Abstract
BACKGROUND:
Premature coronary heart disease (CHD) is a major cause of death in women. We aimed to characterize biomarker profiles of women who developed CHD before and after age 65 years.
METHODS:
In the Women’s Health Study (median follow-up 21.5 years), women were grouped by age and timing of incident CHD: baseline age <65 years with premature CHD by age 65 years (25 042 women; 447 events) and baseline age ≥65 years with nonpremature CHD (2982 women; 351 events). Associations of 44 baseline plasma biomarkers measured using standard assays and a nuclear magnetic resonance (NMR)-metabolomics assay were analyzed using Cox models adjusted for clinical risk factors.
RESULTS:
Twelve biomarkers showed associations only with premature CHD and included lipoprotein(a), which was associated with premature CHD [adjusted hazard ratio (HR) per SD: 1.29 (95% CI 1.17–1.42)] but not with nonpremature CHD [1.09(0.98–1.22)](Pinteraction = 0.02). NMR-measured lipoprotein insulin resistance was associated with the highest risk of premature CHD [1.92 (1.52–2.42)] but was not associated with nonpremature CHD (Pinteraction <0.001). Eleven biomarkers showed stronger associations with premature vs nonpremature CHD, including apolipoprotein B. Nine NMR biomarkers showed no association with premature or nonpremature CHD, whereas 12 biomarkers showed similar significant associations with premature and nonpremature CHD, respectively, including low-density lipoprotein (LDL) cholesterol [1.30(1.20–1.45) and 1.22(1.10–1.35)] and C-reactive protein [1.34(1.19–1.50) and 1.25(1.08–1.44)].
CONCLUSIONS:
In women, a profile of 12 biomarkers was selectively associated with premature CHD, driven by lipoprotein(a) and insulin-resistant atherogenic dyslipoproteinemia. This has implications for the development of biomarker panels to screen for premature CHD.
Introduction
Premature coronary heart disease (CHD) is a major driver of disability and death worldwide. Identifying individuals at risk of premature CHD may guide interventions for cardiovascular primary prevention and reduce the burden of premature CHD (1). However, the biomarker risk profile associated with risk of premature CHD is incompletely defined, thereby limiting efforts to develop biomarker panels for screening and risk prediction.
In the USA, from 1979 to 2011, CHD mortality in women differed by age. Based on the Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research database, in women <55 years, CHD mortality declined by 4.6% annually from 1979 to 1989, remained flat from 1990 to 1999, and declined by 1.0% from 2000 to 2011 (2). By contrast, CHD mortality in older women declined more from 2000 to 2011 (by 4.5% in women aged 55–64 years, and by 5.0% in women ≥65 years (2). In another study using the CDC Prevention Wide-ranging Online Data for Epidemiologic Research database, from 1999 to 2019, premature mortality in women (ages 18–65 years) due to acute myocardial infarction (MI), a key contributor to CHD, declined by 4.3% annually from 1999 to 2011, but by 1.9% annually thereafter (3).
Premature CHD may stem from genetic, lifestyle, and community factors including access to primary prevention, management of acute MI, and secondary prevention (1, 4, 5). Premature CHD may also stem from incompletely characterized risk profiles for cardiovascular primary prevention and suboptimal screening. Previous studies have examined biomarker profiles with non-age-stratified CHD (6–8), reporting overall associations with CHD. By contrast, there is sparse information specifically on the associations of biomarkers with premature CHD, in particular for women, which is often defined as CHD occurring before age 65 years (in men, before age 55 years) (9). To address these knowledge gaps, we evaluated the associations of 44 biomarkers from metabolic, inflammatory, and lipid pathways that were measured with a nuclear magnetic resonance (NMR) metabolomics assay and standard biomarker assays, with premature and nonpremature CHD among 28 024 women in the prospective Women’s Health Study (WHS). The objective of the study was to identify biomarker profiles for premature CHD compared with nonpremature CHD and guide the future development of biomarker panels for premature CHD screening.
Materials and Methods
STUDY DESIGN AND POPULATION
We followed the Strengthening the Reporting of Observational Studies in Epidemiology guideline for cohort studies. The study was approved by the institutional review board at Brigham and Women’s Hospital, Boston, MA.
The study cohort has been described in detail (10, 11). From April 1993 to January 1996, WHS randomized 39 876 apparently healthy women aged ≥45 years separately in a 2 × 2 × 2 factorial design to receive aspirin, vitamin E, and β-carotene or matching placebo, to assess effects on cardiovascular and cancer outcomes (NCT00000479). The trial ended in 2004 with no significant reduction in the primary endpoints for any treatment, and since then, participants have been followed on an observational basis.
Using baseline age (i.e., age at randomization), we identified 2 subcohorts: women with baseline age <65 years followed up for CHD developing before age 65 years (premature CHD), and women with baseline age ≥65 years who were followed up for CHD (nonpremature CHD). In women with baseline age <65 years, women without CHD before age 65 years were censored at the time of the first of the following events: death, reaching age 65 years, or last follow-up. In women with baseline age ≥65 years, women without incident CHD were censored at the time of death or last follow-up.
INCIDENT CHD
Incident CHD was a composite of first MI, percutaneous coronary intervention, coronary artery bypass grafting, or CHD related death. This analysis included CHD through 2016. CHD was confirmed using medical records by a blinded end points committee of physicians (9).
RISK FACTORS
Baseline risk factors were determined as previously reported (12). Participants reported demographic, lifestyle, anthropometric, medical history, medication, and family history information on detailed questionnaires (13).
PLASMA BIOMARKER MEASUREMENTS
At baseline, 28 345 participants provided a blood sample that was processed and stored at −170°C until analysis. We excluded participants without biomarker measurements (n = 321) yielding 28 024 participants. Banked blood samples were used to measure concentrations of lipids/apolipoproteins, lipoprotein subclasses (particle concentration and size), inflammatory biomarkers, and metabolic biomarkers (Supplemental Table 1 in the online Data Supplement) (12–20). Total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides) were enzymatically assessed (Roche Diagnostics), and triglycerides were corrected for endogenous glycerol. Apolipoprotein A-I and apolipoprotein B were measured using turbidimetric assays (Diasorin). Lipoprotein(a) and high-sensitivity C-reactive protein (CRP) were measured with immunoturbidimetric assays using a Hitachi-911 analyzer (Roche Diagnostics). Soluble intracellular adhesion molecule 1 (ICAM-1) was quantified using enzyme-linked immunosorbent assay (R&D Systems). Hemoglobin A1c was measured with an immunoturbidimetric assay; homocysteine was enzymatically assessed using a Hitachi-917 analyzer; and creatinine was assessed through the Jaffe reaction-based rate-blanked method (all, Roche Diagnostics). Estimated glomerular filtration rate (eGFR) was obtained from the 2021 race-free Chronic Kidney Disease Epidemiology collaboration: eGFR = 142 × min [standardized creatinine (mg/dL)/0.7, 1]−0241 × max [standardized creatinine (mg/dL)/0.7, 1]−1.200 × (0.9938)age × 1.012 (female).
The following biomarkers were measured by NMR spectroscopy using the 1H-NMR (400 MHz) LipoProfile-IV platform (LipoScience; now LabCorp) (18): lipoprotein particle concentration and size [LDL, triglyceride-rich lipoprotein (TRL), and HDL], lipoprotein insulin resistance score [LPIR score, derived as a weighted combination of 6 lipoprotein measures (13)], glycan biomarker of N-acetyl side chains of acute-phase proteins (GlycA; a biomarker of posttranslational glycosylation of acute-phase reactants), alanine, citrate, and total branched chain amino acids (BCAAs). Biomarker measurement is further described in the Supplemental Methods.
STATISTICAL METHODS
Statistical analyses were conducted with SAS v.9.4 (SAS Institute Inc.) and heat maps were developed with R-Statistics (R Foundation for Statistical Computing). Statistical comparisons used Student t-tests (continuous variables, expressed as means), Wilcoxon rank-sum tests (continuous variables, expressed as medians), and χ2 tests (categorical variables) to compare baseline characteristics between participants with vs without incident CHD. We fitted separate Cox proportional hazards regression models to estimate hazard ratios [HRs (95% CI)] for incident CHD per SD increment of each biomarker. Time since baseline was used as the time axis. The proportional hazards assumption in Cox models was evaluated by statistical tests and graphical methods using Schoenfeld residuals. SDs were derived from the entire cohort (Supplemental Table 1). Models were adjusted for baseline age, race (non-Hispanic white; other), education (<4 years post high school; Bachelor’s degree; Master’s degree or Ph.D.), postmenopausal status, postmenopausal hormone use, and randomized treatment assignment (model 1). Model 2 included model 1 variables plus physical activity [<7.5 metabolic equivalent (MET)-h/week; ≥7.5 MET-h/week], smoking (never, former, current), body-mass index [normal, overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2)], systolic blood pressure, diabetes, parental history of MI <60 years, and baseline treatment for hypertension or for high cholesterol. The association between variables and incident CHD (premature or nonpremature) was assessed by the P value for that variable (in models 1 and 2), separately for premature and nonpremature CHD. Heterogeneity in the effect of a risk factor on premature vs nonpremature CHD risk was evaluated on the relative risk scale by the P value for interaction (variable × age category) between baseline age categories (<65 years vs ≥ 65 years) in models 1 and 2. In the overall cohort, we conducted principal component analysis of 13 lipoprotein subclasses, generated a correlation matrix, and selected principal components (PCs) with eigenvalue >1.0. The associations of the selected PC with incident CHD (premature or nonpremature) and the P value for interaction (PC × age category) were assessed using models 1 and 2. Statistical significance was established at 2-tailed P < 0.05.
Results
CHD incidence rates per 100 person-years (95% CI) were 0.15 (0.14–0.17) for premature CHD and 0.70 (0.63–0.78) for nonpremature CHD, during overall median follow-up of 21.5 (interquartile range, 19.9 to 22.1) years. Most baseline characteristics differed between those with vs without CHD, separately in the baseline age <65 years and age ≥65 years subcohorts (Tables 1 and 2; Supplemental Table 2). The prevalence of most clinical risk factors was higher in cases vs noncases; however, higher prevalence of parental MI <60 years was observed only within the premature subcohort.
Table 1.
Baseline clinical risk factors according to baseline age and incident CHD status.
| Baseline age <65 years N = 25 042 |
Baseline age ≥65 years N = 2982 |
|||
|---|---|---|---|---|
| CHD N = 447 |
Non-CHD N = 24 595 |
CHD N = 351 |
Non-CHD N = 2631 |
|
| Age, years | 51.9 ± 4.3* | 53.0 ± 5.2 | 69.5 ± 3.7 | 69.2 ± 3.5 |
| Self-reported race, % | ||||
| White | 427 (96.8) | 23 180 (95.0) | 344 (98.6) | 2524 (97.0) |
| Black | 5 (1.1) | 477 (2.0) | 2 (0.6) | 36 (1.4) |
| Hispanic | 2 (0.5) | 269 (1.1) | 2 (0.6) | 17 (0.7) |
| Asian | 5 (1.1) | 358 (1.5) | 0 (0) | 19 (0.7) |
| Other | 2 (0.5) | 110 (0.5) | 1 (0.3) | 6 (0.2) |
| Education, % | * | ** | ||
| <4 years post high school | 288 (65.2) | 13 168 (54.5) | 249 (72.2) | 1676 (64.7) |
| Bachelor’s degree | 93 (21.0) | 5915 (24.5) | 54 (15.7) | 433 (16.7) |
| Master’s degree or Ph.D. | 61 (13.8) | 5076 (21.0) | 42 (12.2) | 480 (18.5) |
| Body-mass index, kg/m2 | 28.4 ± 5.8* | 26.0 ± 5.0 | 26.1 ± 4.8** | 25.4 ± 4.2 |
| Current smoker, n (%) | 136 (30.4)* | 2866 (11.7) | 48 (13.8)** | 216 (8.2) |
| Diabetes, n (%) | 87 (19.5)* | 556 (2.3) | 37 (10.5)* | 90 (3.4) |
| Metabolic syndrome, % | 260 (58.2)* | 5740 (23.4) | 153 (43.7)* | 759 (29.0) |
| Hypertension, % | 188 (42.1)* | 5835 (23.7) | 204 (58.1)* | 1139 (43.3) |
| Hypertension treatment, % | 112 (25.1)* | 2911 (11.8) | 113 (32.2)** | 618 (23.5) |
| Systolic blood pressure | 125 (125–135)* | 125 (115–135) | 135 (125–145)* | 125 (125–145) |
| Physical activity (<7.5 MET-h/week), % | 256 (57.3)** | 11 059 (45.0) | 172 (49.0)* | 1071 (40.7) |
| Parental MI <60 years, n (%) | 118 (26.8)* | 3558 (14.7) | 39 (11.4) | 252 (9.8) |
| Postmenopausal, % | 187 (41.2)** | 12 038 (49.0) | 351 (100) | 2631 (100) |
| Postmenopausal hormone replacement therapy use, % | 100 (22.4)* | 7590 (30.9) | 117 (33.3) | 887 (33.8) |
| Cholesterol lowering treatment, % | 38 (8.5)* | 645 (2.6) | 30 (8.6) | 185 (7.0) |
Data presented as mean ± standard deviation (age and body-mass index), median (interquartile range)(systolic blood pressure), and frequency (%) (others). Baseline age refers to age at randomization. Percentages may not total 100 due to rounding. Student t-tests (variables expressed as means), Wilcoxon rank-sum tests (variables expressed as medians), and χ2 tests (categorical variables) were used. Statistical differences presented for participants with CHD vs non-CHD.
P < 0.001;
P < 0.01; otherwise not significant (P ≥ 0.05).
See the Supplemental Methods for biomarker measurement.
Table 2.
Baseline biomarkers according to baseline age and incident CHD status.
| Baseline age <65 years N = 25 042 |
Baseline age ≥65 years N = 2982 |
|||
|---|---|---|---|---|
| CHD N = 447 |
Non-CHD N = 24 595 |
CHD N = 351 |
Non-CHD N = 2631 |
|
| Lipid/apolipoprotein biomarker concentration | ||||
| Total cholesterol, mg/dL | 224.0 (198.0–252.0)* | 206.0 (182.0–234.0) | 226.0 (201.0–254.0)** | 220.0 (196.0–244.0) |
| LDL cholesterol, mg/dL | 133.5 (111.1–157.2)* | 120.1 (99.4–142.8) | 136.7 (116.1–159.3)** | 130.2 (110.0–152.8) |
| HDL cholesterol, mg/dL | 42.7 (35.6–52.0)* | 52.0 (43.3–62.4) | 47.6 (39.5–59.5)* | 52.1 (42.9–62.7) |
| Total/HDL cholesterol ratio | 5.3 (4.1–6.5)* | 3.9 (3.2–4.9) | 4.6 (3.9–5.7)* | 4.2 (3.4–5.2) |
| Triglycerides, mg/dL | 166.0 (116.0–262.0)* | 116.0 (82.0–173.0) | 154.5 (107.0–214.0)* | 132.0 (93.0–186.0) |
| Trig/HDL cholesterol ratio | 4.0 (2.2–6.5)* | 2.2 (1.4–3.7) | 3.1 (1.9–5.1)* | 2.5 (1.6–4.0) |
| Non-HDL cholesterol, mg/dL | 177.9 (149.5–206.1)* | 152.5 (127.6–180.0) | 178.0 (150.3–201.6)* | 165.4 (141.6–190.9) |
| Apolipoprotein B, mg/dL | 120.0 (97.4–140.6)* | 98.8 (82.8–119.4) | 120.7 (103.8–137.3)* | 111.7 (92.8–129.7) |
| Apolipoprotein A-I, mg/dL | 135.4 (121.1–155.2)* | 149.0 (132.4–167.7) | 147.4 (130.5–169.8) | 150.4 (134.7–170.2) |
| Lipoprotein(a), mg/dL | 14.9 (5.0–54.7)* | 10.4 (4.4–31.8) | 12.2 (4.8–50.0) | 11.9 (4.9–34.3) |
| Lipoprotein biomarker concentration | ||||
| Total LDL particles, nmol/L | 1776.0 (1543.2–2112.0)* | 1549.1 (1313.5–1815.7) | 1852.5 (1580.4–2081.2)* | 1686.9 (1458.6–1938.9) |
| Large LDL particles | 191.6 (50.0–403.6)* | 305.5 (161.7–464.6) | 297.9 (113.7–504.9) | 326.1 (163.8–489.5) |
| Medium LDL particles | 71.4 (0.0–265.8)* | 161.5 (1.3–351.0) | 64.0 (0.0–281.7)** | 130.3 (0.0–325.2) |
| Small LDL particles | 1375.8 (920.0–1796.9)* | 932.5 (674.1–1305.7) | 1338.3 (861.8–1755.3)* | 1073.8 (792.8–1473.7) |
| LDL particle avg. size, nm | 20.7 (20.3–21.1)* | 20.9 (20.6–21.2) | 20.8 (20.4–21.2)*** | 20.9 (20.6–21.2) |
| Total TRL particles, nmol/L | 189.8 (157.2–234.9)* | 164.0 (128.3–205.4) | 195.9 (157.5–243.4)* | 184.1 (145.4–225.6) |
| Very large TRL particles | 0.2 (0.1–0.4)* | 0.1 (0.1–0.2) | 0.1 (0.1–0.3)** | 0.1 (0.1–0.2) |
| Large TRL particles | 3.2 (1.0–6.3)* | 1.5 (0.3–4.2) | 3.0 (0.8–5.2)** | 2.1 (0.5–4.7) |
| Medium TRL particles | 21.0 (13.3–33.2)* | 15.5 (8.3–25.1) | 20.7 (12.3–30.7)** | 17.5 (10.0–27.3) |
| Small TRL particles | 58.7 (33.7–84.5) | 54.8 (33.7–80.8) | 61.1 (37.8–91.9) | 61.4 (36.7–89.4) |
| Very small TRL particles | 98.9 (75.7–134.6)* | 82.9 (57.7–113.0) | 101.9 (71.9–136.6)** | 92.9 (66.5–124.4) |
| TRL cholesterol, mg/dL | 33.3 (27.0–41.7)* | 28.4 (21.8–35.9) | 34.2 (27.2–42.2)* | 31.8 (24.8–39.3) |
| TRL triglycerides, mg/dL | 104.4 (72.9–147.8)* | 80.8 (55.0–116.0) | 99.5 (69.8–141.2)* | 90.7 (63.3–125.7) |
| TRL particle avg. size, nm | 45.4 (39.8–52.0)* | 42.4 (38.5–47.8) | 43.7 (39.8–49.1)*** | 42.8 (39.0–48.0) |
| Total HDL particles, μmol/L | 23.3 (21.1–26.3)* | 24.4 (22.0–27.0) | 24.5 (22.2–27.0) | 24.4 (22.0–27.0) |
| Large HDL particles | 1.4 (0.9–2.2)* | 2.1 (1.3–3.3) | 1.9 (1.2–3.1)*** | 2.1 (1.4–3.3) |
| Medium HDL particles | 4.1 (2.4–6.1)* | 5.4 (3.8–7.3) | 4.3 (2.9–6.5)*** | 4.9 (3.3–6.7) |
| Small HDL particles | 17.3 (15.4–19.4)* | 16.3 (14.0–18.6) | 17.4 (15.2–19.7)** | 16.7 (14.6–19.2) |
| HDL particle avg. size, nm | 8.7 (8.5–8.9)* | 8.9 (8.7–9.2) | 8.8 (8.6–9.1)** | 8.9 (8.7–9.2) |
| Inflammatory biomarker concentration | ||||
| CRP, mg/L | 3.4 (1.5–6.8)* | 2.0 (0.8–4.3) | 2.9 (1.3–5.5)** | 2.3 (1.0–4.5) |
| Fibrinogen, mg/dL | 385.2 (326.2–453.1)* | 347.0 (305.1–397.8) | 392.3 (338.6–445.4)** | 380.4 (333.5–431.5) |
| ICAM-1, ng/mL | 377.9 (326.2–452.5)* | 339.7 (298.7–391.2) | 373.6 (334.2–422.6)** | 360.9 (321.7–408.8) |
| GlycA, μmol/L | 419.0 (371.0–469.0)* | 381.0 (337.0–429.0) | 403.0 (363.0–449.0)** | 389.0 (348.0–433.0) |
| Metabolic biomarker concentration | ||||
| Hemoglobin A1c, % | 5.1 (4.9–5.5)* | 5.0 (4.8–5.2) | 5.1 (5.0–5.4)** | 5.1 (4.9–5.3) |
| LPIR score (0–100) | 59 (38–74)* | 39 (20–60) | 50 (30–68)* | 44 (23–62) |
| Homocysteine, μmol/L | 11.1 (8.9–13.8)** | 10.4 (8.6–12.8) | 11.3 (9.3–14.2) | 11.1 (9.3–13.5) |
| Citrate, μmol/L | 94.5 (80.3–110.2) | 93.2 (77.8–109.7) | 100.9 (85.5–116.6) | 100.0 (84.4–117.7) |
| Total BCAAs, μmol/L | 430.9 (374.0–511.3)* | 401.1 (349.6–460.9) | 406.7 (354.0–475.1)*** | 396.1 (348.5–452.9) |
| Valine, μmol/L | 236.0 (205.0–272.4)* | 219.7 (192.7–250.5) | 224.7 (198.2–261.1)** | 218.9 (192.2–247.1) |
| Leucine, μmol/L | 140.0 (116.2–172.9)* | 131.6 (110.2–154.8) | 133.0 (110.5–155.7) | 129.7 (109.2–153.0) |
| Isoleucine, μmol/L | 58.7 (42.2–76.9)* | 50.4 (38.8–64.0) | 52.8 (41.8–67.5)** | 48.9 (37.7–62.7) |
| Alanine, μmol/L | 419.0 (355.8–500.9)** | 402.6 (334.3–477.0) | 405.3 (341.5–478.4) | 402.6 (336.7–481.6) |
| eGFR, mL/min per 1.73 m2 | 102.2 (90.6–107.8)** | 100.6 (88.2–107.1) | 91.5 (76.9–96.7) | 90.1 (77.1–96.2) |
| Creatinine, mg/dL | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) |
Data presented as median (interquartile range). Baseline age refers to age at randomization. Wilcoxon rank-sum tests were used with statistical differences for participants with CHD vs non-CHD.
P < 0.001;
P < 0.01;
P < 0.05; otherwise not significant (P ≥ 0.05).
SI conversion factors: to convert total cholesterol, HDL-C, and LDL-C to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; lipoprotein(a) to μmol/L, multiply by 0.0357; CRP to nmol/L, multiply by 9.524; fibrinogen to μmol/L, multiply by 0.0294; homocysteine to mg/dL, divide by 7.397; creatinine to μmol/L, multiply by 88.4.
See the Supplemental Methods for biomarker measurement.
Most biomarkers were higher in cases vs noncases; notably, level of lipoprotein(a) was higher only within the premature subcohort.
LIPIDS/APOLIPOPROTEINS
In adjusted models, most lipids (e.g., HDL cholesterol, triglycerides, and non-HDL cholesterol) showed stronger relative risk associations with premature vs nonpremature CHD whereas both total and LDL cholesterol had similar positive association with premature and nonpremature CHD (Figs. 1 and 2; Supplemental Table 3 and Supplemental Fig. 2). Of all lipids, total/HDL cholesterol ratio had the highest relative risk association with premature CHD [1.68 (1.52–1.86)] and nonpremature CHD [1.37 (1.22–1.55)] with stronger association with premature vs nonpremature CHD (Pinteraction < 0.001).
Fig. 1.
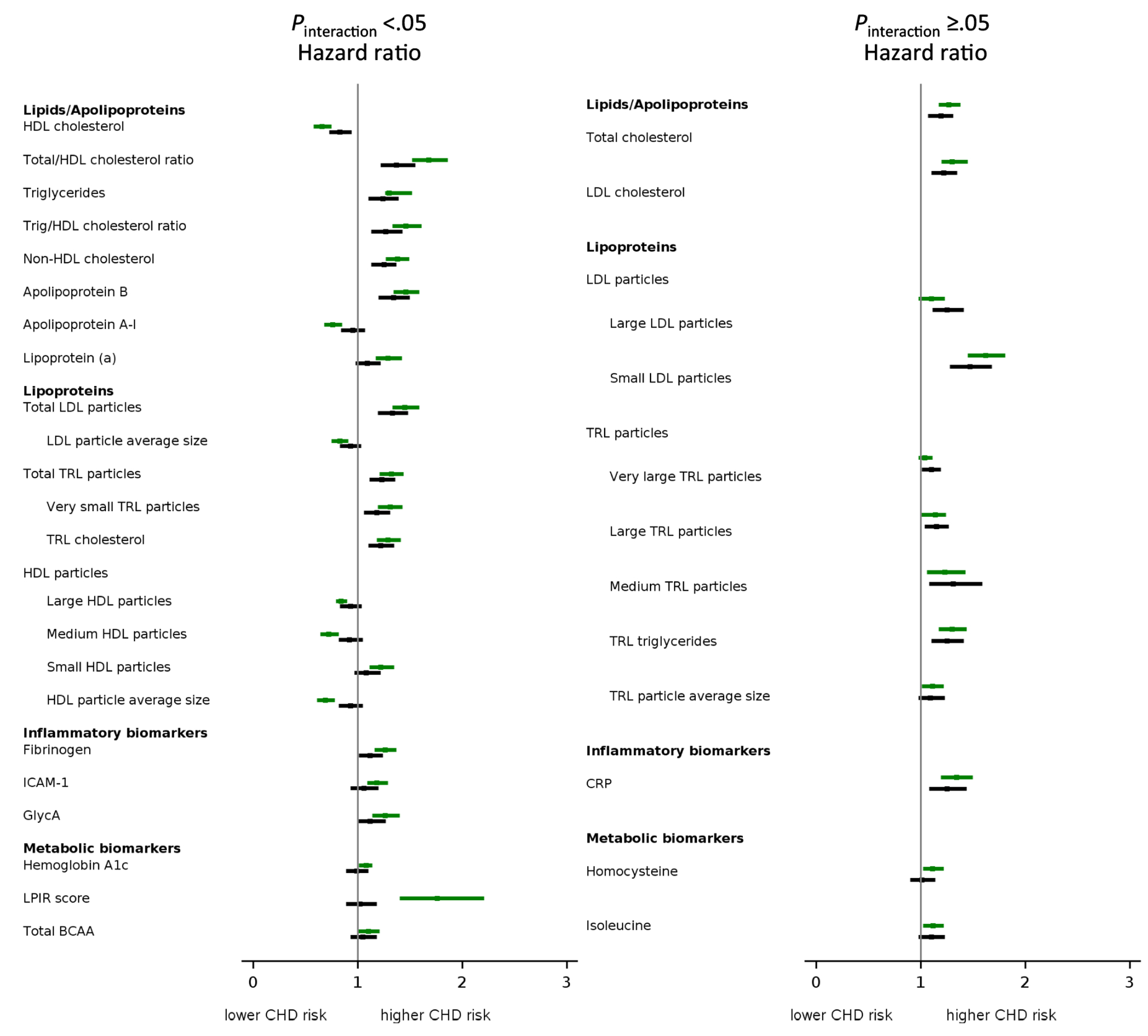
Forest plot of baseline lipids/apolipoproteins, lipoproteins, inflammatory, and metabolic biomarker concentrations in relation to incident CHD. Cox proportional HRs (95% CIs) per SD increment of biomarker concentrations in association with incident premature (upper bar; green color) and nonpremature (lower bar; black color) CHD. Models were adjusted for baseline age, race, education, menopause, postmenopausal hormone use, randomized treatment assignment, physical activity, BMI, smoking, systolic blood pressure, diabetes, parental history of MI < 60 years, baseline treatment for hypertension, or for high cholesterol (model 2) (see Supplemental Tables 3–5). To adjust for confounding among large, medium, and small LDL particles, their models included model 2 variables plus the other LDL subclasses (small, medium, and large LDL particle concentrations). Results are categorized by the probability value of interaction terms for biomarker with baseline age category (<65 years vs ≥ 65 years); Pinteraction < 0.05 (left panel) and Pinteraction ≥ 0.05 (right panel). Biomarkers that showed no association with incident premature and nonpremature CHD are depicted in Supplemental Fig. 1 (medium LDL particles, small TRL particles, total HDL particles, citrate, valine, leucine, alanine, eGFR, and creatinine).
Fig. 2.
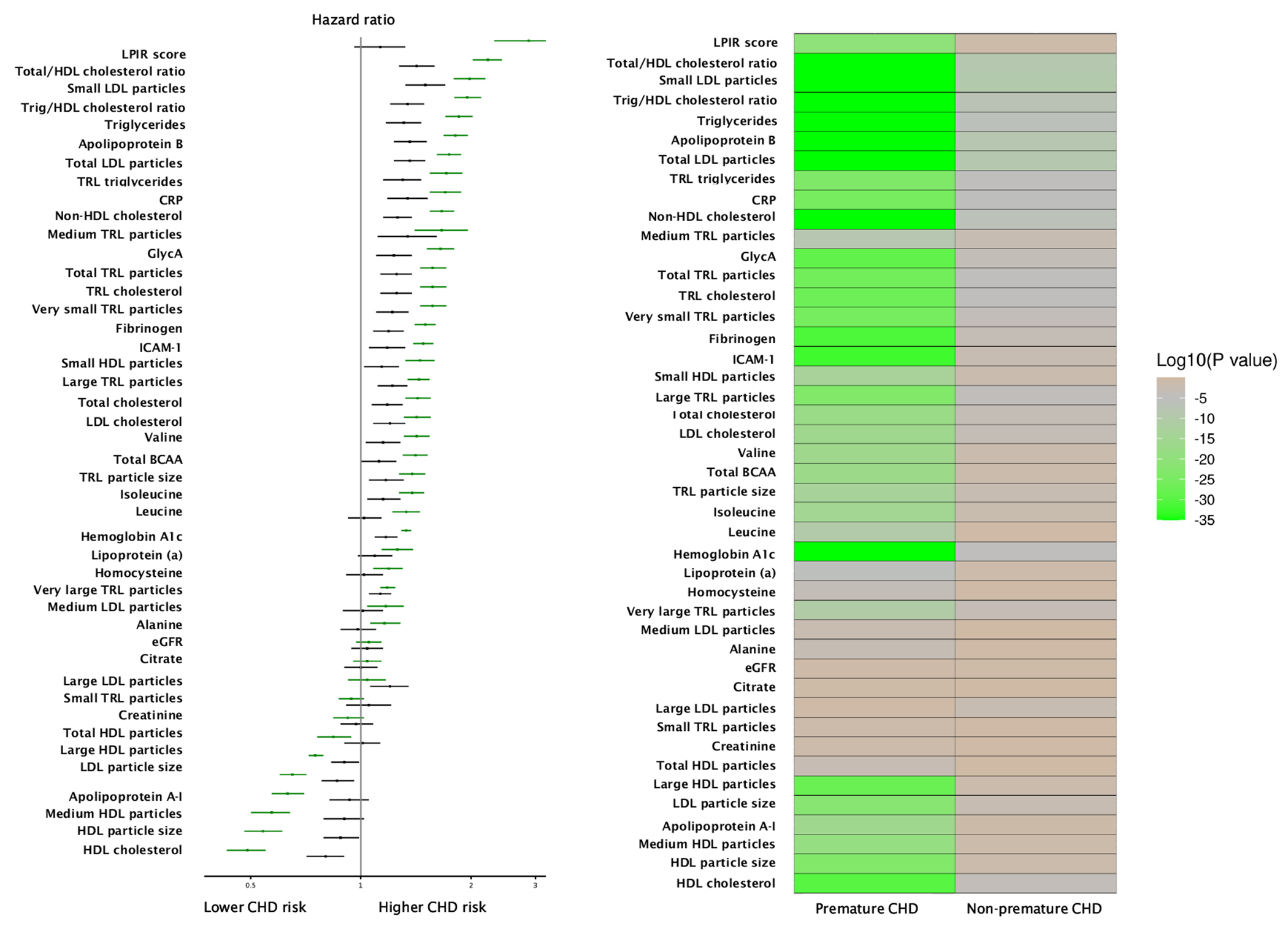
Heat map of baseline lipids/apolipoproteins, lipoproteins, inflammatory, and metabolic biomarker concentrations in relation to incident CHD. Models were adjusted for baseline age, race, education, menopause, postmenopausal hormone use, and randomized treatment assignment (model 1). To adjust for confounding among large, medium, and small LDL particles, their models included model 1 variables plus the other LDL subclasses (small, medium, and large LDL particle concentrations). The left panel shows the Cox proportional HRs (in descending order of magnitude of risk) for risk factors in association with premature (green bars) and nonpremature (black bars) incident CHD. HR for biomarkers based on per SD increment of plasma concentration. The right panel shows the logarithm of P value for the association of risk factors with baseline age category (<65 years vs ≥ 65 years) in descending order of strength of association (from brown to green in color).
Apolipoprotein B had stronger association with premature CHD (1.46 (1.34–1.59)] vs nonpremature CHD [1.34 (1.20–1.50)](Pinteraction = 0.02), whereas apolipoprotein A-I [0.76 (0.68–0.85)](Pinteraction < 0.001) and lipoprotein(a) [1.29 (1.17–1.42)](Pinteraction = 0.02) were only associated with premature CHD. The strengths of association of apolipoprotein B and non-HDL cholesterol with both premature and nonpremature CHD were generally similar.
NMR LIPOPROTEINS
In adjusted models, LDL particle subclasses showed variable relative risk associations with premature and nonpremature CHD (Figs. 1 and 2; Supplemental Table 4 and Supplemental Figs. 1 and 3). Total LDL particle concentration, driven by the concentration of small LDL particles, was associated with both premature and nonpremature CHD but showed somewhat stronger association with premature CHD.
By contrast, LDL subclasses had more complex risk associations. In model 2 that did not adjust for the concentration of medium and small LDL particles but adjusted only for standard risk factors, large LDL particle concentration had inverse association with premature CHD [0.83 (0.75–0.93)] and but not with nonpremature CHD [1.07 (0.96–1.19)](Pinteraction = 0.001); however, further adjusting for the concentration of medium and small LDL particles altered the association of large LDL particle concentration such that it was no longer inversely associated with premature CHD [1.10 (0.98–1.23)] and strengthened the positive association of large LDL with nonpremature CHD [1.25 (1.11–1.41)]. Medium LDL risk associations tracked more closely with large LDL than small LDL. Further adjusting total LDL particle concentration with LDL particle average size, and the converse, did not materially alter the strength of association with premature and nonpremature CHD (model 3 in Supplemental Table 4).
Total TRL particle and TRL triglyceride concentrations were associated with both premature and nonpremature CHD, with stronger associations (Pinteraction = 0.03 for total TRL particle) for premature CHD (Figs. 1 and 2; Supplemental Table 4 and Supplemental Figs. 1 and 3). Notably, variable associations were also seen for TRL subclasses. The stronger association with premature CHD was seen for very small TRL particle concentrations but not different-sized TRLs. TRL cholesterol concentration was associated with higher risk of CHD (both premature and nonpremature) with almost similar magnitude as total TRL particle concentration or TRL triglyceride concentration.
For HDL particles, variable risk associations were noted for premature CHD, and none of the HDL particle subclasses was associated with nonpremature CHD (Figs. 1 and 2; Supplemental Table 4 and Supplemental Figs. 1 and 4). Total HDL particle concentration trended toward an inverse association with premature [0.91 (0.81–1.01)] but not nonpremature CHD, although the interaction was not significant (Pinteraction = 0.11). On the other hand, the sized HDL particle concentrations had stronger associations with premature than nonpremature CHD, with differing directions of association. Specifically, inverse association with premature CHD was noted for large HDL particle concentration [0.84 (0.79–0.90)] and medium HDL particle concentration [0.72 (0.64–0.82)], whereas small HDL particle concentration showed positive association with premature CHD [1.22 (1.11–1.35)].
In the overall cohort, principal component analysis of 13 lipoprotein subclasses yielded 4 PCs that accounted for 66.6% of the cumulative variance (Supplemental Table 5). PC1 (explaining 36.0% of variance in NMR lipoproteins and driven by insulin-resistant atherogenic dyslipoproteinemia) was associated with a higher risk of premature CHD vs nonpremature CHD in models 1 and 2. In model 2, PC3 (driven by medium HDL particles and large LDL particles) showed inverse association with risk of premature CHD, whereas PC2, PC3, and PC4 showed no association with risk of nonpremature CHD (Supplemental Table 6). The selective and/or stronger association of PCs with premature vs nonpremature CHD identify atherogenic patterns associated with premature CHD.
INFLAMMATORY AND METABOLIC BIOMARKERS
Of the inflammatory biomarkers, CRP concentration showed a similar positive association with both premature CHD [1.34 (1.19–1.50)] and nonpremature CHD [1.25 (1.08–1.44)](Pinteraction = 0.15), whereas fibrinogen, ICAM-1, and GlycA concentrations showed stronger positive associations with premature CHD (Figs. 1 and 2; Supplemental Table 7 and Supplemental Figs. 1 and 5).
Biomarkers of dysglycemia and insulin-resistant atherogenic dyslipoproteinemia, such as hemoglobin A1c and LPIR score, showed stronger positive associations with premature CHD, and none was associated with nonpremature CHD (Figs. 1 and 2; Supplemental Table 7 and Supplemental Fig. 6). Of the 44 biomarkers examined, based on model 2, LPIR score was associated with the highest relative risk of incident premature CHD [1.92 (1.52–2.42)] with no association with nonpremature CHD [1.03 (0.88–1.19)](Pinteraction <0.001). Further adjusting LPIR score for baseline diabetes did not alter the association with premature and nonpremature CHD. Hemoglobin A1c was only weakly associated with premature CHD [1.08 (1.01–1.14)]. Most other metabolic biomarkers (alanine, citrate, creatinine, eGFR, leucine, valine) showed no association with premature or nonpremature CHD. The concentration of total BCAAs implicated in the pathway of insulin resistance, in particular isoleucine, showed a positive association with premature CHD.
SENSITIVITY ANALYSES
We conducted 2 sensitivity analyses using models 1 and 2. First, we used age as the time axis (rather than “time to event”) within each CHD group. Second, we restricted the premature CHD group to women with baseline age <60 years to allow at least 5 years of follow-up. In both analyses, the risk associations with incident CHD were generally similar to those in the main analysis (data not shown).
CLINICAL RISK FACTORS
In adjusted models, most clinical risk factors showed stronger associations with premature vs nonpremature CHD (Supplemental Figs. 7 and 8). Diabetes had the highest relative risk association and showed stronger positive association (Pinteraction < 0.001) with premature CHD [6.24 (4.77–8.16)] vs nonpremature CHD [3.31 (2.28–4.81)]. Metabolic syndrome was also associated with greater risk of premature than nonpremature CHD. Current smoking showed similar positive associations with both premature and nonpremature CHD (pinteraction = 0.64). By contrast, history of parental MI <60 years showed a positive association with premature CHD [1.83 (1.48–2.28)] but not nonpremature CHD [1.14 (0.81–1.60)](Pinteraction = 0.04).
SUMMARY OF ASSOCIATIONS
Of 44 biomarkers examined, 12 biomarkers showed association only with premature CHD, 11 biomarkers showed stronger relative risk associations with premature vs nonpremature CHD, 12 biomarkers showed similar associations with premature vs nonpremature, and 9 biomarkers showed no association with CHD. The profile associated with premature CHD was driven predominantly by lipoprotein(a) and insulin-resistant atherogenic dyslipoproteinemia.
Figure 1 displays the associations of biomarkers and clinical risk factors with incident CHD grouped by statistically significant interactions. Supplemental Table 8 groups them by associations with (a) premature CHD only, (b) premature greater than nonpremature CHD, and (c) premature similar to nonpremature CHD.
Discussion
In this study, most biomarkers showed stronger associations with premature vs nonpremature CHD, when compared on the relative hazard scale. Of 44 biomarkers examined, 23 biomarkers showed association only with premature CHD or stronger relative risk association with premature vs nonpremature CHD. These included lipids/apolipoproteins [e.g., non-HDL cholesterol, lipoprotein(a)], lipoproteins (e.g., total LDL particles, TRL triglycerides), inflammatory (e.g., GlycA), and metabolic (e.g., LPIR score) biomarkers. In particular, insulin-resistant atherogenic dyslipoproteinemia and related biomarkers were major determinants of risk of premature CHD, with potential implications for biomarker screening and therapeutic strategies.
The finding that biomarkers of insulin-resistant atherogenic dyslipoproteinemia showed positive associations with premature CHD, but not with nonpremature CHD, was intriguing. We have previously shown that LPIR score was positively associated with incident diabetes both in the presence and absence of statin therapy (13, 21). In the present study, a 1 SD increment in LPIR score was selectively associated with a 1.8–1.9-fold higher relative risk of premature CHD, much greater than the association of 1 SD increment in LDL cholesterol (approximately 40%) or hemoglobin A1c (approximately 10%). LPIR score potentially links insulin resistance and dyslipidemia with future risk of diabetes and premature CHD, a role that requires further investigation.
The selective positive association of lipoprotein(a) with premature CHD is consistent with the stronger associations of apolipoprotein A-I and apolipoprotein B with premature CHD, and the association of lipoprotein(a) with higher CHD risk in non-age-stratified analyses (22, 23). Recent studies have reported the about 80% reduction in lipoprotein(a) levels using antisense oligonucleotides (24, 25). The effect of reducing lipoprotein(a) levels on incident CHD, including premature CHD, is under evaluation in randomized clinical trials.
The association of CRP with premature and nonpremature CHD supports the role of inflammation in CHD (26). The selective association of ICAM-1 with premature, but not nonpremature, CHD, is intriguing as ICAM-1 has been associated with atheroma progression, a process that presumably occurs in premature and nonpremature CHD. We have previously shown that GlycA was associated with higher risk of incident type 2 diabetes (27). The selective association of GlycA with premature, but not nonpremature, CHD is consistent with our finding that diabetes and insulin resistance were major determinants of premature CHD.
In non-age stratified analyses, several studies have reported associations between LDL lipoprotein subclasses and incident CHD (7, 28, 29). The present study builds on these observations and shows stronger associations of total LDL and small LDL particle concentrations with premature vs nonpremature CHD and the selective association of LDL particle average size with premature CHD. Similarly, most TRL particle concentrations were associated with a 1.04–1.31-fold adjusted relative increase in risk of premature and nonpremature CHD, consistent with their atherogenic role. Notably, total and very small TRL particles had stronger association with premature vs nonpremature CHD, potentially related to their role in insulin resistance and fatty acid metabolism.
HDL particles showed different associations with premature and nonpremature CHD. The magnitude of the inverse association of large and medium HDL particle concentrations was similar to that observed for apolipoprotein A-I and HDL cholesterol, suggesting that the inverse association of HDL cholesterol could be related to large and medium HDL particle concentrations. Supporting this is the observation that apolipoprotein A-I was inversely associated with premature, but not nonpremature CHD. The reasons for the selective association of HDL particle concentration with premature CHD are unknown. Recent studies on the inhibition of cholesteryl ester transfer protein, which raises plasma HDL cholesterol concentration, had mixed effects on incident CHD (30), and the effect of cholesteryl ester transfer protein inhibitors on premature CHD requires investigation.
Our observation that biomarkers of insulin-resistant atherogenic dyslipoproteinemia were selectively associated with premature CHD has clinical significance. Obesity prevalence in the USA has been increasing and also increases the risk for insulin resistance and diabetes (31). In this context, improved screening based on more targeted biomarker panels may detect risk of premature CHD and identify younger people for targeted intervention. While this study focused on biomarker profiles, this does not diminish the importance of clinical risk factors such as smoking, metabolic syndrome, and systolic blood pressure (9). Managing these risk factors through lifestyle and/or pharmacologic approaches would be expected to improve the biomarker profile and lower the risk for premature CHD.
Our study has strengths and potential limitations. The generalizability of our findings to other populations (e.g., men, different racial and ethnic groups) requires evaluation. In WHS, limited baseline data on pregnancy complications precluded analysis of these factors. The determinants of sex-based differences require evaluation including whether biomarker profiles differ for men vs women with premature CHD (32, 33). Our study has strengths, including that it was a large prospective study with robust baseline data, and follow-up of prespecified end points. While we characterized biomarkers into different groups of biological pathways, it is important to acknowledge the overlap in these pathways/biomarkers. We compared the biomarkers on a relative risk scale that may differ from population-attributable risks, which depend on both the magnitude of risk association and the prevalence of the risk factors in the population. NMR-measured biomarkers may not be generalizable to measurements using different NMR platforms and/or spectral acquisition protocols, require specialized laboratories, and have associated costs (34, 35). In this study, we chose to include women with baseline age ≥65 years and evaluate the biomarker risk profile for incident CHD. While these women may have had survivorship bias (i.e., no CHD event before age 65 years), it was important to include these women for comparison with the women who experienced premature CHD.
In summary, biomarker risk profiles differed by age of CHD onset in women. In particular, insulin-resistant atherogenic dyslipoproteinemia and related metabolic biomarkers were major determinants of risk of premature CHD, with potential implications for biomarker screening among younger individuals. Future research should build on these findings to evaluate whether these biomarkers can improve screening, prediction, or classification of premature CHD beyond traditional risk factors and guide interventions to reduce the burden of premature CHD.
Supplementary Material
Research Funding:
This work was supported by the National Institutes of Health (CA047988, HL043851, HL080467, HL099355, and UM1CA182913 to Women’s Health Study; K23 MD016230 to S.B. Dugani; K01 HL135342 to O.V. Demler; R01 HL134811, HL160799, HL117861, DK112940, and K24 HL136852 to S. Mora) and the Robert and Elizabeth Strickland Career Development Award, Mayo Clinic (S.B. Dugani).
Disclosures:
S.B. Dugani has received research funding from Dalio Philanthropies for work unrelated to the present study. P.M Ridker has received research grant support from Kowa, Novartis, Amarin, Pfizer, Esperion, NovoNordisk, and the NHLBI; has served as a consultant to Novartis, Flame, Agepha, AstraZeneca, Janssen, Civi Biopharm, Glaxo Smith Kline, SOCAR, Novo Nordisk, Uptton, Omeicos, Health Outlook, Montai Health, New Amsterdam, Boehringer-Ingelheim, Angiowave, RTI, Zomagen, Cytokinetics, Horizon Therapeutics, and Cardio Therapeutics; and receives compensation for service on the Peter Munk Advisory Board (University of Toronto), the Leducq Foundation, Paris FR, and the Baim Institute (Boston, MA). R.J. Glynn has received research grants to the Brigham & Women’s Hospital from AstraZeneca, Kowa, Novartis, and Pfizer. S. Mora has served as a consultant to Pfizer and Quest Diagnostics and has a patent regarding the use of glycoprotein acetylation in relation to colorectal cancer risk.
Role of Sponsor:
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Nonstandard Abbreviations:
- CHD
coronary heart disease
- NMR
nuclear magnetic resonance
- HR
hazard ratio
- LDL
low-density lipoprotein
- MI
myocardial infarction
- WHS
Women’s Health Study
- HDL
high-density lipoprotein
- CRP
high-sensitivity C-reactive protein
- ICAM
intracellular adhesion molecule
- eGFR
estimated glomerular filtration rate
- TRL
triglyceride-rich lipoprotein
- LPIR
lipoprotein insulin resistance
- GlycA
glycan biomarker of N-acetyl side chains of acute-phase proteins
- BCAAs
branched chain amino acids
- MET
metabolic equivalent
Footnotes
Disclaimer: The conclusions do not necessarily represent the views of the funders.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
References
- 1.Stone NJ, Smith SC Jr, Orringer CE, Rigotti NA, Navar AM, Khan SS, et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:819–36. [DOI] [PubMed] [Google Scholar]
- 2.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011. Circulation 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dani SS, Lone AN, Javed Z, Khan MS, Zia Khan M, Kaluski E, et al. Trends in premature mortality from acute myocardial infarction in the United States, 1999 to 2019. J Am Heart Assoc 2022;11:e021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol 2018;15:230–40. [DOI] [PubMed] [Google Scholar]
- 5.Khera AV, Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat Rev Genet 2017;18:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based bruneck study. Circulation 2014;129:1821–31. [DOI] [PubMed] [Google Scholar]
- 7.Mora S, Buring JE, Ridker PM, Cui Y. Association of high-density lipoprotein cholesterol with incident cardiovascular events in women, by low-density lipoprotein cholesterol and apolipoprotein B100 levels: a cohort study. Ann Intern Med 2011;155:742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol 2018;71:620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dugani SB, Moorthy MV, Li C, Demler OV, Alsheikh-Ali AA, Ridker PM, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol 2021;6:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the women’s health study: a randomized controlled trial. JAMA 2005;294:47–55. [DOI] [PubMed] [Google Scholar]
- 11.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the women’s health study: a randomized controlled trial. JAMA 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes Mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation 2014;129:635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the reynolds risk score. JAMA 2007;297:611–9. [DOI] [PubMed] [Google Scholar]
- 16.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N-linked glycoprotein side-chain biomarker, rosuvastatin therapy, and incident cardiovascular disease: an analysis from the JUPITER trial. J Am Heart Assoc 2016;5:e003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobias DK, Lawler PR, Harada PH, Demler OV, Ridker PM, Manson JE, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genom Precis Med 2018;11:e002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad S, Demler OV, Sun Q, Moorthy MV, Li C, Lee I-M, et al. Association of the Mediterranean diet with onset of diabetes in the Women’s Health Study. JAMA Netw Open 2020;3:e2025466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia E, Connelly MA, Matyus SP, Otvos JD, Shalaurova I. High-throughput nuclear magnetic resonance measurement of citrate in serum and plasma in the clinical laboratory. Pract Lab Med 2021;25:e00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. Glyca: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem 2015;61:714–23. [DOI] [PubMed] [Google Scholar]
- 21.Harada PHN, Demler OV, Dugani SB, Akinkuolie AO, Moorthy MV, Ridker PM, et al. Lipoprotein insulin resistance score and risk of incident diabetes during extended follow-up of 20 years: the Women’s Health Study. J Clin Lipidol 2017;11:1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J 2022;43:3925–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2022;42:e48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020;382:244–55. [DOI] [PubMed] [Google Scholar]
- 25.O’Donoghue ML, Rosenson RS, Gencer B, López JAG, Lepor NE, Baum SJ, et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N Engl J Med 2022;387:1855–64. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 27.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2015;35:1544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora S, Glynn RJ, Ridker PM. High-Density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation 2013;128:1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation 2014;129:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armitage J, Holmes MV, Preiss D. Cholesteryl ester transfer protein inhibition for preventing cardiovascular events: JACC review topic of the week. J Am Coll Cardiol 2019;73:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Adult Obesity Facts. https://www.cdc.gov/obesity/data/adult.html (Accessed September 2023). [Google Scholar]
- 32.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol 2014;64:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugani SB, Fabbri M, Chamberlain AM, Bielinski SJ, Weston SA, Manemann SM, et al. Premature myocardial infarction: a community study. Mayo Clin Proc Innov Qual Outcomes 2021;5:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emeasoba EU, Ibeson E, Nwosu I, Montemarano N, Shani J, Shetty VS. Clinical relevance of nuclear magnetic resonance LipoProfile. Front Nucl Med 2022;2:960522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia E, Bennett DW, Connelly MA, Jeyarajah EJ, Warf FC, Shalaurova I, et al. The extended lipid panel assay: a clinically-deployed high-throughput nuclear magnetic resonance method for the simultaneous measurement of lipids and Apolipoprotein B. Lipids Health Dis 2020;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


