation of cytosolic β-catenin by Wnt-1. As the membranous pool contains the majority of β-catenin in Rat-1 fibroblasts, the total cellular β-catenin was not noticeably changed by Wnt-1. Previous studies have shown that Wnt-1 stabilizes β-catenin as monomers and complexes with cadherin or APC in mammary epithelial cells (33). Our studies show Wnt-1 induces a specific accumulation of β-catenin in the cytosol and are consistent with analysis of β-catenin in other established cell lines (23). We also found that β-catenin levels were not altered in response to Wnt-5A expression, consistent with previous reports of different activities for Wnt-1 and Wnt-5A (12, 32, 52). We did not find evidence that Wnt-5A expression could interfere with Wnt-1 signaling in Rat-1 fibroblasts, in contrast to studies of the activity of Wnt-5A in Xenopus (46). It can be speculated that interactions between Wnt-5A and Wnt-1 are different in mammalian tissue culture cell lines from in Xenopus embryos. It is also possible that significant overexpression of Wnt-5A relative to Wnt-1 expression is required to exhibit an inhibitory effect on Wnt-1 activity.
One proposed function of cytosolic β-catenin is interaction with Tcf/Lef family transcription factors, activating downstream Wnt target genes. Tcf/Lef functions are critical in lymphoid cell development and inductive epithelial-mesenchymal cell interactions (49, 50). Recent findings with Xenopus and Drosophila have suggested that Tcf/Lef transcriptional activation is part of the Wnt/wingless signal transduction pathway (8, 26, 38, 48). However, there is no evidence of a direct link between Wnt signal and Tcf/Lef transcription in mammalian cells. Addressing this question, we examined Wnt-1 induction of Tcf/Lef transcriptional activities in Rat-1 fibroblasts. In both transient and stable systems, we observed that Wnt-1 expression results in strong activation of Tcf/Lef transcriptional elements. The observed Wnt-1 induction of Tcf/Lef transcriptional activities is specifically correlated with Wnt-1 induction of β-catenin in Rat-1 cells. Our findings with Rat-1 fibroblasts are consistent with current genetic models (30), pointing to a link between the Wnt signal, β-catenin induction, and Tcf/Lef transcriptional activation.
The biological phenotype of Rat-1 fibroblasts in response to the Wnt-1 signal suggests the presence of mitogenic activities of Wnt-1. Activities of Wnt-1 in mammalian cells have previously been examined by ectopic expression of Wnt-1 in mammary epithelial cells (7, 39) or mouse fibroblasts (5). Similar to previous findings, we observed that ectopic expression of Wnt-1 is sufficient to induce growth postconfluence in quiescent Rat-1 fibroblasts. In contrast to previous studies reporting that Wnt-1 was insufficient to support cell growth in low serum (5), we found that Wnt-1 enhances serum-independent growth of Rat-1 fibroblasts. The mechanism(s) by which Wnt-1 increases Rat-1 cell numbers is these assays is not known. Wnt-1 may act as a mitogen to support cell growth, which is indicated by the fact that Wnt-1-expressing cells showed significant growth postconfluence. However, it may act as a cell survival factor under serum-deprived conditions, hence increasing the number of viable cells. Wnt-5A does not have similar biological effects on Rat-1 fibroblasts, suggesting that the observed phenotype is due to Wnt-1-specific activities and not to a pan-Wnt activity; thus, Wnt-5A serves as an appropriate negative control in our experiments.
The observed growth response to Wnt-1 in Rat-1 fibroblasts is correlated with β-catenin induction and Tcf/Lef transcriptional activation. To explore the role of β-catenin, we generated Rat-1 stable cell lines expressing mutant β-catenin (S37A). Mutation of serine 37 of β-catenin (S37A) was originally identified in melanoma cell lines (22, 28, 42). S37A may interfere with GSK-3β phosphorylation of β-catenin and may lead to stabilization of cytosolic β-catenin (41). However, we found that mutant β-catenin (S37A) did not accumulate in the cytosol in Rat-1 fibroblasts. One interpretation of this finding is that alternate GSK-3β phosphorylation sites remain active and cause rapid degradation of β-catenin (S37A). Alternatively, β-catenin (S37A) may require additional modifications by the Wnt-1 signal to accumulate in the cytosol. Interestingly, β-catenin (S37A) is relatively insensitive to Wnt-1 signal, showing little accumulation in the cytosol following Ad/Wnt-1 infection (data not shown). It is possible, then, that serine 37 may be necessary for additional modifications of β-catenin by the Wnt-1 signal to accumulate in the cytosol.
Expression of β-catenin (S37A), however, results in strong constitutive Tcf/Lef transcriptional activities in Rat-1 cells, in the absence of Wnt-1 signal. The magnitude of activation seen in β-catenin (S37A)-expressing cells was comparable to that seen in Wnt-1-expressing cells. Introducing increasing amounts of β-catenin expression plasmid resulted in increasing transcriptional activation, while introducing increasing amounts of Wnt-1 did not lead to increasing activation. Our data suggest that β-catenin is a rate-limiting intermediate in Wnt-1 induction of Tcf/Lef transcriptional activation.
Constitutive Tcf/Lef transcriptional activity has recently been identified in colon cancer cell lines (22, 28). Mutations in β-catenin or mutations in the APC tumor suppressor gene are associated with constitutive Tcf/Lef transcriptional activity by a β-catenin/Tcf complex in these cell lines. Together, these findings suggest that deregulation of β-catenin or APC can lead to constitutive β-catenin/Tcf transcriptional activation, which may contribute directly to tumorigenesis. One interpretation of our findings is that Wnt-1 induces β-catenin and constitutive Tcf/Lef transcriptional activity, resulting in proliferative responses in Rat-1 fibroblasts.
Interestingly, we found that induction of Tcf/Lef transcriptional activity is not sufficient to alter cell growth and morphology in place of Wnt-1. Although Rat-1/Wnt-1 and Rat-1/β-CatS37A cells show comparable magnitudes of Tcf/Lef transcriptional activation, these cell lines do not show comparable biological phenotypes. One interpretation of these data is that the S37A mutation in β-catenin leads to aberrant transcriptional activity, independent of other potential signaling functions of β-catenin. Western blot analysis of nuclear fractions of Rat-1/β-CatS37A cell lysates showed some localization of β-catenin (S37A) in the nuclei (data not shown). It is thus possible that β-catenin (S37A) can translocate into the nucleus and have increased transcriptional activity. Another interpretation of these data is that Tcf/Lef transcription may be one of many downstream events in the Wnt-1 signal transduction pathway and that Tcf/Lef transcriptional activation alone is not sufficient to elicit proliferative effects in Rat-1 fibroblasts in place of Wnt-1. However, we also recognize that the Tcf/Lef transcriptional activity measured by reporter constructs (22) may be saturable and may not represent endogenous gene activation.
Cytosolic accumulation of β-catenin in response to Wnt-1 may have additional growth-signaling functions aside from Tcf/Lef transcriptional activation. β-Catenin (S37A) is functional in transcriptional activation but does not accumulate in the cytosol or enhance growth. We propose that accumulation of β-catenin may activate Tcf-dependent transcription but may also be required to transmit Wnt-1 growth signals in the cytosol. Modest changes in β-catenin levels may be sufficient to form functional β-catenin/Tcf complex, activating transcription. However, accumulation of a larger pool of cytosolic β-catenin may be necessary for Wnt-1 growth signals, for instance, by interactions with other cytosolic proteins. Considering β-catenin functions in cell adhesion, it is also possible that Wnt-1 expression in Rat-1 cells modulates β-catenin functions in cell adhesion, as reported in other settings (6, 20, 27). Consequently, changes in adhesive properties may alter cellular morphology and growth, eliciting Wnt-1 growth effects. In an alternative model, the Wnt-1 signal transduction pathway may bifurcate at a point upstream of β-catenin. As a result, a secondary signal upstream of β-catenin may contribute to Wnt-1 proliferative effects in Rat-1 fibroblasts. Experiments are in progress to further address the potential signaling events critical to Wnt-1 growth signals.
ACKNOWLEDGMENTS
We thank Eugene Marcantonio, Frank Costantini, Rudy Grosschedl, and Martin Julius for discussions and critical reading of the manuscript, and we thank Martin Julius and Qingyou Yan for construction of retroviral vectors. pTOPFLASH and pFOPFLASH reporter constructs were generously provided by Hans Clevers, β-catenin (S37A) cDNA was generously provided by Steven Byers, and Rat-1 fibroblasts were generously provided by Riccardo Dalla-Favera.
This work was supported by grants to J.K. from the U.S. Army Medical Research and Material Command (USAMRMC) under grant DAMD17-94-J-4069, the American Cancer Society (ACS DB-81), and the Marilyn Bokemeier Sperry Fund and by a predoctoral fellowship to C.S.Y. from the NIH (T32 CA09503).
ADDENDUM IN PROOF
Since submission of this paper, other groups have reported that Wnt-1 can induce Tcf reporter gene transcription (V. Korinek, N. Barker, K. Willert, M. Molenaar, J. Roose, G. Wagenaar, M. Markman, W. Lamers, O. Destree, and H. Clevers, Mol. Cell. Biol. 18:1248–1256, 1998) or a β-catenin–LEF-1 complex (E. Porfiri, B. Rubinfeld, I. Albert, K. Hovanes, M. Waterman, and P. Polakis, Oncogene 15:2833–2839, 1997).
REFERENCES
- 1.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens J, von Kries J P, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 3.Bett A J, Haddara W, Prevec L, Graham F L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhanot P, Brink M, Samos C H, Hsieh J-C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury J M, Niemeyer C C, Dale T C, Edwards P A W. Alterations of the growth characteristics of the fibroblast cell line C3H10T1/2 by members of the Wnt gene family. Oncogene. 1994;9:2597–2603. [PubMed] [Google Scholar]
- 6.Bradley R S, Cowin P, Brown A M C. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown A M C, Wilden R S, Prendergast T J, Varmus H E. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986;46:1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- 8.Brunner E, Peter O, Schweizer L, Basler K. Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- 9.Dickinson M E, Krumlauf R, McMahon A P. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson M E, Stark K L, Krumlauf R, McMahon A P. Misexpression of Wnt-1 in transgenic mouse embryos results in a massive overgrowth of cells in the spinal cord. In: Berns A, editor. Mouse molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. p. 17. [Google Scholar]
- 11.Donehower L A, Godley L A, Aldaz C M, Pyle R, Shi Y P, Pinkel D, Gray J, Bradley A, Medina D, Varmus H E. Deficiency of p53 accelerates mammary tumorigenesis in Wnt-1 transgenic mice and promotes chromosomal instability. Genes Dev. 1995;9:882–895. doi: 10.1101/gad.9.7.882. [DOI] [PubMed] [Google Scholar]
- 12.Du S J, Purcell S M, Christian J L, McGrew L L, Moon R T. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 14.Funayama N, Fagotto F, McCrea P, Gumbiner B M. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Giarre, M., Q. Dong, and A. M. C. Brown. Unpublished data.
- 15.Guger K A, Gumbiner B M. Beta-catenin has Wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- 16.Gumbiner B M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 17.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S, Rubinfeld B, Souza B, Polakis P, Wieschaus E, Levine A J. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates beta-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc Natl Acad Sci USA. 1997;94:242–247. doi: 10.1073/pnas.94.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzlinger D, Qiao J, Cohen D, Ramakrishna N, Brown A M C. Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev Biol. 1994;166:815–818. doi: 10.1006/dbio.1994.1360. [DOI] [PubMed] [Google Scholar]
- 20.Hinck L, Nelson W J, Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B G, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 22.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 23.Lin K, Wang S, Julius M A, Kitajewski J, Moos M, Jr, Luyten F P. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc Natl Acad Sci USA. 1997;94:11196–11200. doi: 10.1073/pnas.94.21.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrea P D, Turck C W, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 25.Miller J R, Moon R T. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 26.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 27.Moon R T, DeMarais A, Olson D J. Responses to Wnt signals in vertebrate embryos may involve changes in cell adhesion and cell movement. J Cell Sci. 1993;106:183–188. doi: 10.1242/jcs.1993.supplement_17.26. [DOI] [PubMed] [Google Scholar]
- 28.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 29.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusse R. A versatile transcriptional effector of Wingless signaling. Cell. 1997;89:321–323. doi: 10.1016/s0092-8674(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 31.Nusse R, Varmus H E. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 32.Olson D J, Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ. 1994;5:197–206. [PubMed] [Google Scholar]
- 33.Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peifer M. Beta-catenin as oncogene: the smoking gun. Science. 1997;275:1752–1753. doi: 10.1126/science.275.5307.1752. [DOI] [PubMed] [Google Scholar]
- 36.Peifer M, Pai L M, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- 37.Perrimon N. Serpentine proteins slither into the Wingless and Hedgehog fields. Cell. 1996;86:513–516. doi: 10.1016/s0092-8674(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 38.Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S C, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–787. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- 39.Rijsewijk F, Deemter L, Wagenaar E, Sonneberg A, Nusse R. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J. 1987;6:127–131. doi: 10.1002/j.1460-2075.1987.tb04729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- 41.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 42.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 43.Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain S H, Masiarz F R, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu H, Julius M A, Giarre M, Zheng Z, Brown A M C, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of β-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 45.Su L-K, Vogelstein B, Kinzler K W. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- 46.Torres M A, Yang-Snyder J A, Purcell S M, DeMarais A A, McGrew L L, Moon R T. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsukamoto A S, Grosschedl R, Guzman R C, Parslow T, Varmus H E. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 47a.Uyttendaele, H., J. V. Soriano, R. Montesano, and J. Kitajewski. Notch 4 and Wnt-1 proteins function to regulate branching morphogenesis of mammary epithelial cells in opposing fashion. Dev. Biol., in press. [DOI] [PubMed]
- 48.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 49.van Genderen C, Okamura R M, Farinas I, Quo R G, Parslow T G, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 50.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald H R, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 51.Whitehead I, Kirk H, Kay R. Expression cloning of oncogenes by retroviral transfer of cDNA libraries. Mol Cell Biol. 1995;15:704–710. doi: 10.1128/mcb.15.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong G T, Gavin B J, McMahon A P. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]