Abstract
This study, which included patients over the age of 18 who were diagnosed with coronavirus disease 2019 (COVID-19) in the emergency clinic, aims to determine the relationship between coagulation parameters and mortality. Epidemiologic data such as age, gender, medical history, vital parameters at emergency department admission, clinical findings, coagulation parameters such as d-dimer, prothrombin time (PT), active partial thromboplastin time (aPTT), international normalized ration (INR), fibrinogen, and platelet were evaluated. Patients with positive computerized tomography (CT) findings and positive polymerase chain reaction (PCR) together were included in the study. It was revealed that d-dimer, fibrinogen, INR, and PT values were higher in the elderly group. It was shown that there was a significant relationship between hospitalization days (ward or intensive care unit) and d-dimer levels. It was observed that d-dimer, fibrinogen elevation was significantly associated with prognosis by increasing mortality, and that platelet and aPTT values were also associated with prognosis and were lower in the mortality group. On the other hand, in receiver operating characteristic (ROC) analysis, the sensitivity and specificity data were 80.3%/80.0% for d-dimer, 70.5%/72.2% for fibrinogen, 58.2%/59.4% for aPTT, and 59.7%/59.2% for platelet, respectively. The overall classification success was 88.6% and mortality prediction success was 37.7% in the regression model of some coagulation parameters (d-dimer, fibrinogen, aPTT, and platelet) which were effective on prognosis. In conclusion, it was determined that d-dimer, fibrinogen, aPTT, and platelet parameters were directly associated with mortality and when these coagulation parameters were used together with the clinical, vital, and demographic data of the patients, the success of mortality prediction increased significantly.
Keywords: Coagulation, COVID-19, Laboratory parameters, Mortality, Prognosis
1. INTRODUCTION
Coronaviruses (CoVs) are known as a large family of viruses that generally affect the upper respiratory tract and have the potential to cause mild, self-limiting infections that are common in the community and more serious infections such as severe acute respiratory syndrome (SARS-2003) and Middle East Respiratory Syndrome (MERS-2012).1 There are subtypes of CoVs that are found in humans and can be easily transmitted from one individual to another. There are also many coronavirus subtypes identified in animals and it is known that these viruses can pass from animals to humans and cause many severe diseases in humans.2
On December 31, 2019, after the first case of acute respiratory syndrome of unknown etiology was reported in Wuhan, Hubei province, Chinese authorities identified a novel coronavirus (SARS-CoV-2), which clinically causes coronavirus disease 2019 (COVID-19) disease. The virus outbreak spread rapidly, infecting millions of people, affecting all continents and causing many deaths.3,4 On January 30, 2020, the World Health Organization (WHO) declared the current viral outbreak to be a public health emergency of international concern.5 Then, on February 11, 2020, this disease was named COVID-19 by WHO. On March 11, 2020, WHO declared COVID-19 a pandemic as the SARS-CoV-2 viral infection spread rapidly in all countries.6
While the entire coronavirus family predominantly affects the respiratory tract, SARS-CoV-2 virus also affects the heart, gastrointestinal system, liver, kidney, and central nervous system, and consequently may lead to multiple organ failure.7,8 Epidemiologic data and studies show that droplets after talking, coughing, or sneezing are the most common mode of transmission. Prolonged contact with an infected person (for at least 15 minutes) and shorter contact with symptomatic individuals are associated with a higher risk of transmission.9 Spread by contact (touching a surface with the virus on it) is another mode of transmission. Transmission can also occur via aerosols (smaller droplets suspended in the air), but it is not yet clear whether this is an important source of infection and route of transmission in humans outside the laboratory setting.10
The COVID-19 pandemic has become an emergency and public health issue in almost every country. According to many studies, the frequency of coagulopathy has increased in patients with severe clinical conditions and disseminated intravascular coagulation (DIC) is frequently observed in these patients. Therefore, it is considered useful to examine coagulation parameters to distinguish severe COVID-19 cases. The clinical presentation of COVID-19-related coagulopathy is usually organ dysfunction and thrombosis, while hemorrhagic events are relatively rare. There is a frequent relationship between the increase in d-dimer and fibrin/fibrinogen products and massive fibrin formation.11
Compared to other viral infections of respiratory origin, COVID-19 disease has a higher frequency of thrombus and clot formation and is generally associated with increased d-dimer levels. This condition is usually accompanied by an increase in prothrombin time (PT) and active partial thromboplastin time (aPTT), which is an indicator of poor prognosis.12 In some studies, d-dimer levels increase in approximately 10% of COVID-19 patients.13 When the physiology of COVID-19-associated coagulopathy is examined, it is thought to occur as a result of a series of complex chain of events such as the release of proinflammatory cytokines, platelet hyperactivation, and endothelial cell damage.14 With the triggering of viral infection, proinflammatory cytokines are activated and the inflammatory response of the immune system is triggered. Following this inflammation and as a result of the upregulated immune system, platelets are activated and natural anticoagulant systems are downregulated.15 High levels of inflammatory cytokines in the bloodstream provide stimulation of macrophages and leukocytes, but also cause the recruitment of these inflammatory cells, especially in the lung tissue.16 In response, infiltrated macrophages and polymorphonuclear neutrophils produce more cytokines and chemokines, which in turn cause other immune cells to be directed toward the lung tissue, further increasing the damage. With further damage, hyperinflammation and cytokine storm occur.17 On the other hand, increased endothelial damage may also be the cause of COVID-19-related coagulopathy. Mechanisms such as Von Willebrand factor (vWF), Toll-like receptors (TLR), and complement activation released from damaged endothelial structures may cause this. As a result of exacerbation of the clinic and progression of the sepsis, life-threatening conditions such as DIC may develop in patients. In a study, the rate of COVID-19-related DIC was reported to be 0.6% in survivors and 71.4% in non-survivors.12
Ranucci et al18 reported the presence of elevated fibrinogen, d-dimer, and interleukin-6 (IL-6) levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Zhou et al19 suggested that high d-dimer and increased PT are important factors associated with COVID-19 mortality. In a study including 388 COVID-19 patients, the rates of ischemic stroke, acute coronary syndrome/myocardial infarction, and DIC were found to be 2.5%, 1.1%, and 2.2%, respectively.20 All these data indicate that there is a close relationship between coagulation parameters and COVID-19 prognosis and that coagulation parameters may be important markers in terms of clinic and mortality. By using these markers as the basis of our study, the clinical, prognosis (mortality), hospitalization periods, and predictive features of the patients will be further defined and will provide important data to the literature to help identify risk groups.
Our study aims to determine the relationship between coagulation parameters (d-dimer, PT, aPTT, international normalized ratio [INR], fibrinogen, and platelet) and mortality in adult patients (patients with both positive polymerase chain reaction [PCR] and computerized tomography [CT] findings) diagnosed with COVID-19 in the Emergency Medicine Clinic.
2. MATERIALS AND METHODS
2.1. Research model
For this study, ethics committee approval numbered 2021/236 was obtained, and epidemiological data such as age, gender, background characteristics, vital parameters at admission to the emergency department, and clinical findings of patients aged 18 years and over who were diagnosed with COVID-19 between March 11, 2020, and April 20, 2021, were evaluated retrospectively. Coagulation parameters d-dimer, PT, aPTT, INR, fibrinogen, and platelet values were analyzed. Patients with both thorax CT findings and PCR positivity were included in the study. The patients were divided into 2 age groups, 18 to 65 and >65 years. The data of 498 patients, 369 in the 18 to 65 years age group and 129 in the >65 years age group, were evaluated. The pneumonia severity index (PSI) scores of the patients were calculated. A categorical distinction was made between the 18 to 65 and >65 age groups according to hospitalization-discharge status at the time of first admission, post-hospitalization prognosis (exitus—discharge with recovery), hospitalization status in the ward or intensive care unit (ICU), and the difference between the mean PSI scores of these groups was investigated.
In our study, survival groups (exitus and recovery), age groups, gender, hospitalization status at first admission, hospitalization unit (ICU or ward), and some clinical conditions were noted, and coagulation parameters were compared between these groups. Models were developed for the effect profile of coagulation parameters on mortality and their relationship with outcome. In model 1, all coagulation parameters were included and their effect profiles were evaluated. In model 2, other quantitative and qualitative parameters were included and parameters with effects on mortality were defined. After determining the parameters with an effect profile on mortality, sensitivity, specificity, and cut-off values were determined, and adapted for clinical use, and diagnostic (predictive) values were determined.
In the next step of the study, coagulation parameters were appropriately combined, and their abilities to classify the overall prognosis (outcome) were analyzed. In logistic regression (LR) analysis, significant and non-significant parameters were noted and their overall classification abilities were shown. Parameters with an impact profile on mortality were similarly combined with each other and their overall classification abilities were defined proportionally. In the last stage, the parameters with an effect profile on mortality were combined with the qualitative parameters that have an effect on mortality, and it was tested whether the combined use of clinical and vital parameter laboratory findings increased the overall classification ability. Thus, the effects of combining coagulation parameters and patient characteristics on mortality prediction were revealed. The confusion, uremia, respiratory rate, blood pressure, age ≥ 65 years (CURB-65) scores of the patients were calculated. A categorical distinction was made between 18 to 65 and >65 age groups according to hospitalization-discharge status at the time of first admission, post-hospitalization prognosis (exitus—discharge with recovery), hospitalization status in the ward or ICU, and the difference between the CURB-65 mean scores of these groups was investigated.
In older ages, the cut-off values of d-dimer may vary depending on age. In patients over 50 years of age, the cut-off value of d-dimer can be considered normal up to the age × 10 limit.21,22 Accordingly, stratified categorization was made and different cut-off values for d-dimer were calculated separately according to 50 years of age.
2.2. Sample size
Hospital Information Management System Records (HIMS) of the University Hospital Faculty of Medicine, epicrisis information in patient files, consultation information, and radiology reports were used to obtain the data. First, the information of patients aged 18 years and over was classified according to the International Statistical Classification of Diseases and Related Health Problems (ICD) diagnosis codes via HIMS, and the obtained data were compared with patient files and epicrisis information. Patients were randomly selected and a total of 498 patients were included in the study.
2.3. Statistical methods
Statistical analyses were performed using the SPSS 21.0 (IBM Inc, Chicago, Illinois) program. Descriptive statistics of numerical and categorical data obtained in the study were analyzed and numerical parameters were expressed as mean ± standard deviation (SD), and categorical variables were expressed as frequency. Kolmogorov–Smirnov test, histogram analyses, and Q–Q plot graphs were used for the conformity of numerical variables to normal distribution. Levene test was used for the analysis of homogeneity properties of numerical parameters. In cases where parametric conditions were met, independent sample t test was used for the comparison of 2 independent groups, and a one-way analysis of variance (ANOVA) test was used for multiple groups. In other cases where parametric assumptions were not met, the Mann-Whitney U test was used to compare 2 independent groups, and the Kruskal–Wallis H test was used to compare multiple independent groups. Tukey honestly significant difference (HSD) was used as a post hoc analysis for the interpretation of the significant results, and the relationships between the groups were analyzed. Binary LR modeling was used to determine prognostic factors on mortality. Box-Tidwell assumptions were met for the parameters used in the LR analysis. Additionally, model compatibility and model quality were checked with the Hosmer and Lemeshow goodness-of-fit test. Significant parameters affecting the prognosis were subjected to ROC analysis and diagnostic data were revealed. Relationships between categorical parameters were determined by Pearson Chi-square analysis. The type I error rate was 5% in the entire study and a P value of <.05 was considered significant.
3. RESULTS
3.1. Distribution of age, gender, comorbidity, and vital signs
Patients were divided into 2 age groups: 18 to 65 years old and ages over 65. A total of 369 (74.41%) patients in the 18 to 65 age group and 129 (25.9%) patients in the >65 age group were assessed and 234 (47%) of the patients were female, whereas 264 (53%) were male (Table 1).
Table 1.
Age and gender characteristics of patients.
| Frequency (N) | Percentage (%) | |
|---|---|---|
| Age | ||
| 18–65 | 369 | 74.1 |
| ≥65 | 129 | 25.9 |
| Gender | ||
| Female | 234 | 47.0 |
| Male | 264 | 53.0 |
Vital signs of patients were examined, and the average values were noted as follows: heart rate (HR) 86.56 beats/min, respiratory rate was 17.43 ± 4.28 breaths/min, partial oxygen saturation (SpO2) was 92.67 ± 8.66%, fever 37.28 ± 0, 83°C, systolic blood pressure (SBP) 119.68 ± 11.79 mm Hg, diastolic blood pressure (DBP) 73.61 ± 7.74 mm Hg, Glasgow Coma Score (GCS) 14.86 ± 0.86, and blood sugar 150.76 ± 55.16 mg/dL. The distribution of vital signs and laboratory results are summarized in Table 2.
Table 2.
Statistical distribution characteristics of vital and coagulation parameters.
| Vital signs | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Vital signs and GCS | |||
| Heart rate (min−1) | 64 | 152 | 86.56 ± 11.83 |
| Respiratory rate (min−1) | 12 | 40 | 17.43 ± 4.28 |
| SpO2 | 45 | 99 | 92.67 ± 8.66 |
| Fever (°C) | 35.5 | 39.4 | 37.28 ± 0.83 |
| Systolic blood pressure (mm Hg) | 70 | 188 | 119.68 ± 11.79 |
| Diastolic blood pressure (mm Hg) | 40 | 115 | 73.61 ± 7.74 |
| GCS | 3 | 15 | 14.86 ± 0.86 |
| Coagulation parameters | |||
| d-Dimer (ng/mL) | 80 | 16200 | 864.49 ± 1423.39 |
| Fibrinogen (ng/dL) | 79 | 1222 | 422.55 ± 147.54 |
| INR | 0.8 | 10.8 | 1.13 ± 0.86 |
| PT (s) | 9,4 | 110.0 | 12.45 ± 5.16 |
| aPTT (s) | 16.3 | 64.0 | 27.76 ± 4.80 |
| Platelet (K/µL) | 9 | 684 | 215.29 ± 86.40 |
aPTT = active partial thromboplastin time, GCS = Glasgow Coma Score, INR = international normalized ration, PT = prothrombin time, SD = standard deviation, SpO2 = partial oxygen saturation.
The most common comorbidities in the patients were diabetes mellitus (DM) (n = 72, 14.5%), hypertension (HT) (n = 112, 22.5%), chronic obstructive pulmonary disease (COPD) (n = 33, 6.6%), and vascular-peripheral-coronary artery disease (n = 37, 7.4%). Considering their comorbidity status, the patients were divided into 2 groups as those with no comorbidity in their history and those with at least 1 comorbidity, and 35.9% of the patients were found to have at least 1 comorbidity. When the distributional relationship between age groups (18–65 and >65 years of age) and the presence of comorbidity (present or absent) was examined, the overall comorbidity rate was seen to be higher in individuals >65 years of age (57.0% vs 43.0%; P < .001) as expected. It was also noted that exitus rates were higher in patients with comorbidities compared to those without any comorbidity (30.7% vs 3.8%; P < .001).
3.2. Pneumonia scoring systems: PSI and CURB-65 scores
The PSI score is a clinical and laboratory-based scoring system that is divided into 5 clinical classes, and these classes are correlated with the severity of pneumonia.23 PSI scores were calculated using a fast and systemically reliable online medical calculation tool, and the scores were calculated and recorded for each patient.24 The PSI scores of the patients did not differ statistically significantly from any other defined groups (P > .05) (Table 3).
Table 3.
Relationship of PSI and CURB-65 scoring with qualitative groups.
| First admission | P | ||
|---|---|---|---|
| Hospitalized | Discharged | ||
| PSI score (mean ± SD) | 81.88 ± 36.4 | 85.25 ± 38.7 | .45 |
| Inpatient outcome (mortality) | P | ||
| Exitus | Recovered | ||
| 83.57 ± 50.52 | 82.62 ± 36.5 | .84 | |
| Hospitalization-unit | P | ||
| Ward | ICU | ||
| 81.82 ± 35.57 | 79.35 ± 33.69 | .75 | |
| Age groups | P | ||
| 18–65 | >65 | ||
| 81.98 ± 35.91 | 84.94 ± 40.10 | .76 | |
| CURB-65 score (mean ± SD) | First admission | P | |
| Hospitalized | Discharged | ||
| 0.72 ± 1.07 | 0.22 ± 0.57 | <.001 | |
| Inpatient outcome (mortality) | P | ||
| Exitus | Recovered | ||
| 2.01 ± 1.24 | 0.37 ± 0.72 | <.001 | |
| Hospitalization-unit * | P | ||
| Ward | ICU | ||
| 0.44 ± 0.77 | 2.39 ± 1.40 | <.001 | |
| Age groups | P | ||
| 18–65 | >65 | ||
| 0.18 ± 0.53 | 1.76 ± 1.07 | <.001 | |
CURB-65 = confusion, uremia, respiratory rate, blood pressure, age ≥ 65 years, ICU = intensive care unit, PSI = Pneumonia Severity Index, SD = standard deviation.
Calculations were carried out for patients who were hospitalized (ward only or ICU only) at the time of first admission.
CURB-65 is a comprehensively researched and validated scoring system for predicting 30-day mortality in patients with pneumonia, consisting of 5 clinical parameters, and 1 point is assigned for each criterion.25 Scoring divides patients into 3 clinical groups according to the score they get, and mortality and the type of treatment are closely related to these groups.26,27
The CURB-65 score was analyzed among specific groups such as prognosis (mortality), hospitalization status, age groups, and hospitalization unit (ward or ICU), and statistical properties were revealed. The mean CURB-65 of the patients who were hospitalized at the time of first admission was 0.72 ± 1.07 and was noted to be significantly higher than those who were discharged (recovered) (P < .001). The mean CURB-65 score of the group who died after hospitalization (2.01 ± 1.24) was found to be significantly higher than those who were discharged with recovery (P < .001). The mean CURB-65 score of the patients who were followed up in ICU was noted as 2.39 ± 1.40 which was significantly higher than the group that received ward admission (P < .001). The mean CURB-65 score of patients over the age of 65 was found to be 1.76 ± 1.07, and it was found to be higher than the 18 to 65 age group (P < .001) (Table 3).
3.3. Outcome: hospitalization and prognosis (mortality)
At the first admission, 129 (25.9%) of the patients were discharged without hospitalization, and the remaining 369 (74.1%) patients were hospitalized. When analyzed specifically according to age groups, 69.9% of the patients in the 18 to 65 age group and 86.1% of the patients in the >65 age group were hospitalized (X2 =12,953, P < .001). When hospitalization and prognosis status were examined, 67 (13.45%) patients died, and 431 (86.55%) patients were fully recovered and discharged after treatment. Forty (59.7%) of the patients who died were female and 27 (40.3%) were male, indicating that there was no significant distribution relationship between gender and mortality (X2 = 1.391, P = .23). Sixty-eight patients were admitted to the ICU, and 338 patients were admitted to the ward. Patients who were followed in ICU were mostly hospitalized between 1 and 5 days (36 patients, 53%), and similarly, patients who were followed up in the ward were hospitalized most between 1 and 5 days (150 patients, 44%).
Considering the prognosis status of hospitalized patients, an examination was made according to death and recovery status, and each group was divided into age groups within itself. Forty-seven (36.4%) of the patients over the age of 65 and 20 (5.4%) of the patients in the 18 to 65 age group died which constituted a significant statistical difference (X2 = 78,961 P < .001).
According to the length of ward hospitalization, parameters were investigated in this regard and a significant difference was observed between the d-dimer averages (P = .009). The d-dimer average was highest in the group of patients who were admitted to the service for 11 to 20 days (984.75 ± 1471.30 ng/mL). No significant difference was observed in other parameters.
The comparison of the coagulation parameters between the groups according to the hospitalization units (ICU or ward) is summarized in Table 4.
Table 4.
Statistical analysis of coagulation parameters according to length of hospital stay (ward or ICU) and pre-defined GCS groups.
| Coagulation parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d-Dimer (ng/mL) | P | Fibrinogen (ng/dL) | P | INR | P | PT (s) | P | aPTT (s) | P | Platelet (K/µL) | P | |
| Mean ± SD | ||||||||||||
| LOSH-ICU | .09 | .13 | .31 | .22 | .86 | .58 | ||||||
| 1–5 d (n = 36) | 1585.89 ± 1799.53 | 479.78 ± 176.24 | 1.15 ± 0.38 | 13.68 ± 4.56 | 27.42 ± 4.83 | 182.19 ± 77.65 | ||||||
| 6–10 d (n = 14) | 1758.46 ± 1391.47 | 567.92 ± 154.78 | 1.17 ± 0.15 | 13.65 ± 1.99 | 27.00 ± 4.26 | 210.50 ± 133.57 | ||||||
| 11–20 d (n = 15) | 2529.40 ± 2385.20 | 580.08 ± 166.80 | 1.09 ± 0.18 | 27.07 ± 6.68 | 27.07 ± 6.68 | 189.53 ± 91.64 | ||||||
| >20 d (n = 3) | 413.50 ± 51.61 | 392.33 ± 183.30 | 4.26 ± 5.65 | 28.90 ± 6.86 | 28.90 ± 6.86 | 247.67 ± 70.49 | ||||||
| LOSH-Ward | .009 | .48 | .32 | .38 | .84 | .11 | ||||||
| 1–5 d (n = 150) | 816.29 ± 1677.74 | 438.28 ± 151.68 | 1.08 ± 0.80 | 11.80 ± 1.49 | 27.09 ± 3.68 | 224.18 ± 96.51 | ||||||
| 6–10 d (n = 112) | 814.43 ± 915.56 | 416.48 ± 136.64 | 1.10 ± 0.43 | 12.56 ± 3.55 | 27.31 ± 4.47 | 218.79 ± 98.12 | ||||||
| >10 d (n = 76) | 984.75 ± 1471.30 | 439.82 ± 151.65 | 1.19 ± 1.07 | 13.59 ± 11.79 | 28.01 ± 6.45 | 204.55 ± 86.12 | ||||||
| GCS | <.001 | .03 | <.001 | <.001 | .15 | .11 | ||||||
| 3–8 (n = 2) | 4449.50 ± 4879.74 | 496.50 ± 161.92 | 0.95 ± 0.02 | - | 22.10 ± 4.10 | 156.00 ± 48.08 | ||||||
| 9–13 (n = 12) | 2427.25 ± 2274.47 | 495.45 ± 154.35 | 1.41 ± 0.60 | 16.46 ± 6.98 | 29.88 ± 6.74 | 170.50 ± 89.79 | ||||||
| 14–15 (n = 484) | 809.34 ± 1339.42 | 420.18 ± 147.09 | 1.12 ± 0.86 | 12.35 ± 5.09 | 27.73 ± 4.73 | 216.66 ± 86.18 | ||||||
aPTT = active partial thromboplastin time, GCS = Glasgow Coma Score, ICU = intensive care unit, INR = international normalized ration, LOSH = length of stay in hospital, PT = prothrombin time, SD = standard deviation.
3.4. Comparison of coagulation parameters regarding specific groups
3.4.1. Age groups
When examined in both age groups, a significant difference was found between the mean values of d-dimer (P < .001), fibrinogen (P < .001), INR (P = .004), and PT (P = .001). The average values of d-dimer (1683.95 ± 2276.72), fibrinogen (505.33 ± 169.58), INR (1.24 ± 1.19), and PT (13.53 ± 9.31) were found to be higher in patients over 65. On the other hand, there was no significant difference between age groups for aPTT and platelet values. The mean hospitalization days of the patients admitted to the ICU did not differ significantly between age groups (P = .57). The mean hospitalization days of the >65 age group were higher (7.98 ± 5.31 days) than the 18 to 65 age group (6.55 ± 4.0), which revealed a significant difference between the hospitalization days and age groups (P = .02) (Table 5).
Table 5.
Comparison of the characteristics of coagulation parameters according to age groups.
| Age groups | Mean ± SD | P | |
|---|---|---|---|
| d-Dimer (ng/mL) | 18–65 (n = 362) | 588.32 ± 818.48 | <.001 |
| >65 (n = 122) | 1683.95 ± 2276.72 | ||
| Fibrinogen (ng/dL) | 18–65 (n = 306) | 393.61 ± 127.15 | <.001 |
| >65 (n = 107) | 505.33 ± 169.58 | ||
| INR | 18–65 (n = 366) | 1.09 ± 0.70 | .004 |
| >65 (n = 128) | 1.24 ± 1.19 | ||
| PT (s) | 18–65 (n = 367) | 12.05 ± 2.31 | .001 |
| >65 (n = 128) | 13.53 ± 9.31 | ||
| aPTT (s) | 18–65 (n = 367) | 27.78 ± 4.08 | .15 |
| >65 (n = 128) | 27.71 ± 6.46 | ||
| Platelet (K/µL) | 18–65 (n = 364) | 217.42 ± 81.83 | .15 |
| >65 (n = 129) | 209.27 ± 98.25 | ||
| LOSH-ICU (d) | 18–65 (n = 26) | 7.04 ± 4.98 | .57 |
| >65 (n = 42) | 8.36 ± 11.32 | ||
| LOSH-ward (d) | 18–65 (n = 245) | 6.55 ± 4.0 | .02 |
| >65 (n = 93) | 7.98 ± 5.31 |
aPTT = active partial thromboplastin time, LOSH = length of stay in hospital, ICU = intensive care unit, INR = international normalized ration, PT = prothrombin time, SD = standard deviation.
3.4.2. GCS groups
Average levels of d-dimer (P < .001), fibrinogen (P = .03), INR (P < .001), and PT (P < .001) showed a significant difference between GCS groups. aPTT and platelet showed no significant difference between the defined groups.
3.4.3. Prognosis (outcome) groups
Demographics, vital signs, coagulation parameters, and duration of hospitalization (ward or ICU) were compared between the 2 groups: exitus and recovery groups. In the examination, the mean values of age, pulse, and respiratory rate were found to be significantly higher in the mortality group (P < .001). Results revealed that the mean fever of patients who died (37.5 ± 0.84) was found to be significantly higher (P = .001). Additionally, average levels of d-dimer, fibrinogen, INR, and PT significantly differed within the prognosis groups (P < .001). The average values of d-dimer (2489.48 ± 2898.50), fibrinogen (525.69 ± 163.44), INR (1.25 ± 1.12), and PT (14.71 ± 12.33) was substantially higher in the exitus groups. On the other hand, SpO2 (P < .001), SBP (P = .002), DBP (P < .001), GCS (P = .001), aPTT (P = .007), and platelet (P < .001) values were found to be significantly lower in the exitus group. In the exitus group, it is recorded that the mean values were 79.82 ± 13.52 for SpO2, 112.33 ± 20.88 for SBP, 68.22 ± 12.31 for DBP, 14.04 ± 2.18 for GCS, 26.78 ± 6.83 for aPTT, and 178.46 ± 80.92 for platelet (Table 6).
Table 6.
Characteristics of the patients at the time of first admission according to the prognosis (mortality) status.
| Parameters | Exitus (n = 67) | Recovered (n = 431) | P |
|---|---|---|---|
| Mean ± SD | |||
| Age (y) | 72.01 ± 14.54 | 48.54 ± 16.35 | <.001 |
| Heart rate (/min) | 95.13 ± 18.29 | 85.23 ± 9.86 | <.001 |
| Respiratory rate (/min) | 20.97 ± 6.69 | 16.88 ± 3.47 | <.001 |
| SpO2 (%) | 79.82 ± 13.52 | 94.67 ± 5.38 | <.001 |
| Fever (°C) | 37.5 ± 0.84 | 37.2 ± 0.82 | .001 |
| SBP (mm Hg) | 112.33 ± 20.88 | 120.82 ± 9.17 | .002 |
| DBP (mm Hg) | 68.22 ± 12.31 | 74.45 ± 6.38 | <.001 |
| GCS (points) | 14.04 ± 2.18 | 14.98 ± 0.14 | .001 |
| d-Dimer (ng/mL) | 2489.48 ± 2898.50 | 603.41 ± 725.13 | <.001 |
| Fibrinogen (ng/dL) | 525.69 ± 163.44 | 404.68 ± 137.17 | <.001 |
| INR | 1.25 ± 1.12 | 1.11 ± 0.81 | <.001 |
| PT (s) | 14.71 ± 12.33 | 12.09 ± 2.54 | <.001 |
| aPTT (s) | 26.78 ± 6.83 | 27.92 ± 4.39 | .007 |
| Platelet (K/µL) | 178.46 ± 80.92 | 221.08 ± 85.89 | <.001 |
| LOSH-ICU (d) | 7.13 ± 6.23 | 10.40 ± 16.40 | .67 |
| LOSH-ward (d) | 5.92 ± 4.43 | 7.07 ± 4.43 | .059 |
aPTT = active partial thromboplastin time, DBP = diastolic blood pressure, GCS = Glasgow Coma Score, ICU = intensive care unit, INR = international normalized ration, LOSH = length of stay in hospital, PT = prothrombin time, SBP = systolic blood pressure, SD = standard deviation, SpO2 = partial oxygen saturation.
3.5. Regression models on mortality
Coagulation parameters, demographic characteristics, vital signs, and some clinical parameters were carried out in LR analysis for prediction of prognosis outcome (model 1), and the model was found to be significant in terms of predicting mortality (−2LL = 243.434 R2 Nagelkerke = 0.382). In the analysis, it was observed that the elevation of d-dimer (P < .001, B = 0.001 and odd ration [OR] >1) and fibrinogen (P < .001, B = 0.004, and OR > 1) were significant in favor of mortality. In addition, aPTT and platelet values have significant effects on prognosis; as aPTT (P = .03, B = −0.099, and OR = 0.906) and platelet (P < .001, B = −0.013, and OR = 0.988) values decreased, it was observed that it was relevant to mortality. However, INR and PT values did not have any effect or predictive capabilities on prognosis (Table 7, model 1).
Table 7.
Coagulation parameters at the time of first admission and their prognostic values that affect mortality.
| Mortality (exitus): effect profile of independent variables | ||||
|---|---|---|---|---|
| Binary LR model 1 | −2LL = 243.434 R2 Nagelkerke = 0.382 |
|||
| B | P | Exp(B) | 95% CI | |
| d-Dimer (ng/mL) | 0.001 | <.001 | 1.001 | 1.000–1.001 |
| Fibrinogen (ng/dL) | 0.004 | <.001 | 1.004 | 1.002–1.007 |
| INR | −0.261 | .640 | 0.770 | 0.258–2.302 |
| PT (s) | 0.118 | .064 | 1.125 | 0.993–1.274 |
| aPTT (s) | −0.099 | .030 | 0.906 | 0.828–0.990 |
| Platelet (K/µL) | −0.013 | <.001 | 0.988 | 0.982–0.993 |
| Mortality (exitus): effect profile of independent variables | ||||
| Binary LR model 2 |
−2LL= 178.281
R2 Nagelkerke = 0.642 |
|||
| Age (y) | 0.094 | <.001 | 1.099 | 1.066–1.132 |
| Gender | 0.372 | .364 | 1.451 | 0.650–3.239 |
| Fever (°C) | 0.243 | .309 | 1.275 | 0.798–2.038 |
| Heart rate (min−1) | 0.010 | .493 | 1.010 | 0.981–1.040 |
| SBP (mm Hg) | −0.002 | .915 | 0.998 | 0.957–1.041 |
| DBP (mm Hg) | −0.037 | .253 | 0.904 | 0.904–1.027 |
| Respiratory rate (min−1) | −0.086 | .038 | 0.918 | 0.847–0.995 |
| SpO2 (%) | −0.137 | <0.001 | 0.872 | 0.834–0.912 |
| GCS (points) | −1.107 | 0.023 | 0.331 | 0.127–0.860 |
aPTT = active partial thromboplastin time, CI = confidence interval, DBP = diastolic blood pressure, Exp(B): odds ratio, GCS = Glasgow Coma Score, INR = international normalized ration, LL = log likehood, LR = logistic regression, PT = prothrombin time, SBP = systolic blood pressure, SpO2 = partial oxygen saturation.
Reference category: recovery group.
The model (***second model), which included patient's demographic characteristics, vital signs, and some clinical parameters was found to be significant in terms of mortality (−2LL = 178.281 R2 Nagelkerke = 0.642). The analysis showed that age, respiratory rate, SpO2, and GCS have significant effects on prognosis. It was observed that age had significant effects on mortality (P < .001, B = 0.094, and OR > 1). On the other hand, respiratory rate (P = .038, B = −0.086, and OR = 0.918), SpO2 (P < .001, B = −0.137, and OR = 0.872), and GCS (P = .023, B =−1.107, and OR = 0.331) have significant effects on prognosis. GCS has been shown to be closely associated with mortality (Table 7, model 2).
3.6. Successes of combining parameters in predicting mortality
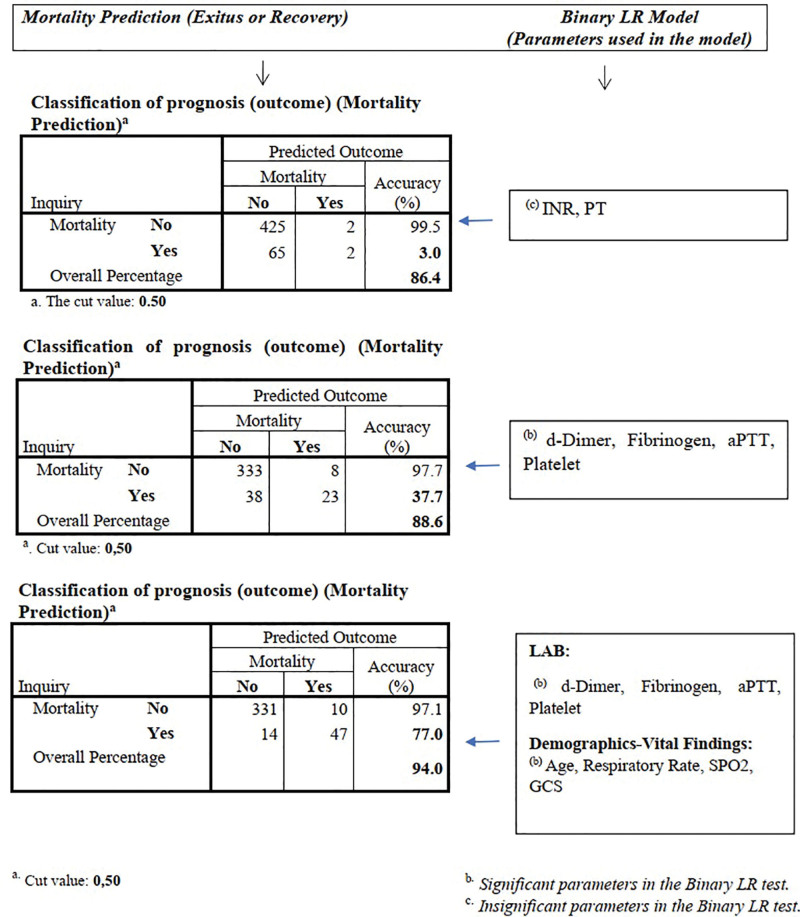
The binary relationship of the parameters used in the study was analyzed statistically, and grouping was made for the parameters regarding their significance. The power of the combined parameters in each group in predicting mortality and their predictive success in percentage were analyzed and compared by schematizing in separate models.
In the model using INR and PT, the mortality prediction rate was 3%, and the overall classification rate (mortality or discharge with recovery) was noted as 86.4% which revealed that the mortality prediction capability of this model was not considered adequate.
On the other hand, the mortality detection rate of the second model obtained by using d-dimer, fibrinogen, aPTT, platelet parameters was 37.7% and the overall prognosis classification (mortality or discharge with recovery) rate was 88.6%, which was found to be more significant than the previous model.
Finally, in the third model in which age, respiratory rate, SpO2, GCS data were included in addition to d-dimer, fibrinogen, aPTT, and platelet parameters; the mortality detection rate was 77.7% and the overall classification rate (mortality or discharge with recovery) was 94.0% which demonstrates that the model is significant and clinically useful in terms of mortality prediction (Fig. 1).
Figure 1.
Combining parameters and investigating their predictive features. aPTT = active partial thromboplastin time, GCS = Glasgow Coma Score, INR = international normalized ration, LR = logistic regression, PT = prothrombin time, SpO2 = partial oxygen saturation.
3.7. ROC analysis and predictive values
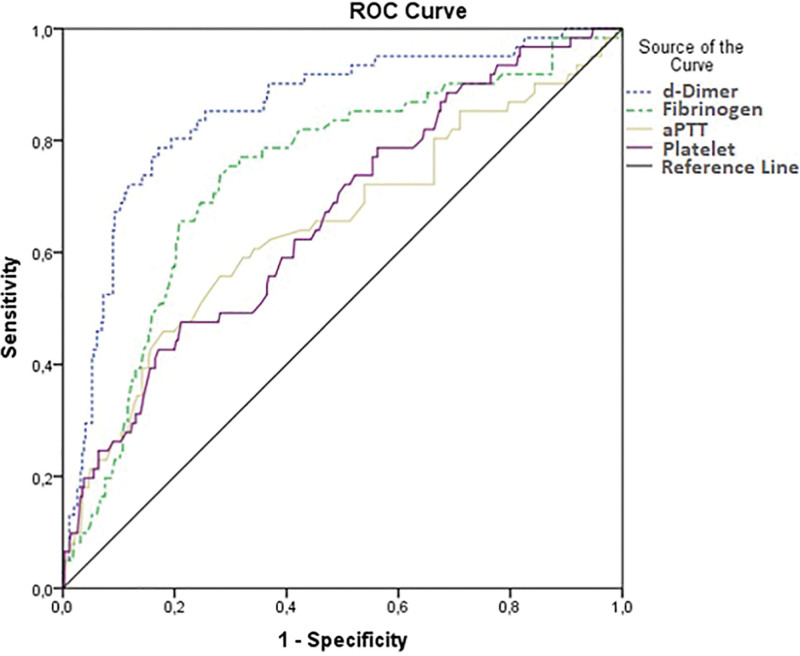
ROC curves of d-dimer, fibrinogen, aPTT, and platelet parameters are shown in Figure 2. Area under curve (AUC), sensitivity, specificity, cut-off, and significance (P) data obtained in ROC analysis were recorded and summarized in Table 8.
Figure 2.
ROC analysis curves of d-dimer, fibrinogen, aPTT, and platelet parameters. aPTT = active partial thromboplastin time, ROC = receiver operating characteristic.
Table 8.
ROC analysis results and the AUC of the significant coagulation parameters regarding mortality prediction.
| AUC (%95 CI) | Cut-off | P | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| d-Dimer (ng/mL) | 0.85 (0.805–0.914) | 698.5 | <.001 | 80.30 | 80.00 |
| Fibrinogen (ng/dL) | 0.73 (0.667–0.806) | 451.5 | <.001 | 70.5 | 72.2 |
| aPTT* (s) | 0.60 (0.518–0.682) | 26.95 | .009 | 58.2 | 59.4 |
| Platelet* (K/µL) | 0.64 (0.569–0.717) | 195.5 | <.001 | 59.7 | 59.2 |
aPTT = active partial thromboplastin time, AUC = area under curve, CI = confidence interval, ROC = receiver operating characteristic.
Lower laboratory results were found to be associated with positive outcome (mortality).
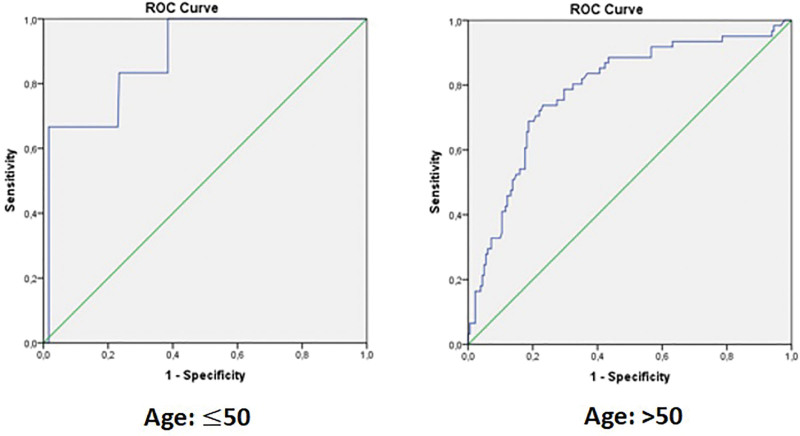
In older ages, the cut-off values of d-dimer may vary depending on age. In patients over 50 years of age, the cut-off value of d-dimer can be considered normal up to the age ×10 limit. In this regard, stratified categorization was made and different cut-off values for d-dimer were calculated separately according to 50 years of age (Fig. 3, Table 9).
Figure 3.
ROC analysis schemes for d-dimer on mortality prediction (for each group categorized by age). ROC = receiver operating characteristic.
Table 9.
ROC analysis results, predictive features, and the AUC of d-dimer (categorized by age and analyzed separately).
| Lab. | Age groups | Mean ± SD | AUC (95% CI) | Cut-off | P | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| d-Dimer (ng/mL) | ≤50 | 36.59 ± 8.70 | 0.886 (0.768–1.000) | 514.00 | .001 | 83.3 | 76.6 |
| >50 | 66.68 ± 10.88 | 0.785 (0.717–0.853) | 930.50 | <.001 | 73.8 | 73.6 |
AUC = area under curve, CI = confidence interval, ROC = receiver operating characteristic, SD = standard deviation.
4. DISCUSSION
The recently emerged COVID-19 disease is a highly contagious viral disease caused by another zoonotic novel coronavirus called SARS-CoV-2.
Several studies have shown that thrombotic complications and coagulopathy are common in COVID-19. In addition, the features of COVID-19-associated coagulopathy differ from those seen with bacterial sepsis-induced coagulopathy and DIC and are usually characterized by increased d-dimer and fibrinogen levels. However, normal or minimally increased values may also be seen initially.
In a study conducted by Satici et al23 in a university hospital in Istanbul, CURB-65 and PSI scores were analyzed in 681 PCR-confirmed COVID-19 patients. The mean age of the sample was 56.9 ± 15.7 years. In the related study, the PSI score was grouped, and no mortality was observed in group I. Significant values were obtained in predicting 30-day mortality with 80% sensitivity and 89% specificity (P < .001) in the PSI score in general, and the 30-day mortality rate of the sample was 8%. The PSI ≥ 4 group showed better sensitivity (80% vs 73%) and specificity (89% vs 85%) compared to those with a CURB-65 score ≥2, with a similar negative predictive value (98% vs 97%). In our study, unlike the study by Celal et al, patients were included in the study based on both PCR positivity and typical CT findings, and PSI and CURB-65 scores were analyzed according to age, hospitalization status, and prognosis groups. When analyzed according to prognosis (exitus or discharge with recovery), hospitalization (ward or ICU), and age groups (18–65 and >65 years), no significant difference was observed in PSI score between the groups. On the other hand, CURB-65 scores were significantly higher in patients who were hospitalized at the time of first admission, compared to those who were discharged, in patients who were hospitalized in the ICU compared to patients who were hospitalized in the ward, and in older patients compared to the younger group (P < .001). In our study, it was seen that the CURB-65 score, rather than the PSI score, was more significant in terms of hospitalization status and prognosis, and was more predictive in terms of mortality.
Zhang et al28 diagnosed 289 patients with positive PCR results at a University hospital in Wuhan with COVID-19 and divided them into 3 groups according to disease severity and clinical outcome: patients who died (group A), patients with severe clinical course (group B) and non-severe patients (group C). By March 28, 2020, all 78 patients with severe clinical course and 162 (56.0%) non-severe patients were discharged and 49 (17.0%) patients died. The patients were divided into 4 groups as 18 to 30, 30 to 49, 50 to 69, 70 years old. It was observed that the mean age of group A was higher than group B (P = .029). Group C was found to consist of younger individuals compared to group B (P < .001). LR model showed that age was a significant risk factor for mortality.28 Our study included patients who had both a positive PCR and a positive thorax CT findings which differs from their study in this regard. The mean length of hospitalization (days) of patients in the ward showed a significant difference between age groups (P = .024) and the mean length of hospitalization was higher in the >65 age group (7.98 ± 5.31 days). On the other hand, when analyzed according to the prognosis groups, the mean age was significantly higher in the exitus group (P < .001). Unlike the results of Zhang et al,28 the mean fever (37.5 ± 0.84) of patients who died was significantly higher in our study (P = .001). In our study, the effect of age on mortality and prognosis is similar to the studies of Zhang et al.28
Güçlü et al29 investigated the effect of COVID-19 on platelet count in 215 COVID-19 patients in a retrospective cohort study conducted in a tertiary education and research hospital between April 1, 2020, and April 15, 2020. Control platelet counts were evaluated as the values measured on the third day of hospitalization. Accordingly, 9 patients were excluded from the study because they were discharged by the third day. According to clinical severity, patients with saturation above 90 and below 90 were considered as moderate and severe patients, respectively. Thrombocytopenia was observed in 54 patients (25.1%) on the day of hospitalization, and in 52 patients (24.1%) on the third day of follow-up. The difference in platelet count between the 2 days was significant in patients with severe and moderate COVID-19 (P < .05).29 In our study, no significant difference was observed in mean platelet values according to age groups, length of stay in the ward, or ICU. In our study, demographics, vital signs, coagulation parameters, and length of hospitalization of the patients were analyzed between the mortality and recovery groups, and the effect of platelet values on prognosis (mortality) was also investigated. In our study, platelet (P < .001) values were significantly lower in the exitus group and were found to be associated with prognosis and mortality.
In their study, Li et al30 investigated the dynamic relationship between d-dimer levels and the progression of COVID-19 in 279 PCR-positive COVID-19 patients at 3 hospitals in Hubei Province, China. Patients were further divided into 3 groups according to their clinical course: mild (n = 136), worsening and slowly recovering (n = 23), severe or mortal (n = 120). The coagulation parameters of the patients were examined for 10 days. High d-dimer levels at admission were associated with a poor prognosis of COVID-19. The d-dimer levels at admission were higher in the first and third groups. D-Dimer levels decreased gradually in the recovering group, but remained high as the disease worsened in the third group. In addition, in the current study, significant differences were found between the three groups in DBP and respiratory rate, which are vital parameters measured at the time of first admission. Respiratory rate was higher in the third group, and DBP was lower in the third group.30 In our study, the patients were divided into 3 groups as GCS 3 to 8, 9 to 13, and 14 to 15, and the relationships of coagulation parameters between these groups were examined. It was observed that d-dimer increased significantly as GCS decreased (P < .001). According to the prognosis (outcome) status, the mean d-dimer was found to be higher in the exitus group and it was shown to have significant effects on prognosis (P < .001, B = 0.001, and OR > 1). In this context, our study is similar to the study by Li et al.30 On the other hand, SBP (P = .002) and DBP (P < .001) were found to be significantly lower in the exitus group when compared between the 2 prognosis groups. The pulse rate was higher in the exitus group, which was found to be significant. In this respect, our study differs from the study of Li et al30 in terms of the results obtained for SBP and pulse rate.
Long et al31 retrospectively investigated the effect of d-dimer and PT on prognosis in a total of 115 patients with PCR-confirmed COVID-19 who were admitted to Wuhan Tianyou Hospital between January 18, 2020, and March 5, 2020. Patients with a hospital stay longer than 14 days were included in the study. The cases with incomplete laboratory data or a hospital stay of less than 14 days were excluded. According to clinical status, patients were categorized into 4 groups: mild cases, moderate cases, severe cases, and critical cases. Significant differences in d-dimer were observed between different clinical groups (P < .05). It was found that high levels of d-dimer were associated with the severity of the disease, and there was a correlation between clinical outcomes and d-dimer levels. There was also a significant difference (P < .05) and positive correlation between PT and aPTT results. Based on the study results, d-dimer, PT, and aPTT levels were also found to be significantly higher in mortality group. In addition, study results showed that d-dimer, one of the fibrin degradation products, gradually increased throughout the disease.31 When both age groups were analyzed in our study, a significant difference was found between the groups in d-dimer (P < .001) and PT (P = .001) values. In addition, aPTT (B = −0.013, P = .03, and OR = 0.98) was also found to have an effect on prognosis (mortality) in our study, similar to the results of Long et al.31 When the parameters were analyzed according to the length of ICU stay and hospitalization unit (ward or ICU), no significant difference was observed in PT between the groups. Similar to the study by Long et al,31 d-dimer and aPTT levels were found to be associated with mortality. On the other hand, PT values had no effect on prognosis. In this respect, our study differs from the study by Long et al.31
In a retrospective study evaluating the correlation between coagulation factors and disease status in 303 patients with COVID-19, Zou et al32 compared coagulation parameters between 2 groups of patients with mild and severe clinical course. Based on demographic characteristics, compared to the mild group, most patients in the severe group were male (76.9% vs 49.8%) and elderly, and 209 of 303 patients had abnormal parameters. Median coagulation parameters in the severe group were higher than those in the mild group: INR (1.04 vs 1.01), PT (13.8 vs 13.4) seconds, aPTT (43.2 vs 39.2) seconds, fibrinogen (4.74 vs 4.33) g/L, and D-dimer (1.04 vs 0.43) µg/mL and the differences between groups were statistically significant (P < .05).32 Although the results obtained in our study were similar, there were also differences. When analyzed in both age groups, significant differences were found between the mean values of d-dimer (P < .001), fibrinogen (P < .001), INR (P = .004), and PT (P = .001). On the other hand, there was no significant difference between age groups in the mean of aPTT and platelet values. In terms of mortality and clinical course, d-dimer, fibrinogen, INR, and PT mean values differed significantly (P < .001). In the exitus group, the mean values of d-dimer (2489.48 ± 2898.50), fibrinogen (525.69 ± 163.44), INR (1.25 ± 1.12), and PT (14.71 ± 12.33) were higher, while aPTT (P = .007) and platelet (P < .001) values were significantly lower. In our study, the coagulation parameters of the patients were subjected to LR analysis to determine the effect on prognosis. The analysis showed that d-dimer and fibrinogen elevation had a significant effect in favor of mortality (P < .001, B = 0.001, and OR > 1). In addition, aPTT and platelet values had significant effects on prognosis, and as aPTT (P = .03, B = −0.099, and OR = 0.906) and platelet (P < .001, B = −0.013, and Exp(B) = 0.988) values decreased, they were found to have effects in favor of mortality. On the other hand, no relationship of gender with prognosis and mortality was found in our study (B = 0.372, P = .36, and OR = 1.451). Our study showed no effect of gender, INR, and PT values on prognosis and in this respect, it differs from the study by Zou et al.32
In 2021, in a study conducted on 267 COVID-19 patients, Esmaeel et al33 compared biochemistry and coagulation parameters on disease severity and investigated their relationship with mortality. In the study, PCR positivity was taken as the diagnostic criteria for COVID-19. In the study, disease severity was divided into 2 groups (severe and non-severe) and evaluated. According to the results, it was noted that deaths were observed only in the severe group. In the study conducted by Esmaeel et al,33 ROC analysis and predictive values were noted for the prediction of the group in which the disease was severe and deaths occurred, and it was observed that AUC = 0.940, sensitivity 76.0%, and specificity 93.0% at d-dimer 2.0 ng/L cut-off value (P < .001). Our study was conducted with a larger sample size of 498 patients and included patients with both PCR positivity and COVID-related thorax CT involvement. When our data were analyzed, in the general ROC analysis for mortality, AUC = 0.850, sensitivity = 80.3%, and specificity = 80.0% at the cut-off value of 698.5 ng/mL, which was different from the study conducted by Esmaeel et al.33 In addition, in our study, stratified categorization was made according to the age limit of 50 and different cut-off and ROC analysis values were obtained and detailed for d-dimer in groups under and above 50 years of age. At the cut-off value of 514 ng/mL for those aged ≤50, the AUC = 0.886 was found to be higher than the general analysis. In the study by Esmaeel et al,33 AUC values and specificity values were higher compared to our study, while the sensitivity value was higher in our study. Since our study included patients with both PCR and thorax CT positive, a more assuring approach was adopted in terms of COVID-19 diagnosis. However, false positivity rates of 5% were reported in PCR positivity.34,35 On the other hand, our study includes a larger sample size. In Esmaeel et al33 study, the fact that the study included only 267 samples and the mortality rate was only 2.99% (n = 8) brings limitations due to unbalanced data in the evaluation of mortality. On the contrary, the rate of mortal patients was higher in our study (13.5%, n = 67). In light of all these data, we think that the difference in the results in our study is due to the larger sample size, the use of a more assuring method in terms of diagnosis and the larger number of mortal patients (more balanced data).
Job et al conducted a study36 on coagulation parameters in 100 COVID-19 patients in 2020. In their study, patients were divided into 3 groups as mild, moderate-severe, and critical, and coagulation parameters were analyzed according to mortality and severity groups. It was observed that d-dimer was associated with disease severity and there was a significant difference between the groups (P < .0001). In addition, d-dimer was found to be associated with mortality on days 1 and 5 and was higher in the mortal patient groups (P = .002). No significant difference was found in fibrinogen, platelets, and aPTT values according to disease severity groups. Similarly, in our study, d-dimer was found to be significantly higher in mortal patients (P < .001). In this respect, our results were similar to the study. In contrast to the study by Job et al, fibrinogen, INR, PT, aPTT, and PLT values differed according to the mortality groups in our study. In addition, it was revealed by LR analysis that d-dimer, fibrinogen, aPTT, and PLT values were directly related to mortality and had predictive properties.
Citu et al37 examined the role of coagulation parameters in COVID-19 prognosis and mortality prediction. In contrast to our investigation, Citu et al37 demonstrated that there were no significant differences in fibrinogen levels between the exitus and survival groups (P = .97). In contrast, our study observed that fibrinogen levels were significantly higher in the exitus group (P < .001), indicating its significance as a predictive marker for mortality. Also in the relevant study, aPTT values were seen to be higher in the exitus group (P = .001), while sensitivity and specificity were seen as 52% and 92%, respectively. In our study, sensitivity and specificity of aPTT were 59.7% and 59.2%, respectively, whereas compared to the investigation conducted by Citu et al,37 the specificity values were significantly lower in our study and differed in this respect. In another study, Altay et al38 investigated the relationship between platelet and d-dimer levels and COVID-19 outcome and demonstrated that platelet levels did not constitute a significant difference according to survival groups (P = .193). On the contrary, in our study, platelet levels were seen to be lower in the exitus group and constituted a significant difference (P < .001).
In our study, a close relationship was observed between age and mortality, and it is thought that increasing age increases the comorbidity rates in patients, and accordingly, comorbidities may have significant effects on mortality. In our results, when the distributional relationship between age groups (18–65 and >65 years) and the presence of comorbidity (present or absent) was analyzed, the comorbidity rate was found to be higher in individuals >65 years of age (57.0% vs 43.0%; P < .001) as expected and the related results support this situation. In addition, the higher rate of exitus in patients with comorbidities compared to those without any comorbidity (30.7% vs 3.8%) also supports this situation.
Our data indicate that there is a significant clinical relationship between coagulation parameters (d-dimer, fibrinogen, aPTT, and platelet) and mortality and that these data can be predictive in regard to mortality. In addition, our results also emphasize the importance of evaluating the patient comprehensively and from a wide perspective (clinical findings, demographics, vital values, etc), not only with laboratory results. In this context, clinicians should use a combination of laboratory and patient characteristics and consider that these parameters have significant effects on mortality and prognosis.
5. CONCLUSION AND RECOMMENDATIONS
In this study, the importance of some coagulation parameters in the clinical course of the disease and between age groups and the relationship between the length of ICU or ward stays in patients with COVID-19 were examined. The results obtained in our study were largely similar to the results obtained in the previous studies.
No significant difference was found between the mean PSI scores between the groups. Contrary to our study, some studies have reported that PSI is more valuable. In our study, there was a significant relationship between CURB-65 and hospitalization unit (ward/ICU), survival and age groups. The fact that CURB-65 was higher in our study, especially in the exitus group, suggests that it may be closely related to mortality and reveals the importance of its clinical use.
In our study, a significant difference was found between the mean values of d-dimer, fibrinogen, INR, and PT according to age groups, which is similar to the results obtained in other studies.
When the parameters were analyzed according to the length of ICU stay, it was observed that the highest d-dimer mean was in the group with 11 to 20 days of ICU stay, but there was no significant statistical difference between the groups. In patients hospitalized in the ward, d-dimer level was highest in the group hospitalized for 11 to 20 days and a significant difference was observed between the groups.
In addition, when classification according to GCS was made in our study, the mean values of d-dimer, fibrinogen, INR, and PT were significantly different between GCS groups. On the other hand, elevated d-dimer (B = 0.001 and P < .001) and fibrinogen (B = 0.004 and P < .001) were significantly associated with mortality. These results emphasize the clinical importance of these parameters in terms of mortality prediction and prognosis evaluation.
In our study, the mean values of age, pulse rate, respiratory rate, and fever were also significantly higher in the exitus group. The mean values of d-dimer, fibrinogen, INR, and PT also differed significantly. In this context, the results show that clinical, vital, and demographic parameters of patients are as important as laboratory parameters and should be taken into consideration in patient evaluation.
The binary relationship of the parameters used in the study on mortality was statistically analyzed and grouping was made for the parameters that were and were not significant on prognosis (outcome). In the LR model obtained using d-dimer, fibrinogen, aPTT, platelet parameters, the mortality detection rate was 37.7%, and the overall classification (exitus-discharged with recovery) rate was 88.6%, which was more significant than the previous model. In another model, in which age, respiratory rate, SpO2, GCS data were included in addition to d-dimer, fibrinogen, aPTT, and platelet parameters, the mortality detection rate was 77.7% and the overall classification rate was 94.0%, which was a highly significant modeling method in terms of mortality prediction. As a result, when clinical, vital, and demographic data are taken into account in addition to coagulation parameters, survival prediction increases and becomes more clinically useful. The results also emphasize the importance of evaluating the patient comprehensively and from a wide perspective.
The fact that our study was conducted retrospectively also caused some limitations. Some limitations of our study are that laboratory parameters may vary over time during clinical follow-up, patients’ clinical conditions may vary from day to day, and coagulation parameters may have a dynamic structure. More dynamic and comprehensive prospective studies that involve long-term patient follow-up, detailed monitoring of coagulation parameters over multiple days, and correlation of coagulation parameters with other clinical and laboratory data may demonstrate different results and may demonstrate more objective predictive values.
ETHICAL APPROVAL
In this study, the approval numbered 2021/236 was obtained from the Faculty of Medicine of Selcuk University Non-Invasive Clinical Research Ethics Committee.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
REFERENCES
- [1].Tan W, Zhao X, Ma X, et al. A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019−2020. China CDC Wkly 2020;2(4):61–62. [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization (WHO). WHO’s response to COVID-19 - 2021 Annual Report. 2021. Available from: https://covid19.who.int/.
- [3].Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol 2020;30(3):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control 2021;49(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro Surveill 2020;25(5):200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed 2020;91(1):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016;24(6):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lewis D. Is the coronavirus airborne? Experts can’t agree. Nature 2020;580(7802):175. [DOI] [PubMed] [Google Scholar]
- [11].Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost 2020;18(9):2103–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA 2021;326(17):1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Becker RC. COVID-19 update: COVID-19-associated coagulopathy. J Thromb Thrombolysis 2020;50(1):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Esmon CT. The interactions between inflammation and coagulation. Br J Haematol 2005;131(4):417–430. [DOI] [PubMed] [Google Scholar]
- [16].Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18(7):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Douma RA, Tan M, Schutgens RE, et al. Using an age-dependent D-dimer cut-off value increases the number of older patients in whom deep vein thrombosis can be safely excluded. Haematologica 2012;97(10):1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Robert-Ebadi H, Robin P, Hugli O, et al. Impact of the age-adjusted D-dimer cutoff to exclude pulmonary embolism. Circulation 2021;143(18):1828–1830. [DOI] [PubMed] [Google Scholar]
- [23].Satici C, Demirkol MA, Sargin Altunok E, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis 2020;98:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Medhesap. PSI skoru hesaplama. 2021. Available from: https://www.medhesap.com/psi-skoru-hesaplama/. Accessed August 27, 2021.
- [25].Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010;65(10):878–883. [DOI] [PubMed] [Google Scholar]
- [26].Lim WS, Baudouin S, George R, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl 3):iii1–iii55. [DOI] [PubMed] [Google Scholar]
- [27].Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200(7):e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang JJ, Cao YY, Tan G, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy 2021;76(2):533–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Güçlü E, Kocayiğit H, Okan HD, et al. Effect of COVID-19 on platelet count and its indices. Rev Assoc Med Bras (1992) 2020;66(8):1122–1127. [DOI] [PubMed] [Google Scholar]
- [30].Li Y, Zhao K, Wei H, et al. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol 2020;190(1):e24–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Long H, Nie L, Xiang X, et al. D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. Biomed Res Int 2020;2020:6159720–6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zou Y, Guo H, Zhang Y, et al. Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China. Biosci Trends 2020;14(4):285–289. [DOI] [PubMed] [Google Scholar]
- [33].Esmaeel HM, Ahmed HA, Elbadry MI, et al. Coagulation parameters abnormalities and their relation to clinical outcomes in hospitalized and severe COVID-19 patients: prospective study. Sci Rep 2022;12(1):13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Healy B, Khan A, Metezai H, Blyth I, Asad H. The impact of false positive COVID-19 results in an area of low prevalence. Clin Med (Lond) 2021;21(1):e54–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Braunstein GD, Schwartz L, Hymel P, Fielding J. False positive results with SARS-CoV-2 RT-PCR tests and how to evaluate a RT-PCR-positive test for the possibility of a false positive result. J Occup Environ Med 2021;63(3):e159–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].S B MJ, Chacko B, Selvarajan S, et al. Biomarkers of coagulation, endothelial, platelet function, and fibrinolysis in patients with COVID-19: a prospective study. Sci Rep 2024;14(1):2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Citu C, Burlea B, Gorun F, et al. Predictive value of blood coagulation parameters in poor outcomes in COVID-19 patients: a retrospective observational study in Romania. J Clin Med 2022;11(10):2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Altay N, Karahan MA, Atlas A. The effectiveness of platelet and D-dimer levels in predicting prognosis in intensive care patients diagnosed with COVID-19. Harran Üniversitesi Tip Fakültesi Dergisi 2022;19(3):493–498. [Google Scholar]