Abstract
Background
The psychological well‐being of lung cancer patients is critical in‐patient care but frequently overlooked.
Methods
This study, employing a cross‐sectional, questionnaire‐based design, aimed to elucidate the prevalence of depressive and anxiety symptoms among lung cancer patients and identify associated risk factors. Participants' demographic, medical history, disease stage, and pathology were systematically collected. Psychological assessment was conducted using the general anxiety disorder‐7 (GAD‐7), patient health questionnaire‐9 (PHQ‐9), and hospital anxiety and depression scale (HADS). Statistical analyses were performed using SPSS software (version 25.0).
Results
Out of 294 distributed questionnaires, 247 lung cancer patients were included in the final analysis, with an average completion time of 9.08 min. Notably, 32.4% exhibited depressive symptoms, while 30% displayed signs of anxiety. A significant correlation was found between both depressive and anxiety symptoms and a history of tobacco and alcohol consumption. Specifically, increased nicotine dependence and greater cumulative tobacco use were linked to higher rates of depressive symptoms, whereas cumulative alcohol consumption was associated with increased risks of anxiety symptoms.
Conclusion
The study affirms the feasibility of GAD‐7, PHQ‐9, and HADS as screening tools for depressive and anxiety symptoms in lung cancer patients. It further highlights tobacco and alcohol consumption as significant risk factors for poor psychological health in this population.
Keywords: anxiety, depression, lung cancer, psychology, Psycho‐Oncology
We use a self‐assessment questionnaire to screen patients with lung cancer for depressive and anxiety symptoms. About one third of patients were with depressive or anxiety symptoms. Smoking and drinking were risk factors for the psychological symptoms.
INTRODUCTION
Patients with cancer are significantly more susceptible to psychological distress than the general population. Studies reveal that the prevalence of major depression is approximately 14.9%, which is in stark contrast to the 6.7% prevalence found within the general US population. 1 , 2 Anxiety disorders are also notably prevalent among cancer patients, with an average reported rate of 10.3%. 1 Such levels of distress can adversely affect treatment adherence and overall outcomes. 3 , 4 , 5 , 6 Recognizing and managing psychosocial distress through screening and intervention is thus recommended throughout the treatment process. 7 However, the practical application of psychological care, from screening to treatment and follow‐up, often falls short. Surveys have indicated that stress screening is conducted in fewer than half of U.S. healthcare institutions, 8 and the majority of patients experiencing distress do not receive the mental health care they require. 9 , 10 , 11 Even when psychosocial care is provided, follow‐up is frequently lacking. 12 To bridge this gap, the integration of systematic screening into daily clinical practice is crucial. In this study, we attempted to assess the feasibility of using the general anxiety disorder 7 (GAD‐7), patient health questionnaire PHQ‐9 and the hospital and depression scale (HADS) as screening methods. We also discovered the risk factors for depression and anxiety in lung cancer patients.
METHODS
Participants
The study cohort comprised patients attending the inpatient thoracic cancer department at the Cancer Hospital, Chinese Academy of Medical Sciences, from February 1 to June 30, 2022. An inclusive approach was adopted, inviting participants from both conventional and specialist clinics. The clinic annually serves approximately 1000 patients; however, due to the COVID‐19 pandemic, patient attendance decreased by an estimated 20% in 2022. Consequently, 300 patients were solicited for the study.
Eligibility criteria included: (1) being 18 years of age or older, (2) a confirmed cytological or pathological diagnosis of primary bronchial lung cancer encompassing both small cell and non‐small cell lung cancer across all stages, (3) consent to information collection, and (4) the capacity to complete the questionnaire. Patients were excluded if they (1) had pre‐existing mental health diagnoses prior to their lung cancer diagnosis, or (2) had confirmed brain metastases that may induce mental symptoms. The recruitment process is detailed in Figure 1.
FIGURE 1.
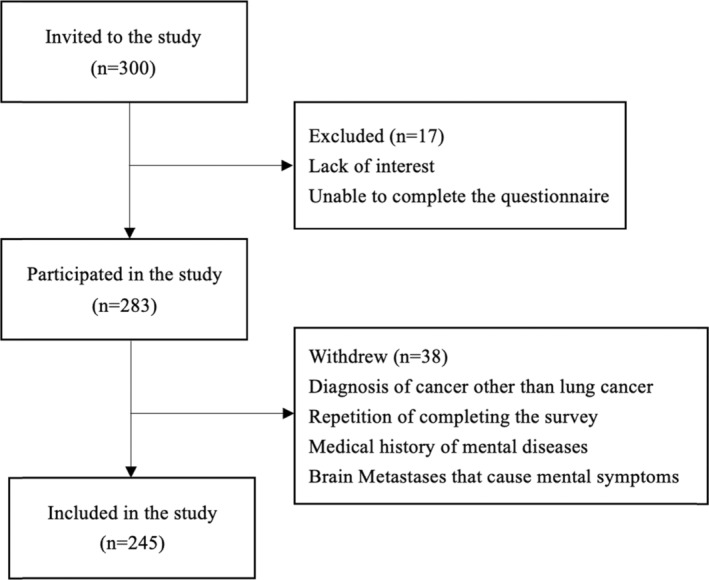
Flow chart of participant recruitment.
Design of questionnaire
Data were gathered via an electronic questionnaire which included a consent form. The questionnaire captured: (1) basic demographic information such as age, height, weight, and educational level, (2) personal medical history including depression, diabetes, hypertension, (3) details of lung cancer pathology and stage and (4) a history of tobacco and alcohol use, cumulative consumption levels, and nicotine dependence as measured by the Fagerström test for nicotine dependence (FNTD). 13 , 14 Anxiety and depressive level were assessed by general anxiety disorder 7 (GAD‐7).
Levels of anxiety and depression were evaluated using the general anxiety disorder‐7 (GAD‐7), 15 patient health questionnaire‐9 (PHQ‐9) 16 and hospital anxiety and depression scale (HADS). 17 These instruments are widely recognized and validated as effective screening tools in both the general population and among individuals with cancer and other physical ailments. 18 , 19 , 20 , 21 , 22 , 23 , 24 The questionnaire used for the study is appended.
Statistical analysis
Statistical analyses were performed using SPSS software version 25.0, with a p‐value of ≤0.05 denoting statistical significance. Descriptive statistics were computed for the baseline characteristics.
For the identification of participants with anxiety and depressive symptoms, a GAD‐7 score of ≥7 or a HADS‐anxiety score of ≥8 was indicative of anxiety symptoms, while a PHQ‐9 score of ≥10 or a HADS‐depression score of ≥8 suggested depressive symptoms. These cutoff values were selected based on their demonstrated optimal balance of sensitivity and specificity in previous studies. 19 , 21 , 22 , 25
Comparative analyses of categorical variables between participants with and without anxiety or depressive symptoms were performed using Pearson's chi‐squared test. Continuous variables were analyzed using the Kruskal‐Wallis H test, the Mantel–Haenszel test for linear trends, and Kendall's tau‐b correlation analysis.
RESULTS
Out of 300 patients approached, 283 (94.3%) completed the questionnaire, with an average completion time of 9.08 min; 192 (67.80%) finished within 10 min. The demographic and clinical characteristics of the 245 study participants included: a predominance of males (72%), over half with education below high school level (52.7%), a quarter with a previous malignancy (24.9%), the majority diagnosed with non‐small cell lung cancer (84.9%), and malignancy presenting at stages III to IV (84.5%). Notable lifestyle factors were a history of alcohol (20.8%) and tobacco use (31.0%). These details are summarized in Table 1.
TABLE 1.
Patient characteristics.
| Frequency | Percent (%) | ||
|---|---|---|---|
| Gender | Male | 177 | 72 |
| Education | <High school | 129 | 52.7 |
| High school | 67 | 27.3 | |
| >High school | 49 | 20.0 | |
| Medical History of Malignancy | Yes | 61 | 24.9 |
| Family history of depression | Yes | 2 | 0.8 |
| No | 241 | 98.4 | |
| Unknown | 2 | 0.8 | |
| Histology | Small cell | 32 | 13.1 |
| Non‐small cell | 208 | 84.9 | |
| Undiagnosed | 1 | 0.4 | |
| Unknown | 1 | 0.4 | |
| Stage | I | 12 | 4.9 |
| II | 12 | 4.9 | |
| III | 117 | 47.8 | |
| IV | 90 | 36.7 | |
| Undiagnosed | 4 | 1.6 | |
| Unknown | 5 | 2.0 | |
| Medical history of diabetes | Yes | 23 | 9.4 |
| Medical history of hypertension | Yes | 47 | 19.2 |
| Medical history of craniocerebral injury | Yes | 4 | 1.6 |
| Medical history of epilepsy | Yes | 2 | 0.8 |
| Medical history of periodic paralysis | Yes | 0 | 0 |
| Medical history of cardiovascular disease | Yes | 22 | 9.0 |
| Medical history of metabolic and endocrine diseases | Yes | 17 | 6.9 |
| Obesity (BMI > =28) | Yes | 25 | 10.2 |
| History of liquor use | Never or Seldom | 196 | 79.2 |
| Liquor abuse before diagnosis (used to drink liquor >100 g/day, 3 days/week) | 51 | 20.8 | |
| History of smoking | Never smoker | 158 | 63.7 |
| Used to smoke | 76 | 31.0 | |
| Smoking | 13 | 5.3 | |
| Nicotine dependence level calculated with FTND | Very low | 18 | 7.3 |
| Low | 27 | 11.0 | |
| Moderate | 9 | 3.7 | |
| High | 24 | 9.8 | |
| Very high | 11 | 4.5 | |
| Anxiety level calculated by GAD7 | Minimal anxiety | 194 | 79.2 |
| Mild anxiety | 40 | 16.3 | |
| Moderate anxiety | 4 | 1.6 | |
| Moderate to severe anxiety | 4 | 1.6 | |
| Severe anxiety | 3 | 1.2 | |
| Depression level calculated by PHQ‐9 | Minimal depression | 159 | 64.9 |
| Mild depression | 53 | 21.6 | |
| Moderate depression | 21 | 8.6 | |
| Severe depression | 12 | 4.9 |
Abbreviations: BMI, body mass index; FTND, Fagerstrom test for nicotine dependence.
Prevalence of psychological symptoms
A significant proportion of the cohort displayed psychological symptoms, with 32.4% exhibiting depressive symptoms and 30% showing signs of anxiety. A notable overlap was observed, with 62.4% of patients with anxiety also identified with depressive symptoms (p < 0.0001).
Univariate analysis of psychological symptoms
Univariate analysis revealed several risk factors for depressive symptoms: small cell lung cancer pathology (OR = 2.71, p = 0.010), stage IV disease (OR = 2.09, p = 0.011), and histories of diabetes (OR = 2.50, p = 0.032), hypertension (OR = 3.74, p < 0.0001), alcohol (OR = 5.19, p < 0.0001), and tobacco use (OR = 3.31, p < 0.0001) (Table 2).
TABLE 2.
Univariate analysis of factors in depressive symptoms.
| Variable | Participants with depressive symptoms | Participants without depressive symptoms | X2 | p‐value |
|---|---|---|---|---|
| Gender | 0.650 | 0.258 | ||
| Male | 60 | 117 | ||
| Female | 19 | 49 | ||
| Obesity (BMI > 28) | 0.002 | 0.965 | ||
| Yes | 8 | 17 | ||
| No | 71 | 149 | ||
| Pathological type | 7.035 | 0.010* | ||
| Small cell lung cancer (SCLC) | 17 | 15 | ||
| Non‐small cell lung cancer (NSCLC) | 61 | 147 | ||
| Stage | 6.829 | 0.011* | ||
| Stage I–III | 38 | 103 | ||
| Stage IV | 39 | 51 | ||
| History of malignancy | 3.046 | 0.057 | ||
| Yes | 25 | 36 | ||
| No | 54 | 130 | ||
| History of diabetes | 4.534 | 0.032* | ||
| Yes | 12 | 11 | ||
| No | 67 | 155 | ||
| History of hypertension | 16.644 | <0.0001* | ||
| Yes | 27 | 20 | ||
| No | 52 | 146 | ||
| History of liquor use | 27.046 | <0.0001* | ||
| Yes | 32 | 19 | ||
| No | 47 | 147 | ||
| History of tobacco use | 18.469 | <0.0001* | ||
| Yes (including used to smoke and smoking) | 44 | 44 s | ||
| No | 35 | 122 |
Note. Analogously, anxiety symptoms were associated with small cell lung cancer (OR = 4.12, p < 0.0001), stage IV disease (OR = 2.72, p = 0.011), and history of malignancy (OR = 4.22, p < 0.0001), diabetes (OR = 2.85, p = 0.016), hypertension (OR = 2.80, p = 0.002), alcohol (OR = 3.57, p < 0.0001), and tobacco use (OR = 3.52, p < 0.0001) (Table 3).
Abbreviations: BMI, body mass index; OR, odds ratio.
TABLE 3.
Univariate analysis of factors in anxiety symptoms.
| Variable | Participants with anxiety symptoms | Participants without anxiety symptoms | X2 | p‐value |
|---|---|---|---|---|
| Gender | 0.100 | 0.760 | ||
| Male | 52 | 125 | ||
| Female | 21 | 47 | ||
| Obesity (BMI > 28) | 0.051 | 1.000 | ||
| Yes | 7 | 18 | ||
| No | 66 | 154 | ||
| Pathological type | 14.405 | <0.0001* | ||
| Small cell lung cancer (SCLC) | 19 | 13 | ||
| Non‐small cell lung cancer (NSCLC) | 54 | 154 | ||
| Stage | 11.908 | 0.001* | ||
| Stage I–III | 30 | 111 | ||
| Stage IV | 38 | 52 | ||
| History of malignancy | 23.231 | <0.0001* | ||
| Yes | 33 | 28 | ||
| No | 40 | 144 | ||
| History of diabetes | 5.964 | 0.018* | ||
| Yes | 12 | 11 | ||
| No | 61 | 161 | ||
| History of hypertension | 9.961 | 0.002 | ||
| Yes | 23 | 24 | ||
| No | 50 | 148 | ||
| History of liquor use | 16.177 | <0.0001* | ||
| Yes | 27 | 24 | ||
| No | 46 | 148 | ||
| History of tobacco use | 19.688 | <0.0001* | ||
| Yes (including used to smoke and smoking) | 42 | 47 | ||
| No | 31 | 125 |
History of tobacco use and dependence on nicotine were positively correlated with severity of depressive symptoms
A history of tobacco use and nicotine dependence were significantly associated with depressive symptoms (OR = 3.31, p < 0.0001, Figure 2c). To verify the connection between tobacco use and depressive symptoms, we compared the distribution of PHQ‐9 score of three groups of never smokers, participants who used to smoke and were now smoking using the Kruskal‐Wallis H test, whose results identified substantial differences in PHQ‐9 scores among never smokers and former smokers (H = 72.112, p < 0.001), as presented in Figure 3a.
FIGURE 2.
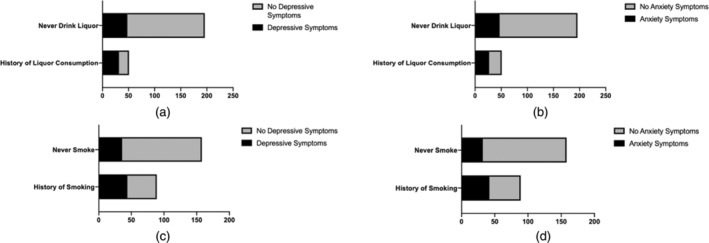
The association of history of liquor/tobacco consumption and depressive/anxiety symptoms. (a) History of liquor consumption was a risk factor for depressive symptoms (OR = 5.19, p < 0.0001). (b) History of liquor consumption was a risk factor for anxiety symptoms (OR = 3.57, p < 0.0001). (c) History of smoking was a risk factor for depressive symptoms (OR = 3.31, p < 0.0001). (d) History of smoking was a risk factor for anxiety symptoms (OR = 3.52, p < 0.0001).
FIGURE 3.
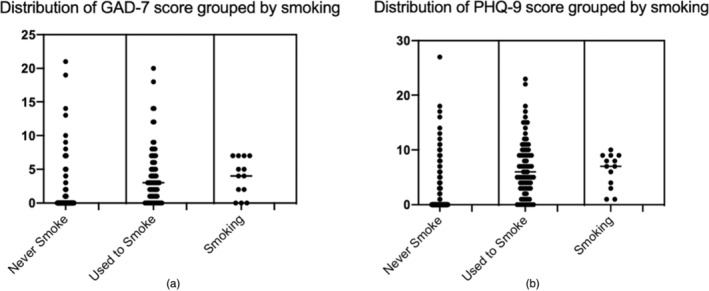
Distribution of GAD‐7/PHQ‐9 score grouped by patients who never smoke/used to smoke/are smoking. (a) Severity of anxiety symptoms assessed by GAD‐7 is significantly higher in participants who used to smoke (H = 72.112, p < 0.001). (b) Severity of depressive symptoms assessed by PHQ‐9 is significantly higher in participants who used to smoke (H = 57.199, p < 0.001).
Although no significant difference was observed between former and current smokers, possibly due to the small number of current smokers, depressive symptoms correlated with nicotine dependence as measured by the FNTD (χ2 = 6.470, p = 0.011). The severity of depressive symptoms, as evaluated by the PHQ‐9, showed a moderate positive correlation with nicotine dependence (Pearson's R = 0.271, p = 0.011), validated by a Kendall's tau‐b correlation coefficient of 0.186 (p < 0.030).
History of tobacco use and dependence on nicotine were correlated with severity of anxiety symptoms
The data revealed that a history of smoking was significantly associated with anxiety symptoms (OR = 3.52, p < 0.0001, as illustrated in Figure 2d). To further examine the relationship between tobacco use and anxiety symptoms, a comparison of GAD‐7 scores was conducted using the Kruskal‐Wallis H test among never smokers, former smokers, and current smokers. The results demonstrated a significant difference in the distribution among these groups (H = 57.199, p < 0.001). Notably, positive correlation was observed between the severity of anxiety symptoms, as indicated by GAD‐7 scores, and nicotine dependence assessed by the FNTD score (Kendall's tau‐b correlation coefficient = 0.186, p < 0.030, Figure 4a).
FIGURE 4.
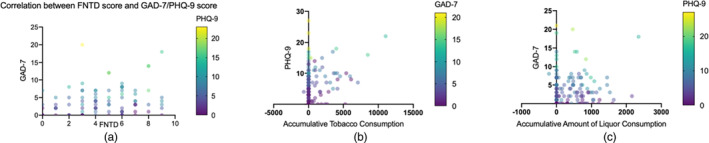
Correlation of dependence on nicotine (assessed by FNTD score), accumulative tobacco and liquor consumption with severity of anxiety and depressive symptoms (assessed by GAD‐7 and PHQ‐9, respectively). (a) Severity of anxiety symptoms represented by GAD‐7 score is positively correlated with dependence of nicotine assessed by FNTD score (Kendall's tau‐b correlation coefficient = 0.186, p < 0.030). (b) Accumulative amount of tobacco consumption is positively correlated with severity of depressive symptoms, which is assessed by PHQ‐7 (Kendall's tau‐b = 0.401, p < 0.0001). (c) Accumulative amount of liquor consumption is positively correlated with severity of anxiety symptoms (Kendall's tau‐b = 0.260, p < 0.0001).
Accumulative consumption of tobacco and alcohol was positively correlated with depressive symptoms and anxiety symptoms
Consistent with findings on nicotine, cumulative tobacco consumption was positively correlated with depressive symptom severity (Kendall's tau‐b = 0.401, p < 0.0001, Figure 4b). Furthermore, univariate analysis indicated that a history of alcohol consumption significantly contributed to both depressive (OR = 5.19, p < 0.0001) and anxiety symptoms (OR = 3.57, p < 0.0001), with accumulative alcohol intake positively related to the severity of anxiety symptoms (Kendall's tau‐b = 0.260, p < 0.0001, Figure 4c).
DISCUSSION
Patients with lung cancer demonstrated a commendable level of cooperation during the completion of the questionnaires designed to assess their psychological well‐being. The vast majority of patients were able to independently complete the questionnaires in less than 10 min, indicating the practicality of the GAD‐7, PHQ‐9, and HADS as screening tools for depressive and anxiety symptoms. The validity of these tools, particularly their sensitivity and specificity, would benefit from further confirmation through face‐to‐face diagnostic interviews.
The study identified a considerable prevalence of psychological symptoms among the participants, with 32.4% and 30% experiencing depressive and anxiety symptoms, respectively. Multiple risk factors for these conditions were revealed, although common variables such as age, gender, and education level did not exhibit a significant impact in this cohort, in contrast to previous studies.
For the first time it was discovered that history of tobacco and liquor consumption was related to worse mental state of lung cancer patients, with both more severe depressive and anxiety symptoms. Moreover, patients tended to have more depressive and anxiety symptoms with a higher level of nicotine dependence and accumulative amount of consumption of tobacco and alcohol. This is a possible mechanism regarding why patients with history of smoking or alcohol abuse tend to have a worse prognosis.
For the first time, our research identified a correlation between the history of tobacco and alcohol consumption and an exacerbated mental state in lung cancer patients, marked by heightened depressive and anxiety symptoms. This association was compounded by increased levels of nicotine dependence and cumulative substance consumption. Such findings suggest a potential mechanism underlying the observed poorer prognosis in patients with a history of smoking or alcohol abuse 26 , 27 However, the impact of cessation on mental health remains inconclusive due to the limited sample size of current and former smokers and drinkers in this study.
Contrary to the common assumption that a history of smoking is not an independent risk factor for psychological symptoms due to associated stigma with lung cancer, a previous study indicated no significant stigma difference between smokers and nonsmokers. 28 Our findings suggest that the psychological symptoms are correlated with the quantity of substance consumption, which is unlikely to be solely attributable to stigma. We propose that the history of smoking and alcohol consumption are independent risk factors for depressive and anxiety symptoms, although a larger sample size is necessary for confirmation.
The findings are consistent with the established roles of smoking and addiction to alcohol as risk factors for depression. 29 Cigarette smoking induced depression through oxidative damage and inflammation. 30 , 31 Similar mechanisms were indicated by experiments in mice with lung cancer. 32 Alcohol exposure regulates gut microbiota and mediates anxiety and depression through NLRP3‐mediated neuroinflammation. 33 , 34
This analysis not only illuminates the potential mechanisms affecting mental well‐being but also provides valuable insights for clinical practice. Depression and anxiety significantly affect patient prognosis, and there is a concerted effort to alleviate such distress. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 Established practices, such as early palliative care that incorporates psychological support, have been shown to enhance quality of life. 44 Nevertheless, the optimal approach to palliative care remains unclear, 45 , 46 and the psychological assistance currently offered by most oncologists falls short of the needs of patients with depression or anxiety. For instance, at CHCA, group discussions among patients, nurses, and clinicians are employed as a form of psychosocial support; yet this approach may overlook individuals at high risk of psychological distress who require more specialized, individualized interventions. The questionnaire‐based screening method proposed in this study could aid in the identification and referral process.
The present study is not without limitations. Notably, there was a disproportionate representation of late‐stage (III–IV) patients (84.7%), attributable to the recruitment strategy at the inpatient thoracic cancer department at CHCA. Additionally, the potential for nonresponse bias was identified. To mitigate these biases, future studies should aim to broaden the recruitment scope. The use of self‐administered psychological questionnaires such as the PHQ‐9 and GAD‐7, in the absence of face‐to‐face assessments by psychologists, may also lead to inaccuracies if participants do not respond truthfully or patiently. Therefore, subsequent validation of these screening methods should involve structured interviews with psychologists to fully ascertain their effectiveness.
In conclusion, the general anxiety disorder‐7 (GAD‐7), patient health questionnaire‐9 (PHQ‐9), and hospital anxiety and depression scale (HADS) have demonstrated their feasibility as effective tools for screening depressive and anxiety symptoms in lung cancer patients. This study has substantiated the history of tobacco and alcohol consumption as significant risk factors for both depressive and anxiety symptoms within this patient population. Moreover, it has been observed that a higher level of nicotine dependence and greater cumulative tobacco consumption are linked to an increased occurrence of depressive symptoms. Similarly, a substantial relationship has been established between the cumulative consumption of alcohol and the exacerbation of anxiety symptoms.
AUTHOR CONTRIBUTIONS
Conceptualization: J.D. and X.Z.; Methodology: B.C.; Validation: H.R. and Y.C.; Formal analysis: Y.C. and H.Z.; Investigation: Y.C.; Resources: Z.M., X.Z. and J.D.; Data curation: H.Z.; Writing—original draft preparation: Y.C.; Writing—review and editing: H.Z. and J.D.; Visualization: B.C. and H.R.; Supervision: H.Z. and X.Z.; Project administration: Z.M., X.Z. and J.D.; Funding acquisition: Z.M. and J.D.; All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Institute of Psychology, Chinese Academy Sciences.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study.
ACKNOWLEDGMENTS
We thank Beijing Cancer Foundation for its financial support.
Chau YF, Zhou H, Chen B, Ren H, Ma Z, Zhang X, et al. Screening for depression and anxiety in lung cancer patients: A real‐world study using GAD‐7 and HADS . Thorac Cancer. 2024;15(13):1041–1049. 10.1111/1759-7714.15287
Yi Fung Chau and Huixia Zhou contributed equally to this work.
Contributor Information
Xiangyang Zhang, Email: zhangxy@psych.ac.cn.
Jianchun Duan, Email: duanjianchun79@163.com.
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to principles of confidentiality.
REFERENCES
- 1. Mitchell AJ et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, hematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncol. 2011;12(2):160–174. [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC et al. Prevalence, Severity, and Comorbidity of 12‐Month DSM‐IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin C et al. Breast cancer oral anti‐cancer medication adherence: a systematic review of psychosocial motivators and barriers. Breast Cancer Res Treat. 2017;165(2):247–260. [DOI] [PubMed] [Google Scholar]
- 4. Mausbach BT, Schwab RB, Irwin SA. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta‐analysis. Breast Cancer Res Treat. 2015;152(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pirl WF et al. Depression and survival in metastatic non‐small‐cell lung cancer: effects of early palliative care. J Clin Oncol. 2012;30(12):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Batty GD et al. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gladly A . The Commission on Cancer (CoC) of the American College of Surgeons (ACS). 2020.
- 8. Lazenby M et al. Supporting commission on cancer‐mandated psychosocial distress screening with implementation strategies. J Oncol Pract. 2015;11(3):e413–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krebber A‐MH et al. Screening for psychological distress in follow‐up care to identify head and neck cancer patients with untreated distress. Support Care Cancer. 2016;24(6):2541–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fallowfield L et al. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84(8):1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. NCCN . Clinical Practice Guidelines in Oncology (NCCN Guidelines): Cancer‐Related Fatigue. Version 2.2022. National Comprehensive Cancer Network, Plymouth Meeting, Montgomery County, Pennsylvania, United States; 2022. [Google Scholar]
- 12. Deshields T et al. Psychosocial staffing at National Comprehensive Cancer Network member institutions: data from leading cancer centers. Psycho‐Oncol. 2016;25(2):164–169. [DOI] [PubMed] [Google Scholar]
- 13. Heatherton TF et al. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 14. Pomerleau CS, Majchrzak MJ, Pomerleau OF. Nicotine dependence and the Fagerström Tolerance Questionnaire: a brief review. J Subst Abuse. 1989;1(4):471–477. [PubMed] [Google Scholar]
- 15. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 18. Baker AM et al. Test Performance Characteristics of the AIR, GAD‐7, and HADS‐Anxiety Screening Questionnaires for Anxiety in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2018;15(8):926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Esser P et al. The Generalized Anxiety Disorder Screener (GAD‐7) and the anxiety module of the Hospital and Depression Scale (HADS‐A) as screening tools for generalized anxiety disorder among cancer patients. Psychooncology. 2018;27(6):1509–1516. [DOI] [PubMed] [Google Scholar]
- 20. Marrie RA et al. The validity and reliability of screening measures for depression and anxiety disorders in multiple sclerosis. Mult Scler Relat Disord. 2018;20:9–15. [DOI] [PubMed] [Google Scholar]
- 21. Snijkers JTW et al. Examining the optimal cutoff values of HADS, PHQ‐9 and GAD‐7 as screening instruments for depression and anxiety in irritable bowel syndrome. Neurogastroenterol Motil. 2021;33(12):e14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo C, Huang X. Hospital anxiety and depression scale exhibits good consistency but shorter assessment time than Zung self‐rating anxiety/depression scale for evaluating anxiety/depression in non‐small cell lung cancer. Medicine. 2021;100(8):e24428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinz A et al. Assessment of depression severity with the PHQ‐9 in cancer patients and in the general population. BMC Psychiatry. 2016;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy P et al. Identification of depression in diabetes: the efficacy of PHQ‐9 and HADS‐D. Br J Gen Pract. 2010;60(575):e239–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manea L, Gilbody S, McMillan D. Optimal cut‐off score for diagnosing depression with the Patient Health Questionnaire (PHQ‐9): a meta‐analysis. Cmaj. 2012;184(3):E191–E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McMenamin ÚC, McCain S, Kunzmann AT. Do smoking and alcohol behaviors influence GI cancer survival? Best Pract Res, Clin Gastroenterol. 2017;31(5):569–577. [DOI] [PubMed] [Google Scholar]
- 27. Walter V et al. Smoking and survival of colorectal cancer patients: systematic review and meta‐analysis. Ann Oncol. 2014;25(8):1517–1525. [DOI] [PubMed] [Google Scholar]
- 28. Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16(3):264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kunugi H. Depression and lifestyle: Focusing on nutrition, exercise, and their possible relevance to molecular mechanisms. Psychiatry Clin Neurosci. 2023;77(8):420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holitzki H et al. Health effects of exposure to second‐ and third‐hand marijuana smoke: a systematic review. CMAJ Open. 2017;5(4):E814–e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Onor IO et al. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int J Environ Res Public Health. 2017;14(10):1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu BP et al. The combination of chronic stress and smoke exacerbated depression‐like changes and lung cancer factor expression in A/J mice: Involve inflammation and BDNF dysfunction. PLoS One. 2022;17(11):e0277945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yao H et al. Gut microbiota regulates chronic ethanol exposure‐induced depressive‐like behavior through hippocampal NLRP3‐mediated neuroinflammation. Mol Psychiatry. 2023;28(2):919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li J et al. CB2R activation ameliorates late adolescent chronic alcohol exposure‐induced anxiety‐like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain, Behav, Immun. 2023;110:60–79. [DOI] [PubMed] [Google Scholar]
- 35. Arrieta O et al. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non‐small cell lung cancer. Ann Surg Oncol. 2013;20(6):1941–1948. [DOI] [PubMed] [Google Scholar]
- 36. Jung JY et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health‐related quality of life in lung cancer survivors. Psychooncology. 2018;27(2):465–470. [DOI] [PubMed] [Google Scholar]
- 37. Krebber AM et al. Stepped care targeting psychological distress in head and neck cancer and lung cancer patients: a randomized, controlled trial. Ann Oncol. 2016;27(9):1754–1760. [DOI] [PubMed] [Google Scholar]
- 38. Ostuzzi G et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2018;4(4):Cd011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta‐analysis. Psychol Med. 2010;40(11):1797–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Polański J et al. Intensity of Anxiety and Depression in Patients with Lung Cancer in Relation to Quality of Life. Adv Exp Med Biol. 2018;1023:29–36. [DOI] [PubMed] [Google Scholar]
- 41. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta‐analysis. Cancer. 2009;115(22):5349–5361. [DOI] [PubMed] [Google Scholar]
- 42. Semple C et al. Psychosocial interventions for patients with head and neck cancer. Cochrane Database Syst Rev. 2013;7:Cd009441. [DOI] [PubMed] [Google Scholar]
- 43. Walker J et al. Different independent associations of depression and anxiety with survival in patients with cancer. J Psychosom Res. 2020;138:110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Temel JS et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 45. Gärtner J et al. Early palliative care: Pro, but please be precise! Oncol Res Treat. 2019;42(1–2):11–18. [DOI] [PubMed] [Google Scholar]
- 46. von Blanckenburg P, Leppin N. Psychological interventions in palliative care. Curr Opin Psychiatry. 2018;31(5):389–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to principles of confidentiality.


