Abstract
Background:
The U.S. Preventive Services Task Force recently issued an updated draft recommendation statement to initiate breast cancer (BC) screening at age 40, reflecting well-documented disparities in BC mortality that disproportionately impact younger Black women. This study applied a novel approach to identify hotspots of BC diagnosed before age 50 and/or at an advanced stage to improve BC detection within these communities.
Methods:
Cancer registry data for 3,497 women with invasive BC diagnosed or treated between 2012 and 2020 at the Helen F. Graham Cancer Center & Research Institute (HFGCCRI) and who resided in the HFGCCRI catchment area, defined as New Castly County, Delaware, were geocoded and analyzed with spatial intensity. Standardized incidence ratios stratified by age and race were calculated for each hotspot.
Results:
Four hotspots were identified, two for BC diagnosed before age 50, one for advanced BC, and one for advanced BC diagnosed before age 50. Younger Black women were overrepresented in these hotspots relative to the full catchment area.
Conclusions:
The novel use of spatial methods to analyze a community cancer center catchment area identified geographic areas with higher rates of BC with poor prognostic factors and evidence that these areas made an outsized contribution to racial disparities in BC.
Impact:
Identifiying and prioritizing hotspot BC communities for community outreach and engagement activities designed to improve BC detection has the potential to reduce the overall burden of BC and narrow racial disparities in BC.
INTRODUCTION
In 2023, the U.S. Preventive Services Task Force (Task Force) issued an updated draft recommendation statement on screening for breast cancer (BC)(1). The most significant change from the prior 2016 Task Force statement (2) was the recommendation that all women initiate BC screening at age 40, down from 50. Early detection reduces BC mortality by detecting tumors at a more treatable stage (3–5). Initiating BC screening earlier aligns Task Force recommendations with guidelines from other national health organizations (e.g., National Comprehensive Cancer Center Network)(6), although other important differences remain between guidelines (e.g., screening interval). Of the multiple guidelines, Task Force recommendations directly impact which preventive services third-party payers are required to cover with no cost share to patients (7). The Task Force recommendation to initiate screening earlier was informed by increasing and well-documented racial disparities in BC mortality. Compared to White women, Black women experience a 40% higher BC mortality rate overall and a twofold higher BC mortality rate among women under age 50 (8). Among Black women, more than one in five BC cases (vs. approximately one in seven for White women) and more than one in four advanced-stage BC cases (vs. one in five for White women) are diagnosed before age 50 (9). These statistics and simulation studies highlight the potential for the updated Task Force BC screening guidelines to improve early detection for younger Black women (10,11).
Realizing the full potential benefit of Task Force recommendations could be limited by disparities in access to screening mammography. Common barriers to BC screening that disparately impact younger Black women include transportation, economic factors (e.g., limited paid time off, uncertainty about costs), childcare, and reservations about screening (e.g., mistrust, pain) (12–15). These barriers vary geographically, with less access to BC screening observed in communities with fewer mammography facilities (12,13), lower insurance rates (16), less access to primary care (17), socioeconomic disadvantage (18), and residential segregation (19). Deploying interventions to improve screening uptake in geographic areas with worse BC outcomes aligns with the National Cancer Institute (NCI) mandate for NCI-designated Cancer Centers to address disparities via community outreach and engagement within their catchment areas (20,21).
As a first step toward this end, the primary objective of this study was to apply novel statistical methods to identify geographic areas with elevated rates of BC diagnosed before age 50 and/or at an advanced stage within a cancer center catchment area (i.e., “hotspots”). The secondary objective was to assess whether hotspots contribute to Black-White disparities. This study analyzed the catchment area for the Helen F. Graham Cancer Center & Research Institute (HFGCCRI), a community cancer center in Delaware. We focused on a community cancer center because upwards of 90% cancer care in the US takes place in the community setting (22,23). Consistent with this finding, the HFGCCRI provides care to approximately 85% of the breast cancer cases in its catchment area (24), even though this region has been attributed to another NCI-designated cancer center catchment area (25,26). We focused on Delaware for several reasons. First, Delaware is generally representative of the US along demographic and rural-urban characteristics (27,28). Regarding land use, 32.3% of the Delaware population lives in a rural area compared to 31.8% of the US population. Demographically, 20.6% of the adult female population in Delaware is Black and 71.7% is White compared to 12.4% and 74.4% for the US, respectively. Adult women under age 50 make up 54.2% of the Delaware population compared to 56% of the US population. Second, Delaware leads the US for the incidence of late stage BC among women under age 50 (statecancerprofiles.cancer.gov), the subpopulation of BC patients for whom earlier detection could offer the greatest potential benefit. Third, Delaware leads the US in the incidence of triple-negative BC (TNBC) (29,30), an aggressive BC subtype that disproportionately impacts younger Black women (31) and accounts for the majority of the Black-White disparity in BC mortality (32). Applying this approach to a Delaware community cancer center catchment area can inform and prioritize local public health initiatives while illustrating a methodology that can be applied more broadly to other cancer center catchment areas.
MATERIALS AND METHODS
STUDY DESIGN AND POPULATION
This single-institution retrospective study was conducted with data from a cohort of BC patients who were treated at the HFGCCRI and reside in its catchment area, defined as New Castle County, Delaware. The HFGCCRI provides care to more than 600 analytic breast cancer cases annually (24), or cases that were diagnosed and/or received the first course of treatment at the HFGCCRI (33). As reported previously, based on data provided by the Delaware Cancer Registry, cases treated at the HFGCCRI represented 85% of all BC cases treated in the catchment area and were not significantly different from New Castle County BC cases not treated at the HFGCCRI for age, race, BC subtype, and stage (24). The HFGCCRI is a part of ChristianaCare and the study was reviewed and approved by the ChristianaCare Institutional Review Board (CCC# 43040) and conducted in accordance with the U.S. Common Rule. The need for consent was waived.
Patient records came from the HFGCCRI cancer registry for 3,497 adult female New Castle County residents age 20 years and older who were diagnosed with invasive breast cancer between 2012 and 2020. The HFGCCRI cancer registry identifies cases via multiple means, including an ongoing review of records from pathology and treatment areas (e.g., new patients in radiation oncology). At the end of each reporting year, a Disease Index review of International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes is conducted to identify potentially missed cases. Breast cancer cases are identified with an ICD-10-CM code of C50 (malignant neoplasm of breast). This time period was selected to maximize the study population. Prior to 2012, not all the BC characteristics necessary for staging purposes were consistently contained within the cancer registry. Given the disruptions to BC screening and diagnosis imposed by the Coronavirus Disease 2019 (COVID-19) beginning in 2020 (34), we compared the distribution of BC cases by age and stage in 2020 to prior years and did not observe a significant difference.
MEASURES
Patient measures included age at diagnosis, race, ethnicity, residential address, BC stage, and insurance status (private, Medicare, or Medicaid), which were abstracted from the HFGCCRI registry and the ChristianaCare electronic health record. BC cases were classified by age as <50 or ≥50, consistent with other reports (8), and as advanced if the American Joint Committee on Cancer prognostic pathologic stage was IIA or higher, consistent with the recommendation by Kerlikowske and colleagues based on a comparison of 3 different BC classifications in over 50,000 women with invasive BC (35). For cases that received neoadjuvant chemotherapy (e.g., in the treatment of TNBC), we used the clinical stage for classification, consistent with current practices (36). Patient addresses were geocoded to latitude and longitude coordinates with ArcGIS Desktop 10.8, yielding a 95% match rate. As reported elsewhere (37), unmatched patients did not differ from matched patients on demographic or clinical characteristics.
A special note regarding the ethnicity measure is warranted. Ethnicity is collected during the registration process and prior research conducted at our health system showed that Hispanic/Latina patients are often misclassified as non-Hispanic (White) (38). This issue has been well documented across US health care databases (39) and represents an important barrier to identifying disparities by ethnicity (40). The North American Association of Central Cancer Registries (NAAACR) developed an algorithm to improve the identification of Hispanic/Latino patients (41). Unfortunately, we did not have access to the necessary datapoints (e.g., birthplace) to implement this algorithm. Therefore, we reported on the ethnicity data we had available, recognizing this likely represented an underestimate of the true Hispanic/Latina population of BC patients.
STATISTICAL ANALYSIS
Descriptive statistics were used to characterize BC patients by age and stage. Next, the geographic distribution of BC cases were characterized within the HFGCCRI catchment area with the method of spatial intensity. Spatial intensity is a spatial statistical function defined to represent the expected number of events per unit area at a location, which is often estimated using the kernel density approach, hence the alias spatial kernel density estimation (KDE) (42,43). Spatial intensity calculates a distance-weighted count of events per unit area, with events here representing BC cases. A fine mesh of generic locations is placed over the study region, spatial intensity estimated at each location, and the results mapped to display continuous spatial variation in concentrations of events (i.e., not aggregated to geographic units). The spatial intensity estimates were edge-corrected to better accommodate estimation near the catchment area boundary (44). The bandwidth parameter of the intensity function controls the level of smoothing. An adaptive bandwidth (i.e., varies by location) was used for this application given the variation in population density and BC cases, ranging from more dense in the northern part of the county to less dense in the rural, southern part of the county (45,46).
Spatial intensity was estimated for the binary stratifications of age, stage, and age/stage, defined as follows: 1) <50 vs. ≥50, 2) advanced vs not advanced, and 3) <50/advanced vs. all other cases. Next, the ratio of intensities by these age, stage, and age/stage categories (e.g. spatial intensity of age <50 BC cases relative to the spatial intensity of age ≥50 BC cases), were used to nonparametrically estimate and visualize spatial variation in risk for each comparison, an approach common in spatial epidemiologic applications and often expressed as log relative risk (24,43,47). The log-transform is used in an effort to make the relative risk surface more symmetric while also reducing the influence of outlying values on visualization.35,38 Tolerance regions, denoting areas with significantly higher risk for cases classified by the age, stage, and age/stage categories were identified using asymptotic methods and labeled as ‘hotspots’ (37,45). These hotspots represent areas where the ratios of BC cases by age, stage, and age/stage were significantly greater (p<.05) than the ratio observed across the full catchment area. The demographic and clinical characteristics of BC cases by age, race, and stage in each hotspot were examined.
To complement the hotspot analysis, population demographics by age and race were obtained for New Castle County at the Census block group level from the 2010 decennial Census (48) and standardized incidence ratios (SIRs) were calculated for each hotspot based on catchment area BC rates and adjusting for age and race (49). Hotspot population totals were estimated by prorating block group totals based on the pecent of their area contained within the identified hostpsot boundaries. The SIRs provide estimates of the ratio of the observed to expected number of BC cases.
DATA AVAILABILITY
The data generated in this study are not publicly available and cannot be shared to protect the privacy of the individuals included in this study. The measures in this study, including residential address and clinical data, are protected health information that if made available could comporomise patient confidentiality.
RESULTS
Table 1 reports the demographic and clinical characteristics for the 3,497 identified invasive BC cases. Approximately 18% of these cases were among women under 50, 44% were advanced, and 10% were among women under 50 and advanced. Among the cases diagnosed before 50, approximately 25% were <40 and 75% 40–49. Black women were moderately overrepresented among <50, advanced, and the <50/advanced groups, accounting for 25.9%, 26.5% and 28.6% of these cases, respectively, compared to 22.6% of all BC cases. Conversely, White women were underrepresented for these groups, accounting for 66.9%, 69.3%, and 64.3% of these cases, respectively, compared to 73.5% of all cases. Patients belonging to the Other/Unknown racial group represented <4% of all cases. With regard to ethnicity, consistent with the limitations noted above, Hispanic women appeared to be underrepresented overall. Only 2.5% of the BC patients in this cohort were identified as Hispanic, much lower than the approximately 10% of the population that Hispanics represent in New Castle County. Even accounting for lower BC incidence among Hispanic (vs. non-Hispanic) women,(50) these findings appear to confirm that a meaningful proportion of Hispanic women were misclassified as non-Hispanic. To the extent that these descriptive statistics can be interpreted, recognizing that misclassification may vary by patient subgroup, Hispanic women appeared to be overrepresented among the <50, advanced, and <50/advanced groups. Finally, about half of patients were covered through private insurance, a third via Medicare, and 3% by Medicaid.
Table 1.
Characteristics of HFGCCRI breast cancer patients in New Castle County, Delaware (2012–2020)*
| Age >= 50 (N=2868) | |||||||
|---|---|---|---|---|---|---|---|
| Age at Diagnosis | |||||||
| Mean (SD) | 41.3 (6.2) | 43.7 (4.9) | 42.4 (5.8) | 66.5 (11.1) | 67.0 (10.0) | 66.8 (10.5) | 62.4 (13.5) |
| Race, n % | |||||||
| Black | 100 (28.6) | 63 (22.6) | 163 (25.9) | 307 (25.8) | 322 (19.2) | 629 (21.9) | 792 (22.6) |
| White | 225 (64.3) | 196 (70.3) | 421 (66.9) | 842 (70.8) | 1309 (78.0) | 2151 (75.0) | 2572 (73.5) |
| Other/Unknown | 25 (7.1) | 20 (7.2) | 45 (7.2) | 40 (3.4) | 48 (2.9) | 88 (3.1) | 133 (3.8) |
| Ethnicity, n % | |||||||
| Hispanic | 21 (6.0) | 13 (4.7) | 34 (5.4) | 19 (1.6) | 34 (2.0) | 53 (1.8) | 87 (2.5) |
| Non Hispanic | 328 (93.7) | 266 (95.3) | 594 (94.4) | 1164 (97.9) | 1641 (97.7) | 2805 (97.8) | 3399 (97.2) |
| Unknown Ethnicity | 1 (0.3) | 0 (0.0) | 1 (0.2) | 6 (0.5) | 4 (0.2) | 10 (0.3) | 11 (0.3) |
| Race & Ethnicity, n % | |||||||
| Black-Non Hispanic | 98 (28.0) | 63 (22.6) | 161 (25.6) | 306 (25.7) | 321 (19.1) | 627 (21.9) | 788 (22.5) |
| Black-Hispanic | 1 (0.3) | 0 (0.0) | 1 (0.2) | 1 (0.1) | 0 (0.0) | 1 (0.0) | 2 (0.1) |
| Black-Unknown Ethnicity | 1 (0.3) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.1) | 1 (0.0) | 2 (0.1) |
| White-Non Hispanic | 207 (59.1) | 183 (65.6) | 390 (62.0) | 821 (69.0) | 1275 (75.9) | 2096 (73.1) | 2486 (71.1) |
| White-Hispanic | 18 (5.1) | 13 (4.7) | 31 (4.9) | 16 (1.3) | 33 (2.0) | 49 (1.7) | 80 (2.3) |
| White-Unknown Ethnicity | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.4) | 1 (0.1) | 6 (0.2) | 6 (0.2) |
| Other/Unknown-Non Hispanic | 23 (6.6) | 20 (7.2) | 43 (6.8) | 37 (3.1) | 45 (2.7) | 82 (2.9) | 125 (3.6) |
| Other/Unknown-Hispanic | 2 (0.6) | 0 (0.0) | 2 (0.3) | 2 (0.2) | 1 (0.1) | 3 (0.1) | 5 (0.1) |
| Other/Unknown-Unknown Ethnicity | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 2 (0.1) | 3 (0.1) | 3 (0.1) |
| Insurance, n % | |||||||
| Medicaid | 26 (7.4) | 6 (2.2) | 32 (5.1) | 48 (4.0) | 24 (1.4) | 72 (2.5) | 104 (3.0) |
| Medicare | 7 (2.0) | 8 (2.9) | 15 (2.4) | 490 (41.2) | 740 (44.1) | 1230 (42.9) | 1245 (35.6) |
| Private | 304 (86.9) | 245 (87.8) | 549 (87.3) | 545 (45.8) | 730 (43.5) | 1275 (44.5) | 1824 (52.2) |
| Unknown Payer | 13 (3.7) | 20 (7.2) | 33 (5.2) | 106 (8.9) | 185 (11.0) | 291 (10.1) | 324 (9.3) |
HFGCCRI = Helen F. Graham Cancer Center & Research Institute; Adv = advanced stage, defined as stage ≥IIA; Non-Adv = non-advanced stage, defined as stage I
Hotspot Detection
Figure 1 visualizes each of the hotspots described below. Table 2 reports demographic and clinical characteristics for the BC cases within each of the four identified hotspots. Table 3 lists the age and race demographics for the adult (≥ 18 years old) female population in each of the identified hotspots.
Figure 1.
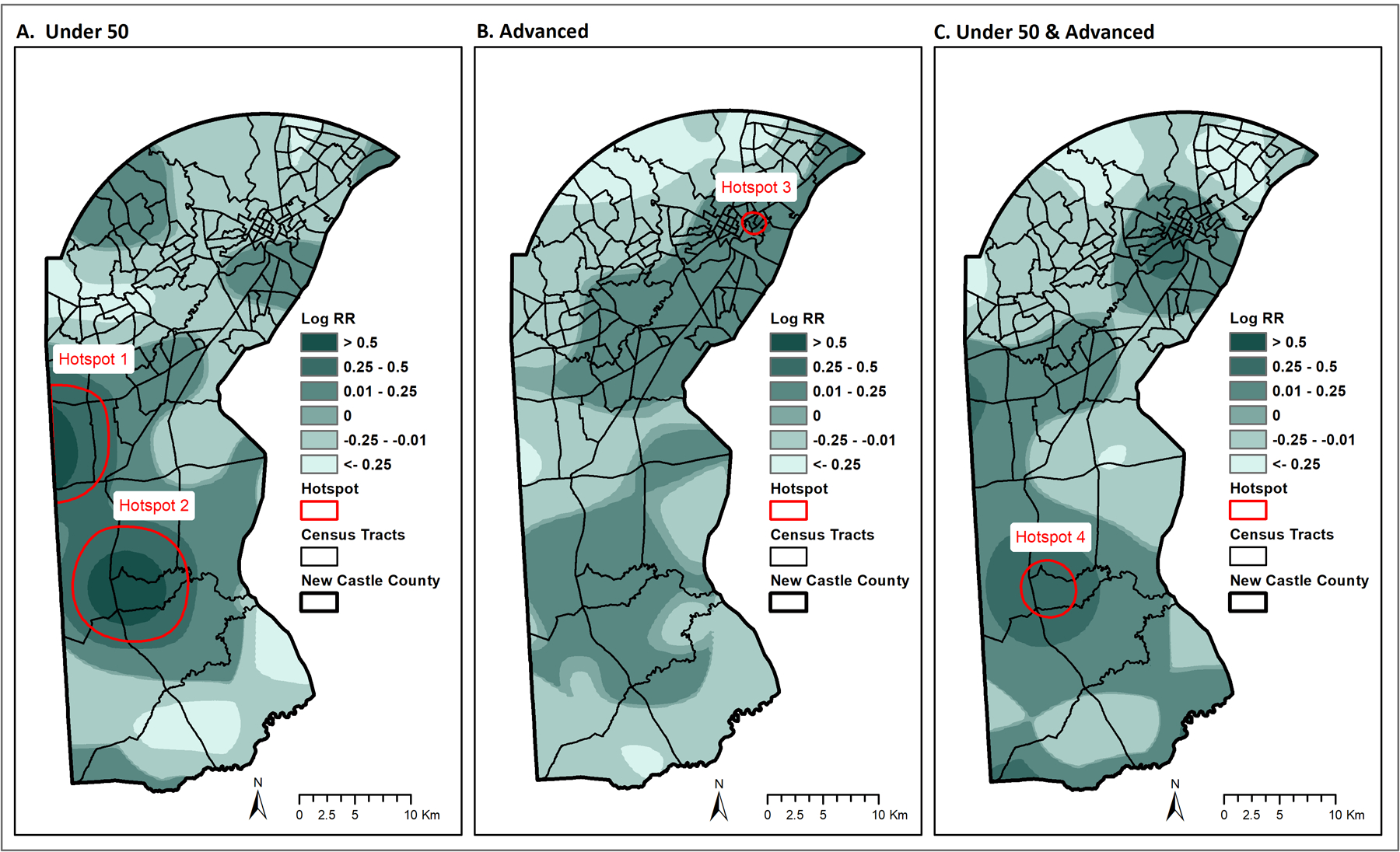
Spatial variation in risk of BC by age and stage and detected hotspots for New Castle County, DE (2012–2020). A. Hotspots for BC diagnosed before age 50. B. Hotspot for BC diagnosed at an advanced stage. C. Hotspot for BC diagnosed under age 50 and at an advanced stage. Spatial variation is visualized as the log relative risk for each type of hotspot, with red outlines representing regions with statistically significant higher risk.
Table 2.
Characteristics of breast cancer patients in identified hotspots, New Castle County, DE (2012–2020)*
| < 50 (N=29) | >= 50 (N=84) | Total | < 50 (N=48) | >= 50 (N=143) | Total | Adv (N=38) | Non-Adv (N=31) | Total | <50 & Adv (N=18) | Others (N=86) | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at Diagnosis | ||||||||||||
| Mean (SD) | 43.2 (5.1) | 63.6 (8.9) | 58.4 (12.0) | 43.6 (4.2) | 67.9 (10.1) | 61.8 (13.8) | 58.9 (13.5) | 64.7 (14.5) | 61.5 (14.2) | 43.8 (3.4) | 61.9 (13.1) | 58.8 (13.8) |
| Race, n % | ||||||||||||
| Black | 9 (31.0) | 17 (20.2) | 26 (23.0) | 17 (35.4) | 35 (24.5) | 52 (27.2) | 37 (97.4) | 29 (93.5) | 66 (95.7) | 8 (44.4) | 22 (25.6) | 30 (28.8) |
| White | 18 (62.1) | 65 (77.4) | 83 (73.5) | 29 (60.4) | 104 (72.7) | 133 (69.6) | 1 (2.6) | 2 (6.5) | 3 (4.3) | 8 (44.4) | 62 (72.1) | 70 (67.3) |
| Other/Unknown | 2 (6.9) | 2 (2.4) | 4 (3.5) | 2 (4.2) | 4 (2.8) | 6 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.1) | 2 (2.3) | 4 (3.8) |
| Stage, n % | ||||||||||||
| Advanced | 15 (51.7) | 28 (33.3) | 43 (38.1) | 26 (54.2) | 62 (43.4) | 88 (46.1) | 38 (100.0) | 0 (0.0) | 38 (55.1) | 18 (100.0) | 33 (38.4) | 51 (49.0) |
| Non-Advanced | 14 (48.3) | 56 (66.7) | 70 (61.9) | 22 (45.8) | 81 (56.6) | 103 (53.9) | 0 (0.0) | 31 (100.0) | 31 (44.9) | 0 (0.0) | 53 (61.6) | 53 (51.0) |
Adv = advanced stage, defined as stage ≥IIA; Non-Adv = non-advanced stage, defined as stage I
Table 3.
Census Population Summary for the Hotspots in New Castle County, DE (2012–2020)*
| Hotspot 1 (Pop=5969) | Hotspot 2 (Pop=7727) | Hotspot 3 (Pop=4658) | Hotspot 4 (Pop=3789) | County (Pop=216453) | |
|---|---|---|---|---|---|
| Race Group, n (%) | |||||
| Black | 1088 (18.2) | 1861 (24.1) | 4127 (88.6) | 1086 (28.7) | 49881 (23.0) |
| White | 4277 (71.7) | 5344 (69.2) | 347 (7.4) | 2407 (63.5) | 147541 (68.2) |
| Others | 603 (10.1) | 521 (6.7) | 184 (4.0) | 295 (7.8) | 19031 (8.8) |
| Age Group, n (%) | |||||
| <50** | 3830 (64.2) | 4990 (64.6) | 2888 (62.0) | 2537 (67.0) | 124341 (57.4) |
| >=50 | 2139 (35.8) | 2737 (35.4) | 1770 (38.0) | 1251 (33.0) | 92112 (42.6) |
Population data is rounded to integer for presentation purpose
Inclusive of the female adult population (≥ 18 years old)
Hotspot 1, located in the western part of the county, corresponding to a region known as Glasgow, represents a geographic area with a significantly greater ratio of <50 (N=29) to ≥50 (N=84) BC cases than observed in the catchment area. In this hotspot, 25.7% of the cases were <50 (vs. 18% countywide). More than half of the <50 cases in this hotspot were diagnosed at an advanced stage. Black women were overrepresented in this group, accounting for 31% of <50 BC cases, compared to 28.6% of <50 BC cases countywide. Black women represent 18.2% of the general population in this hotspot.
Hotspot 2 was located in the more southern part of the county, encircling the town of Middletown. Similar to hotspot 1, hotspot 2 represented a geographic area with a significantly greater ratio of <50 (N=48) to ≥50 (N=143) BC cases. Also similar to hotspot 1, more than half of the <50 cases were diagnosed at an advanced stage. In this hotspot, Black women were again overrepresented, accounting for 33.6% of the <50 BC cases (vs. 28.6% countywide). Black women represented 24.1% of the general population in this hotspot.
Hotspot 3 was located in the northern part of the county, covering a part of the city of Wilmington. This hotpsot represented a geographic area with a significantly greater ratio of advanced (N=38) to non-advanced (N=31) BC cases. In this hotspot, 55% of cases were diagnosed at an advanced stage, compared to 44% for the county. All but 3 of the 69 cases in hotspot 3 were Black (95.7%), greater than even the very high degree of residential segregation in this hotspot where the general population is 88.6% Black.
Hotspot 4 was located in the southern part of the county, covering a part of Middetown, and enclosed entirely within hotspot 2. This hotspot represents a geographic area with a significantly greater ratio of <50/advanced BC cases (N=18) to all other BC cases (i.e., ≥50 and/or non-advanced; N=86), representing 17% of cases (vs. 10% countywide). Black women accounted for 44.4% of the <50/advanced group in this hotspot, compared to 28.6% of these cases countywide. Black women represented 28.7% of the general population in this hotspot.
Hotspot SIRs
Table 4 presents the observed, expected, and the age-race adjusted SIRs (observed divided by expected ratios) for the BC cases in the four hotspots. In hotspots 1 and 2, where higher rates of <50 BC cases were observed, the adjusted SIR was 1.32 (95% CI: 1.08, 1.57) and 1.72 (95% CI: 1.47, 1.96), indicating that there were 32% and 72% more <50 BC cases, respectively, than would be expected based on the underlying population. When stratified by age and race, the largest SIR ratios for both hotspots was for the <50 Black group, where approximately 2.5 more cases were observed than expected.
Table 4.
Standardized Incidence Ratios in Identified Hotspots in New Castle County, DE (2020)*
| Obs | Exp | SIR (CI) | Obs | Exp | SIR (CI) | Obs | Exp | SIR (CI) | Obs | Exp | SIR (CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Race Adjusted | 113 | 85.40 | 1.32 (1.08, 1.57) | 191 | 111.18 | 1.72 (1.47, 1.96) | 69 | 74.43 | 0.93 (0.71, 1.15) | 104 | 52.28 | 1.99 (1.61, 2.37) |
| <50 Black | 9 | 3.65 | 2.47 | 17 | 6.64 | 2.56 | 15 | 13.11 | 1.14 | 12 | 3.76 | 3.19 |
| <50 White | 18 | 14.38 | 1.25 | 29 | 17.75 | 1.63 | 1 | 1.17 | 0.85 | 21 | 8.49 | 2.47 |
| <50 Other/Unknown | 2 | 1.40 | 1.42 | 2 | 1.26 | 1.59 | 0 | 0.39 | 0.00 | 2 | 0.72 | 2.79 |
| >=50 Black | 17 | 13.11 | 1.30 | 35 | 19.72 | 1.77 | 51 | 54.61 | 0.93 | 18 | 12.25 | 1.47 |
| >=50 White | 65 | 49.92 | 1.30 | 104 | 63.56 | 1.64 | 2 | 4.03 | 0.50 | 49 | 25.80 | 1.90 |
| >=50 Other/Unknown | 2 | 2.94 | 0.68 | 4 | 2.24 | 1.79 | 0 | 1.12 | 0.00 | 2 | 1.25 | 1.60 |
Obs = observed cases; Exp = expected cases. SIR = standardized incidence ratio, CI= 95% confidence interval; SIRs were estimated based on catchment area BC rates and adjusted for age and race
In hotspot 3, where higher rates of advanced BC cases were observed, the adjusted SIR was 0.93 (95% CI: 0.71, 1.57). Unlike the other hotspots that were defined either solely (hotspots 1 and 2) or in part (hotspot 4) by age, hotspot 3 was defined entirely by stage. A non-significant adjusted SIR for hotspot 3 indicates that the total number of BC cases were consistent with population expectations (i.e., adjusting for age and race), whereas the ratio of the spatial intensities results reported above indicate that the ratio of advanced to non-advanced cases in this geographic area was significantly higher than observed countywide. However, within the <50 Black group specifically, the SIR was 1.14, indicating there were 14% more BC cases than expected.
In hotspot 4, where higher rates of <50/advanced BC cases were observed, the SIR was 1.99 (95% CI: 1.61, 2.37), indicating that approximately twice as many <50/advanced BC cases were observed as expected. Higher rates of cases were observed for all groups, with the largest SIRs observed for Black (3.19) and White (2.47) women <50.
DISCUSSION
This study applied a novel spatial approach to identify BC hotspots defined by age and stage for a community cancer center catchment area in Delaware, the state that leads the US for the incidence of late stage BC among women under 50 (50) and other important BC disparities (30). Whereas other approaches to hotspot detection have estimated the spatial intensity of advanced cancer cases (51), the approach employed in this study considered the ratio of spatial intensities for BC by age and stage. This approach controls for several potential confounders (e.g., population density) and offers greater confidence for identifying geographic areas that have a statistically significant higher-than-expected number of early-onset and/or advanced BC cases. Utilizing this approach, four BC hotspots were identified in the catchment area, two of which were defined by age, one by stage, and one by age and stage.
In the two hotspots defined just by age (hotspots 1 and 2), more than a quarter of cases were diagnosed before age 50. In both of these hotspots, Black women were even more overrepresented than observed for the full catchment area, accounting for nearly a third of these cases. Approximately 2.5 as many cases among Black women under 50 were observed in these hotspots as would be expected, providing evidence that these hotspots contribute to the overall racial disparity in BC. These findings underscore the importance of implementing the Task Force recommendations to initiate BC screening at age 40 for women who reside in these hotspots, notably younger Black women. For women under 40, the Task Force recommends that primary care clinicians should conduct BC risk assessments to determine if a referral to genetic counseling would be appropriate based on personal and family history (52). Implementing this recommendation also has particular relevance for women under 50 who reside in these hotspots, as approximately 25% of BC cases under 50 were diagnosed in women under 40.
In the hotspot defined by stage, more than half of the BC cases were diagnosed at an advanced stage and approximately 97% of the cases were Black. This hotspot covers a portion of Wilmington, the largest city in the catchment area. For comparison, approximately 58% of Wilmington’s residents are Black (53) and 89% of the general population who reside within the hotspot are Black, indicating that even given very high rates of residential segregation within this section of Wilmington, Black women were still overrepresented. Factors related to structural racism, including persistent trends in segregation, poverty, and the cumulative exposure to multiple harmful exposures contribute to worse BC outcomes and other health disparities in this area (54–56). Thus, improving BC early detection in this hotspot will likely require multilevel interventions.
In the hotspot defined by age and stage, which was enclosed within a larger hotspot defined just by age within the town of Middletown, nearly one in five of the BC cases were diagnosed at an advanced stage among women under 50. As with other hotspots, Black women were even more overrepresented in this group than they were across the catchment area, with more than three times as many observed cases as expected. The Middletown finding was unexpected because, compared to Wilmington, it is a smaller and much more affluent town (53) with relatively good access to mammography facilities (19). That said, Middletown has undergone rapid population growth in recent decades, with its population sextupling and diversifying over the last 30 years primarily through in-migration (57). This raises new questions about whether compositional or contextual effects apply (58). That is, the Middletown hotspots may be a compositional artifact of some risk factor shared by a disproportionate share of the in-migrating population (e.g., prior exposure or ancestry). Alternatively, a contextual factor inherent to Middletown may have contributed to the observed pattern, such as toxic environmental exposures or, given the rapid population growth, inadequate primary care resources to facilitate access to mammography facilities. To address these questions, new research is currently underway taking a model-based approach to describe the spatial variation in BC risk by evaluating a range of patient (e.g., history of screening) and environmental (e.g., primary care access) factors.
There are several study limitations. First, single-site designs often have limited external validity, which is by definition true for the detection of hotspots in a single cancer center catchment area. However, given the representativeness of the population demographics and land use in this catchment area, these methods may have broad applicability to other cancer center catchment areas. Second, given privacy protections established by the Delaware Cancer Registry, the data for this study came from only those patients diagnosed or treated at the HFGCCRI, increasing the risk of a biased sample. This risk is mitigated by the large share of patients in the catchment area who received their care at the HFGCCRI and a comparison that showed that cancer center and state registry cases did not differ by demographic or clinical characteristics (24). Nevertheless, it remains unclear if the sample was representative in terms of insurance coverage or spatially representative of all cases in the catchment area, notably within the hotspots. Third, it cannot be assumed that higher rates of BC diagnosed before age 50 or at an advanced stage reflect inadequate screening in the identified hotspots. Even with annual screening mammorgraphy beginning at age 40, aggressive forms of BC can emerge in the interval between screenings, a particular concern for women with higher lifetime risk of BC (59). Clarifying this issue in future research by considering patient-level measures of screening history and BC risk factors (e.g., family history) will be important for the development of effective interventions. And fourth, when estimating the ratio of the observed to expected number of BC cases in each hotspot stratified by both age and race, the number of cases in several of the cells were relatively small. This raises concerns about unstable SIR estimates. This concern may be compounded by any inaccuracies in the underlying Census data.
In addition to these limitations, important sociodemographic data were not available for this study population, including measures of socioeconomic status (SES) and ethnicity. Past research has found an association between SES and risk of advanced-stage BC diagnoses (60). While less is known about the relationship between SES and the incidence of BC diagnosed before age 50, several modifiable BC risk factors that have links to have SES may impact the development of early-onset BC (61). Unfortunately, measures of SES are not routinely collected at the HFGCCRI. The secondary objective of this study was to assess whether hotspots contribute to Black-White disparities in BC mortality (8). Extending these analyses to ethnicity may have produced important findings but was limited by the available patient demographic data. Hispanic women are more likely to be diagnosed with BC before age 50, at an advanced stage, and both under 50 and at an advanced stage (9). Hispanic women are also more likely to be diagnosed with more aggressive BC subtypes and experience delays in the treatment of BC (62). While Hispanic women as a whole have lower BC incidence and mortality rates (8), reporting such statistics without regard to race, ancestry, nativity, enclave residence, and time in the US masks important heterogeneity (62–64). With higher quality ethnicity data, spatial methods can help identify communities where Hispanic women are at greater risk for disparate BC outcomes.
In conclusion, the novel application of advanced spatial methods for the identification of BC hotspots can help to prioritize communities for targeted community outreach and engagement as a form of precision health (65). These methods can be applied to other cancer center catchment areas and for other cancer types where area-level factors may impact early detection (e.g., colorectal cancer) (66). The findings from this study further showed that younger Black women were even more overrepresented in the BC hotspots than was observed for the entire catchment area. This raises the promising prospect that prioritizing hotspots has the potential to both reduce the overall burden of BC and narrow racial disparities.
Acknowledgements
This project was supported by NIGMS (P20 GM103446) from the NIH and the State of Delaware (to S.D. Siegel).
Footnotes
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- 1.U.S. Preventive Services Task Force. Breast Cancer: Screening [Internet]. [cited 2023 Apr 24]. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/breast-cancer-screening-adults
- 2.Siu AL. Screening for breast cancer: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2016;164(4):279–96. [DOI] [PubMed] [Google Scholar]
- 3.Monticciolo DL. Current Guidelines and Gaps in Breast Cancer Screening. J Am Coll Radiol [Internet]. 2020;17(10):1269–75. Available from: 10.1016/j.jacr.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Duffy SW, Tabár L, Yen AMF, Dean PB, Smith RA, Jonsson H, et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer. 2020;126(13):2971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy SW, Tabár L, Yen AMF, Dean PB, Smith RA, Jonsson H, et al. Beneficial effect of consecutive screening mammography examinations on mortality from breast cancer: A prospective study. Radiology. 2021;299(3):541–7. [DOI] [PubMed] [Google Scholar]
- 6.NCCN Guidelines (R) for Breast Cancer Screening and Diagnosis, Version 1.2022 - June2, 2022 [Internet]. Available from: www.NCCN.org
- 7.U.S. Preventive Services Task Force. Procedure Manual Appendix I. Congressional Mandate Establishing the U.S. Preventive Services Task Force [Internet]. [cited 2022 Nov 16]. Available from: https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual/procedure-manual-appendix-i
- 8.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin [Internet]. 2022 Oct 3;0(0):1–18. Available from: https://onlinelibrary.wiley.com/doi/10.3322/caac.21754 [DOI] [PubMed] [Google Scholar]
- 9.Hendrick RE, Monticciolo DL, Biggs KW, Malak SF. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127(23):4384–92. [DOI] [PubMed] [Google Scholar]
- 10.Chapman CH, Schechter CB, Cadham CJ, Trentham-Dietz A, Gangnon RE, Jagsi R, et al. Identifying Equitable Screening Mammography Strategies for Black Women in the United States Using Simulation Modeling. Ann Intern Med. 2021;174(12):1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Kharazmi E, Fallah M. Race and Ethnicity–Adjusted Age Recommendation for Initiating Breast Cancer Screening. JAMA Netw Open [Internet]. 2023 Apr 19;6(4):e238893. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2803948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jewett PI, Gangnon RE, Elkin E, Hampton JM, Jacobs EA, Malecki K, et al. Geographic access to mammography facilities and frequency of mammography screening. Ann Epidemiol [Internet]. 2018. Feb;28(2):65–71.e2. Available from: http://journals.sagepub.com/doi/10.1177/028418519403500407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young SG, Ayers M, Malak SF. Mapping mammography in Arkansas: Locating areas with poor spatial access to breast cancer screening using optimization models and geographic information systems. J Clin Transl Sci. 2020;4(5):437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mootz A, Arjmandi F, Dogan BE, Evans WP. Health Care Disparities in Breast Cancer: The Economics of Access to Screening, Diagnosis, and Treatment. J Breast Imaging. 2020;2(6):524–9. [DOI] [PubMed] [Google Scholar]
- 15.Aleshire ME, Adegboyega A, Escontrías OA, Edward J, Hatcher J. Access to Care as a Barrier to Mammography for Black Women. Policy, Polit Nurs Pract. 2021;22(1):28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangka FK, Subramanian S, Mobley LR, Hoover S, Wang J, Hall IJ, et al. Racial and ethnic disparities among state Medicaid programs for breast cancer screening. Prev Med (Baltim) [Internet]. 2017;102:59–64. Available from: 10.1016/j.ypmed.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry J The Relationship between the Supply of Primary Care Physicians and Measures of Breast Health Service Use. J Women’s Heal. 2017;26(5):511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castaldi M, Smiley A, Kechejian K, Butler J, Latifi R. Disparate access to breast cancer screening and treatment. BMC Womens Health [Internet]. 2022;22(1):1–10. Available from: 10.1186/s12905-022-01793-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster JL, Goldstein ND, Rowland JP, Tuite CM, Siegel SD. A catchment and location-allocation analysis of mammography access in Delaware, US: implications for disparities in geographic access to breast cancer screening. Breast Cancer Res [Internet]. 2023. Nov 8;25(1):137. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36909545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paskett ED, Hiatt RA. Catchment Areas and Community Outreach and Engagement : The New Mandate for NCI-Designated Cancer Centers. 2018;517–20. [DOI] [PubMed] [Google Scholar]
- 21.Manne SL, Knott CL, Berger A, Champion VL, Chrischilles E, Fitzgibbon ML, et al. Current Approaches to Serving Catchment Areas in Cancer Centers: Insights from the Big Ten Cancer Research Consortium Population Science Working Group. Cancer Epidemiol Biomarkers Prev. 2023;32(4):465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clauser SB, Johnson MR, O’Brien DM, Beveridge JM, Fennell ML, Kaluzny AD. Improving clinical research and cancer care delivery in community settings: Evaluating the NCI community cancer centers program. Implement Sci. 2009;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green LA, Fryer GE, Yawn BP, Lanier D, Dovey SM. The Ecology of Medical Care Revisited. N Engl J Med. 2001;344(26):2021–5. [DOI] [PubMed] [Google Scholar]
- 24.Siegel SD, Brooks MM, Sims-Mourtada J, Schug ZT, Leonard DJ, Petrelli N, et al. A Population Health Assessment in a Community Cancer Center Catchment Area: Triple-Negative Breast Cancer, Alcohol Use, and Obesity in New Castle County, Delaware. Cancer Epidemiol Biomarkers Prev [Internet]. 2021 Nov 4;(16):cebp.1031.2021. Available from: http://cebp.aacrjournals.org/lookup/doi/10.1158/1055-9965.EPI-21-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DelNero PF, Buller ID, Jones RR, Tatalovich Z, Vanderpool RC, Ciolino HP, et al. A National Map of NCI-Designated Cancer Center Catchment Areas on the 50th Anniversary of the Cancer Centers Program. Cancer Epidemiol Biomarkers Prev [Internet]. 2022 May 4;31(5):965–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35101903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Cathment Areas of NCI-Designated Cancer Centers [Internet]. Available from: https://gis.cancer.gov/ncicatchment/
- 27.USDS Economic Research Service. Rural Definitions: Delaware State-Level Map [Internet]. 2007. Available from: https://www.ers.usda.gov/data-products/rural-definitions/
- 28.U.S. Census Bureau. Decennial Census by Decade. US Dep Commer. 2010; [Google Scholar]
- 29.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung H, Wiese D, Jatoi I, Jemal A. State Variation in Racial and Ethnic Disparities in Incidence of Triple-Negative Breast Cancer Among US Women. JAMA Oncol [Internet]. 2023 Mar 2;30144:1–5. Available from: https://jamanetwork.com/journals/jamaoncology/fullarticle/2802138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard FM, Olopade OI. Epidemiology of Triple-Negative Breast Cancer. Cancer J [Internet]. 2021. Jan;27(1):8–16. Available from: https://journals.lww.com/10.1097/PPO.0000000000000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jatoi I, Sung H, Jemal A. The Emergence of the Racial Disparity in U.S. Breast-Cancer Mortality. N Engl J Med [Internet]. 2022 Jun 18; Available from: http://www.nejm.org/doi/10.1056/NEJMp2200244 [DOI] [PubMed] [Google Scholar]
- 33.Kirtane K, Zhao Y, Amorrortu RP, Fuzzell LN, Vadaparampil ST, Rollison DE. Demographic disparities in receipt of care at a comprehensive cancer center. Cancer Med [Internet]. 2023 Jun 28;12(12):13687–700. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cam4.5992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richman I, Tessier-Sherman B, Galusha D, Oladele CR, Wang K. Breast cancer screening during the COVID-19 pandemic: moving from disparities to health equity. J Natl Cancer Inst. 2023;115(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerlikowske K, Bissell MCS, Sprague BL, Buist DSM, Henderson LM, Lee JM, et al. Advanced Breast Cancer Definitions by Staging System Examined in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2021;113(7):909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrd DR, Brierley JD, Baker TP, Sullivan DC, Gress DM. Current and future cancer staging after neoadjuvant treatment for solid tumors. CA Cancer J Clin. 2021;71(2):140–8. [DOI] [PubMed] [Google Scholar]
- 37.Siegel SD, Brooks MM, Lynch SM, Sims-Mourtada J, Schug ZT, Curriero FC. Racial disparities in triple negative breast cancer: toward a causal architecture approach. Breast Cancer Res [Internet]. 2022 Dec 1;24(1):37. Available from: 10.21203/rs.3.rs-1177963/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks M, Axe M, Ortiz J, Papas M. From data flaws to data-driven practices: using continuous evaluation and knowledge transfer to improve the collection of patient race, ethnicity, and language. Acad Heal Annu Res Meet. 2019; [Google Scholar]
- 39.Johnson JA, Moore B, Hwang EK, Hickner A, Yeo H. The accuracy of race & ethnicity data in US based healthcare databases: A systematic review. Am J Surg [Internet]. 2023. Oct;226(4):463–70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002961023001976 [DOI] [PubMed] [Google Scholar]
- 40.Magaña López M, Bevans M, Wehrlen L, Yang L, Wallen GR. Discrepancies in Race and Ethnicity Documentation: a Potential Barrier in Identifying Racial and Ethnic Disparities. J Racial Ethn Heal Disparities [Internet]. 2017;4(5):812–8. Available from: 10.1007/s40615-016-0283-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NAACR Race and Ethnicity Work Group. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.2]. Springfield (IL); 2011. [Google Scholar]
- 42.Bithell JF. An application of density estimation to geographical epidemiology. Stat Med [Internet]. 1990. Jun;9(6):691–701. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2218172 [DOI] [PubMed] [Google Scholar]
- 43.Kelsall JE, Diggle PJ. Kernel estimation of relative risk. Bernoulli. 1995;1:3–16. [Google Scholar]
- 44.Zheng P, Durr PA, Diggle P. Edge-correction for spatial kernel smoothing methods - when is it necessary? In: Durr PA, Martin SW, editors. GISVET’04: second International Conference of the Applications of GIS and Spatial Analysis to Veterinary Science. Ontario: Veterinary Laboratores Agency; 2004. p. 36–8. [Google Scholar]
- 45.Davies TM, Hazelton ML. Adaptive kernel estimation of spatial relative risk. Stat Med [Internet]. 2010 Oct 15;29(23):2423–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20603814 [DOI] [PubMed] [Google Scholar]
- 46.Davies TM, Jones K, Hazelton ML. Symmetric adaptive smoothing regimens for estimation of the spatial relative risk function. Comput Stat Data Anal [Internet]. 2016. Sep;101:12–28. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016794731630024X [Google Scholar]
- 47.Kelsall JE, Diggle PJ. Non-parametric estimation of spatial variation in relative risk. Stat Med. 1995;14(21–22):2335–42. [DOI] [PubMed] [Google Scholar]
- 48.U.S. Census Bureau. Total population 2010 (Table ID: P12). Available from: https://data.census.gov/table?g=050XX00US10003,10003$1500000&d=DEC+Summary+File+1&tid=DECENNIALSF12010.P12
- 49.Breslow NE, Day NE. Statistical Methods in Cancer Research. Volume 1 -- The Analysis of Case-Control Studies [Internet]. Lyon: nternational Agency for Research on Cancer; (IARC Scientific Publications No. 32); 1980. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123864543004024 [PubMed] [Google Scholar]
- 50.Cronin KA, Scott S, Firth AU, Sung H, Henley SJ, Sherman RL, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer [Internet]. 2022 Oct 27; Available from: https://onlinelibrary.wiley.com/doi/10.1002/cncr.34479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou K, Thompson LK, Liu L, Terrault NA, Cockburn MG. Geographic hotspot detection for late-stage hepatocellular carcinoma: novel approach to cancer control. Cancer Causes Control [Internet]. 2022;33(5):701–10. Available from: 10.1007/s10552-022-01555-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA -Related Cancer. JAMA [Internet]. 2019 Aug 20;322(7):652. Available from: https://jamanetwork.com/journals/jama/fullarticle/2748515 [DOI] [PubMed] [Google Scholar]
- 53.U.S. Census Bureau. 2014–2018 American Community Survey 5-Year Estimates [Internet]. 2020. Available from: https://www.census.gov/programs-surveys/acs/technical-documentation/table-and-geography-changes/2018/5-year.html
- 54.Siegel SD, Brooks MM, Berman JD, Lynch SM, Sims‐Mourtada J, Schug ZT, et al. Neighborhood factors and triple negative breast cancer: The role of cumulative exposure to area‐level risk factors. Cancer Med [Internet]. 2023 Mar 14;(January):1–13. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegel SD, Brooks M, Ragozine-Bush HE, Schnoll RA, Curriero FC. The co-occurrence of smoking and alcohol use disorder in a hospital-based population: Applying a multimorbidity framework using geographic information system methods. Addict Behav [Internet]. 2021 Jul;118(November 2020):106883. Available from: 10.1016/j.addbeh.2021.106883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegel SD, Brooks M, Bourke J, Curriero FC. Reducing exposure to tobacco retailers with residential zoning policy: insights from a geospatial analysis of Wilmington, Delaware. Cities Heal [Internet]. 2021 Jun 16;00(00):1–13. Available from: 10.1080/23748834.2021.1935141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Institute for Public Administration. Town of Middletown Delaware 2022 Comprehensive Plan [Internet]. 2022. Available from: https://www.middletown.delaware.gov/media/Middletown-Comp-Plan-FINAL-1-24-23.pdf
- 58.Oakes JM. The (mis)estimation of neighborhood effects: Causal inference for a practicable social epidemiology. Soc Sci Med. 2004;58(10):1929–52. [DOI] [PubMed] [Google Scholar]
- 59.Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. npj Breast Cancer [Internet]. 2017. Apr 13;3(1):12. Available from: 10.1038/s41523-017-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy D, Du DY. Socioeconomic and Racial Disparities in Cancer Stage at Diagnosis, Tumor Size, and Clinical Outcomes in a Large Cohort of Women with Breast Cancer, 2007–2016. J Racial Ethn Heal Disparities. 2021;8(4):990–1001. [DOI] [PubMed] [Google Scholar]
- 61.Daly AA, Rolph R, Cutress RI, Copson ER. A Review of Modifiable Risk Factors in Young Women for the Prevention of Breast Cancer. Breast Cancer Targets Ther [Internet]. 2021. Apr;Volume 13:241–57. Available from: https://www.dovepress.com/a-review-of-modifiable-risk-factors-in-young-women-for-the-prevention--peer-reviewed-article-BCTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Champion CD, Thomas SM, Plichta JK, Parrilla Castellar E, Rosenberger LH, Greenup RA, et al. Disparities at the Intersection of Race and Ethnicity: Examining Trends and Outcomes in Hispanic Women With Breast Cancer. JCO Oncol Pract [Internet]. 2022. May;18(5):e827–38. Available from: https://ascopubs.org/doi/10.1200/OP.20.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fejerman L, Ramirez AG, Napoles AM, Gomez SL, Stern MC. Cancer Epidemiology in Hispanic Populations: What Have We Learned and Where Do We Need to Make Progress? Cancer Epidemiol Biomarkers Prev. 2022;31(5):932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shariff-Marco S, Von Behren J, Reynolds P, Keegan THM, Hertz A, Kwan ML, et al. Impact of social and built environment factors on body size among breast cancer survivors: The pathways study. Cancer Epidemiol Biomarkers Prev. 2017;26(4):505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynch SM, Wiese D, Ortiz A, Sorice KA, Nguyen M, González ET, et al. Towards precision public health: Geospatial analytics and sensitivity/specificity assessments to inform liver cancer prevention. SSM - Popul Heal. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayhand KN, Handorf EA, Ortiz AG, Gonzalez ET, Devlin A, Sorice KA, et al. Effect of Neighborhood and Individual-Level Socioeconomic Factors on Colorectal Cancer Screening Adherence. Int J Environ Res Public Health [Internet]. 2021 Apr 21;18(9):4398. Available from: https://www.mdpi.com/1660-4601/18/9/4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are not publicly available and cannot be shared to protect the privacy of the individuals included in this study. The measures in this study, including residential address and clinical data, are protected health information that if made available could comporomise patient confidentiality.


