Abstract
Introduction
The DOLAM trial revealed that switching from triple antiretroviral therapy (three-drug regimen; 3DR) to dolutegravir plus lamivudine (two-drug regimen; 2DR) was virologically non-inferior to continuing 3DR after 48 weeks of follow-up. Weight increased with 2DR relative to 3DR but it did not impact on metabolic parameters.
Methods
Multiomics plasma profile was performed to gain further insight into whether this therapy switch might affect specific biological pathways. DOLAM (EudraCT 201500027435) is a Phase 4, randomized, open-label, non-inferiority trial in which virologically suppressed persons with HIV treated with 3DR were assigned (1:1) to switch to 2DR or to continue 3DR for 48 weeks. Untargeted proteomics, metabolomics and lipidomics analyses were performed at baseline and at 48 weeks. Univariate and multivariate analyses were performed to identify changes in key molecules between both therapy arms.
Results
Switching from 3DR to 2DR showed a multiomic impact on circulating plasma concentration of N-acetylmuramoyl-L-alanine amidase (Q96PD5), insulin-like growth factor-binding protein 3 (A6XND0), alanine and triglyceride (TG) (48:0). Correlation analyses identified an association among the up-regulation of these four molecules in persons treated with 2DR.
Conclusions
Untargeted multiomics profiling studies identified molecular changes potentially associated with inflammation immune pathways, and with lipid and glucose metabolism. Although these changes could be associated with potential metabolic or cardiovascular consequences, their clinical significance remains uncertain. Further work is needed to confirm these findings and to assess their long-term clinical consequences.
Introduction
Antiretroviral drugs have improved over time, becoming more effective, simpler and better tolerated. However, for some patients current ART may still be challenging.1 Despite better tolerability, some patients may experience direct toxicities with contemporary antiretrovirals. The risk for most potential toxicities is usually cumulative and may further depend on specific individual factors. Beyond direct short-term harm, antiretroviral toxicities may have a long-term impact on the development or progression of comorbidities. Medications for comorbidities may further increase the risk of significant interactions with some antiretrovirals. Switching antiretrovirals for reasons other than virological failure is a common and evolving strategy in clinical practice.2
The effects of switching to dolutegravir plus lamivudine (a two-drug regimen; 2DR) in adults suppressed on triple therapy have been assessed in several observational studies or single-arm trials and three large randomized clinical trials.3–9 The results of all these studies support that switching to 2DR in selected adults with HIV is virologically non-inferior and as safe as continuing triple ART (three-drug regimen; 3DR).
Whether switching from 3DR to 2DR might be associated with better long-term tolerability due to the reduction of antiretroviral drug burden has not been proven yet.10 Conversely, integrase inhibitors in general, including dolutegravir, have been associated with higher risk of weight gain, diabetes, hypertension and cardiovascular disease, although the not entirely consistent data leave many unknowns.11
In the DOLAM (EudraCT 201500027435) study and SALSA (NCT00295620) study, switching to 2DR was associated with weight gain at 48 weeks compared with continuing 3DR but there were no differences in lipid parameters between arms.8 It was the discontinuation of tenofovir disoproxil fumarate that was associated with 48 week increases in total cholesterol and weight in persons switched to 2DR.8
Omics approaches have emerged as robust and powerful tools for a better understanding of immunometabolism in HIV pathogenesis. Proteomics-based approaches offer a high-throughput method not only to identify biomarkers for diagnostic antigens and therapeutic targets but also to investigate mechanisms of drug action in persons with HIV.12–14 Along with proteomics, metabolomics and lipidomics technologies have yielded new insights into the key role of cellular metabolism in the activity of immune cells and the treatment-induced metabolic derangements in the context of HIV infection.15–17
An untargeted multiomics plasma profile, including proteomics, metabolomics and lipidomics data, was pre-planned as a substudy of the DOLAM study to gain insight on whether some immunometabolic pathways might be affected by this therapy switch. The untargeted approach was selected for a more comprehensive and systemic analysis of both unknown and known plasma compounds to discover new biomarkers to be selected for more accurate quantitation and annotation in future studies (targeted).
Methods
Study design
The DOLAM study is a Phase 4, multicentre, randomized, controlled with active treatment, open-label, parallel clinical trial. The study was done at six major HIV clinics in Catalonia (Spain). Institutional review boards from all participating centres and the Spanish Agency of Medicines and Medical Devices approved the study. Participants were randomly assigned to continue their current 3DR (control arm) or to switch to 2DR once daily (dual-therapy arm). Major results of the DOLAM study have already been published.8
Study participants and data collection
Consecutive asymptomatic adults (≥18 years old) with HIV on stable (defined as at least the previous 12 months) 3DR including two NRTIs plus either a boosted PI, an NNRTI, or an integrase inhibitor with sustained viral suppression (defined by plasma HIV RNA < 50 copies/mL in two or more consecutive determinations during at least the 12 months before inclusion; blips up to 200 copies/mL were admissible) were invited to participate. For women of childbearing age, a negative pregnancy test within 10 days before randomization into the study was required. Exclusion criteria were: pregnancy, lactation or planned pregnancy during the study period; prior virological failure (defined as plasma HIV RNA ≥ 50 copies/mL in two consecutive tests or >500 copies/mL in one test) to regimens containing lamivudine, emtricitabine or integrase inhibitors; any mutation conferring resistance to lamivudine, emtricitabine or integrase inhibitors; CD4 nadir <200 cells/µL; any disease or history of disease that might confound the results of the study or pose additional risk to participant’s treatment; and chronic hepatitis B, defined by a positive hepatitis B surface antigen (HBsAg) result at screening. Written informed consent was obtained from all eligible participants before randomization.
Participants were visited at baseline and every 12 weeks until completing at least 48 weeks of follow-up. At baseline and at 48 weeks, 10 mL EDTA blood samples were collected after at least 8 h of fasting and processed immediately; plasma samples were split into 1 mL aliquots and stored at −80°C for deferred multiomics studies.
Determination of the proteomic profile
Detailed information about protein extraction and identification can be found in the Supplementary Methods (available as Supplementary data at JAC Online). MS analyses were performed on an LTQ-Orbitrap Velos Pro from Thermo Fisher by an enhanced FT-resolution MS spectrum (R = 30 000 FHMW) followed by a data-dependent FT-MS/MS acquisition (R = 15 000 FHMW, 40% NCE HCD) from the 10 most intense parent ions with a charge state rejection of one and dynamic exclusion of 0.5 min. Protein identification/quantification was performed on Proteome Discoverer software v.1.4.0.288 (Thermo Fisher Scientific, CA, USA) by Multidimensional Protein Identification Technology (MudPIT) combining the two raw data files obtained from each sample. For protein identification, all MS and MS/MS spectra were analysed using the Mascot search engine (v.2.5). The workflow was set up using two different Mascot nodes combining a Homo sapiens database (74 449 entries) and a contaminants database (247 entries), both searches assuming trypsin digestion. Two missed cleavages were allowed and an error of 0.02 Da for FT-MS/MS fragmentation mass and 10.0 ppm for an FT-MS parent ion mass were allowed. TMT-10plex was set as quantification modification and oxidation of methionine and acetylation of N-termini were set as dynamic modifications, whereas carbamidomethylation of cysteine was set as static modification. The false discovery rate (FDR) and protein probabilities were calculated by Percolator. For protein quantification, the ratios between each TMT label against 126-TMT label were used and quantification results were normalized based on the protein median. The results are a ratio of reporter ion abundance and are dimensionless.
Determination of the metabolomic profile (analytical method)
Detailed information about the analytical method for metabolomic identification can be found in the Supplementary Methods. Samples were analysed on a 7200 GC-qTOF from Agilent Technologies (Santa Clara, CA, USA). The chromatographic separation was based on the Fiehn method.18 Targeted compounds were identified using pure standards with a mass accuracy of 20 ppm and different internal standards were used to correct signal response. Chromatographic peaks were deconvoluted using Unknowns Analysis software (version B.09.00, from Agilent) based on the exact mass. Identification of compounds was tentatively made by comparing the mass spectra and retention time of all detected compounds with the Fiehn 2013 Mass Spectral RTL Library and the National Institute of Standards and Technology (NIST) library 11 (2014) also using the Unknowns software. The identity of the main compounds was confirmed with commercial pure standards. After direct (with pure standards) or putative (with a library) identification of metabolites, these were semiquantified in terms of internal standard response ratio. For this relative quantification, the area of specific fragments for each metabolite was divided by the area of its specific internal standard to provide a reliable, accurate and reproducible relative concentration of metabolites.
Determination of the lipidomic profile (analytical method)
Lipid extraction procedure can be found in the Supplementary Methods. The identification of lipid species was performed using the Agilent MassHunter Profinder B.08 software. First, a feature extraction deconvolution was made; accurate mass and tandem mass spectrum, when available, was then matched to Metlin-PCDL (2017) from Agilent containing more than 40 000 metabolites and lipids, allowing a mass error of 20 ppm and a score higher than 80 for isotopic distribution. To ensure the tentative characterization, the chromatographic behaviour of pure standards for each family and corroboration with the Lipid Maps database (www.lipidmaps.org) was used to ensure their putative identification. Afterwards, matched entities were selected to perform a targeted MS/MS acquisition on the LC-QTOF-MS instrument to corroborate the identification. Lipid species were then semiquantified in terms of internal standard response ratio using one internal standard for each lipid family.
Statistical analysis
To further delineate semiquantitative differences between groups due to the switching effect, a ratio between data from samples at 48 weeks and data from baseline timepoints was performed in each participant before statistical analyses. Only those proteins, metabolites and lipids that were present in >60% of the samples in at least one of the experimental groups were considered. In addition, log2 transformations were applied to the data (proteomics, lipidomics and metabolomics) for variance stabilization, data range compression and making the data more normally distributed. This transformation was performed by Mass Profiler Professional software v.15.1 from Agilent Technologies. Regression model such as partial least squares discriminant (PLSD) was performed from compounds in each comparison and random forest (RF) analyses were performed to determine those molecules with higher accuracy to differentiate both groups. An unpaired t-test (Mann–Whitney U-test) was then performed between ratios (48 weeks/baseline) from experimental groups (control and case). In each comparison, its significant level (P value) was corrected for multiple testing using the FDR with the Benjamin–Hochberg procedure and a P value cut-off of <0.05 was applied. Associations between quantitative variables were evaluated using the Spearman correlation test and receiver operating characteristic (ROC) curves were employed to confirm the statistical relevance of molecules in the switching from 3DR to 2DR. The protein network was constructed with the online String software (version 11.5).
Statistical analyses were performed using SPSS (version 21.0, SPSS Inc., Chicago, IL, USA), and graphical representations were generated with GraphPad Prism (version 5.0, GraphPad Inc., San Diego, CA, USA), MetaboAnalyst 5.0 software and Open Office software. The results were considered significant at P values < 0.05.
Ethics
Institutional review boards from all participating centres and the Spanish Agency of Medicines and Medical Devices granted ethical approval of the study. Written informed consent was obtained from all eligible participants before randomization.
Results
Patient characteristics
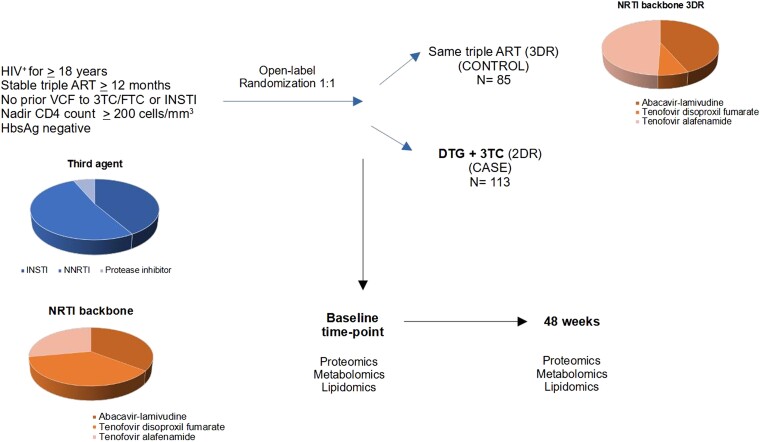
There were 198 (75%) out of 265 participants with valid samples for this substudy, 85 receiving 3DR and 113 receiving 2DR.8,9 Their baseline characteristics were like those of the main study. Participants were predominantly male (158; 84%) with a median (IQR) age of 45 (38–52) years. Roughly similar proportions were taking abacavir (35%), tenofovir disoproxil fumarate (37%) or tenofovir alafenamide (28%) before study entry (Table 1, Figure 1).
Table 1.
Baseline demographic and clinical characteristics
| Triple therapy (3DR) | Dolutegravir plus lamivudine (2DR) | |
|---|---|---|
| Age, years | 47 (39–51) | 44 (37–53) |
| Sex at birth | ||
| Female | 12 (14) | 17 (16) |
| Male | 72 (86) | 86 (84) |
| BMI, kg/m2 | 24 (23–26) | 25 (23–27) |
| Suspected route of HIV transmission | ||
| Male-to-male sex | 62 (74) | 71 (68) |
| Heterosexual sex | 13 (15) | 19 (18) |
| Injection drug | 4 (5) | 6 (6) |
| Other/unknown | 3 (7) | 7 (8) |
| CD4 and CD8 measurements | ||
| CD4 cells/µL | 746 (553–919) | 698 (577–938) |
| CD4, % | 35 (31–40) | 36 (31–43) |
| CD8 cells/µL | 795 (591–931) | 731 (502–959) |
| CD8, % | 36 (31–43) | 35 (27–42) |
| CD4:CD8 ratio | 0.88 (0.72–1.02) | 0.92 (0.69–1.09) |
Data are reported as n (%) or median (IQR).
Figure 1.
Study design. The study cohort comprised 198 PLHIV on stable triple ART for more than 12 months and with nadir CD4 T cell count greater or equal to 200 cells/mm3. Participants were assigned to a treatment group using computer-generated randomization. 3TC, lamivudine; DTG, dolutegravir; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; NRTI, nucleoside or nucleotide reverse transcriptase inhibitor; VCF, virological failure. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Switching from 3DR to 2DR is associated with the p53 signalling pathway
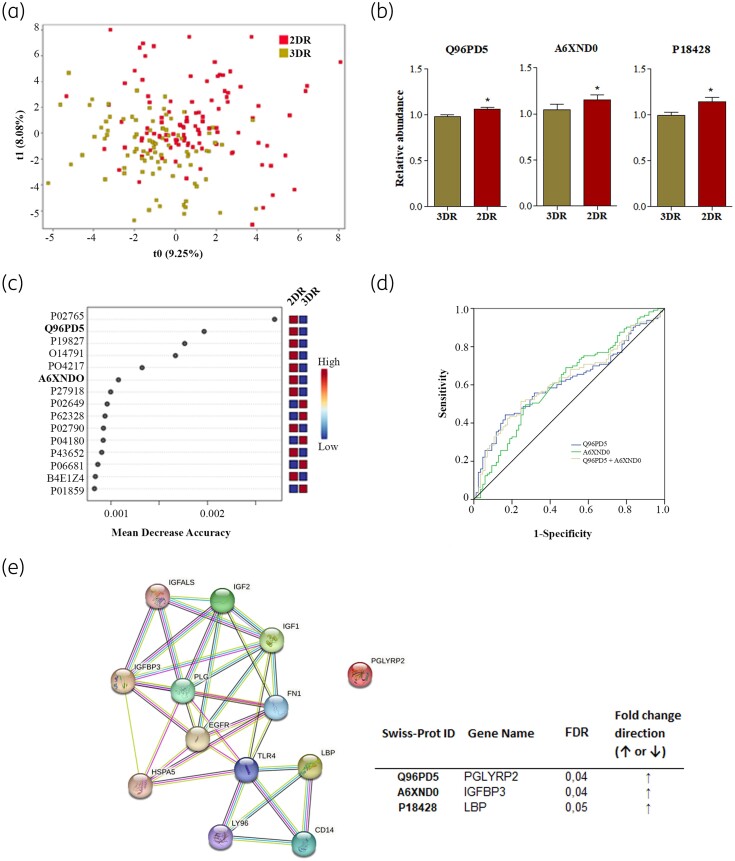
A total of 136 proteins were identified in plasma samples (Table S1) and the ratio between data from samples at 48 weeks and data from basal timepoint was compared between 3DR and 2DR groups. The PLSD regression model showed a group-average effect, although the two groups overlapped (Figure 2a). Case–control samples were then compared using a non-parametric test corrected by multiple test (FDR < 0.05), and 3 of 136 proteins were found to be statistically different between 3DR and 2DR groups (Figure 2b). N-acetylmuramoyl-L-alanine amidase (Q96PD5) (P = 0.035), insulin-like growth factor-binding protein 3 (IGFBP3) (A6XND0) (P = 0.043) and LPS-binding protein (LBP) (P18428) (P = 0.05) had elevated plasma concentrations in the 2DR group compared with the 3DR group. Furthermore, Q96PD5 and A6XND0 were consistent with those proteins that had the highest discriminatory power between groups (random forest analysis) (Figure 2c). Thus, by using a combination of univariate and multivariate approaches, Q96PD5 and A6XND0, which showed a positive correlation (ρ 0.247, P < 0.001), were discovered as the main proteins significantly increased in therapy simplification. These proteins were subjected to an ROC curve analysis to confirm their statistical relevance in the switching from 3DR to 2DR (Figure 2d). Both Q96PD5 and A6XND0 yielded significant AUC values (0.616, P = 0.005 and 0.613, P = 0.04, respectively) and the combination of both proteins resulted in an AUC of 0.624 (0.547–0.702, P = 0.003). Network mapping identified an interconnecting cluster between LBP and A6XND0 (confidence score 0.4) and STRING database analysis confirmed the interaction between switching from 3DR to 2DR and proteins associated with the p53 signalling pathway (FDR = 0.03, impact 0.05).
Figure 2.
Changes in plasma proteins when switching from 3DR to 2DR. (a) A PLSD regression model plot is shown based on relative protein levels. (b) Circulating levels of the three proteins with significant expression among groups by univariate analysis corrected by multiple tests (FDR) results for positive mode features (FDR ≤ 0.05). (c) RF analysis. The top 15 metabolites with the highest discriminatory power between both groups are listed. (d) ROC curve for proteins selected based on the combination of univariate and multivariate analyses. AUC for Q96PD5 was 0.616 (95% CI 0.538–0.694), AUC for A6XND0 was 0.613 (95% CI 0.534–0.693) and AUC for the combination of both proteins (Q96PD5+A6ZND0) was 0.624 (95% CI 0.547–0.702). (e) STRING database analysis confirmed the interaction between switching from 3DR to 2DR and proteins associated with the p53 signalling pathway (FDR = 0.03, impact 0.05). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Switching from 3DR to 2DR is associated with increased alanine and TG 48:0
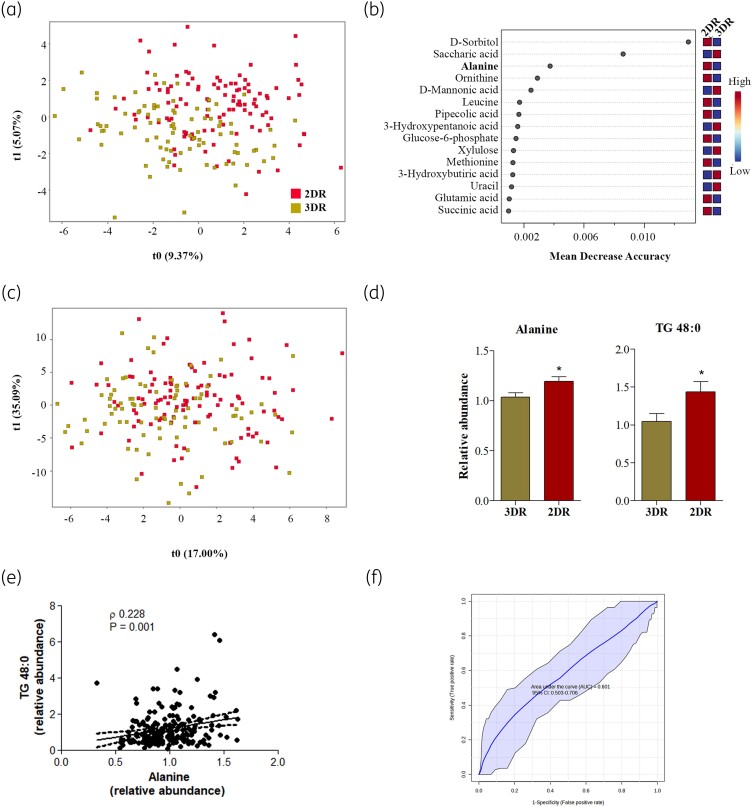
A total of 97 metabolites were identified in plasma samples (Table S2) and as previously performed with proteomics data, the ratio between metabolomics data from samples at 48 weeks and data from basal timepoint was compared between 3DR and 2DR groups. PLSD analysis also showed a group-average effect in the relative concentration of metabolites (Figure 3a). The univariate non-parametric test corrected by multiple test identified five metabolites statistically different between 3DR and 2DR groups; relative to 3DR, two were significantly decreased (2-hydroxybutyric acid, P < 0.001; and benzoic acid, P = 0.03) and three were significantly increased (2-hydroxy isobutyric acid, P < 0.001; nonanoic acid, P = 0.04; and alanine, P = 0.05) in 2DR. However, the multivariate analysis revealed alanine only as the main metabolite significantly increased by therapy simplification (Figure 3b and c). Of interest, circulating alanine levels positively correlated with A6XND0 levels (ρ = 0.141, P = 0.018).
Figure 3.
Changes in plasma metabolites and lipid species of switching from 3DR to 2DR. (a) A PLSD regression model plot is shown based on relative metabolite levels. (b) RF analysis. The top 15 metabolites with the highest discriminatory power between both groups are listed. (c) A PLSD regression model plot is shown based on relative lipid species levels. (d) Circulating differences between the significant metabolite and lipid species by univariate analysis corrected by multiple tests (FDR ≤ 0.05). (e) Correlation analysis between alanine (metabolite) and TG 48:0 (lipid species). The Spearman (ρ) correlation coefficient and P value are indicated inside the graphical representation. (f) ROC curve model for the combination of alanine and TG 48:0 to evaluate the feature of these molecules in the therapy simplification. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Regarding lipidomics analysis, a total of 117 lipid species were identified in plasma samples and the regression model (PLSD) also showed a group-average effect in the relative concentration of lipid species (Figure 3a). However, the univariate analysis corrected by multiple tests (FDR) only identified TG 48:0 as significantly increased in the 2DR group compared with the 3DR group (P = 0.018) (Figure 3c). The first number indicates the acyl carbon atoms, and the second number indicates the number of unsaturations.
Thus, alanine and TG 48:0 were analysed in combination to evaluate their association in therapy simplification. Correlation analyses confirmed a positive association between circulating alanine and TG 48:0 levels (ρ = 0.2134, P = 0.003) (Figure 3d) and the combination of both features were evaluated using the ROC curve, obtaining an AUC of 0.601 (95% CI 0.503–0.706) (Figure 3e).
No significant association was found between proteins and metabolites identified in the switching from 3DR to 2DR.
Discussion
Similar to other randomized clinical trials such as TANGO or SALSA, the DOLAM study showed that switching from 3DR to 2DR in selected virologically suppressed adults with HIV is virologically non-inferior and as safe as continuing 3DR.7,9 However, dolutegravir has been associated with weight increase in both naive and treated persons with HIV and there are concerns that it could also be associated with diabetes and cardiovascular disease.19,20 In the DOLAM study, persons living with HIV (PLHIV) assigned to 2DR significantly gained more weight and there were more overweight or obese persons at 48 weeks relative to those continuing 3DR, but we were unable to detect significant changes in body fat, lean mass or bone mineral density (BMD) between arms.8 Taking advantage of omic approaches for a better understanding of cellular metabolism, we attempted to explore if there were any plasma molecular alterations affected by the switch from 3DR to 2DR.
The combination of untargeted proteomics, metabolomics (polar compounds) and lipidomics evidenced that switching from 3DR to 2DR was related to a few circulating metabolomic perturbations. Specifically, the multiomic approach identified two proteins, one metabolite and one lipid differentially expressed in the 2DR group compared with 3DR. Correlation analyses identified an association among the up-regulation of these four molecules in the 2DR arm.
At 48 weeks of follow-up, participants receiving 2DR showed increased plasma concentration of Q96PD5 and A6XND0 compared with 3DR. Q96PD5 is a protein encoded by the peptidoglycan recognition protein 2 (PGLYRP2) gene, a gene from the peptidoglycan recognition proteins (PGLYRPs) family. PGLYRPs are proteins that are conserved from insects to mammals, acting in inflammation and immune responses independently of their bactericidal and enzymatic activities.21 Specifically, PGLYRP2 has shown both anti-inflammatory and pro-inflammatory properties, probably related to its link to the transcription factor family nuclear factor κB (NF-κB).22–25 NF-κB represents a family of inducible transcription factors involved in the regulation of genes belonging to immune and inflammatory pathways (including cytokines, chemokines and inflammasome) whose expression has been linked to the p53 signalling pathway.24,26 Our results suggested a possible association between the differentially expressed proteins associated with therapy simplification and the p53 signalling pathway. Of interest, a recent study also showed a positive correlation between PGLYRP2 and ApoB/A1 in patients with systemic lupus erythematosus (SLE), which suggested a potential role of PGLYRP2 in the dyslipidaemia and cardiovascular disease risks in SLE patients.27 In the present study, no association was found between Q96PD5 and the lipid species differentially identified among groups (TG 48:0), but Q96PD5 was shown to be significantly related to A6XND0, whose gene expression (IGFBP3) was previously related to lipid metabolism.28 In serum, IGFBP-3 is the most abundant protein of the IGFBP family that functions in an IGF-dependent (delivery of IGF and activation of IGF downstream signalling) manner, as well as in an IGF-independent (interaction with proteins) manner.29 Although some studies unveiled its role in metabolic regulation, the exact role of IGFBP3 in glucose and lipid metabolism remains unclear, even in PLHIV.29,30 Some studies suggested that serum IGFBP3 could be involved in HIV disease progression and related to insulin resistance, but on the other hand, 3 months of treatment with IGF-I/IGFBP-3 improved whole-body glucose, glucose tolerance and fasting TGs in men living with HIV with excess abdominal adiposity and insulin resistance.31–33 Based on our results, the role of an increase in plasma IGFBP3 concentration in the 2DR arm remains uncertain as no significant metabolic alterations were clinically observed between 3DR and 2DR groups at 48 weeks of therapy randomization.8 However, it is important to highlight that our results suggested a positive relationship between A6XND0 and plasma alanine levels, and between plasma alanine levels and TG (48:0). At the molecular level, participants in the 2DR group showed increased plasma concentrations in both circulating plasma alanine and TG (48:0) compared with the 3DR group. Increased circulating alanine concentrations could indicate an alteration in the glucose-alanine cycle in which alanine can be synthesized from pyruvate deriving from skeletal muscle through the enzymatic reaction of ALT and then transported to the liver to be used for gluconeogenesis. High circulating alanine concentration has been related to the risk of incident type II diabetes while reduced rates of glucose-alanine cycling have been associated with the regulation of hepatic mitochondrial oxidation.34,35 Regarding the monoacid triglyceride TG (48:0), previous studies have considered the saturated TG (48:0) as a key lipid species in biomarker panels related to liver-fat content.36,37
This study had limitations. The multiomic analysis was performed from baseline to 48 weeks after randomization, a period in which potential metabolic consequences of weight gain had not been apparent. Multiomics data integration can bring several problems and requires several attention to combine high-throughput data obtained from different molecular layers. To reduce the heterogeneity across the three different omics technologies applied and the difficulty of interpreting multilayered system models derived from the combination of omics data and non-omic data (clinical data), single analysis was preferred for the present work focused on the antiretroviral simplification. Performing a multiomic analysis after a longer follow-up would be of great interest. As a strength, this study provides novel and valuable information, as no multiomic analysis investigating changes after switching from 3DR to 2DR has been previously published to our knowledge.
Conclusions
In summary, 48 weeks of dolutegravir plus lamivudine (2DR) represents a reduction in antiretroviral drug burden that is virologically non-inferior, safe and without apparent clinical metabolic derangement compared with continuing triple ART (3DR). Switching from 3DR to 2DR, however, was related to some plasma metabolomics perturbations, in which four soluble compounds were involved. Plasma changes in Q96PD5, A6XND0, alanine and TG (48:0) might be associated with the activation of inflammation and immune pathways but also with an alteration in lipid and glucose metabolism. Although these changes could be associated with potential metabolic or cardiovascular consequences, their clinical significance remains uncertain. Further work is needed to confirm these findings and to assess their long-term clinical consequences.
Supplementary Material
Acknowledgements
This study would not have been possible without the collaboration of all the patients, medical and nursery staff, and data managers who have taken part in the project. We want to particularly acknowledge the support of the HIV BioBank, which is integrated into the Spanish AIDS Research Network and all collaborating centres, for the generous contribution of clinical samples for the present work.
Contributor Information
Elisa de Lazzari, Hospital Clinic - IDIBAPS, Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Universitat de Barcelona, Barcelona, Spain.
Eugenia B Negredo, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Lluita contra les Infeccions, Hospital Universitari Germans Trias i Pujol, Badalona, Universitat Autònoma de Barcelona, Barcelona, Spain.
Pere Domingo, Infectious Diseases Unit, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Juan M Tiraboschi, Hospital Universitario de Bellvitge, Barcelona, Spain.
Esteve Ribera, Hospital Universitario de la Vall d’Hebron, Barcelona, Spain.
Nadia Abdulghani, Hospital Arnau de Vilanova, Lleida, Spain.
Verònica Alba, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Infection and Immunity Research Group (INIM), Institut Investigació Sanitària Pere Virgili (IISPV), Tarragona, Spain; Hospital Universitari de Tarragona Joan XXIII, Tarragona, Spain; Universitat Rovira i Virgili (URV), Tarragona, Spain.
Salvador Fernández-Arroyo, Eurecat, Centre Tecnològic de Catalunya, Centre for Omic Sciences, Joint Unit Eurecat-Universitat Rovira i Virgili, Unique Scientific and Technical Infrastructure (ICTS), 43204 Reus, Spain.
Consuelo Viladés, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Infection and Immunity Research Group (INIM), Institut Investigació Sanitària Pere Virgili (IISPV), Tarragona, Spain; Hospital Universitari de Tarragona Joan XXIII, Tarragona, Spain; Universitat Rovira i Virgili (URV), Tarragona, Spain.
Joaquim Peraire, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Infection and Immunity Research Group (INIM), Institut Investigació Sanitària Pere Virgili (IISPV), Tarragona, Spain; Hospital Universitari de Tarragona Joan XXIII, Tarragona, Spain; Universitat Rovira i Virgili (URV), Tarragona, Spain.
Jose M Gatell, Universitat de Barcelona, Barcelona, Spain; ViiV Healthcare, Barcelona, Spain.
Jose L Blanco, Hospital Clinic - IDIBAPS, Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain.
Francesc Vidal, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Infection and Immunity Research Group (INIM), Institut Investigació Sanitària Pere Virgili (IISPV), Tarragona, Spain; Hospital Universitari de Tarragona Joan XXIII, Tarragona, Spain; Universitat Rovira i Virgili (URV), Tarragona, Spain.
Anna Rull, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Infection and Immunity Research Group (INIM), Institut Investigació Sanitària Pere Virgili (IISPV), Tarragona, Spain; Hospital Universitari de Tarragona Joan XXIII, Tarragona, Spain; Universitat Rovira i Virgili (URV), Tarragona, Spain.
Esteban Martinez, Hospital Clinic - IDIBAPS, Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III (ISCIII), Madrid, Spain; Universitat de Barcelona, Barcelona, Spain.
Funding
This research was funded by the Instituto de Salud Carlos III (ISCIII) through the project (PI19/01337) and co-funded by the European Union. Anna Rull is supported by a grant from IISPV through the project ‘2019/IISPV/05’ (Boosting Young Talent), by Grupo de Estudio del SIDA (GeSIDA) from Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica through the ‘III Premio para Jóvenes Investigadores 2019’ and by the Instituto de Salud Carlos III (ISCIII) under grant agreement ‘CP19/00146’ through the Miguel Servet Program. Esteban Martínez was the recipient of an intensification grant from Hospital Clínic - IDIBAPS, Barcelona, during 2023.
Transparency declarations
Esteban Martínez has received funds for research from ViiV Healthcare. Jose M. Gatell is a full-time employee of and owns stock in ViiV as Senior Global Medical Director since 1 May 2018. All other authors: none to declare.
Author contributions
Funding body: Esteban Martinez. Study concept and design: Elisa de Lazzari, Anna Rull, Francesc Vidal, Esteban Martinez. Patient selection and clinical data acquisition: Eugenia Negredo, Pere Domingo, Juan M. Tiraboschi, Esteve Ribera, Nadia Abdulghani, Consuelo Viladés, Joaquim Peraire, Jose M. Gatell, Jose L. Blanco, Joaquim Peraire, Francesc Vidal, Esteban Martinez. Sample preparation, and biomarker analysis: Anna Rull, Verònica Alba, Salvador Fernández-Arroyo. Statistical analysis and interpretation of data: Elisa de Lazzari. Writing of the manuscript: Anna Rull, Esteban Martinez. Critical revision of the manuscript for relevant intellectual content: all authors. Supervision and visualization: all authors. All authors approved the final version of the article, including the authorship list.
Data availability
The datasets used and analysed during the current study may be made available by the corresponding author upon reasonable request.
Supplementary data
Supplementary Methods, Figures S1 to S3 and Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Panel de expertos de GeSIDA y División de control de VIH, ITS, Hepatitis virales y Tuberculosis. Plan Nacional sobre el Sida . Documento de consenso de GeSIDA/Plan Nacional sobre el Sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (January 2022 update). 2022. GuiaGeSIDAPlanNacionalSobreElSidaRespectoAlTratamientoAntirretroviralEnAdultosInfectadosPorElVirusDeLaInmunodeficienciaHumanaActualizacionEnero2022.pdf(gesida-seimc.org).
- 2. Wood BR. Switching antiretroviral therapy in the setting of virologic suppression: a why and how-to guide. Infect Dis Clin North Am 2019; 33: 693–705. 10.1016/j.idc.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 3. Maggiolo F, Gulminetti R, Pagnucco L et al. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis 2017; 17: 215. 10.1186/s12879-017-2311-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hidalgo-Tenorio C, Cortés LL, Gutiérrez A et al. DOLAMA study: effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients. Medicine (Baltimore) 2019; 98: e16813. 10.1097/MD.0000000000016813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldin G, Ciccullo A, Rusconi S et al. Long-term data on the efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multi-centre cohort of HIV-1-infected, virologically suppressed patients. Int J Antimicrob Agents 2019; 54: 728–34. 10.1016/j.ijantimicag.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 6. Joly V, Burdet C, Landman R et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL). J Antimicrob Chemother 2019; 74: 739–45. 10.1093/jac/dky467 [DOI] [PubMed] [Google Scholar]
- 7. Osiyemi O, De Wit S, Ajana F et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis 2022; 75: 975–86. 10.1093/cid/ciac036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rojas J, de Lazzari E, Negredo E et al. Efficacy and safety of switching to dolutegravir plus lamivudine versus continuing triple antiretroviral therapy in virologically suppressed adults with HIV at 48 weeks (DOLAM): a randomised non-inferiority trial. Lancet HIV 2021; 8: e463–73. 10.1016/S2352-3018(21)00100-4 [DOI] [PubMed] [Google Scholar]
- 9. Llibre JM, Brites C, Cheng CY et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis 2023; 76: 720–9. 10.1093/cid/ciac130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel R, Evitt L, Mariolis I et al. HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther 2021; 10: 2051–70. 10.1007/s40121-021-00522-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diggins CE, Russo SC, Lo J. Metabolic consequences of antiretroviral therapy. Curr HIV/AIDS Rep 2022; 19: 141–53. 10.1007/s11904-022-00600-6 [DOI] [PubMed] [Google Scholar]
- 12. Venkatesh A, Gil C, Fuentes M et al. A perspective on proteomics of infectious diseases. Proteomics Clin Appl 2018; 12: e1700139. 10.1002/prca.201700139 [DOI] [PubMed] [Google Scholar]
- 13. deFilippi C, Toribio M, Wong LP et al. Differential plasma protein regulation and statin effects in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients utilizing a proteomics approach. J Infect Dis 2020; 222: 929–39. 10.1093/infdis/jiaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toribio M, Fitch KV, Stone L et al. Assessing statin effects on cardiovascular pathways in HIV using a novel proteomics approach: analysis of data from INTREPID, a randomized controlled trial. EBioMedicine 2018; 35: 58–66. 10.1016/j.ebiom.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curran A, Rull A, Navarro J et al. Lipidomics reveals reduced inflammatory lipid species and storage lipids after switching from EFV/FTC/TDF to RPV/FTC/TDF: a randomized open-label trial. J Clin Med 2020; 9: 1246. 10.3390/jcm9051246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villumsen SO, Benfeitas R, Knudsen AD et al. Integrative lipidomics and metabolomics for system-level understanding of the metabolic syndrome in long-term treated HIV-infected individuals. Front Immunol 2022; 12: 742736. 10.3389/fimmu.2021.742736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liebenberg C, Luies L, Williams AA. Metabolomics as a tool to investigate HIV/TB co-infection. Front Mol Biosci 2021; 8: 692823. 10.3389/fmolb.2021.692823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kind T, Wohlgemuth G, Lee DY et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem 2009; 81: 10038–48. 10.1021/ac9019522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jayedi A, Rashidy-Pour A, Soltani S et al. Adult weight gain and the risk of cardiovascular disease: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Clin Nutr 2020; 74: 1263–75. 10.1038/s41430-020-0610-y [DOI] [PubMed] [Google Scholar]
- 20. Jayedi A, Soltani S, Motlagh SZ et al. Anthropometric and adiposity indicators and risk of type 2 diabetes: systematic review and dose-response meta-analysis of cohort studies. BMJ 2022; 376: e067516. 10.1136/bmj-2021-067516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dziarski R, Gupta D. Review: mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun 2010; 16: 168–74. 10.1177/1753425910366059 [DOI] [PubMed] [Google Scholar]
- 22. Saha S, Jing X, Park SY et al. Peptidoglycan recognition proteins protect mice from experimental colitis by promoting normal gut flora and preventing induction of interferon-gamma. Cell Host Microbe 2010; 8: 147–62. 10.1016/j.chom.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park SY, Gupta D, Hurwich R et al. Peptidoglycan recognition protein Pglyrp2 protects mice from psoriasis-like skin inflammation by promoting regulatory T cells and limiting Th17 responses. J Immunol 2011; 187: 5813–23. 10.4049/jimmunol.1101068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saha S, Qi J, Wang S et al. PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe 2009; 5: 137–50. 10.1016/j.chom.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li X, Wang S, Wang H et al. Differential expression of peptidoglycan recognition protein 2 in the skin and liver requires different transcription factors. J Biol Chem 2006; 281: 20738–48. 10.1074/jbc.M601017200 [DOI] [PubMed] [Google Scholar]
- 26. Schneider G, Krämer OH. NFκb/p53 crosstalk—a promising new therapeutic target. Biochim Biophys Acta 2011; 1815: 90–103. 10.1016/j.bbcan.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 27. Li H, Meng D, Jia J et al. PGLYRP2 as a novel biomarker for the activity and lipid metabolism of systemic lupus erythematosus. Lipids Health Dis 2021; 20: 95. 10.1186/s12944-021-01515-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eggert ML, Wallaschofski H, Grotevendt A et al. Cross-sectional and longitudinal relation of IGF1 and IGF-binding protein 3 with lipid metabolism. Eur J Endocrinol 2014; 171: 9–19. 10.1530/EJE-13-1017 [DOI] [PubMed] [Google Scholar]
- 29. Shrivastav SV, Bhardwaj A, Pathak KA et al. Insulin-like growth factor binding protein-3 (IGFBP-3): unraveling the role in mediating IGF-independent effects within the cell. Front Cell Dev Biol 2020; 8: 286. 10.3389/fcell.2020.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim HS. Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism. Ann Pediatr Endocrinol Metab 2013; 18: 9–12. 10.6065/apem.2013.18.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strickler HD, Fazzari M, Kovacs A et al. Associations of insulin-like growth factor (IGF)-I and IGF-binding protein-3 with HIV disease progression in women. J Infect Dis 2008; 197: 319–27. 10.1086/524848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanley TL, Fourman LT, Zheng I et al. Relationship of IGF-1 and IGF-binding proteins to disease severity and glycemia in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2021; 106: e520–33. 10.1210/clinem/dgaa792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao MN, Mulligan K, Tai V et al. Effects of insulin-like growth factor (IGF)-I/IGF-binding protein-3 treatment on glucose metabolism and fat distribution in human immunodeficiency virus-infected patients with abdominal obesity and insulin resistance. J Clin Endocrinol Metab 2010; 95: 4361–6. 10.1210/jc.2009-2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen S, Akter S, Kuwahara K et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci Rep 2019; 9: 7010. 10.1038/s41598-019-43431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petersen KF, Dufour S, Cline GW et al. Regulation of hepatic mitochondrial oxidation by glucose-alanine cycling during starvation in humans. J Clin Invest 2019; 129: 4671–5. 10.1172/JCI129913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mayo R, Crespo J, Martínez-Arranz I et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun 2018; 2: 807–20. 10.1002/hep4.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orešič M, Hyötyläinen T, Kotronen A et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia 2013; 56: 2266–74. 10.1007/s00125-013-2981-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study may be made available by the corresponding author upon reasonable request.