Abstract
Background:
Ultrafine particles (UFPs) are unregulated air pollutants abundant in aviation exhaust. Emerging evidence suggests that UFPs may impact lung health due to their high surface area-to-mass ratio and deep penetration into airways. This study aimed to assess long-term exposure to airport-related UFPs and lung cancer incidence in a multiethnic population in Los Angeles County.
Methods:
Within the California Multiethnic Cohort, we examined the association between long-term exposure to airport-related UFPs and lung cancer incidence. Multivariable Cox proportional hazards regression models were used to estimate the effect of UFP exposure on lung cancer incidence. Subgroup analyses by demographics, histology and smoking status were conducted.
Results:
Airport-related UFP exposure was not associated with lung cancer risk [per one IGR HR = 1.01, 95% CI: 0.97-1.05] overall and across race/ethnicity. A suggestive positive association was observed between a one IQR increase in UFP exposure and lung squamous cell carcinoma (SCC) risk [HR = 1.08, 95% CI: 1.00-1.17] with a Phet for histology=0.05. Positive associations were observed in 5-year lag analysis for SCC [HR = 1.12, CI: 1.02-1.22] and large cell carcinoma risk [HR = 1.23, CI: 1.01-1.49] with a [Phet for histology = 0.01].
Conclusions:
This large prospective cohort analysis suggests a potential association between airport-related UFP exposure and specific lung histologies. The findings align with research indicating that UFPs found in aviation exhaust may induce inflammatory and oxidative injury leading to SCC.
Impact:
These results highlight the potential role of airport-related UFP exposure in the development of lung SCC.
Keywords: air pollution, airport-related ultrafine particles, lung cancer, squamous cell carcinoma
Introduction
The health effects associated with particles less than or equal to 2.5 μm in aerodynamic diameter (PM2.5) have been well studied, leading to its classification as a Group 1 carcinogen and the establishment of air quality standards and routine air monitoring.1-3 There is now an increasing body of experimental evidence indicating that ultrafine particles (UFPs), particles with an aerodynamic diameter of 0.1 μm or less, may be a driving factor for the adverse effect of PM2.5 on lung health. This may be related to the higher surface area-to-mass ratio of UFPs compared to larger particles, which allows UFPs to adsorb higher amounts of toxic pollutants and provides greater surface area for interaction with cell membranes.4 Additionally, UFPs may be able to penetrate deeper into airways and remain longer in the lung parenchyma, which may result in more severe damage than larger particles.5-8 To our knowledge, three cohort studies, all using land-use regression models to estimate ambient UFP exposures have reported mixed results between UFP and lung cancer associations. In the Los Angeles Ultrafines Study (1995-2017), historical UFP exposure was modestly associated with overall lung cancer risk (HR = 1.03, 95% CI: 0.99-1.08 per 10,000 particles/cm3) after adjusting for smoking and other confounders.9 A Dutch cohort study using data from Statistics Netherlands found that UFP exposure determined at the midpoint of the follow-up period (2013-2019) was positively associated with lung cancer mortality (HR = 1.038, 95% CI: 1.028-1.048) when individual and area-level socioeconomic status were considered.10 In the Ontario Population Health and Environment Cohort (ONPHEC), ambient UFP exposure from 1996 through 2012 was not significantly associated with incidence of overall lung cancer after a mean follow-up of 14.6 years, adjusted for comorbidities (HR = 1.00, 95% CI: 0.97-1.04); this study also lacked information on smoking.11 While airport-related pollution contributed to the overall ambient UFP in these studies, none specifically examined lung cancer risk in relation to airport-related UFPs, which differ from roadway-related UFPs in that the former contain larger concentrations of certain organophosphate esters and heavy metals that may be associated with adverse health outcomes.12 Studies in Rome,13 Amsterdam,14 and Los Angeles County,15 have documented how the major airports in these areas contribute to potentially harmful urban UFP concentrations reaching as far as 10 km away. At the Seattle-Tacoma Airport, investigators reported that plumes containing UFPs from descending aircraft impacted a larger area than UFPs from roadways, with UFP-containing plumes affecting communities 15 km downwind within 15-20 minutes travel time.16 Additionally, in our prior research in the Multiethnic Cohort (MEC), airport-related UFPs from the Los Angeles airport (LAX) were found to be associated with an increased risk for brain cancer.17
In this study, we examined the association between long-term exposure to airport-related UFPs and incidence of lung cancer among MEC participants residing in California from 1993 through 2013. This investigation sought to provide new insights on UFPs and lung cancer risk in a large, racially, ethnically and socioeconomically diverse study population residing in Los Angeles County, a U.S. region with high levels of air pollution.18
Materials and Methods
Study Participants & Characteristics
The MEC is a prospective cohort study of over 215,000 men and women aged 45-75 at enrollment from 1993-1996. Detailed descriptions of the cohort have been published elsewhere.19 Briefly, participants of five self-reported racial and ethnic groups (African American, Japanese American, Latino, Native Hawaiian, and White) were recruited from either California (primarily Los Angeles County), or Hawaii. Participants were mailed a comprehensive 26-page baseline questionnaire that assessed demographics, medical and reproductive history, medication use, family history of various cancers, smoking history, physical activity and an extensive quantitative food frequency questionnaire. Participants were followed for an invasive lung cancer diagnosis by regular linkages with the California Cancer Registry and Hawaii Tumor Registry (both Surveillance, Epidemiology, and End Results Program registries), and also for vital status by regular linkages with the National Death Index and state death certificate files. Lung cancer histology was obtained from the cancer registries and cell-types were classified according to the framework provided by Lewis et al20 (Table 2 footnotes). We limited our subgroup analysis by histologic subtypes to the major groups of SCC, small cell carcinoma, adenocarcinoma, and large cell carcinoma.
Table 2.
Association between Airport-Related UFP and Lung Cancer Risk Overall by Sex, Race and Ethnicity, and Histology among California MEC participants, 1993-2013
| Cases (Cohort) | HR | 95% CI | Phet | Men | Women | Phetc | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | |||||||
| All Lung Cancer | 2,183 (71,387) | 1.01 | (0.97-1.05) | 1.02 (0.97-1.08) | 1.00 (0.96-1.05) | ||
| By Race/Ethnicity | |||||||
| African American | 1,032 (23,874) | 1.01 | (0.96-1.06) | [0.99]a | 1.02 (0.96-1.08) | 1.01 (0.95-1.07) | 0.96 |
| Japanese American | 257 (9,286) | 1.04 | (0.84-1.27) | 1.23 (1.00-1.51) | 0.72 (0.51-1.03) | 0.01 | |
| Latino | 524 (29,151) | 1.02 | (0.93-1.12) | 0.97 (0.87-1.09) | 1.10 (0.98-1.24) | 0.19 | |
| White | 368 (8,970) | 1.03 | (0.93-1.14) | 1.12 (0.98-1.29) | 0.98 (0.87-1.10) | 0.15 | |
| By Histology | |||||||
| Adenocarcinomad | 785 (69,989) | 1.00 | (0.94-1.07) | [0.05] b | 1.02 (0.94-1.11) | 1.00 (0.92-1.07) | 0.28 |
| Squamous Celle | 446 (69,650) | 1.08 | (1.00-1.17) | 1.10 (1.02-1.20) | 1.05 (0.95-1.17) | 0.52 | |
| Small Cellf | 211 (69,415) | 0.89 | (0.78-1.02) | 0.82 (0.65-1.04) | 0.97 (0.82-1.16) | 0.35 | |
| Large Cellg | 91 (69,295) | 0.96 | (0.80-1.16) | 0.75 (0.53-1.05) | 1.13 (0.90-1.42) | 0.02 | |
Note: bold indicates p ≤ 0.05; models were stratified by age at cohort entry and adjusted for smoking intensity and duration, family history of lung cancer, education, marital status, occupation, neighborhood socioeconomic status (nSES), non-steroidal anti-inflammatory drug (NSAID) use, body mass index (BMI), drinking, physical activity, energy intake, red meat intake, and processed meat intake, as well as race/ethnicity and sex where applicable.
Abbreviations: HR = Hazard Ratio; CI = Confidence Interval, UFP = ultrafine particles. HR represents the increase in lung cancer per each IQR change of UFP, where IQR is specific to each analytic sample; unit=0.002296 (any in grid).
Probability of heterogeneity by race/ethnicity
Probability of heterogeneity by histology
Probability of heterogeneity by sex within subgroup (i.e. race/ethnicity: African American or histology: adenocarcinoma)
ICD-O-3 morphology codes that were classified as Adenocarcinoma: 8015, 8050, 8140-1, 8143-5, 8147, 8190, 8201, 8211, 8250-5, 8260, 8290, 8310, 8320, 8323, 8333, 8401, 8440, 8470-1, 8480-1, 8490, 8503, 8507, 8550, 8570-2, 8574, 8576
ICD-O-3 morphology codes that were classified as Squamous Cell Carcinoma: 8051-2, 8070-6, 8078, 8083-4, 8090, 8094, 8120, 8123
ICD-O-3 morphology codes that were classified as Small Cell Carcinoma: 8002, 8041-5
ICD-O-3 morphology codes that were classified as Large Cell Carcinoma: 8012-4, 8021, 8034, 8082
Participants eligible for this study included only California MEC participants who completed a baseline questionnaire, did not have lung cancer prior to cohort entry (i.e., reported on baseline questionnaire or through linkage with the tumor registry), and with geocodable addresses (n=105,359). We excluded participants with missing smoking information (n=7,974), residence coordinates that were outside the LAX Airport UFP exposure grid (n=18,139), or had >50% imputed UFP exposure level (n=7,762). The final sample size for this analysis was 71,387 participants. Our previous work on airport-related UFPs17 showed similar distributions of baseline characteristics between included and excluded CA MEC participants. These characteristics included sex, age, education, occupation, smoking history, and BMI. A greater proportion of included participants had a neighborhood SES in the lowest quintile versus the highest quintile, which reflects the lower SES of neighborhoods nearby LAX airport.
Address Geocoding & Socioeconomic Status
The MEC maintains accurate and up-to-date residential histories (i.e., addresses over study period) on all participants using periodic follow-up questionnaires, mailed newsletters, and linkages to databases and registries. Addresses over the course of the study were geocoded to latitude/longitude coordinates based on parcel or street centroids for UFP exposure assessment and appended to U.S. Census block groups as follows: baseline addresses (1993-1996 addresses) to the 1990 block groups, 1997-2005 addresses to the 2000 block groups, and 2006-2013 addresses to the 2010 block groups. A composite index of neighborhood socioeconomic status (nSES) at the level of census block group was created based on a principal component analysis of seven census SES indicators for each decennial Census year: education, median household income, percent living 200% below poverty level, percent blue-collar workers, percent older than 16 years in workforce without job, median rent, and median house value.21,22 This nSES index was assigned to participants’ block group at baseline (time of cohort entry) and time of event. nSES was categorized into quintiles based on the distribution across census block groups in Los Angeles County.
Assessment of Ultrafine Particle Exposure
UFP concentrations from LAX flight activity were estimated using the Environmental Protection Agency’s recommended American Meteorological Society/Environmental Protection Agency Regulatory Dispersion Model (AERMOD).17 This model considered hourly changes in meteorology, such as wind speed and direction, atmospheric stability and mixing height, and also considered hourly variations in flight activity within a 53 km by 43 km grid at a spatial resolution of 1 km.23 The 1 km grid resolution was found to be sufficient in capturing the spatial distribution of airport UFPs resulting from the broad effects of landing jets, which can produce plumes several hundred to thousand meters wide as they touch down, as opposed to take-offs and taxiing of jets, which tend to produce impacts much nearer downwind of the airport. In a prior investigation, the model results showed good agreement with real-time, mobile measurements taken over seven days along six transects downwind of LAX, adjusted for non-LAX UFP contributions from vehicular traffic (Pearson R2 of 0.71 and a mean absolute percentage error of 6%).15 Several other studies have similarly found LAX to be a significant contributor to UFP concentrations, adjusted for traffic-related sources, with reports in one study showing that daily contribution to UFP from the LAX was about 11 times greater than those of surrounding freeways.24,25
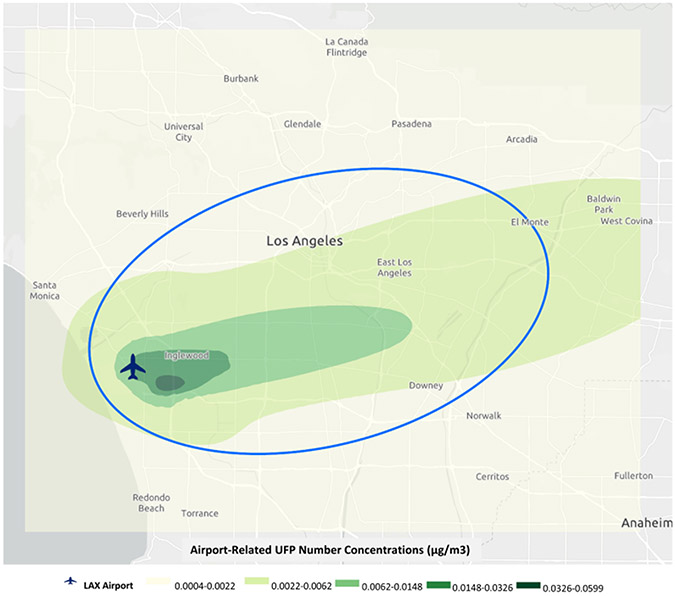
For the participants in our study, monthly UFP data at each centroid of 1 km by 1 km grids (based on specific latitude/longitude coordinates) were used to generate annual UFP trend maps and continuous kriging surfaces in order to assign monthly UFP exposures to participants’ residential histories. As a result, cumulative UFP exposure averages were estimated for the study period for MEC participants. As described previously,17 the impact zone of LAX was defined as an oval aligned with areas of high airport-related UFP concentrations in order to focus our analyses on areas most directly impacted by the airport, which included the highest UFP concentrations (Figure 1). We conducted four analyses based on varying numbers of participants with UFP measures. The first two analyses included participants with any addresses (2,183 lung cancer cases among n=71,387) within the UFP exposure grid, or all addresses (2,068 cases among n=64,871) within the UFP exposure grid, and the third and fourth analyses included participants with any addresses (1,599 cases among n=51,625) within the impact zone, or with all addresses (1,389 cases among n=41,348) within the impact zone. Results from these analyses showed largely comparable associations, and we present findings from “any addresses in the grid” as this included the largest number of participants. Exposure to combustion-related co-pollutants nitrogen dioxide (NO2) and particulate matter with aerodynamic diameter equal or less than 10 μm and 2.5 μm (PM10, PM2.5) have been linked to lung cancer,26,27 and were assessed using an established kriging interpolation approach that has been described previously.28
Figure 1: Impact Zone of Airport-Related Ultrafine Particle Exposure near Los Angeles International Airport.
Airport-related UFP exposure estimates (μg/m3) for a 53 km 43 km grid area around the Los Angeles International Airport (1993–2013). Natural breaks were used to classify five UFP exposure categories, displayed in gradations of green. The impact UFP zone was defined as an oval with an aspect ratio of 2:1 aligned along with the orientation of the airport runways and predominant daytime wind direction, with one, long-axis edge aligned with the upwind airport property line. The long axis represented the distribution of maximum centerline UFP concentrations for all the July months between 1993 and 2013. The natural breaks were used to facilitate the visualization of UFP number concentrations that had a highly right-skewed distribution.
Statistical Analysis
Cox proportional hazards regression models with monthly time-varying exposure variables were used to estimate the effect of UFP exposure on lung cancer incidence. This model used calendar month/year as the time variable and defined a series of risk sets based on month/year at diagnosis of each lung cancer event (index case) using age at cohort entry (one-year age groups) as a stratum variable. Each risk set included all MEC participants who were alive and uncensored at the time of lung cancer diagnosis. For each member of each risk set (including the index case) based on their residential history, the average air pollutant exposure was computed for the period between the time of cohort entry (month/year) and the time of lung cancer diagnosis of the index case. This average exposure was used as the independent variable.
Based on a favored hypothesis of repeated oxidative and inflammatory insults leading to stepwise oncogenic mutations,29,30 it was expected that any association between UFPs and lung cancer would occur as a function of long-term exposure. To address this latency period, a 5-year-lagged sensitivity analysis was conducted to exclude UPF exposures within 5 years of the censor data.
Multivariable adjusted models included race and ethnicity, sex, family history of lung cancer in first degree relatives (yes or no), education level (high school or less, some college, college graduate, or graduate school), marital status (married, single, or separated/divorced/widowed), work history (six categories that combined industries and occupations and if participants had been employed in a specific job for 10 years or more, if any), longest worked occupation type (office work only, labor/craft only, or both), nSES (quintiles), nonsteroidal anti-inflammatory drug (NSAID) use (yes or no), body mass index (BMI) (underweight: <18.5 kg/m2, normal weight: 18.5-24.9 kg/m2, overweight: 25-29.9 kg/m2 or obese: ≥30 kg/m2), smoking status (never, current, or former), daily alcohol intake (non-drinker, 1 drink per day, 2 or more drinks per day), moderate or vigorous physical activity (hours/week, none, quartiles), energy intake (kcal/day, quintiles), red meat intake (g/day, quintiles), and processed red meat intake (g/day, quintiles).31 Smoking was incorporated into our model by calculating smoking duration in pack-years while also considering quitting probabilities dependent on participant time in the study, average number of cigarettes per day, race and ethnicity, as well as interaction of race and ethnicity with cigarettes per day as described in past studies.31 Minimal models that adjusted only for race and ethnicity, sex, smoking intensity (based on pack-years by age 50 and years without smoking), duration and smoking cessation were compared to the full model and showed similar associations (Table 3).
Table 3.
Five-Year-Lagged Analysis of Airport-Related UFP Association with Lung Cancer Risk by Histology, Sex, and Race/Ethnicity among CA MEC Participants, 1993-2013
| Cases (Cohort) |
HR | 95% CI | Phet | Men | Women | Phetc | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | |||||||
| By Race/Ethnicity | |||||||
| [0.89]a | |||||||
| African-American | 730 (21,669) | 1.02 | (0.96-1.08) | 1.07 (0.98-1.16) | 0.99 (0.91-1.07) | 0.25 | |
| Japanese | 191 (8,813) | 1.04 | (0.81-1.33) | 1.25 (0.93-1.68) | 0.72 (0.43-1.18) | 0.09 | |
| Latino | 407 (27,521) | 1.01 | (0.91-1.13) | 1.00 (0.87-1.14) | 1.06 (0.88-1.26) | 0.58 | |
| White | 266 (8,268) | 1.08 | (0.97-1.21) | 1.22 (1.01-1.48) | 1.05 (0.90-1.22) | 0.20 | |
| By Histology | |||||||
| [0.05]b | |||||||
| Adenocarcinomad | 594 (65,364) | 0.98 | (0.91-1.06) | 1.04 (0.93-1.16) | 0.95 (0.85-1.05) | 0.18 | |
| Squamous Celle | 323 (65,093) | 1.12 | (1.02-1.22) | 1.19 (1.07-1.32) | 1.03 (0.89-1.20) | 0.46 | |
| Small Cellf | 150 (64,920) | 0.95 | (0.81-1.11) | 0.87 (0.67-1.14) | 1.02 (0.84-1.25) | 0.59 | |
| Large Cellg | 45 (64,815) | 1.23 | (1.01-1.49) | 0.94 (0.60-1.48) | 1.44 (1.15-1.82) | 0.02 | |
Note: bold indicates p < 0.05; Models were stratified by age at cohort entry and adjusted for smoking intensity and duration, family history of lung cancer, education, marital status, occupation, neighborhood socioeconomic status (nSES), non-steroidal anti-inflammatory drug (NSAID) use, body mass index (BMI), drinking, physical activity, energy intake, red meat intake, and processed meat intake, as well as sex and race/ethnicity where applicable.
Abbreviations: HR = Hazard Ratio; CI = Confidence Interval, UFP = ultrafine particles. HR represents the increase in lung cancer per each interquartile range (IQR) change of UFP, where IQR is specific to each analytic sample; unit=0.002296 (any in grid).
Probability of heterogeneity by race/ethnicity
Probability of heterogeneity by histology
Probability of heterogeneity by sex
ICD-O-3 morphology codes that were classified as Adenocarcinoma: 8015, 8050, 8140-1, 8143-5, 8147, 8190, 8201, 8211, 8250-5, 8260, 8290, 8310, 8320, 8323, 8333, 8401, 8440, 8470-1, 8480-1, 8490, 8503, 8507, 8550, 8570-2, 8574, 8576
ICD-O-3 morphology codes that were classified as Squamous Cell Carcinoma: 8051-2, 8070-6, 8078, 8083-4, 8090, 8094, 8120, 8123
ICD-O-3 morphology codes that were classified as Small Cell Carcinoma: 8002, 8041-5
ICD-O-3 morphology codes that were classified as Large Cell Carcinoma: 8012-4, 8021, 8034, 8082
Hazard ratios (HRs) and 95% confidence intervals (CIs) for lung cancer risk per interquartile range (IQR) increase in airport-related UFP were calculated (IQR unit=6,700 particles/cm3). We also conducted co-pollutant analysis by mutually adjusting for kriging derived measures of gaseous (e.g., NO2) and PM (e.g., PM10, PM2.5) pollutants which are estimates of largely regional air pollution exposures obtained from routine continuous air monitoring data in California.10 The proportional hazards assumption for each pollutant in a model was checked with all covariates by graphing Schoenfeld residuals against time, and no violation was found.
Subgroup analyses were conducted to assess differences in effect estimates by sex, race and ethnicity, and smoking status. A global simultaneous test of interaction based on the Wald test was used to assess heterogeneity of effects for each pollutant and subgroup. To test for differences in associations by histology, focusing on SCC, small cell carcinoma, adenocarcinoma, and large cell carcinoma, a competing risk analysis using a Lunn-McNeil augmentation approach32,33 was conducted such that each histology is fit by a cause-specific model in a separate stratum. Parameter estimates across histological cell types were compared using the Wald test. Sensitivity analysis examined a five-year lag of UFP exposure. All p-values were two-sided with a statistical significance level of 0.05. Analyses were performed using SAS 9.2 statistical software (SAS Institute, Cary, NC).
Data Availability
The Multiethnic Cohort investigators and institutions affirm their intention to share the research data consistent with all relevant NIH resource/data sharing policies. Data requests should be submitted through Multiethnic Cohort online data request system at https://www.uhcancercenter.org/for-researchers/mec-data-sharing.
Results
Our study included 71,387 eligible participants in the California MEC (41,413 females and 29,974 males); 33.4% identified as African American, 13.0% as Japanese American, 40.8% as Latino, and 12.6% as White. Approximately 65.3% of participants had a BMI categorized as overweight or obese (BMI>25 kg/m2). A family history of lung cancer was reported by 5.2% of participants. Additionally, 17.9% and 36.2% were current or former smokers, respectively. Over the study period, the majority of participants (64.2%) remained at their baseline residence, and the mean follow-up time was 16.4 years (SD: 5.5 years). Further information and distributions of demographic and behavioral factors including education, marital status, employment, nSES, NSAID use, alcohol intake, physical activity, and dietary intake are presented in Table 1. Further breakdown of baseline participant characteristics by race/ethnicity can be found in Supplemental Table 1. Correlation analyses of UFP with kriged-based air pollutants across the follow-up period showed that UFP was negatively correlated with PM10 (R = −0.19), followed by NO2 (R = −0.11), and PM2.5 (R = −0.09) while UFP was positively correlated with NOx (R = 0.08), followed by CO (R = 0.06), and O3 (R = 0.04).
Table 1.
Study Characteristics of California Multiethnic Cohort (MEC) Participants at Baseline, 1993-1996a
| Overall | Women | Men | ||||
|---|---|---|---|---|---|---|
| Total Participants |
% | Total Participants |
% | Total Participants |
% | |
| Total | 71,387 | 41,413 | 29,974 | |||
| Mean age at entry (SD) | 60.1 (8.30) | 61.1 (8.20) | ||||
| Race/Ethnicity | ||||||
| African American | 23,874 | 33.4 | 15,589 | 37.6 | 8,285 | 27.6 |
| Hawaiian | 106 | 0.10 | 45 | 0.10 | 61 | 0.20 |
| Japanese American | 9,286 | 13.0 | 4,818 | 11.6 | 4,468 | 14.9 |
| Latino | 29,151 | 40.8 | 15,273 | 36.9 | 13,878 | 46.3 |
| White | 8,970 | 12.6 | 5,688 | 13.7 | 3,282 | 10.9 |
| Family History of Lung Cancer | ||||||
| No | 67,675 | 94.8 | 39,005 | 94.2 | 28,670 | 95.6 |
| Yes | 3,712 | 5.20 | 2,408 | 5.80 | 1,304 | 4.40 |
| Educationb | ||||||
| ≤ High School | 36,608 | 51.3 | 21,622 | 52.2 | 14,986 | 50.0 |
| Some College | 20,674 | 29.0 | 12,018 | 29.0 | 8,656 | 28.9 |
| College Graduate | 7,247 | 10.2 | 3,871 | 9.30 | 3,376 | 11.3 |
| Graduate/Professional | 6,368 | 8.90 | 3,597 | 8.70 | 2,771 | 9.20 |
| Marital Statusb | ||||||
| Married | 42,560 | 59.6 | 20,567 | 49.7 | 21,993 | 73.4 |
| Separated/Divorced/Widowed | 22,682 | 31.8 | 17,115 | 41.3 | 5,567 | 18.6 |
| Single | 5,381 | 7.50 | 3,236 | 7.80 | 2,145 | 7.20 |
| Employment in a Manufacturing Enterprise & Occupational Category | ||||||
| No & Office | 30,967 | 43.4 | 20,245 | 48.9 | 10,722 | 35.8 |
| No & Labor/Craft | 9,377 | 13.1 | 4,770 | 11.5 | 4,607 | 15.4 |
| No & Office/Labor/Craft | 18,425 | 25.8 | 13,180 | 31.8 | 5,245 | 17.5 |
| Yes & Office | 2,932 | 4.10 | 738 | 1.80 | 2,194 | 7.30 |
| Yes & Labor/Craft | 7,662 | 10.7 | 2,021 | 4.90 | 5,641 | 18.8 |
| Yes & Office/Labor/Craft | 2,024 | 2.80 | 459 | 1.10 | 1,565 | 5.20 |
| NSAIDa useb | ||||||
| No | 26,793 | 37.5 | 14,334 | 34.6 | 12,459 | 41.6 |
| Yes | 40,721 | 57.0 | 24,624 | 59.5 | 16,097 | 53.7 |
| BMI,c kg/m2b | ||||||
| Underweight | 879 | 1.20 | 705 | 1.70 | 174 | 0.60 |
| Normal | 23,415 | 32.8 | 14,005 | 33.8 | 9,410 | 31.4 |
| Overweight | 29,156 | 40.8 | 14,476 | 35.0 | 14,680 | 49.0 |
| Obese | 17,470 | 24.5 | 11,906 | 28.7 | 5,564 | 18.6 |
| Smoking Status | ||||||
| Never | 32,781 | 45.9 | 23,724 | 57.3 | 9,057 | 30.2 |
| Current Smoker | 12,747 | 17.9 | 6,366 | 15.4 | 6,381 | 21.3 |
| Former Smoker | 25,859 | 36.2 | 11,323 | 27.3 | 14,536 | 48.5 |
| Alcohol Intakeb | ||||||
| Non-Drinker | 36,357 | 50.9 | 24,879 | 60.1 | 11,478 | 38.3 |
| 1 Drink per Day | 21,424 | 30.0 | 11,606 | 28.0 | 9,818 | 32.8 |
| 2 or More Drinks per Day | 10,522 | 14.7 | 3,105 | 7.50 | 7,417 | 24.7 |
| Physical Activity, hours of moderate or vigorous activity/dayb | ||||||
| No: 0 | 5,739 | 8.00 | 3,300 | 8.00 | 2,439 | 8.10 |
| Quartile 1: 0.11-0.32 (M); 0.11-0.32 (F)d | 12,625 | 17.7 | 7,069 | 17.1 | 5,556 | 18.5 |
| Quartile 2: 0.36-0.71 (M); 0.36-0.57 (F) | 18,866 | 26.4 | 10,289 | 24.8 | 8,577 | 28.6 |
| Quartile 3: 0.82-1.43 (M); 0.713-1.18 (F) | 15,691 | 22.0 | 9,189 | 22.2 | 6,502 | 21.7 |
| Quartile 4: 1.54-13.29 (M); 1.21-13.29 (F) | 16,419 | 23.0 | 10,170 | 24.6 | 6,249 | 20.8 |
| Baseline Neighborhood SES (nSES)b,e | ||||||
| Quintile 1 - Low | 19,386 | 27.2 | 11,621 | 28.1 | 7,765 | 25.9 |
| Quintile 2 | 19,308 | 27.0 | 11,321 | 27.3 | 7,987 | 26.6 |
| Quintile 3 | 14,129 | 19.8 | 8,112 | 19.6 | 6,017 | 20.1 |
| Quintile 4 | 11,969 | 16.8 | 6,783 | 16.4 | 5,186 | 17.3 |
| Quintile 5 - High | 6,592 | 9.20 | 3,575 | 8.60 | 3,017 | 10.1 |
| Number of residential addresses per participant | ||||||
| 1 | 45,819 | 64.2 | 19,897 | 66.4 | 25,922 | 62.6 |
| 2 | 13,482 | 18.9 | 5,361 | 17.9 | 8,121 | 19.6 |
| 3 | 6,966 | 9.8 | 2,780 | 9.3 | 4,186 | 10.1 |
| 4+ | 5,120 | 7.2 | 1,936 | 6.5 | 3,184 | 7.7 |
| Years in cohort study | ||||||
| Mean years (SD) | 16.4 (5.5) | 15.7 (5.8) | 16.9 (5.2) | |||
NSAID = non-steroidal anti-inflammatory drug
Subcategory percentages do not add up to 100% due to missing values
BMI = body mass index
M = male, F = female
nSES = neighborhood socioeconomic status
The associations between airport-related UFP and lung cancer risk overall, by race and ethnicity and histology for men and women are presented in Table 2. For overall lung cancer, no association was observed with UFP (HR = 1.01, 95% CI: 0.97-1.05) for all subjects combined or separately by racial and ethnic group. In subgroup anaysis by sex across race and ethnicity, UFP was associated with an increased lung cancer risk among Japanese American men (HR = 1.23, 95% CI: 1.00-1.51) but not among Japanese American women (Phet for sex=0.01). In contrast, UFP was not associated with lung cancer risk among Latino men but was associated with elevated risk among Latino women (HR = 1.10, 95% CI: 0.98-1.24) that was not statistically significant (Phet for sex=0.19). For SCC, a per unit increase in the IQR of airport-related UFP exposure was associated with an increased risk (HR = 1.08, 95% CI: 1.00-1.17) that was borderline statistically significant. A statistically significant positive association between UFP and SCC was observed in men (HR = 1.10, 95% CI: 1.02-1.20) and also positively associated in women (HR = 1.05, 95% CI: 0.95-1.17). UFP exposure was not associated with risk for the other histology types (Phet for histology = 0.05) (Table 2) except for a suggestive difference in the association between large cell carcinoma risk and UFP in men and women (Phet for sex=0.02). In Supplemental Table 2, a per unit increase in the IQR of UFP exposure was associated with a statistically significant increased risk of SCC among current smokers (HR = 1.11, 95% CI: 1.01-1.22) with a suggestive association among former smokers (HR = 1.06, 95% CI 0.92-1.21) but not among never smokers; however, this latter result was based on only 17 lung cancer cases who identified as never smokers. No associations with UFP exposures were observed for the remaining histologies by smoking status (Supplemental Table 3).
Adjustment for co-pollutants PM2.5, PM10, and NO2 were conducted by lung cancer histology (Supplemental Table 4). The association between airport-related UFP exposure and increased SCC risk remained borderline significant when adjusted for PM2.5 (HR = 1.08, 95% CI: 1.00-1.17) and was borderline statistically significant when adjusted for PM10 exposure (HR = 1.07, 95% CI: 1.00-1.16) and NO2 (HR = 1.06, 95% CI: 0.98-1.14). In a sensitivity analysis by 5-year lagged UFP exposure (Table 3) which reduced the number of events by 27%, results remained similar showing a statistically significant increased risk of SCC and large cell carcinoma in association with UFP exposure but not for the other lung histologies (Phet for histology = 0.01). In addition, an analysis of the association between airport-related UFP and lung cancer risk, stratified by follow-up from 1993 to 2013, showed no differences in the associations between the “1993 to 2003” or “2004 to 2013” groups for all lung cancer as well as histologic subtypes (Supplemental Table 5).
Discussion
In this large prospective cohort analysis in the MEC, we observed a possible association between airport-related UFP exposure and risk of SCC. In models stratified by sex, this result remained statistically significant among males and was borderline statistically significant among females. Similar patterns of association with SCC risk were observed in current and former smokers, adjusted for smoking intensity, duration, and lung cancer risk factors. In models further adjusted for co-pollutants (PM2.5, PM10 and NO2), elevated risk of SCC associated with UFP exposure remained for PM2.5 and was borderline statistically significant for PM10 and NO2. Additionally, in 5-year lagged exposure analysis, the associations of UFP with SCC and large cell carcinoma were statistically significant but the number of events were modest, particularly for large cell carcinoma. These histology-specific results require confirmation, however they suggest that airport-related UFP may independently increase the risk of specific lung histologies.
The observed findings with risk of SCC are supported by recent research that found diesel-associated UFPs to upregulate TNF-α signaling via NF-κB and induce genes associated with SCC and chemical carcinogenesis5. While a paucity of research exists on jet engine emissions, which are a major component of airport-related UFPs, jet exhaust has been associated with similar health effects and is biophysically similar to diesel exhaust, as both are comprised of volatile organic compounds, transition metals, and particulate matter consisting of an inorganic carbon core with associated polycyclic aromatic hydrocarbons.15 These study results are additionally supported by findings that the extent of inflammatory and oxidative injury caused by UFPs may be more potent than that of larger particles due to greater retention in the lung parenchyma and increased total surface area enabling transport of larger toxin loads.4,34,35 It is well-known that such injury leads to SCC by stepwise progression to increasingly dysmorphic cellular features.30 It should be noted that lung SCC has been traditionally associated with central lesions located proximally in the tracheobronchial tree, whereas UFPs’ small size predisposes them to deposit deeper in the respiratory tract.34 Further, SCC is the lung cancer histological subtype most strongly associated with smoking, and both SCC and smoking rates have fallen since the 1990s.20 At the same time that these rates have fallen, an increase in the presentation of peripheral lung SCC lesions has been noted.36,37 Clinicopathologic features of peripheral SCC have also been found to be distinct from central SCC (i.e. differing frequency of EGFR and KRAS mutations, clinical presentation, and prognosis).28,38 Based on the current evidence, a potential implication is that UFP-associated SCC may be more likely to appear peripherally compared to smoking-associated SCC.
Our findings add to the three previously published cohort studies on this topic, which differed from our study in the assessment of UFP and in the covariates that were available for consideration in the analysis. Our null results for overall lung cancer incidence are consistent with the Canadian ONPHEC study’s observation that overall lung cancer incidence was not associated with intra-urban UFP exposures.11,39 However, the present study found 5-year lagged airport-related UFP exposure to be associated with risk of SCC and large cell carcinoma. Our finding on SCC is compatible with findings of a modest association of 1.03 (95% CI: 0.92-1.15) between 10-year lagged ambient UFP exposure and SCC risk in the Los Angeles Ultrafines Study.9 We found no significant association between ambient UFP exposure and small cell carcinoma or adenocarcinoma, consistent with the small cell carcinoma null results in the Los Angeles Ultrafines study (results on large cell were not presented) but differed from their finding of a positive association between ambient UFP exposure and adenocarcinoma among men (HR = 1.09, 95% CI: 1.00-1.18).9 Our studies also differed in UFP assessment methodology. We focused on airport-related UFPs and used AERMOD, a dispersion model to capture airplane emissions.15 In contrast, the Los Angeles Ultrafines study was focused on UFP exposure from all sources, and usedS land use regression modeling to capture small-scale spatial variations in urban settings.9
Although the Dutch cohort study found a statistically significant association between ambient intra-urban UFP exposure and lung cancer mortality, information on histological subtypes were not available.10 Our co-pollutant results align with those of the Dutch cohort study, which found ambient UFPs to be independently associated with lung cancer mortality in the presence of NO2, PM2.5, or PM10.10
The ONPHEC, similar to the Dutch cohort study, assessed intra-urban rather than airport-related UFP levels. Additionally, while the Dutch cohort study attempted to indirectly adjust for missing smoking data, neither the Dutch cohort nor ONPHEC had individual level data on smoking behavior, and other relevant covariates.11 As the present study lacked sufficient power to assess UFP-SCC association among non-smokers, future studies should ascertain whether UFP exposure differs by individual-level smoking history. Overall, our study evaluated associations by lung cancer histology and a specific source of UFP exposure that has not been examined in-depth by previous cohort studies.
The present study’s strengths include its population-based design, which enables greater external validity of our findings based on its large, racially, ethnically and socioeconomically diverse composition. Further, the availability of detailed individual-level data on health behaviors allowed us to account for lung cancer risk factors, including changing patterns of smoking behavior over time.31 The extensive residential histories of MEC participants also allowed us to capture UFP exposures that varied over time and locations. This study’s extended follow-up of up to 21 years enabled us to assess long-term prospective exposures. We were unable to assess historical exposure prior to cohort entry that may be relevant for the period of lung cancer development. Finally, the present study benefited from finer UFP exposure measurements, temporally and spatially, which are pertinent given the time and spatial-varying nature of UFP exposure.
Several limitations of this study should also be considered. Assessment of ambient airport-related UFP exposure was based on participants’ residential history, and we lacked information on exposures that may have occurred at work, while commuting, or in other outdoor locations. Relevant to the present study, previous research has noted that this may lead to underestimation of particulate matter exposure for those who live in less dense areas (such as suburban areas of Los Angeles County) and overestimation of exposure for those who live in urban centers or near roadways, particularly if individuals commute to work in areas with significantly different exposure levels.40 Our ability to address latency period between UFP exposure and lung cancer was limited due to available data, though we did conduct a 5-year-lagged sensitivity analysis and found a change from borderline and no association to statistically significant associations for SCC and large cell carcinoma, respectively. The average latency period between UFP exposure and lung cancer may be similar to that of other pollutants such as PM2.5, with prior research showing increased association between PM2.5 and lung cancer at 10 to 15 year lags compared to one to five year lags.41 While analyses were controlled for socioeconomic factors such as education, marital status, occupation category, and nSES, we lacked information on income and our information on occupational exposure was relatively crude. Among the types of lung cancer, SCC is the most strongly related to smoking.42 The stronger association between airport-related UFP and SCC observed among current smokers could theoretically reflect synergy between UFP and cigarette smoking, but could also reflect residual confounding by smoking. The observed gender difference in lung cancer risk among Japanese Americans, with borderline association between UFP and SCC among Japanese American men only, may also be a sign of residual confounding by smoking. There is a greater difference in smoking rates by gender among Asians compared to other races/ethnicities in the United States20 and higher smoking rates among Japanese individuals compared to other Asian subgroups.43 Thus, the large gender difference in smoking rates among Japanese Americans may explain the observed difference in association between UFP and SCC within this ethnic group (Phet by sex=0.01). Finally, given the limited number of SCC cases among never smokers in our sample, robust assessment of the association between UFP and SCC was not possible among never smokers.
The present study found a suggestive positive association between airport-related UFP exposure and risk of SCC. Despite its close relationship with roadway and aviation emissions, UFP pollution remains an unregulated and understudied form of air pollution, with few human studies evaluating this relationship longitudinally. Our results warrant further histology-specific investigations in other large epidemiologic cohorts to replicate these findings.
Supplementary Material
Acknowledgements
This work was supported by National Institute of Environmental Health Sciences grant R01ES026171 (C. Tseng, T.V. Larson, J. Yang, S.L. Park, J. Wu, S. Shariff-Marco, P.P. Inamdar, M.C. DeRouen, V.W. Setiawan, D.O. Stram, B. Ritz, S. Fruin, A.H. Wu, I. Cheng), National Cancer Institute grant U01 CA164973 (L.L. Marchand) and T32CA229110 (U. Ihenacho), National Institute of Environmental Health Sciences Environmental Exposures, Host Factors, and Human Disease grant P30 ES0070480 (A.H. Wu), and California Air Resource Board contract 04–323 (B. Ritz). The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or decision to submit the article for publication.
Abbreviations:
- CIs
95% confidence intervals
- BMI
body mass index
- HRs
Hazard ratios
- IQR
interquartile range
- LAX
Los Angeles airport
- MEC
Multiethnic Cohort
- nSES
neighborhood socioeconomic status
- NSAID
nonsteroidal anti-inflammatory drug
- ONPHEC
Ontario Population Health and, Environment Cohort
- SCC
squamous cell carcinoma
- UFP
ultrafine particle
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.US EPA O. National Ambient Air Quality Standards (NAAQS) for PM. Published April 13, 2020. Accessed June 18, 2023. https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm
- 2.WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. World Health Organization; 2021. Accessed June 18, 2023. http://www.ncbi.nlm.nih.gov/books/NBK574594/ [PubMed] [Google Scholar]
- 3.Ambient Outdoor Ultrafine Particulate Matter and Lung Cancer Risk - NCI. Published July 15, 2015. Accessed June 18, 2023. https://dceg.cancer.gov/research/what-we-study/ambient-outdoor-matter
- 4.Lin S, Ryan I, Paul S, et al. Particle surface area, ultrafine particle number concentration, and cardiovascular hospitalizations. Environ Pollut Barking Essex 1987. 2022;310:119795. doi: 10.1016/j.envpol.2022.119795 [DOI] [PubMed] [Google Scholar]
- 5.Grilli A, Bengalli R, Longhin E, et al. Transcriptional profiling of human bronchial epithelial cell BEAS-2B exposed to diesel and biomass ultrafine particles. BMC Genomics. 2018;19(1):302. doi: 10.1186/s12864-018-4679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med. 2020;52(3):311–317. doi: 10.1038/s12276-020-0403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sannolo N, Lamberti M, Pedata P. [Human health effects of ultrafine particles]. G Ital Med Lav Ergon. 2010;32(4 Suppl):348–351. [PubMed] [Google Scholar]
- 8.U.S. EPA. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-19/188, 2019 [Google Scholar]
- 9.Jones RR, Fisher JA, Hasheminassab S, et al. Outdoor Ultrafine Particulate Matter and Risk of Lung Cancer in Southern California. Am J Respir Crit Care Med. Published online October 19, 2023. doi: 10.1164/rccm.202305-0902OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouma F, Janssen NA, Wesseling J, et al. Long-term exposure to ultrafine particles and natural and cause-specific mortality. Environ Int. 2023;175:107960. doi: 10.1016/j.envint.2023.107960 [DOI] [PubMed] [Google Scholar]
- 11.Weichenthal S, Bai L, Hatzopoulou M, et al. Long-term exposure to ambient ultrafine particles and respiratory disease incidence in Toronto, Canada: a cohort study. Environ Health Glob Access Sci Source. 2017;16(1):64. doi: 10.1186/s12940-017-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendtsen KM, Bengtsen E, Saber AT, Vogel U. A review of health effects associated with exposure to jet engine emissions in and around airports. Environ Health. 2021;20(1):10. doi: 10.1186/s12940-020-00690-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stafoggia M, Cattani G, Forastiere F, Di Menno di Bucchianico A, Gaeta A, Ancona C Particle number concentrations near the Rome-Ciampino city airport. Atmos Environ. 2016;147:264–273. doi: 10.1016/j.atmosenv.2016.09.062 [DOI] [Google Scholar]
- 14.Keuken MP, Moerman M, Zandveld P, Henzing JS, Hoek G. Total and size-resolved particle number and black carbon concentrations in urban areas near Schiphol airport (the Netherlands). Atmos Environ. 2015;104:132–142. doi: 10.1016/j.atmosenv.2015.01.015 [DOI] [Google Scholar]
- 15.Hudda N, Gould T, Hartin K, Larson TV, Fruin SA. Emissions from an international airport increase particle number concentrations 4-fold at 10 km downwind. Environ Sci Technol. 2014;48(12):6628–6635. doi: 10.1021/es5001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin E, Xiang J, Gould TR, et al. Distinct Ultrafine Particle Profiles Associated with Aircraft and Roadway Traffic. Environ Sci Technol. 2021;55(5):2847–2858. doi: 10.1021/acs.est.0c05933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu AH, Fruin S, Larson TV, et al. Association between airport-related ultrafine particles and risk of malignant brain cancer: a multiethnic cohort study. Cancer Res. 2021;81(16):4360–4369. doi: 10.1158/0008-5472.CAN-21-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Most Polluted Places to Live ∣ State of the Air. Accessed April 23, 2023. https://www.lung.org/research/sota/key-findings/most-polluted-places
- 19.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–357. doi: 10.1093/oxfordjournals.aje.a010213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883–2892. doi: 10.1002/cncr.28749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control CCC. 2001;12(8):703–711. doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Schupp CW, Harrati A, Clarke C, Keegan THM, Gomez SL. Developing an Area-Based Socioeconomic Measure from American Community Survey Data. Cancer Prevention Institute of California, Fremont, California; 2014. [Google Scholar]
- 23.Wing SE, Larson TV, Hudda N, Boonyarattaphan S, Fruin S, Ritz B. Preterm Birth among Infants Exposed to in Utero Ultrafine Particles from Aircraft Emissions. Environ Health Perspect. 2020;128(4):47002. doi: 10.1289/EHP5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RR, Hoek G, Fisher JA, et al. Land use regression models for ultrafine particles, fine particles, and black carbon in Southern California. Sci Total Environ. 2020;699:134234. doi: 10.1016/j.scitotenv.2019.134234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirmohammadi F, Sowlat MH, Hasheminassab S, Saffari A, Ban-Weiss G, Sioutas C. Emission rates of particle number, mass and black carbon by the Los Angeles International Airport (LAX) and its impact on air quality in Los Angeles. Atmos Environ. 2017;151:82–93. doi: 10.1016/j.atmosenv.2016.12.005 [DOI] [Google Scholar]
- 26.Badida P, Krishnamurthy A, Jayaprakash J. Meta analysis of health effects of ambient air pollution exposure in low- and middle-income countries. Environ Res. 2023;216(Pt 4):114604. doi: 10.1016/j.envres.2022.114604 [DOI] [PubMed] [Google Scholar]
- 27.Cui P, Huang Y, Han J, Song F, Chen K. Ambient particulate matter and lung cancer incidence and mortality: a meta-analysis of prospective studies. Eur J Public Health. 2015;25(2):324–329. doi: 10.1093/eurpub/cku145 [DOI] [PubMed] [Google Scholar]
- 28.Cheng I, Yang J, Tseng C, et al. Traffic-related Air Pollution and Lung Cancer Incidence: The California Multiethnic Cohort Study. Am J Respir Crit Care Med. 2022;206(8):1008–1018. doi: 10.1164/rccm.202107-1770OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu S, Zhu W, Thompson P, Hannun YA. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 2018;9(1):3490. doi: 10.1038/s41467-018-05467-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakrar RM, Pennycuick A, Borg E, Janes SM. Preinvasive disease of the airway. Cancer Treat Rev. 2017;58:77–90. doi: 10.1016/j.ctrv.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 31.Stram DO, Park SL, Haiman CA, et al. Racial/Ethnic Differences in Lung Cancer Incidence in the Multiethnic Cohort Study: An Update. J Natl Cancer Inst. 2019;111(8):811–819. doi: 10.1093/jnci/djy206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 33.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; 2000. doi: 10.1007/978-1-4757-3294-8 [DOI] [Google Scholar]
- 34.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev. 2008;26(4):339–362. doi: 10.1080/10590500802494538 [DOI] [PubMed] [Google Scholar]
- 35.Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res Mol Mech Mutagen. 2005;592(1):119–137. doi: 10.1016/j.mrfmmm.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 36.Krimsky W, Muganlinskaya N, Sarkar S, et al. The changing anatomic position of squamous cell carcinoma of the lung – a new conundrum. J Community Hosp Intern Med Perspect. 2016;6(6): 10.3402/jchimp.v6.33299. doi: 10.3402/jchimp.v6.33299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung YE, Cho U, Lee KY. Peripheral type squamous cell carcinoma of the lung: clinicopathologic characteristics in comparison to the central type. J Pathol Transl Med. 2020;54(4):290–299. doi: 10.4132/jptm.2020.05.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomashefski JF, Connors AF, Rosenthal ES, Hsiue IL. Peripheral vs central squamous cell carcinoma of the lung. A comparison of clinical features, histopathology, and survival. Arch Pathol Lab Med. 1990;114(5):468–474. [PubMed] [Google Scholar]
- 39.Ostro B, Hu J, Goldberg D, et al. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ Health Perspect. 2015;123(6):549–556. doi: 10.1289/ehp.1408565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tayarani M, Rowangould G. Estimating exposure to fine particulate matter emissions from vehicle traffic: Exposure misclassification and daily activity patterns in a large, sprawling region. Environ Res. 2020;182:108999. doi: 10.1016/j.envres.2019.108999 [DOI] [PubMed] [Google Scholar]
- 41.Coleman NC, Burnett RT, Ezzati M, Marshall JD, Robinson AL, Pope CA. Fine Particulate Matter Exposure and Cancer Incidence: Analysis of SEER Cancer Registry Data from 1992–2016. Environ Health Perspect. 2020;128(10):107004. doi: 10.1289/EHP7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbone F, Bovenzi M, Cavallieri F, Stanta G. Cigarette smoking and histologic type of lung cancer in men. Chest. 1997;112(6):1474–1479. doi: 10.1378/chest.112.6.1474 [DOI] [PubMed] [Google Scholar]
- 43.Mukherjea A, Wackowski OA, Lee YO, Delnevo CD. Asian American, Native Hawaiian and Pacific Islander tobacco use patterns. Am J Health Behav. 2014;38(3):362–369. doi: 10.5993/AJHB.38.3.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Multiethnic Cohort investigators and institutions affirm their intention to share the research data consistent with all relevant NIH resource/data sharing policies. Data requests should be submitted through Multiethnic Cohort online data request system at https://www.uhcancercenter.org/for-researchers/mec-data-sharing.