Abstract
Background
Elderly patients undergoing surgery are prone to cognitive decline known as perioperative neurocognitive disorders (PND). Several studies have shown that the microglial activation and the decrease of short-chain fatty acids (SCFAs) in gut induced by surgery may be related to the pathogenesis of PND. The purpose of this study was to determine whether microglia and short-chain fatty acids were involved in cognitive dysfunction in aged rats.
Methods
Male wild-type Wistar rats aged 11–12 months were randomly divided into control group (Ctrl: Veh group), propionic acid group (Ctrl: PA group), exploratory laparotomy group (LP: Veh group) and propionic acid + exploratory laparotomy group (LP: PA group) according to whether exploratory laparotomy (LP) or PA pretreatment for 21 days was performed. The motor ability of the rats was evaluated by open field test on postoperative day 3 (POD3), and then the cognitive function was evaluated by Y-maze test and fear conditioning test. The expression of IL-1β, IL-6, RORγt and IL-17A mRNA in hippocampus was detected by RT-qPCR, the expression of IL-17A and IL-17RA in hippocampus was detected by Western blot, and the activation of microglia was detected by immunofluorescence.
Results
The PND rat model was successfully established by laparotomy. Compared with Ctrl: Veh group, the body weight of LP: Veh group decreased, the percentage of spontaneous alternations in Y maze decreased (P < 0.001), and the percentage of freezing time in contextual fear test decreased (P < 0.001). Surgery triggers neuroinflammation, manifested as the elevated levels of the inflammatory cytokines IL-1β (P < 0.001) and IL-6 (P < 0.001), the increased expression of the transcription factor RORγt (P = 0.0181, POD1; P = 0.0073, POD5)and major inflammatory cytokines IL-17A (P = 0.0215, POD1; P = 0.0071, POD5), and the increased average fluorescence intensity of Iba1 (P < 0.001, POD1; P < 0.001, POD5). After PA preconditioning, the recovery of rats in LP: PA group was faster than that in LP: Veh group as the body weight lost on POD1 (P = 0.0148) was close to the baseline level on POD5 (P = 0.1846), and they performed better in behavioral tests. The levels of IL-1β (P < 0.001) and IL-6 (P = 0.0035) inflammatory factors in hippocampus decreased on POD1 and the average fluorescence intensity of Iba1 decreased (P = 0.0024, POD1; P < 0.001, POD5), representing the neuroinflammation was significantly improved. Besides, the levels of RORγt mRNA (P = 0.0231, POD1; P = 0.0251, POD5) and IL-17A mRNA (P = 0.0208, POD1; P = 0.0071, POD5) in hippocampus as well as the expression of IL-17A (P = 0.0057, POD1; P < 0.001, POD5) and IL-17RA (P = 0.0388) decreased.
Conclusion
PA pretreatment results in reduced postoperative neuroinflammation and improved cognitive function, potentially attributed to the regulatory effects of PA on Th17-mediated immune responses.
Keywords: Perioperative neurocognitive disorder, Propionic acid, Th17 cells, IL-17A, Neuroinflammation, Microglia
1. Introduction
Perioperative neurocognitive disorders (PND) commonly observed in elderly patients undergoing general anesthesia [1]. The incidence of PND ranged from 41 % to 75 % on POD7 and from 18 % to 45 % on the 3rd month after operation. PND is associated with suboptimal functional recovery and increased mortality following major surgery [2,3]. Surgical interventions can trigger acute systemic inflammation, subsequently leading to neuroinflammation and synaptic dysfunction, thereby contributing to hippocampal-dependent cognitive impairment [[4], [5], [6]]. Despite numerous studies highlighting pathological alterations linked to PND [7,8], effective clinical strategies for prevention or treatment remain elusive.
T helper 17 cells (Th17 cells) are a distinct subset of CD4+ T cells, independent of Th1 and Th2 cells. They predominantly reside in the lamina propria of intestinal mucosa and mesenteric lymph nodes, playing a crucial role in acquired immune response, autoimmune diseases, and transplant immune rejection. The transcription factor retinoic acid-related orphan receptor γt (RORγt) and signal transducer and activator of transcription 3 (STAT3) are specifically expressed by Th17 cells [9], regulating the differentiation of intestinal T cells into Th17 cells to significantly alleviate the neuroinflammation caused by their infiltration in multiple sclerosis and experimental encephalomyelitis models [10,11]. IL-17 is primarily produced by Th17 cells, with six isoforms known as IL-17A∼IL-17F [12]. Among them, IL-17A is considered as one of the pro-inflammatory factors contributing to the pathogenesis of central nervous system (CNS) inflammatory diseases [13,14]. The IL-17 receptor family consists of five members from IL-17RA to IL-17RE, of which IL-17RA serves as a common receptor subunit for all subtypes [15]. The signal transduction of IL-17A occurs through the heterodimer formed by IL-17RA and IL-17RC receptor subunits [16]. Although there is ongoing debate regarding neuronal expression of the IL-17A receptor within CNS [[17], [18], [19]], microglia clearly express IL-17RA [20,21]. Given its pivotal role in neuroinflammation and neurodegeneration, we hypothesize that through interaction with microglia, IL-17A may contribute to the development and progression of PND.
Short chain fatty acids (SCFAs) are saturated fatty acids consisting of 1–6 carbon atoms in their chain length. They are the primary byproducts of dietary fiber fermentation by Escherichia coli. Depending on different fiber content, approximately 500–600 mmol SCFAs are produced daily in the intestine [22], with acetic acid, propionic acid (PA) and butyric acid being the most abundant among them [23]. Large amounts of studies have demonstrated that SCFAs can upregulate tight junction protein expression and increase the transmembrane electrical resistance (TEER) to enhance intestinal barrier function, regulate Th17/Treg balance in the colon, and maintain immune homeostasis [[24], [25], [26], [27]]. The interaction between SCFAs and gut-brain pathways can directly or indirectly modulate physiological processes associated with neural function, learning, memory, and emotion [28]. Although PA is less abundant than acetic acid in the intestine, some experiments have shown its effective inhibition of abnormal immune activation in both the intestine and CNS. In Th17 cells specifically, PA inhibits IL-17A expression thereby ameliorating autoimmune inflammation within CNS. These findings provide valuable insights into how PA regulates tolerance versus adverse immune reactions.
Our study aimed to investigate the potential of PA in regulating Th17 pathogenicity, reducing IL-17A secretion, and improving neuroinflammation in PND model, with the goal of providing novel insights for PND prevention and treatment.
2. Materials and methods
2.1. Animals
Male Wistar rat about 12 months old were provided by the Laboratory Animal Center of Nanjing Medical University. Laboratory conditions were maintained at 22 °C ± 2 °C with a relative humidity of 60 % ± 5 % and on a 12-h light/dark cycle. Food and water are plentiful for these animals.
2.2. Drugs
Rats were pretreated for 21 days with sodium propionate (150 mM) in the drinking water or pH and sodium-matched water, and both were changed every three days [29].
2.3. Exploratory laparotomy
The rat was placed in a transparent semi-closed respiratory loop box, which was prefilled with 7 % sevoflurane +30 % oxygen. When the righting reflex disappeared, the rat was placed in the right lateral position and the eyes were smeared with terramycin eye ointment. The anesthetic concentration was adjusted to 3 % sevoflurane +30 % oxygen to maintain anesthesia. Apply lidocaine gel to the wound for analgesia. After abdominal disinfection, a 5 cm long incision was made along the midline of the abdomen to explore the abdominal organs in a clockwise direction slowly for 10 min, and to ensure that the direction of the intestines did not change to prevent intestinal torsion. During the operation, part of the intestinal tract about 10 cm was carefully pulled out of the abdominal cavity, gently squeezed with wet cotton swabs or gloves for 30–60 s. After the exploration, the muscle and skin were sutured with 5-0 and 4-0 sterile silk threads, respectively. Finally, the rat was placed back in the home cage for recovering. A heating plate was used to maintain the body temperature during operation and recovery period.
2.4. Behavioral tests
Open field test (OFT) was used to detect the motor ability of mice. The animals were placed in the center of the bottom of the box (100 cm × 100 cm × 40 cm). The rats were allowed to explore freely for 10 min. The movement track was observed, and the moving distance was recorded. The equipment of Y-maze test consisted of three equally long arms (50 cm × 10 cm × 8 cm) labeled A, B, and C. The sequence of the animals entering each arm within 8 min was recorded. Alternation is defined as consecutive entry into three arms, such as (ABC, BCA, or CAB). Then calculate the percentage = alternation/(sum of arm passes - 2) × 100 %. The fear conditioning (FC) test included training and test periods. Rats were placed into the Skinner box for 180 s and then received sound stimulation (80 dB, 3000 Hz) for 30 s. Foot shock (0.8 mA) was performed during the last 2 s of sound stimulation. Then the rats were returned to their home cage. Contextual fear test was performed 24 h after training. The rats were placed into the Skinner box without any stimulus for 5 min. After 6 h, cued fear test was performed. The rats were placed into the Skinner box with deferent decoration and presented with the same sound used in the training period for 3 min. Calculate the percentage of freezing time (the state without any other behavior except respiratory movement) to observation time in each period.
2.5. Western blot
After extraction of hippocampal protein, loading 20 μg of protein into the 12.5 % SDS-PAGE gel for electrophoresis. Then the isolated protein was transferred to the PVDF membrane. After blocking the membrane with 5 % bovine serum albumin for 2 h at room temperature (RT), the target protein was incubated with primary mouse anti-GAPDH antibody (1:8000, Proteintech), rabbit anti-IL-17A antibody (1:1000, Bioss), rabbit anti-IL-17RA antibody (1:1000, Abcam) overnight at 4 °C. The next day, the membranes were washed with TBST and incubated with secondary antibodies for 2 h at RT. Protein bands were then detected by chemiluminescence, and the intensities were measured on ImageJ software (National Institutes of Health, USA).
2.6. Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was hippocampus using Trizol reagent, according to the manufacturer's instructions (Invitrogen, USA). Reverse transcription to cDNA was performed using HiScript III RT SuperMix for qPCR reagents (Vazyme, China). All gene transcripts were analyzed using the SYBR qPCR Master Mix (Vazyme industry, China) and detected using an ABI 7500 fast instrument (Applied Biosystems, CA). The relative mRNA expression in each sample was displayed as 2−ΔΔCT values and was representative of at least three independent replicates. The following primer sequences were used for the real-time PCR analysis:
RORγt Forward: TGCAAGACTCATCGACAAGG.
Reverse: TCAGAGGGCTGAAGGAAGTAGA.
IL-17A Forward: ATTCCATCCATGTGCCTGAT.
Reverse: GAGTCCAGGGTGAAGTGGAA.
IL-1β Forward: GCAATGGTCGGGACATAGTT.
Reverse: GACTTGGCAGAGGACAAAGG.
IL-6 Forward: ACCACCCACAACAGACCAGT.
Reverse: AACGGAACTCCAGAAGACCA.
GAPDH Forward: TGTGAACGGATTTGGCCGTA.
Reverse: GATGGTGATGGGTTTCCCGT.
2.7. Immunofluorescence
After deep anesthesia with sevoflurane, the rats were perfused with precooled PBS and 4 % PFA. Brain tissue was immediately collected, then fixed with 4 % PFA overnight and dehydrated with 30 % sucrose overnight at 4 °C. The collected specimens were cut into 30 μm thick coronal sections. After rinsing with PBS, the brain slices were infiltrated with PBS containing 0.1 % Triton X-100 (PBT) for another 30 min, and then blocked with 10 % fetal bovine serum for 2 h. Next, these brain slices were incubated with primary antibodies (rabbit anti-Iba1, 1:500, Wako) at 4 °C. The next day, the secondary antibodies were incubated up for 2 h at RT. Nuclei were stained with DAPI (Sigma). Finally, brain sections were photographed under Olympus laser confocal microscope and analyzed on ImageJ.
2.8. Statistics
GraphPad Prism 9.3 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analyses. Multiple-group comparisons were performed using two-way analysis of variance (ANOVA) tests followed by Tukey's test. Unpaired two-tailed Student's t tests were used for comparisons of two conditions. Pearson simple correlation and linear correlation were used to estimate the correlation between the two variables. Data are expressed as mean ± SEM. P < 0.05 were considered significant.
3. Results
3.1. Operation leads to cognitive impairment related to hippocampus in rats
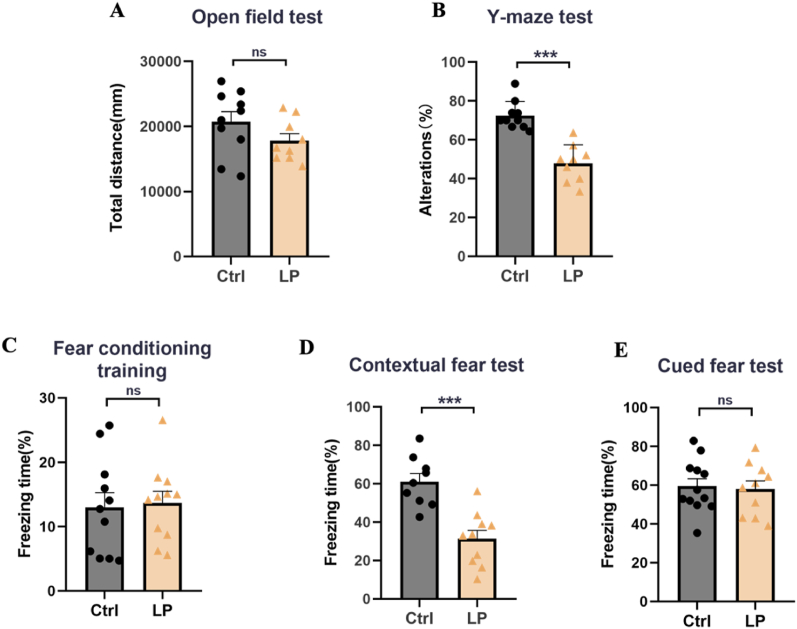
We first performed exploratory laparotomy on rats in the LP: Veh group and evaluated whether their motor ability was affected by OFT on POD3. The results showed that there was no significant difference in the total movement distance between Ctrl: Veh group and LP: Veh group (Fig. 1A). Next, we evaluated the cognitive function of rats. The results of the Y-maze test showed that the working memory ability of the rats in the LP: Veh group decreased as a percentage of the number of spontaneous alternations in the total number of arm entry (Fig. 1B). FC test was conducted on POD4. There was no significant difference between the two groups at the baseline level during the training phase (Fig. 1C). In contextual fear teat, the percentage of freezing time in the LP: Veh group was significantly lower than that in the Ctrl: Veh group (Fig. 1D), while there was no difference between the two groups in the cued fear test (Fig. 1E), indicating that the operation damaged the hippocampus related cognitive function.
Fig. 1.
Operation-induced cognitive impairment related to hippocampus. A. Total distance of OFT. B. The percentage of spontaneous alternation in Y-maze test. C-E. Percentage of freezing time in FC training, contextual fear test and cued fear test. n = 9∼12. Data are presented as the mean ± SEM. ns: P > 0.05, ***: P < 0.001.
3.2. PA preconditioning accelerates postoperative rehabilitation of PND rats and improves hippocampal cognitive dysfunction
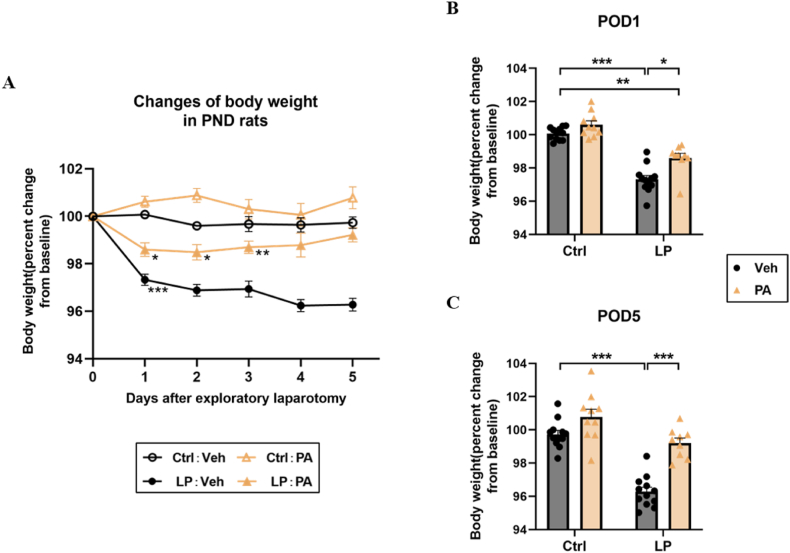
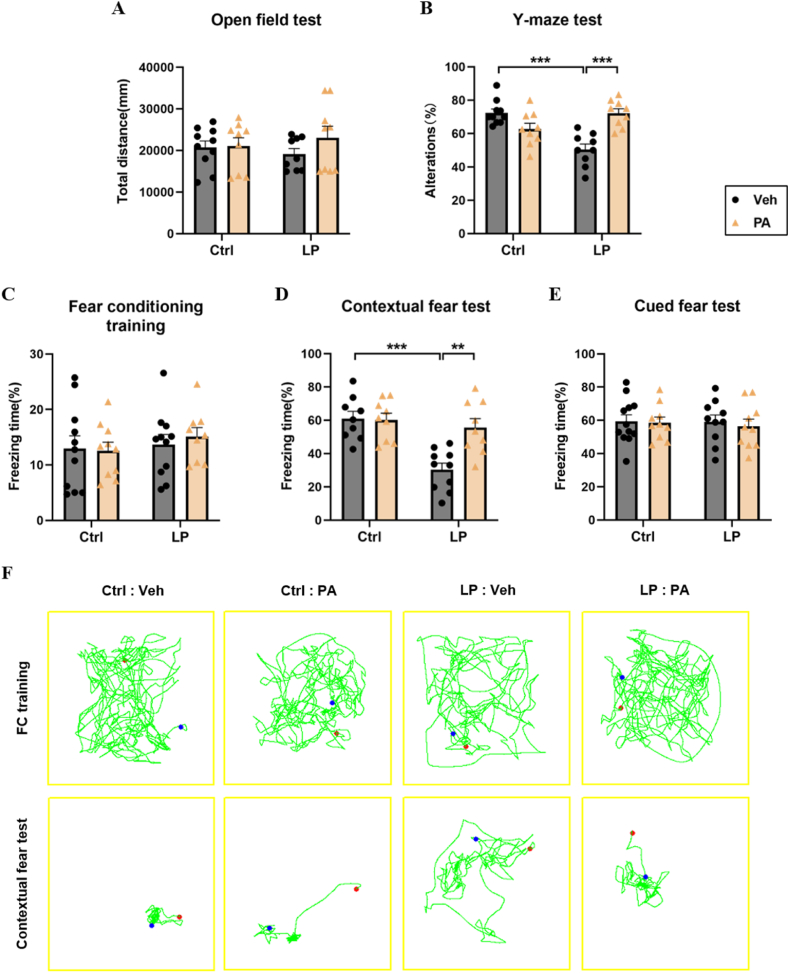
To explore the therapeutic effect, we conducted PA pretreatment for 21 days in rats before operation. By monitoring the weight of rats, we found that on the first day after operation, the weight of rats in the LP: Veh group and the LP: PA group decreased significantly compared with the baseline before operation. On POD5, the weight of rats in the LP: PA group returned to the baseline level, while the weight of rats in the LP: Veh group always showed a downward trend (Fig. 2A–C), indicating that PA, as an effective factor of intestinal flora, may promote the recovery of intestinal function after operation. The results of OFT showed that the motor ability of the four groups of rats was similar (Fig. 3A). On this basis, the cognitive function was further evaluated. The percentage of spontaneous alternation in the Y-maze test of rats in LP: Veh group was significantly lower than that in Ctrl: Veh group and Ctrl: PA group (Fig. 3B). After fear conditioning training, the percentage of freezing time in the situational fear test of LP: Veh group was also significantly lower than that in Ctrl: Veh group and Ctrl: PA group; The percentage of spontaneous alternation and the percentage of freezing time of rats in the LP: PA group were significantly higher than those in the Ctrl: Veh group and the Ctrl: PA group (Fig. 3C–F). Above results show that PA pretreatment can prevent cognitive dysfunction caused by surgery.
Fig. 2.
Effect of PA pretreatment on postoperative weight change of rats. A. Line chart of body weight change during 1–5 days after operation (taking preoperative body weight as the baseline). B. Relative changes of body weight of rats on POD1; C. Relative changes of body weight on POD5. n = 9∼12. Data are presented as the mean ± SEM. *: P < 0.05, **: P < 0.01, ***: P < 0.001.
Fig. 3.
PA preconditioning improves the impairment of hippocampus related memory function in rats after operation. A. Total distance of OFT. B. The percentage of spontaneous alternation in Y-maze test. C-E. Percentage of freeing time in FC training, contextual fear test, and cued fear test; F. Representative trajectories of FC training and contextual fear test. N = 9–12. Data are presented as the mean ± SEM. **: P < 0.01, ***: P < 0.001.
3.3. PA preconditioning attenuates neuroinflammatory response in PND rats
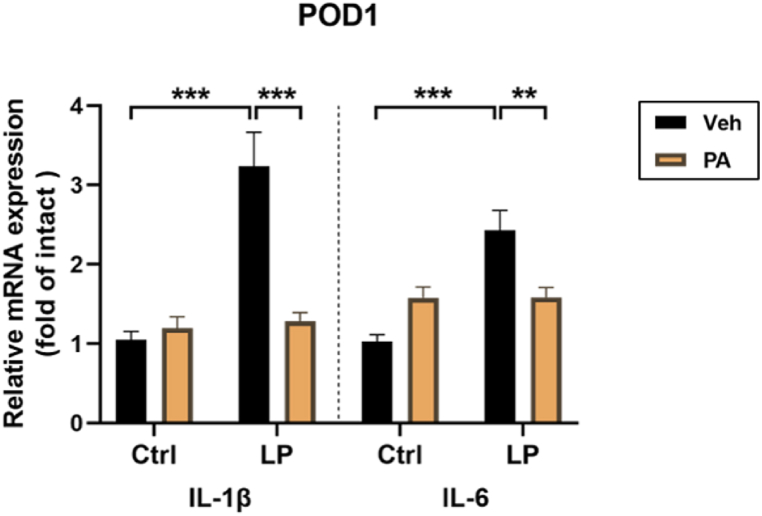
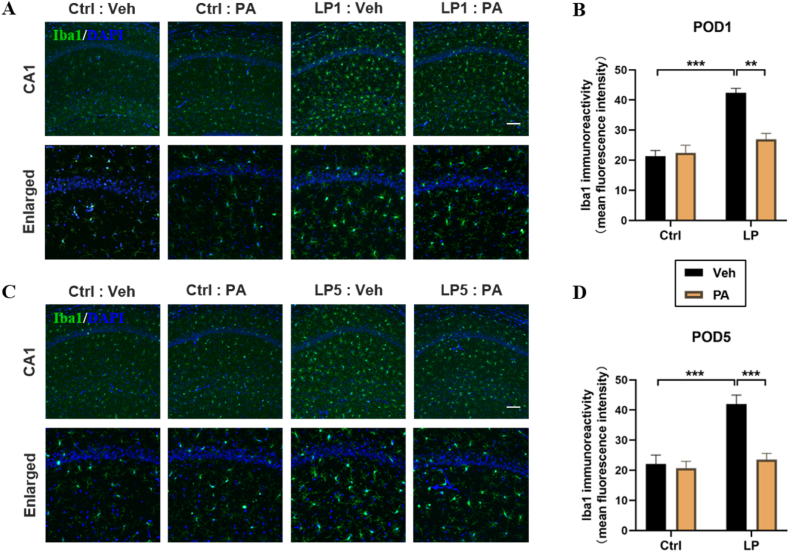
Neuroinflammatory response is considered as one of the important mechanisms leading to PND. The results of RT-qPCR showed that the levels of IL-1β and IL-6 in hippocampus were up-regulated on POD1, while PA preconditioning significantly decreased the expression of inflammatory factors (Fig. 4). The activation of microglia is one of the important signs of neuroinflammatory response. After immunofluorescence staining of frozen sections of rat brain tissue, it was found that the quantity of Iba1 positive staining cells in hippocampal CA1 region of LP: Veh group was significantly higher than that of Ctrl: Veh group and Ctrl: PA group on POD1(Fig. 5A–B) and POD5(Fig. 5C–D), accompanied by cell body thickening, which was partially reversed by PA pretreatment. These results suggest that PA preconditioning can improve the cognitive impairment of PND rats by reducing postoperative neuroinflammatory response.
Fig. 4.
Effect of PA pretreatment on the level of inflammatory factors in hippocampus. n = 6, **: P < 0.01, ***: P < 0.001.
Fig. 5.
Effect of PA preconditioning on the activation of hippocampal microglia. A&C. Representative images of Iba1 staining in hippocampal CA1 region on POD1 and POD5. B&D. Statistical map of mean fluorescence intensity of Iba1 in hippocampal CA1 region on POD1 and POD5. n = 6. Data are presented as the mean ± SEM. **: P < 0.01, ***: P < 0.001. The scale is 100 μm.
3.4. PA pretreatment reduced the infiltration of peripheral Th17 cells in the central nervous system and the level of IL-17A in the hippocampus
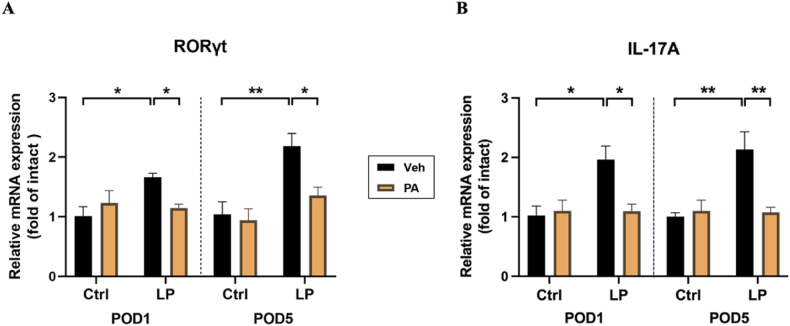
To further explore the possible mechanism of PA pretreatment in reducing neuroinflammation, we first detected the expression of Th17 cell-specific transcription factors and characteristic cytokines in the hippocampus at the transcriptional level. The results of RT-qPCR showed that compared with Ctrl: Veh group and Ctrl: PA group, the expression of RORγt mRNA and IL-17 mRNA in hippocampus of LP: Veh group increased, while PA preconditioning decreased the expression of both on POD1(Fig. 6A) and POD5 (Fig. 6B).
Fig. 6.
Effect of PA preconditioning on the number and function of Th17 cells in hippocampus. A. the expression of ROR γt mRNA in hippocampus. B. the expression of IL-17A mRNA in hippocampus. n = 6. Data are presented as the mean ± SEM. *: P < 0.05, **: P < 0.01.
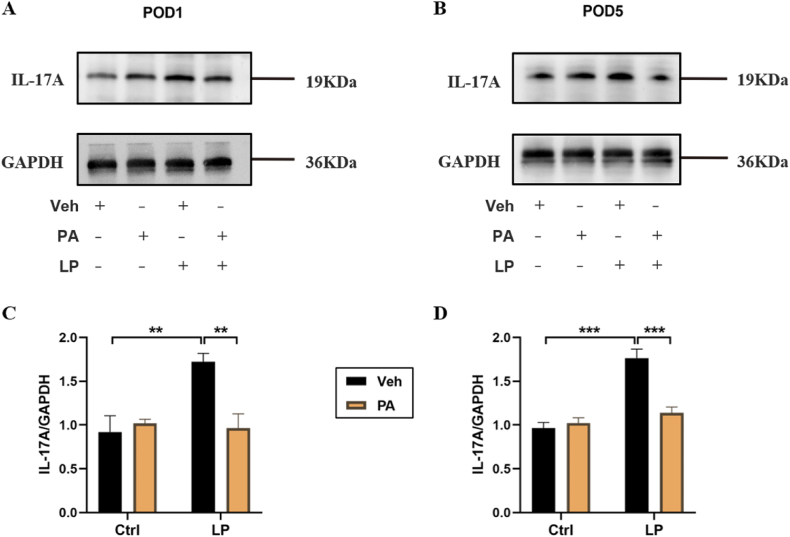
Next, we detected the expression of major cytokines IL-17A in Th17 cells at the protein level. WB results showed that the hippocampal IL-17A level in LP: Veh group was significantly higher than that in Ctrl: Veh group and Ctrl: PA group, while the hippocampal IL-17A level in LP: PA group was significantly lower than that in LP: Veh group on both POD1(Fig. 7A–C) and POD5 (Fig. 7B–D).
Fig. 7.
Effect of PA preconditioning on the level of IL-17A protein in hippocampus. A. hippocampal IL-17A representative protein band on POD1. B. hippocampal IL-17A representative protein band on POD5. C. hippocampal IL-17A protein expression statistical chart on POD1. D. IL-17A protein expression statistical chart on POD5. n = 6. Data are presented as the mean ± SEM. **: P < 0.01, ***: P < 0.001.
3.5. PA preconditioning down-regulates the expression of IL-17RA in hippocampus
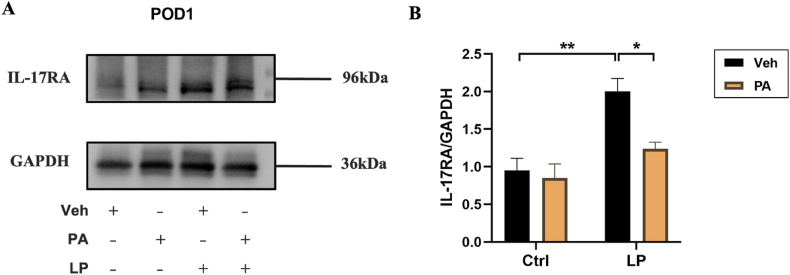
Finally, we detected the level of IL-17RA in hippocampus. The results showed that the expression of IL-17RA in LP: Veh group was significantly higher than that in Ctrl: Veh group and Ctrl: PA group, while that in LP: PA group was decreased (Fig. 8A–B). We speculated that after Th17 cells infiltrated into the central nervous system, they would overexpress IL-17A, activate microglia through IL-17A/IL-17RA signals, and promote the occurrence and development of neuroinflammation, which could be reversed by PA pretreatment.
Fig. 8.
Exepression of IL-17RA protein after PA preconditioning. A. hippocampal IL-17RA representative protein band on POD1. B. hippocampal IL-17RA protein expression statistical chart on POD1. n = 6. Data are presented as the mean ± SEM. *: P < 0.05, **: P < 0.01.
4. Discussion
In this study, we utilized a PND rat model to explore the correlation between PA pretreatment and postoperative cognitive impairment. We found that preoperative supplementation of PA promoted the recovery of intestinal function of rats after surgery. More importantly, it alleviated neuroinflammation and hippocampus-dependent memory damage caused by surgical trauma, which may be related to the regulatory effects of PA on Th17 cell-mediated function. By reducing the infiltration of Th17 cells in CNS as well as lowering the expression of IL-17A and the activation of microglia mediated by IL-17A/IL-17RA, neurocognitive function could be improved.
The induction of PND by exploratory laparotomy is a widely used research method [[30], [31], [32]] and highly reconstructs clinical scenarios. Therefore, we adopted this method to establish an animal model of PND. Our Y-maze and FC tests showed that exploratory laparotomy resulted in significant cognitive impairment. The hippocampus dependent memory in rats is particularly sensitive to surgical shocks [[33], [34], [35]], therefore, we chose the hippocampus as the target for molecular biology experimental analysis. In contextual fear test, we observed that the percentage of freezing time in the LP: Veh group was significantly lower than that in the Ctrl: Veh group, while the results in cued fear test were opposite. This may be due to cued fear is exclusively associated with the amygdala [36], while contextual fear is strongly related to hippocampus and basolateral amygdala complex [37]. Hippocampus is also crucial to the generation of spontaneous alternation behavior. Research shows that hippocampal injury can reduce spontaneous alternation behavior [38], but the enhancement of neuroplasticity or signal in the hippocampus can increase spontaneous alternation behavior [39]. Our Y-maze test results indicated that PA pretreatment can improve the reduction of spontaneous alternation behavior caused by surgery.
Microglia are innate immune cells in the CNS, accounting for 10–15 % of glia [40]. They are involved in the development of CNS and help maintain dynamic equilibrium of tissues by supporting neuron survival, cell death and synapse formation [41]. Surgery may activate the originally silent microglia and promote the release of inflammatory factors into CNS, thus triggering neuroinflammation. It is reported that inhibiting microglia can reduce the level of IL-1β and IL-6, thereby inhibiting neuroinflammation and enhancing neurocognitive function [42]. IL-1β plays an important role in PND, and the innate immune response induced by peripheral surgery triggers IL-1β in the hippocampus mediated inflammatory processes, which is one of the foundations of cognitive impairment [43,44]. Our results showed that PA pretreatment inhibited the activation of microglia and the expression of these inflammatory factors, thereby improving the cognitive function damage caused by neuroinflammation.
Among the six isoforms of IL-17, no other subtypes have been found to be expressed or related signaling pathways in CNS except IL-17A [45]. In vivo and in vitro experiments show that Th17 cells can infiltrate into the brain [46,47], while IL-17A can destroy the blood–brain barrier (BBB) [48]. Intrathecal injection of Aβ-42 peptides was used to establish an AD rat model, and it was found that after Th17 cells entered the central nervous system, the permeability of BBB increased, and the levels of IL-17A and RORγt in the hippocampus, cerebrospinal fluid, and serum increased [49]. With the destruction of BBB integrity, more Th17 cells migrate to brain parenchyma, producing more IL-17A and leading to serious neuronal dysfunction [50,51]. The presence of Th17 cells in CNS promotes the activation of astrocyte and microglia and amplifies neuroinflammation by targeting resident glia cells [52,53]. Neutralizing IL-17A can slow down the progress of neuroinflammation by reducing the production of pathogenic cytokines. Studies have shown that the addition of IL-17A to the co-culture of microglia and neurons can cause the activation of microglia and the death of neurons. Interestingly, only when microglia exist, IL-17A can aggravate the loss of neurons, while inhibiting the IL-17A receptor on microglia weakens these effects [54]. There may be some communication network between glia and Th17 cells, and in-depth understanding of this interaction may provide a new therapeutic approach for neuroinflammation.
Microbial host communication is currently receiving increasing attention, particularly the bidirectional communication between the gut microbiota and CNS, known as the gut-brain axis [55,56]. The communication between gut and brain can be established through the modulation of metabolites on BBB and the systemic immune effect mediated by the intestinal endocrine factors and microbial metabolites (such as SCFAs, tryptophan metabolites, phytoestrogen and bile acid metabolites) [57]. SCFAs are a diverse class of metabolites generated through the fermentation of dietary fiber by the gut microbiota. Acetic acid, propionic acid (PA), and butyric acid represent the most abundant SCFAs, with molar ratios of 60:20:20 [58]. Numerous in vitro [[59], [60], [61]], in vivo [62,63] and clinical [[64], [65], [66]] studies have semonstrated the regulatory role of propionic acid on T cells. Exogenous supplementation of PA can increase the activity and quantity of Treg cells while reducing the quantity of Th17 cells and inhibiting their pathogenicity. Additionally, it slightly suppresses Th1 cells by inhibiting histone deacetylase [67]. A consistent finding among studies investigating the microbiota composition in MS patients is a decrease in the populations of bacteria that produce SCFAs [[68], [69], [70]], suggesting potential physiological implications. Further clinical studies have revealed lower levels of PA in both feces and plasma of MS patients compared to healthy controls [[71], [72], [73], [74]]; however the correlation between acetic acid and butyric acid is weak. Similarly, reduced SCFAs levels were observed in the serum of PD patients compared with healthy controls; notably, only PA level exhibited correlations with the Unified Parkinson's Disease Rating Scale (UPDRs) part III score, Mini-mental State Examination (MMSE) score, and Hamilton Depression Scale (HAMD) score [75]. Surgical interventions significantly decreased gut microbiota production of SCFAs in mice according to animal experiments [76]. Furthermore, a separate animal study has confirmed that PA exerted neuroprotective effects on streptozocin (STZ)-induced type 1 diabetes mellitus (T1DM) mice through modulation of phosphoinositide 3-kinase (PI3K)/serine-threonine protein kinase (Akt)/endothelial nitrogen monoxide synthase (eNOS) signaling pathway [77]. However, the impact of PA on PND remains ambiguous, and preliminary exploration efforts have been conducted, constituting the novelty of this study.
This study has several limitations: Firstly, the concentration of PA in CNS was not detected. Secondly, an experimental group receiving intrathecal injection of IL-17A neutralizing antibody as a parallel control for assessing the therapeutic effect of PA was not included. In addition, further investigation using Th17 cells-knockout mice is necessary to elucidate the role of Th17 cells in the occurrence and development of PND. In the future, we will continue to explore the preventive and therapeutic effects and mechanisms underlying PA in PND with the aim of translating relevant research findings into clinical practice.
5. Conclusion
In summary, our study suggests a significant decrease in the intestinal content of PA in PND rats. Preconditioning with PA alleviated postoperative neuroinflammation and enhanced cognitive function in rats. This mechanism may be attributed to the regulatory effects of PA on Th17 cell-mediated immune function. Our findings present a novel therapeutic strategy for treating neuroinflammation-induced cognitive impairment. However, further investigations employing advanced tools such as chemical genetics or optogenetic manipulations are warranted to validate our findings.
Ethics statement
This experimental protocol was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (No. IACUC-2102026). All animal procedures were performed in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) and the National Research Council's Guide for the Care and Use of Laboratory Animals.
Funding
This work was supported by the Provincial Key R&D Program (Social Development) of Science and Technology Department of Jiangsu Province, China (No. SBE2021741263); the 789 Talents Training Program of the Second Affiliated Hospital of Nanjing Medical University (No. 789ZYRC080236).
Data available statement
The authors confirm that the relevant data supporting the findings of this study are available within the article.
CRediT authorship contribution statement
Hong-yu Dai: Writing – original draft, Investigation, Methodology, Validation, Formal analysis, Data curation. Ze-xin Zhang: Writing – original draft, Investigation, Visualization. Cheng Tan: Methodology, Data curation. Xian Xian: Methodology, Data curation. Dong Ji: Formal analysis. Jing Yang: Formal analysis. Jie Sun: Writing – review & editing, Conceptualization, Supervision. Hao Yao: Writing – review & editing, Conceptualization, Funding acquisition, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28817.
Contributor Information
Jie Sun, Email: dgsunjie@hotmail.com.
Hao Yao, Email: yaohao@njmu.edu.cn.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Evered L., Silbert B., Knopman D.S., et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129(5):872–879. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 2.Deo H., West G., Butcher C., Lewis P. The prevalence of cognitive dysfunction after conventional and computer-assisted total knee replacement. Knee. 2011;18(2):117–120. doi: 10.1016/j.knee.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Skvarc D.R., Berk M., Byrne L.K., et al. Post-Operative Cognitive Dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev. 2018;84:116–133. doi: 10.1016/j.neubiorev.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Terrando N., Monaco C., Ma D., et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. U. S. A. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan H., Cao J., Zhang J., Zuo Z. Critical role of inflammatory cytokines in impairing biochemical processes for learning and memory after surgery in rats. J. Neuroinflammation. 2014;11:93. doi: 10.1186/1742-2094-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vizcaychipi M.P., Watts H.R., O'Dea K.P., et al. The therapeutic potential of atorvastatin in a mouse model of postoperative cognitive decline. Ann. Surg. 2014;259(6):1235–1244. doi: 10.1097/SLA.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Wang Y., Wu H., et al. Postoperative cognitive dysfunction: current developments in mechanism and prevention. Med Sci Monit. 2014;20:1908–1912. doi: 10.12659/MSM.892485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrando N., Eriksson L.I., Ryu J.K., et al. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011;70(6):986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annunziato F., Cosmi L., Liotta F., et al. Defining the human T helper 17 cell phenotype. Trends Immunol. 2012;33(10):505–512. doi: 10.1016/j.it.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Reinhold D., Bank U., Täger M., et al. DP IV/CD26, APN/CD13 and related enzymes as regulators of T cell immunity: implications for experimental encephalomyelitis and multiple sclerosis. Front. Biosci. 2008;13:2356–2363. doi: 10.2741/2849. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Tao Y., Troiani L., Markovic-Plese S. Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+ T cells derived from patients with multiple sclerosis. J. Immunol. 2011;187(6):3431–3437. doi: 10.4049/jimmunol.1100580. [DOI] [PubMed] [Google Scholar]
- 12.McGeachy M.J., Cua D.J., Gaffen S.L. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becher B., Segal B.M. T(H)17 cytokines in autoimmune neuro-inflammation. Curr. Opin. Immunol. 2011;23(6):707–712. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waisman A., Hauptmann J., Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129(5):625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 15.Nesmond S., Muller C., Le Naour R., et al. Characteristic pattern of IL-17RA, IL-17RB, and IL-17RC in Monocytes/Macrophages and Mast cells from patients with bullous pemphigoid. Front. Immunol. 2019;10:2107. doi: 10.3389/fimmu.2019.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Bechara R., Zhao J., et al. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019;20(12):1594–1602. doi: 10.1038/s41590-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segond von Banchet G., Boettger M.K., König C., et al. Neuronal IL-17 receptor upregulates TRPV4 but not TRPV1 receptors in DRG neurons and mediates mechanical but not thermal hyperalgesia. Mol. Cell. Neurosci. 2013;52:152–160. doi: 10.1016/j.mcn.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Li K., Zhu L., et al. Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol. 2013;14:20. doi: 10.1186/1471-2172-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawanokuchi J., Shimizu K., Nitta A., et al. Production and functions of IL-17 in microglia. J. Neuroimmunol. 2008;194(1–2):54–61. doi: 10.1016/j.jneuroim.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Das Sarma J., Ciric B., Marek R., et al. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J. Neuroinflammation. 2009;6:14. doi: 10.1186/1742-2094-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kebir H., Kreymborg K., Ifergan I., et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergman E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990;70(2):567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 23.Macfarlane S., Macfarlane G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003;62(1):67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 24.Arpaia N., Campbell C., Fan X., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furusawa Y., Obata Y., Fukuda S., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 26.Kelly C.J., Zheng L., Campbell E.L., et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal Epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith P.M., Howitt M.R., Panikov N., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 29.Smith P.M., Howitt M.R., Panikov N., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu L.L., Ji M.H., Zhang H., et al. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav. Immun. 2016;51:109–118. doi: 10.1016/j.bbi.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Pan K., Chen L., et al. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J. Neuroinflammation. 2016;13(1):268. doi: 10.1186/s12974-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawano T., Eguchi S., Iwata H., Tamura T., Kumagai N., Yokoyama M. Impact of preoperative environmental enrichment on prevention of development of cognitive impairment following abdominal surgery in a rat model. Anesthesiology. 2015;123(1):160–170. doi: 10.1097/ALN.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 33.Hovens I.B., Schoemaker R.G., van der Zee E.A., Absalom A.R., Heineman E., van Leeuwen B.L. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav. Immun. 2014;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Le V., Kurnutala L., SchianodiCola J., et al. Premedication with intravenous ibuprofen improves recovery characteristics and stress response in adults undergoing laparoscopic cholecystectomy: a randomized controlled trial. Pain Med. 2016;17(6):1163–1173. doi: 10.1093/pm/pnv113. [DOI] [PubMed] [Google Scholar]
- 35.Katsumi Y., Racine A.M., Torrado-Carvajal A., et al. The Role of Inflammation after Surgery for Elders (RISE) study: Examination of [11C]PBR28 binding and exploration of its link to post-operative delirium. Neuroimage Clin. 2020;27 doi: 10.1016/j.nicl.2020.102346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dityatev A.E., Bolshakov V.Y. Amygdala, long-term potentiation, and fear conditioning. Neuroscientist. 2005;11(1):75–88. doi: 10.1177/1073858404270857. [DOI] [PubMed] [Google Scholar]
- 37.Chaaya N., Battle A.R., Johnson L.R. An update on contextual fear memory mechanisms: transition between Amygdala and Hippocampus. Neurosci. Biobehav. Rev. 2018;92:43–54. doi: 10.1016/j.neubiorev.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Stoneham E.T., McHail D.G., Boggs K.N., et al. Functional perturbation of forebrain principal neurons reveals differential effects in novel and well-learned tasks. Brain Res. 2017;1671:1–13. doi: 10.1016/j.brainres.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Z.P., Dai D., Wei P.J., et al. Effects of cordycepin on spontaneous alternation behavior and adenosine receptors expression in hippocampus. Physiol. Behav. 2018;184:135–142. doi: 10.1016/j.physbeh.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Nayak D., Roth T.L., McGavern D.B. Microglia development and function. Annu. Rev. Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subhramanyam C.S., Wang C., Hu Q., Dheen S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019;94:112–120. doi: 10.1016/j.semcdb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Feng X., Valdearcos M., Uchida Y., et al. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2(7) doi: 10.1172/jci.insight.91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrientos R.M., Frank M.G., Hein A.M., et al. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav. Immun. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cibelli M., Fidalgo A.R., Terrando N., et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waisman A., Hauptmann J., Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129(5):625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 46.IL-17A is associated with the breakdown of the blood-brain barrier in relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2019;332:147–154. doi: 10.1016/j.jneuroim.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Kebir H., Kreymborg K., Ifergan I., et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system infammation. Nat. Med. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huppert J., Closhen D., Croxford A., et al. Cellular mechanisms of IL17-induced blood-brain barrier disruption. FASEB J. 2010;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Ke K.F., Liu Z., et al. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of aβ1-42-induced Alzheimer's disease model rats. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zenaro E., Pietronigro E., Della Bianca V., et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015;21(8):880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 51.Yin Y., Wen S., Li G., Wang D. Hypoxia enhances stimulating efect of amyloid beta peptide (25–35) for interleukin 17 and T helper lymphocyte subtype 17 upregulation in cultured peripheral blood mononuclear cells. Microbiol. Immunol. 2009;53(5):281–286. doi: 10.1111/j.1348-0421.2009.00120.x. [DOI] [PubMed] [Google Scholar]
- 52.Prajeeth C.K., Kronisch J., Khorooshi R., et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflammation. 2017;14(1):204. doi: 10.1186/s12974-017-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy A.C., Lalor S.J., Lynch M.A., Mills K.H. Infltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2010;24(4):641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z., Qiu A.W., Huang Y., et al. IL-17A exacerbates neuroinfammation and neurodegeneration by activating microglia in rodent models of Parkinson's disease. Brain Behav. Immun. 2019;81:630–645. doi: 10.1016/j.bbi.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 55.Tilocca B., Pieroni L., Soggiu A., et al. Gut-brain Axis and neurodegeneration: state-of-the-Art of Meta-Omics sciences for microbiota characterization. Int. J. Mol. Sci. 2020 J;21(11):4045. doi: 10.3390/ijms21114045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maslowski K.M., Mackay C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cummings J.H., Hill M.J., Bone E.S., et al. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 1979;32(10):2094–2101. doi: 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- 59.Furusawa Y., Obata Y., Fukuda S., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 60.Smith P.M., Howitt M.R., Panikov N., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tayyeb J.Z., Popeijus H.E., Mensink R.P., et al. Short-chain fatty acids (except hexanoic acid) lower NF-kB transactivation, which rescues inflammation-induced decreased apolipoprotein A-I transcription in HepG2 cells. Int. J. Mol. Sci. 2020;21(14):5088. doi: 10.3390/ijms21145088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma H., Tao W., Zhu S. T lymphocytes in the intestinal mucosa: defense and tolerance. Cell. Mol. Immunol. 2019;16(3):216–224. doi: 10.1038/s41423-019-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haghikia A., Jörg S., Duscha A., et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43(4):817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Duscha A., Gisevius B., Hirschberg S., et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell. 2020;180(6):1067–1080.e16. doi: 10.1016/j.cell.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 65.Meyer F., Seibert F.S., Nienen M., et al. Propionate supplementation promotes the expansion of peripheral regulatory T-Cells in patients with end-stage renal disease. J. Nephrol. 2020;33(4):817–827. doi: 10.1007/s40620-019-00694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su X., Yin X., Liu Y., et al. Gut dysbiosis contributes to the imbalance of Treg and Th17 cells in graves' disease patients by propionic acid. J. Clin. Endocrinol. Metab. 2020;105(11) doi: 10.1210/clinem/dgaa511. [DOI] [PubMed] [Google Scholar]
- 67.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 68.Cekanaviciute E., Yoo B.B., Runia T.F., et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berer K., Gerdes L.A., Cekanaviciute E., et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jangi S., Gandhi R., Cox L.M., et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016;7 doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng Q., Junli Gong, Liu X., et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem. Int. 2019;129 doi: 10.1016/j.neuint.2019.104468. [DOI] [PubMed] [Google Scholar]
- 72.Takewaki D., Suda W., Sato W., et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2020;117(36):22402–22412. doi: 10.1073/pnas.2011703117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park J., Wang Q., Wu Q., Mao-Draayer Y., Kim C.H. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019;9(1):8837. doi: 10.1038/s41598-019-45311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trend S., Leffler J., Jones A.P., et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci. Rep. 2021;11(1):5244. doi: 10.1038/s41598-021-84881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu G., Jiang Z., Pu Y., et al. Serum short-chain fatty acids and its correlation with motor and non-motor symptoms in Parkinson's disease patients. BMC Neurol. 2022 Jan 7;22(1):13. doi: 10.1186/s12883-021-02544-7. 10.1186/s12883-021-02544-7IF: 2.6 Q3 . PMID: 34996385; PMCID: PMC8740341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang X.L., Gu X.Y., Zhou X.X., et al. Intestinal dysbacteriosis mediates the reference memory deficit induced by anaesthesia/surgery in aged mice. Brain Behav. Immun. 2019;80:605–615. doi: 10.1016/j.bbi.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 77.Wu Q., Dong J., Bai X., et al. Propionate ameliorates diabetes-induced neurological dysfunction through regulating the PI3K/Akt/eNOS signaling pathway. Eur. J. Pharmacol. 2022 Jun 15;925 doi: 10.1016/j.ejphar.2022.174974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.