Highlights
-
•
The frequency and severity of adverse reactions to the mRNA COVID-19 vaccine series that included the last bivalent booster were examined.
-
•
The anti-spike IgG titers were measured after each vaccination.
-
•
The bivalent booster led to fewer and less severe adverse reactions than the preceding monovalent doses.
-
•
Previous post-vaccination fever increased the risk of subsequent fever, but subsequent fever was overall rare.
-
•
An independent correlation was found between post-vaccination fever and IgG titers for each dose.
Keywords: SARS-CoV-2, Vaccine, Reactogenicity, Antibody, Booster
Abstract
Background
SARS-CoV-2 mRNA vaccination, recognized for high immunogenicity, frequently induces adverse reactions, especially fever. We previously reported a correlation between post-vaccination fever and specific antibody responses to the primary series and first booster. We herein report changes in adverse reactions and the correlation between post-vaccination fever and antibody responses across successive vaccinations, from monovalent to bivalent mRNA vaccines.
Methods
This cohort study was conducted at a Japanese hospital to investigate adverse reactions to the monovalent primary, first booster, and BA.4/5 bivalent BNT162b2 vaccinations. Local and systemic reactions were reported through a self-reporting diary after each dose. The spike-specific IgG titers were measured following each vaccination.
Results
Across 727 vaccinations in the vaccine series, the bivalent booster induced fewer adverse reactions than earlier doses. Fever ≥ 38.0 °C was significantly less frequent in the bivalent booster (12.3 %) compared to the primary series and monovalent booster (22.0 %, 26.2 %, p < 0.001). Reaction severity was also reduced in the bivalent booster. In the analysis of 70 participants with complete data for all doses, post-vaccination fever ≥ 38.0 °C exhibited the highest relative risk (RR) among all solicited reactions throughout the vaccine series (RR: 5.24 [95 % CI: 2.40–11.42] for monovalent and 6.24 [95 % CI: 2.14–18.15] for bivalent). The frequency of fever ≥ 38.0 °C after all doses was 8.6 % (6/70), with no fever ≥ 39.0 °C across all vaccinations. A high-grade post-vaccination fever was correlated with higher IgG titers, with multivariate analyses confirming this correlation as independent for each dose and unaffected by previous post-vaccination fever.
Conclusions
The bivalent mRNA vaccine booster showed fewer and milder adverse reactions than the monovalent doses. Although vaccinees with a history of post-vaccination fever were more likely to experience fever after a subsequent dose, such recurrences were infrequent. A correlation between post-vaccination fever and increased IgG titers was identified for each vaccination, irrespective of post-vaccination fever history.
1. Introduction
The mRNA-based COVID-19 vaccine developed by BioNTech and Pfizer (BNT162b2) has initially demonstrated an efficacy of 95 % in preventing symptomatic COVID-19 [1] and exhibited strong immunogenicity, evidenced by robust specific antibody responses [2], [3]. However, alongside the high immunogenicity, this type of vaccine has been shown to induce a higher frequency of adverse reactions, particularly fever, compared to other viral vaccines [4]. In our prior cohort studies that investigated adverse reactions after the two-dose primary series and the first booster (third dose) of the BNT162b2 monovalent vaccine, over 20 % of the participants experienced a fever of ≥ 38.0 °C post-vaccination [5], [6], a rate substantially higher than the approximately 1 % reporting such a response after the influenza vaccine [7]. These studies also identified a positive association between post-vaccination fever and vaccine-induced spike-specific IgG titers. For instance, in the primary series, the geometric mean of the IgG titers was 7,186 AU/mL for participants without fever (body temperature < 37.0 °C), nearly doubling to 13,035 AU/mL for those with fever above 38.0 °C [5]. This pattern was also observed for the monovalent booster [6], suggesting a potential link between post-vaccination fever and the strong immunogenicity of COVID-19 mRNA vaccines.
Following the administration of monovalent mRNA vaccines, bivalent mRNA vaccine boosters targeting the Omicron BA.4/5 sublineages were authorized in response to the emergence of antigenically divergent Omicron variants [8]. The Centers for Disease Control and Prevention (CDC) has reviewed the frequency and severity of adverse events post-bivalent boosters, finding them comparable to those reported post-monovalent boosters [9]. However, comprehensive real-world data tracking individual-level trends in adverse reactions across successive vaccinations, including the bivalent booster, remains limited.
In this cohort study, extending our previous research on the primary series and monovalent booster, we prospectively examined the frequency and severity of adverse reactions to the second booster (fourth) dose with the BNT162b2 BA.4/5 bivalent mRNA vaccine, assessing these reactions with a standardized scale. Sequential monitoring of the same cohort throughout the vaccine series allowed us to track changes in adverse reactions at an individual level after each administered dose. Furthermore, the spike-specific IgG titers were measured after the bivalent booster for analysis of its correlation with post-vaccination fever following it and the preceding doses.
2. Participants and methods
2.1. Participants
This study included healthcare workers at Fukuoka City Hospital in Japan who had received three doses of the BNT162b2 monovalent vaccine (including the two-dose primary series and the first booster), followed by the BNT162b2 BA.4/5 bivalent booster. The criteria for inclusion in the analysis required participants to have completed questionnaires regarding solicited adverse reactions and to have available data on the anti-spike IgG titers measured post-bivalent booster administration.
2.2. Participant consent statement
All participants provided written informed consent before undergoing any of the study procedures. The study was approved by the ethics review board of Fukuoka City Hospital (approval number 254).
2.3. Outcomes
The primary outcomes include assessing the frequency and severity of adverse reactions to the bivalent booster compared to those from preceding monovalent vaccinations, as well as the analysis of reaction recurrence across successive doses. Secondary outcomes include 1) examining the correlation between adverse reactions and anti-spike IgG titers; and 2) comparing adverse reactions based on SARS-CoV-2 infection history. The analysis related to infection history will be detailed in the Supplementary Materials.
2.4. Demographic characteristics, reactogenicity, and antipyretic medications
Participant background information was collected by a web-based questionnaire. Solicited adverse reactions were reported daily for seven days after the bivalent booster through a web-based self-reporting diary. The solicited data were as follows: 1) local reactions (pain at the injection site, redness, and swelling), and 2) systemic events (fever, fatigue, headache, chills, vomiting, diarrhea, muscle pain, and joint pain). Axillary body temperature was measured twice daily, morning and night, and additionally whenever the participant felt feverish. The highest body temperature during the seven days was used for analysis. Duration of fever was defined as the number of days with fever ≥ 38.0 °C after vaccination. The severity of all solicited adverse reactions was categorized into four grades (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, potentially life threatening), in accordance with the Food and Drug Administration (FDA) guidelines, as detailed in Table s1 [10]. The use of antipyretics was at the participant’s discretion. We collected information on self-medicated antipyretics taken within 24 h before and seven days after vaccination. This included the name of the antipyretic, dosage, and reason for use on self-medicated antipyretics was collected daily with the adverse reaction data.
Previously collected data on adverse reactions and anti-spike IgG titers following the primary series and monovalent booster were used in the analysis. The method of data collection for the monovalent booster was identical to that used in this study [6]. For the primary series, notable methodological differences included a shorter post-vaccination period, five days, for reporting adverse reactions, compared to seven days for the bivalent booster. Additionally, an originally defined subjective scaling method was used to evaluate severity of all reactions except fever [5].
2.5. Serological testing
Serum samples were collected twice, before and approximately one month after the bivalent booster. The quantitative levels of IgG antibodies for the receptor binding domain of the S1 subunit of the viral spike protein were measured using the SARS-CoV-2 IgG II assay and SARS-CoV-2 IgG assay (Abbott Laboratories Co., Ltd., Park, IL, USA).
2.6. Statistical analysis
The anti-spike IgG titers were log-transformed for analysis. Statistical measures including median, interquartile range (IQR), mean, relative risk (RR), geometric mean titer (GMT), fold change, and 95 % confidence interval (CI) were computed. Group differences were assessed using Student's t-test, Wilcoxon rank-sum test, ANOVA, chi-square test, or Fisher's exact test, depending on the data characteristics. Correlation coefficients were calculated using Spearman’s rank correlation test. Multivariate linear regression models were used to assess the correlation between post-vaccination fever and anti-spike IgG titers, incorporating variables such as occurrences of fever ≥ 38.0 °C following the primary series, monovalent booster, and bivalent booster, along with age and sex. The level of significance was set at < 5 %, two-sided. All analyses were performed using the SAS software package, release 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Demographic characteristics and anti-spike IgG titers
This study involved 114 participants who received the bivalent booster, predominantly female (76.3 %) with a median age of 43 (Table 1). All participants were immunocompetent and Japanese. There were 296 instances of vaccination in the primary series and 317 for the monovalent booster, resulting in a total of 727 instances across all vaccine series (Fig. s1). The age and sex distributions were consistent across these cohorts. A notable difference was observed in SARS-CoV-2 infection history: 41.2 % of those in the bivalent booster group reported previous infections, a significantly higher proportion than the 0 % in the primary series and 2.5 % in the monovalent booster group (p < 0.001).
Table 1.
Anti-spike IgG titers and adverse reactions after the primary series, monovalent booster, and bivalent booster.
| Primary series (N = 296) | Monovalent booster (N = 317) | Bivalent booster (N = 114) | p-value | ||
|---|---|---|---|---|---|
| Participant background | |||||
| Age, median (IQR, range) | 40 (30.5–47.5, 20–70) | 41 (33–48, 21–71) | 43 (35–49, 20–69) | 0.142 | |
| Sex (female, %) | 221 (74.7) | 231 (72.9) | 87 (76.3) | 0.745 | |
| Previous SARS-CoV-2 infection (%) | 0 (0) | 8 (2.5) | 47 (41.2) | < 0.001 | |
| Anti-spike IgG titers | |||||
| Before vaccination, GMT (95 % CI), AU/mL | − | 589 (543–640) | 5,506a (4,257–7,119) | < 0.001b | |
| After vaccination, GMT (95 % CI), AU/mL | 9,093 (8,393–9,849) | 16,971 (15,700–18,340) | 24,871 (21,558–28,695) | < 0.001 | |
| Local reactions (%) | |||||
| Pain at injection site | 278 (93.9) | 314 (99.1) | 110 (96.5) | 0.002 | |
| Redness | 61 (20.6) | 109 (34.4) | 27 (23.7) | < 0.001 | |
| Swelling | 122 (41.2) | 151 (47.6) | 46 (40.4) | 0.198 | |
| Systemic reactions (%) | |||||
| Fever | ≥ 38.0°C | 65 (22.0) | 83 (26.2) | 14 (12.3) | 0.009 |
| Duration, mean (95 % CI), days | 0.25 (0.19–0.31) | 0.31 (0.25–0.38) | 0.12 (0.06–0.18) | 0.003 | |
| Fatigue | 247 (83.5) | 269 (84.9) | 90 (79.0) | 0.347 | |
| Headache | 189 (63.9) | 215 (67.8) | 67 (58.8) | 0.202 | |
| Chills | 146 (49.3) | 167 (52.7) | 45 (39.5) | 0.054 | |
| Vomiting | N/A | 12 (3.8) | 3 (2.6) | 0.768b | |
| Diarrhea | 38 (12.8) | 40 (12.6) | 8 (9.3) | 0.222 | |
| Muscle Pain | 175 (59.1) | 271 (85.5) | 81 (71.1) | < 0.001 | |
| Joint Pain | 142 (48.0) | 172 (54.3) | 45 (39.5) | 0.021 | |
| Antipyretic use | |||||
| Before vaccination | 1 (0.3) | 20 (6.3) | 8 (7.0) | < 0.001 | |
| After vaccination | 152 (51.4) | 146 (46.1) | 56 (49.1) | 0.422 | |
IQR; inter-quartile range, GMT; geometric mean titer, CI; confidence interval, N/A; not available.
Data on the IgG titers before the bivalent booster were available from 86 participants.
Comparison between the monovalent and bivalent boosters.
A significant increase in post-vaccination IgG titers was noted for each successive dose. The GMTs of IgG were 9,093 AU/mL (95 % CI, 8,393–9,849) following the primary series, 16,971 AU/mL (95 % CI, 15,700–18,340) after the monovalent booster, and 24,871 AU/mL (95 % CI, 21,558–28,695) post-bivalent booster (p < 0.001). This increasing trend in IgG titers was consistent, even among vaccinees without a previous SARS-CoV-2 infection, increasing from 16,726 AU/mL (95 % CI, 15,463–18,088) post-monovalent booster to 21,242 AU/mL (95 % CI, 17,951–25,130) post-bivalent booster.
3.2. Adverse reaction frequency to the bivalent booster compared with the primary series and monovalent booster
The frequency of adverse reactions after the bivalent booster was assessed for comparison to those following the primary series and the monovalent booster (Table 1). Generally, the frequency of adverse reactions post-bivalent booster was found to be comparable to or lower than those reported for the preceding doses. Significant differences were noted in all local reactions except swelling, and some systemic reactions such as fever ≥ 38.0 °C, duration of fever, muscle pain, and joint pain. Notably, fever ≥ 38.0 °C incidence post-bivalent booster was markedly lower (12.3 %) compared to the primary series (22.0 %) and monovalent booster (26.2 %) (p < 0.001). The mean duration of fever was also shorter following the bivalent booster. Due to their low occurrence, vomiting and diarrhea were excluded from further analyses.
In the primary series, only one participant used antipyretics before vaccination, accounting for 0.3 % of the total. For the monovalent and bivalent boosters, the proportions of participants who used antipyretics before vaccination were 6.3 % and 7.0 %, respectively. No significant differences were observed in the frequency of all solicited adverse reactions, including fever, due to the use of antipyretics before vaccination (Table s2).
3.3. Comparison of adverse reaction severity after the bivalent and monovalent boosters
The adverse reaction severity after the monovalent and bivalent boosters was subsequently compared (Table 2). The mean severity grade for all solicited adverse reactions, except pain at the injection site and swelling, was significantly lower for the bivalent booster. This was especially notable for fever: the mean severity grade post-bivalent booster was 0.21 (95 % CI, 0.09–0.33), less than half of that observed for the monovalent booster (0.44 [95 % CI, 0.35–0.54], p = 0.003).
Table 2.
Comparison of adverse reaction severity between the monovalent and bivalent boosters.
| Monovalent booster (N = 317) | Bivalent booster (N = 114) | p-value | ||
|---|---|---|---|---|
| Local reactions (%) | ||||
| Pain at injection site | Mild (grade 1) | 127 (40.1) | 50 (43.9) | 0.480 |
| Moderate (grade 2) | 167 (52.7) | 52 (45.6) | 0.196 | |
| Severe (grade 3) | 20 (6.3) | 8 (7.0) | 0.694 | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 1.64 (1.58–1.71) | 1.56 (1.44–1.69) | 0.234 | |
| Redness | Mild (grade 1) | 96 (30.3) | 26 (22.8) | 0.129 |
| Moderate (grade 2) | 13 (4.1) | 1 (0.9) | 0.126 | |
| Severe (grade 3) | 0 (0) | 0 (0) | N/A | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 0.38 (0.32–0.45) | 0.25 (0.16–0.33) | 0.006 | |
| Swelling | Mild (grade 1) | 125 (39.4) | 39 (34.2) | 0.325 |
| Moderate (grade 2) | 26 (8.2) | 7 (6.1) | 0.478 | |
| Severe (grade 3) | 0 (0) | 0 (0) | N/A | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 0.56 (0.49–0.63) | 0.46 (0.35–0.58) | 0.178 | |
| Systemic reactions (%) | ||||
| Fever | 38.0 to 38.4°C (grade 1) | 45 (14.2) | 7 (6.1) | 0.024 |
| > 38.4 to 38.9°C (grade 2) | 25 (7.9) | 4 (3.5) | 0.110 | |
| > 38.9 to 40.0°C (grade 3) | 11 (3.5) | 3 (2.6) | 1.000 | |
| > 40.0°C (grade 4) | 3 (1.0) | 0 (0) | 0.569 | |
| Grade, mean (95 % CI) | 0.44 (0.35–0.54) | 0.21 (0.09–0.33) | 0.003 | |
| Fatigue | Mild (grade 1) | 100 (31.6) | 35 (30.7) | 0.868 |
| Moderate (grade 2) | 114 (36.0) | 46 (40.4) | 0.406 | |
| Severe (grade 3) | 54 (17.0) | 9 (7.9) | 0.018 | |
| Grade 4 | 1 (0.3) | 0 (0) | 1.000 | |
| Grade, mean (95 % CI) | 1.56 (1.45–1.66) | 1.35 (1.18–1.52) | 0.044 | |
| Headache | Mild (grade 1) | 91 (28.7) | 34 (29.8) | 0.822 |
| Moderate (grade 2) | 93 (29.3) | 27 (23.7) | 0.248 | |
| Severe (grade 3) | 31 (9.8) | 6 (5.3) | 0.140 | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 1.17 (1.06–1.28) | 0.93 (0.76–1.10) | 0.026 | |
| Chills | Mild (grade 1) | 65 (20.5) | 30 (26.3) | 0.199 |
| Moderate (grade 2) | 70 (22.1) | 14 (12.3) | 0.024 | |
| Severe (grade 3) | 32 (10.1) | 1 (0.9) | 0.002 | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 0.95 (0.83–1.07) | 0.54 (0.40–0.67) | < 0.001 | |
| Muscle Pain | Mild (grade 1) | 130 (41.0) | 50 (43.9) | 0.597 |
| Moderate (grade 2) | 118 (37.2) | 25 (21.9) | 0.003 | |
| Severe (grade 3) | 23 (7.3) | 6 (5.3) | 0.467 | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 1.37 (1.28–1.46) | 1.04 (0.88–1.19) | < 0.001 | |
| Joint Pain | Mild (grade 1) | 81 (25.6) | 30 (26.3) | 0.873 |
| Moderate (grade 2) | 71 (22.4) | 12 (10.5) | 0.006 | |
| Severe (grade 3) | 20 (6.3) | 3 (2.6) | 0.134 | |
| Grade 4 | 0 (0) | 0 (0) | N/A | |
| Grade, mean (95 % CI) | 0.89 (0.79–1.00) | 0.55 (0.41–0.70) | < 0.001 | |
| Use of medication | ||||
| Antipyretic use | No | 171 (53.9) | 58 (50.9) | 0.574 |
| Yes | 146 (46.1) | 56 (49.1) | ||
N/A; not available.
3.4. Difference in adverse reactions to bivalent booster based on SARS-CoV-2 infection history
Of the 114 participants who received the bivalent booster, 47 (41.2 %) reported a history of SARS-CoV-2 infection before vaccination. Analyzing the adverse reactions to the bivalent booster based on infection history, the incidence of fever ≥ 38.0 °C, although not statistically significant, was lower for those with a history of infection (6.4 %) compared to those without (16.4 %, p = 0.108) (Table s3). The severity of fever was significantly milder in those with a history of infection, with a mean severity grade of 0.09 (95 % CI: −0.02 to 0.19), compared to 0.30 (95 % CI: 0.11 to 0.48) for those without (p = 0.047). Notably, regardless of previous infection history, the bivalent booster demonstrated a lower incidence and severity of fever compared to the monovalent booster, which had a 26.2 % fever incidence and a mean severity grade of 0.44, as indicated in Table 1, Table 2. No significant differences were observed in the incidence or severity of other adverse reactions.
3.5. Recurrence of adverse reactions following successive vaccinations
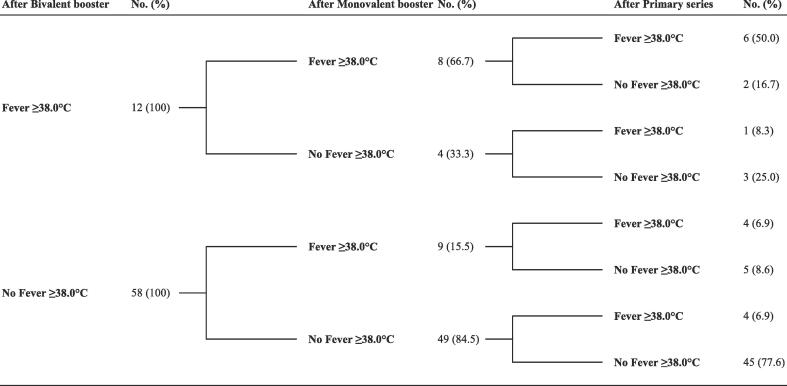
Using the data of 70 participants with complete records of adverse reactions for all doses, we analyzed the likelihood of a subsequent adverse reaction based on the incidence after a preceding dose (Table 3). The RRs for all solicited adverse reactions, where data were available, consistently exceeded 1.0. Statistically significant RRs for both the monovalent and bivalent boosters were observed for fever ≥ 38.0 °C, chills, and joint pain. Notably, the RRs for fever were the highest across the vaccine series, at 5.24 following the monovalent and 6.24 after the bivalent booster. A comprehensive observation across the vaccine series revealed that 50 % (6/12) of participants who experienced post-vaccination fever after the bivalent booster had also reported fever following all preceding vaccinations (Fig. 1). However, these participants constituted only 8.6 % (6/70) of the total cohort. Importantly, no recurrent cases of a subsequent high-grade fever (≥39.0 °C, grade 3 or 4) were observed following either booster.
Table 3.
Risk ratios for adverse reactions based on the incidences of a corresponding reaction to a previous vaccination.
|
Corresponding reaction after monovalent booster |
Incidence of a corresponding reaction after monovalent booster, % | RR | 95 % CI |
Corresponding reaction after bivalent booster |
Incidence of a corresponding reaction after bivalent booster, % | RR | 95 % CI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | |||||||||||||
| Local reactions | After primary series | After monovalent booster | ||||||||||||||
| Pain at injection site | Present | 64 | 0 | 100.0 | 1.20 | 0.84–1.72 | Present | 67 | 2 | 97.1 | N/A | N/A | ||||
| Absent | 5 | 1 | 83.3 | Reference | Reference | Absent | 0 | 1 | 0.0 | |||||||
| Redness | Present | 7 | 7 | 50.0 | 1.87 | 0.95–3.68 | Present | 9 | 13 | 40.9 | 1.79 | 0.87–3.68 | ||||
| Absent | 15 | 41 | 26.8 | Reference | Reference | Absent | 11 | 37 | 22.9 | Reference | Reference | |||||
| Swelling | Present | 18 | 13 | 58.1 | 1.42 | 0.88–2.29 | Present | 20 | 14 | 58.8 | 1.93 | 1.09–3.39 | ||||
| Absent | 16 | 23 | 41.0 | Reference | Reference | Absent | 11 | 25 | 30.6 | Reference | Reference | |||||
| Systemic reactions | ||||||||||||||||
| Fever | ≥ 38.0°C | Present | 10 | 5 | 66.7 | 5.24 | 2.40–11.42 | Present | 8 | 9 | 47.1 | 6.24 | 2.14–18.15 | |||
| Absent | 7 | 48 | 12.7 | Reference | Reference | Absent | 4 | 49 | 7.5 | Reference | Reference | |||||
| ≥ 39.0°C | Present | 0 | 1 | 0.0 | N/A | N/A | Present | 0 | 1 | 0.0 | N/A | N/A | ||||
| Absent | 1 | 68 | 1.4 | Reference | Reference | Absent | 3 | 66 | 4.3 | Reference | Reference | |||||
| Fatigue | Present | 55 | 4 | 93.2 | 1.71 | 0.99–2.94 | Present | 51 | 10 | 83.6 | 1.88 | 0.90–3.94 | ||||
| Absent | 6 | 5 | 54.5 | Reference | Reference | Absent | 4 | 5 | 44.4 | Reference | Reference | |||||
| Headache | Present | 37 | 7 | 84.1 | 1.82 | 1.18–2.81 | Present | 31 | 18 | 63.3 | 1.48 | 0.86–2.53 | ||||
| Absent | 12 | 14 | 46.2 | Reference | Reference | Absent | 9 | 12 | 42.9 | Reference | Reference | |||||
| Chills | Present | 18 | 10 | 64.3 | 1.69 | 1.05–2.71 | Present | 21 | 13 | 61.8 | 3.18 | 1.55–6.50 | ||||
| Absent | 16 | 26 | 38.1 | Reference | Reference | Absent | 7 | 29 | 19.4 | Reference | Reference | |||||
| Muscle Pain | Present | 30 | 4 | 88.2 | 1.13 | 0.92–1.40 | Present | 43 | 15 | 74.1 | 1.11 | 0.72–1.71 | ||||
| Absent | 28 | 8 | 77.8 | Reference | Reference | Absent | 8 | 4 | 66.7 | Reference | Reference | |||||
| Joint Pain | Present | 22 | 8 | 73.3 | 2.26 | 1.37–3.71 | Present | 20 | 15 | 57.1 | 1.82 | 1.03–3.21 | ||||
| Absent | 13 | 27 | 32.5 | Reference | Reference | Absent | 11 | 24 | 31.4 | Reference | Reference | |||||
RR, relative risk; CI, confidence interval; N/A, not available.
Fig. 1.
Comprehensive analysis across vaccine series for post-vaccination fever incidence based on previous fever response. This flowchart illustrates a comprehensive analysis of fever incidence (≥38.0℃) following the bivalent mRNA vaccine booster, structured according to previous fever responses to earlier vaccinations. It traces the fever history, beginning with the most recent bivalent booster and looking back through the monovalent booster to the primary series.
3.6. Correlation between adverse reactions and anti-spike IgG titers
This section examines correlations between the solicited adverse reactions and anti-spike IgG titers (Table 4). Univariate analyses revealed that post-vaccination fever—in terms of both instances exceeding 38.0 °C and duration—was the only reaction significantly correlated with the IgG titers across the entire vaccine series, distinguishing it from other reactions. This indicates a consistent link between post-vaccination fever and increased IgG titers. However, it should be noted that the correlation between the duration of fever and the IgG titers was weak in either dose, with correlation coefficients ranging from 0.237 to 0.288. Analysis of fold changes in the IgG titers showed no significant correlation with either local or systemic reactions.
Table 4.
Correlation between adverse reactions and anti-spike IgG titers.
|
Primary series |
p-value |
Monovalent booster |
p-value | Fold change, mean (95 % CI) | p-value |
Bivalent booster |
p-value | Fold change, mean (95 % CI) | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | GMT (95 % CI), AU/mL | No. (%) | GMT (95 % CI), AU/mL | No. (%) | GMT (95 % CI), AU/mL | |||||||||||||
| Local reactions | ||||||||||||||||||
| Pain at injection site | No | 18 (6.1) | 8,502 (5,281–13,687) | 0.759 | 3 (1.0) | 14,818 (4,032–54,450) | 0.738 | 23.89 (10.88–52.44) | 0.658 | 2 (2.3) | 18,690 (416–839,267) | 0.660 | 3.08 (0.48–19.68) | 0.630 | ||||
| Yes | 278 (93.9) | 9,131 (8,426–9,895) | 314 (99.1) | 16,990 (15,711–18,378) | 28.84 (26.58–31.30) | 84 (97.7) | 24,033 (20,202–28,596) | 4.38 (3.51–5.46) | ||||||||||
| Redness | No | 124 (79.4) | 8,892 (8,106–9,757) | 0.285 | 208 (65.6) | 16,893 (15,353–18,587) | 0.873 | 28.0 (25.30–31.0) | 0.353 | 66 (76.7) | 23,302 (19,489–27,868) | 0.599 | 4.35 (3.40–5.58) | 0.961 | ||||
| Yes | 61 (20.6) | 9,901 (8,486–11,553) | 109 (34.4) | 17,120 (14,949–19,602) | 30.35 (26.52–34.75) | 20 (23.3) | 25,948 (16,259–41,400) | 4.30 (2.67–6.93) | ||||||||||
| Swelling | No | 174 (58.8) | 8,925 (8,000–9,954) | 0.585 | 166 (52.4) | 17,045 (15,262–19,041) | 0.905 | 30.0 (26.95–33.38) | 0.296 | 50 (58.1) | 27,002 (22,009–33,128) | 0.092 | 4.21 (3.15–5.62) | 0.736 | ||||
| Yes | 122 (41.2) | 9,337 (8,304–10,498) | 151 (47.6) | 16,885 (15,125–18,854) | 27.52 (24.32–31.14) | 36 (41.9) | 20,160 (15,031–27,046) | 4.53 (3.23–6.35) | ||||||||||
| Systemic reactions | ||||||||||||||||||
| Fever | < 38.0°C | 231 (78.0) | 8,222 (7,528–8,983) | < 0.001 | 234 (73.8) | 15,318 (14,022–16,738) | < 0.001 | 27.61 (25.20–30.26) | 0.088 | 77 (89.5) | 22,423 (18,797–26,749) | 0.029 | 4.36 (3.44–5.53) | 0.890 | ||||
| ≥ 38.0°C | 65 (22.0) | 12,993 (11,069–15,248) | 83 (26.2) | 22,646 (19,561–26,218) | 32.39 (27.29–38.43) | 9 (10.5) | 41,115 (22,830–74,063) | 4.15 (2.64–6.52) | ||||||||||
| Duration | r = 0.288a | < 0.001 | r = 0.237a | < 0.001 | r = 0.103a | 0.067 | r = 0.244a | 0.009 | r = -0.010a | 0.928 | ||||||||
| Fatigue | No | 49 (16.6) | 6,433 (5,092–8,125) | 0.001 | 48 (15.1) | 13,571 (11,089–16,607) | 0.017 | 24.47 (19.20–31.19) | 0.095 | 17 (19.8) | 25,722 (17,993–36,771) | 0.671 | 5.71 (3.20–10.20) | 0.211 | ||||
| Yes | 247 (83.5) | 9,739 (8,972–10,571) | 269 (84.9) | 17,660 (16,241–19,204) | 29.63 (27.21–32.28) | 69 (80.2) | 23,464 (19,271–28,569) | 4.06 (3.21–5.12) | ||||||||||
| Headache | No | 107 (36.2) | 8,024 (6,953–9,260) | 0.020 | 102 (32.2) | 14,883 (13,152–16,842) | 0.022 | 28.35 (24.58–32.70) | 0.797 | 35 (40.7) | 20,716 (15,004–28,602) | 0.197 | 4.75 (3.37–6.69) | 0.496 | ||||
| Yes | 189 (63.9) | 9,759 (8,876–10,730) | 215 (67.8) | 18,059 (16,372–19,920) | 29.0 (26.27–32.02) | 51 (59.3) | 26,351 (21,837–31,798) | 4.08 (3.06–5.43) | ||||||||||
| Chills | No | 150 (50.7) | 7,588 (6,778–8,494) | < 0.001 | 150 (47.3) | 14,911 (13,421–16,562) | 0.002 | 26.72 (23.67–30.18) | 0.086 | 51 (59.3) | 24,121 (19,884–29,255) | 0.901 | 4.53 (3.35–6.13) | 0.639 | ||||
| Yes | 146 (49.3) | 10,947 (9,845–12,176) | 167 (52.7) | 19,063 (17,049–21,311) | 30.78 (27.62–34.30) | 35 (40.7) | 23,567 (17,073–32,531) | 4.08 (2.99–5.57) | ||||||||||
| Muscle pain | No | 121 (40.9) | 7,997 (7,032–9,095) | 0.008 | 46 (14.5) | 17,167 (14,151–20,831) | 0.904 | 29.25 (23.21–36.89) | 0.872 | 24 (27.9) | 20,946 (13,140–33,389) | 0.452 | 4.91 (2.93–8.22) | 0.486 | ||||
| Yes | 175 (59.1) | 9,936 (8,983–10,990) | 271 (85.5) | 16,936 (15,552–18,446) | 28.71 (26.33–31.31) | 62 (72.1) | 25,142 (21,375–29,573) | 4.14 (3.28–5.23) | ||||||||||
| Joint pain | No | 154 (52.0) | 8,422 (7,487–9,473) | 0.049 | 145 (45.7) | 16,413 (14,682–18,353) | 0.441 | 27.52 (24.20–31.28) | 0.313 | 55 (64.0) | 22,361 (17,832–28,041) | 0.305 | 4.45 (3.37–5.87) | 0.763 | ||||
| Yes | 142 (48.0) | 9,879 (8,878–10,993) | 172 (54.3) | 17,454 (15,649–19,463) | 29.91 (26.96–33.18) | 31 (36.1) | 26,872 (20,735–34,826) | 4.15 (2.90–5.96) | ||||||||||
| Antipyretic use | ||||||||||||||||||
| Before vaccination | No | 295 (99.7) | 9,097 (8,397–9,858) | N/A | 297 (93.7) | 17,065 (15,743–18,501) | 0.581 | 26.55 (22.91–30.78) | 0.700 | 80 (93.0) | 23,233 (19,404–27,816) | 0.234 | 4.46 (3.57–5.58) | 0.355 | ||||
| Yes | 1 (0.3) | N/A | 20 (6.3) | 15,599 (11,474–21,203) | 36.93 (3.06–445.96) | 6 (7.0) | 34,706 (22,151–54,375) | 3.00 (0.96–9.43) | ||||||||||
| After vaccination | No | 144 (48.7) | 8,549 (7,577–9,645) | 0.141 | 171 (53.9) | 16,669 (15,087–18,420) | 0.627 | 28.48 (25.40–31.93) | 0.777 | 41 (47.7) | 22,116 (16,757–29,188) | 0.391 | 4.56 (3.39–6.12) | 0.672 | ||||
| Yes | 152 (51.4) | 9,638 (8,668–10,715) | 146 (46.1) | 17,326 (15,321–19,593) | 29.15 (25.98–32.72) | 45 (52.3) | 25,639 (20,716–31,725) | 4.15 (3.0–5.74) | ||||||||||
GMT, geometric mean titer; CI, confidence interval; N/A, not available.
r values refer to the Spearman’s correlation coefficient.
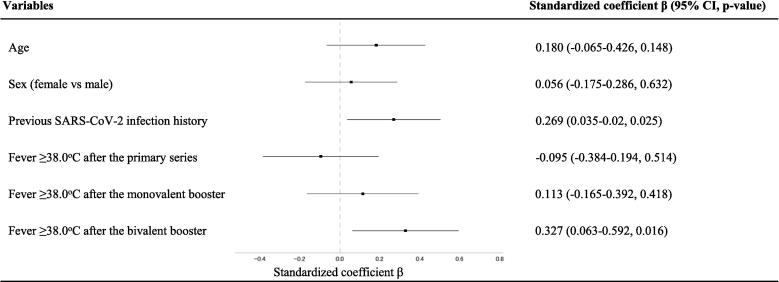
As detailed in Section 3.5, an association was found between the incidence of post-vaccination fever and its recurrence following a subsequent dose. This pattern raises the possibility that the observed correlation between post-vaccination fever and vaccine-induced IgG titers might be influenced by a history of previous post-vaccination fever. To evaluate this, we conducted a multivariate analysis, incorporating fever ≥ 38.0 °C occurrences post-primary series, monovalent booster, and bivalent booster as variables, with post-bivalent booster IgG titers as the outcome. Adjusting for age, sex, and SARS-CoV-2 infection history, the analysis showed that fever ≥ 38.0 °C following the bivalent booster was independently correlated with increased post-booster IgG titers (p = 0.016, standardized regression coefficient beta 0.327 [95 % CI: 0.063–0.592]), irrespective of previous fever occurrences (adjusted R2 0.126) (Fig. 2). Similarly, an independent correlation between post-vaccination fever and IgG titers was also identified in both the primary series and monovalent booster (Figs. s2 and s3).
Fig. 2.
Multivariate analysis of the correlation between anti-spike IgG titers post-bivalent mRNA vaccine booster and fever after the primary series, monovalent booster, and bivalent booster. The data of 70 participants were available for inclusion in the analysis. Forest plots depict the results of a multivariate regression analysis examining the correlation between anti-spike IgG titers post-bivalent mRNA vaccine booster and fever after the primary series, monovalent booster, and bivalent booster. The standardized coefficients β with 95 % Confidence Intervals (CI) and p-values represent the strength and direction of the association between post-bivalent booster IgG titers and the listed variables, after adjusting for covariates. The dotted vertical line denotes the point of no association (β = 0), with coefficients to the right that suggest a positive association and to the left suggesting a negative association.
4. Discussion
In this longitudinal cohort study, we monitored adverse reactions across 727 cumulative instances of individuals receiving successive SARS-CoV-2 mRNA vaccinations, including the primary series, monovalent booster, and BA.4/5 bivalent booster. We found a significant reduction in adverse reactions following the bivalent booster, with fever ≥ 38.0 °C occurring in 12.3 %, lower than 22.0 % and 26.2 % after the primary series and monovalent booster, respectively. These frequencies were comparable with data from the Ministry of Health, Labour and Welfare (MHLW) of Japan that reported fever ≥ 38.0 °C frequencies of 21.3 % for the primary series, 21.1 % for the monovalent booster, and 10.4 % for the bivalent booster [11]. It also aligns with the phase 2/3 BNT162b2 vaccine trial, which showed decreasing fever ≥ 38.0 °C incidences of 16.4 %, 8.7 %, and 4.9 % for the respective doses [12], [13], although these are generally lower than those observed in our study and the MHLW data from Japan. This consistency across various datasets suggests that the reduction in adverse reaction frequency with successive doses might be an inherent characteristic of COVID-19 mRNA vaccines. Additionally, our study indicates reduced severity of most solicited reactions for the bivalent booster compared to the monovalent booster. These findings are important for anticipating adverse reactions to future COVID-19 mRNA vaccine boosters. Starting in fall 2023, the FDA has recommended the use of an updated monovalent Omicron XBB.1.5-containing mRNA vaccine [14]. Our findings suggest that future successive vaccinations, including the XBB.1.5-adapted booster, might not induce more frequent or severe adverse reactions than observed in earlier doses.
Vaccine safety surveillance conducted by public health agencies and pharmaceutical companies typically involves large but distinct populations for each vaccine dose [11], [12], [13]. Such surveillance limits tracking individual-level changes in adverse reactions across vaccine series. Of note, our study monitored adverse reactions to the COVID-19 mRNA vaccine series within the same cohort, providing detailed data on sequential vaccinations for each participant. To our knowledge, this is the first study to longitudinally track changes in adverse reactions following the primary series, the wild-type monovalent booster, and the BA.4/5 bivalent booster. A notable finding was that fever above 38.0 °C had the highest RRs for the recurrence of the same adverse reaction after subsequent vaccination throughout the vaccine series, suggesting a strong association between previous post-vaccination fever and the likelihood of fever after subsequent doses. Nonetheless, repeated fever across all doses was rare, occurring in only 8.6 % (6/70) of our participants, with no instances of repeated high-grade fevers ≥ 39.0 °C. Furthermore, as described above, we observed a trend of reduced adverse reactions with each successive dose. Consequently, while a history of post-vaccination fever may suggest a potential for fever recurrence following a subsequent dose, such a history should not be a significant concern for individuals considering future SARS-CoV-2 mRNA vaccinations.
Although vaccine-related adverse reactions are generally viewed as unfavorable, there is a hypothesis suggesting that such reactions might indicate a successful immune response to COVID-19 mRNA vaccines [15]. Supporting this hypothesis, several studies have demonstrated associations between certain adverse reactions and increases in spike-specific and neutralizing antibody titers following SARS-CoV-2 mRNA vaccinations [16], [17]. Our previous research has further shown that among the solicited reactions, post-vaccination fever consistently exhibited an independent correlation with the anti-spike IgG titers in both the primary series and the monovalent booster dose [5], [6]. Extending these findings in this follow-up study of the bivalent booster, we identified a similar correlation between post-vaccination fever and anti-spike IgG titers. We also confirmed that this correlation remains independent for each vaccine dose, irrespective of previous fever episodes (Fig. 2, Figs. s2 and s3). Furthermore, in a related study of the same cohort, we noted an inverse correlation between anti-spike IgG titers and the risk of breakthrough infection during the Omicron-predominant period [18]. Taken together, these findings imply that fever following a SARS-CoV-2 mRNA vaccination may be linked to robust antibody production, potentially leading to enhanced protection against SARS-CoV-2 infection. However, the observed trend of reducing post-vaccination fever incidence, even as vaccine-induced IgG titers increase with each successive vaccination, prompts questions about the ongoing correlation between post-vaccination fever and specific antibody responses to upcoming booster doses. Further research is essential to determine whether this correlation will continue in subsequent vaccinations, particularly with the introduction of the novel XBB.1.5-adapted vaccine, which differs antigenically from the existing wild-type mRNA vaccines.
This study has several limitations. First, it involved a small cohort of young, healthy Japanese healthcare workers from a single hospital, potentially limiting the generalizability of our findings to broader populations. However, the observed adverse reaction trends were similar to those reported in larger studies [11], [12], [13], supporting the relevance of our results. Second, the bivalent booster group had a larger proportion of individuals with a prior SARS-CoV-2 infection compared to earlier vaccine groups, which could influence reactions and antibody responses. To address this, separate analyses were conducted based on prior infection history, which revealed a reduction in reactions with successive vaccinations, regardless of infection history. Multivariate analysis was also employed to adjust for the influence of prior infection history on the correlation between post-vaccination fever and anti-spike IgG titers. Third, the use of antipyretics was at the discretion of the participants, with 6–7 % reporting their use before receiving the monovalent and bivalent boosters. Although concerns exist that pre-vaccination antipyretic use might influence the emergence of adverse reactions, our previous research found no significant differences in the frequency of adverse reactions, including fever, based on antipyretic use before the monovalent booster [19]. Similar results were observed with the bivalent booster in this study as shown in Table s2, suggesting that the presence of participants who used antipyretics before vaccination likely had a minimal impact on the primary outcome. Finally, the anti-spike IgG titers measured in this study are specific to the wild-type strain, representing only part of the immunogenicity of the BA.4/5 bivalent mRNA vaccine. Despite this, we consistently found a correlation between post-vaccination fever and these IgG titers in the bivalent booster, similar to that observed with the wild-type monovalent vaccine doses. Additionally, these IgG titers have been shown to correlate not only with neutralizing antibody titers against the wild-type strain but also against the Omicron BA.5 variant [20], [21]. The specificity of this assay is unlikely to considerably affect the validity of our findings regarding the potential link between fever after SARS-CoV-2 mRNA vaccination and specific antibody responses.
5. Conclusions
In this longitudinal cohort study examining adverse reactions to the monovalent mRNA vaccine doses and the subsequent BA.4/5 bivalent mRNA vaccine booster, we confirmed a reduction in both the frequency and severity of adverse reactions following the second booster with the bivalent vaccine, compared to those after the primary series and the first monovalent booster. Our sequential analysis of individual-level changes in adverse reactions across the vaccine series indicates that a history of post-vaccination fever is associated with an increased likelihood of fever recurrence following a subsequent dose. However, it is important to note that recurrent fever was relatively infrequent among our participants, with no recurrent cases of a subsequent high-grade fever exceeding 39.0 °C reported. Additionally, we consistently observed a positive correlation between post-vaccination fever and vaccine-induced spike-specific IgG titers for each dose, and notably, this correlation was unaffected by any fever experienced after preceding doses.
Funding
This work was supported by our own resources.
CRediT authorship contribution statement
Naoki Tani: Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Hideyuki Ikematsu: Writing – review & editing, Project administration, Methodology, Conceptualization. Haruka Watanabe: Writing – review & editing. Takeyuki Goto: Writing – review & editing. Yuki Yanagihara: Investigation. Yasuo Kurata: Investigation. Yukiko Harada: Writing – review & editing, Investigation. Takahiko Horiuchi: Writing – review & editing, Supervision. Koichi Akashi: Writing – review & editing, Supervision. Nobuyuki Shimono: Writing – review & editing, Supervision. Yong Chong: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all of the staff members of Fukuoka City Hospital for their support for sample collection and data reduction.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100489.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 2020; 383:2603–2615. Available at: Doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed]
- 2.Busà R., Sorrentino M.C., Russelli G., et al. Specific anti-SARS-CoV-2 humoral and cellular immune responses after booster dose of BNT162b2 Pfizer-BioNTech mRNA-based vaccine: Integrated study of adaptive immune system components. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.856657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Ibarguengoitia ME, Rivera-Salinas D, Hernández-Ruíz YG, et al. Effect of the third dose of BNT162b2 vaccine on quantitative SARS-CoV-2 spike 1–2 IgG antibody titers in healthcare personnel. PLoS One 2022; 17:e0263942-. Available at: Doi: 10.1371/journal.pone.0263942. [DOI] [PMC free article] [PubMed]
- 4.Kim MS, Jung SY, Ahn JG, et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: A pharmacovigilance analysis using WHO international database. J Med Virol 2022; 94:1085–1095. Available at: Doi: 10.1002/jmv.27424. [DOI] [PMC free article] [PubMed]
- 5.Tani N., Chong Y., Kurata Y., et al. Relation of fever intensity and antipyretic use with specific antibody response after two doses of the BNT162b2 mRNA vaccine. Vaccine. 2022;40:2062–2067. doi: 10.1016/j.vaccine.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tani N, Ikematsu H, Goto T, et al. Correlation of Postvaccination Fever With Specific Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 BNT162b2 Booster and No Significant Influence of Antipyretic Medication. Open Forum Infect Dis 2022; 9:ofac493. Available at: Doi: 10.1093/ofid/ofac493. [DOI] [PMC free article] [PubMed]
- 7.Pillsbury A.J., Glover C., Jacoby P., et al. Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia. BMJ Open. 2018;8:e023263. doi: 10.1136/bmjopen-2018-023263. http://bmjopen.bmj.com/content/8/10/e023263.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA. 2.12. 1, BA. 4, and BA. 5. New England Journal of Medicine 2022; 387:86–88. [DOI] [PMC free article] [PubMed]
- 9.Hause A.M., Marquez P., Zhang B., et al. Safety monitoring of bivalent COVID-19 mRNA vaccine booster doses among persons aged≥ 12 years—United States, August 31–October 23, 2022. Morb Mortal Wkly Rep. 2022;71:1401. doi: 10.15585/mmwr.mm7144a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Available at: https://www.fda.gov/media/73679/download. Accessed on January 1, 2024. [DOI] [PubMed]
- 11.Survey of Health Status Following Booster Vaccination with Bivalent Omicron-adapted Vaccine. Available at: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_kenkoujoukyoutyousa_bivalent.html. Accessed on January 1, 2024.
- 12.BNT162b2 VRBPAC Briefing Document. Available at: https://www.fdanews.com/ext/resources/files/2021/VRBPAC-091721-Meeting-Briefing.pdf?1631743724. Accessed on January 1, 2024.
- 13.COMIRNATY® (COVID-19 Vaccine mRNA) suspension for injection for intramuscular use. Available at: https://labeling.pfizer.com/ShowLabeling.aspx?id=16351&format=pdf. Accessed on January 1, 2024.
- 14.Updated COVID-19 Vaccines for Use in the United States Beginning in Fall 2023. Available at: https://www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2023. Accessed on January 1, 2024.
- 15.Sprent J., King C. COVID-19 vaccine side effects: The positives about feeling bad. Sci Immunol. 2021;6:eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. The Lancet regional health Europe 2021; :100208. [DOI] [PMC free article] [PubMed]
- 17.Siangphoe U, Baden LR, El Sahly HM, et al. Associations of Immunogenicity and Reactogenicity After Severe Acute Respiratory Syndrome Coronavirus 2 mRNA-1273 Vaccine in the COVE and TeenCOVE Trials. Clinical Infectious Diseases 2023; 76:271–280. Available at: Doi: 10.1093/cid/ciac780. [DOI] [PMC free article] [PubMed]
- 18.Tani N., Ikematsu H., Goto T., et al. Correlation between specific antibody response to wild-type BNT162b2 booster and the risk of breakthrough infection with omicron variants: Impact of household exposure in hospital healthcare workers. Vaccine. 2023 doi: 10.1016/j.vaccine.2023.09.051. [DOI] [PubMed] [Google Scholar]
- 19.Tani N., Ikematsu H., Goto T., et al. No significant influence of pre-vaccination antipyretic use on specific antibody response to a BNT162b2 vaccine booster against COVID-19. Vaccine X. 2022;12 doi: 10.1016/j.jvacx.2022.100224. https://www.sciencedirect.com/science/article/pii/S2590136222000845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bormann M, Brochhagen L, Alt M, et al. Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA. 1 and Omicron-BA. 5. Front Immunol 2023; 14. [DOI] [PMC free article] [PubMed]
- 21.Goto T., Chong Y., Tani N., et al. Distinct features of SARS-CoV-2 humoral immunity against Omicron breakthrough infection. Vaccine. 2023;41:7019–7025. doi: 10.1016/j.vaccine.2023.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.