Highlights
-
•
Epidemiological and mechanistic studies have linked cardiac risk to epilepsy.
-
•
Arrhythmias, myocardial infarction, heart failure, and sudden death affect PWE.
-
•
Echocardiography is a tool to analyze cardiac structure and function.
-
•
The full potential of new echocardiographic technology has not been explored in PWE.
-
•
Risk stratification should evolve to better select individuals with epilepsy at risk.
Keywords: Epilepsy, Heart, Echocardiography, Myocardial strain, Atrial fibrillation
Abstract
Epilepsy is an increasing global neurological health issue. Recently, epidemiological and mechanistic studies have raised concern about cardiac involvement in individuals with epilepsy. This has resulted in the “epileptic heart” concept. Epidemiological data linking epilepsy to cardiovascular disease indicate an increased risk for ventricular and atrial arrhythmias, myocardial infarction, heart failure, and sudden death among individuals with epilepsy. Pathways of this interaction comprise increased prevalence of traditional cardiac risk factors, genetic abnormalities, altered brain circuitry with autonomic imbalance, and antiseizure medications with enzyme-inducing and ionic channel-blocking proprieties. Pathophysiological findings in the atria and ventricles of patients with epilepsy are discussed. Echocardiographic findings and future applications of this tool are reviewed. A risk stratification model and future studies on cardiac risk assessment in individuals with epilepsy are proposed.
Introduction
Epilepsy is an increasing global neurological health issue affecting 50 to 70 million people worldwide, mainly in low- and middle-income countries [1], [2]. In the last few years, epidemiological and mechanistic studies have raised concern about cardiac involvement in individuals with epilepsy. This has resulted in the “epileptic heart” concept proposed by Verrier and colleagues in 2020 [3]. In their original definition, the epileptic heart is defined as “a heart and coronary vasculature damaged by chronic epilepsy as a result of repeated surges in catecholamines and hypoxemia leading to electrical and mechanical dysfunction”, and this has also been supported by other authors [3], [4].
In this paper, we will review epidemiological data linking epilepsy to cardiovascular disease, and we will discuss pathways of this interaction and pathophysiological findings in the atria and ventricles of patients with epilepsy. Finally, we will propose risk stratification and provide directions for future studies on this topic.
Epidemiological evidence of epilepsy-heart interactions
Individuals with epilepsy are at increased risk of premature death. This occurs due to accidents, injuries, infections, suicide, and sudden death, among others. The standardized mortality ratio, which is defined as the ratio of the observed numbers of deaths in the study population (epilepsy) to the expected number of deaths estimated by standardization to the reference population (without epilepsy), in population-based studies has ranged between 1.6 and 3.0 in adults with epilepsy and 6.4 to 7.5 in children with epilepsy [5]. Among patients with uncontrolled seizures, the risk of premature death is even higher, reaching 9.3 to 13.4 times higher compared to seizure-free patients [5]. In a review published in 2021, Surges et al concluded that sudden cardiac death and acute myocardial infarction (MI) are responsible for nearly 15 % of deaths in individuals with epilepsy [6]. Additionally, these patients have a three- to sixfold higher risk of sudden cardiac death than the general population and are 8 years younger at the time of cardiovascular death [6], [7], [8].
In the Oregon Sudden Unexpected Death study, individuals with epilepsy had a 4.4 % rate of death per year, which is approximately 4.5-fold higher than sudden unexpected death in epilepsy (SUDEP). In two-thirds of these cases, typical seizure manifestations occurring just prior to the sudden death were not reported or observed [8].
Mounting evidence has indicated an increased risk of myocardial infarction, arrhythmias, or sudden death days to months following seizures or an epilepsy diagnosis. Rossi and colleagues studied 1,270,304 patients seeking medical attention for arrhythmias, including cardiac arrest. They found that previous hospitalization or emergency visits for seizure or status epilepticus were associated with increased risk for future arrhythmic outcomes as soon as the first day to 180 days after the seizure [9]. In another report, Cheng and colleagues found that hazard ratios for MI, arrhythmia, or sudden death among 5411 individuals with epilepsy were 1.71, 2.11, and 1.83, respectively, compared to controls, and these ratios were significant as early as 1 to 2 years after epilepsy diagnosis [10]. Recently, Wang and colleagues studied 2699 individuals with epilepsy in the UK Biobank from 2006 to 2021 and found that 11 % of these patients had cardiac arrhythmias, compared to 7.9 % in controls (11). They had a 36 % increased risk of any cardiac arrhythmia, 26 % of atrial fibrillation (AF), 87 % of bradyarrhythmias, and 80 % of ventricular arrhythmias. Interestingly, these findings were associated with carbamazepine and valproic acid prescriptions and were not altered by polygenic risk scores [11].
Cardiovascular disease (including coronary artery disease, myocardial infarction, and angina pectoris, among others) was reported in 21 % of individuals with epilepsy 18 years old or older, compared to 11.7 % of individuals without epilepsy. That represents a 75 % higher prevalence in that age group, and in individuals between 45 and 64 years, the increased prevalence was even higher, ranging from 122 % [12].
Possible pathways mediating cardiovascular risk in individuals with epilepsy
Cardiac comorbidities and risk factors, such as obesity, physical inactivity, smoking status, psychological distress, and socioeconomic factors, have a higher prevalence in individuals with epilepsy, being reported in 62 to 82 % of those individuals [13], [14], [15], [16].
In an age, sex, and body mass index-matched study, individuals with epilepsy without cardiovascular disease were subjected to a maximal treadmill test and compared to a control group. Individuals with epilepsy had greater chronotropic incompetence, less exercise duration, and less physical fitness [17]. They achieved 1.7 less metabolic equivalent of task (MET), which is associated with cardiovascular and all-cause death in population studies. For each reduction in 1 MET there is a 12 to 15 % increase in cardiovascular and all-cause death [18], [19].
Besides the classical risk factors, epilepsy per se and acute seizures also play a role in these heart-brain interactions, and chronic and acute autonomic nervous system involvement have been shown [17], [20], [6]. The increased sympathetic tone in chronic epilepsy, and especially during seizures, promotes catecholaminergic toxicity, myocardial ischemia, and inflammation with subtle myocardial injury and cardiac electrical disturbances, promoting acute or chronic heart conditions, such as arrhythmias, ischemia, or heart failure [3], [4], [21]. Cardiac fibrosis and an altered cardiac channel phenotype (an acquired form of cardiac channelopathies) following seizures have also been shown in animal models [22], [23].
Genetics in epilepsy has demonstrated some conditions of inherited “brain-cardiac” channelopathies. In these cases, both epilepsy and cardiac arrhythmias (such as the long QT syndrome) are possible manifestations, being one additional mode of epilepsy-heart interaction [24], [25].
Brain circuitry involvement in epilepsy could also be related to increased cardiovascular risk. Tawakol and colleagues reported that, in a cohort of 293 patients submitted to an 18F-fluorodexoyglucose PET/CT with a median follow-up of 3.7 years, amygdalar activity was related to bone marrow activity, arterial wall inflammation, and a hazard ratio of 1.59 for cardiovascular events. In that study, perceived stress was related to amygdalar activity [26]. The amygdalar function has also been associated with decreased left ventricle ejection fraction, cardiac perfusion abnormalities in women [27], and risk for takotsubo cardiomyopathy [28], [29]. The amygdala has pronounced projections to the cardiovascular and respiratory areas of the parabrachial pons and the periaqueductal gray matter, and involvement of the amygdala and other brain regions in epilepsy has been linked to altered heart rate variability (HRV) and SUDEP risk [30], [31], [32]. Melo and colleagues showed that HRV in patients with pharmacoresistant mesial temporal lobe epilepsy is independently associated with longer disease duration and changes in the glutamate subunits of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in the amygdala and anterior hippocampus. In these patients, the same sites of AMPA receptor subunits are highly sensitive to glucocorticoids [30], suggesting that in addition to the effects on the heart and the body, the stress-related hormones released during seizures may also have a feedback modulation of the amygdala and HRV [33].
One additional pathway of cardiac involvement in epilepsy is through its treatment. Anti-seizure medication (ASM) is the most important treatment option for epilepsy and has been associated with increased cardiovascular risk, arrhythmias, dyslipidemia, and death [7], [11], [34], [35], [36], [37], [38]. Enzyme-inducing ASM was associated with a 21 % increase in ischemic heart disease or stroke among more than 30 thousand patients with epilepsy with 25 years of follow-up. This relationship was even more significant depending on the duration of exposure and medication dose [37]. Enzyme-inducing ASMs are associated with increased levels of C-reactive protein, lipoprotein (a), homocysteine, and lipids [34], [35]. Additionally, the ionic channel blockage properties of some ASM have also been linked to increased cardiovascular risk [7], [11], [36]. Sodium channel blockade is associated with an increased risk of sudden cardiac death, total mortality, and non-fatal cardiac arrest, as shown in two landmark trials in individuals with coronary artery disease and heart failure, the CAST (Cardiac Arrhythmia Suppression) and CASH (Cardiac Arrest Study Hamburg) trials [39], [40].
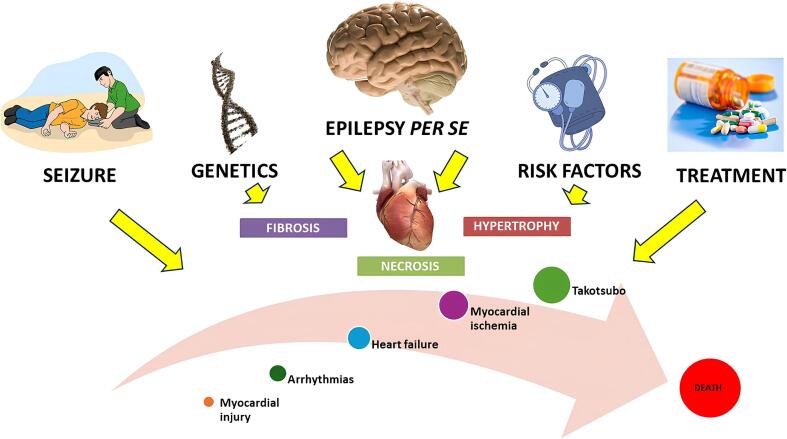
Summing up these different pathways, we conclude that epilepsy has a very peculiar influence on the heart (Fig. 1). It acts as a risk factor for cardiac disease (through its chronic influence in the heart, with autonomic imbalance, altered genetics and brain circuitry, and accumulation of subtle myocardial injuries) and acts as a trigger for cardiovascular events due to catecholaminergic toxicity, inflammation, and ischemia secondary to seizures [41].
Fig. 1.
Pathways of brain–heart interactions in individuals with epilepsy. Epilepsy acts as a risk factor for cardiac disease and a trigger for acute cardiac outcomes. Individuals with epilepsy have an increased prevalence of cardiac risk factors, autonomic imbalance, altered genetics, and brain circuitry, and are exposed to antiseizure medication. These factors, through catecholaminergic toxicity, inflammation, accelerated atherosclerosis and ischemia, can lead to an increased risk of arrhythmias, myocardial infarction and injury, heart failure, and sudden death.
Functional and structural findings in the heart of individuals with epilepsy – Echocardiography studies
Whereas multiple reports focused on electrocardiographic changes in individuals with chronic or uncontrolled seizures, we will focus on the echocardiogram. Echocardiography is an established tool in cardiovascular medicine and in general medical practice. It can, non-invasively, analyze the anatomical structure and function of the heart. Variables obtained with the echo analysis are important to classify the different phenotypes of heart failure regarding its ejection fraction (reduced, preserved, and mildly reduced ejection fraction), etiologies (hypertrophic, dilated, restrictive, non-compacted, and arrhythmogenic, for example), analyze valvular and segmental contractile function, investigate pericardial conditions, sudden cardiac risk, and cardiac damage secondary to extracardiac disease [42].
In a recently published systematic review and meta-analysis, Liu and colleagues selected 21 echocardiographic studies in individuals with epilepsy (8 in adults, 11 in children, and 2 in both populations) from more than 4,000 studies [43]. They performed a meta-analysis of 10 prospective case-control studies with 515 patients with epilepsy and 445 controls. They found that patients with epilepsy, compared to controls, had decreased left ventricle ejection fraction (MD: −1.80; 95 % CI: −3.56 to − 0.04; p = 0.045); greater mitral A-wave velocity, which is related to atrial pump and late ventricular diastolic filling phase (MD: 4.73; 95 % CI: 1.87–7.60; p = 0.001); prolonged isovolumetric relaxation time (IVRT), which is related to the first phase of diastole (MD: 10.18; 95 % CI: 2.05–18.32; p = 0.014) and increased E/e' ratio, which is related to left ventricle filling pressure (MD: 0.39; 95 % CI: 0.06–0.71; p = 0.019). They did not find differences in other echocardiographic variables such as fractional shortening, mitral E wave velocity, mitral E/A ratio, mitral E wave deceleration time, tissue Doppler e' wave velocity, left atrial diameter, or left ventricle mass, volumes, diameter, and thickness between people with epilepsy and healthy controls [43].
In a study with adult patients with convulsive status epilepticus, 56 % (18 out of 32) had a 20 % or more decrease in left ventricular ejection fraction in serial exams (0, 6, and 48 h after hospitalization) [44].
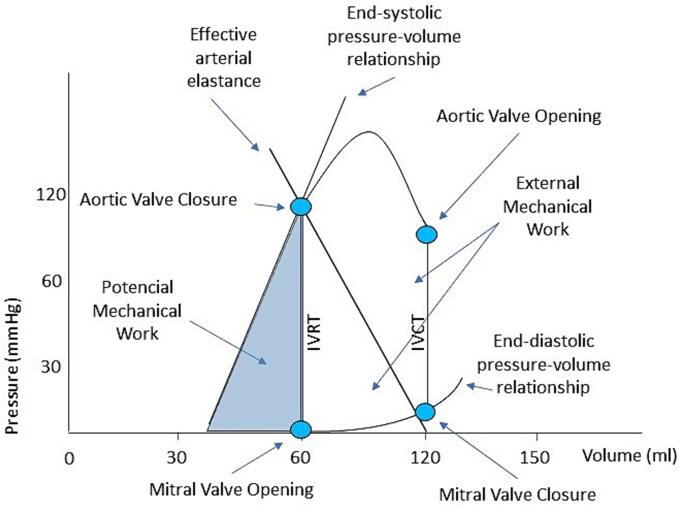
Further echocardiographic-derived data, however, is yet to be fully explored. Subtle alterations in cardiac physiology evaluated by pressure–volume curves can be non-invasively assessed by echocardiography (Fig. 2). The end-diastolic pressure–volume relationship (EDPVR) is related to myocardial stiffness (β), and the end-systolic pressure–volume relationship (ESPVR) represents a load-independent measure of ventricle contractility, which differs from ejection fraction, called end-systolic elastance (Ees) [45], [46]. The cardiac pressure–volume curves unmask preload, afterload, diastolic compliance, and contractility, and the area inside the cardiac pressure–volume curve is related to mechanical work [45]. A downward shift of the ESPVR or an upward shift of the EDPVR can lead to different heart failure phenotypes [45].
Fig. 2.
Cardiac pressure–volume curve. Left ventricle diastolic filling starts with mitral valve opening. After mitral valve closure, isovolumetric contraction (IVCT) begins. The aortic valve opening starts the ejection phase, which ends with the aortic valve closure. Isovolumetric relaxation (IVRT) lowers left ventricle pressure leading to mitral valve opening. The cardiac pressure–volume curves unmask preload, afterload, diastolic compliance, and contractility. The end-systolic and end-diastolic pressure–volume relationship can be assessed and explain the different heart failure phenotypes.
Our group has demonstrated increased left ventricle stiffness, left ventricle filling pressure, and left atrial volume in 30 patients with temporal lobe epilepsy (TLE) without cardiovascular diseases compared to controls with similar age, sex, body mass index, and cardiac risk factors. In multiple regressions, autonomic dysfunction explained 52 % of stiffness, and carbamazepine treatment and polytherapy with ASM additionally explained 6 % each [46]. In the Gutenberg Health Study, a population study with nearly 15,000 individuals, myocardial stiffness (β) was non-invasively assessed by echocardiography and showed an exponential association with all-cause mortality [47]. Increased myocardial stiffness is related to myocyte stiffness, extracellular matrix deposition, and fibrosis (49). These findings, together with inflammation, abnormalities in the sarcomeric filament, titin, and its phosphorylation, are related to heart failure with preserved ejection fraction (HFpEF). Intracellular calcium handling is also important in the pathophysiology of HFpEF and can, additionally, contribute to arrhythmias with delayed afterdepolarizations [48]. At the other end of the cardiac pressure–volume curve, end-systolic elastance, arterial-effective elastance, and ventricle-arterial coupling were similar in individuals with epilepsy and controls [45]. Increased awareness of cardiac conditions and heart failure risk in individuals with epilepsy should be applied [49], [50]. Dodge and colleagues have shown that, among 9,646 individuals with epilepsy followed up from 2005 to 2018, the hazard ratio for future heart failure was 1.56 compared to controls without epilepsy [50]. In that sense, we must acknowledge the initial signs of heart failure in individuals with epilepsy.
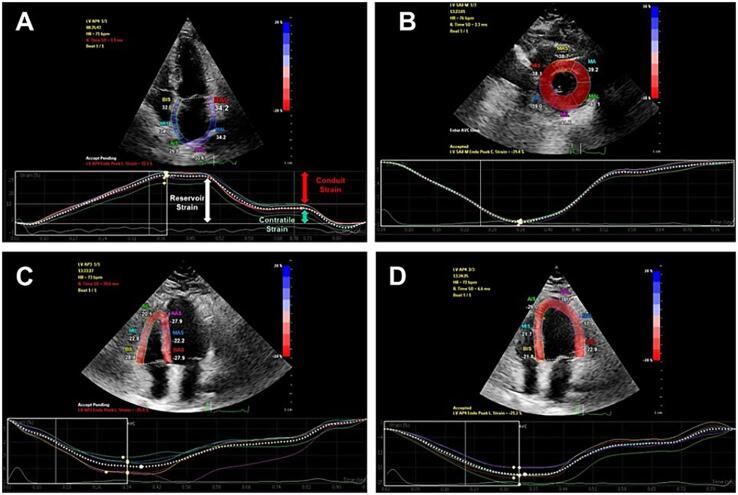
A few studies in epilepsy have uncovered myocardial strain in echocardiography. The word strain comes from physics and refers to the deformation produced by a force. Myocardial strain is the deformation (fiber shortening and elongation) of the heart myocytes during contraction and relaxation, respectively, which occurs in longitudinal, radial, and circumferential directions (Fig. 3) [51]. Myocardial strain has gained interest since it has additional prognostic value over left ventricle ejection fraction in many situations, such as heart failure with preserved or reduced ejection fraction, valvular heart disease, cardiotoxicity (as seen in oncologic patients), and even in asymptomatic individuals [51].
Fig. 3.
Applicability of myocardial strain. A – Left atrium longitudinal strain; B – Left ventricular circumferential strain; C – Right ventricular longitudinal strain; D – Left ventricle longitudinal strain.
Çelik and colleagues were the first to report global longitudinal strain in 60 children with epilepsy (without seizures in the preceding 6 months) compared to controls. Although left ventricle ejection fraction and chamber volumes were similar between the two groups, individuals with epilepsy had lower global longitudinal strain and different variables related to diastolic function, such as mitral A wave, E/A ratio, E/e' ratio, and IVRT [52]. Schreiber and colleagues, studying children with pharmacoresistant epilepsy, found a decreased longitudinal and circumferential left ventricle strain but a similar ejection fraction compared to controls [53]. In that study, however, they used only the apical four-chamber view to measure longitudinal strain and not the apical two-, three-, and four-chamber view, and regarding circumferential strain, they measured the left ventricle short axis at the level of papillary muscles, but not at the mitral and apical levels, as would be more appropriate. A third study in children found, not only a reduced left ventricle strain, but also a reduced right ventricle strain in children with epilepsy with refractory (n = 30) or well-controlled seizures (n = 30) compared to controls (n = 30) [54]. One small study in adult patients with epilepsy (mean age of 28 years), analyzing only 5 among 21 patients with epilepsy, found no differences in left ventricle longitudinal strain compared to controls [55].
Echocardiographic markers of increased risk of sudden cardiac death have been reported from the Atherosclerosis Risk in Communities (ARIC) study and the Cardiovascular Health Study (CHS), among 2383 and 5366 individuals, respectively [56]. Six echocardiographic markers were identified: reduced left ventricular ejection fraction (hazard ratio: 3.07 (2.29–4.11)); mitral annular calcification (hazard ratio: 1.85 (1.36–2.52)); mitral E/A > 1.5 (versus mitral E/A 0.7–1.5 − hazard ratio: 1.64 (1.07–2.51)); mitral E/A < 0.7 (versus mitral E/A 0.7–1.5 − hazard ratio: 1.52 (1.14–2.02)); left ventricular mass (hazard ratio: 1.30 (1.15–1.48) for each 1 standard deviation increase); and left atrial diameter (hazard ratio: 1.15 (1.02–1.30) for each 1 standard deviation increase) [56]. A small study with 30 individuals with temporal lobe epilepsy and 30 controls found that seventeen (56.6 %) individuals with epilepsy had a total of 22 of these risk markers versus 11 (36.7 %) controls with 12 markers with a p-value of 0.12 and 0.07 for the number of individuals with any echo risk marker and the total number of altered risk markers among groups, respectively [57].
Gaps in knowledge and future directions
At this point, we have more questions than answers regarding heart-brain interactions in epilepsy. It seems reasonable to use what we learn from cardiology as a starting point from which we can select those individuals with epilepsy with increased cardiovascular risk for further evaluation.
Echocardiography has many more tools to offer in this challenging situation. We are now starting to have a closer look at the other cardiac chambers, such as the right ventricle and the atria (Fig. 4). Atrial pathologies in individuals with epilepsy deserve special attention. Desai and colleagues showed that 23.9 % of 1.4 million hospitalized patients with epilepsy had some form of cardiac arrhythmia, and, in that cohort, atrial fibrillation (AF) was present in 9.7 %, making it the most common arrhythmia in patients with epilepsy. Ventricular tachycardia and sudden cardiac arrest were present in 1 % and 1.4 %, respectively [58]. A previous report on P-wave heterogeneity (PWH) measured in electrocardiography, a marker of atrial electrical dispersion, found that individuals with epilepsy have PWH levels similar to a more than 20 years older population with AF. AF is the most prevalent sustained arrhythmia in adults, and it is commonly associated with structural heart disease, including heart failure [59]. Early signs of impaired atrial mechanics and altered anatomy evaluated by echocardiography can predict heart failure and future AF risk [60]. Simple measures such as atrial volume and atrial strain are not yet properly and routinely assessed in individuals with epilepsy.
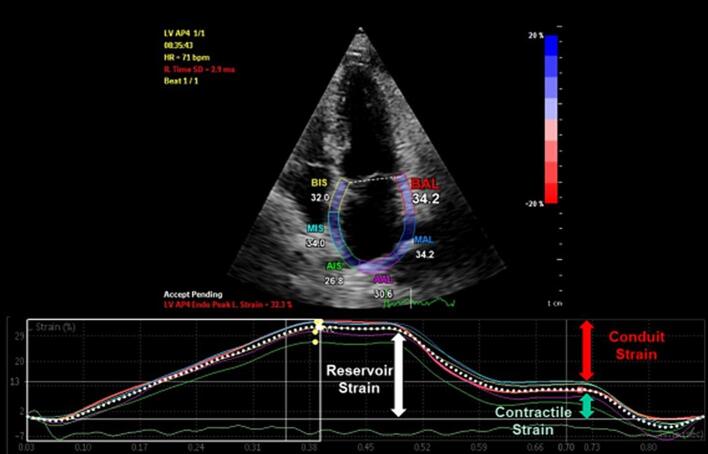
Fig. 4.
Left atrium strain. Left atrium strain has been increasingly recognized as an important measure for the assessment of heart failure with preserved ejection fraction (HFpEF) and arrhythmia risk. Atrium strain is evaluated in its three components: reservoir strain, related to atrial filling and stretching before mitral valve opening, conduit strain, related to passive atrial emptying, and contractile strain, related to atrial systole.
Other echocardiographic tools yet to be explored in individuals with epilepsy are 3-d echo, with its increased precision in chamber volumes and function quantification, stress echo, used to uncover initial left ventricle filling abnormalities as seen in early heart failure with preserved ejection fraction (HFpEF), or to assess ischemic cardiomyopathy, and myocardial work, which analyzes the strain-pressure curves and has proven to be a less load-dependent variable to assess myocardial contractility [61]. Cardiac mechanical dispersion and electromechanical coupling in individuals with epilepsy is another promising approach. A small study with 19 individuals with epilepsy and 21 controls showed that electrocardiographic markers of diastolic electrical dispersion, such as the QT interval and the T-peak-to-T-end (TpTe) interval, were related to left ventricular mass, left atrial volume, and the mitral E/A ratio, all echocardiographic markers associated with diastolic dysfunction [62].
There are, however, additional points that need further clarification. Which patients with epilepsy would benefit the most from having an electrocardiogram, echo, or a cardiology consult? Will these methods change the clinical management of patients? Possibly, we should try to clinically identify those patients who are at increased cardiac risk and those who need closer follow-up and/or aggressive cardiac risk factor treatment. For example, patients with uncontrolled seizures, cardiac complaints, or an altered risk factor profile should be submitted for a detailed clinical, electrocardiographic, and echocardiographic evaluation. In that sense, it is important to highlight that Verrier and colleagues took the first step and proposed a clinical syndromic approach to account for the “epileptic heart“ risk and diagnosis [63]. They proposed that individuals with chronic epilepsy with or without drug resistance should be evaluated for clinical symptoms such as effort intolerance, chest pain, irregular pulse, and palpitations, and for electrical and echocardiographic markers of autonomic, functional, or anatomical cardiac compromise [63].
Repurposing of drugs well known for their safety and protective profile in cardiology, such as beta-blockers or SLGT2 inhibitors, may prove to be a future strategy for lowering the cardiovascular risk in individuals with epilepsy and increased cardiovascular risk. To accomplish this, we need longitudinal studies to understand disease progression, prove the prognostic significance of electrocardiographic and echocardiographic changes in epilepsy, and confirm if these changes are modifiable or not with medical intervention [43].
Conclusion
In this article, we reviewed the epidemiological data linking epilepsy and cardiovascular disease. We discussed its possible pathways, from increased prevalence of traditional cardiac risk factors, chronic and acute autonomic changes with catecholaminergic toxicity and ischemia, altered brain circuitry, genetics, and use of drugs that may harm the heart and vessels in that population. We summarized data from echocardiographic studies in individuals with epilepsy, recognizing that much is yet to be done, such as looking at the structure and function of other heart chambers and using state-of-the-art measures to look further and deeper at the epileptic heart. Finally, some immediate questions must be addressed to optimize resources and gain knowledge on how to improve outcomes in individuals with epilepsy.
Declaration of generative AI and AI-assisted technologies in the writing process
We declare that we did not use any kind of AI technology to accomplish this manuscript.
CRediT authorship contribution statement
Guilherme Loureiro Fialho: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ramsés Miotto: Writing – review & editing, Visualization. Márcia Tatsch Cavagnollo: Writing – review & editing, Visualization. Hiago Murilo Melo: Writing – review & editing, Visualization. Peter Wolf: Writing – review & editing, Validation. Roger Walz: Writing – review & editing, Visualization. Katia Lin: Writing – review & editing, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Guilherme Loureiro Fialho, Email: fialhog@gmail.com.
Katia Lin, Email: linkatia@uol.com.br.
References
- 1.Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54(2):185–191. doi: 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- 2.Fisher R.S., Cross H., D’Souza C., French J.A., Haut S., Higurashi N., et al. 2017 International League Against Epilepsy classifications of seizures and epilepsy are steps in the right direction. Epilepsia. 2019;60:1040–1044. doi: 10.1111/epi.15052. [DOI] [PubMed] [Google Scholar]
- 3.Verrier R.L., Pang T.D., Nearing B.D., Schachter S.C. The epileptic heart: concept and clinical evidence. Epilepsy Behav. 2020;105 doi: 10.1016/j.yebeh.2020.106946. [DOI] [PubMed] [Google Scholar]
- 4.Fialho G.L., Wolf P., Walz R., Lin K. Epilepsy and ultra-structural heart changes: The role of catecholaminergic toxicity and myocardial fibrosis. What can we learn from cardiology? Seizure. 2019 Oct;71:105–109. doi: 10.1016/j.seizure.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Thurman D.J., Logroscino G., Beghi E., Hauser W.A., Hesdorffer D.C., Newton C.R., et al. Epidemiology Commission of the International League Against Epilepsy. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017 Jan;58(1):17–26. doi: 10.1111/epi.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surges R., Shmuely S., Dietze C., Ryvlin P., Thijs R.D. Identifying patients with epilepsy at high risk of cardiac death: signs, risk factors and initial management of high risk of cardiac death. Epileptic Disord. 2021;23(1):17–39. doi: 10.1684/epd.2021.1254. [DOI] [PubMed] [Google Scholar]
- 7.Bardai A., Blom M.T., van Noord C., Verhamme K.M., Sturkenboom M.C.J.M., Tan H.L. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart. 2015;101:17–22. doi: 10.1136/heartjnl-2014-305664. [DOI] [PubMed] [Google Scholar]
- 8.Stecker E.C., Reinier K., Uy-Evanado A., Teodorescu C., Chugh H., Gunson K., et al. Relationship between seizure episode and sudden cardiac arrest in patients with epilepsy. Circ Arrhythm Electrophysiol. 2013;6(5):912–916. doi: 10.1161/CIRCEP.113.000544. [DOI] [PubMed] [Google Scholar]
- 9.Rossi K.C., Gursky J.M., Pang T.D., Dhamoon M.S. Seizures and status epilepticus may be risk factor for cardiac arrhythmia or cardiac arrest across multiple time frames. Epilepsy Behav. 2021 Jul;120 doi: 10.1016/j.yebeh.2021.107998. [DOI] [PubMed] [Google Scholar]
- 10.Cheng C.Y., Hsu C.Y., Wang T.C., Liu C.Y., Yang Y.H., Yang W.H. Risk of cardiac morbidities and sudden death in patients with epilepsy and no history of cardiac disease: a population-based nationwide study. Mayo Clin Proc. 2021;96(4):964–974. doi: 10.1016/j.mayocp.2020.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Huang P., Yu Q., Lu J., Liu P., Yang Y., et al. Epilepsy and long-term risk of arrhythmias. Eur Heart J. 2023 Sep 14;44(35):3374–3382. doi: 10.1093/eurheartj/ehad523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zack M., Luncheon C. Adults with an epilepsy history, notably those 45–64 years old or at the lowest income levels, more often report heart disease than adults without an epilepsy history. Epilepsy Behav. 2018;86:208–210. doi: 10.1016/j.yebeh.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selassie A.W., Wilson D.A., Martz G.U., Smith G.G., Wagner J.L., Wannamaker B.B. Epilepsy beyond seizure: a population-based study of comorbidities. Epilepsy Res. 2014 Feb;108(2):305–315. doi: 10.1016/j.eplepsyres.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Zack M.M., Luncheon C. Adults with an epilepsy history, especially those 45years or older, those with lower family incomes, and those with a history of hypertension, report a history of stroke five times as often as adults without such a history-2010, 2013, and 2015 U.S. National Health Interview Survey. Epilepsy Behav. 2018 Jun;83:236–238. doi: 10.1016/j.yebeh.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Kobau R., Zahran H., Thurman D.J., Zack M.M., Henry T.R., Schachter S.C., et al. Centers for Disease Control and Prevention (CDC). Epilepsy surveillance among adults–19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008 Aug 8;57(6):1–20. [PubMed] [Google Scholar]
- 16.Strine T.W., Kobau R., Chapman D.P., Thurman D.J., Price P., Balluz L.S. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005 Jul;46(7):1133–1139. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- 17.Fialho G.L., Pagani A.G., Walz R., Wolf P., Lin K. Maximal/exhaustive treadmill test features in patients with temporal lobe epilepsy: Search for sudden unexpected death biomarkers. Epilepsy Res. 2017 Jul;133:83–88. doi: 10.1016/j.eplepsyres.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Myers J., Prakash M., Froelicher V., Do D., Partington S., Atwood J.E. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002 Mar 14;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 19.Kodama S., Saito K., Tanaka S., Maki M., Yachi Y., Asumi M., et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009 May 20;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 20.Lotufo P.A., Valiengo L., Benseñor I.M., Brunoni A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012 Feb;53(2):272–282. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 21.Pang T.D., Nearing B.D., Verrier R.L., Schachter S.C. T-wave heterogeneity crescendo in the surface EKG is superior to heart rate acceleration for seizure prediction. Epilepsy Behav. 2022 May;130 doi: 10.1016/j.yebeh.2022.108670. [DOI] [PubMed] [Google Scholar]
- 22.Powell K.L., Liu Z., Curl C.L., Raaijmakers A.J.A., Sharma P., Braine E.L., et al. Altered cardiac structure and function is related to seizure frequency in a rat model of chronic acquired temporal lobe epilepsy. Neurobiol Dis. 2021 Nov;159 doi: 10.1016/j.nbd.2021.105505. [DOI] [PubMed] [Google Scholar]
- 23.Li M.C.H., O’Brien T.J., Todaro M., Powell K.L. Acquired cardiac channelopathies in epilepsy: evidence, mechanisms, and clinical significance. Epilepsia. 2019;60:1753–1767. doi: 10.1111/epi.16301. [DOI] [PubMed] [Google Scholar]
- 24.Chahal C.A.A., Salloum M.N., Alahdab F., Gottwald J.A., Tester D.J., Anwer L.A., et al. Systematic review of the genetics of sudden unexpected death in epilepsy: potential overlap with sudden cardiac death and arrhythmia-related genes. J Am Heart Assoc. 2020 Jan 7;9(1):e012264. doi: 10.1161/JAHA.119.012264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagnall R.D., Crompton D.E., Petrovski S., Lam L., Cutmore C., Garry S.I., et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016 Apr;79(4):522–534. doi: 10.1002/ana.24596. [DOI] [PubMed] [Google Scholar]
- 26.Tawakol A., Ishai A., Takx R.A., Figueroa A.L., Ali A., Kaiser Y., et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017 Feb 25;389(10071):834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiechter M., Roggo A., Burger I.A., Bengs S., Treyer V., Becker A., et al. Association between resting amygdalar activity and abnormal cardiac function in women and men: a retrospective cohort study. Eur Heart J Cardiovasc Imaging. 2019 Jun 1;20(6):625–632. doi: 10.1093/ehjci/jez047. [DOI] [PubMed] [Google Scholar]
- 28.Templin C., Hänggi J., Klein C., Topka M.S., Hiestand T., Levinson R.A., et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J. 2019 Apr 14;40(15):1183–1187. doi: 10.1093/eurheartj/ehz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radfar A., Abohashem S., Osborne M.T., Wang Y., Dar T., Hassan M.Z.O., et al. Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome. Eur Heart J. 2021 May 14;42(19):1898–1908. doi: 10.1093/eurheartj/ehab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melo H.M., de Carvalho C.R., Hoeller A.A., Marques J.L.B., Linhares M.N., Lopes M.W., et al. AMPAr GluA1 Phosphorylation at Serine 845 in Limbic System Is Associated with Cardiac Autonomic Tone. Mol Neurobiol. 2021 Apr;58(4):1859–1870. doi: 10.1007/s12035-020-02272-y. [DOI] [PubMed] [Google Scholar]
- 31.Legouhy A., Allen L.A., Vos S.B., Oliveira J.F.A., Kassinopoulos M., Winston G.P., et al. Volumetric and microstructural abnormalities of the amygdala in focal epilepsy with varied levels of SUDEP risk. Epilepsy Res. 2023 May;192 doi: 10.1016/j.eplepsyres.2023.107139. [DOI] [PubMed] [Google Scholar]
- 32.Allen L.A., Vos S.B., Kumar R., Ogren J.A., Harper R.K., Winston G.P., et al. Cerebellar, limbic, and midbrain volume alterations in sudden unexpected death in epilepsy. Epilepsia. 2019 Apr;60(4):718–729. doi: 10.1111/epi.14689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopes M.W., Leal R.B., Guarnieri R., Schwarzbold M.L., Hoeller A., Diaz A.P., et al. A single high dose of dexamethasone affects the phosphorylation state of glutamate AMPA receptors in the human limbic system. Transl Psychiatry. 2016 Dec 13;6(12):e986. doi: 10.1038/tp.2016.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mintzer S., Yi M., Hegarty S., Maio V., Keith S. Hyperlipidemia in patients newly treated with anticonvulsants: A population study. Epilepsia. 2020 Feb;61(2):259–266. doi: 10.1111/epi.16420. [DOI] [PubMed] [Google Scholar]
- 35.Mintzer S., Trinka E., Kraemer G., Chervoneva I., Werhahn K.J. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid-lowering agents in the elderly. Epilepsia. 2018 Oct;59(10):1899–1907. doi: 10.1111/epi.14554. [DOI] [PubMed] [Google Scholar]
- 36.Zaccara G., Lattanzi S., Brigo F. Cardiac adverse effects of antiseizure medications. Expert Opin Drug Saf. 2022 May;21(5):641–652. doi: 10.1080/14740338.2022.2023128. [DOI] [PubMed] [Google Scholar]
- 37.Josephson C.B., Wiebe S., Delgado-Garcia G., Gonzalez-Izquierdo A., Denaxas S., Sajobi T.T., et al. Association of Enzyme-Inducing Antiseizure Drug Use With Long-term Cardiovascular Disease. JAMA Neurol. 2021 Nov 1;78(11):1367–1374. doi: 10.1001/jamaneurol.2021.3424. [DOI] [PubMed] [Google Scholar]
- 38.Lee-Lane E., Torabi F., Lacey A., Fonferko-Shadrach B., Harris D., Akbari A., et al. Epilepsy, antiepileptic drugs, and the risk of major cardiovascular events. Epilepsia. 2021 Jul;62(7):1604–1616. doi: 10.1111/epi.16930. [DOI] [PubMed] [Google Scholar]
- 39.Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989 Aug 10;321(6):406-12. https://doi.org/10.1056/NEJM198908103210629. [DOI] [PubMed]
- 40.Siebels J., Cappato R., Rüppel R., Schneider M.A., Kuck K.H. Preliminary results of the Cardiac Arrest Study Hamburg (CASH) CASH Investigators Am J Cardiol. 1993 Nov 26;72(16):109F–F113. doi: 10.1016/0002-9149(93)90973-g. [DOI] [PubMed] [Google Scholar]
- 41.Fialho G.L., Wolf P., Walz R., Lin K. Trigger, risk factor, and self-organizing criticality - One more piece of the puzzle to explain increased mortality in epilepsy? Epilepsy Behav. 2021 Sep;122 doi: 10.1016/j.yebeh.2021.108123. [DOI] [PubMed] [Google Scholar]
- 42.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015 Jan;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z., Thergarajan P., Antonic-Baker A., Chen Z., Sparks P.B., Lannin N.A., et al. Cardiac structural and functional abnormalities in epilepsy: A systematic review and meta-analysis. Epilepsia Open. 2023 Mar;8(1):46–59. doi: 10.1002/epi4.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belcour D., Jabot J., Grard B., Roussiaux A., Ferdynus C., Vandroux D., et al. Prevalence and Risk Factors of Stress Cardiomyopathy After Convulsive Status Epilepticus in ICU Patients. Crit Care Med. 2015 Oct;43(10):2164–2170. doi: 10.1097/CCM.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 45.Fialho G.L., Wolf P., Walz R., Lin K. Left ventricle end-systolic elastance, arterial-effective elastance, and ventricle-arterial coupling in Epilepsy. Acta Neurol Scand. 2021 Jan;143(1):34–38. doi: 10.1111/ane.13334. [DOI] [PubMed] [Google Scholar]
- 46.Fialho G.L., Wolf P., Walz R., Lin K. Increased cardiac stiffness is associated with autonomic dysfunction in patients with temporal lobe epilepsy. Epilepsia. 2018 Jun;59(6):e85–e90. doi: 10.1111/epi.14084. [DOI] [PubMed] [Google Scholar]
- 47.Schwarzl M., Ojeda F., Zeller T., Seiffert M., Becher P.M., Munzel T., et al. Risk factors for heart failure are associated with alterations of the LV end-diastolic pressure-volume relationship in non-heart failure individuals: data from a large-scale, population-based cohort. Eur Heart J. 2016 Jun 14;37(23):1807–1814. doi: 10.1093/eurheartj/ehw120. [DOI] [PubMed] [Google Scholar]
- 48.Nagueh S.F. Heart failure with preserved ejection fraction: insights into diagnosis and pathophysiology. Cardiovasc Res. 2021 Mar 21;117(4):999–1014. doi: 10.1093/cvr/cvaa228. [DOI] [PubMed] [Google Scholar]
- 49.Terman S.W., Aubert C.E., Hill C.E., Skvarce J., Burke J.F., Mintzer S. Cardiovascular disease risk, awareness, and treatment in people with epilepsy. Epilepsy Behav. 2021 Apr;117 doi: 10.1016/j.yebeh.2021.107878. [DOI] [PubMed] [Google Scholar]
- 50.Doege C., Luedde M., Kostev K. Epilepsy is associated with an increased incidence of heart failure diagnoses. Epilepsy Behav. 2021 Dec;125 doi: 10.1016/j.yebeh.2021.108393. [DOI] [PubMed] [Google Scholar]
- 51.Amzulescu M.S., De Craene M., Langet H., Pasquet A., Vancraeynest D., Pouleur A.C., et al. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20(6):605–619. doi: 10.1093/ehjci/jez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Çelik S.F., Baratalı E., Güven A.S., Torun Y.A. Left ventricular myocardial deformation abnormalities in seizure-free children with epilepsy. Seizure. 2018 Oct;61:153–157. doi: 10.1016/j.seizure.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Schreiber J.M., Frank L.H., Kroner B.L., Bumbut A., Ismail M.O., Gaillard W.D. Children with refractory epilepsy demonstrate alterations in myocardial strain. Epilepsia. 2020 Oct;61(10):2234–2243. doi: 10.1111/epi.16652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaki E.R., Mohamed L.A., Agiba N.A., Mohammed S.A.A., Mansour M.I.S., Habib S.A., et al. Relation between Seizure Control and Ventricular Functions in Epileptic Children. NeuroQuantology. 2023 April;20(4):1098–1101. doi: 10.48047/NQ.2022.20.4.NQ22334. [DOI] [Google Scholar]
- 55.González A., Haugaa K.H., Brekke P.H., Hopp E., Ørn S., Alvestad S., et al. Cardiac Structure and Function in Epilepsy Patients with Drug-Resistant Convulsive Seizures. Case Rep Neurol. 2022 Mar 10;14(1):88–97. doi: 10.1159/000522237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konety S.H., Koene R.J., Norby F.L., Wilsdon T., Alonso A., Siscovick D., et al. Echocardiographic predictors of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. Circ Cardiovasc Imaging. 2016 Aug;9(8) doi: 10.1161/CIRCIMAGING.115.004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fialho G.L., Pagani A.G., Wolf P., Walz R., Lin K. Echocardiographic risk markers of sudden death in patients with temporal lobe epilepsy. Epilepsy Res. 2018 Feb;140:192–197. doi: 10.1016/j.eplepsyres.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 58.Desai R., Rupareliya C., Patel U., Naqvi S., Patel S., Lunagariya A., et al. Burden of arrhythmias in epilepsy patients: a nationwide inpatient analysis of 1.4 million hospitalizations in the United States. Cureus. 2017;9(8):e1550. doi: 10.7759/cureus.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fialho G.L., Pang T.D., Kong W.Y., Tran A.P., Yu C.G., Rodriguez I.D., et al. Individuals with chronic epilepsy have elevated P-wave heterogeneity comparable to patients with atrial fibrillation. Epilepsia. 2023 Sep;64(9):2361–2372. doi: 10.1111/epi.17686. [DOI] [PubMed] [Google Scholar]
- 60.Bandera F., Mollo A., Frigelli M., Guglielmi G., Ventrella N., Pastore M.C., et al. Cardiac imaging for the assessment of left atrial mechanics across heart failure stages. Front Cardiovasc Med. 2022 Jan;13(8) doi: 10.3389/fcvm.2021.750139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roemer S., Jaglan A., Santos D., Umland M., Jain R., Tajik A.J., et al. The utility of myocardial work in clinical practice. J Am Soc Echocardiogr. 2021 Aug;34(8):807–818. doi: 10.1016/j.echo.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Fialho G.L., Verrier R.L., D'Avila A., Melo H.M., Wolf P., Walz R., et al. Dual assessment of abnormal cardiac electrical dispersion and diastolic dysfunction for early detection of the epileptic heart condition. J Electrocardiol. 2023 May–Jun;78:69–75. doi: 10.1016/j.jelectrocard.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Verrier R.L., Pang T.D., Nearing B.D., Schachter S.C. The Epileptic Heart: A clinical syndromic approach. Epilepsia. 2021 Aug;62(8):1780–1789. doi: 10.1111/epi.16966. [DOI] [PubMed] [Google Scholar]