Abstract
Nicotinamide adenine dinucleotide (NAD+) is an essential coenzyme involved in many pathophysiological processes. Supplementation with NAD+ and its precursors has been demonstrated as an emerging therapeutic strategy for the diseases. NAD+ also plays an important role in the reproductive system. Here, we summarize the function of NAD+ in various reproductive diseases and review the application of NAD+ and its precursors in the preservation of reproductive capacity and the prevention of embryonic malformations. It is shown that NAD+ shows good promise as a therapeutic approach for saving reproductive capacity.
Keywords: Nicotinamide adenine dinucleotide, NAD+, NAD+ precursors, Female reproduction, Ovarian diseases, Gynecologic tumor
Graphical abstract
Highlights
-
•
NAD+ precursors supplementation or depletion inhibition offers a non-invasive gynecological disease treatment.
-
•
Supplementation with NAD+ rescue ovarian aging, PCOS and assisted reproduction.
-
•
Supplementation with NAD+ rescue spontaneous abortions and congenital malformations caused by NAD+ deficiency.
1. Introduction
Fertility is a unique physiological function of the female. The number of oocytes in mammals is fixed from the beginning of embryonic development and remains essentially constant after birth. In general, after the age of 35, the quantity and quality of oocytes gradually decline and fertility is also threatened [1]. In addition, reproductive endocrine disorders such as polycystic ovary syndrome (PCOS) and tumors of the reproductive system also pose a massive damage to women's fertility. Currently, hormone replacement therapy (HRT) and assisted reproductive technology (ART) are mainly used to improve the pregnancy rate of women, but both have limitations [2,3]. Therefore, the search for new therapeutic breakthroughs to preserve fertility in women of reproductive age is the focus and difficulty of current research.
Nicotinamide adenine dinucleotide (NAD+) is a redox cofactor and enzyme substrate (its reduced form is NADH) involved in biochemical processes such as glycolysis, the tricarboxylic acid (TCA) cycle, and mitochondrial oxidative phosphorylation (OXPHOS), and is essential for mitochondrial function and metabolism, ATP synthesis and storage, DNA repair, and epigenetics [4]. It is involved in many physiopathological processes including tumors, aging, oxidative stress, and inflammation, and is an important factor in human health and longevity [5,6]. NAD+ also plays a significant role in the reproductive system. With age, NAD+ levels decrease in reproductive system organs such as the ovary and uterus, which can be reversed by the intervention of NAD+ precursors [7,8]. NAD+ is a promising treatment for preserving fertility.
In this study, we reviewed the role of NAD+ in female reproduction diseases, highlighted the protective effect of NAD+ precursor supplementation on fertility and other diseases that are related to females, and aim to provide new perspectives for therapeutic research in female reproduction.
2. Biosynthesis of NAD+
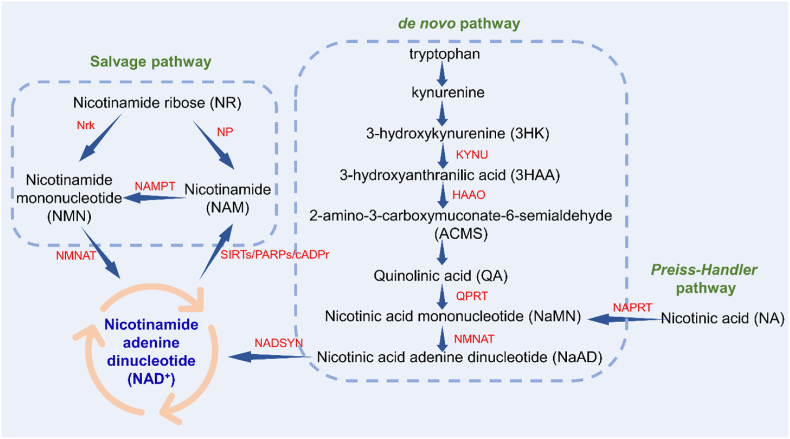
NAD+ was originally considered as an auxiliary component to enhance alcoholic fermentation, and the process of the rise and fall of NAD+ has been described in detail in the review by Luis et al. [9]. In mammals, NAD+ is synthesized from four precursors tryptophan (Trp), nicotinic acid (NA), nicotinamide ribose (NR), and nicotinamide (NAM) via different biosynthetic pathways, including the de novo pathway, the Preiss-Handler pathway, and the salvage pathway (Fig. 1).
Fig. 1.
The biosynthesis process of NAD+.
The de novo pathway begins with dietary-derived Trp. Trp produces quinolinic acid (QA) through a series of catabolism in the kynurenine pathway. Among them, 3-hydroxyanthraninlic acid oxygenase (HAAO) and kynureninase (KYUN) play important roles in embryonic development and congenital malformations [10], which we will describe in detail later. QA is converted to nicotinic acid mononucleotide (NaMN) by quinolinic acid phosphate ribosyltransferase (QPRT). NaMN adenyltransferases (NMNATs) catalyze the conversion of NaMN to nicotinic acid adenine dinucleotide (NaAD). NaAD is then converted to NAD+ by glutamine-dependent NAD+ synthase (NADSYN) [11]. In the Preiss-Handler pathway, NA is converted to NaMN by nicotinic acid phosphoribosyltransferase (NAPRT) and then converted to NAD+ [12]. The salvage pathway is the main source of cellular NAD+ synthesis and involves the conversion of NAM to nicotinamide mononucleotide (NMN) via the action of nicotinamide phosphate ribosyltransferase (NAMPT), the rate-limiting enzyme of the NAD+ biosynthetic pathway. NMN is then catalysed by nicotinamide mononucleotide adenyltransferase (NMNAT) to form NAD+. Simultaneously, NAD+ can be converted back to NAM depending on the enzyme substrate [13]. NR is converted to NMM or NAM via the nicotinamide ribokinase (Nrk) or nucleoside phosphorylase (NP), respectively, and then into the salvage pathway [14].
Three subcellular compartments of NAD+ have been identified - mitochondria, nucleus, and cytoplasm - and mitochondrial NAD+ plays a key role in the energy production pathway [15]. NAD+ acts as a hydrogen acceptor, allowing electron transfer for redox reactions, which leads to ATP production in the mitochondria. Decreased intracellular NAD+ levels lead to decreased ATP levels, which ultimately leads to cell death through energy depletion [16].
3. Extracellular NAD + precursors
NAD+ precursors are important forms of exogenous NAD+ supplementation, including Trp, NR, NA, NAM, etc., mainly contained in natural vegetables, fruits, meat, eggs, fish, such as broccoli, cabbage, avocados, beef, etc. [17]. To prevent the adverse effects of NA or Trp deficiency such as pellagra, the recommended daily intake of NA for adult men and women is recommended to be 16 and 14 mg per day, respectively [18]. NAD+ precursors show clinical potential in many metabolic and age-related diseases, and there have been a very large number of preclinical and clinical studies on the regulation of NAD + homeostasis by supplementation with NAD + precursors.
NMN is one of the NAD+ intermediates and is a reaction product of NAMPT. NMN has been shown to be effective in attenuating age-related physiological decline in mice when given long-term (12 months) with no significant side effects [19]. NMN also plays a pivotal role in a series of diseases such as cardiovascular and cerebrovascular diseases, diabetes, obesity, and alcoholic liver disease, which may be related to the reversal of mitochondrial homeostasis, reactive oxygen species (ROS) production, and DNA repair [20].
NR is an NAD+ precursor present in milk and is already available as a nutraceutical. Oral supplementation with NR increases NAD+ levels in several tissues and improves mitochondrial function and stem cell regeneration potential [21,22]. In addition, NR is currently considered a suitable precursor compared to other NAD+ precursors. A clinical study showed that long-term NR supplementation was well tolerated and effective in stimulating NAD+ metabolism in healthy middle-aged and elderly people, with a selective improvement trend in cardiovascular-related parameters such as blood pressure, aortic stiffness, etc., and more importantly, did not cause serious side effects [23].
Both NA and NAM are water-soluble forms of vitamin B3. High cellular NAD+ levels are achieved by NA supplementation to maintain poly (ADP-ribosylation) and SIRTs activity, enhance DNA repair, and chromosome stability [24]. In human cells, NAM is readily converted to NMN and then to NAD+ via the salvage pathway. Inhibition of NAMPT can eliminate most of the effects of NAM [25].
In addition to the NAD+ precursors mentioned above, studies on the novel precursors dihydronicotinamide ribose (NRH) and reduced nicotinamide mononucleotide (NMNH) have gradually increased in recent years. NRH is a reduced form of NR, a new and very efficient fifth pathway for NAD+ synthesis. Unlike NR, which is phosphorylated to NMN by Nrk, NRH is phosphorylated by adenosine kinase (AK) and converted to NMNH and subsequently to NADH and NAD+ [26,27]. NRH can improve mitochondrial function and regulate oxidative stress [28]. NMNH is the reduced form of NMN, which is converted to NAD+ by NMNAT and inhibits glycolysis, the TCA cycle, and cell growth [29]. Surprisingly, NRH and NMNH increased NAD+ levels to a much greater extent than NR and NMN [29,30].
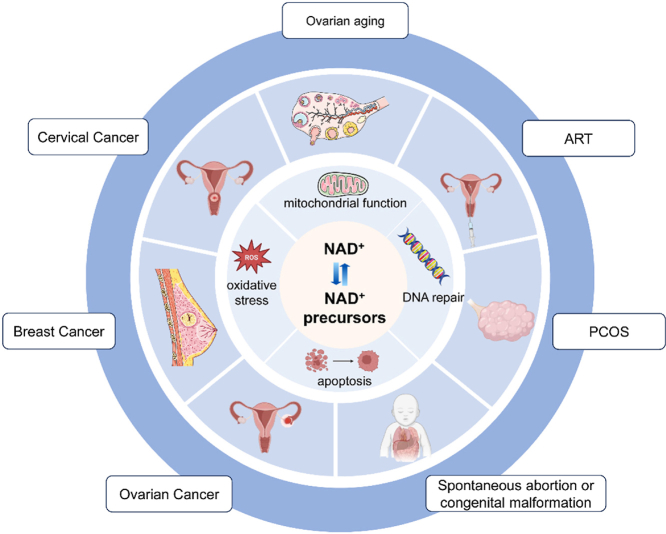
4. Effects of NAD+ and its precursors on reproductive disorders
The quality of the oocyte, the microenvironment of the follicle, and the interactions between the reproductive systems all contribute to female infertility. Since NAD+ is involved in a wide range of biological processes, disruption of the NAD+ pool in the reproductive system has implications for ovarian aging, PCOS, embryonic development, and tumors. And a large body of evidence suggests that supplementation of NAD+ precursors and intervention of NAD+ biosynthesis have beneficial effects on related diseases, showing great potential in protecting women's health and improving female reproduction.
4.1. Ovarian aging
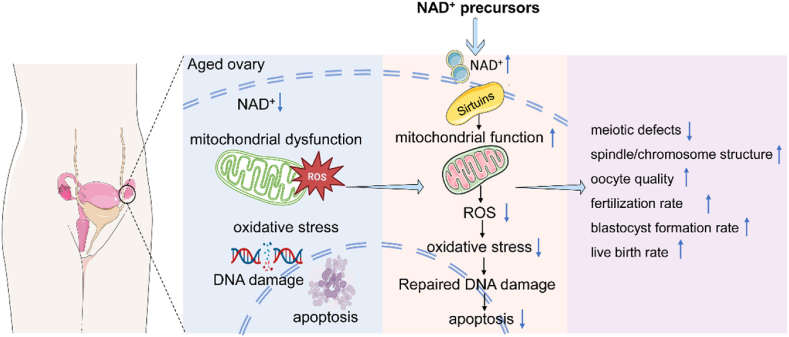
Age-related decline in oocyte quantity and quality is a major obstacle in the treatment of infertility in middle-aged women. Studies of ovarian NAD+ levels using cycling assays and ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) have shown that, as in other organs, NAD+ levels were reduced in aging oocytes, as evidenced by a 50–70 % reduction in NAD+ levels in the ovaries of 8-month-old mice compared to 2-month-old mice. For 12-month-old mice, NAD+ levels in the ovaries were approximately 20–30 % of the levels in 2-month-old mice. In contrast, NADH levels increased progressively with age and the NAD+/NADH ratio decreased [31]. Bertolo et al. found a decrease in NAD(P)H levels in 12-month-old aged mice, but this decrease occurred only in oocytes and not in the entire ovary [32]. Studies conducted on other species such as porcine follicles [33], equine follicles [34], and humans [35] have shown similar results with age-related decreases in NAD+. All these results indicate that NAD+ is closely related to ovarian aging and that the maintenance of NAD+ levels in the follicles are necessary to ensure normal ovarian development.
Maintenance of follicular NAD+ levels can be achieved by supplementation with NAD+ precursors. Supplementation with NAD+ precursors (such as NMN, NR, NA, and NAM) protects the first meiosis of aged oocytes and reduces the incidence of defects in spindle components such as spindle and chromosome and aneuploidy while improving the number of pregnancies and live births in aged mouse (Table 1) [32,[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. Most of the mechanisms that NAD+ precursors protecting ovarian preservation are through improving mitochondrial function, decreasing ROS levels, reducing oxidative damage, inhibiting DNA damage, and apoptosis (Fig. 2). Miao et al. [37] performed single-cell transcriptome analysis of oocytes from young, aged and NMN-added aged mice. The results showed that genes related to oxidative phosphorylation pathway, mitochondrial membrane fraction, mitochondrial respiratory chain, microtubules, spindle and DNA repair were mis-expressed in aged oocytes compared to young oocytes, but these were restored in NMN-added aged oocytes. All these pathways or biological processes are highly relevant to mitochondrial function. A constant number of follicles in the primordial follicle pool and oocytes in prolonged germinal vesicle (GV) phase while waiting for meiosis to resume make them susceptible to DNA damage and meiosis-related damage, with the accumulation of reactive oxygen species ROS being a major cause of such damage. Mitochondrial DNA (mtDNA) is more vulnerable to ROS attack [49]. Oocytes accumulate more mtDNA mutations during aging. mtDNA mutation accumulation reduces female fertility by impairing the NAD+/NADH redox status of oocytes [36]. The genes for mitochondrial fusion (Opa1, Mfn1, and Mfn2) and fission (Drp 1) were misexpressed in aged oocytes, but were restored to levels comparable to those in young oocytes after NMN supplementation [37]. Mitochondrial dysfunction and excess ROS lead to early apoptosis and DNA damage. After NAD+ precursor supplementation, DNA damage was recovered as indicated by the DNA damage markers γ-H2AX and annexin-V staining [39]. When mitochondrial function is restored, the oocyte can obtain energy to drive normal development. Supplementation with NAD+ precursors allows the recovery of hindered meiotic defects, such as the repair of errors in the spindle and chromosome. This was also demonstrated by the simultaneous detection of actin [39,40].
Table 1.
Supplementation with NAD + precursors improve ovarian aging and fertility outcomes.
| Intervention | Species | Mechanisms | Fertility outcomes | Ref |
|---|---|---|---|---|
| NMN | Mice | rescues of the NADH/NAD redox state in oocytes damaged by mtDNA mutations | improved the first litter rate | [36] |
| Mice | protects the first meiosis and reduces spindle assembly defects and the incidence of aneuploidy | oocyte quality, follicle dynamics, embryo development, and live birth rates were recovered | [32] | |
| Mice | restores meiotic maturation in aged oocytes, improves mitochondrial function, reduces ROS levels, and inhibits DNA damage and apoptosis | enhanced sperm binding ability of aged oocytes, improved fertilization ability, and early embryonic development of aged oocytes | [37] | |
| Mice | up-regulates the level of mitochondrial metabolism and autophagy, increases the antioxidant capacity of follicles, and reduces oxidative damage | improved endocrine function, increased follicular reserve, and delayed fertility decline | [38] | |
| Mice | improves mitochondrial function and oxidative stress, reduces DNA damage and early apoptosis | – | [39] | |
| Pig | enhances mitochondrial function and inhibits oxidative stress-induced apoptosis | improved the fertilization rate of aged oocytes | [40] | |
| Pig | restores meiotic defects, enhances mitochondrial function, and inhibits ROS, DNA damage, and apoptosis | promoted oocyte development and early embryonic development | [41] | |
| Pig | restores mitochondrial function eliminates excess ROS, and rescues meiotic defects | – | [42] | |
| NR | Mice | improves mitochondrial function | increased ovulation potential and live birth rate | [31] |
| Mice | reduces ROS levels, DNA damage, and apoptosis | improved fertilization potential and embryo development potential | [43] | |
| NA | Mares | improves maternal age-related meiotic defects | oocyte-matured blastocysts contain more cells | [34] |
| Mice | improves maternal age-related meiotic defects and oocyte oxidative stress | – | [44] | |
| Mice | inhibits apoptosis | – | [45] | |
| Pig | – | improve blastocyst formation rate | [46] | |
| NAM | Pig | – | blastocysts contain more cells | [46] |
| Mice | inhibits fragmentation and spindle elongation and minimizes acetylation of α-tubulin during oocyte senescence | – | [47] | |
| Mice | reduces ROS levels and oxidative stress | promoted oocyte maturation and improved fertilization rate | [48] |
Fig. 2.
NAD + precursors protect the ovary by improving mitochondrial function, lowering ROS levels, reducing oxidative damage, and inhibiting DNA damage and apoptosis.
Mitigation of ovarian aging by supplementation with NAD+ precursors has attracted attention as a potential geriatric protection strategy, which can maintain ovarian health and improve female fertility. However, NAD+ precursor supplementation appears to be a process that requires long-term adherence, and short-term studies appear to be less effective than long-term studies using NAD+ precursors in drinking water [50]. Stringer J et al. [51]exposed mice to γ-irradiation or cyclophosphamide 8 days after NMN supplementation and collected ovaries and serum for analysis on day 12. The results showed that γ-irradiation and cyclophosphamide significantly reduced the number of primordial follicles, but NMN supplementation did not prevent follicle loss. Compared to similar trials, the intervention time of Stringer J et al. ‘s study was shorter, and other studies were mostly 2 months, half a year, or even up to a year, and they stopped NMN treatment after chemotherapy. These may be the reasons why their study showed that NMN could not compensate for ovarian tissue damage caused by γ-irradiation or cyclophosphamide.
4.2. ART
For women without the need for infertility treatment, HRT is usually used when symptoms are related to ovarian aging or premature ovarian failure. For women who wish to have children, estrogen and progesterone supplementation cannot salvage the quality of damaged oocytes. In contrast to natural conception, ART such as artificial insemination and in vitro fertilization-embryo transfer, can help improve the success rate of pregnancy.
Oocyte quality deteriorates with time after ovulation, affecting the outcome of early embryonic development during ART. NAD+ levels in post-ovulatory mouse oocytes were reduced during in vitro culture, and supplementation with NAD+ precursors prevented this reduction, improved the oocyte quality and early embryonic development potential of post-ovulatory oocytes, and effectively increased the fertilization rate of older oocytes, thus potentially improving the success rate of ART. Supplementation with NA improved in vitro porcine oocyte maturation and blastocyst formation, while supplementation with NAM improved the germinal vesicle breakdown (GVBD) rate and increased the maturation and developmental potential [46]. NMN supplementation significantly promoted the blastocyst formation rate of fertilized oocytes in aged mice, improved the fertilization capacity of aged oocytes, facilitated their subsequent embryonic development, and ameliorated infertility in mtDNA mutant female mice [36,37]. NR restored the quality of MII oocytes and enhanced the early embryonic development potential of post-ovulatory oocytes by alleviating mitochondrial dysfunction and maintaining normal spindle/chromosome structure, probably by reducing ROS levels in post-ovulatory oocytes and decreasing DNA damage and apoptosis [43].
4.3. PCOS
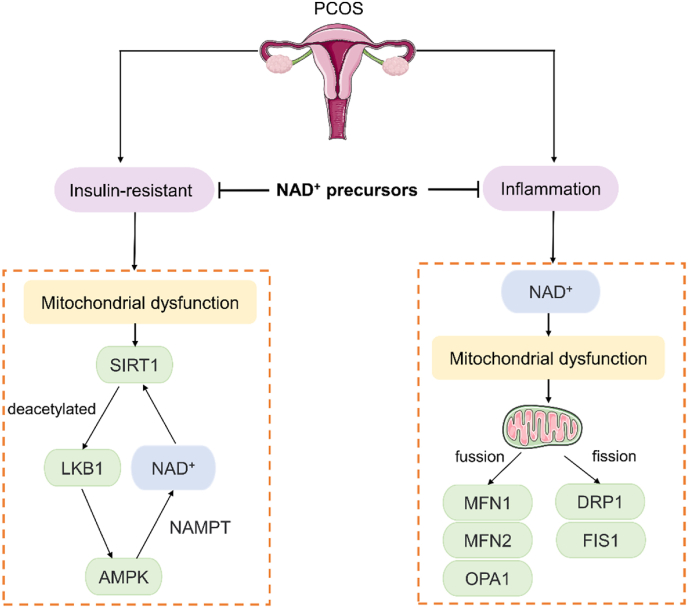
PCOS is an inflammatory, systemic female reproductive endocrine disorder that manifests primarily as hyperandrogenism, leading to a range of health problems such as infertility, obesity, and insulin resistance, and is considered the most common cause of anovulatory infertility [52]. Inadequate NAD+ pools in the oocytes of patients with PCOS may affect follicle and oocyte development. A cross-sectional study found that the tryptophan-kynurenine pathway was dysregulated in patients with PCOS and showed a positive correlation with clinical indicators of PCOS such as luteinizing hormone, anti-Müllerian hormone, and fasting insulin [53]. Patients with PCOS are prone to metabolic disturbances related to glucose and insulin levels, and the increased capacity of oocytes to consume glucose may be related to the production of NAD(P)H or purine precursors via pentose phosphate pathway, NMN increased muscle insulin sensitivity and corrected hyperinsulinemia, obesity and hepatic steatosis [54].
Regarding the pathogenesis of PCOS, it is currently believed to be closely related to insulin resistance, inflammation and hyperandrogenemia. SIRT1, a NAD + -dependent histone deacetylase, regulates metabolism and regulates oxidative stress. NAD+ deficiency may cause dysfunction of NAD+ catabolic enzymes such as SIRTs, particularly mitochondrial function, and intracellular signaling [55]. AMP-activated protein kinase (AMPK) also plays an important role in the regulation of glucose-lipid metabolism, insulin sensitivity, and inflammatory response, both of which are synergistic. In insulin-resistant states, SIRT1 is decreased in patients with PCOS, and liver kinase B1 (LKB1) is deacetylated by SIRT1 and translocated to the cytoplasm to phosphorylate and activate AMPK [56]. AMPK increases the intracellular NAD+/NADH ratio indirectly activating SIRT1 [57]. Meanwhile, the effect of SIRT1 on deacetylation requires the cofactor NAD+, and NAMPT is the rate-limiting enzyme of the NAD+ synthesis pathway. NAMPT enhances SIRT1 activity by promoting NAD+ biosynthesis and induces steroidogenesis in bovine ovarian granulosa cells. AMPK activation significantly upregulates NAMPT expression, which enhances SIRT1 activity [58]. The AMPK-SIRT1 pathway enhances oocyte and embryo quality through anti-inflammatory, anti-apoptotic, and antioxidant effects [59,60]. The co-occurrence of hyperandrogenemia and hyperinsulinemia is one of the major causes of metabolic problems such as insulin resistance in PCOS. 17,20-lyase, and 17-hydroxylase (CYP17A1) are rate-limiting enzymes in androgen production, and insulin and LH induce ovarian androgen production by raising CYP17A1. N1-methylnicotinamide and NAM significantly activated AMPK-CYP17A1, limited the production of androgens, and reversed the hyperandrogenic state of PCOS [61].
Several clinical studies have shown a low-grade chronic inflammatory state in the peripheral blood and ovarian microenvironment of PCOS patients [[62], [63], [64], [65]]. NAD+ levels are decreased in granulosa cells (GCs) of patients with PCOS, which is accompanied by the activation of inflammation. Xie et al. summarized the relationship between NAD+ and inflammation in detail [66]. The intracellular inflammatory response disrupts mitochondrial structure and function, leading to oxidative stress, impaired cellular metabolism and compromised cell proliferation. As the level of inflammation increases, inflammation reduces NAD+ levels and damages the mitochondrial homeostasis apparatus, leading to abnormal mitochondrial metabolism [67]. Metabolic and karyotype analysis revealed that NAD(P)H was higher in the oocytes of patients with PCOS, and these observed NAD(P)H were mainly derived from cytoplasm and mitochondria [68]. Mitochondrial activity was significantly correlated with oocyte NAD(P)H content, and oocytes with a larger proportion of active mitochondria tended to have higher NAD(P)H levels [68]. Wang et al. [69]showed that decreased NAD + levels in PCOS patients were accompanied by inflammatory activation of GCs. And after LPS treatment of KGN cells, ROS content was significantly increased in LPS-treated KGN cells compared with controls. It has also been shown that in PCOS GCs, the mRNA levels of mitochondrial fusion-related genes (including MFN1, MFN2, and OPA1) are all downregulated, but the mRNA levels of mitochondrial fission-related genes DRP1 and FIS1 are upregulated, the mitochondrial membrane potential is decreased, and ATP production is significantly reduced [69]. Supplementation with NR increased NAD+ levels in GCs and rescued inflammation associated with impaired OXPHOS and mitochondrial dysfunction [69]. In a word, NAD+ precursors rescue PCOS by improving insulin resistance, inflammation and mitochondrial function (Fig. 3).
Fig. 3.
NAD + precursors rescue PCOS by improving insulin resistance, inflammation and mitochondrial function.
4.4. Spontaneous abortion or congenital malformation
Embryo survival and development depend on the provision of adequate levels of NAD+ during pregnancy. Studies investigating human dietary NA requirements have shown that women need more NA during pregnancy to maintain more complex physical changes [70]. NAD+ deficiency during pregnancy has been identified as the cause of congenital malformations in recurrent spontaneous abortions due to genetic disruption of the nicotinamide synthesis pathway. Early mouse embryos are dependent on maternal contributions of NAD+ until the liver can synthesize it. Restriction of maternal intake of NAD+ precursors leads to embryonic loss and multiple congenital malformations in wild-type mice. Therefore, there is an elevated risk of NAD+ deficiency during pregnancy, when an adequate supply of NAD+ is required to ensure normal embryonic development [71]. Birth defects resulting from insufficient NAD+ synthesis during embryogenesis are called congenital NAD+ deficiency and most commonly affect the heart, kidneys, vertebrae and limbs [72]. Mice were fed with a vitamin - and Trp-free NAD+ precursor diet, and different concentrations of Trp were added to drinking water. The results showed that the maintenance of pregnancy increased, early embryonic necrosis and live embryo malformation were reduced in a concentration-dependent manner, and organ malformations mainly occurred in fingers, bones, and hearts [73]. It has also been shown that NAD+ deficiency causes malformations in the development of the umbilical cord and body wall, mainly manifested as body wall closure defects, amniotic band development and consequences, and umbilical artery development and anomalies [74].
The de novo pathway is affected by biallelic mutations in HAAO, KYNU, and NADSYN1. HAAO encodes HAAO, KYNU encodes KYNU, and NADSYN1 encodes NAD synthetase 1 enzyme (NADSYN1). All of them are enzymes involved in the kynurenine pathway and can affect embryonic development by disrupting the synthesis of NAD+, and these variants are responsible for congenital malformations and abortions in humans and mice (Table 2). HAAO and KYNU variants have been identified in studies of gene sequencing in families with multiple congenital disorders [10]. Genetic testing of a patient with Catel-Manzke syndrome, which is characterized by micrognathia and hyperphalangism, has identified possible recessive loss-of-function or sub-polymorphic variants in KYNU, including deletions of exons 1–8 and three very rare missense variants, and metabolomics analysis of the patient's urine and plasma supports canine urease deficiency [75]. VCRL syndrome (Vertebral, Cardiac, Renal, and Limb Defect Syndrome) is a congenital organ defect characterized by congenital vertebral malformations and other organ defects, and genetic loss of KYNU and HAAO has been associated with VCRL syndrome [76]. However, if adequate NAD+ levels are maintained through dietary supplementation, there is no impact on embryonic development [10,73,77]. Biallelic mutations in the NADSYN1 have also been reported as a cause of VCRL syndrome [78]. NADSYN1 encodes the final enzyme in the de novo pathway, the yeast homolog protein Qns1, which converts NaAD to NAD+ [79]. Analysis of NADSYN1 in patients with congenital vertebral malformations (CVM) revealed eight variants in nine unrelated patients, including two truncated variants and six missense variants. All enrolled patients had multiple organ defects involving the heart, kidneys, limbs, or liver, as well as intraspinal malformations [80]. As demonstrated in HAAO- or KYNU-deficient mice, defects induced by NADSYN1 mutations can be rescued by dietary supplements enriched with NAD + precursors [79]. SLC6A19 is the major apical transporter of free Trp in the gut and proximal tubules of the kidney. Studies have shown that maternal deficiency of SLC6A19 if Trp is restricted in the diet, leads to retention of Trp by intestinal and renal absorption, and the embryo receives insufficient Trp and other NAD+ precursors, thereby increasing embryonic mortality and congenital malformations. According to the Genome Aggregation Database (gnomAD), approximately 1 in 578 people carry the predicted loss-of-function variant in SLC6A19. Female carriers of this SLC6A19 mutation may be susceptible to poor pregnancy outcomes if there is insufficient Trp or vitamin B3 to produce NAD+ during pregnancy [81].
Table 2.
Congenital NAD + deficiency-related gene pathogenic variants, malformations, and Metabolism or NAD+ levels.
| Gene | Area | Pathogenic variants | Malformations | Metabolism or NAD+ levels | Ref |
|---|---|---|---|---|---|
| HAAO | Australia; United States |
①homozygous for a c.483dupT variant, leading to a stop codon (p.D162*) ②homozygous for a c.558G→A variant, leading to a stop codon (p.W186*) |
congenital vertebral and heart malformations; postnatal growth and cognitive defects |
upstream: 3HAA was elevated 64-fold/385-fold; downstream: levels of NAD+ and NAD(H) were one-third to one-quarter of the levels in the other family members; NA supplementation prevented the disruption of embryogenesis |
[10] |
| Europe | ①II.8 [c.128G > A p. (Arg43Lys)]; [c.141C > A p. (His47 Gln)] ②II.1 [c.43del p. (Arg15Glyfs*99)]; [c.43del p. (Arg15Glyfs*99)] ③II.3 [c.301G > T p. (Gly101Trp)]; [c.323G > A p. (Arg108 Gln)] |
diverse congenital heart defects; limb defects; vertebral anomalies |
reduced growth and total NAD+ levels | [72] | |
| China | HAAO rs3816183 TT phenotype: homozygous risk for rs3816183 | toward anterior/middle hypospadias | – | [82] | |
| KYNU | Australia; United States |
①homozygous for a c.170-1G→T splicing variant, leading to a stop codon downstream (p.V57Efs*21); ②heterozygous for KYNU variants c.468 T→A and c.1045_1051delTTTAAGC, each variant results in a stop codon (p.Y156* and p.F349Kfs*4, respectively). |
congenital vertebral and heart malformations; postnatal growth and cognitive defects |
upstream:3HK (161 times the other family members); downstream: levels of NAD+ and NAD(H) were one-seventh of the levels in the other family members; NA supplementation prevented the disruption of embryogenesis |
[10] |
| Europe | ①II.2 [c.788 A > G p. (His263Arg)]; [c.788 A > G p. (His263Arg)] ②II.1 [c.616G > A; p. (Glu206Lys)]; [c.616G > A; p. (Glu206Lys)]; ③II.4 [c.361_363del p. (Lys121del)]; [c.1035 T > A p. (Ser345Arg)]; ④II.2 [c.489 del p. (Ala164Profs*26)]; [c.1282C > T p. (Arg428Trp)] |
limb defects; congenital heart defects; mild facial dysmorphism |
reduced total NAD+ levels | [72] | |
| Europe | ①c.1282C > T (p.Arg428Trp) ②c.989G > A (p.Arg330Gln) ③c.326G > C (p.Trp109Ser) |
Catel-Manzke syndrome | kynureninase deficiency and xanthurenic aciduria | [75] | |
| Germany | 863 bp deletion (g.del 142954376-g.142955239): loss of exon 5
|
VCRL | urine xanthurenic acid was elevated | [76] | |
| Somalia | c.593 A > G substitution leading to a threonine-to-alanine (T198A) shift | no symptoms of niacin deficiency | xanthurenic aciduria | [83] | |
| NADSYN1 | France | ①c.1717G > A, p. (Ala573 Thr) (PP5 +8, PM2 +2, PP4 +1, BP1 -1) (paternally inherited); c.644 T > C, p. (Leu215Pro) (PP3 +4, PM2 +2, PM3 +2, PP4 +1, BP1 -1) (maternally inherited); ②c.1717G > A, p. (Ala573 Thr) |
VCRL | – | [78] |
| Australia | ①II.1 c.1717G > A (p.Ala573Thr) and c.1717G > A (p.Ala573Thr); ②II.2 c.1717G > A (p.Ala573Thr) and c.1717G > A (p.Ala573Thr); ③II.1 c.1717G > A (p.Ala573Thr) and c.1819 del (p.Val607Trpfs∗30); ④II.4 c.145 T > C (p.Cys49Arg) and c.395G > T (p.Trp132Leu); ⑤II.1 c.735 T > A (p.Cys 245∗) and c.1839C > G (p.Tyr613∗) |
defects of the heart, kidney, and vertebrae | NAD+ levels are severely reduced | [79] | |
| China | ①c.232G > A (p.Val78Ile) ②c.709G > A (p.Gly237Arg) ③c.861delT (p.Arg288GlufsTer14) ④c.1037G > A (p.Arg346Gln) ⑤c.1216C > T (p.Arg406Ter) ⑥c.1511G > A (p.Arg504Gln) ⑦c.1762G > A (p.Glu588Lys) ⑧c.2083G > A (p.Glu695Lys) |
CVM | – | [80] | |
| United States | ①c.395G > T (p.Trp132Leu), (paternally inherited), c.85+1G > C, (maternally inherited); ②c.1717G > A (p.Ala573Thr) and c.2014C > T (p.Arg 672*) |
cardiac and vertebral defects | – | [84] | |
| Denmark | c.1717G > A (p.Ala573Thr) | skeletal malformations; aortic stenosis |
NAD+ pool was lower in the patient; NAD+ levels increased after 10 weeks of oral NAM supplementation |
[85] |
Under the influences of genes and environment, NAD+ synthesis is impaired and NAD+ deficiency is easy to lead to adverse pregnancy outcomes such as abortion and embryo malformation. If the innate gene is damaged, it can be supplemented with NAD+ precursors as a means, rather than directly increasing the maternal NAD + level through the de novo pathway.
4.5. Reproductive tumors
The treatment of tumors includes surgery, radiotherapy, and chemotherapy, etc. Radiotherapy and chemotherapy can easily cause ovarian toxicity, leading to the depletion of primordial follicles and affecting female fertility. There is growing evidence that tumor cells require up-regulation of NAD+ biosynthesis to meet their growth requirements. As NAD+ is involved in a variety of biological processes such as mitochondrial function, DNA repair, and epigenetics, which are the basis for cancer cell proliferation and differentiation, sustained NAD+ production is a hallmark of many tumor types [86].
4.5.1. Ovarian cancer (OC)
OC is one of the leading causes of cancer death in women, with a five-year survival rate of less than 45 % [87]. Detection of circulating NAD+ metabolism-related genes (NMRG) in patients with OC showed that most of them were strongly associated with OC prognosis, and they were significantly enriched in pathways related to NA and NAM metabolism and NAD+ metabolism [88]. The levels of tumor suppressor TP53 correlated with several NMRGs, and were positively correlated with NADK, NAXDs, NMRK2, NT5C2, NT5C1B, PARP16, PARP4, PARP8, QPRT, RNLS, SIRT1, SIRT3, SIRT5, and negatively correlated with NAXE [88]. These results indicate that NAD+ plays an important role in OC, and NMRG is a potential biomarker for OC, which may provide a biological target for the diagnosis and treatment of OC.
BRCA1 is a tumor suppressor gene that plays a key role in many cellular processes. BRCA1 mRNA expression reduction mediated by promoter hypermethylation was found to be inversely correlated with intracellular NAD + levels in OC cells. Overexpression of intracellular BRCA1 effectively reduced NAD + levels in A2780, HO-8910, and ES2 cells, as well as in primary non-BRCA1-mutant and BRCA1-mutant OC cells. Knockdown of BRCA1 increased NAD + levels in the above three cells, and the mechanism may be related to the regulation of intracellular NAMPT and restriction of NAD+ synthesis [89]. Meanwhile, BRCA1 inactivity mediates loss of H3K9ac enrichment around the core promoter inhibiting DNA methyltransferase 1 (DNMT1) transcription, which causes upregulation of DNMT1 levels, as well as increased NAD+ levels in non-BRCA1 mutant OC cells [90].
NAMPT is overexpressed in human tumors which helps maintain NAD+ stores and promotes OXPHOS, ATP production, protein synthesis, and DNA repair [[91], [92], [93]]. The NAD+/NADH ratio was significantly reduced with the small molecule NAMPT inhibitors FK866 or GMX1778 [94]. When FK866 and cisplatin were administered together, the addition of NAMPT inhibitor significantly delayed tumor recurrence and improved in vivo survival in epithelial ovarian cancer (EOC) mice compared with the cisplatin administered alone. The mechanism may be through the regulation of senescence-related secretory phenotype (SASP) in an NF-κB-dependent manner, and supplementation of NMN significantly increased SASP gene expression [95].
4.5.2. Breast cancer (BC)
BC is the most common cancer in women. Yang et al. [96]used ssGSEA to calculate NAD+ biosynthesis scores of patients in the TCGA database and found that NAD+ biosynthesis scores were significantly higher in BC tissues than in normal breast tissues, and that NAD+ biosynthesis activity correlated with BC prognosis and immune microenvironment. Patients with high NAD+ biosynthesis scores had a poor prognosis, high immune infiltration, high immunogenicity, and elevated PD-L1 expression and may benefit more from immunotherapy. The extracellular form of NAMPT is called visfatin, which regulates NAD+ concentration inside and outside BC cancer cells and also induces proliferation. The mechanism is related to the up-regulation of SIRT1 activity and the deacetylation of p53 [97]. Supplementation with NMN promoted SIRT1 activity, leading to deacetylation and functional inactivation of p66Shc and inhibition of epithelial-mesenchymal transition, thereby inhibiting tumor growth in triple-negative BC mice [98]. NAMPT-mediated NAD+ salvage pathway contributes to BC promotion. Inhibition of NAMPT with miR-206, miR-381, miR-154, and miR-613 significantly reduced the malignancy of MCF-7 and MDA-MB-231 cells [[99], [100], [101], [102]]. Treatment with NA or NAM enhanced the NAD+/NADH ratio in MDA-MB-435 and MDA-MB-231 cells. In the subsequent mechanistic study, NA or NAM treatment activated tumor cell autophagy in vitro and in vivo, reduced the expression of autophagy-expressing gene p62, and decreased AKT/mTORC1 signaling [103]. Measurement of NAMPT and p73 levels in BC cell lines revealed that human BC cell lines have high NAMPT and low p73 levels and that NMN reverses the low NAD+ levels caused by the NAMPT inhibitor FK866 and regulates autophagic p73 stability [104].
4.5.3. Cervical cancer (CC)
CC is the second most common female cancer. Patients with CC have significantly lower NAD+ compared to healthy volunteers [105]. Fluorescence lifetime imaging microscopy (FLIM) was used to analyze NAD(P)H in exfoliated cervical cells from healthy volunteers and patients with different degrees of cancerization. The results showed that cancer cells have a relatively short average fluorescence lifetime and less protein-bound NAD(P)H ratio and are more prone to glycolysis than OXPHOS compared with normal cells [106]. Chen et al. [107] identified 293 NAD+ metabolic-related genes and 21 prognostic NAD+ metabolic-related genes in 39 CC patients: ADAMTS10, ANGPTL5, APCDD1L, CCDC85A, CGREF1, CHRDL2, CRP, DENND5B, EFS, FGF8, P4HA3, PCDH20, PCDHAC2, RASGRF2, S100P, SLC19A3, SLC6A14, TESC, TFPI, TNMD, ZNF229, and for the first time described SLC19A3 as a prognostic signature through NAD + metabolism, providing clinical prognostic biomarkers and therapeutic targets for CC. Immunohistochemical analysis of NAMPT in cancerous tissues showed that the level of NAMPT increased with the grade of cervical intraepithelial neoplasia and squamous cell carcinoma [108]. Compared with adjacent normal tissues, CD38 is highly expressed in CC tissues and can enhance the proliferation and inhibit apoptosis of CC cells by regulating the PI3K/AKT/mTOR pathway and affecting mitochondrial function [[109], [110], [111]]. After HeLa cells were treated with NAM, the proliferation of cancer cells was inhibited, and ROS accumulation and glutathione (GSH) consumption were significantly increased at relatively high concentrations, indicating that NAM induces apoptosis through oxidative stress and intrinsic mitochondrial apoptosis pathways [112].
4.5.4. The use of PARP inhibitors (PARPi) in reproductive system tumors
The PARPs are a family of NAD + -dependent deacetylases that regulate a variety of cellular processes and play a wide range of roles in the cellular response to DNA damage, of which PARP1 is the most widely studied [113]. As the level of DNA damage increases, the DNA repair enzyme PARP1 consumes and depletes NAD+. Li et al. [114]showed that aged mice had lower PARP1 activity and a reduced response to DNA damage. NMN treatment restored reduced PARP1 activity and DNA damage responses in aged mice, indicating that these responses depend on NAD+ levels. PARP1 deficiency in mice was shown to accelerate aging and lead to premature manifestations of spontaneous carcinogenesis. Strategies for treating malignant tumors such as radiotherapy and photodynamic therapy induce DNA damage and destroy cancer cells. However, the cell's repair mechanism repairs some of the damaged DNA, which may reduce the efficiency of treatment of tumors. Specifically, PARP senses DNA damage and catalyzes the transfer of ADP-ribose or PAR to itself and other receptor proteins by binding to DNA SSBs, using NAD+ as a substrate, and then recruiting DNA repair proteins and other proteins to the DNA damage site to aid in DNA repair [115,116]. In contrast, PARPi leads to the accumulation of SSBs in DNA through multiple cell cycle checkpoint inhibitory pathways (e.g., ATM/CHK2/p53, ATR/CHK1/WEE1) and even to the development of double-stranded DNA breaks (DSBs), which ultimately kill the tumor cells [117]. Therefore, patients may benefit from the combination of conventional treatment of tumors and PARPi.
BRCA1 and BRCA2 are two key tumor suppressors [118]. Patients carrying germline mutations in BRCA1 or BRCA2 are sensitive to PARPi because they have specific types of DNA repair defects [119]. Ovarian cancer has been found to be one of the common BRCA mutation-associated malignancies, with approximately 18 % of epithelial ovarian cancer cases due to inherited mutations in the BRCA1/2 genes, and approximately 5–10 % of patients with breast cancer having an inherited loss of function in one or both of the BRCA1 and BRCA2 genes [120,121]. Currently, the FDA has approved olaparib, niraparib and rucaparib for the treatment and management of ovarian cancer with BRCA mutations in combination with first-line platinum-based chemotherapeutic agents to block the repair of DNA damage caused by such agents. Two PARP inhibitors, olaparib and talazoparib, are used for the treatment of breast cancer with BRCA1/2 mutations. PARPi trials for cervical cancer are currently more limited than for ovarian and breast cancer, focusing on preclinical as well as phase I trials of veriparib [119]. As it stands, although PARPi is approved for the treatment of ovarian, breast and cervical cancers, among others, only a small number of patients have benefited from PARPi monotherapy, which only inhibits tumor growth. Resistance and toxicity to PARPi remains an important area of exploration.
5. Conclusion
There is an increasing amount of research on NAD+, especially in the fields of aging and tumor research, which affects almost all biological processes. At the same time, the role of NAD+ in the reproductive system has attracted public attention, and many studies have been published in many gynecological fields such as ovarian aging, ART, PCOS, abortion and malformation, and gynecological tumors. Supplementation of NAD+ precursors or inhibition of NAD+ depletion could indeed provide a non-invasive approach to clinical salvage of most gynecologic diseases, which may provide a new strategy for the treatment of gynecological diseases. However, most of these studies are based on animal experiments, and the safety and toxicity of NAD+ are not considered. The pathogenesis of NAD+ involved in these diseases is still unclear. It is worth further exploration to determine the safety of NAD+ precursors, develop new inhibitors, improve the related mechanism research, and further translate the current research into clinical research.
Funding
This work was supported by Scientific Research Fund of Hunan Provincial Education Department (22A0256), Scientific Research Fund of Hunan Administration of Traditional Chinese Medicine (C2022008), Hunan Provincial Innovation Foundation for Postgraduate (CX20220788), Key research and development Project of Hunan Province (2023SK2050).
CRediT authorship contribution statement
Lan Li: Writing – original draft, Methodology, Data curation. Xin Zhou: Data curation. Wene Liu: Project administration, Formal analysis. Zhen Chen: Supervision, Funding acquisition. Xiaoqin Xiao: Writing – review & editing, Funding acquisition, Conceptualization. Guiming Deng: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Xiaoqin Xiao, Email: Xqaxy99@163.com.
Guiming Deng, Email: dengguiming@hnucm.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Sauer M.V. Reproduction at an advanced maternal age and maternal health. Fertil. Steril. 2015;103:1136–1143. doi: 10.1016/j.fertnstert.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Vigneswaran K., Hamoda H. Hormone replacement therapy – current recommendations. Best Pract. Res. Clin. Obstet. Gynaecol. 2022;81:8–21. doi: 10.1016/j.bpobgyn.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira B.L., Ataman L.M., Rodrigues J.K., Birchal T.S., Reis F.M. Restricted access to assisted reproductive technology and fertility preservation: legal and ethical issues. Reprod. Biomed. Online. 2021;43:571–576. doi: 10.1016/j.rbmo.2021.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Kordowitzki P., Ho W.-H.J., Listijono D.R. Nicotinamide adenine nucleotide—the fountain of youth to prevent oocyte aging? Cells. 2021;10:2441. doi: 10.3390/cells10092441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zapata‐Pérez R., Wanders R.J.A., van Karnebeek C.D.M., Houtkooper R.H. NAD+ homeostasis in human health and disease. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaku K., Okabe K., Nakagawa T. NAD metabolism: implications in aging and longevity. Ageing Res. Rev. 2018;47:1–17. doi: 10.1016/j.arr.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q., Cong L., Wang Y., Luo X., Li H., Wang H., Zhu J., Dai S., Jin H., Yao G., Shi S., Hsueh A.J., Sun Y. Increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging. Free Radic. Biol. Med. 2020;156:1–10. doi: 10.1016/j.freeradbiomed.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Vrablik T.L., Huang L., Lange S.E., Hanna-Rose W. Nicotinamidase modulation of NAD+ biosynthesis and nicotinamide levels separately affect reproductive development and cell survival in C. elegans. Development. 2009;136:3637–3646. doi: 10.1242/dev.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajman L., Chwalek K., Sinclair D.A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metabol. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi H., Enriquez A., Rapadas M., Martin E.M.M.A., Wang R., Moreau J., Lim C.K., Szot J.O., Ip E., Hughes J.N., Sugimoto K., Humphreys D.T., McInerney-Leo A.M., Leo P.J., Maghzal G.J., Halliday J., Smith J., Colley A., Mark P.R., Collins F., Sillence D.O., Winlaw D.S., Ho J.W.K., Guillemin G.J., Brown M.A., Kikuchi K., Thomas P.Q., Stocker R., Giannoulatou E., Chapman G., Duncan E.L., Sparrow D.B., Dunwoodie S.L. NAD deficiency, congenital malformations, and niacin supplementation. N. Engl. J. Med. 2017;377:544–552. doi: 10.1056/NEJMoa1616361. [DOI] [PubMed] [Google Scholar]
- 11.Castro-Portuguez R., Sutphin G.L. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: targeting tryptophan metabolism to promote longevity and healthspan. Exp. Gerontol. 2020;132 doi: 10.1016/j.exger.2020.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/S0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 13.Guo X., Tan S., Wang T., Sun R., Li S., Tian P., Li M., Wang Y., Zhang Y., Yan Y., Dong Z., Yan L., Yue X., Wu Z., Li C., Yamagata K., Gao L., Ma C., Li T., Liang X. NAD+ salvage governs mitochondrial metabolism, invigorating natural killer cell antitumor immunity. Hepatology. 2022 doi: 10.1002/hep.32658. [DOI] [PubMed] [Google Scholar]
- 14.Belenky P., Racette F.G., Bogan K.L., McClure J.M., Smith J.S., Brenner C. Nicotinamide riboside promotes Sir 2 silencing and extends lifespan via Nrk and urh1/pnp1/meu1 pathways to NAD+ Cell. 2007;129:473–484. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Cambronne X.A., Stewart M.L., Kim D., Jones-Brunette A.M., Morgan R.K., Farrens D.L., Cohen M.S., Goodman R.H. NAD+ biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braidy N., Berg J., Clement J., Khorshidi F., Poljak A., Jayasena T., Grant R., Sachdev P. Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxidants Redox Signal. 2019;30:251–294. doi: 10.1089/ars.2017.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poddar S.K., Sifat A.E., Haque S., Nahid N.A., Chowdhury S., Mehedi I. Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule. Biomolecules. 2019;9:34. doi: 10.3390/biom9010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute of Medicine (US . Pantothenic Acid, Biotin, and Choline. National Academies Press (US); Washington (DC): 1998. Standing committee on the scientific evaluation of dietary reference intakes and its panel on folate, other B vitamins, and choline, dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12.http://www.ncbi.nlm.nih.gov/books/NBK114310/ [PubMed] [Google Scholar]
- 19.Mills K.F., Yoshida S., Stein L.R., Grozio A., Kubota S., Sasaki Y., Redpath P., Migaud M.E., Apte R.S., Uchida K., Yoshino J., Imai S. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metabol. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong W., Mo F., Zhang Z., Huang M., Wei X. Nicotinamide mononucleotide: a promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Front. Cell Dev. Biol. 2020;8:246. doi: 10.3389/fcell.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Ryu D., Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 22.Cantó C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A.A., Auwerx J. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet induced obesity. Cell Metabol. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martens C.R., Denman B.A., Mazzo M.R., Armstrong M.L., Reisdorph N., McQueen M.B., Chonchol M., Seals D.R. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 2018;9:1286. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidele K., Beneke S., Bürkle A. The NAD+ precursor nicotinic acid improves genomic integrity in human peripheral blood mononuclear cells after X-irradiation. DNA Repair. 2017;52:12–23. doi: 10.1016/j.dnarep.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Hwang E.S., Song S.B. Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules. 2020;10:687. doi: 10.3390/biom10050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Zhang N., Zhang G., Sauve A.A. NRH salvage and conversion to NAD+ requires NRH kinase activity by adenosine kinase. Nat. Metab. 2020;2:364–379. doi: 10.1038/s42255-020-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciarlo E., Joffraud M., Hayat F., Giner M.P., Giroud-Gerbetant J., Sanchez-Garcia J.L., Rumpler M., Moco S., Migaud M.E., Cantó C. Nicotinamide riboside and dihydronicotinic acid riboside synergistically increase intracellular NAD+ by generating dihydronicotinamide riboside. Nutrients. 2022;14:2752. doi: 10.3390/nu14132752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonavane M., Hayat F., Makarov M., Migaud M.E., Gassman N.R. Dihydronicotinamide riboside promotes cell-specific cytotoxicity by tipping the balance between metabolic regulation and oxidative stress. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Luo C., Li T., Zhang W., Zong Z., Liu X., Deng H. Reduced nicotinamide mononucleotide (NMNH) potently enhances NAD+ and suppresses glycolysis, the TCA cycle, and cell growth. J. Proteome Res. 2021;20:2596–2606. doi: 10.1021/acs.jproteome.0c01037. [DOI] [PubMed] [Google Scholar]
- 30.Chini C.C.S., Peclat T.R., Gomez L.S., Zeidler J.D., Warner G.M., Kashyap S., Mazdeh D.Z., Hayat F., Migaud M.E., Paulus A., Chanan-Khan A.A., Chini E.N. Dihydronicotinamide riboside is a potent NAD+ precursor promoting a pro-inflammatory phenotype in macrophages. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.840246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q., Cong L., Wang Y., Luo X., Li H., Wang H., Zhu J., Dai S., Jin H., Yao G., Shi S., Hsueh A.J., Sun Y. Increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging. Free Radic. Biol. Med. 2020;156:1–10. doi: 10.1016/j.freeradbiomed.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Bertoldo M.J., Listijono D.R., Ho W.-H.J., Riepsamen A.H., Goss D.M., Richani D., Jin X.L., Mahbub S., Campbell J.M., Habibalahi A., Loh W.-G.N., Youngson N.A., Maniam J., Wong A.S.A., Selesniemi K., Bustamante S., Li C., Zhao Y., Marinova M.B., Kim L.-J., Lau L., Wu R.M., Mikolaizak A.S., Araki T., Le Couteur D.G., Turner N., Morris M.J., Walters K.A., Goldys E., O'Neill C., Gilchrist R.B., Sinclair D.A., Homer H.A., Wu L.E. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep. 2020;30:1670–1681.e7. doi: 10.1016/j.celrep.2020.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollard C.-L., Younan A., Swegen A., Gibb Z., Grupen C.G. Insights into the NAD+ biosynthesis pathways involved during meiotic maturation and spindle formation in porcine oocytes. J. Reprod. Dev. 2022;68:216–224. doi: 10.1262/jrd.2021-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollard C.-L., Gibb Z., Clulow J., Ruiz A., Sheridan A., Bahrami M., Swegen A., Grupen C.G. Supplemental nicotinic acid elevates NAD+ precursors in the follicular fluid of mares. Animals. 2022;12:1383. doi: 10.3390/ani12111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venturas M., Yang X., Kumar K., Wells D., Racowsky C., Needleman D.J. Metabolic imaging of human cumulus cells reveals associations among metabolic profiles of cumulus cells, patient clinical factors, and oocyte maturity. Fertil. Steril. 2021;116:1651–1662. doi: 10.1016/j.fertnstert.2021.07.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L., Lin X., Tang H., Fan Y., Zeng S., Jia L., Li Y., Shi Y., He S., Wang H., Hu Z., Gong X., Liang X., Yang Y., Liu X. Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD+ redox. Aging Cell. 2020;19 doi: 10.1111/acel.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao Y., Cui Z., Gao Q., Rui R., Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.107987. [DOI] [PubMed] [Google Scholar]
- 38.Huang P., Zhou Y., Tang W., Ren C., Jiang A., Wang X., Qian X., Zhou Z., Gong A. Long-term treatment of Nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J. Nutr. Biochem. 2022;101 doi: 10.1016/j.jnutbio.2021.108911. [DOI] [PubMed] [Google Scholar]
- 39.Y. Jiang, D. Wang, C. Zhang, Y. Jiao, Y. Pu, R. Cheng, C. Li, Y. Chen, Nicotinamide mononucleotide restores oxidative stress-related apoptosis of oocyte exposed to benzyl butyl phthalate in mice, Cell Proliferation n/a (n.d.) e13419. 10.1111/cpr.13419.. [DOI] [PMC free article] [PubMed]
- 40.Miao Y., Cui Z., Zhu X., Gao Q., Xiong B. Supplementation of nicotinamide mononucleotide improves the quality of postovulatory aged porcine oocytes. J. Mol. Cell Biol. 2022;14 doi: 10.1093/jmcb/mjac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song M., Li Y., Zhou Y., Yan J., Zhou X., Gao Q., Miao Y., Xiong B. Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress. J. Anim. Sci. Biotechnol. 2022;13:68. doi: 10.1186/s40104-022-00716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miao Y., Li X., Shi X., Gao Q., Chen J., Wang R., Fan Y., Xiong B. Nicotinamide mononucleotide restores the meiotic competency of porcine oocytes exposed to ethylene glycol butyl ether. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.628580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Wang H., Xu J., Zeng X., Sun Y., Yang Q. Nicotinamide riboside supplementation ameliorated post-ovulatory oocyte quality decline. Reproduction. 2023;165:103–111. doi: 10.1530/REP-22-0095. [DOI] [PubMed] [Google Scholar]
- 44.Wu X., Hu F., Zeng J., Han L., Qiu D., Wang H., Ge J., Ying X., Wang Q. NMNAT2‐mediated NAD+ generation is essential for quality control of aged oocytes. Aging Cell. 2019;18 doi: 10.1111/acel.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S., Sun M., Yu L., Wang Y., Yao Y., Wang D. Niacin inhibits apoptosis and rescues premature ovarian failure. Cell. Physiol. Biochem. 2018;50:2060–2070. doi: 10.1159/000495051. [DOI] [PubMed] [Google Scholar]
- 46.Pollard C.-L., Gibb Z., Hawdon A., Swegen A., Grupen C.G. Supplementing media with NAD+ precursors enhances the in vitro maturation of porcine oocytes. J. Reprod. Dev. 2021;67:319–326. doi: 10.1262/jrd.2021-080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee A.R., Kishigami S., Amano T., Matsumoto K., Wakayama T., Hosoi Y. Nicotinamide: a class III HDACi delays in vitro aging of mouse oocytes. J. Reprod. Dev. 2013;59:238–244. doi: 10.1262/jrd.2012-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Z., Yang J., Yang G., Feng T., Zhang X., Chen Y., Feng R., Qian Y. Effects of nicotinamide on follicular development and the quality of oocytes. Reprod. Biol. Endocrinol. 2022;20:70. doi: 10.1186/s12958-022-00938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittler R. ROS are good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Bertoldo M.J., Paris V.R., Gook D.A., Edwards M.C., Wu K., Liang C.J.J., Marinova M.B., Wu L.E., Walters K.A., Gilchrist R.B. Impact of nicotinamide mononucleotide on transplanted mouse ovarian tissue. Reproduction. 2021;161:215–226. doi: 10.1530/REP-20-0539. [DOI] [PubMed] [Google Scholar]
- 51.Stringer J., Groenewegen E., Liew S.H., Hutt K. NMN does not protect the ovarian reserve from cancer treatments. Reproduction. 2020;159:105–113. doi: 10.1530/REP-19-0337. [DOI] [PubMed] [Google Scholar]
- 52.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Wang S., Mu L., Zhang C., Long X., Zhang Y., Li R., Zhao Y., Qiao J. Abnormal activation of tryptophan-kynurenine pathway in women with polycystic ovary syndrome. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.877807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aflatounian A., Paris V.R., Richani D., Edwards M.C., Cochran B.J., Ledger W.L., Gilchrist R.B., Bertoldo M.J., Wu L.E., Walters K.A. Declining muscle NAD+ in a hyperandrogenism PCOS mouse model: possible role in metabolic dysregulation. Mol. Metabol. 2022;65 doi: 10.1016/j.molmet.2022.101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H., Zhao Y., Li T., Li M., Li J., Li R., Liu P., Yu Y., Qiao J. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic. Biol. Med. 2015;86:295–307. doi: 10.1016/j.freeradbiomed.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Xu X.J., Gauthier M.-S., Hess D.T., Apovian C.M., Cacicedo J.M., Gokce N., Farb M., Valentine R.J., Ruderman N.B. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. JLR (J. Lipid Res.) 2012;53:792. doi: 10.1194/jlr.P022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cantó C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reverchon M., Rame C., Bunel A., Chen W., Froment P., Dupont J. VISFATIN (NAMPT) improves in vitro IGF1-induced steroidogenesis and IGF1 receptor signaling through SIRT1 in bovine granulosa cells. Biol. Reprod. 2016;94:54. doi: 10.1095/biolreprod.115.134650. [DOI] [PubMed] [Google Scholar]
- 59.Wu M., Zhang J., Gu R., Dai F., Yang D., Zheng Y., Tan W., Jia Y., Li B., Cheng Y. The role of Sirtuin 1 in the pathophysiology of polycystic ovary syndrome. Eur. J. Med. Res. 2022;27:158. doi: 10.1186/s40001-022-00746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao X., Chen L., Cai L., Ge S., Deng X. Regulatory effects of the AMPKα-SIRT1 molecular pathway on insulin resistance in PCOS mice: an in vitro and in vivo study. Biochem. Biophys. Res. Commun. 2017;494:615–620. doi: 10.1016/j.bbrc.2017.09.154. [DOI] [PubMed] [Google Scholar]
- 61.Nejabati H.R., Samadi N., Shahnazi V., Mihanfar A., Fattahi A., Latifi Z., Bahrami-asl Z., Roshangar L., Nouri M. Nicotinamide and its metabolite N1-Methylnicotinamide alleviate endocrine and metabolic abnormalities in adipose and ovarian tissues in rat model of Polycystic Ovary Syndrome. Chem. Biol. Interact. 2020;324 doi: 10.1016/j.cbi.2020.109093. [DOI] [PubMed] [Google Scholar]
- 62.Palomba S., Falbo A., Chiossi G., Orio F., Tolino A., Colao A., La Sala G.B., Zullo F. Low-grade chronic inflammation in pregnant women with polycystic ovary syndrome: a prospective controlled clinical study. J. Clin. Endocrinol. Metab. 2014;99:2942–2951. doi: 10.1210/jc.2014-1214. [DOI] [PubMed] [Google Scholar]
- 63.Özay A.C., Özay Ö.E. The importance of inflammation markers in polycystic ovary syndrome. Rev. Assoc. Med. Bras. 2021;67:411–417. doi: 10.1590/1806-9282.20200860. [DOI] [PubMed] [Google Scholar]
- 64.Xiong Y., Liang X., Yang X., Li Y., Wei L. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;159:148–150. doi: 10.1016/j.ejogrb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y., Liu H., Li Z., Fan H., Yan X., Liu X., Xuan J., Feng D., Wei X. The release of peripheral immune inflammatory cytokines promote an inflammatory cascade in PCOS patients via altering the follicular microenvironment. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.685724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie N., Zhang L., Gao W., Huang C., Huber P.E., Zhou X., Li C., Shen G., Zou B. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Targeted Ther. 2020;5:227. doi: 10.1038/s41392-020-00311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waddell J., Khatoon R., Kristian T. Cellular and mitochondrial NAD homeostasis in health and disease. Cells. 2023;12:1329. doi: 10.3390/cells12091329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris S.E., Maruthini D., Tang T., Balen A.H., Picton H.M. Metabolism and karyotype analysis of oocytes from patients with polycystic ovary syndrome. Hum. Reprod. 2010;25:2305–2315. doi: 10.1093/humrep/deq181. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., Yang Q., Wang H., Zhu J., Cong L., Li H., Sun Y. NAD+ deficiency and mitochondrial dysfunction in granulosa cells of women with polycystic ovary syndrome. Biol. Reprod. 2021;105:371–380. doi: 10.1093/biolre/ioab078. [DOI] [PubMed] [Google Scholar]
- 70.Palawaththa S., Islam R.M., Illic D., Rabel K., Lee M., Romero L., Leung X.Y., Karim MdN. Effect of maternal dietary niacin intake on congenital anomalies: a systematic review and meta-analysis. Eur. J. Nutr. 2022;61:1133–1142. doi: 10.1007/s00394-021-02731-9. [DOI] [PubMed] [Google Scholar]
- 71.Mark P.R., Dunwoodie S.L. Viewing teratogens through the lens of nicotinamide adenine dinucleotide (NAD+) Birth Defects Research. 2022;114:1313–1323. doi: 10.1002/bdr2.2089. [DOI] [PubMed] [Google Scholar]
- 72.Szot J.O., Slavotinek A., Chong K., Brandau O., Nezarati M., Cueto-González A.M., Patel M.S., Devine W.P., Rego S., Acyinena A.P., Shannon P., Myles-Reid D., Blaser S., Mieghem T.V., Yavuz-Kienle H., Skladny H., Miller K., Riera M.D.T., Martínez S.A., Tizzano E.F., Dupuis L., Stavropoulos D.J., McNiven V., Mendoza-Londono R., Elliott A.M., Phillips R.S., Chapman G., Dunwoodie S.L. New cases that expand the genotypic and phenotypic spectrum of congenital NAD deficiency disorder. Hum. Mutat. 2021;42:862–876. doi: 10.1002/humu.24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cuny H., Rapadas M., Gereis J., Martin E.M.M.A., Kirk R.B., Shi H., Dunwoodie S.L. NAD deficiency due to environmental factors or gene–environment interactions causes congenital malformations and miscarriage in mice. Proc. Natl. Acad. Sci. U. S. A. 2020;117:3738–3747. doi: 10.1073/pnas.1916588117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mark P.R. NAD+ deficiency in human congenital malformations and miscarriage: a new model of pleiotropy. Am. J. Med. Genet. 2022;188:2834–2849. doi: 10.1002/ajmg.a.62764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biallelic variants in KYNU cause a multisystemic syndrome with hand hyperphalangism. Bone. 2020;133 doi: 10.1016/j.bone.2019.115219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schüle I., Berger U., Matysiak U., Ruzaike G., Stiller B., Pohl M., Spiekerkoetter U., Lausch E., Grünert S.C., Schmidts M. A homozygous deletion of exon 5 of KYNU resulting from a maternal chromosome 2 isodisomy (UPD2) causes catel-manzke-syndrome/VCRL syndrome. Genes. 2021;12:879. doi: 10.3390/genes12060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vander Heiden M.G. Metabolism and congenital malformations — NAD's effects on development. N. Engl. J. Med. 2017;377:509–511. doi: 10.1056/NEJMp1707487. [DOI] [PubMed] [Google Scholar]
- 78.A.-M. M, J. C, P.-B. V, P. O, T. R, E. T, M. C, T. A, C.-D. V, A.-B. T, B. G Clinical heterogeneity of NADSYN1-associated VCRL syndrome. Clin. Genet. 2023 doi: 10.1111/cge.14328. [DOI] [PubMed] [Google Scholar]
- 79.Szot J.O., Campagnolo C., Cao Y., Iyer K.R., Cuny H., Drysdale T., Flores-Daboub J.A., Bi W., Westerfield L., Liu P., Leung T.N., Choy K.W., Chapman G., Xiao R., Siu V.M., Dunwoodie S.L. Bi-Allelic mutations in NADSYN1 cause multiple organ defects and expand the genotypic spectrum of congenital NAD deficiency disorders. Am. J. Hum. Genet. 2020;106:129–136. doi: 10.1016/j.ajhg.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin J., Zhao L., Zhao S., Li S., Zhao Z., Chen Z., Zheng Z., Shao J., Niu Y., Li X., Zhang J.T., Wu Z., Wu N. Disruptive NADSYN1 variants implicated in congenital vertebral malformations. Genes. 2021;12:1615. doi: 10.3390/genes12101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cuny H., Bozon K., Kirk R.B., Sheng D.Z., Bröer S., Dunwoodie S.L. Maternal heterozygosity of Slc6a19 causes metabolic perturbation and congenital NAD deficiency disorder in mice. Dis Model Mech. 2022;16 doi: 10.1242/dmm.049647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y., Fu W., Fu K., Zuo X., Jia W., Wang N., Zhang Y., Liu G., Deng F. HAAO rs3816183 polymorphisms [T] increase anterior/middle hypospadias risk in southern han Chinese population. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.842519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christensen M., Duno M., Lund A.M., Skovby F., Christensen E. Xanthurenic aciduria due to a mutation in KYNU encoding kynureninase. J. Inherit. Metab. Dis. 2007;30:248–255. doi: 10.1007/s10545-007-0396-2. [DOI] [PubMed] [Google Scholar]
- 84.Kortbawi H., Ames E., Pritchard A., Devine P., van Ziffle J., Slavotinek A. Further description of two patients with biallelic variants in NADSYN1 in association with cardiac and vertebral anomalies. Am. J. Med. Genet. 2022;188:2479–2484. doi: 10.1002/ajmg.a.62765. [DOI] [PubMed] [Google Scholar]
- 85.Erbs E., Brasen C.L., Lund A.M., Rasmussen M. Adult patient diagnosed with NADSYN1 associated congenital NAD deficiency and analysis of NAD levels to be published in: European Journal of Medical Genetics. Eur. J. Med. Genet. 2023;66 doi: 10.1016/j.ejmg.2023.104698. [DOI] [PubMed] [Google Scholar]
- 86.Ghanem M.S., Monacelli F., Nencioni A. Advances in NAD-lowering agents for cancer treatment. Nutrients. 2021;13:1665. doi: 10.3390/nu13051665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lheureux S., Braunstein M., Oza A.M. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA A Cancer J. Clin. 2019;69:280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 88.Lin L., Chen L., Xie Z., Chen J., Li L., Lin A. Identification of NAD+ metabolism-derived gene signatures in ovarian cancer prognosis and immunotherapy. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.905238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D., Chen N.-N., Cao J.-M., Sun W.-P., Zhou Y.-M., Li C.-Y., Wang X.-X. BRCA1 as a nicotinamide adenine dinucleotide (NAD)-dependent metabolic switch in ovarian cancer. Cell Cycle. 2014;13:2564–2571. doi: 10.4161/15384101.2015.942208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang Y.-Y., Bi F.-F., Zhou Y.-M., Sun W.-P., Li C.-Y., Liu Q., Zhao Y., Li D. Nicotinamide adenine dinucleotide (NAD) may affect DNA methyltransferase 1 through regulation of BRCA1 in ovarian cancer. Am. J. Cancer Res. 2015;5:1199–1206. [PMC free article] [PubMed] [Google Scholar]
- 91.Navas L.E., Blanco-Alcaina E., Suarez-Martinez E., Verdugo-Sivianes E.M., Espinosa-Sanchez A., Sanchez-Diaz L., Dominguez-Medina E., Fernandez-Rozadilla C., Carracedo A., Wu L.E., Carnero A. NAD pool as an antitumor target against cancer stem cells in head and neck cancer. J. Exp. Clin. Cancer Res. 2023;42:55. doi: 10.1186/s13046-023-02631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang B., Hasan M.K., Alvarado E., Yuan H., Wu H., Chen W.Y. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene. 2011;30:907–921. doi: 10.1038/onc.2010.468. [DOI] [PubMed] [Google Scholar]
- 93.Gujar A.D., Le S., Mao D.D., Dadey D.Y.A., Turski A., Sasaki Y., Aum D., Luo J., Dahiya S., Yuan L., Rich K.M., Milbrandt J., Hallahan D.E., Yano H., Tran D.D., Kim A.H. An NAD+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E8247–E8256. doi: 10.1073/pnas.1610921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sampath D., Zabka T.S., Misner D.L., O'Brien T., Dragovich P.S. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol. Therapeut. 2015;151:16–31. doi: 10.1016/j.pharmthera.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 95.T N., L L., T F., J Z., N F., S W., Km A., O I., Av K., D S., Ki N., Ja B., Z S., Hy T., Dw S., G D., R Z. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 2019;21 doi: 10.1038/s41556-019-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y., Wang Z., He M., Diao L., Yu B., Li D. NAD+ biosynthesis metabolism predicts prognosis and indicates immune microenvironment for breast cancer. Pathol. Oncol. Res. 0. 2023 doi: 10.3389/pore.2023.1610956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Behrouzfar K., Alaee M., Nourbakhsh M., Gholinejad Z., Golestani A. Extracellular NAMPT/visfatin causes p53 deacetylation via NAD production and SIRT1 activation in breast cancer cells. Cell Biochem. Funct. 2017;35:327–333. doi: 10.1002/cbf.3279. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Y., Luo Z., Gong Y., Fu Y., Luo Y. NAD+ supplementation limits triple-negative breast cancer metastasis via SIRT1-P66Shc signaling. Oncogene. 2023;42:808–824. doi: 10.1038/s41388-023-02592-y. [DOI] [PubMed] [Google Scholar]
- 99.Hesari Z., Nourbakhsh M., Hosseinkhani S., Abdolvahabi Z., Alipour M., Tavakoli-Yaraki M., Ghorbanhosseini S.S., Yousefi Z., Jafarzadeh M., Yarahmadi S. Down-regulation of NAMPT expression by mir-206 reduces cell survival of breast cancer cells. Gene. 2018;673:149–158. doi: 10.1016/j.gene.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 100.Alizadeh-Fanalou S., Hosseinkhani S., Nazarizadeh A., Ezzati-Mobaser S., Hesari Z., Aziminezhadan P., Abdolvahabi Z., Abolmaali M., Tavakoli-Yaraki M., Nourbakhsh M. MiR-613 promotes cell death in breast cancer cells by downregulation of nicotinamide phosphoribosyltransferase and reduction of NAD. DNA Cell Biol. 2021;40:1026–1036. doi: 10.1089/dna.2021.0330. [DOI] [PubMed] [Google Scholar]
- 101.Bolandghamat Pour Z., Nourbakhsh M., Mousavizadeh K., Madjd Z., Ghorbanhosseini S.S., Abdolvahabi Z., Hesari Z., Ezzati Mobasser S. Suppression of nicotinamide phosphoribosyltransferase expression by miR-154 reduces the viability of breast cancer cells and increases their susceptibility to doxorubicin. BMC Cancer. 2019;19:1027. doi: 10.1186/s12885-019-6221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bolandghamat Pour Z., Nourbakhsh M., Mousavizadeh K., Madjd Z., Ghorbanhosseini S.S., Abdolvahabi Z., Hesari Z., Mobaser S.E. Up-regulation of miR-381 inhibits NAD+ salvage pathway and promotes apoptosis in breast cancer cells. EXCLI J. 2019;18:683–696. doi: 10.17179/excli2019-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santidrian A.F., Matsuno-Yagi A., Ritland M., Seo B.B., LeBoeuf S.E., Gay L.J., Yagi T., Felding-Habermann B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J. Clin. Invest. 2013;123:1068–1081. doi: 10.1172/JCI64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharif T., Ahn D.-G., Liu R.-Z., Pringle E., Martell E., Dai C., Nunokawa A., Kwak M., Clements D., Murphy J.P., Dean C., Marcato P., McCormick C., Godbout R., Gujar S.A., Lee P.W.K. The NAD+ salvage pathway modulates cancer cell viability via p73. Cell Death Differ. 2016;23:669–680. doi: 10.1038/cdd.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Comes R., Mustea I. The levels of NAD and NADH in blood of patients with cancer. Neoplasma. 1976;23:451–455. [PubMed] [Google Scholar]
- 106.Ji M., Zhong J., Xue R., Su W., Kong Y., Fei Y., Ma J., Wang Y., Mi L. Early detection of cervical cancer by fluorescence lifetime imaging microscopy combined with unsupervised machine learning. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms231911476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen A., Jing W., Qiu J., Zhang R. Prediction of cervical cancer outcome by identifying and validating a NAD+ metabolism-derived gene signature. J. Personalized Med. 2022;12:2031. doi: 10.3390/jpm12122031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vora M., Alattia L.A., Ansari J., Ong M., Cotelingam J., Coppola D., Shackelford R. Nicotinamide phosphoribosyl transferase a reliable marker of progression in cervical dysplasia. Anticancer Res. 2017;37:4821–4825. doi: 10.21873/anticanres.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liao S., Xiao S., Chen H., Zhang M., Chen Z., Long Y., Gao L., Zhu G., He J., Peng S., Xiong W., Zeng Z., Li Z., Zhou M., Li X., Ma J., Wu M., Xiang J., Li G., Zhou Y. CD38 enhances the proliferation and inhibits the apoptosis of cervical cancer cells by affecting the mitochondria functions. Mol. Carcinog. 2017;56:2245–2257. doi: 10.1002/mc.22677. [DOI] [PubMed] [Google Scholar]
- 110.Liao S., Xiao S., Zhu G., Zheng D., He J., Pei Z., Li G., Zhou Y. CD38 is highly expressed and affects the PI3K/Akt signaling pathway in cervical cancer. Oncol. Rep. 2014;32:2703–2709. doi: 10.3892/or.2014.3537. [DOI] [PubMed] [Google Scholar]
- 111.Liao S., Liang L., Yue C., He J., He Z., Jin X., Luo G., Zhou Y. CD38 is involved in cell energy metabolism via activating the PI3K/AKT/mTOR signaling pathway in cervical cancer cells. Int. J. Oncol. 2020;57:338–354. doi: 10.3892/ijo.2020.5040. [DOI] [PubMed] [Google Scholar]
- 112.Feng Y., Wang Y., Jiang C., Fang Z., Zhang Z., Lin X., Sun L., Jiang W. Nicotinamide induces mitochondrial-mediated apoptosis through oxidative stress in human cervical cancer HeLa cells. Life Sci. 2017;181:62–69. doi: 10.1016/j.lfs.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Dasovich M., Leung A.K.L. PARPs and ADP-ribosylation: deciphering the complexity with molecular tools. Mol. Cell. 2023;83:1552–1572. doi: 10.1016/j.molcel.2023.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li J., Bonkowski M.S., Moniot S., Zhang D., Hubbard B.P., Ling A.J.Y., Rajman L.A., Qin B., Lou Z., Gorbunova V., Aravind L., Steegborn C., Sinclair D.A. A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355:1312–1317. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eustermann S., Wu W.-F., Langelier M.-F., Yang J.-C., Easton L.E., Riccio A.A., Pascal J.M., Neuhaus D. Structural basis of detection and signaling of DNA single-strand breaks by human PARP-1. Mol. Cell. 2015;60:742–754. doi: 10.1016/j.molcel.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.C. Kim, X.-D. Wang, Y. Yu, PARP1 inhibitors trigger innate immunity via PARP1 trapping-induced DNA damage response, Elife 9 (n.d.) e60637. 10.7554/eLife.60637.. [DOI] [PMC free article] [PubMed]
- 117.Li M., Yu X. The role of poly(ADP-ribosyl)ation in DNA damage response and cancer chemotherapy. Oncogene. 2015;34:3349–3356. doi: 10.1038/onc.2014.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jonsson P., Bandlamudi C., Cheng M.L., Srinivasan P., Chavan S.S., Friedman N.D., Rosen E.Y., Richards A.L., Bouvier N., Selcuklu S.D., Bielski C.M., Abida W., Mandelker D., Birsoy O., Zhang L., Zehir A., Donoghue M.T.A., Baselga J., Offit K., Scher H.I., O'Reilly E.M., Stadler Z.K., Schultz N., Socci N.D., Viale A., Ladanyi M., Robson M.E., Hyman D.M., Berger M.F., Solit D.B., Taylor B.S. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571:576–579. doi: 10.1038/s41586-019-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ragupathi A., Singh M., Perez A.M., Zhang D. Targeting the BRCA1/2 deficient cancer with PARP inhibitors: clinical outcomes and mechanistic insights. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1133472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen S., Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Couch F.J., Nathanson K.L., Offit K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science. 2014;343:1466–1470. doi: 10.1126/science.1251827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.