Key Points
-
•
Cooccurring mutational profile and not allelic ratio determines clinical outcomes for patients with FLT3-ITD.
-
•
Therapy intensification improves survival for patients with FLT3-ITD; however, those with cooccurring poor-risk mutations still fare poorly.
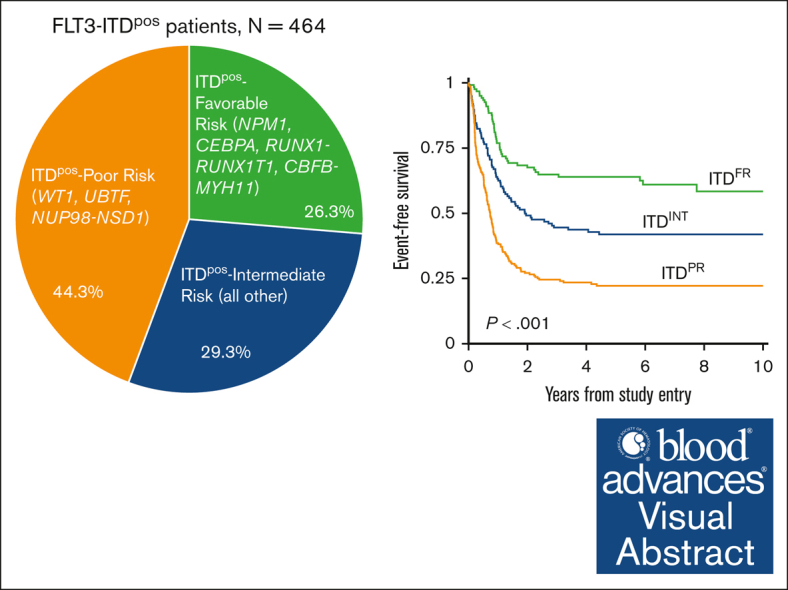
Visual Abstract
Abstract
We sought to define the cooccurring mutational profile of FLT3-ITD–positive (ITDpos) acute myeloid leukemia (AML) in pediatric and young adult patients and to define the prognostic impact of cooperating mutations. We identified 464 patients with FLT3-ITD mutations treated on Children’s Oncology Group trials with available sequencing and outcome data. Overall survival, event-free survival (EFS), and relapse risk were determined according to the presence of cooccurring risk stratifying mutations. Among the cohort, 79% of patients had cooccurring alterations across 239 different genes that were altered through mutations or fusions. Evaluation of the prognostic impact of the cooccurring mutations demonstrated that patients with ITDpos AML experienced significantly different outcomes according to the cooccurring mutational profile. Patients with ITDpos AML harboring a cooccurring favorable-risk mutation of NPM1, CEBPA, t(8;21), or inv(16) experienced a 5-year EFS of 64%, which was significantly superior to of 22.2% for patients with ITDpos AML and poor-risk mutations of WT1, UBTF, or NUP98::NSD1 as well to 40.9% for those who lacked either favorable-risk or poor-risk mutation (ITDpos intermediate; P < .001 for both). Multivariable analysis demonstrated that cooccurring mutations had significant prognostic impact, whereas allelic ratio had no impact. Therapy intensification, specifically consolidation transplant in remission, resulted in significant improvements in survival for ITDpos AML. However, patients with ITDpos/NUP98::NSD1 continued to have poor outcomes with intensified therapy, including sorafenib. Cooccurring mutational profile in ITDpos AML has significant prognostic impacts and is critical to determining risk stratification and therapeutic allocation. These clinical trials were registered at www.clinicaltrials.gov as NCT00002798, NCT00070174, NCT00372593, and NCT01371981.
Introduction
Mutations in FLT3, specifically internal tandem duplications (FLT3-ITD), occur in 10% to 30% of pediatric and young adult acute myeloid leukemia (AML) cases.1, 2, 3, 4, 5, 6 FLT3-ITD mutations are associated with adverse prognosis, and allelic ratio (AR) is reportedly a mediating factor, with patients with high AR (HAR) FLT3-ITD having very poor survival when treated with chemotherapy alone.2,7 Thus, AR has been used for risk stratification by many cooperative groups and in trials across age groups. Intensive consolidation with hematopoietic stem cell transplantation (HCT) improves survival for patients with HAR FLT3-ITD.7, 8, 9, 10 FLT3 mutations have been effectively targeted with FLT3 inhibitors (FLT3i) for therapeutic intervention, with improved outcomes with the addition of FLT3i to chemotherapy and as maintenance after HCT.11, 12, 13, 14, 15, 16
Despite intensive therapy with HCT and FLT3i therapy, many patients with FLT3-ITD still experience relapse.8,17 Even among low AR (LAR) and HAR subgroups, the outcomes are heterogeneous, with many patients with LAR failing to achieve cure and many patients with HAR relapsing despite therapy intensification.8,11,18 Thus, we hypothesized that factors beyond AR may be able to refine prognosis in pediatric and young adult patients with FLT3-ITD AML. In a large cohort of patients with FLT3-ITD, we sought to interrogate the mutational spectrum and to evaluate retrospectively the prognostic impact of additional mutations, specifically those that may otherwise be used for risk stratification, and in the context of AR. We also evaluated the outcomes of patients with FLT3-ITD across treatment trials and in the context of intensified and targeted therapy with the use of HCT in first complete remission (CR1) and FLT3i.
Materials and methods
Patients and treatments
Our cohort included 3033 pediatric and young adult patients (aged 1 month-29 years) with de novo AML enrolled on successive clinical trials from the Children’s Cancer Group (CCG)/Children’s Oncology Group (COG; CCG2961, [ClinicalTrials.gov identifier: NCT00002798; n = 610], COG AAML03P1 [ClinicalTrials.gov identifier: NCT00070174; n = 270], COG AAML0531 [ClinicalTrials.gov identifier: NCT00372593; n = 924], and COG AAML1031 [ClinicalTrials.gov identifier: NCT01371981; n = 1229]). Treatment protocol details have been described previously.12,19, 20, 21, 22 FLT3-ITD was used in the risk stratification of some patients on AAML0531 after an amendment, and for all patients on AAML1031 with an AR of >0.4 considered high risk, and who were allocated to HCT in CR1 if a donor was available. Additionally, on AAML1031 those same patients were also eligible to receive the FTL3i sorafenib in combination with chemotherapy and as post-HCT maintenance. Protocols were approved by the institutional review boards at each participating center. All studies were conducted in accordance with the Declaration of Helsinki.
Mutational analysis
Diagnostic bone marrow or peripheral blood from patients was tested for FLT3-ITD, NPM1, CEBPA, WT1, and NUP98::NSD1 mutations and conventional karyotyping was performed on all patients with available specimen. Testing for the NUP98::NSD1 fusion, which can be cryptic, was performed on all FLT3-ITD samples from CCG2961, COG AAML03P1, and COG AAML0531 using reverse transcription polymerase chain reaction, as previously described, whereas all samples on COG AAML1031 had fusion detected by genomic sequencing.23 Additionally, specimens underwent comprehensive sequencing with either targeted-capture sequencing using a panel of 338 genes (n = 788), whole-genome sequencing (n = 329), and/or transcriptome sequencing (n = 1782).24 Among FLT3-ITD cases, samples underwent at least 1, and in some cases multiple, sequencing methodologies including targeted-capture (n = 125), whole-genome (n = 32), or transcriptome (n = 328) sequencing for identification of cooperating mutations and fusions (supplemental Figure 1). Determination of FLT3-ITD AR was performed after polymerase chain reaction amplification as previously described.7
Statistical methods
Patients were defined as being in CR if they had <5% blasts and absence of extramedullary disease after completion of first induction course. In cases for which measurable residual disease (MRD) data were available, remission without evidence of MRD was defined as <0.1% blasts in the bone marrow detected by flow cytometry. The Kaplan-Meier method was used to estimate survival outcomes.25 Overall survival (OS) was defined as time from study entry to death; and event-free survival (EFS) was defined as time from study entry until death, induction failure, or relapse of any type. Disease-free survival (DFS) was defined as time from the end of induction 1 for patients in CR until relapse or death from any cause; and relapse rate (RR) was defined as time from end of induction 1 for patients in CR to relapse, for which deaths in the absence of relapse were considered competing events.26 The significance of predictor variables was tested using log-rank statistic for OS, EFS, and DFS and Gray statistic for RR.27,28 Outcome estimates at 5 years were summarized with their corresponding log-log 95% confidence intervals (CI). For analyses that violated the proportional hazards assumption, a direct comparison (landmark analysis) between the 5-year estimates was summarized instead of the log-rank statistic. Patients lost to follow-up were censored at the time of last contact. The significance of observed difference in proportions was analyzed by the χ2 test between patient groups, and the Fisher exact test was used if the data were sparse. The Kruskal-Wallis test was used to determine the significance between differences in medians of groups. Cox proportional hazards models were used to estimate hazard ratios for multivariable analyses of OS and EFS.29 Competing risk regression models were used to estimate the subgroup hazard ratios for multivariable analyses of RR.30 Patients receiving HCT in CR were analyzed as a time-varying covariate.
Results
Patient characteristics
Of 3033 patients, FLT3-ITD mutations were identified in 464 (15.3%) patients treated on the following trials: CCG2961 (n = 74), AAML03P1 (n = 30), AAML0531 (n = 149), and AAML1031 (n = 211). Patients with a FLT3-ITD mutation (ITDpos) were older than patients who did not have FLT3-ITD (non-ITD; median age 13.2 vs 9.1 years [P < .001]) and had higher diagnostic white blood cell counts and blast percentage (supplemental Table 1).
Mutational profile
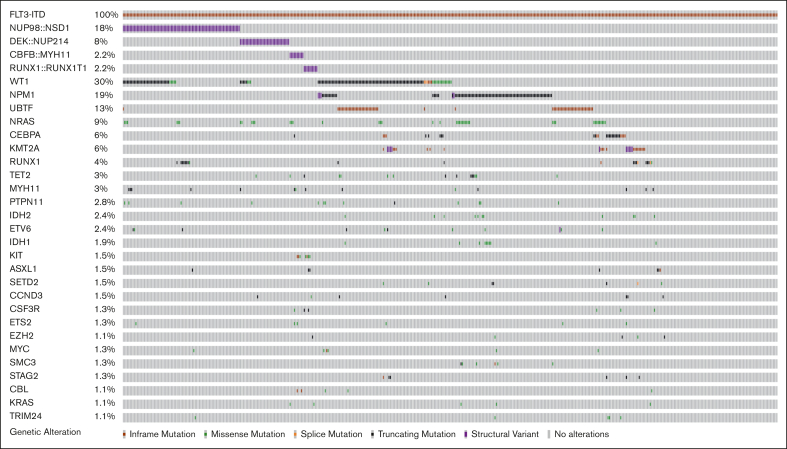
Among 464 patients with ITDpos AML, cooccurring alterations were identified in 79% of the cohort in 239 distinct genes; 217 with single gene mutations and 22 altered by fusions; the median number of cooccurring mutations per patient was 3 (range, 0-25). A heterogeneous mutational profile was observed, with cooccurring missense and truncating mutations, copy number variants, as well as fusions detected (Figure 1). The most common cooccurring alterations were detected in WT1 (n = 141, 30.4%), NPM1 (n = 85, 18.3%; mutations, n = 81 and fusions, n = 4), and NRAS (n = 42, 9.1%). WT1 and NPM1 mutations were significantly more common in patients with ITDpos vs those with non-ITD, 30.7% vs 7.2% and 18.7% vs 6.3%; P < .001 for both. In addition, among patients with known results, we found UBTF alterations and KMT2A-partial tandem duplications significantly more common among patients with ITDpos vs those with non-ITD, 15.7% vs 10.1% and 3% vs 1.2%; P < .001 for both,. The most common fusions involved the nucleoporin (NUP) genes with NUP98::NSD1 (n = 83, 17.9%) and DEK::NUP214/t(6;9) (n = 35, 7.5%), these were also significantly more common in patients with ITDpos vs those without (P ≤ .001 for both). Trisomy 8 was the most common recurring cytogenetic abnormality (n = 58, 12.5%) and significantly more common than in patients with non-ITD (P < .001, supplemental Table 1).
Figure 1.
Cooccuring alterations in pediatric and young adult FLT3-ITD AML. Genes with alterations, including missense and truncating mutations and fusions with a frequency of >1%.
Outcomes for ITDpos vs non-ITD
Patients with ITDpos had significantly inferior end of induction I CR and higher MRD rates compared with patients with non-ITD (supplemental Table 1). Evaluations of outcomes across the entire cohort demonstrated that ITDpos status was associated with inferior outcomes compared with non-ITD status; 5-year EFS of 39.0% (95% CI, 34.4-43.5) vs 47.7% (95% CI, 45.7-49.6; P < .001) and OS of 53.8% (95% CI, 49.0-58.3) vs 63.3% (95% CI, 61.3-65.1; P < .001; supplemental Figure 2). Changes were made to therapy across the different treatment eras and studies; specifically, patients with HAR ITDpos were designated as being at high risk and were recommended for HCT in CR1 after an amendment to AAML0531, and those on AAML1031, in which they also were eligible to receive sorafenib. Evaluation according to treatment trial demonstrated that outcomes for patients with ITDpos improved significantly from CCG2961 to AAML1031, with a 5-year EFS of 26.9% (95% CI, 17.2-37.5) vs 46.5% (95% CI, 39.5-53.1; P = .007), and a corresponding drop in RR from 62.1% (95% CI, 46.4-74.4) to 32.7% (95% CI, 25.0-40.6); P = .002; supplemental Figure 3). In the 3 earlier studies, EFS and OS were significantly inferior for patients with ITDpos, with a trend toward higher RR than for non-ITD; however, in AAML1031, outcomes were similar for patients with ITDpos and those with non-ITD (supplemental Figure 3).
Impact of cooccuring mutations on outcome
We stratified patients with ITDpos overall according to presence of cooccuring mutations. We initially evaluated the outcome of patients with ITDpos with mutations that have been previously recognized to be associated with either favorable-risk [NPM1, CEBPA, RUNX1::RUNX1T1/t(8;21), and CBFB::MYH11/inv(16)/t(16;16)] or high-risk [NUP98::NSD1, DEK::NUP214/t(6;9)] disease. We also evaluated the outcome of patients with a cooccurring WT1 or UBTF because both of these are reportedly associated with inferior outcomes in FLT3-ITD AML.31, 32, 33 Patients with ITDpos with both NPM1 and WT1 mutations were included in the WT1 cohort. There was also overlap of WT1 and UBTF alterations, and those with both were included in the WT1 cohort; thus, patients in the UBTF cohort lacked WT1. Outcomes (EFS, OS, and RR) varied significantly for patients with ITDpos according to their cooccuring mutational profile and those lacking any of the above mutations (supplemental Figure 4). Based on outcomes according to these cooccurring mutations, we subsequently grouped patients with ITDpos into 3 distinct groups for subsequent analyses. Patients with NPM1, CEBPA, RUNX1::RUNX1T1, or CBFB::MYH11 and who lacked a cooccuring mutation that was considered to be unfavorable were grouped together for subsequent analyses and classified as favorable-risk ITD (ITDFR; n = 122; 26.3%). In contrast, WT1 and UBTF mutations and NUP98::NSD1 fusions were found to associated with adverse outcomes, and we found that 44.3% of patients with ITDpos (n = 206) had a cooccurring poor-risk (PR) mutation (ITDPR). The remaining 29.3% (n = 136) of patients with ITDpos lacked the above risk stratifying mutations and were defined as ITDpos intermediate (ITDINT). Our analyses found that patients with ITDpos with cooccurring DEK::NUP214 had significantly improved outcomes compared with those in the ITDPR cohort. While Although this group overall, regardless of ITD status, has been associated with unfavorable outcomes in prior studies but improved with HCT in CR1,34,35 nearly half of patients with DEK::NUP214 in our analysis received HCT in CR. Thus, for our subsequent analyses, patients with DEK::NUP214 were classified as ITDINT.
Among the ITDpos cohort, patients were stratified according to the cooccurring risk mutations of ITDFR, ITDINT, and ITDPR (supplemental Table 2). Analysis by cooccurring mutational group demonstrated significantly different CR rates: ITDFR, 91.6% vs ITDINT, 70.1% vs ITDPR, 49.7% (P < .001). CR1 rates were similar among the ITDFR vs ITDWT-FR cohorts (91.6% vs 87.9%, P = .238) and among ITDINT vs ITDWT-INT cohorts (70.1% vs 72.5%, P = .556). Analysis according to end of induction I MRD-negative status demonstrated similar findings among the risk-defined cohorts: ITDFR, 87.6% vs ITDINT, 54.9% vs ITDPR, 31.6% (P < .001). Again, no significant difference was observed between those in the ITDFR vs ITDWT-FR cohorts (87.6% vs 84.2%, P = .378).
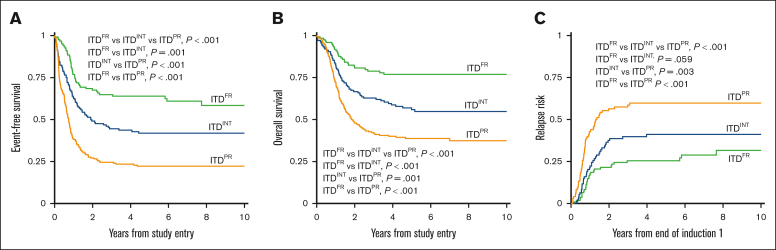
Analysis of outcomes for patients with ITDpos demonstrated striking differences when stratified according to the cooccurring risk-stratifying mutations. Patients with ITDFR experienced superior outcomes compared with those with ITDINT and ITDPR (P < .001 for both OS and EFS; Figure 2). Notably, patients with ITDPR experienced outcomes that were significantly inferior to both those with ITDFR and those with ITDINT. This inferior EFS was driven by relapse, with patients with ITDPR experiencing significantly higher RR than those with ITDINT (P = .003) and those with ITDFR (P < .001; Figure 2). Outcomes of the ITDFR cohort compared with patients without ITD with the same cooccurring FR features (non-ITDFR) were nearly identical (EFS: 64.0% [95% CI, 54.6-71.9] vs 65.1% [95% CI, 61.9-68.1], P = .547), as were those for ITDINT vs non-ITD without risk-stratifying lesions (non-ITDINT; EFS: 41.9% [95% CI, 33-4-50.1] vs 38.4% [95% CI, 35.9-40.9], P = .230). There were also no significant outcome differences among those in the ITDPR cohort compared with those in the non-ITD with cooccurring PR (non-ITDPR) cohort, although there was a signal of inferior outcomes the patients with ITDPR (EFS: 22.2% [95% CI, 16.7-28.2] vs 29.7% [95% CI, 22.1-37.6], P = .065; Table 1; supplemental Figure 5).
Figure 2.
Outcomes for patients with ITDpos according to cooccurring risk groups of FR mutation, INT, or PR mutations. (A) 5-year EFS, (B) 5-year OS, and (C) 5-year relapse risk.
Table 1.
Outcomes for patients without FLT3-ITD and those with FLT3-ITDpos according to cooccurring mutation risk groups
| Non-FLT3-ITD |
FLT3-ITDpos |
P value | |||
|---|---|---|---|---|---|
| N | %, 95 CI | N | %, 95 CI | ||
| FR mutations | |||||
| 5-year OS | 931 | 81.5%, 78.9%-83.9% | 122 | 76.9%, 68.1%-83.5% | .357 |
| 5-year EFS | 931 | 65.1%, 61.9%-68.1% | 122 | 64.0%, 54.6%-71.9% | .547 |
| 5-year relapse risk from EOI1 | 807 | 25.3%, 22.3%-28.4% | 109 | 25.5%, 17.6%-34.1% | .506 |
| INT risk mutations | |||||
| 5-year OS | 1502 | 53.2%, 50.6%-55.8% | 136 | 55.9%, 46.8%-63.9% | .372 |
| 5-year EFS | 1502 | 38.4%, 35.9%-40.9% | 136 | 41.9%, 33.4%-50.1% | .230 |
| 5-year relapse risk from EOI1 | 1064 | 47.4%, 44.3%-50.4% | 94 | 41.1%, 30.9%-51.0% | .104 |
| PR mutations | |||||
| 5-year OS | 136 | 49.1%, 40.2%-57.4% | 206 | 38.7%, 31.8%-45.5% | .093 |
| 5-year EFS | 136 | 29.7%, 22.1%-37.6% | 206 | 22.2%, 16.7%-28.2% | .065 |
| 5-year relapse risk from EOI1 | 90 | 53.5%, 42.4%-63.3% | 98 | 59.8%, 49.2%-63.9% | .323 |
Cooccurring mutation risk groups stratified according to favorable (NPM1, CEBPA, RUNX1-RUNX1T1, CBFB-MYH11), poor (WT1, UBTF, NUP98-NSD1), and intermediate (all other) risk mutations.
EOI1, endo of induction 1.
Impact of AR
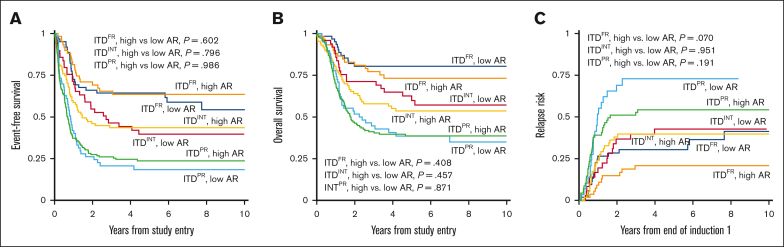
We evaluated the impact of AR among the different cooccurring risk mutation groups with a cutoff of >0.4 and ≤0.4 considered HAR and LAR, respectively, to align with designated cutoffs of AAML0531 and AAML1031. The ITDPR group had a higher prevalence of HAR (70.4%) vs LAR (29.6%) disease and had significantly higher prevalence of HAR disease than ITDFR and ITDINT subgroups (P < .001). In contrast, patients with ITDFR had nearly equivalent prevalence of HAR vs LAR (49.2% vs 50.8%) and the prevalence of HAR disease was significantly less in patients with FR compared with those with non-FR disease (49.2% vs 67.8%, P < .001). Analysis in each of the ITDpos subgroups (FR, INT, and PR) found no significant differences in EFS, OS, or RR in patients with LAR vs those with HAR (Figure 3). Multivariable regression analysis demonstrated that cooccurring mutational profile but not AR affected outcomes (Table 2).
Figure 3.
Outcomes for patients with LAR ITDpos (≤0.4) vs HAR ITDpos (>0.4) according to cooccurring risk group. (A) 5-year EFS, (B) 5-year OS, (C) 5-year relapse risk from end of induction 1.
Table 2.
Multivariable regression analysis for EFS, OS, and RR
| EFS |
OS |
Relapse risk end course 1 |
||||||
|---|---|---|---|---|---|---|---|---|
| n | HR (95% CI) | P value | HR (95% CI) | P value | n | HR (95% CI) | P value | |
| ITDFR | 118 | 1 | 1 | 105 | 1 | |||
| ITDINT | 134 | 1.91 (1.30-2.78) | .001 | 2.07 (1.3-3.31) | .002 | 92 | 1.74 (1.07-2.84) | .027 |
| ITDPR | 202 | 3.70 (2.61-5.24) | <.001 | 3.48 (2.26-5.37) | <.001 | 95 | 3.87 (2.44-6.14) | <.001 |
| LAR | 166 | 1 | 1 | 112 | 1 | |||
| HAR | 288 | 1.25 (0.83-1.45) | .097 | 1.29 (0.94-1.76) | .117 | 180 | 1.17 (0.79-1.73) | .431 |
| Chemotherapy treatment | 256 | 1 | 1 | 160 | 1 | |||
| Gemtuzumab ozogamicin treatment | 110 | 1.10 (0.83-1.45) | .526 | 1.06 (0.77-1.47) | .723 | 70 | 0.69 (0.44-1.10) | .118 |
| Sorafenib + HCT in CR1 (arm C AAML1031) | 88 | 0.63 (0.43-0.93) | .019 | 0.81 (0.52-1.26) | .355 | 62 | 0.30 (0.15-0.61) | .001 |
| HCT in CR not received | 291 | 1 | 1 | 163 | 1 | |||
| HCT in CR received (TVC) | 163 | 0.60 (0.43-0.83) | .002 | 0.62 (0.44-0.87) | .006 | 129 | 0.57 (0.37-0.90) | .016 |
Multivariable regression analysis for EFS, OS, and RR according to cooccurring risk mutation group (FR, INT, PR), LAR (≤0.4) vs HAR (>0.4), treatment received, and HCT in CR as TVC.
Patients in the chemotherapy treatment group included patients on CCG2961, AAML0531 arm A, and AAML1031 arm A/B, patients in the gemtuzumab ozogamicin treatment group included patients treated on AAML03P1 and AAML0531 arm B, and patients in the sorafenib + HCT in CR1 group were those on AAML1031 arm C.
HR, hazard ratio; TVC, time-varying covariate.
Impact of treatment intensification with HCT and sorafenib
Analysis of outcomes according to treatment trial demonstrated overall improvements in survival in patients with ITDpos. Multivariable analysis with treatment analyzed according to the type of therapy received (eg, chemotherapy, gemtuzumab ozogamicin, sorafenib and HCT, and HCT alone) demonstrated the significant impact of specific interventions in patients with ITDpos. We found that patients treated on arm C of AAML1031 (sorafenib + HCT in CR1) had improved EFS and RR, and that HCT in CR on its own also resulted in significant improvements in OS, EFS, and RR (Table 2). Given our findings for patients with DEK-NUP214 in the cohort overall, we analyzed outcomes specifically for patients with DEK-NUP214 who received HCT in CR1, and they achieved a 5-year DFS of 84.6% (95% CI, 51.2-94.9).
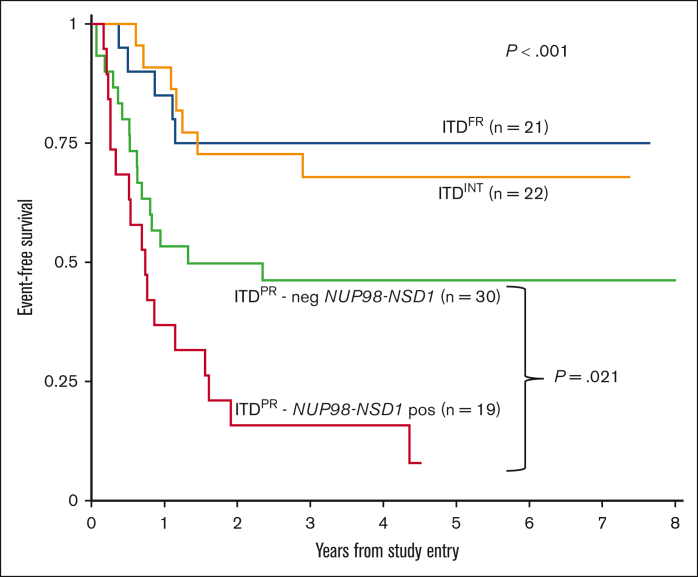
Although outcomes improved overall for patients with ITDpos and were comparable with those for patients with ITDWT treated on AAML1031 and that we saw benefit of intensification approaches on arm C with sorafenib and HCT in CR1, we found significant outcome differences according to cooccurring mutations. Among patients with ITDpos treated on arm C, differences among cooccurring mutational risk groups persisted, with a 5-year EFS of 75.0% (95% CI, 50.0-88.7) for ITDFR vs 67.9% (95% CI, 44.1-83.2) for ITDINT vs 30.8% (95% CI, 19.0-33.5) for ITDPR (P < .001), with similar findings in OS and RR (supplemental Table 3). With continued inferior outcomes for patients with PR, we sought to determine whether any of the PR subgroups experienced differential benefit from therapy intensification. We found that patients with NUP98::NSD1 continued to experience poor outcomes despite these intensifications in therapy, with a 5-year EFS of 7.9% (95% CI, 0.7-27.7) vs 46.2% (95% CI, 27.9-62.7; P = .021) in the group of patients with ITDPR who did not harbor a NUP98::NSD1 (Figure 4); similar trends were seen for OS and RR (supplemental Figure 6). Analysis of the patients with ITDFR treated on AAML1031 found no differences according to treatment arm/intensity, with patients who received chemotherapy on arm A/B having similar outcomes to those treated with sorafenib and HCT in CR on arm C (supplemental Table 4). We subsequently compared outcomes for patients with ITDFR HAR who were risk stratified to HCT in CR1 on AAML1031 or AAML0531 with those of patients treated on earlier studies (CCG2961, AAML03P1, and before amendment on AAML0531) in which AR was not used as a risk stratifying feature and found no differences in outcomes (supplemental Table 5).
Figure 4.
EFS for patients with ITDpos treated on arm C of AAML1031 with sorafenib and HCT in CR1 according to cooccurring risk groups (FR, INT, and PR) and those with PR mutations further stratified according to presence of NUP98-NSD1 fusion.
Discussion
Our findings demonstrate that, in FLT3-ITD AML, cooccurring mutations significantly affect treatment responses and prognosis. We demonstrate that the cooccurring mutational profile, not AR, is the most important prognostic feature in ITDpos AML and that, in the setting of incorporation of mutational profile, AR loses its prognostic significance. We show that presence of a cooccurring FR mutation in patients with ITDpos identifies a cohort with favorable outcomes that may not require HCT in CR1. Concurrent NPM1 is generally considered a risk-modifying feature in FLT3-ITD AML in adults; however, findings regarding the impact on outcome are varied, especially when accounting for the impact of AR.8,36, 37, 38, 39, 40 Favorable survival has been reported in a small cohort of pediatric patients with dual ITD/NPM1 in the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05 study.41 Cooccurrence of ITD and CEBPA and core binding factor (CBF) fusions have rarely been reported in adults,42,43 but in our pediatric cohort we observed a nontrivial overlap with these lesions, highlighting the importance of recognizing favorable cooperating events outside of NPM1. Future studies that prospectively evaluate the outcomes of patients with ITDFR HAR with appropriate response to initial therapy treated with chemotherapy alone will help more definitively define the outcomes of these patients. Although FLT3-ITD may not act as the leukemia initiating event in patients with FR, biologically there is likely an effect that may derive benefit from FLT3i. For patients with dual FLT3-ITD/NPM1, a trend toward improved outcomes with midostuarin on the RATIFY trial has been shown, as well as improved outcomes with sorfenib when it was also used as post-HCT mainteance.39,44
Our findings demonstrate that cooperating mutational status and not AR affects outcomes for patients with ITDpos. The prognostic impact of diagnostic AR has been subject to inconsistency, with cooperative groups and clinical trials designating variable cutoffs of HAR vs LAR.7,8,11 Determination of AR is affected by multiple factors including blast percentage and assay. Notably, FLT3i therapy, thus far, has resulted in therapeutic benefit across a wide range of ARs, including what has been considered lower ARs.11,16,45 Further studies are important to determine whether AR may be important in predicting which patients derive the most benefit from FLT3i therapy. We found that HAR disease was more prevalent among patients with ITDPR and ITDINT, thus AR may, in some situations, serve as a surrogate for other higher-risk disease features. Importantly, our findings show that that pediatric patients with ITDpos without a cooccurring FR lesion should be allocated to HCT in CR1 regardless of AR. This aligns with recent European Society for Blood and Marrow Transplation recommendations in adult AML.46
Treatment advances for patients with ITDpos including the incorporation of gemtuzumab ozogamicin, FLT3i therapy, and allogeneic HCT in CR1 have been shown to result in incremental improvements in survival.8,10,11,16,47,48 Our findings support this; specifically, we show that HCT in CR1 and the combination of sorafenib and HCT in CR1 resulted in significantly improved outcomes in a multivariable analysis. However, our study also highlights that, among patients with ITDpos, those in the ITDPR group have generally continued to experience significantly inferior outcomes compared with those with other cooccurring mutations; importantly, among this group, the gains have been uneven. Our findings regarding the prognostic impact of patients with ITDpos/DEK::NUP214 being classified as having an INT and not a PR lesion, likely reflects the beneficial response to intensified therapy, specifically HCT in CR1 that this group experiences; thus, they should still receive intensified therapy and can achieve quite good outcomes with this therapy. Earlier studies have suggested that patients with DEK::NUP214 experienced improved outcomes when FLT3-ITD HAR started being used as a risk-stratifying lesion and those patients were allocated to HCT in CR1.34 Our findings align with a recent study in adults with ITDpos/DEK::NUP214 AML that found HCT in CR1 significantly improved outcomes compared with chemotherapy.49
We demonstrate early dismal responses to therapy and poor survival in NUP98::NSD1 AML. This supports recent findings that FLT3-ITD cooccurring with WT1, UBTF, or NUP98-NSD1 is associated with significantly inferior prognosis.23,31, 32, 33,50 Although there is significant overlap in WT1 and UBTF among patients with ITDpos, we show that poor outcome was seen in patients with mutant UBTF independent of WT1 status. Our findings highlight the particularly dismal responses to therapy and poor survival that has persisted despite therapy intensification among patients with NUP98-NSD1 fusion. This, to our knowledge, is the first analysis of response of patients with ITDpos/NUP98::NSD1 to FLT3i, and we show that sorafenib failed to have any benefit. Our findings suggest that, overall, FLT3 inhibition is not an effective target for therapeutic intervention in NUP98::NSD1 AML. The unique biology of this group manifests clinically as poor responses to chemotherapy, including FLT3i. Our findings support previous studies demonstrating distinct gene expression profile for NUP98::NSD1 AML.50,51 Understanding the biology of this group may provide insights into potential targets for intervention.52,53 Novel strategies are needed and should be prioritized early in therapy for these patients. The cohort of patients with ITDPR with WT1 and UBTF alterations continued to have comparatively inferior outcomes to the ITDFR and ITDINT cohorts but were improved compared with those of patients with NUP98::NSD1. Further studies are needed to determine the relative degree of benefit of FLT3i in other PR subgroups.
The inclusion of patients across multiple studies receiving different treatments is a limitation of our study because there were significant evolutions in treatment for ITDpos AML over the study period. Some of the patients with cooccurring FR mutations and HAR treated on the later studies would have received HCT, which may have affected outcomes. However, inclusion of multiple studies allowed us to compare the impact of treatment changes, specifically intensification efforts with HCT consolidation and FLT3i. Our study did include post hoc analyses because outcome of FLT3-ITD AML was not a major aim of the studies except for patients with HAR ITDpos treated on AAML1031. However, given the frequency of the FLT3-ITD mutations in pediatric AML, a larger cohort than is generally included in a study was required to study the cooccurring mutational subgroups. Independent validation in additional cohorts is needed to validate our findings, and future studies that prospectively evaluated risk stratified treatments among patients with ITDpos will be important to confirm these findings.
We demonstrate that the incorporation of comprehensive cooccurring mutational profiling is the most critical factor in refining prognosis and appropriate risk and therapeutic stratification for patients with ITDpos and should be used instead of AR in determining risk allocation. We also show that therapy intensification, specifically the use of sorafenib and HCT in CR1 has resulted in significant improvements in outcome for patients with ITDpos. Although FLT3-ITD has generally been considered a high-risk feature for which HCT in CR1 is needed, we demonstrate that patients with cooccurring FR lesions may not require this degree of intensification. Additionally, although some patients with ITDpos AML greatly benefit from therapy intensification and can achieve very good outcomes, to date, patients with NUP98::NSD1 fusions have not benefited from approaches, and further efforts to study the early intervention of novel and targeted therapies are urgently needed.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
This study was supported by the National Cancer Institute’s National Clinical Trial Network Operations Center (grant N), the National Cancer Institute’s National Clinical Trial Network Statistics & Data Center (grant U10CA180899), the National Cancer Institute (grant R01CA114563; to S.M.), and the St. Baldrick's Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: K.T. and S.M. conceived of, and initiated, the study; R.B.G. and T.A.A. performed statistical analysis and helped generate figures; J.L.S., R.E.R., A.L., B.J.H., D.K., L.R., and J.H.P. performed genomic analyses; B.L., T.M.C., A.S.G., E.A.K., R.A., and J.A.P. oversaw the clinical trials and contributed data; K.T. wrote the initial manuscript draft; and K.T. and S.M. edited the manuscript with input from all authors.
Footnotes
The data generated for this study have been deposited in the Database of Genotypes and Phenotypes (dbGaP, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000465.v21.p8) under the study ID phs000465.v21.p8 and are also available at the National Cancer Institute’s Genomic Data Commons (https://portal.gdc.cancer.gov/projects/TARGET-AML) under the TARGET-AML project.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Meshinchi S, Woods WG, Stirewalt DL, et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood. 2001;97(1):89–94. doi: 10.1182/blood.v97.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Zwaan CM, Meshinchi S, Radich JP, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102(7):2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 3.Rubio P, Campos B, Digiorge JA, et al. NPM1, FLT3 and CEBPA mutations in pediatric patients with AML from Argentina: incidence and prognostic value. Int J Hematol. 2016;104(5):582–590. doi: 10.1007/s12185-016-2064-5. [DOI] [PubMed] [Google Scholar]
- 4.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 5.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 6.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 7.Meshinchi S, Alonzo TA, Stirewalt DL, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. 2006;108(12):3654–3661. doi: 10.1182/blood-2006-03-009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlenk RF, Kayser S, Bullinger L, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–3449. doi: 10.1182/blood-2014-05-578070. [DOI] [PubMed] [Google Scholar]
- 9.Lin PH, Lin CC, Yang HI, et al. Prognostic impact of allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia patients with internal tandem duplication of FLT3. Leuk Res. 2013;37(3):287–292. doi: 10.1016/j.leukres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Ho AD, Schetelig J, Bochtler T, et al. Allogeneic stem cell transplantation improves survival in patients with acute myeloid leukemia characterized by a high allelic ratio of mutant FLT3-ITD. Biol Blood Marrow Transplant. 2016;22(3):462–469. doi: 10.1016/j.bbmt.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard JA, Alonzo TA, Gerbing R, et al. Sorafenib in combination with standard chemotherapy for children with high allelic ratio FLT3/ITD+ acute myeloid leukemia: a report from the Children's Oncology Group Protocol AAML1031. J Clin Oncol. 2022;40(18):2023–2035. doi: 10.1200/JCO.21.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlenk RF, Weber D, Fiedler W, et al. Midostaurin added to chemotherapy and continued single agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840–851. doi: 10.1182/blood-2018-08-869453. [DOI] [PubMed] [Google Scholar]
- 14.Brunner AM, Li S, Fathi AT, et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br J Haematol. 2016;175(3):496–504. doi: 10.1111/bjh.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN) J Clin Oncol. 2020;38(26):2993–3002. doi: 10.1200/JCO.19.03345. [DOI] [PubMed] [Google Scholar]
- 16.Erba HP, Montesinos P, Kim HJ, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10388):1571–1583. doi: 10.1016/S0140-6736(23)00464-6. [DOI] [PubMed] [Google Scholar]
- 17.Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30(7):735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 18.Hitzler JK, He W, Doyle J, et al. Outcome of transplantation for acute myelogenous leukemia in children with Down syndrome. Biol Blood Marrow Transplant. 2013;19(6):893–897. doi: 10.1016/j.bbmt.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children's Oncology Group Phase 3 Trial for untreated pediatric acute myeloid leukemia: a report from the Children's Oncology Group. Blood. 2008;111(3):1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. 2012;118(3):761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 22.Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: a report from the Children's Oncology Group. Haematologica. 2020;105(7):1879–1886. doi: 10.3324/haematol.2019.220962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostronoff F, Othus M, Gerbing RB, et al. NUP98/NSD1 and FLT3/ITD coexpression is more prevalent in younger AML patients and leads to induction failure: a COG and SWOG report. Blood. 2014;124(15):2400–2407. doi: 10.1182/blood-2014-04-570929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolouri H, Farrar JE, Triche T, Jr., et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. 2018;24(1):103–112. doi: 10.1038/nm.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 26.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons, Inc; 2002. [Google Scholar]
- 27.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 28.Peto R, Peto J. Asymptomatically efficient rank invariant test procedures. J Roy Stat Soc. 1972;135(2):185–207. [Google Scholar]
- 29.Cox DR. Regression models and life-tables. J Roy Stat Soc B. 1972;34(2):187–202. [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 31.Niktoreh N, Walter C, Zimmermann M, et al. Mutated WT1, FLT3-ITD, and NUP98-NSD1 fusion in various combinations define a poor prognostic group in pediatric acute myeloid leukemia. J Oncol. 2019;2019 doi: 10.1155/2019/1609128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umeda M, Ma J, Huang BJ, et al. Integrated genomic analysis identifies UBTF tandem duplications as a recurrent lesion in pediatric acute myeloid leukemia. Blood Cancer Discov. 2022;3(3):194–207. doi: 10.1158/2643-3230.BCD-21-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaburagi T, Shiba N, Yamato G, et al. UBTF-internal tandem duplication as a novel poor prognostic factor in pediatric acute myeloid leukemia. Genes Chromosomes Cancer. 2023;62(4):202–209. doi: 10.1002/gcc.23110. [DOI] [PubMed] [Google Scholar]
- 34.Tarlock K, Alonzo TA, Moraleda PP, et al. Acute myeloid leukaemia (AML) with t(6;9)(p23;q34) is associated with poor outcome in childhood AML regardless of FLT3-ITD status: a report from the Children's Oncology Group. Br J Haematol. 2014;166(2):254–259. doi: 10.1111/bjh.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Beya M, Labopin M, Maertens J, et al. Allogeneic stem cell transplantation in AML with t(6;9)(p23;q34);DEK-NUP214 shows a favourable outcome when performed in first complete remission. Br J Haematol. 2020;189(5):920–925. doi: 10.1111/bjh.16433. [DOI] [PubMed] [Google Scholar]
- 36.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 37.Pratcorona M, Brunet S, Nomdedeu J, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–2738. doi: 10.1182/blood-2012-06-431122. [DOI] [PubMed] [Google Scholar]
- 38.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dohner K, Thiede C, Jahn N, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135(5):371–380. doi: 10.1182/blood.2019002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaguchi M, Yamaguchi H, Najima Y, et al. Prognostic impact of low allelic ratio FLT3-ITD and NPM1 mutation in acute myeloid leukemia. Blood Adv. 2018;2(20):2744–2754. doi: 10.1182/bloodadvances.2018020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimada A, Iijima-Yamashita Y, Tawa A, et al. Risk-stratified therapy for children with FLT3-ITD-positive acute myeloid leukemia: results from the JPLSG AML-05 study. Int J Hematol. 2018;107(5):586–595. doi: 10.1007/s12185-017-2395-x. [DOI] [PubMed] [Google Scholar]
- 42.Faber ZJ, Chen X, Gedman AL, et al. The genomic landscape of core-binding factor acute myeloid leukemias. Nat Genet. 2016;48(12):1551–1556. doi: 10.1038/ng.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duployez N, Marceau-Renaut A, Boissel N, et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia. Blood. 2016;127(20):2451–2459. doi: 10.1182/blood-2015-12-688705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao R, Zhang Y, He J, et al. Impact of genetic patterns on sorafenib efficacy in patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation: a multi-center, cohort study. Signal Transduct Target Ther. 2023;8(1):348. doi: 10.1038/s41392-023-01614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abou Dalle I, Ghorab A, Patel K, et al. Impact of numerical variation, allele burden, mutation length and co-occurring mutations on the efficacy of tyrosine kinase inhibitors in newly diagnosed FLT3- mutant acute myeloid leukemia. Blood Cancer J. 2020;10(5):48. doi: 10.1038/s41408-020-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bazarbachi A, Bug G, Baron F, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2020;105(6):1507–1516. doi: 10.3324/haematol.2019.243410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarlock K, Alonzo TA, Gerbing RB, et al. Gemtuzumab ozogamicin reduces relapse risk in FLT3/ITD acute myeloid leukemia: a report from the Children's Oncology Group. Clin Cancer Res. 2016;22(8):1951–1957. doi: 10.1158/1078-0432.CCR-15-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 49.Kayser S, Hills RK, Luskin MR, et al. Allogeneic hematopoietic cell transplantation improves outcome of adults with t(6;9) acute myeloid leukemia: results from an international collaborative study. Haematologica. 2020;105(1):161–169. doi: 10.3324/haematol.2018.208678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiba N, Ichikawa H, Taki T, et al. NUP98-NSD1 gene fusion and its related gene expression signature are strongly associated with a poor prognosis in pediatric acute myeloid leukemia. Genes Chromosomes Cancer. 2013;52(7):683–693. doi: 10.1002/gcc.22064. [DOI] [PubMed] [Google Scholar]
- 51.Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118(13):3645–3656. doi: 10.1182/blood-2011-04-346643. [DOI] [PubMed] [Google Scholar]
- 52.Kivioja JL, Thanasopoulou A, Kumar A, et al. Dasatinib and navitoclax act synergistically to target NUP98-NSD1(+)/FLT3-ITD(+) acute myeloid leukemia. Leukemia. 2019;33(6):1360–1372. doi: 10.1038/s41375-018-0327-2. [DOI] [PubMed] [Google Scholar]
- 53.Mohanty S, Jyotsana N, Sharma A, et al. Targeted inhibition of the NUP98-NSD1 fusion oncogene in acute myeloid leukemia. Cancers (Basel) 2020;12(10):2766. doi: 10.3390/cancers12102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.