Abstract
The regulation of human immunodeficiency virus type 1 (HIV-1) gene expression involves a complex interplay between cellular transcription factors, chromatin-associated proviral DNA, and the virus-encoded transactivator protein, Tat. Here we show that Tat transactivates the integrated HIV-1 long terminal repeat (LTR), even in the absence of detectable basal promoter activity, and this transcriptional activation is accompanied by chromatin remodeling downstream of the transcription initiation site, as monitored by increased accessibility to restriction endonucleases. However, with an integrated promoter lacking both Sp1 and NF-κB sites, Tat was unable to either activate transcription or induce changes in chromatin structure even when it was tethered to the HIV-1 core promoter upstream of the TATA box. Tat responsiveness was observed only when Sp1 or NF-κB was bound to the promoter, implying that Tat functions subsequent to the formation of a specific transcription initiation complex. Unlike Tat, NF-κB failed to stimulate the integrated transcriptionally silent HIV-1 promoter. Histone acetylation renders the inactive HIV-1 LTR responsive to NF-κB, indicating that a suppressive chromatin structure must be remodeled prior to transcriptional activation by NF-κB. Taken together, these results suggest that Sp1 and NF-κB are required for the assembly of transcriptional complexes on the integrated viral promoter exhibiting a continuum of basal activities, all of which are fully responsive to Tat.
In vivo studies of gene activation have revealed that changes in chromatin are often associated with transcriptional activation (reviewed in references 21, 30, and 44). For example, in erythroid cells where each globin gene is sequentially expressed during development, the human β-globin locus is hypersensitive to nucleases (19, 22). In contrast, the entire β-globin locus is condensed into a nuclease-resistant chromatin structure in nonerythroid tissues, where the genes are not transcribed. Thus, transcriptionally active chromatin domains differ from the bulk of the genome in their susceptibility to digestion by nucleases. Detailed studies of nuclease-hypersensitive sites have suggested that interactions of sequence-specific DNA binding factors with chromatin alter the canonical nucleosomal structure (for example, see references 2, 7, and 59). These conformational changes generate transcriptionally competent chromatin templates that are accessible to general transcription factors and the RNA polymerase II. In other instances, complexes such as SWI-SNF, NURF, or RSC utilize ATP-dependent nucleosome remodeling mechanisms for efficient transcription activation (reviewed in reference 54). These results, along with the biochemical and genetic studies of yeast histones (29, 42), support the conclusion that nucleosomes exert a repressor effect on a variety of genes and remodeling of chromatin structure is a part of the transcription activation process.
Because retroviruses such as human immunodeficiency virus type 1 (HIV-1) must integrate a DNA copy of their genome into the chromosomal DNA of newly infected cells, it is necessary to characterize the nucleosomal organization of the integrated viral promoter to understand the mechanism(s) associated with the induction of viral transcription. In this regard, we have previously described several cloned human cell lines containing stably integrated copies of the HIV-1 long terminal repeat (LTR) linked to a reporter gene (15). Analyses of the chromatin structure of these integrated HIV-1 templates, using nuclease hypersensitivity and restriction endonuclease accessibility, indicated that the LTR is incorporated into two distinct nucleosomal regions, separated by a nuclease-hypersensitive region containing enhancer and basal promoter elements. The putative nucleosome located upstream of the enhancer appears to be invariably resistant to nuclease digestion. In contrast, the chromatin associated with sequences immediately downstream of the transcription start site is accessible to nucleases in a transcriptionally active HIV-1 LTR. However, when these downstream sequences are incorporated into a nuclease-resistant nucleosome as a consequence of mutagenesis, the promoter becomes inactive. These observations suggest that the organization of chromatin downstream of the transcription initiation site reflects the functional state of the promoter.
The HIV-1 enhancer and promoter are composed of two NF-κB and three Sp1 binding sites located upstream of a canonical TATA box. Binding sites for USF, Ets, and LEF-1 are located adjacent to these sequences and have also been reported to modulate HIV-1 transcription (25, 29, 51). We recently reported that cis-acting sequences, located downstream of the transcription start site and including AP-1, AP-3-like, Sp1, and DBF1 binding sites, are also able to regulate the activity of the viral promoter in the context of chromatin (15, 16). Moreover, HIV-1 encodes a potent transcriptional activator, Tat, that is required for viral gene expression and the production of progeny virions. Tat activates transcription directed by HIV-1 LTR by binding to an RNA structure, designated TAR, which is present at the 5′ termini of all viral transcripts. The region of TAR located between residues +19 and +42 folds into a bulge-stem-loop structure that functions in a position- and orientation-dependent manner (4, 47, 50). However, the precise mechanism by which Tat binding to TAR results in the activation of the HIV-1 promoter has not yet been elucidated. Whether the higher steady-state levels of HIV-1 RNA induced by Tat are a result of increased elongation rates or the stimulation of both transcription initiation and elongation is presently unresolved. An elongation mechanism, consistent with Tat acting on nascent RNA transcripts, is supported by steady-state RNA analyses in transient transfection assays, transactivation experiments conducted in cell-free systems, and some studies of viral replication (20, 25, 26, 36).
In this study, changes in the chromatin structure associated with the transcriptional activation of the integrated HIV-1 promoter have been examined. We show that when the integrated HIV-1 promoter exhibits high levels of basal activity, it can be activated by both NF-κB and Tat. In contrast, Tat, but not NF-κB, is able to activate the integrated, transcriptionally silent HIV-1 promoter, and this transactivation is accompanied by remodeling of the chromatin structure downstream of the transcription initiation site, as assayed by increased accessibility to endonucleases. Treatment of cells carrying the inactive HIV-1 promoter with the histone deacetylase inhibitor trichostatin A (TSA) results in NF-κB activation, suggesting that the histone acetylation increases the accessibility of the chromatin-associated LTR to components of the transcription machinery, thereby stimulating the levels of both basal and activated transcription. Taken together, these results suggest that low levels of HIV-1 expression are maintained by a suppressive chromatin structure which is remodeled during transcriptional activation.
MATERIALS AND METHODS
Plasmid constructs and expression vectors.
The HIV-1 wild-type LTR-chloramphenicol acetyltransferase (CAT) construct has been previously described (15). The mutations introduced in the DS-, KBmt- and IRF-LTRs were generated by oligonucleotide-mediated substitutions (49). In the DS-LTR, the downstream binding sites for AP-1, AP-3-like, DBF1, and Sp1 transcription factors were deleted and replaced with an unrelated 120-bp sequence containing the AvaI, EheI, and XbaI restriction sites as indicated in Fig. 5C. In the KBmt-LTR, the nucleotide substitutions which changed the two NF-κB sites from AGGGACTTTCCGCTGGGGACTTTC (wild type) to ATCTACAGACCGCTGTCTACAGAC (mutated) are underlined. In the IRF-LTR, the sequence between nucleotides (nt) 298 (AvaI site) and 410 (upstream of the TATA box) were deleted and replaced with a DNA fragment containing six repeats of the interferon-stimulated regulatory element sequence GAAAGCGAAAG. All of the HIV-1 LTR-derived constructs were inserted upstream of the CAT gene between the SphI and XbaI restriction sites of the previously described pLCH plasmid DNA (15).
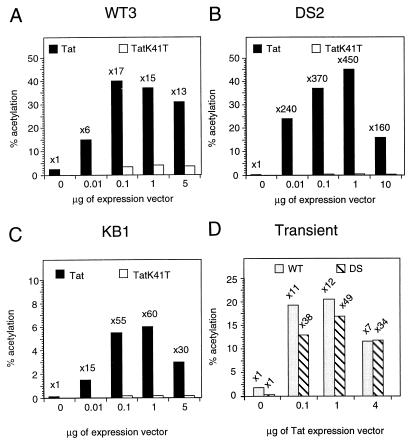
FIG. 5.
Tat strongly transactivates the integrated HIV-1 LTR. (A to C) Titration of CAT activities in WT3 (A), DS2 (B), and KB1 (C) cell clones transfected with either pSV-Tat (Tat) or pSV-TatK41T (TatK41T). (D) HeLa cells were transiently transfected with WT- or DS-LTR-CAT reporter plasmid and increasing amounts of Tat expression vector as indicated at the bottom. At 24 h posttransfection, CAT activity was determined in cell lysates and expressed as percent acetylated chloramphenicol (% acetylation). The bars show the results from a representative transfection experiment; the numbers above the bars indicate average fold induction.
The expression vectors used in these studies included pSV-Tat101 (27), pSV-TatK41T, (derived from pSV-Tat101 and containing a lysine-to-threonine substitution at position 41; graciously provided by Julie A. Brown, National Cancer Institute, Bethesda, Md.), pSV-Sp1 (48), NF-κB p50 and p65 (6), and pCMV-IRF2/p65 (34). To construct expression vectors for interferon regulatory factor 2 (IRF2)-Tat fusion proteins, a DNA fragment encoding the IRF2 DNA binding domain (34) was generated by PCR and subcloned either in frame upstream of the tat gene at the BglII site in pSV-Tat101 to create IRF2 fused to the N terminus of Tat or in frame with C terminus of Tat at the ApaI site to create the Tat-IRF2 fusion. The later fusion construct was inserted into the mammalian expression vector pcDN3.1/Myc-His (Invitrogen). All plasmids were prepared by alkaline lysis and purified through a Qiagen column as recommended by the manufacturer (Qiagen).
Cell culture, transfections, and CAT assays.
The selection of HeLa cell clones stably transfected with LTR-CAT constructs was performed as described previously (15). Cell clones containing integrated copies of LTR-CAT constructs were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 100 U of penicillin-streptomycin per ml, 2 mM l-glutamine, and 150 μg of hygromycin B (Calbiochem) per ml.
Transient transfections of HeLa cell clones were performed by using the DOSPER liposomal transfection reagent as specified by the manufacturer (Boehringer Mannheim). Briefly, subconfluent cells in 60-mm-diameter plates were transfected with 5 μg of total plasmid DNA and 25 μl of DOSPER in 2.5 ml of serum-free Opti-MEM (Life Technologies) for 6 h at 37°C. For CAT assays, cells were harvested 24 h posttransfection and assayed as described previously (16). The acetylated and nonacetylated forms of [14C]chloramphenicol were quantitated with a Fuji phosphorimager, and percent acetylation was determined. In each case, at least two independent transfections were performed.
RNase protection assay.
Total cellular RNA was isolated from unstimulated subconfluent cells or 12 h posttransfection, using RNAsol B (Tel-Test Inc.). Purified RNA (10 or 20 μg) was incubated overnight at 45°C with radiolabeled probe (approximately 106 cpm) in a reaction mixture containing 20 μl of hybridization buffer (100 mM sodium citrate, 300 mM sodium-acetate [pH 6.4], 1 mM EDTA, 80% formamide). The reaction mixtures were digested with RNases A and T1, and subsequently analyzed by electrophoresis and autoradiography as described previously (15). For RNase protection assays with a β-actin probe, 10 μg of RNA was used per reaction.
Treatment of cells with TSA and induction of endogenous NF-κB.
In CAT assays, cells were treated with 25 nM phorbol myristate acetate (PMA) plus 1 μM ionomycin, 10 ng of tumor necrosis factor alpha (TNFα) per ml, or 0.8 μM TSA (histone deacetylase inhibitor) for 36 h (induction of NF-κB) or were first treated with 0.8 μM TSA for 12 h, in the presence of 25 nM PMA plus 1 μM ionomycin or 10 ng of TNFα per ml, for an additional 24 h. At 36 h postinduction, CAT activity in cell lysates was determined.
For assays of chromatin accessibility to restriction endonucleases, cells were either untreated or treated with 25 nM PMA plus 1 μM ionomycin for 4 h or 0.8 μM TSA for 18 h or were first treated with 0.8 μM TSA for 18 h followed by exposure to 25 nM PMA plus 1 μM ionomycin for an additional 4 h. Nuclei were prepared and digested with restriction endonucleases in the appropriate buffer for 30 min at 37°C as described previously (15).
Restriction endonuclease digestion of nuclei from transfected cells.
Subconfluent HeLa cell clones cultured in 10-cm-diameter plates were either maintained in DMEM or transfected with 5 to 7 μg of the appropriate expressing vectors (see figure legends) supplemented with 5 μg of carrier plasmid DNA and 70 μl of the DOSPER transfection reagent, premixed in 0.5 ml of buffer as recommended by the manufacturer (Boehringer Mannheim), and then added to cells in 6.5 ml of Opti-MEM (Life Technologies). Nuclei were prepared from untransfected or transfected cells 12 later and digested with restriction endonucleases in the appropriate buffer for 30 min at 37°C as described previously (15). Purified DNA was subsequently digested to completion with BglI and HincII.
Southern blotting and indirect end labeling.
Digested DNA (20 to 30 μg) was electrophoresed through 1% agarose gels in 1× Tris-borate-EDTA, transferred to a Nylon Plus membrane (Qiagen), and hybridized with [32P]dCTP-labeled DNA probes for 2.5 h at 65°C in Quick-Hyb buffer (Stratagene). The blots were washed four times with 2× SSC (150 mM NaCl, 15 mM sodium citrate [pH 7])–0.1% sodium dodecyl sulfate (SDS), washed two times with 1× SSC–0.2% SDS at 65°C, and autoradiographed.
RESULTS
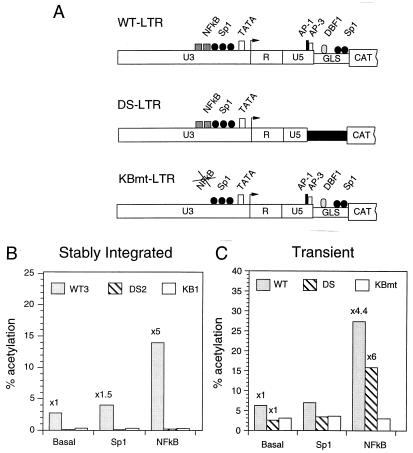
Most previously published studies evaluating the regulation of HIV-1 gene expression have used transient transfection of mammalian cells or in vitro transcription assays. In few reports assessing the influence of chromatin structure on HIV-1 promoter function, the expression of full-length proviral DNA in chronically infected ACH2 and U1 cells was examined (56, 57). The presence of both cellular and viral regulatory proteins during activation of the viral promoter has complicated the analysis of how these different factors contribute to HIV-1 gene expression. To examine the roles of cellular transcription factors such as NF-κB and Sp1 or the virus-encoded Tat transactivator in the transcription activation process, we have developed a cloned HeLa cell system, consisting of integrated copies of the wild-type or mutagenized HIV-1 LTR linked to a CAT reporter gene (Fig. 1A). The derived HeLa cell clones, containing one or two integrated copies of the viral LTR, exhibit a continuum of basal transcriptional activities (fully active to inactive). As previously reported (15), the integrated full-length wild-type HIV-1 LTR (WT-LTR) and its associated adjacent downstream regulatory sequences exhibit appreciable levels of basal activity (Fig. 1B, clone WT3). When the two NF-κB sites were inactivated by point mutations, the basal activity of the integrated promoter was reduced 10- to 30-fold (Fig. 1B, clone KB1). Furthermore, when the adjacent downstream regulatory sequences that include the AP-1, AP-3-like, Sp1, and DBF1 binding sites are deleted (Fig. 1A, DS-LTR) or inactivated by point mutations (15), the basal activity of the integrated HIV-1 promoter is barely detectable (Fig. 1B, clone DS2). Similar results were obtained with pools of several cell clones carrying each of the LTR-CAT constructs described above (data not shown). Thus, the basal transcriptional activity of the chromatin-associated integrated HIV-1 LTR is regulated by both the upstream enhancer and promoter sequences as well as cis-acting elements situated downstream of the transcription start site.
FIG. 1.
Effects of NF-κB and Sp1 on the activity of integrated HIV-1 LTRs. (A) Schematic representation of the WT-, KBmt-, and DS-LTR CAT constructs. The LTR (U3, R, and U5) and gag leader sequence (GLS) are represented by open rectangles. Binding sites for the NF-κB, Sp1, TFIID (TATA box), AP-1, AP-3-like, and DBF1 transcription factors are shown. The transcription initiation site is indicated by the arrow at the junction between in U3 and R sites. In the DS-LTR, the regulatory elements downstream of the transcription start site (AP-1, AP-3, DBF1, and the downstream Sp1) were deleted and an unrelated sequence (filled rectangle) was inserted between U5 and the CAT gene. (B) CAT activities in unstimulated (Basal) or transfected (1 μg of Sp1 or NF-κB expression vector) WT3, KB1, and DS2 cells. (C) HeLa cells were transiently transfected with WT-, DS- or KBmt-LTR-CAT reporter plasmid alone (Basal) or along with 1 μg of vector expressing Sp1 or NF-κB as indicated at the bottom. At 24 h posttransfection, CAT activity was determined in cell lysates and expressed as percent acetylated chloramphenicol (% acetylation). The bars show the results from a representative transfection experiment; the numbers above the bars indicate average fold induction.
NF-κB modestly stimulates the transcriptionally active integrated HIV-1 promoter but not the inactivate chromatin-repressed promoter.
To investigate whether the transcriptional activators NF-κB and Sp1 could stimulate transcription directed by the integrated viral promoter, WT3, KB1, and DS2 cells were each transfected with NF-κB- or Sp1-expressing vectors. As shown in Fig. 1B, the expression of NF-κB increased the levels of LTR-driven CAT activity fivefold in WT3 cells. Increased Sp1 expression, however, had little effect on CAT activity. As expected, NF-κB failed to stimulate basal levels of CAT activity in KB1 cells carrying the integrated LTR with mutations affecting the NF-κB sites (Fig. 1B). Surprisingly, the expression of NF-κB or Sp1 in DS2 cells, which carry the integrated transcriptionally inactive HIV-1 promoter with intact NF-κB sites, failed to induce increased CAT activity (less than twofold). In contrast, the transiently transfected DS-LTR, but not the KBmt-LTR, was fully activated by NF-κB (Fig. 1C). These data suggest that NF-κB is able to activate only HIV-1 LTRs directing basal levels of transcription (i.e., in WT3 or transiently transfected cells). However, NF-κB failed to stimulate transcription in the DS2 cells, in which the HIV-1 promoter is inactive, presumably because of a suppressive chromatin structure.
TSA treatment of DS2 cells renders the inactive HIV-1 promoter responsive to NF-κB.
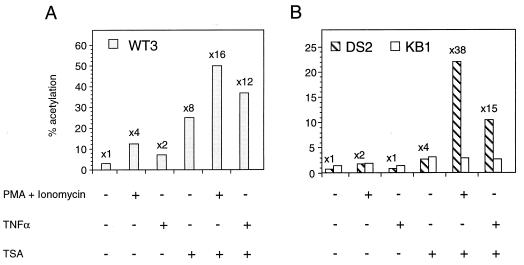
Acetylation of histones has long been linked to transcriptionally active domains in chromatin (reviewed in references 35 and 55). Because the acetylation of nucleosomes may alter histone-DNA interactions and facilitate the binding of transcriptional regulatory proteins to the HIV-1 LTR, we examined whether TSA, a known inhibitor of histone deacetylase (55, 61), would affect the basal and inducible activity of the integrated viral promoter. WT3, KB1, and DS2 cells were first treated with 0.8 μM TSA for 12 h in the presence or absence of either PMA plus ionomycin or TNFα, both potent inducers of endogenous NF-κB, for an additional 24 h. The results shown in Fig. 2A demonstrated that exposure of WT3 cells to either PMA plus ionomycin or TNFα modestly increased (2- to 4-fold) CAT expression, and treatment of these cells with TSA alone or TSA in combination with PMA plus ionomycin or TNFα greatly increased (8- to 16-fold) the levels of LTR-driven CAT expression. As expected, exposure of KB1 cells to PMA plus ionomycin or TNFα failed to stimulate CAT expression (Fig. 2B), whereas TSA treatment stimulated the levels of CAT activity in KB1 cells about two- to threefold.
FIG. 2.
Synergistic activation of the integrated HIV-1 promoter by treatment of cells with histone deacetylase inhibitor TSA and NF-κB inducers. WT3, DS2, and KB1 cells were untreated (−) or treated (+) with PMA plus ionomycin, TNFα, and/or TSA as indicated at the bottom. At 36 h postinduction, CAT activity was determined in cell lysates and expressed as percent acetylated chloramphenicol (% acetylation). The bars show the results from a representative transfection experiment; the numbers above the bars indicate average fold induction.
In contrast to the results obtained with WT3 cells, treatment of DS2 cells with PMA plus ionomycin or TNFα had little effect on CAT expression (Fig. 2B). Similarly, treatment with TSA alone induced CAT activity about fourfold. However, the combination of TSA plus NF-κB stimulation (exposure to PMA plus ionomycin or TNFα) resulted in synergistic activation (15- to 38-fold) of CAT expression. These results suggest that acetylation of histones induced by TSA activated LTR-driven CAT expression, strongly implicating chromatin as a potent suppressor of HIV-1 transcription in DS2 cells.
The chromatin structure of the HIV-1 LTR changes in response to NF-κB activation in WT3 cells but not in KB1 and DS2 cells.
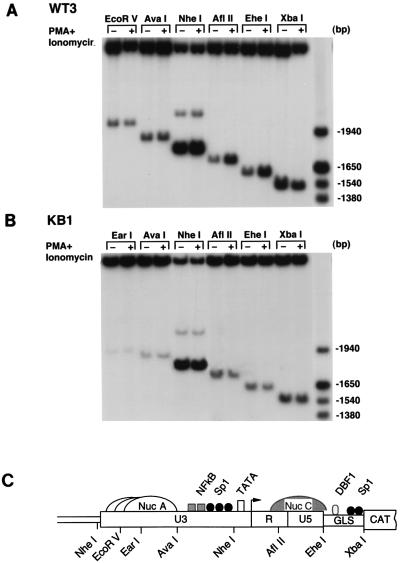
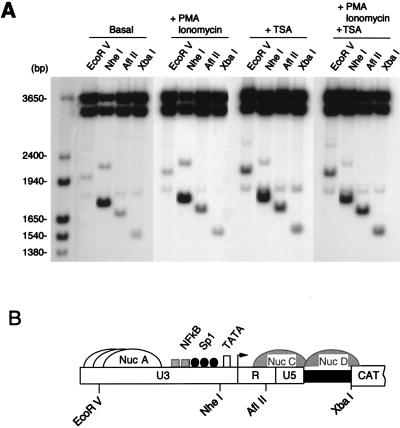
In eukaryotic cells, DNA that is assembled in chromatin and transcriptional activation has been often associated with chromatin remodeling, as detected by nuclease-hypersensitive sites analysis (2, 21). As previously reported (15), the HIV-1 LTR in unstimulated WT3 cells is packaged into two potential nucleosomes, Nuc A and Nuc C, separated by a large nuclease-hypersensitive region encompassing the enhancer and promoter (Fig. 3C). Nuc A, extending over the 3′ portion of U3, is relatively resistant to nuclease digestion, whereas Nuc C, located downstream of the promoter, exhibits some sensitivity to both micrococcal nuclease and restriction endonucleases. Alterations in the nucleosomal structure of the integrated wild-type HIV-1 LTR following a 4-h exposure to PMA plus ionomycin was assessed with restriction endonuclease accessibility as described in Materials and Methods. The results of this analysis (Fig. 3A) revealed greater endonuclease sensitivity in stimulated cells of the Nuc C region encompassing the AflII and EheI sites (compare + and − lanes). Digestions with the other enzymes were essentially unchanged (EcoRV, AvaI, and NheI) or slightly decreased (XbaI).
FIG. 3.
NF-κB stimulation induces increased accessibility of the integrated HIV-1 WT-LTR to restriction endonucleases. (A and B) Nuclei isolated from WT3 and KB1 cells maintained in DMEM (−) or stimulated with PMA plus ionomycin (+) were digested with the restriction enzymes indicated at the top. DNA was then purified, digested to completion with BglI and HincII, and analyzed by indirect end labeling. A molecular weight size marker was prepared by digesting a previously described LTR-CAT plasmid, pLCH (15), with several restriction enzymes. (C) Schematic representation of the putative nucleosomal structure and partial restriction map of WT-LTR. Nuc A and Nuc C are positioned relative to micrococcal nuclease and restriction enzyme cleavage sites (11). Nuc A is shown as three adjacent and overlapping structures to indicate that it has not been precisely positioned. Nuc C is represented by a hatched structure to indicate that its accessibility to endonucleases is increased following stimulation with PMA plus ionomycin. GLS, gag leader sequence.
Accessibility of the integrated KBmt-LTR template to restriction endonuclease was also evaluated in uninduced and PMA-plus-ionomycin-stimulated KB1 cells (Fig. 3B). As expected, the EarI, AvaI, AflII, and EheI sites, located in the Nuc A and Nuc C regions, are inefficiently cleaved in uninduced KB1 cells, whereas the NheI site, situated in the promoter region, is readily digested. Unlike the wild-type LTR in PMA-induced WT3 cells, the cleavage pattern of the KBmt-LTR in PMA-treated KB1 cells is virtually the same as in the untreated cells. Taken together, these results demonstrate that NF-κB induces transcriptional activation of the wild-type HIV-1 LTR (Fig. 1 and 2) and localized chromatin remodeling. In contrast, an integrated LTR lacking binding sites for NF-κB is refractory to NF-κB stimulation, and its chromatin structure remains unchanged following NF-κB induction.
Although the integrated LTR in DS2 cells contains two intact NF-κB sites (Fig. 1A, middle), the expression of NF-κB following either transfection (Fig. 1) or exposure of cells to PMA plus ionomycin or TNFα (Fig. 2B) failed to activate LTR-directed CAT activity. To ascertain whether treatment of DS2 cells with PMA plus ionomycin might still induce changes in the chromatin structure of DS-LTR, a restriction endonuclease analysis was carried out. As shown in Fig. 4, the digestion pattern was either unchanged (EcoRV, NheI, and XbaI) or slightly increased (AflII) in cells treated with PMA plus ionomycin compared to untreated cells (basal). These results suggest that increased accessibility of the Nuc C to restriction endonucleases (e.g., in PMA-stimulated WT3 cells) correlates with elevated transcriptional activity, while in the absence of detectable transcription activation (e.g., in DS2 cells), the expression of NF-κB is unable to induce chromatin remodeling.
FIG. 4.
TSA treatment of DS2 cells enhances endonuclease accessibility of the integrated LTR. Nuclei isolated from DS2 cells maintained in DMEM (Basal) or treated with either PMA plus ionomycin, TSA, or a combination of both PMA, ionomycin, and TSA, as indicated, were digested with the restriction enzymes indicated at the top. DNA was then purified, digested to completion with BglI and HincII, and analyzed by indirect end labeling. (B) Schematic representation of the putative nucleosomal structure and partial restriction map of DS-LTR. Nuc C and Nuc D are represented by a hatched structure to indicate that their accessibility to endonucleases is increased following treatment with TSA.
TSA treatment of DS2 cells enhances endonuclease accessibility of the integrated HIV-1 LTR.
The results shown in Fig. 2B demonstrate that the histone deacetylase inhibitor TSA, alone or in combination with inducers of NF-κB (PMA-ionomycin or TNFα), strongly stimulated CAT activity directed by the inactive LTR present in DS2 cells. To determine whether the chromatin structure of the HIV-1 LTR in DS2 cells has been altered following exposure to TSA alone or in combination with PMA plus ionomycin, a restriction endonuclease accessibility assay was performed. As shown in Fig. 4, the sensitivity of the HIV-1 LTR to endonucleases was greater in TSA-treated cells than in untreated cells (basal). The cleavage by EcoRV, AflII, and XbaI was significantly increased in both TSA alone and TSA-PMA-ionomycin-induced DS2 cells. Although, the precise mechanism mediating this chromatin alteration is unknown, the simplest interpretation is that the observed increase in accessibility to endonucleases is due to acetylation of histones amino-terminal tails (36, 57, 63). The resulting alteration of chromatin structure allows the DS-LTR to respond to NF-κB and leads to strong transcriptional activation (Fig. 2B). These results indicate that nucleosomal structure of the integrated DS-LTR effectively blocks basal and NF-κB-induced transcription and histone acetylation alleviates this chromatin-mediated repression.
Unlike NF-κB, Tat strongly stimulates the chromatin-repressed HIV-1 promoter.
We next examined if the Tat responsiveness of the integrated HIV-1 LTR is similarly affected by the level of basal transcriptional activity. WT3, DS2, and KB1 cells were transfected with increasing amounts of a Tat expression vector. As shown in Fig. 5, a concentration-dependent stimulation of CAT by Tat was observed in the three cloned HeLa cell lines. When levels of basal transcription were relatively high (WT3 cells [Fig. 5A]) or low (KB1 cells [Fig. 5C]), a 17- to 60-fold increase of CAT activity occurred. However, a significantly greater (450-fold) activation was observed with DS2 cells, which produced virtually no detectable CAT in the absence of Tat (Fig. 5B). To further demonstrate that the observed activation was Tat specific, a Tat expression vector containing mutation affecting the activation domain (K41T) was transfected into the three cell clones. In agreement with previous reports (31), Tat (K41T) failed to activate the three integrated HIV-1 LTRs. Thus, Tat strongly stimulates integrated HIV-1 LTR-directed CAT expression; its transactivation capacity is not influenced by the levels of basal promoter activity or mutations affecting the NF-κB sites.
Transcriptional activation by Tat correlates with chromatin remodeling downstream of the transcription initiation site.
Much of our understanding of the mechanism underlying Tat transactivation comes from transient transfections or cell-free transcription assays in which the DNA template is chromatin free. Therefore, to assess the effects of Tat expression on the chromatin structure of the integrated HIV-1 promoter, WT3, KB1, and DS2 cells were transfected with vectors expressing the wild-type or mutated Tat protein. Twelve hours following transfection, nuclei were isolated and digested by various endonucleases as described in Materials and Methods.
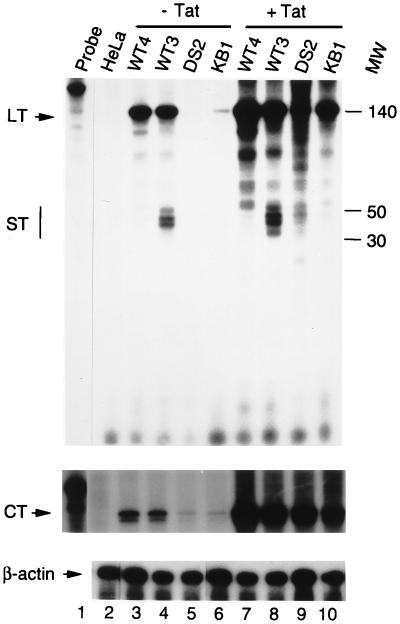
As a control for the steady-state levels of RNA synthesis directed by the integrated HIV-1 promoter prior or 12 h after transfection of Tat expression vector, total RNA was isolated and analyzed by RNase protection assay using two radiolabeled DNA probes: (i) an LTR probe designed to detect both promoter-proximal short transcripts and long transcripts, initiated at the authentic transcriptional start site and elongated up to 140 nt; and (ii) a CAT probe for detection of mRNAs that extend 680 nt downstream of the initiation site (Fig. 6). In absence of Tat, both short and long RNA transcripts were synthesized in the WT3 and WT4 HeLa cell clones, containing integrated, transcriptionally active, wild-type HIV-1 LTRs. Substantial increases of all three classes of RNA were induced within 12 h by Tat in these two cell lines. In agreement with the CAT assay results (which were carried out 24 h after transfection), the RNA protection analysis (performed 12 h after transfection) indicated that the inactive LTR in DS2 cells produced very little RNA (detected only by phosphorimaging) in the absence of Tat. In the presence of Tat, however, transcription was markedly stimulated (Fig. 6; compare lanes DS2 [− and + Tat]). This response to Tat induction confirms the existence of very small amounts of TAR-containing transcripts, presumably products of deficiently processive transcriptional complexes. Thus, in DS2 cells, the inactive HIV-1 promoter is associated with an elongation-incompetent RNA polymerase II that transcribes extremely low amounts of TAR RNA that are, nonetheless, fully responsive to Tat when it is subsequently expressed (Fig. 5 and 6).
FIG. 6.
Tat stimulates transcription directed by the integrated HIV-1 LTR. RNase protection assays showing the levels of RNA synthesis in untransfected (− Tat) or transfected (+ Tat) HeLa cell clones carrying integrated copies of the WT-LTR (WT3 and WT4), the DS-LTR (DS2), or the KBmt-LTR (KB1). The probes used were (i) an HIV-1 LTR probe that detects both the short (ST) and long (LT) transcripts, (ii) a CAT probe to detect CAT transcripts that extend 680 nt downstream of the transcription start site (CT), and (iii) a β-actin probe that detects cellular actin mRNAs. Molecular weights (MW) are indicated in nucleotides.
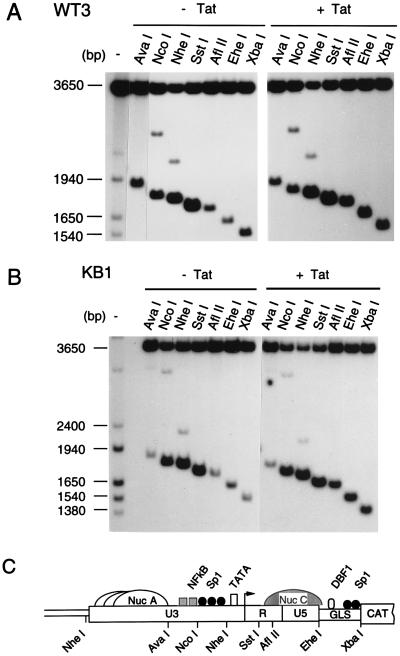
The accessibility of the HIV-1 LTR, in nuclei prepared from WT3 cells, to various restriction endonucleases was examined in the presence and absence of Tat (Fig. 7). As previously reported (16), several of the restriction enzyme sites associated with the integrated wild-type HIV-1 LTR were cleaved in the absence of Tat (Fig. 7A, left). Interestingly, the expression of Tat in WT3 cells resulted in increased digestion at the AflII and EheI sites without affecting the cleavage pattern of endonucleases sites, located further upstream of the LTR (Fig. 7A, upper NcoI and NheI bands). The increased accessibility of the AflII, EheI, and, to a lesser extent, XbaI sites was also confirmed by phosphoimager analysis (not shown).
FIG. 7.
Accessibility of the integrated HIV-1 WT- and KBmt-LTRs to restriction endonucleases in the presence or absence of Tat. (A and B) Nuclei isolated from WT3 (A) and KB1 (B) cells maintained in DMEM (− Tat) or transfected with a Tat expression vector (+ Tat) were digested with the restriction enzymes indicated at the top. DNA was then purified, digested to completion with BglI and HincII and analyzed by indirect end labeling. A molecular weight size marker was prepared by digesting a previously described LTR-CAT plasmid, pLCH (15), with several restriction enzymes. (C) Schematic representation of the putative nucleosomal structure and partial restriction map of WT-LTR. Nuc C is represented by a hatched structure to indicate that its accessibility to endonucleases is increased following the expression of Tat protein. GLS, gag leader sequence.
The sensitivity of the integrated KBmt-LTR template to restriction endonuclease digestion was also evaluated in the presence and absence of Tat (Fig. 7B). In uninduced KB1 cells, the AflII, EheI, and XbaI sites, located in the Nuc C region, are inefficiently cleaved, whereas the NcoI, NheI, and SstI restriction sites, situated in the promoter region, are readily digested. Following Tat transactivation, the AflII, EheI, and XbaI sites become highly accessible to endonucleases, consistent with the remodeling of chromatin, while digestion with NcoI and NheI in the promoter region or upstream (upper bands in the NcoI and NheI lanes) of the LTR were unchanged.
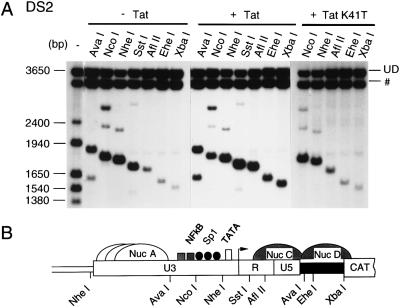
The effect of Tat on the chromatin structure of LTR was even more apparent when the transcriptionally inactive HIV-1 promoter in DS2 cells was examined (Fig. 8). In unstimulated DS2 cells, cleavage at the AflII, EheI, and XbaI sites is quite inefficient, consistent with the presence of stable nucleosomes associated with this region of the LTR and adjacent downstream sequences (Fig. 8B). Following Tat transfection, however, increased accessibility to AflII, EheI, and XbaI sites was observed, while the cleavage by NcoI and NheI, within the LTR and at other sites in the chromatin template, did not change appreciably (Fig. 8A). The HIV-1 LTR present in DS2 cells was designed to contain two AvaI sites; one is situated immediately 5′ of the enhancer/promoter in U3, and the other is located within sequences downstream of U5 (Fig. 8B). This combination of AvaI sites permits a more accurate estimation of restriction enzyme accessibility of the Nuc C region in transcriptionally active or inactive chromatin templates. As shown in Fig. 8A, the downstream AvaI site (represented by the lower of the two AvaI bands), located in Nuc C region, is efficiently digested only following Tat transactivation, indicating that the chromatin downstream of the HIV-1 promoter undergoes remodeling in the presence of Tat.
FIG. 8.
Chromatin changes associated with Tat expression in DS2 cells. (A) Nuclei isolated from cloned DS2 HeLa cells maintained in DMEM (− Tat) or transfected with either a Tat expression vector (+ Tat) or the Tat(K41T) expression vector (+ Tat K41T) were digested with the restriction enzymes indicated at the top. DNA was then purified, digested to completion with BglI and HincII, and analyzed by indirect end labeling. UD, undigested parental fragment; #, an unrelated band that hybridizes to the probe but lacks any HIV-1 LTR sequences. (B) Schematic representation of the putative nucleosomal structure of DS-LTR. Nuc C and Nuc D are represented as hatched structures to indicate that their structure is remodeled during Tat activation.
We also tested whether the mutated Tat(K41T) protein, capable of binding to TAR but transcriptionally inactive, is able to alter chromatin structure. The expression of Tat(K41T) in DS2 cells had no effect on either accessibility to the AflII, EheI, and XbaI sites, or cleavage by NcoI and NheI, compared to untransfected cells (Fig. 8A, right). Similarly, the expression of Tat(K41T) in WT3 and KB1 cells had no effect on the digestion of the integrated LTRs by any of the restriction endonucleases tested (not shown). These results suggest that (i) expression of a functional Tat is associated with the alteration of chromatin structure downstream of the HIV-1 promoter and (ii) the binding of Tat to TAR [as occurs with Tat(K41T)] fails to induce chromatin remodeling.
Binding of Tat to DNA upstream of the HIV-1 core promoter does not activate transcription or alter chromatin structure.
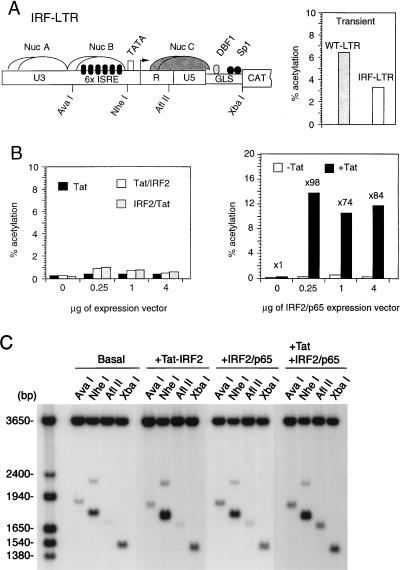
Although Tat binding to the TAR element is not dependent on upstream promoter sequences, the inactivation of NF-κB and Sp1 by point mutations completely abolishes Tat responsiveness (3, 14a). To ascertain whether binding of functional Tat protein upstream the HIV-1 TATA box might activate transcription or trigger chromatin remodeling in the absence of Sp1 and NF-κB, we created a recombinant LTR construct in which the two NF-κB and three Sp1 sites were deleted and replaced with a tandem array of six binding sites for the IRF family of factors (Fig. 9A). In transient transfection assays, this IRF-LTR exhibited relatively high levels of basal activity but was not significantly stimulated by Tat (∼2-fold), despite the presence of an intact TAR element (data not shown). To target Tat to the IRF-LTR, two expression plasmids consisting of the DNA binding domain of IRF2 fused at the C terminus (Tat-IRF2) or N terminus (IRF2-Tat), respectively, of Tat were constructed. Tat as well as both Tat-IRF2 fusion proteins readily activated the integrated wild-type HIV-1 promoter (data not shown). In contrast, neither Tat nor the Tat-IRF2 fusion proteins significantly affected the basal levels of CAT expression directed by the integrated IRF-LTR (Fig. 9B, left). To ascertain whether Tat tethered to the core promoter via IRF binding sites could induce chromatin remodeling in the absence of transcriptional activation, the chromatin structure of the integrated IRF-LTR prior to and following transfection of the Tat-IRF2 expression plasmid was analyzed by the restriction endonuclease accessibility assay (Fig. 9C). With the exception of slightly increased AvaI cleavage, possibly reflecting the binding of the Tat-IRF2 fusion protein adjacent to the AvaI site, the digestion pattern obtained was essentially unchanged (Fig. 9C; compare Basal and +Tat-IRF2). This experiment indicates that when Tat is tethered to the HIV-1 core promoter, it is unable to either activate transcription or induce chromatin remodeling downstream of the transcriptional start site.
FIG. 9.
Transcriptional activation and chromatin remodeling of the IRF-LTR. (A) Schematic representation of the putative nucleosomal structure of the IRF-LTR (14a). Nuc A, Nuc B, and Nuc C are represented as overlapping structures to indicate that their positions have not been precisely determined. The Nuc C segment is hatched to indicate that this region of chromatin is accessible to AflII when the promoter is active. GLS, gag leader sequence. CAT activities in HeLa cells transiently transfected with the WT- or IRF-LTR-CAT reporter plasmid are shown at the right. (B) Left, HeLa cells carrying a single copy of the IRF-LTR were transfected with increasing amounts of Tat, Tat-IRF2, or IRF2-Tat expression vector, and CAT activities in cell lysates were determined. Right, IRF-LTR containing cells were transfected with increasing amounts of a vector expressing the IRF2-p65 fusion protein alone (−Tat) or along with 1 μg of Tat expressing vector (+Tat), and CAT activities were determined. (C) Cells carrying the IRF-LTR template were maintained in DMEM (Basal) or transfected with the indicated expression vectors. At 12 h posttransfection, nuclei were isolated and digested with AvaI, NheI, AflII, and XbaI. DNA was purified and analyzed by indirect end labeling.
What is required to restore Tat responsiveness?
In an attempt to restore the Tat responsiveness to the IRF-LTR, an expression plasmid consisting of the IRF2 DNA binding domain fused to the NF-κB p65 activation domain (34) was cotransfected with Tat into the cloned HeLa cell line carrying integrated IRF-LTR. In the presence of the IRF2-p65 fusion protein plus Tat, CAT activity increased 98-fold (Fig. 9B, right); transfection of the IRF2-p65 expression vector alone, however, resulted in only a 2- to 4-fold elevation of CAT. Taken together, these experiments demonstrate that the transcriptional complexes assembled on an integrated HIV-1 promoter in the presence of Sp1 (Fig. 5 and 6, KBmt-LTR) or NF-κB (Fig. 9B, right) are responsive to Tat, whereas transcriptional complexes recruited by IRF1 and IRF2 transcription factors (Fig. 9B, basal activity) are refractory to Tat activation.
The chromatin structure of the integrated IRF-LTR was also examined in the presence of Tat and the IRF2-p65 fusion protein. As shown in Fig. 9C, the accessibility of the IRF-LTR to AvaI, NheI, AflII, and XbaI was unchanged in cells transfected with IRF-p65 alone compared to the untransfected cells (basal). In contrast, when the IRF2-p65 fusion protein and Tat were coexpressed, digestion at the AflII site increased significantly (Fig. 9C, +Tat +IRF2/p65). Digestion by AvaI was also enhanced about twofold, presumably reflecting the binding of IRF2-p65 to a region of the LTR adjacent to the AvaI cleavage site (Fig. 9A). These data are yet another example linking chromatin remodeling downstream of the HIV-1 promoter with Tat-induced activation of RNA synthesis.
DISCUSSION
In this report, we have shown that when the integrated HIV-1 promoter exhibits basal activity (as in WT3 cells), it is responsive to both NF-κB and Tat stimulation. In contrast, when the promoter is assembled into a suppressive chromatin structure (as in DS2 cells), it is strongly activated by Tat but not NF-κB. Treatment of cells with histone deacetylase inhibitor TSA renders the inactive promoter responsive to NF-κB, suggesting that chromatin exerts a repressive effect on HIV-1 basal transcription and NF-κB-dependent activation. In this case, histone acetylation may increase the accessibility of the chromatin-associated LTR to components of the transcription machinery facilitating both basal and activated transcription.
Role of NF-κB and Sp1 in the establishment of transcriptionally competent HIV-1 promoter in the context of chromatin.
The cellular transcription factors Sp1 and NF-κB are required for HIV-1 LTR-directed transcription (3, 20, 25) and productive virus infections (9, 45). The p65 NF-κB subunit and Sp1 protein have previously been shown to cooperatively activate transcription from transiently transfected DNA (44) and chromatin templates in vitro (43). Our results indicate that exogenously added Sp1 had little if any effect on the integrated HIV-1 LTR, suggesting that the constitutive levels of Sp1 production in cultured HeLa cells are not limiting. In contrast, NF-κB modestly (four- to fivefold) activates an integrated HIV-1 LTR exhibiting basal levels of transcription. However, if the promoter is assembled into a repressive chromatin structure (as in DS2 cells), increased expression of p65 NF-κB subunit is unable to stimulate RNA synthesis. This failure to activate the chromatin-suppressed HIV-1 LTR cannot be due to promoter inaccessibility since the NF-κB and Sp1 binding sites are occupied in vivo (14) and are invariably situated in an open chromatin structure, regardless of the basal promoter activity (15, 45). Moreover, it has been recently shown that both NF-κB and Sp1 are able to bind HIV-1 nucleosomal DNA, at least in vitro (43, 53). On the basis of these observations and the results reported here, the inactive HIV-1 promoter reported to be present in certain types of infected cells (10–12, 18) may reside in chromosomal structure that is the functional equivalent of the integrated DS-LTR. Such a promoter may contain bound Sp1 and NF-κB components (such as the p50 homodimer) that direct the assembly of a complex capable of transcriptional initiation but only inefficient elongation. Thus, the roles of Sp1 and NF-κB are to assemble transcriptional complexes on the integrated HIV-1 promoter exhibiting a continuum of basal activities, depending primarily on the site of integration and the local chromatin structure.
Histone acetylation potentiates NF-κB-dependent activation of the integrated HIV-1 promoter.
The results shown in Fig. 2 suggest that the accumulation of acetylated histones induced by TSA treatment stimulated basal and NF-κB-dependent transcription directed by the integrated HIV-1 promoter in both WT3 and DS2 cells. Therefore, the transcriptionally silent HIV-1 LTR in DS2 cells was neither defective nor irreversibly inactive, since TSA treatment resulted in a significant stimulation of the basal promoter activity (4-fold) and a synergistic activation by NF-κB (38-fold). Similar results have been reported when HIV-1-infected cells or cells carrying stably integrated HIV-1 LTR were treated with the histone deacetylase inhibitors n-butyrate (32, 33), TSA, and trapoxin (56). These observations indicate that nucleosomes are able to inhibit the transcriptional activity of the HIV-1 promoter, and histone acetylation may partially alleviate chromatin-mediated suppression. Consistent with this interpretation, we have shown that treatment of cells with TSA enhanced also the accessibility of the chromatin to restriction endonucleases (Fig. 4). Thus, acetylation of histone may facilitate the interactions of transcription factors with the viral LTR, thereby allowing the recruitment of RNA polymerases for efficient elongation on a chromatin template.
Tat transactivation of the integrated HIV-1 promoter involves both transcription stimulation and chromatin remodeling.
Several previous reports have shown that exogenous Tat activates HIV-1 gene expression in infected cells (1, 17), but the effect of Tat on the chromatin structure of the integrated HIV-1 LTR was not specifically examined. The results presented here demonstrate that Tat is a potent activator of the integrated HIV-1 promoter even when the basal transcriptional activity is quite low as well as in the absence of histone deacetylase inhibitors. Tat transactivation is accompanied by a local chromatin remodeling downstream of the transcription initiation site (Nuc C region [Fig. 3]), as monitored by increased accessibility to restriction endonucleases. However, similar chromatin alterations (Fig. 3) were observed when NF-κB was induced in cells carrying the wild-type HIV-1 promoter (e.g., WT3 cells) or when DS2 cells were treated with the histone deacetylase inhibitor TSA (Fig. 4). In both cases, the integrated promoters were transcriptionally activated, suggesting that chromatin remodeling downstream of the transcription initiation site may simply be a consequence of increased RNA synthesis.
The elongation capacity of RNA polymerase II can be regulated by a variety of mechanisms, including the recruitment of general elongation factors (TFIIF, TFIIS, P-TEFb, ELL, and elongin), phosphorylation of the carboxy-terminal domain (CTD) of polymerase, modulation of the catalytic activity of the polymerase, and the alteration of chromatin structure (reviewed in reference 54). Numerous reports suggest that Tat utilizes some of these mechanisms to stimulate RNA polymerase II processivity. First, Tat has been shown to stimulate or recruit CTD kinases (5, 23, 41, 60, 62) to the transcription initiation complex, resulting in the phosphorylation of the CTD and thereby converting the RNA polymerase II from a nonprocessive to a processive enzyme (37, 39, 40). Second, Tat has also been reported to interact directly with the RNA polymerase II in vitro (13, 38), which might induce conformational changes of the polymerase and allow the subsequent recruitment of factors required for efficient elongation. Finally, our results indicate that Tat transactivation is accompanied by chromatin remodeling downstream of the transcription start site (Fig. 7 to 9). Chromatin remodeling and the stimulation of RNA polymerase II processivity would facilitate the reinitiation of transcription, resulting in increased levels of steady-state RNA detected in presence of Tat (Fig. 6). Such a model would be consistent with reports showing that nucleosomes increase the pausing of RNA polymerase II in vitro (8, 24), and the release in vitro and in vivo of stalled RNA polymerases, as documented for a variety of inducible genes, including the human c-myc and the Drosophila and human hsp70 genes (8, 30, 46), required the expression of specific activators. Similarly, nucleosomes may inhibit HIV-1 LTR-directed gene expression either by blocking access of the promoter to components of transcription machinery or by impeding efficient elongation. Tat alleviates this block to elongation by increasing the processivity of RNA polymerase through the phosphorylation of CTD and perhaps by facilitating recruitment of chromatin remodeling activity. Together, these effects of Tat on elongation and chromatin remodeling account for most of the 450-fold stimulation of CAT activity observed in the Tat-transfected DS2 cells (Fig. 5B). In transient transfection assays, Tat stimulated CAT activity driven by DS-LTR only 30- to 50-fold (Fig. 5D). A simple interpretation of this result is that transiently transfected DNA does not assemble into a functional chromatin structure which, in the case of the stably integrated DS-LTR, suppresses the basal promoter activity. Therefore, the 30- to 50-fold stimulation of CAT activity observed in transient transfections may represent primarily transcriptional stimulation and not chromatin remodeling. In marked contrast to the observed Tat activation, treatment of DS2 cells with TSA, which led to alterations in chromatin structure (Fig. 4), stimulated CAT activity only fourfold (Fig. 2). However, our experiments (Fig. 9) clearly show that Tat itself does not directly alter chromatin structure. A major unanswered question is whether Tat and or Tat-associated factors, via their interactions with TAR, target chromatin remodeling activities to the HIV-1 promoter, thereby creating a more permissive chromatin environment for transcription, or whether Tat stimulates the phosphorylation of RNA polymerase II, which binds additional chromatin-modifying coactivators, such as the SWI-SNF complex (54, 58), or histone acetyltransferases and then induces chromatin remodeling.
In summary, we have described a potentially useful model system to investigate the roles of chromatin structure, cellular transcription factors, and the virus-encoded Tat protein in regulating proviral gene expression in HIV-1-infected cells. In the context of the integrated HIV-1 LTR, Sp1 and NF-κB are required to assemble transcriptional complexes exhibiting a continuum of basal activities, depending primarily on the local chromatin structure, but which are primed to be fully responsive to Tat. The transactivation properties of Tat are not affected by the chromatin structure or levels of basal transcriptional activity. Unlike the case for Tat, activation by NF-κB is strongly inhibited by suppressive chromatin structure and is dependent on levels of basal promoter activity.
ACKNOWLEDGMENTS
We are grateful to Guido Franzoso and Uli Siebenlist for providing the NF-κB expression vectors, John Hiscott for the IRF2-p65 expression vector, Julie Brown for pSV-TatK41T, and Bachoti Rao for DNA sequencing.
REFERENCES
- 1.Adams M, Sharmeen L, Kimpton J, Romeo J M, Garcia J V, Peterlin B M, Groudine M, Emerman M. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc Natl Acad Sci USA. 1994;91:3862–3866. doi: 10.1073/pnas.91.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer T K, Lefebvre P, Wolford R G, Hager G L. Transcription factor loading on the MMTV promoter: a bidominal mechanism for promoter activation. Science. 1992;255:1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- 3.Berkhout B, Gatignol A, Rabson A B, Jeang K-T. TAR-independent activation of the HIV-1 LTR: evidence that Tat requires specific regions of the promoter. Cell. 1990;62:757–767. doi: 10.1016/0092-8674(90)90120-4. [DOI] [PubMed] [Google Scholar]
- 4.Berkhout B, Silverman R H, Jeang K-T. Tat transactivates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 5.Blau J, Xiao H, McCracken S, O’Hare P, Greenblatt J, Bentley D. Three functional classes of transcriptional activation domains. Mol Cell Biol. 1996;16:2044–2055. doi: 10.1128/mcb.16.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bours V, Burd P R, Brown K, Villalobos J, Park S, Ryseck R P, Bravo R, Kelly K, Siebenlist U. A novel mitogen-inducible gene product related to p50/p105 NF-κB participates in transactivation through a kB site. Mol Cell Biol. 1992;12:685–695. doi: 10.1128/mcb.12.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyes J, Felsenfeld G. Tissue-specific factors additively increase the probability to the all-or-none formation of a hypersensitive site. EMBO J. 1996;15:2496–2507. [PMC free article] [PubMed] [Google Scholar]
- 8.Brown S A, Imbalzano A N, Kingston R E. Activator-dependent regulation of transcriptional pausing on nucleosomal templates. Genes Dev. 1996;10:1479–1490. doi: 10.1101/gad.10.12.1479. [DOI] [PubMed] [Google Scholar]
- 9.Chen B K, Feinberg M B, Baltimore D. The κB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J Virol. 1997;71:5495–5504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun T-W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, Kuo Y, Brookmeyer R, Zeiger M, Barditch-Crovo P, Siliciano R F. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 11.Chun T-W, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano R F. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 12.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci A S, Folks T M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 13.Cujec T P, Cho H, Maldonado E, Meyer J, Reinberg D, Peterlin B M. The human immunodeficiency virus transactivator Tat interacts with the RNA polymerase II holoenzyme. Mol Cell Biol. 1997;17:1817–1823. doi: 10.1128/mcb.17.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demarchi F, D’agaro P, Falaschi A, Giacca M. In vivo footprinting analysis of constitutive and inducible protein-DNA interactions at the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1993;67:7450–7460. doi: 10.1128/jvi.67.12.7450-7460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.El Kharroubi, A. Unpublished data.
- 15.El Kharroubi A, Martin M A. cis-acting sequences located downstream of the human immunodeficiency virus type 1 promoter affect its chromatin structure and transcriptional activity. Mol Cell Biol. 1996;16:2958–2966. doi: 10.1128/mcb.16.6.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Kharroubi A, Verdin E. Protein-DNA interactions within DNase I-hypersensitive sites located downstream of the HIV-1 promoter. J Biol Chem. 1994;269:19916–19924. [PubMed] [Google Scholar]
- 17.Feinberg M B, Baltimore D, Frankel A D. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci USA. 1991;88:4045–4049. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folks T M, Justement J, Kinter A, Dinarello C A, Fauci A S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 19.Forrester W G, Takegawa S, Papayannopoulou T, Stamatoyannopoulos G. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin expressing hybrids. Nucleic Acids Res. 1987;15:10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaynor R B. Regulation of HIV-1 gene expression by the transactivator protein Tat. Curr Top Microbiol Immunol. 1995;193:51–77. doi: 10.1007/978-3-642-78929-8_3. [DOI] [PubMed] [Google Scholar]
- 21.Gross D, Garrard W T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- 22.Groudine M, Kohwi-Shigematsu T, Gelinas R, Stamatoyannopoulos G, Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the β-globin gene locus. Proc Natl Acad Sci USA. 1983;80:7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunits of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izban M G, Luse D S. Transcription on nucleosomal templates by RNA polymerase II in vitro: inhibition of elongation with enhancement of sequence-specific pausing. Genes Dev. 1991;5:683–696. doi: 10.1101/gad.5.4.683. [DOI] [PubMed] [Google Scholar]
- 25.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 26.Kao H, Sumimoto H, Pognonec P, Chen C-H, Rosen C A, Roeder R G. HIV-1 Tat acts as a processivity factor in vitro in conjunction with cellular elongation factors. Genes Dev. 1992;6:655–666. doi: 10.1101/gad.6.4.655. [DOI] [PubMed] [Google Scholar]
- 27.Kao S Y, Calman A F, Luciw P A, Peterlin B M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- 28.Kim J Y, Gonzalez-Scarano F, Zeichner S L, Alwine J C. Replication of type 1 human immunodeficiency viruses containing linker substitution mutations in the -201 to -130 region of the long terminal repeat. J Virol. 1993;67:1658–1662. doi: 10.1128/jvi.67.3.1658-1662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornberg R D, Lorch Y. Chromatin structure and transcription. Annu Rev Cell Biol. 1992;8:563–587. doi: 10.1146/annurev.cb.08.110192.003023. [DOI] [PubMed] [Google Scholar]
- 30.Krumm A, Hickey L B, Groudine M. Promoter-proximal pausing of RNA polymerase II defines a general rate-limiting step after transcription initiation. Genes Dev. 1995;9:559–572. doi: 10.1101/gad.9.5.559. [DOI] [PubMed] [Google Scholar]
- 31.Kuppuswamy M, Subramanian T, Chinnadurai G. Multiple functional domains of Tat, the transactivator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989;17:3551–3561. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laughlin M A, Chang G Y, Oakes J W, Gonzalez-Scarano F, Pomerantz R J. Sodium butyrate stimulation of HIV-1 gene expression: a novel mechanism of induction independent of NF-κB. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:332–339. [PubMed] [Google Scholar]
- 33.Laughlin M A, Zeichner S, Kolson D, Alwine J C, Seshamma T, Pomerantz R J, Gonzalez-Scarano F. Sodium butyrate treatment of cells latently infected with HIV-1 results in the expression of unspliced viral RNA. Virology. 1993;196:496–505. doi: 10.1006/viro.1993.1505. [DOI] [PubMed] [Google Scholar]
- 34.Lin R, Mustafa A, Nguyen H, Gewert D, Hiscott J. Mutational analysis of interferon (IFN) regulatory factors 1 and 2: effects on the induction of IFN-β gene expression. J Biol Chem. 1994;269:17542–17549. [PubMed] [Google Scholar]
- 35.Loidl P. Histone acetylation: facts and questions. Chromosoma. 1994;103:441–449. doi: 10.1007/BF00337382. [DOI] [PubMed] [Google Scholar]
- 36.Marciniak R A, Sharp P A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991;10:4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall N F, Peng J, Xie Z, Price D H. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- 38.Mavankal G, Ou S-H I, Oliver H, Sigman D, Gaynor R B. Human immunodeficiency virus type 1 and 2 Tat proteins specifically interact with RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:2089–2094. doi: 10.1073/pnas.93.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien T, Hardin S, Greenleaf A, Lis J T. Phosphorylation of RNA polymerse II C-terminal domain and transcriptional elongation. Nature. 1994;370:75–77. doi: 10.1038/370075a0. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto H, Sheline C T, Corden J L, Jones K A, Peterlin B M. Trans-activation by human immunodeficiency virus Tat protein requires the C-terminal domain of RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:11575–11579. doi: 10.1073/pnas.93.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its corboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 42.Paranjape S M, Kamakaka R T, Kadonaga J T. Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem. 1994;63:265–297. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- 43.Pazin M J, Sheridan P L, Cannon K, Cao Z, Keck J G, Kadonaga J T, Jones K A. NF-κB-mediated chromatin reconfiguration and transcriptional activation of the HIV-1 enhancer in vitro. Genes Dev. 1996;10:37–49. doi: 10.1101/gad.10.1.37. [DOI] [PubMed] [Google Scholar]
- 44.Perkins N D, Edwards N L, Duckett C S, Agranoff A B, Schmid R M, Nabel G J. A cooperative interaction between NF-κB and Sp1 is required for HIV-1 enhancer activation. EMBO J. 1993;12:3551–3558. doi: 10.1002/j.1460-2075.1993.tb06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross E K, Buckler-White A J, Rabson A B, Englund G, Martin M A. Contribution of NF-κB and Sp1 binding motifs to the replicative capacity of human immunodeficiency type 1: distinct patterns of viral growth are determined by T-cell types. J Virol. 1991;65:4350–4358. doi: 10.1128/jvi.65.8.4350-4358.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rougvie A E, Lis J T. The RNA polymerase II Molecule at the 5′ end of the uninduced hsp70 gene of D. melanogaster is transcriptionally engaged. Cell. 1988;54:795–804. doi: 10.1016/s0092-8674(88)91087-2. [DOI] [PubMed] [Google Scholar]
- 47.Roy S, Delling U, Chen C-H, Rosen C A, Sonenberg N. A bulge structure in HIV-1 TAR RNA is required for Tat binding and Tat-mediated transactivation. Genes Dev. 1990;4:1365–1373. doi: 10.1101/gad.4.8.1365. [DOI] [PubMed] [Google Scholar]
- 48.Saffer J D, Jackson S P, Thurston S J. SV40 stimulates expression of the transacting factor Sp1 at the mRNA level. Genes Dev. 1990;4:659–666. doi: 10.1101/gad.4.4.659. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 50.Selby M J, Bain E S, Luciw P A, Peterlin B M. Structure, sequence, and position of the stem-loop in tar determine transcriptional elongation by Tat through the HIV-1 long terminal repeat. Genes Dev. 1989;3:547–558. doi: 10.1101/gad.3.4.547. [DOI] [PubMed] [Google Scholar]
- 51.Sheridan P L, Sheline C T, Cannon K, Voz M L, Pazin M J, Kadonaga J T, Jones K A. Activation of the HIV-1 enhancer by the LEF-1 HMG protein on nucleosome-assembled DNA in vitro. Genes Dev. 1996;9:2090–2104. doi: 10.1101/gad.9.17.2090. [DOI] [PubMed] [Google Scholar]
- 52.Shilatifard A, Conoway J W, Conaway R C. Mechanism and regulation of transcriptional elongation and termination by RNA polymerase II. Curr Opin Genet Dev. 1997;7:199–204. doi: 10.1016/s0959-437x(97)80129-3. [DOI] [PubMed] [Google Scholar]
- 53.Steger D J, Workman J L. Stable co-occupancy of transcription factors and histones at the HIV-1 enhancer. EMBO J. 1997;16:2463–2472. doi: 10.1093/emboj/16.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsukiyama T, Wu C. Chromatin remodeling and transcription. Curr Opin Genet Dev. 1997;7:182–191. doi: 10.1016/s0959-437x(97)80127-x. [DOI] [PubMed] [Google Scholar]
- 55.Turner B, O’Neill L. Histone acetylation in chromatin and chromosomes. Semin Cell Biol. 1995;6:229–236. doi: 10.1006/scel.1995.0031. [DOI] [PubMed] [Google Scholar]
- 56.VanLint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 57.Verdin E, Paras P, Jr, Van Lint C. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 1993;12:3249–3259. doi: 10.1002/j.1460-2075.1993.tb05994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson C J, Chao D M, Imbalzano A N, Schnitzler G R, Kingston R E, Young R A. RNA polymerase II holoenzyme contains SWI/SNF regulators involved in chromatin remodeling. Cell. 1996;84:235–244. doi: 10.1016/s0092-8674(00)80978-2. [DOI] [PubMed] [Google Scholar]
- 59.Workman J L, Taylor I C, Kingston R E. Activation domains of stably bound GAL4 derivatives alleviate repression of promoters by nucleosomes. Cell. 1991;64:533–544. doi: 10.1016/0092-8674(91)90237-s. [DOI] [PubMed] [Google Scholar]
- 60.Yang X, Herrmann C H, Rice A P. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J Virol. 1996;70:4576–4584. doi: 10.1128/jvi.70.7.4576-4584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshida M, Horinouchi S, Beppu T. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Q, Sharp P A. Tat-SF1: cofactor for stimulation of transcriptional elongation by HIV-1 Tat. Science. 1996;274:605–610. doi: 10.1126/science.274.5287.605. [DOI] [PubMed] [Google Scholar]