Abstract
Historically, investigators have not differentiated between patients with and without hemorrhagic transformation (HT) in large core ischemic stroke at risk for life-threatening mass effect (LTME) from cerebral edema. Our objective was to determine whether LTME occurs faster in those with HT compared to those without. We conducted a two-center retrospective study of patients with ≥ 1/2 MCA territory infarct between 2006 and 2021. We tested the association of time-to-LTME and HT subtype (parenchymal, petechial) using Cox regression, controlling for age, mean arterial pressure, glucose, tissue plasminogen activator, mechanical thrombectomy, National Institute of Health Stroke Scale, antiplatelets, anticoagulation, temperature, and stroke side. Secondary and exploratory outcomes included mass effect-related death, all-cause death, disposition, and decompressive hemicraniectomy. Of 840 patients, 358 (42.6%) had no HT, 403 (48.0%) patients had petechial HT, and 79 (9.4%) patients had parenchymal HT. LTME occurred in 317 (37.7%) and 100 (11.9%) had mass effect-related deaths. Parenchymal (HR 8.24, 95% CI 5.46–12.42, p < 0.01) and petechial HT (HR 2.47, 95% CI 1.92–3.17, p < 0.01) were significantly associated with time-to-LTME and mass effect-related death. Understanding different risk factors and sequelae of mass effect with and without HT is critical for informed clinical decisions.
Subject terms: Stroke, Neurology, Risk factors
Introduction
Life-threatening mass effect (LTME) is a critical condition in which space-occupying cerebral edema, with or without hemorrhagic transformation (HT) compresses crucial midline structures. LTME is observed in up to 40% of patients with non-revascularized Middle Cerebral Artery (MCA) ischemic stroke1. This condition, defined as the significant shift of brain structures due to increased intracranial pressure, leads to neurologic decline, disability, and up to an 80% mortality rate2 Despite these grave outcomes, decompressive hemicraniectomy (DHC) can significantly reduce mortality rates, though it is associated with considerable morbidity, necessitating careful patient selection.
The evolution of stroke treatment, particularly with the advent of mechanical thrombectomy, has significantly impacted management strategies and outcomes for acute ischemic stroke patients3. Yet, despite advancements, the differentiation between LTME resulting solely from cerebral edema versus LTME compounded by HT remains underexplored. This distinction is crucial, as the pathophysiological mechanisms underlying these conditions differ: cerebral edema typically results from fluid accumulation within brain tissues, leading to increased intracranial pressure, while HT involves bleeding into brain areas already damaged by ischemia, potentially exacerbating the mass effect and complicating clinical management. Moreover, it remains unclear to what extent HT associated with MT differs from HT in the absence of revascularization. These distinctions remain relevant even as MT eligibility has expanded in recent years, for those who remain ineligible.
Prior studies have delved into risk factors associated with LTME post-stroke4–6, but there remains a gap in literature specifically addressing the nuances between LTME secondary to cerebral edema and those instances compounded by HT. Differentiating between HT subtypes (parenchymal, petechial, none) is essential, as they have varying implications on treatment decisions and prognosis for this critically ill population. In light of this, our study aims to (1) describe the characteristics of HT subtypes in patients with large core MCA infarct and (2) examine whether parenchymal HT is associated with accelerated time to LTME and worse hospital outcomes compared to patients without HT.
Methods
The study was approved to be conducted by the Mass General Brigham Institutional Review Board (2017P002564). All methods were performed in accordance with the relevant guidelines and regulations set forth by the Institutional Review Board. Due to the retrospective nature of the study, the Mass General Brigham Institutional Review Board waived the need of obtaining informed consent.
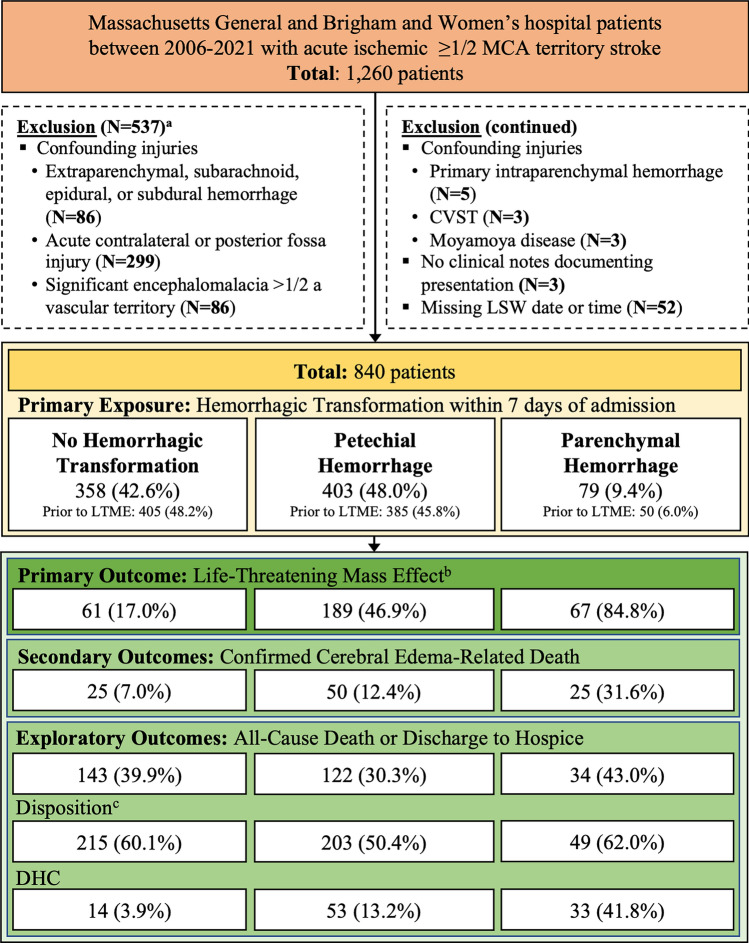
We conducted a two-center retrospective cohort study of adult patients with acute ischemic MCA stroke from the Brigham and Women’s and Massachusetts General Hospital admitted between 2006 and 2021 within 7 days of last seen well. We screened for acute MCA stroke using a previously described, highly sensitive Natural Language Processing algorithm7. All patients with possible large acute MCA stroke were confirmed to have a unilateral stroke size ≥ 1/2 of the MCA territory using direct visualization of radiology images on either CT or MR after acute interventions like tPA or MT by designated, trained, M.D members of the research team (SC, BB, CJO). Exclusion criteria are described in Fig. 1.
Figure 1.
Study sample selection diagram. Patient flow sheet of inclusion and exclusion criteria. N (%) or Median [25th–75th]. CVST Cerebral Venous Sinus Thrombosis, DHC Decompressive Hemicraniectomy, LSW Last Seen Well, MCA Middle Cerebral Artery. aPatients may meet multiple exclusion criteria. bHemorrhagic transformation classification occurs within 7 days of LSW. cLong-term care, hospice, or death.
We collected clinical information from the electronic medical record using the Research Data Patient Registry and Enterprise Data Warehouse. Imaging related variables were collected by visual review by a trained team member (SC, BB, CJO) after establishing inter-rater-reliability (SC, BB, CJO). Detailed information on data collection and quality control for variables and outcomes of interest are included in the Supplementary Methods. We prepared this report according to Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
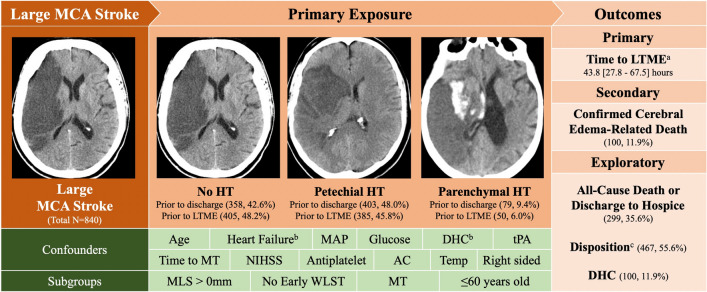
The primary outcome was time-to-LTME, defined as time from last seen well until imaging showed midline shift (MLS) measured at the level of the septum pellucidum of 5 mm or DHC (see Supplemental Methods for more detailed methodology). Under normal circumstances, the septum pellucidum is aligned centrally, indicating that the pressures in the two halves of the brain are balanced. MLS at the septum pellucidum indicates that there is some form of pressure differential across the two cerebral hemispheres. We chose 5 mm as an indicator of significant MLS as it has been used in other studies to predict malignant edema8. Also similar to other studies6,9–11, we used a composite clinical and radiographic outcome to identify life-threatening mass effect as it allows the capture of patients who clinically were felt to go on to have cerebral edema but otherwise would be censored by not having imaging confirmed substantive MLS. The secondary outcome was mass effect-related death, an outcome derived from chart review and team consensus, accounting for stroke size with associated MLS of at least 5 mm, temporality (either within traditional timelines of 2–5 days but screened up to two weeks for possible delayed edema cases), and lack of other possible causes. Further details are described in the supplementary analysis. We conducted exploratory analyses of all-cause death or discharge to hospice, disposition at discharge (long-term care, hospice, or death versus rehabilitation or home) and presence or absence of DHC only (Fig. 2).
Figure 2.
Study overview. Schematic of Exposures, Confounders and Subgroups for Primary, Secondary and Exploratory Outcomes. Representative images of no HT (42.6% of cohort), petechial HT (48% of cohort) and parenchymal HT (9.4% of cohort) shown below. N, % or Median [25th-75th]. AC Anticoagulation, DHC decompressive hemicraniectomy, HT hemorrhagic transformation, LTME life-threatening mass effect, MAP mean arterial blood pressure, MCA middle cerebral artery, MLS midline shift at septum pellucidum, MT mechanical thrombectomy, NIHSS national institute of health stroke score, tPA tissue plasminogen activator, WLST withdrawal of life sustaining therapy. aTime to LTME (MLS ≥ 5 mm or DHC)-only hemorrhagic transformation prior to LTME was considered. Hemorrhagic transformation classification within 7 days. bHeart failure and DHC were additional included covariates for the all-cause death and disposition models. cLong-term Care, Hospice, or Death. Images W:70, L:30.
The primary exposure of HT subtype was defined as patients with no HT, petechial HT only, or parenchymal HT (with or without petechiae). Grading of petechial and parenchymal HT occurred through direct visualization according to the European Co-operative Acute Stroke Study (ECASS II) classification scheme12 on head computed tomography (CT) or Magnetic Resonance Imaging by a trained MD (SC). Multivariable model covariates were selected by reviewing the literature and included age, admission mean arterial pressure, admission glucose, intravenous tissue plasminogen activator (tPA), occurrence and time to mechanical thrombectomy (MT), National Institute of Health Stroke Scale (NIHSS), prior antiplatelet use, prior anticoagulation use, temperature, and right-sided stroke, which has been previously associated with a trend toward increased odds of LTME13. For the secondary outcomes of death and disposition, DHC and heart failure were additional model covariates5,14–16.
Statistical analysis
We summarized patient characteristics at admission with means and standard deviations or frequencies and proportions. We used one-way analyses of variance or chi-square tests where appropriate to test for differences between the HT subtype groups.
For time-to-LTME, we used Cox regression. We considered HT subtype as a time-dependent covariate progressing from no HT to either petechial HT only or parenchymal HT. For LTME analysis, HT was only considered present if it occurred prior to or concurrent with LTME. LTME occurrence could be censored by death or discharge.
We used multivariable logistic regression for other outcomes, treating our primary exposure of HT subtype as a three-level variable with no HT as the reference group. We report odds ratios (OR) and p-values from Wald chi-square test on the coefficients. For DHC analysis, HT was only considered present if it occurred prior to DHC. For discharge outcomes, HT was considered present if it occurred within 7 days of stroke. We similarly report hazard ratios (HR) and p-values.
We performed sensitivity analyses of patients with at least some degree of MLS, early WLST within 24 h who would not have undergone subsequent imaging or care, patients who underwent MT, and patients under 60 years of age to mitigate potential biases conferred by stroke size, early withdrawal of life sustaining therapy (WLST), those who underwent MT, and age on outcome. We also performed a sensitivity analysis using HT classified only by CTs, as MR can have an increased sensitivity in detecting blood products.
We set the significance threshold at =0.05/2 = 0.025 using a Bonferroni correction17 to address the multiple endpoint multiplicity issue and maintain the overall type I error at 0.05 across the association of parenchymal HT and time-to-LTME and mass effect-related death. Other analyses used =0.05 and are hypothesis-generating only.
To handle missing values for structured variables (Supplementary Table 2), we employed multiple imputation by chained equations using the MICE package in R. For all analyses, we used RStudio Version 1.3.959 (RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC) for statistical analyses. Further statistical details are available in Supplementary Methods.
Results
Table 1 and Supplementary Table 7 describe admission characteristics of the 840 eligible patients (mean age 67.9, 48.2% female). Mean NIHSS was 17.2 ± 6.2, and mean Alberta Stroke Program Early CT Score (ASPECTS) was 4.4 ± 2.9. 41.3% of patients had strokes related to cardioembolism, 11.4% had strokes related to large artery atherosclerosis, 7.1% had strokes related to other determined etiology and 36.5% had stroke of undetermined etiology. Three-hundred and thirty-seven patients (40.1%) received intravenous tPA, and 142 (16.9%) received MT. In patients with MT, relevant door-to-needle and revascularization when available are included in Supplementary Table 1. Seventy patients (8.3%) were made comfort measures only within 24 h. Three-hundred and seventeen patients (37.7%) experienced LTME. One hundred (11.9%) received a DHC. One hundred patients had (11.9%) mass effect-related deaths. Two-hundred and forty-six patients (29.3%) of patients died of any cause or went to hospice by discharge. One hundred and sixty-eight patients (20.0%) went to long-term care, 342 (40.8%) went to rehabilitation, and 30 (3.6%) went home by discharge (Table 2).
Table 1.
Baseline characteristics.
| Variable | Total n = 840 | Hemorrhagic transformation subtypesa | |||
|---|---|---|---|---|---|
| No hemorrhage | Petechial | Parenchymal | p-value | ||
| n = 358 (42.6) | n = 403 (48.0) | n = 79 (9.4) | |||
| Demographics | |||||
| Age | 67.9 (15.5) | 69.7 (15.9) | 66.5 (15.3) | 66.5 (13.7) | 0.01 |
| Female | 405 (48.2) | 195 (54.5) | 182 (45.2) | 28 (35.4) | < 0.01 |
| Race | 0.48 | ||||
| White | 649 (77.3) | 286 (79.9) | 304 (75.4) | 59 (74.7) | – |
| Black | 53 (6.3) | 21 (5.9) | 27 (6.7) | 5 (6.3) | – |
| Asian | 33 (3.9) | 10 (2.8) | 17 (4.2) | 6 (7.6) | – |
| Otherb | 105 (12.5) | 41 (11.5) | 55 (13.6) | 9 (11.4) | – |
| Past medical history | |||||
| Hypertension | 598 (71.2) | 274 (76.5) | 267 (66.3) | 57 (72.2) | < 0.01 |
| Atrial fibrillation | 396 (47.1) | 176 (49.2) | 185 (45.9) | 35 (44.3) | 0.58 |
| Heart failure | 248 (29.5) | 118 (33.0) | 113 (28.0) | 17 (21.5) | 0.09 |
| Prior stroke | 93 (11.1) | 54 (15.1) | 33 (8.2) | 6 (7.6) | < 0.01 |
| Home medication | |||||
| Antiplatelets | 246 (29.3) | 112 (31.3) | 111 (27.5) | 23 (29.1) | 0.53 |
| Anticoagulants | 98 (11.7) | 40 (11.2) | 46 (11.4) | 12 (15.2) | 0.59 |
| statins | 247 (29.4) | 103 (28.8) | 115 (28.5) | 29 (36.7) | 0.33 |
| LSW to presentation (hours) | 7.34 [3.93–17.8] | 6.35 [3.87–16.2] | 7.83 [4.02–21.6] | 9.50 [5.25–19.2] | 0.10 |
| TOAST stroke subtypec | 0.58 | ||||
| Large artery atherosclerosis | 96 (11.4) | 33 (9.2) | 54 (13.4) | 9 (11.4) | |
| Cardioembolic | 347 (41.3) | 153 (42.7) | 159 (39.5) | 35 (44.3) | |
| Small vessel occlusion | 0 (0.0) | 0 (0.0 ) | 0 (0.0) | 0 (0.0) | |
| Other known cause of stroke | 60 (7.1) | 28 (7.8) | 29 (7.2) | 3 (3.8) | |
| Unknown cause of stroke | 307 (36.5) | 131 (36.6) | 145 (36) | 31 (39.2) | |
| Right sided stroke | 435 (51.8) | 173 (48.3) | 217 (53.8) | 45 (57.0) | 0.20 |
| Admission ASPECTS | 4.4 (2.9) | 4.6 (3.0) | 4.3 (2.9) | 4.2 (2.9) | 0.25 |
| NIHSS | 17.2 (6.2) | 17.2 (6.5) | 17.3 (6.0) | 17.2 (6.1) | 0.97 |
| Occlusion location | 335 (39.9) | 142 (39.7) | 165 (40.9) | 28 (35.4) | 0.52 |
| Internal carotid artery | 151 (45.1) | 64 (45.1) | 75 (45.5) | 12 (42.9) | – |
| M1 | 129 (38.5) | 55 (38.7) | 61 (37.0) | 13 (46.4) | – |
| M2 | 51 (15.2) | 23 (16.2) | 25 (15.2) | 3 (10.7) | – |
| M3 or M4 | 4 (1.2) | 0 (0.0) | 4 (2.4) | 0 (0.0) | – |
| Acute intervention | |||||
| Tissue plasminogen activator | 337 (40.1) | 126 (35.2) | 173 (42.9) | 38 (48.1) | 0.03 |
| Mechanical thrombectomy | 142 (16.9) | 43 (12.0) | 72 (17.9) | 27 (34.2) | < 0.01 |
| Admission labs | |||||
| HbA1c (mmol/mol) | 6.2 (1.3) | 6.1 (1.2) | 6.4 (1.4) | 6.4 (1.5) | 0.04 |
| Glucose (mg/dL) | 150.8 (65.2) | 145.7 (58.0) | 155 (71.2) | 152.5 (62.7) | 0.14 |
| White blood cell count (K/UL) | 11.8 (7.6) | 11.8 (5.4) | 12.0 (9.6) | 11.6 (4.1) | 0.89 |
| Creatinine (mg/DdL) | 1.1 (0.8) | 1.1 (0.8) | 1.1 (0.7) | 1.2 (1.1) | 0.15 |
| Sodium (mEq/L) | 138.1 (3.6) | 137.8 (3.7) | 138.2 (3.6) | 138.8 (3.1) | 0.07 |
| Admission vital signs | |||||
| Systolic blood pressure (mmHg) | 149.2 (29.8) | 149.1 (30.6) | 149.1 (29.2) | 150 (29.7) | 0.97 |
| Pulse (beats per minute) | 82.7 (21.1) | 82.8 (21.7) | 83.9 (21.6) | 78.1 (17.2) | 0.36 |
| Temperature (Fahrenheit) | 97.9 (1.2) | 97.6 (0.9) | 98.0 (1.3) | 98.2 (1.4) | 0.07 |
Mean (SD), Median [25th–75th], or No. (%).
ASPECTS alberta stroke program early CT score, LSW last seen well, MGB mass general Brigham, NIHSS national institutes of health stroke scale, toast- trial of org 10,172 in acute stroke treatment.
aHemorrhagic transformation classified by any HT within 7 days of LSW.
bOther Races, includes American Indian, Alaska Native, Native Hawaiian, Pacific Islander, Not Recorded, Not given or Unknown.
cToast Classification: Of the 305 patients with stroke of Unknown Etiology, 42 had evidence of cardioembolic and large artery atherosclerosis or other known cause of stroke; Of 62 patients with other known cause of stroke, 24 had carotid dissection.
Table 2.
Cohort Outcomes.
| Variable | Total n = 840 | Hemorrhagic transformation subtypes | p | ||
|---|---|---|---|---|---|
| No hemorrhage | Petechial | Parenchymal | |||
| n = 358 (42.6) | n = 403 (48.0) | n = 79 (9.4) | |||
| Mass effect | |||||
| Midline shift | |||||
| Midline shift > 0 mm | 603 (71.8) | 190 (53.1) | 339 (84.1) | 74 (93.7) | < 0.01 |
| Midline shift ≥ 5 mm | 295 (35.1) | 54 (15.1) | 178 (44.2) | 63 (79.7) | < 0.01 |
| Maximum midline shift (mm) | 6.2 (4.7) | 4.5 (2.9) | 6.5 (4.2) | 9.6 (4.7) | < 0.01 |
| Treatment | |||||
| Osmotic therapy | 282 (33.6) | 81 (22.6) | 157 (39.0) | 44 (55.7) | < 0.01 |
| Decompressive hemicraniectomy | 100 (11.9) | 14 (3.9) | 53 (13.2) | 33 (41.8) | < 0.01 |
| WLST within 24 h | |||||
| DNR or DNI | 187 (22.3) | 102 (28.5) | 68 (16.9) | 17 (21.5) | < 0.01 |
| Comfort measure only | 70 (8.3) | 43 (12.0) | 18 (4.5) | 9 (11.4) | < 0.01 |
| Outcomes | |||||
| Time to LTMEa (hours) | 43.8 [27.8–67.5] | 46.7 [29.1–67.9] | 49.6 [31.0–73.5] | 31.8 [24.8–47.0] | < 0.01 |
| Life threatening mass effectb | 317 (37.7) | 61 (17.0) | 189 (46.9) | 67 (84.8) | < 0.01 |
| Confirmed mass effect-related death | 100 (11.9) | 25 (7.0) | 50 (12.4) | 25 (31.6) | < 0.01 |
| All-cause death or hospice | 299 (35.6) | 143 (39.9) | 122 (30.3) | 34 (43.0) | < 0.01 |
Mean (SD), Median [25th–75th], or No. (%).
DNI do not intubate, DNR Do not resuscitate, LTME Life-threatening mass effect, WLST withdrawal of life sustaining therapy.
aHemorrhagic transformation classified by any HT within 7 days of LSW.
bOf 317 patients who had LTME, 283 (89.3%) met definition by MLS 5, and 10.7% met definition through Decompressive Hemicraniectomy first.
Three-hundred and fifty-eight patients (42.6%) had no HT, 403 (48.0%) had petechial HT, and 79 (9.4%) had parenchymal HT within the first 7 days of admission. ECASS II subtype breakdown is included in the supplementary methods. Thirty-eight petechial HT (9.4%), and 29 (36.7%) parenchymal HT occurred after LTME, and were not included in the LTME survival analysis. Sixteen new petechial HT (4.0%), and 22 (27.8%) new parenchymal HT occurred after DHC, and were not included in the exploratory DHC analysis. (Supplementary Table 8).
Differences between non-HT, petechial HT, and parenchymal HT included age, female sex, hypertension, prior stroke, Hba1c, tPA, and MT (Table 1). Median time-to-petechial HT was 39.8 h and median time to parenchymal HT was 25.3 h. Petechial HT could and did occur prior to parenchymal HT in 29 of 79 patients. Median time-to-LTME occurred at 46.7 [25th-75th range 29.1–67.9], 49.6 [31.0–73.5], and 31.8 [24.8–47.0] hours in patients with no, petechial, and parenchymal HT, respectively. Time-to-death did not appear substantially different between HT subtypes, though length of stay appeared longer in patients with hemorrhage (8 [4–13] v. 9 [6–16] v. 12 [6–19] days) (Supplementary Table 10). Univariable associations are included in Supplementary Table 4.
In the multivariable Cox model, parenchymal (HR 8.24, 95% CI 5.46–12.4, p < 0.01) and petechial (2.47 [1.92–3.17], p < 0.01) HT were significantly associated with time-to-LTME (Table 3). Parenchymal HT was associated with 9.25 [4.35–19.7] (p < 0.01) odds of mass effect-related death, and 4.39 [1.77–10.9] (p < 0.01) odds of DHC. Petechial HT was associated with two-fold odds of mass effect-related death (OR 2.18, CI [1.27–3.72], p < 0.01), but not DHC. There was no significant difference between HT subtype and all-cause death or discharge to hospice or discharge disposition, though there was a trend to significance for HT (p = 0.07) (Table 4).
Table 3.
Time-to-LTME multivariable Cox proportional hazard regression (n = 840a).
| Variables | Time to life-threatening mass effectb | |
|---|---|---|
| HR (95 CI) | p | |
| Parenchymal hemorrhage | 8.24 (5.46–12.4) | < 0.01 |
| Petechial hemorrhage | 2.47 (1.92–3.17) | < 0.01 |
Abb.: LTME, Life-Threatening Mass Effect; CI, Confidence Interval; HR, Hazard Ratio.
Primary exposure: parenchymal HT; Secondary exposure: petechial HT.
aFor the survival analyses, patients were classified into HT subtype based on images concurrent or prior to LTME. Four hundred and five had no HT, 385 had petechial only, and 50 had parenchymal HT.
bMidline shift ≥ 5 mm or decompressive hemicraniectomy; hemorrhagic transformation classification within 7 days.
Table 4.
Multivariable logistic regression for secondary and exploratory outcomes (n = 840).
| Confirmed ME-related death | All-cause death or hospice | Dispositiona | DHCb | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95 CI) | p | OR (95 CI) | p | OR (95 CI) | p | OR (95 CI) | p | |
| Parenchymal | 9.25 (4.35–19.7) | < 0.01 | 1.82 (0.96–3.45) | 0.07 | 1.56 (0.86–2.84) | 0.14 | 4.39 (1.77–10.9) | < 0.01 |
| Petechial | 2.18 (1.27–3.72) | < 0.01 | 0.75 (0.53–1.06) | 0.10 | 0.77 (0.55–1.08) | 0.13 | 1.55 (0.91–2.63) | 0.10 |
Hemorrhagic transformation classification within 7 days.
ME mass effect, CI confidence interval, DHC decompressive hemicraniectomy, OR odds ratio.
aLong-term care, hospice, or death v. rehabilitation or home.
bFor the DHC analysis, patients were classified into HT subtype based on images prior to of concurrent with DHC. Three-hundred and eighty had no HT, 402 had petechial only, and 57 had parenchymal HT.
We observed that parenchymal HT had significant HRs of LTME in all subgroups, and mass effect-related death (Supplementary Table 5). The association of petechial HT with secondary and exploratory outcomes in most subgroups were non-significant. Complete results are included in Supplementary Tables 4 and 5.
Discussion
In patients with large MCA stroke, we found that parenchymal and petechial HT was associated with shorter time-to-LTME and increased risk of mass effect-related death. Our observation that patients with parenchymal or petechial HT progress more rapidly toward LTME may be in part due to the natural timeline of HT. Median time to symptomatic HT after tPA administration has been shown to be 18.25 h18, and may accelerate swelling from ischemia-related cerebral edema, which traditionally is thought to peak at 48–72 h after stroke onset1. We also found that patients with parenchymal and petechial HT had higher maximum MLS and were more likely to receive hyperosmolar therapy and DHC. While other studies have reported that the most severe ECASS II parenchymal HT subtype (PH-2) patients were associated with clinical deterioration and outcome19,20, it has not been previously established that parenchymal HT hastens the course of LTME and radiographic MLS. We found that petechial hemorrhage is also associated with a higher HR of LTME (p < 0.01) which in addition to our observation that petechial HT was associated with mass effect-related death (p < 0.01), may be contrary evidence to previous reports that petechial HT is not associated with poor outcome19–21. However, while we observed that petechial HT had a significant increased HR of LTME (p < 0.01), the longer LTME time compared to the no HT group is somewhat counterintuitive, as is the trend toward decreased all-cause death at discharge. We posit that patients with petechial HT had later petechial HT (median time-to-petechial HT 39.8 h v. 25.3 h) and subsequent LTME than their parenchymal counterparts, but was still associated with an increased HR of LTME once it occurred. Regarding the conflicting trend toward decreased all-cause death, we hypothesize that location and extent of petechial HT may impact its association with outcome, and may warrant further study to determine benign and malignant petechial phenotypes.
Parenchymal (p < 0.01) and petechial (p < 0.01) HT were significantly associated with mass effect-related death. This is consistent with the high rate of LTME (which is closely associated with mass effect-related death) in patients with parenchymal HT (84.8%) and petechial HT (46.9%) compared to none (17.0%). Our observation that HT was associated with mass effect-related death is consistent with the post hoc analysis of the ECASS finding of close to 50% mortality associated with PH-2 patients19. In our study, death or hospice by discharge was 43.0% for patients with any parenchymal HT, and 58.1% in patients with PH-2. Parenchymal HT was also associated with increased odds of receiving DHC in all subgroups except patients who received MT (p = 0.08).
Neither patients with parenchymal nor petechial HT were significantly more likely to experience all-cause death or hospice than those with no HT, though parenchymal HT trended toward significance. While the rate of all cause-death was slightly lower in patients with petechial HT, it was nonsignificant. The lack of a statistical association may be due to the high mortality in all groups given the severity of ischemic stroke; death due to causes outside of mass effect may occur similarly between the three groups. It is also possible that DHC may have resulted in decreasing the mortality relative to the no HT group, as there was a marked difference in the percent of patients who received DHC (42 v. 4% of parenchymal HT v. no HT). This potential rationale would be in line with prior studies showing DHC can decrease death from 80 to 20%2,22–25. We posit that DHC may have occurred more frequently because parenchymal HT tends to occur within the guideline-recommended < 48 h from stroke onset and appeared to occur in slightly younger patients (mean of 66.5 v. 69.7 years of age). Of our 246 deaths by discharge that 100 (40.7% of deaths) were confirmed due to mass effect, 90 (36.6%) were indeterminate, and 55 (22.4%) were confirmed from other causes. Disposition was also not significantly different in patients with parenchymal or petechial HT.
Similar to other studies, HT appeared to be associated with male sex, MT, temperature, stroke side, Asian race (parenchymal), HbA1c (petechial), and age (petechial) in our cohor26–31. HT is a known risk factors of tPA, in which symptomatic HT occurs in 2–7% of patients16.
Increased HT risk after MT is thought to be secondary to reperfusion injury of damaged tissue15. It is notable that in our cohort there are more parenchymal HT patients with MT than petechial or no HT patients (34.2 v. 17.9 v. 12%). It is possible that over the course of the study period technical improvements in MT procedures and patient selection may result in less HT in a “MT rich” era, after multiple studies demonstrating its benefit3. Delays in MT, either due to patient presentation time or door-to-needle time also raises concern for increased HT risk. In our population, we did not note substantive differences between door to needle time—in fact there was a slight increase in door to needle time for the no HT population (7.4 v. 6.2 v. 5.3 h). We expect that because the period of our study was largely before the adoption of MT policies that included either late-presenter and/or larger core infarcts32–34 that small differences between MT times did not substantively impact HT occurrence.
Regarding other associations we observed, acute hyperglycemia is associated with the development of cerebral edema, which may lead to worsened vascular compression and HT predilection6. HbA1c as a marker of chronic hyperglycemia may be reflective of diabetic microangiopathy associated with increased vulnerability to hemorrhage35. Hyperthermia has been shown to be associated with blood–brain barrier disruption in animal models36, and studies show that impaired cardiovascular regulation due to right-sided stroke37,38 may contribute to overall increased edema, injury, and potentially HT formation31.
Unlike other studies which found that amount of hypodensity was associated with HT26, we did not find a significant difference in stroke volume (as approximated by baseline ASPECTS score) and parenchymal HT. The observed differences between our study’s findings and others could partially be attributed to our predefined large-volume stroke population of interest ( 1/2 MCA territory) as well as our three-group differentiation of HT instead of the traditional HT v. non-HT. Stroke size as a factor for HT might not be as important when all sizes of the stroke in the groups are more than half the MCA territory. Other notable findings include that patients with parenchymal HT received DHC later than patients without HT (median time-to-DHC of 40.4 v 34.9 h, respectively), which may suggest that the occurrence of HT prompts life-saving heroic measures when it occurs even outside of the customary 48 h window. Furthermore, less than a third (31.5%) of MCA stroke patients were 60 years or younger. This is a clinically meaningful observation, given that standard guidelines provide strong recommendations for preemptive DHC in patients 60 only1,39,40. Given that more than two-thirds of patients are older than 60, and therefore do not have strong recommendations for practice, it may be worth considering therapeutic trials that focus specifically on an older cohort, contrary to most existing studies.
Prior well-designed clinical trials have led to post hoc investigations of HT and outcome, but only in broad cohorts with any stroke size after receiving medical or mechanical intervention15,16. The association of HT to outcomes in patients at risk from LTME due to large core stroke, especially after more regular use of acute stroke interventions including tPA and MT is less well characterized. Distinguishing the course of patients with different HT subtypes in large stroke is clinically relevant because they may have a different timeline for their peak MLS, respond differently to clinical interventions, and ultimately go on to have different outcomes. This is especially important in the era in which there is increasing evidence supporting acute interventions in patients with large core infarct33,41.
We recognize several important limitations of our study. It is a retrospective, observational study and therefore residual confounding cannot be excluded. Our cohort came from two hospital systems from a single region in which the majority of the population is White and generalizability is uncertain. We also acknowledge the limitations of our composite outcome, MLS 5 mm or DHC. Age and atrophy and anatomy can influence how devastating a given amount of midline shift can be. However, we used 5 mm as it has previously been used as indicator of severe cerebral edema because is highly associated with mortality and need for DHC4,6,42. We also adjusted for age as a covariate, though we were not able to for atrophy or anatomy. While DHC is an important marker for clinical judgement of life-threatening mass effect, we recognize that there is heterogeneity in its timing between clinical providers, centers, and over time. Despite its limitations, our composite outcome enables a feasible way to capture of timing of a devastating complication in a large dataset and to determine whether different variables like HT have different courses and outcomes. To amass a sufficient sample size, our retrospective study included patients over a long time-period in which there were developments that could alter clinical behavior, including regular use of MT. We found 33.6% of patients prior to 2015 had LTME, versus 46.5% thereafter. We posit that along with increasing use of MT, there may have been less early WLST. While the methods of intervention, particularly concerning MT, have evolved significantly, we believe that data on patients from before the widespread adoption of MT remains important and applicable to current practices. This is particularly true as not all patients, especially those from lower socio-economic backgrounds, are able to receive MT promptly or before major injuries occur, thus limiting the full benefits of this treatment.
HT was determined by imaging review, and petechial HT can be better seen on magnetic resonance imaging, which may have led to differences in how petechial HT was classified in patients with CTs only. Moreover, some studies had available susceptibility weighted imaging whereas others only had T2*-weighted gradient-echo sequence, which may have led to some heterogeneity in how small petechial hemorrhage was detected. However, our results were consistent when using only CTs to classify hemorrhages (Supplementary Table 4). Not all patients had an NIHSS. To mitigate this, we created inferred NIHSS from physical exams, possibly leading to artificially lower NIHSS (see Supplementary Methods). While we did not have precise stroke/hemorrhage volume, Demestere’s group reported that patients with ASPECTS < 7 reliably identified core volumes of > 70 cc43, suggesting that our mean ASPECTS score of 4.4 ± 2.9 is well within traditionally defined large MCA stroke volumes used in other studies44. Moreover, a random sampling of 30 patients using an ABC/2 method45 demonstrated that median volume was 122 cc3 (Supplementary Methods). There were no scheduled time points of imaging, however, the median frequency of imaging data within the first 48 and 72 h was 8.16 [3.97–16.4] and 10.6 [4.98–19.9] hours respectively, suggesting that most patients were closely observed to detect our endpoint (Supplementary Methods).
We did not have data on functional outcome either on discharge or 3 months, or neurologic deterioration. Early WLST within 24 h occurred in 22.3% of patients. It is important to acknowledge the bias conferred by WLST may confound our results, obfuscating the risk factors that may lead to unobserved MLS, and potentially misclassifying patients who may have otherwise survived their injury and subsequent mass effect. Finally, we were limited in exploring the association of delays to recanalization due to small sample sizes and because we did not have consistent available data for our whole cohort.
Despite these limitations, the strengths of our study are notable. We meticulously gathered and ascertained a comprehensive set of clinical, laboratory, and radiographic data from a broad cohort of patients across various hospital centers, ensuring a robust dataset. This extensive collection allowed us to classify cerebral edema phenotypes based on hemorrhagic transformation (HT) subtypes, providing novel insights into the distinct risk factors and outcomes associated with each HT category.
We found that patients with large MCA stroke who experience parenchymal HT are more likely to rapidly progress to LTME, and have high mortality, despite a higher rate of DHC. We also observed that patients with petechial HT were also at higher risk of quicker progression and mass effect related death than patients with no HT. Understanding the different clinical courses of HT subtypes in patients with large MCA stroke is important for clinical decision making and prognostication.
Supplementary Information
Acknowledgements
This research was presented at the American Neurological Association (ANA) Conference in October 2022. We would like to acknowledge the assistance of Leigh Ann Mallinger who assisted with manuscript preparation.
Author contributions
CJO, DMG, and SMS conceived the idea of the presented analyses and designed the overall study. JEP, ISYK, YZ, LY organized and collected the patient data for analysis. ISYK, BB, CJO, SC, KMS, LC, HS, KM, MC, OB performed manual reviews of patient notes to extract various features such as stroke onset times, medications, procedures, and outcomes. BB developed code for automated feature extraction from radiology reports. SC, BB, CJO manually reviewed and radiographic images to measure swelling and determine radiographic outcomes. JEP performed statistical analysis and interpretation. LT, QH, EJB, JD provided oversight and review of the statistical analysis. JEP, ISYK, CJO drafted the manuscript. BB, LT, SMS, EJB, JD, DMG helped with reviews and revisions. CJO provided overall study direction and critical review. All authors contributed to the article and approved the submitted version.
Funding
CJO receives support from NIH/NINDS K23NS116033; AHA 23CDA1041762. EJB receives support from R01 HL092577; American Heart Association AF AHA 18SFRN34110082. LT receives support from AHA 18SFRN34150007. DMG receives support from R01 NS102574. SMS receives support from R01 EY024019.
Data availability
Deidentified data can be made available upon request. For access, please email the lead and corresponding authors (JEP, CJO) with an explanation of your intended use. We will review your request, and if approved, provide data within 7 days. We reserve the right to withhold identifiable information such as external patient identifiers, dates of birth, and patient notes. All dates may be consistently shifted back in time up to 364 days so they do not reflect the actual dates, but preserve the intervals between them.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-60635-0.
References
- 1.Wijdicks EF, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222–1238. doi: 10.1161/01.str.0000441965.15164.d6. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, et al. 'Malignant' middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 1996;53(4):309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 3.Yarbrough CK, et al. Endovascular thrombectomy for anterior circulation stroke: Systematic review and meta-analysis. Stroke. 2015;46(11):3177–3183. doi: 10.1161/STROKEAHA.115.009847. [DOI] [PubMed] [Google Scholar]
- 4.Wu S, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke. 2018;49(12):2918–2927. doi: 10.1161/STROKEAHA.118.022001. [DOI] [PubMed] [Google Scholar]
- 5.Miao J, et al. Predictors of malignant cerebral edema in cerebral artery infarction: A meta-analysis. J. Neurol. Sci. 2019;409:116607. doi: 10.1016/j.jns.2019.116607. [DOI] [PubMed] [Google Scholar]
- 6.Ong CJ, et al. Enhanced detection of edema in malignant anterior circulation stroke (EDEMA) score: A risk prediction tool. Stroke. 2017;48(7):1969–1972. doi: 10.1161/STROKEAHA.117.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong C, et al. New methods of natural language processing using machine learning methods to identify ischemic stroke presence, acuity, and location from clinical radiology reports. Mach. Learn. Healthc. 2019 doi: 10.1371/journal.pone.0234908. [DOI] [Google Scholar]
- 8.Pullicino PM, et al. Mass effect and death from severe acute stroke. Neurology. 1997;49(4):1090–1095. doi: 10.1212/WNL.49.4.1090. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y, et al. External validation and modification of the EDEMA score for predicting malignant brain edema after acute ischemic stroke. Neurocrit. Care. 2020;32(1):104–112. doi: 10.1007/s12028-019-00844-y. [DOI] [PubMed] [Google Scholar]
- 10.Kimberly WT, et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: A secondary analysis of the MR CLEAN trial. JAMA Neurol. 2018;75(4):453–461. doi: 10.1001/jamaneurol.2017.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foroushani HM, et al. Quantitative serial CT imaging-derived features improve prediction of malignant cerebral edema after ischemic stroke. Neurocrit. Care. 2020;33(3):785–792. doi: 10.1007/s12028-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guenego A, et al. Hemorrhagic transformation after stroke: Inter- and intrarater agreement. Eur. J. Neurol. 2019;26(3):476–482. doi: 10.1111/ene.13859. [DOI] [PubMed] [Google Scholar]
- 13.Li J, et al. Stroke lateralization in large hemisphere infarctions: Characteristics, stroke-related complications, and outcomes. Front. Neurol. 2021;12:774247. doi: 10.3389/fneur.2021.774247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacke W, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–51. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 15.Thoren M, et al. Effect of recanalization on cerebral edema in ischemic stroke treated with thrombolysis and/or endovascular therapy. Stroke. 2020;51(1):216–223. doi: 10.1161/STROKEAHA.119.026692. [DOI] [PubMed] [Google Scholar]
- 16.Yaghi S, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(12):e343–e361. doi: 10.1161/STR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 18.Chen PM, et al. Timing of symptomatic intracerebral hemorrhage after rt-PA treatment in ischemic stroke. Neurol. Clin. Pract. 2019;9(4):304–308. doi: 10.1212/CPJ.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger C, et al. Hemorrhagic transformation of ischemic brain tissue: Asymptomatic or symptomatic? Stroke. 2001;32(6):1330–1335. doi: 10.1161/01.STR.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 20.Fiorelli M, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30(11):2280–2284. doi: 10.1161/01.STR.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 21.Trouillas P, von Kummer R. Classification and pathogenesis of cerebral hemorrhages after thrombolysis in ischemic stroke. Stroke. 2006;37(2):556–561. doi: 10.1161/01.STR.0000196942.84707.71. [DOI] [PubMed] [Google Scholar]
- 22.Juttler E, et al. DESTINY II: Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery II. Int. J. Stroke. 2011;6(1):79–86. doi: 10.1111/j.1747-4949.2010.00544.x. [DOI] [PubMed] [Google Scholar]
- 23.Juttler E, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): A randomized, controlled trial. Stroke. 2007;38(9):2518–2525. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]
- 24.Vahedi K, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 25.Hofmeijer J, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 2009;8(4):326–333. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 26.Larrue V, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32(2):438–441. doi: 10.1161/01.STR.32.2.438. [DOI] [PubMed] [Google Scholar]
- 27.Marsh EB, et al. Predicting hemorrhagic transformation of acute ischemic stroke: Prospective validation of the HeRS score. Medicine (Baltimore) 2016;95(2):e2430. doi: 10.1097/MD.0000000000002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paciaroni M, et al. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc. Dis. 2009;28(2):119–123. doi: 10.1159/000223436. [DOI] [PubMed] [Google Scholar]
- 29.Zhang G, et al. Hemoglobin A1c predicts hemorrhagic transformation and poor outcomes after acute anterior stroke. Eur. J. Neurol. 2018;25(12):1432–e122. doi: 10.1111/ene.13747. [DOI] [PubMed] [Google Scholar]
- 30.Leira R, et al. A higher body temperature is associated with haemorrhagic transformation in patients with acute stroke untreated with recombinant tissue-type plasminogen activator (rtPA) Clin. Sci. (Lond.) 2012;122(3):113–9. doi: 10.1042/CS20110143. [DOI] [PubMed] [Google Scholar]
- 31.Rastogi V, et al. Hemispheric differences in malignant middle cerebral artery stroke. J. Neurol. Sci. 2015;353(1–2):20–7. doi: 10.1016/j.jns.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Nogueira RG, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 33.Sarraj A, et al. Trial of endovascular thrombectomy for large ischemic strokes. N. Engl. J. Med. 2023;388(14):1259–1271. doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 34.Albers GW, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N. Engl. J. Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acampa M, et al. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int. J. Cardiol. 2017;243:466–470. doi: 10.1016/j.ijcard.2017.03.129. [DOI] [PubMed] [Google Scholar]
- 36.Urakawa M, et al. Blood-brain barrier disturbance following localized hyperthermia in rats. Int. J. Hyperthermia. 1995;11(5):709–18. doi: 10.3109/02656739509022502. [DOI] [PubMed] [Google Scholar]
- 37.Meyer S, et al. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport. 2004;15(2):357–61. doi: 10.1097/00001756-200402090-00029. [DOI] [PubMed] [Google Scholar]
- 38.Yoon BW, et al. Cerebral hemispheric lateralization in cardiac autonomic control. Arch. Neurol. 1997;54(6):741–4. doi: 10.1001/archneur.1997.00550180055012. [DOI] [PubMed] [Google Scholar]
- 39.Powers WJ, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 40.Juttler E, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N. Engl. J. Med. 2014;370(12):1091–100. doi: 10.1056/NEJMoa1311367. [DOI] [PubMed] [Google Scholar]
- 41.Huo X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N. Engl. J. Med. 2023;388(14):1272–1283. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 42.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N. Engl. J. Med. 1986;314(15):953–8. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- 43.Demeestere J, et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017;88(24):2248–2253. doi: 10.1212/WNL.0000000000004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomalla GJ, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke. 2003;34(8):1892–9. doi: 10.1161/01.STR.0000081985.44625.B6. [DOI] [PubMed] [Google Scholar]
- 45.Sims JR, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72(24):2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data can be made available upon request. For access, please email the lead and corresponding authors (JEP, CJO) with an explanation of your intended use. We will review your request, and if approved, provide data within 7 days. We reserve the right to withhold identifiable information such as external patient identifiers, dates of birth, and patient notes. All dates may be consistently shifted back in time up to 364 days so they do not reflect the actual dates, but preserve the intervals between them.