Abstract
Normothermic ex vivo lung perfusion (EVLP) can resuscitate marginal lung allografts to increase organs available for transplantation. During normothermic perfusion, cellular metabolism is more active compared to subnormothermic perfusion, creating a need for an oxygen (O2) carrier in the perfusate. As an O2 carrier, red blood cells (RBCs) are a scarce resource and are susceptible to hemolysis in perfusion circuits, thus releasing cell-free hemoglobin (Hb), which can extravasate into the tissue space, thus promoting scavenging of nitric oxide (NO) and oxidative tissue damage. Fortunately, polymerized human Hb (PolyhHb) represents a synthetic O2 carrier with larger molecular diameter compared to Hb, preventing extravasation, and limiting adverse reactions. In this study, a next-generation PolyhHb-based perfusate was compared to both RBC and asanguinous perfusates in a rat EVLP model. During EVLP, the pulmonary arterial pressure and pulmonary vascular resistance were both significantly higher in lungs perfused with RBCs, which is consistent with RBC hemolysis. Lungs perfused with PolyhHb demonstrated greater oxygenation than those perfused with RBCs. Post-EVLP analysis revealed that the PolyhHb perfusate elicited less cellular damage, extravasation, iron tissue deposition, and edema than either RBCs or colloid control. These results show promise for a next-generation PolyhHb to maintain lung function throughout EVLP.
Keywords: Red Blood Cell Substitute, Hemoglobin Based Oxygen Carrier, Oxygen Therapeutic, Polymerized Human Hemoglobin, Normothermic Ex Vivo Lung Perfusion, Normothermic Machine Perfusion
INTRODUCTION
In the United States, there are almost 108,000 patients on the organ transplant waiting list, yet only 39,000 transplants were performed leading to 11,000 patients being removed from the waiting list due to death or sickness.1 A primary reason for the disparity is a limited number of available donors and a substantial portion of organs are deemed not viable for transplantation.2,3 More than 80% of donor lung allografts are declined due to poor organ quality.4,5 The best way to decrease the number of patients on the organ waiting list is to increase organ availability for transplantation, either through increasing donation and advocacy, or increasing donor organ yield through improvements in organ quality. One strategy to expand the donor pool requires evaluation and rehabilitation of organs from extended criteria donors (ECD); however, ECD grafts are associated with a higher risk of ischemia-reperfusion injury leading to primary graft dysfunction and reduced organ viability.6–8 Due to a rising demand for organ transplantation and a critical donor organ shortage, the need to fill this gap has increased the use of ECD and donation after cardiac death (DCD) organs for transplantation to lower patient mortality.9
Normothermic machine perfusion (NMP) has shown promise in organ storage and preservation to improve the viability of transplanted organs.10,11 Studies have shown that NMP can resuscitate ECD organs to pre-transplant quality comparable to non-marginal organs for subsequent transplantation.5,12 In the lung, NMP is more commonly known as ex vivo lung perfusion (EVLP). Perfusates used in EVLP typically have a colloid-based solution and may include red blood cells (RBCs).13–15 The addition of RBCs to perfusates has been established as clinical standard,13 as it provides the necessary oxygenation to maintain cellular metabolism, especially under normothermic conditions. Unfortunately, there is a limited supply of donated RBCs, and therefore allocation of these materials outside of emergency medicine should be limited. This provides an opportunity to apply a more readily available RBC substitute to EVLP, especially if the RBC substitute is synthesized from hemoglobin (Hb) purified from expired RBCs that are not fit for transfusion. By utilizing expired RBCs for the purpose of Hb purification, we can turn this waste product into an essential raw material to synthesize the RBC substitute.
Hemoglobin (Hb)-based O2 carriers (HBOCs) have shown promise as viable RBC substitutes, which utilize the native ability of Hb to carry and offload O2 to surrounding tissues.16,17 Of all the different types of HBOCs being studied, polymerized Hb (PolyHb) is an attractive HBOC, due its ability to be synthesized at a large scale.18 Previous attempts at developing commercialized PolyHbs have failed due to complications associated with vasoconstriction, high blood pressure, and cardiac arrest.17,19 These adverse events were the result of cell-free Hb and low molecular weight (MW) Hb polymers (< 500 kDa) in the HBOC formulation that extravasate out of the circulation into the tissue space, leading to nitric oxide (NO) scavenging and subsequent vasoconstriction, systemic hypertension, and oxidative tissue injury.20,21 Elimination of these low MW Hb species from the NMP perfusate are hypothesized to mitigate these deleterious side-effects for a product that has already shown promise in kidney and liver NMP.22–25
In this study, we sought to use a preclinically relevant, validated, lung DCD model and EVLP platform to assess the ability of a novel PolyhHb perfusate to meet organ metabolic demands and evaluate organ quality as compared to RBC and asanguinous perfusates.
MATERIALS AND METHODS
All procedures were humanely performed according to the NIH and the National Research Council’s Guide for the Humane Care and Use of Laboratory Animals and with the approval of The Ohio State University Institutional Animal Care and Use Committee (IACUC Protocol #2012A00000135-R2).
PolyhHb Synthesis and Purification
The PolyhHb described in this study was synthesized at the pilot scale as outlined in Cuddington et al.18 In summary, Hb was purified from expired human RBC units generously donated by Canadian Blood Services (Ottawa, Canada) using tangential flow filtration (TFF). In a 30 L bioreactor (Ace Glass, Vineland, NJ), 480 g of human Hb at 20 mg/mL was circulated and partially deoxygenated over a gas-liquid contactor (G420, 3M, Saint Paul, MN) with a nitrogen sweep gas, and subsequent bolus injection of sodium dithionite (Sigma Aldrich, St. Louis, MO). The material was then crosslinked at a 30:1 glutaraldehyde (GA, Sigma) to Hb molar ratio over 4 hours and quenched with a 7:1 molar ratio of sodium cyanoborohydride (Sigma) to GA. Following the reaction, the material was purified using a two-stage TFF system. The first stage consisted of a 0.2 μm polyethersulfone (PES) TFF module (Repligen, Waltham, MA), which removed large MW material and contaminants. The second stage consisted of a 500 kDa polysulfone (PS) TFF module (Repligen), where the material was excipient exchanged with a lactated Ringer’s solution (pH 7.4) to remove unreacted reagents, cell-free hHb, and low MW PolyhHb material. The final product was concentrated to 10 g/dL over the second stage module and stored at −80°C.
Perfusate Formulation
Perfusates were formulated using William’s E Medium (A12176–01, Gibco, ThermoFisher Scientific, Waltham, MA) as the primary colloid solution to a final volume of 165 mL. For the HBOC perfusate, PolyhHb was diluted to a final concentration of 3.7 ± 0.1 g/dL similar to previous HBOC NMP studies.23,26,27 25 wt% human serum albumin (HSA, OctaPharma Plasma) was added to achieve a final concentration of 3% HSA by weight. For the RBC-based perfusate, Sprague-Dawley rat RBCs (Innovative Research Inc, Novi, MI) were diluted to a final hematocrit of 15%, in line with clinical protocols.14,15 The asanguinous control consisted of 4% HSA in William’s media. The HBOC perfusate and asanguinous control were sterile filtered through a 0.2 μm membrane. The perfusates were brought to 37°C and pH 7.4 using tromethamine (Fisher Scientific, Pittsburgh, PA) buffer before beginning EVLP. The final perfusate compositions are shown below in Table 1. Each perfusate was prepared individually for each of the 6 perfusions from the same batch of PolyhHb.18
Table 1.
Biophysical properties of the various perfusates used in this study.
| Perfusate | [Hb] (g/dL) | [HSA] (wt%) | Osmolarity (mOsm) | Viscosity (cP) | COP (mm Hg) |
|---|---|---|---|---|---|
| PolyhHb | 3.7 ± 0.1 | 3.0 | 335 ± 11 | 3.3 ± 0.4 | 17.2 ± 0.4 |
| Colloid | N/A | 4.0 | 303 ± 10 | 1.6 ± 0.1 | 16.5 ± 0.1 |
| RBCs | 5.3 ± 0.2 | N/A | 291 ± 6 | 3.6 ± 0.2 | 2.0 ± 0.1 |
Protein Quantification
For both RBC and PolyhHb perfusates, the cyanomethemoglobin assay28 was used to determine total Hb concentration and percentage of methemoglobin (metHb). For cell-free Hb in RBC-based perfusates, the supernatant was analyzed following centrifugation. To determine the total Hb concentration in the pelleted RBCs, RBCs were lysed via several freeze thaw cycles followed by dilution in phosphate buffer (PB) (3.75 mM, pH 7.4). The lysed RBCs were centrifuged, and the supernatant assayed for the total Hb concentration.
Perfusate Biophysical Characterization
The MW distribution was estimated with size exclusion high performance liquid chromatography (SEC-HPLC) using an Acclaim SEC-1000 column (Thermo Scientific, Waltham, MA) on a Thermo Scientific UHPLC System. The Soret peak absorbance of Hb was monitored at 413 nm. Perfusate viscosity was measured at a shear rate of 160 s−1 using a DV3T-CP viscometer (Brookfield AMETEK, Middleboro, MA) with a CSA-40Z cup and spindle. Osmolarity was measured with a Gonotech 010 freezing point osmometer (Gonotech GmbH, Berlin, Germany) and colloid osmotic pressure (COP) was measured with a Wescor 4420 Colloid Osmometer (Wescor, Logan, UT).
Ex Vivo Lung Perfusion (EVLP)
Eighteen male Sprague-Dawley rats (n=6 per group, average body weight = 311.5 ± 55.2 g, average age = 84.2 ± 22.4 days) were purchased from Envigo (Indianapolis, IN) and housed under pathogen-free conditions at The Ohio State University Animal Facility. Detailed procedures to procure the heart-lung bloc and integrate the organ into the perfusion circuit have been reported in the literature.29 Briefly, rats were fully anesthetized by a ketamine (100 mg/kg, Zeotis, Parsippany-Troy Hills, NJ) and xylazine (10 mg/kg, Covertus, Portland, ME) injection via intraperitoneal injection to achieve an appropriate plane of anesthesia then shaved and positioned for lung procurement. The incision was started by cutting the abdomen to open the peritoneum. Heparin (100 IU/kg, Hospira, Lake Forest, IL) was injected via the inferior vena cava (IVC) and circulated for 10 minutes. Rats were then connected to the ventilator via tracheostomy and exsanguinated by cutting the IVC. The thoracic cavity was opened, and the pulmonary vein and left atrium were cannulated via transapical approach. The heart-lung bloc was removed from the chest cavity with the lung-no-touch technique29 before being connected to the ex vivo perfusion system (Figure 1, Harvard Apparatus, Holliston, MA).
Figure 1. Diagram of the EVLP circuit.

The sweep gas across the oxygenator is composed of 8%/6%/86% CO2/O2/N2. Samples were taken both before and after the lung over a period of 120 min. A heat exchanger maintains the perfusate reservoir and flow path at 37°C.
The perfusate was maintained at normothermia via an in-circuit heat exchanger and circulated over a gas contactor with a sweep gas composed of 8%/6%/86% CO2/O2/N2. Lungs were ventilated for 2 hours or until the pulmonary arterial pressure (PA pressure) exceeded 100 cm H2O. Ventilation was performed with ambient air at 60 bpm and tidal volume of 4 mL/kg of rat with a positive end expiratory pressure (PEEP) of 2 cm H2O. The perfusion flow rate was set at 20% of estimated cardiac output (75 mL/kg of rat).29–32 Perfusate samples entering and exiting the lung were collected every 30 minutes and snap frozen until analysis.
Post-EVLP Analysis
Lactate dehydrogenase (LDH) released into the perfusate was measured using a LDH cytotoxicity detection kit (Clontech Laboratories, Mountain View, CA) following the manufacturer’s instructions. The right inferior lobe was used for wet-to-dry ratio determination. The lobe was weighed immediately upon perfusion termination for wet weight, dried at 60°C for 48 hr, and then weighed again for the dry weight.
Lung Histology
Tissue Ferrozine Assay
Frozen lung samples were homogenized in 10 volume eq. of ddH2O. A 1 mL aliquot of homogenate was incubated (60 min., 50ºC) with 500 μL of a solution containing 1 M HCl (ACROS Organics, Geel, Belgium) and 10% trichloroacetic acid (Sigma-Aldrich). Samples were then centrifuged (15 min., 8,000g) and 375 μL of supernatant was aliquoted. To reduce ferric iron, 125 μL of 20 mg/mL ascorbic acid (Sigma) was added to the samples. To quantitate ferrous iron, 100 μL of a solution containing 1 mg/mL FerroZine™ (ACROS Organics) and 1.5 M sodium acetate (Sigma) was added to the sample. Samples were allowed to develop for 30 minutes, after which the absorbance at 562 nm was measured using a BioTek Synergy HTX plate reader (BioTek, Broadview, IL).
Immunohistochemistry
Paraffin embedded tissue blocks were prepared, and tissue was sectioned (5-micron thickness) by the University of Maryland Baltimore Histology Core. Sections were dewaxed and hydrated in graded ethanol percentages. Heat-mediated antigen retrieval was performed using pH 6.0 citrate buffer (Sigma). The solution was brought to a boil, and then allowed to cool for 30 min. Once cool, sections were incubated (60 min., RT) with 3% horse serum (Fisher). Sections were then incubated (overnight, 4ºC) with an antibody against the Hb α-chain (1:300, Abcam, Ab92492). A biotinylated secondary antibody was used for Hb α-chain staining, sections were incubated (30 min., RT) with (1:300, Invitrogen, 31820). Sections were then incubated (30 min., RT) with avidin and biotinylated horseradish peroxidase (VECTASTAIN® Elite® ABC Kit, Vector Laboratories), and incubated (3 min., RT) with 3,3’-diaminobenzidine and H2O2 (SIGMAFAST™, Sigma). Sections were then counterstained in hematoxylin (Gil no. 2, Fisher).
Bright Field Microscopy
All images were obtained using a Leica DM4-B TL bright field microscope (Leica, Wetzlar, Germany) and captured with LAS X software at 50× and 630× total magnification (final magnification = (objective magnification) × 10× (lens magnification)) using a 5× and 63× objective with oil emersion at 22.02 milliseconds of exposure. Scale bars represent 500 microns (50× final magnification) and 30 microns (630× final magnification). Tissues were stained by one individual, and imaged by a second individual who was blinded to the slide’s identification.
Statistical Analysis
All statistical analysis was done using RStudio (Version 1.4.1106, RStudio, Inc., Boston, MA) including packages ggpubr v0.6.0 and Rmisc v1.5.1. Analysis was performed using the ANOVA comparison of means with multiple comparisons performed using a t.test between sample groups. Significance was reported to an α value of 0.05. In all figures, an asterisk (*) is used to indicate significance between groups. A pound sign (#) is used to indicate significance within a group compared to its initial value.
RESULTS
Full biophysical characterization of the PolyhHb used in this study can be found in Cuddington et al.18 The total concentration of PolyhHb remained constant throughout the course of perfusion compared to the initial timepoint (p > 0.05), while the total concentration of Hb from intact RBCs in the RBC perfusate decreased significantly with time compared to the initial timepoint (p < 0.05) as shown in Figure 2A. Figure 2B shows the cell-free Hb concentration in the RBC perfusate increase initially, indicating hemolysis, but stabilized after 30 minutes; concentrations at all timepoints was statistically higher than the initial timepoint (p < 0.05). PolyhHb experienced slower auto-oxidation compared to the RBC perfusate (Figure 2C), with only a 2% increase in metHb throughout EVLP. However, this change was not statistically significant (p > 0.05). To validate that the PolyhHb was not extravasating, SEC-HPLC was run on the PolyhHb perfusate shown in Figure 2D, where the pre- and post-EVLP curves overlap perfectly.
Figure 2.

A) Concentration of total Hb and PolyhHb in RBC and HBOC-based perfusates, respectively, throughout EVLP. B) Concentration of cell-free Hb in the RBC perfusate. C) The percent change in perfusate metHb during EVLP. D) Representative SEC-HPLC chromatogram at 413 nm for the PolyhHb perfusate before and after perfusion. Hb, hemoglobin; PolyhHb, polymerized human hemoglobin; RBC, red blood cell; NEVLP, normothermic ex vivo lung perfusion; MetHb, methemoglobin. Significance was reported to an α value of 0.05. An asterisk (*) is used to indicate significance between groups. A pound sign (#) is used to indicate significance within a group compared to its initial value.
Figure 3A shows the amount of O2 that the lung delivered to the perfusion circuit. After 30 minutes of EVLP, lungs perfused with PolyhHb delivered significantly more O2 than lungs perfused with RBCs (p < 0.05). At 90 minutes of EVLP, PolyhHb perfused lungs delivered more O2 than those perfused with the colloid control (p < 0.05). Additionally, by 60 minutes, lungs perfused with RBCs and the control experienced a significant decrease in oxygenation compared to their initial values (p < 0.05) (Figure 3B). The lungs perfused with PolyhHb never experienced a statistically significant (p > 0.05) decrease in oxygenation compared to their initial timepoint.
Figure 3.

A) Partial pressure of O2 (pO2) of the perfusate exiting the lung. B) Amount of O2 delivered to the perfusate by the lung. C) Change in the partial pressure of CO2 (pCO2) of the perfusate across the lung, or the Delta PaO2. PolyhHb, polymerized hemoglobin; RBC, red blood cell. Significance was reported to an α value of 0.05. An asterisk (*) is used to indicate significance between groups. A pound sign (#) is used to indicate significance within a group compared to its initial value.
In addition to facilitating O2 delivery, lungs are responsible for carbon dioxide (CO2) clearance. Figure 3C shows the change in CO2 tension across the heart-lung bloc over time. There was no significant (p > 0.05) difference between groups after the initial time point; however, both the PolyhHb and colloid control perfusates exhibited lessened CO2 clearance after 60 minutes (p < 0.05) compared to the initial timepoint. However, the RBC perfusate did not experience this decline (p > 0.05). The colloid control was the only perfusate that saw a reduction in both O2 and CO2 gas exchange properties.
Beyond gas exchange, organ health on the circuit can be evaluated by monitoring physiological parameters throughout EVLP. PA pressure is a critical metric of lung function, and PA pressures above 98 cm H2O is a disqualifying event for continuation of EVLP. All three perfusates had an increase in PA pressure by 60 minutes (Figure 4A). Lungs perfused with RBCs experienced significantly (p < 0.05) higher PA pressures, with three lungs being removed from the circuit after 60 minutes upon exceeding 98 cm H2O.
Figure 4.

A) PA pressure over the course of EVLP. B) PVR over time. PA, pulmonary artery; PolyhHb, polymerized human hemoglobin; RBC, red blood cell; PVR, pulmonary vascular resistance. Significance was reported to an α value of 0.05. An asterisk (*) is used to indicate significance between groups. A pound sign (#) is used to indicate significance within a group compared to its initial value.
The trends displayed by the PA pressure were mimicked by the pulmonary vascular resistance (PVR) (Figure 4B). In both cases, the lungs perfused with RBCs exhibited higher physiological responses compared to lungs perfused with either PolyhHb (p < 0.05) or the control (p < 0.05). Additionally, RBC perfused lungs were the only group to increase significantly (p < 0.05) from the initial timepoint.
The change in lung weight over time is shown in Figure 5A. For RBCs, the lung weight spikes at 60 min skewed by the three non-viable lungs. Lungs perfused with PolyhHb or the control never displayed a significant (p > 0.05) increase in lung weight. The wet-to-dry ratio of the right inferior lobe is shown in Figure 5B. PolyhHb perfused lungs accumulated significantly (p < 0.05) less edema than lungs perfused with either RBCs or the asanguinous control. Despite other poor metrics, lungs perfused with RBCs did not accumulate significantly (p > 0.05) more edema than lungs perfused with the colloid control.
Figure 5.

A) Change in lung weight over time. B) The wet/dry ratio for the three perfusates. PolyhHb, polymerized human hemoglobin; RBC, red blood cell. Significance was reported to an α value of 0.05. An asterisk (*) is used to indicate significance between groups.
Figure 6 shows the change in LDH during EVLP. The PolyhHb perfusate was the only group to not have significantly (p > 0.05) higher LDH release compared to the 30-minute timepoint. PolyhHb also demonstrated significantly less LDH release compared to the RBC perfusate by 60 minutes (p < 0.05) and the control perfusate by 90 minutes (p < 0.05).
Figure 6.
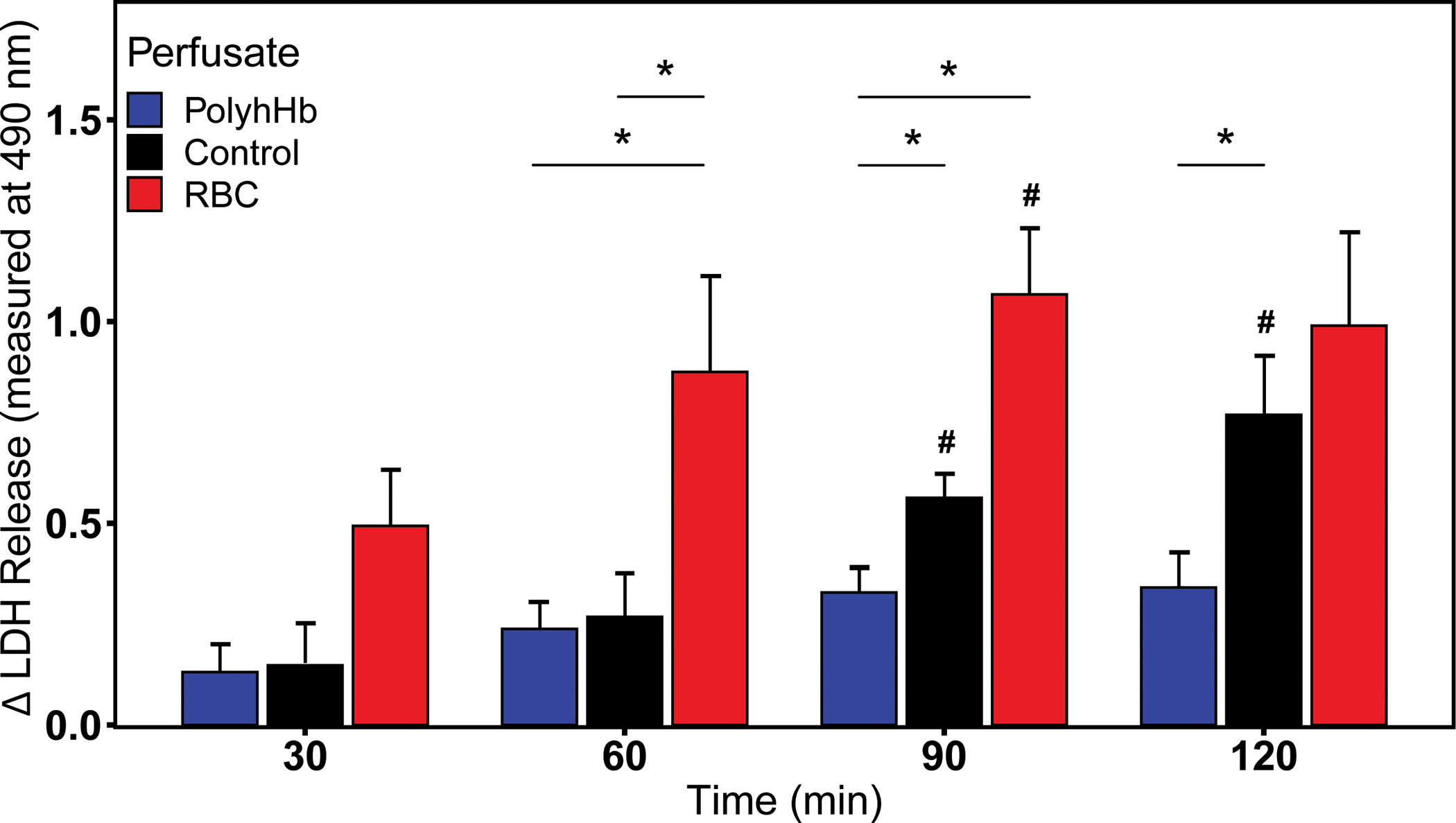
The change in LDH level of the various perfusates during the perfusion. Differences are in optical density (OD) at 490 nm. LDH, lactate dehydrogenase; PolyhHb, polymerized human hemoglobin; RBC, red blood cell. Significance was reported to an α value of 0.05. An asterisk (*) is used to indicate significance between groups. A pound sign (#) is used to indicate significance within a group compared to its initial value.
Lung tissue iron was quantified as a parameter of Hb degradation following perfusion. RBC perfused tissue retained visually greater erythrocytes than control or PolyhHb perfused lungs prior to tissue homogenization and FerroZine™ assay analysis. Based on this assay, RBC lung perfusion resulted in an iron concentration of 1.63 ± 0.540 μg/gram of lung tissue. RBC perfused tissue contained residual iron concentrations that were significantly greater than both PolyhHb (p < 0.05) and control (p < 0.05) perfusions (Figure 7A).
Figure 7. Analysis of total lung iron and Hb localization.

(A) Lung tissue iron as measured by the ferrozine assay shows the mean ± S.D. for total iron quantified from tissue perfused with PolyhHb, control and RBCs (n=6/group). (B) Hb immunohistochemistry of lung tissue sections after perfusion with PolyhHb at (a) 5× and (b) 63× objective magnification (50x and 630x final magnification, final magnification = 10x lens x objective (5x or 63x) showing adventitia α-Hb immunoreactivity (black asterisk). Lung tissue perfusion with the control is shown in (c) at 50× final magnification and (d) at 630× final magnification. Lung tissue perfused with RBCs is shown in (e) at 50× final magnification indicating adventitia α-Hb immunoreactivity (black asterisk) and (f) 630× final magnification with vessel lumen α-Hb immunoreactivity localized to the endothelium (white asterisks). Scale bars represent 500 microns (50× magnification, images b, d, f) and 30 microns (63× objective magnification, images a, c, e). Note, scale bars shown on first images for 50x and 630x final magnification apply to all images their rows, respectively. All data is represented as mean ± S.D. for n=6 biological replicates per group. Statistical analysis was performed using a One- way ANOVA with a Holm-Sidak’s multiple comparisons test based on a normal distribution of data (Kolmogorov-Smirnov test). Significance was set at a p < 0.05, (*). All analyses were performed using GraphPad Prism 9.1.1 (San Diego, California).
For all images, regions of interest correspond to representative vessels in tissue sections from each group. In these sections, Hb from RBCs or PolyhHb would be expected to accumulate within the intravascular lumen or regions (adventitial-vasculature and macrophages) surrounding pre- and post-capillary pulmonary vessels. Immunohistochemistry, specific to the α-Hb chain showed minimal immunoreactivity in PolyhHb perfused tissue at 5× objective magnification (50× final magnification), but adventitial α-Hb immunoreactivity (*) was observed at 63× objective magnification (630× final magnification), Figure 7 B, a and b, respectively. Control perfused tissue did not reveal visual α-Hb immunoreactivity within or around vessels at 5× or 63× magnification, shown in Figure 7 B, c and d, respectively. RBC perfused tissue showed diffuse α-Hb immunoreactivity within the vascular lumen of multiple vessels (*) at 50× final magnification and at 630× final magnification near the vascular endothelium Figure 7 B, e and d, respectively.
DISCUSSION
There is a significant disparity between the number of patients waiting for organ transplants and the number of organs available and deemed viable for transplant.1–3 Normothermic EVLP is one approach that focuses on expanding the donor pool by facilitating assessment and rehabilitation of lungs for subsequent transplantation. This work reports on the application of an HBOC in EVLP as an alternative to RBCs when blood is in limited supply or unavailable.
HBOCs are an attractive alternative to RBCs in perfusion circuits since they can effectively deliver O2 to tissues to maintain metabolic functions crucial to graft viability.16,17 The HBOC presented in this work, PolyhHb, was designed to be optimal for EVLP, including a moderate oxygen affinity,18 large MW,18 low auto-oxidation rate,18 and extended shelf life compared to donated RBCs.33 The use of an HBOC in organ perfusion may facilitate better usage of donated RBC units for emergency medicine. Hence, this study compared the performance of a PolyhHb-based perfusate with that of RBC and colloid perfusates in rat EVLP.
The first major consideration of any perfusate used in EVLP is the material’s stability. In this study, rat RBCs were prone to hemolysis. This was observed in Figures 2A and B with a decrease in the total Hb contained within intact RBCs and corresponded with a spike in acellular Hb. Despite maintaining a steady concentration of cell-free Hb in the perfusate after 30 minutes, this was not due to a decrease in hemolysis, but rather indicative of cell-free Hb extravasation into the tissue space, confirmed with iron staining in Figure 7. The presence of acellular Hb is problematic as Hb scavenges NO resulting in vasoconstriction, systemic hypertension, and oxidative tissue injury.20,21 These adverse effects were observed during EVLP as the PA pressure increased significantly for the RBC group compared to the other perfusate solutions.
In contrast, the PolyhHb-based perfusate demonstrated improved stability in the EVLP circuit compared to the RBC perfusate. Unlike the cell-free Hb released from lysed RBCs, there was no evidence of PolyhHb tissue extravasation as there was no decrease in perfusate PolyhHb concentration during perfusion, no presence of iron in lung tissue, or increase in PA pressure. In addition, Figure 2D shows complete overlap of HPLC elution curves of the perfusate before and after EVLP, demonstrating the stability of the PolyhHb during perfusion.
Another marker of stability for perfusates containing Hb, is auto-oxidation of Hb into its’ non-O2 carrying equivalent metHb. Reduced auto-oxidation of PolyhHb would facilitate extended perfusions and reduce the rate of production of reactive oxygen species.34 The PolyhHb used in this study exhibited lower auto-oxidation rates compared to acellular Hb and previous generations of commercial HBOCs,18 overcoming a major shortcoming of commercial HBOCs seen in prior applications of NMP.35,36 The low auto-oxidation rate of the PolyhHb was confirmed with a minimal increase in metHb, comparable to that of cell-free Hb derived from the RBC perfusates, thus further contributing to the stability of PolyhHb for EVLP.
In addition to maintaining the stability of the perfusate, improving the health of the allograft is the goal of EVLP. The organ metrics monitored in this study indicate graft performance and viability by demonstrating the efficacy of gas exchange and presence of cellular stress. PolyhHb is an attractive perfusate option because it utilizes Hb’s native ability to bind and release O2 and other gaseous ligands without the cytotoxicity associated with acellular Hb. The pO2 of the post-bloc perfusate demonstrated the graft’s ability to supply O2 to the system. Lungs perfused with PolyhHb had a higher post-bloc pO2 and sustained O2 delivery over EVLP (p > 0.05) when compared to RBCs, indicating improved graft health and function.
Cellular stress was monitored throughout EVLP via LDH release and changes in lung weight. LDH production is a consequence of aerobic glycolysis37 in response to trauma or inflammation38 and may indicate perfusion failure. Monitoring LDH production, allows discernment of cellular stress and to what capacity. Throughout EVLP, the control and RBC-based perfusates had a significant increase in LDH compared to PolyhHb (p < 0.05). This increase in stress ultimately lead to increases in lung weight and edema formation. Performing EVLP with PolyhHb as a perfusate elicited less cellular stress and cell death compared to either RBC or asanguinous perfusates, which may ultimately increase available ECD lungs for successful transplantation.
CONCLUSION
This study used a novel PolyhHb in EVLP to support organ integrity. Traditionally, EVLP has been performed with either a colloid or RBC-based perfusate. Both options have shortcomings in preserving graft viability during EVLP. Previous generations of PolyhHbs have caused detrimental side-effects due to the presence of cytotoxic cell-free Hb and low MW Hb polymers. Improvements to the synthesis of PolyhHb yields a product that is less likely to elicit the deleterious effects observed in previous generations of PolyhHbs. Our PolyhHb demonstrated improved lung oxygenation and overall graft health by eliciting less edema, extravasation, iron deposition, and cellular damage. PolyhHb opens the possibility of performing EVLP without the need for RBCs while still meeting the metabolic demand of DCD lung grafts.
Conflicts of Interest and Source of Funding:
Dr. Whitson is partially supported through National Institutes of Health (NIH) National Heart Lung and Blood Institute grant R01HL143000. Dr. Palmer is supported through NIH grants R01HL126945, R01EB021926, R01HL131720, and R01HL138116 and U.S. Army Medical Research and Materiel Command grant W81XWH1810059. Dr. Black is supported through NIH grant R01DK123475. This research was generously supported through The Jewel and Frank Benson Family Endowment and The Jewel and Frank Benson Research Professorship. A patent application was filed on the polymerized hemoglobin described in this work.
REFERENCES
- 1.Organ Procurement & Transplantation Network (OPTN) Metrics Dashboard:, 2020. Available at: https://insights.unos.org/OPTN-metrics/. Accessed March 30, 2023.
- 2.2019 Annual Report:, 2019.
- 3.OPTN/SRTR 2019 Annual Data Report: Introduction American Journal of Transplantation 21: 11–20, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER: Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation The Journal of Heart and Lung Transplantation 19: 1199–1204, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Liu M, et al. : Normothermic Ex Vivo Lung Perfusion in Clinical Lung Transplantation New England Journal of Medicine 364: 1431–1440, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Boteon YL, Afford SC: Machine perfusion of the liver: Which is the best technique to mitigate ischaemia-reperfusion injury? World J Transplant 9: 14–20, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akande O, Chen Q, Toldo S, Lesnefsky EJ, Quader M: Ischemia and reperfusion injury to mitochondria and cardiac function in donation after circulatory death hearts - An experimental study PLoS One 15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CF: Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury Tzu Chi Med J 30: 209–215, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israni AK, Zaun D, Rosendale JD, Schaffhausen C, Mckinney W, Snyder JJ: OPTN/SRTR 2019 Annual Data Report: Deceased Organ Donors American Journal of Transplantation 21: 567–604, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Whitson BA, Black SM: Organ assessment and repair centers: The future of transplantation is near World J Transplant 4: 40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockmann J, Reddy S, Coussios C, et al. : Normothermic perfusion: A new paradigm for organ preservation Ann Surg 250: 1–6, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q, Huang S, Wang D, et al. : Does ischemia free liver procurement under normothermic perfusion benefit the outcome of liver transplantation? Ann Transplant 23: 258–267, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warnecke G, Raemdonck D Van, Smith MA, et al. : Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation phase 3 study 6: 357–367, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjöberg T: Transplantation of lungs from non–heart-beating donors after functional assessment ex vivo Ann Thorac Surg 76: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Machuca TN, Cypel M, Keshavjee S: Advances in Lung Preservation Surgical Clinics of North America 93: 1373–1394, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Shonaka T, Matsuno N, Obara H, et al. : Impact of human-derived hemoglobin based oxygen vesicles as a machine perfusion solution for liver donation after cardiac death in a pig model PLoS One 14, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Palmer AF: Hemoglobin-based oxygen carrier and convection enhanced oxygen transport in a hollow fiber bioreactor Biotechnol Bioeng 102: 1603–1612, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Cuddington CT, Wolfe SR, Belcher DA, et al. : Pilot scale production and characterization of next generation high molecular weight and tense quaternary state polymerized human hemoglobin Biotechnol Bioeng 119: 3447–3461, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore EE, Moore FA, Fabian TC, et al. : Human Polymerized Hemoglobin for the Treatment of Hemorrhagic Shock when Blood Is Unavailable: The USA Multicenter Trial J Am Coll Surg 208: 1–13, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Bucci E, Kwansa H, Koehler RC, Matheson B: Development of zero-link polymers of hemoglobin, which do not extravasate and do not induce pressure increases upon infusion Artificial Cells, Blood Substitutes, and Biotechnology 35: 11–18, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaer CA, Deuel JW, Schildknecht D, et al. : Haptoglobin Preserves Vascular Nitric Oxide Signaling during Hemolysis Am J Respir Crit Care Med 193: 1111–1122, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matton APM, Burlage LC, van Rijn R, et al. : Normothermic machine perfusion of donor livers without the need for human blood products Liver Transplantation 24: 528–538, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahboub P, Aburawi M, Karimian N, et al. : The efficacy of HBOC-201 in ex situ gradual rewarming kidney perfusion in a rat model Artif Organs 44: 81–90, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laing RW, Bhogal RH, Wallace L, et al. : The Use of an Acellular Oxygen Carrier in a Human Liver Model of Normothermic Machine Perfusion Transplantation 101: 2746–2756, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharjee RN, Patel SVB, Sun Q, et al. : Renal Protection Against Ischemia Reperfusion Injury: Hemoglobin-based Oxygen Carrier-201 Versus Blood as an Oxygen Carrier in Ex Vivo Subnormothermic Machine Perfusion Transplantation 104: 482–489, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Beal EW, Kim JL, Reader BF, et al. : [D-Ala 2, D-Leu 5 ] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion Journal of Surgical Research 241: 323–335, 2019 [DOI] [PubMed] [Google Scholar]
- 27.White CW, Hasanally D, Mundt P, et al. : A whole blood-based perfusate provides superior preservation of myocardial function during ex vivo heart perfusion Journal of Heart and Lung Transplantation 34: 113–121, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Drabkin DL, Austin JH: SPECTROPHOTOMETRIC STUDIES II. PREPARATIONS FROM WASHED BLOOD CELLS; NITRIC OXIDE HEMOGLOBIN AND SULFHEMOGLOBIN Journal of Biological Chemistry 112: 51–65, 1935 [Google Scholar]
- 29.Nelson K, Bobba C, Eren E, et al. : Method of Isolated Ex Vivo Lung Perfusion in a Rat Model: Lessons Learned from Developing a Rat EVLP Program Journal of Visualized Experiments, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik AB, Kaplan JE, Saba TM: Reference sample method for cardiac output and regional blood flow determinations in the rat J Appl Physiol 40: 472–475, 1976 [DOI] [PubMed] [Google Scholar]
- 31.Davies B, Morris T: Physiological parameters in laboratory animals and humans Pharm Res 10: 1093–1095, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Eren E, Black SM, Reader BF, et al. : Novel Polymerized Human Serum Albumin For Ex Vivo Lung Perfusion ASAIO Journal, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams AT, Lucas A, Muller CR, et al. : Resuscitation From Hemorrhagic Shock With Fresh and Stored Blood and Polymerized Hemoglobin Shock 54: 464–473, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balaji SN, Trivedi V: Extracellular Methemoglobin Mediated Early ROS Spike Triggers Osmotic Fragility and RBC Destruction: An Insight into the Enhanced Hemolysis During Malaria Indian Journal of Clinical Biochemistry 27: 178–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng F, Kassa T, Jana S, et al. : Comprehensive Biochemical and Biophysical Characterization of Hemoglobin-Based Oxygen Carrier Therapeutics: All HBOCs Are Not Created Equally Bioconjug Chem 29: 1560–1575, 2018 [DOI] [PubMed] [Google Scholar]
- 36.de Vries Y, Matton APM, Nijsten MWN, et al. : Pretransplant sequential hypo- and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin-based oxygen carrier perfusion solution American Journal of Transplantation 19: 1202–1211, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberti M V, Locasale JW: The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41: 211–218, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Certo M, Tsai C-H, Pucino V, Ho P-C, Mauro C: Lactate modulation of immune responses in inflammatory versus tumour microenvironments Nat Rev Immunol 21: 151–161, 2021 [DOI] [PubMed] [Google Scholar]


