Abstract
Background and Aims
Tofacitinib (TFB) appears to be effective in the treatment of ulcerative colitis (UC); however, available real-world studies are limited by cohort size. TFB could be an option in the treatment of acute severe ulcerative colitis (ASUC). We aimed to investigate efficacy and safety of TFB in moderate-to-severe colitis and ASUC.
Methods
This retrospective, international cohort study enrolling UC patients with ≥6-week follow-up period was conducted from February 1 to July 31, 2022. Indications were categorized as ASUC and chronic activity (CA). Baseline demographic and clinical data were obtained. Steroid-free remission (SFR), colectomy, and safety data were analyzed.
Results
A total of 391 UC patients (median age 38 [interquartile range, 28-47] years; follow-up period 26 [interquartile range, 14-52] weeks) were included. A total of 27.1% received TFB in ASUC. SFR rates were 23.7% (ASUC: 26.0%, CA: 22.8%) at week 12 and 41.1% (ASUC: 34.2%, CA: 43.5%) at week 52. The baseline partial Mayo score (odds ratio [OR], 0.850; P = .006) was negatively associated with week 12 SFR, while biologic-naïve patients (OR, 2.078; P = .04) more likely achieved week 52 SFR. The colectomy rate at week 52 was higher in ASUC group (17.6% vs 5.7%; P < .001) and decreased with age (OR, 0.94; P = .013). A total of 67 adverse events were reported, and 17.9% resulted in cessation of TFB. One case of thromboembolic event was reported.
Conclusions
TFB is effective in both studied indications. TFB treatment resulted in high rates of SFR in the short and long terms. Higher baseline disease activity and previous biological therapies decreased efficacy. No new adverse event signals were found.
Keywords: tofacitinib, acute severe ulcerative colitis, moderate-to severe ulcerative colitis
Key Messages.
What is already known?
In real-life cohorts, data are lacking regarding the efficacy and safety of the orally administered tofacitinib in ulcerative colitis; therefore, we conducted an international, multicenter cohort study. Efficacy is already shown in patients with chronic activity; however, there is a paucity of data on use of tofacitinib in acute severe ulcerative colitis (ASUC). In addition, our data confirm that biologic-naïve patients have better response to the therapy. Based on our data, tofacitinib without other risk factors does not increase thromboembolic events.
What is new here?
Based on our results, tofacitinib is efficacious in ASUC colitis as well. Higher baseline clinical activity, older age, and male sex may decrease efficacy.
How can this study help patient care?
Tofacitinib could be used as a rescue treatment in ASUC. Tofacitinib admission should be considered as a first-line treatment.
Introduction
Advances in therapeutic targets, monitoring tactics, and biologic agents with targeted mechanisms of action have not yet brought the therapeutic breakthrough in the treatment of ulcerative colitis (UC) that we had hoped for. This is partly explained by the lack of predictive markers, and thus inadequate therapeutic choice; partly by the limited use of the multiomic approach, but especially for newer therapies; and partly by the insufficient number of patients, and thus incomplete data.
The orally administered small molecule innovative targeted tofacitinib (TFB) is effective as a monotherapy in biologic-naïve UC patients, but due to its cost and the different funding rules between countries, it is used more frequently in the second line.1,2 Based on meta-analyses, the pan-JAK inhibitor TFB3 appears to be effective in UC also in a higher therapeutic line.2,4,5 TFB is a fast-acting treatment, with short half-life; consequently, in addition to chronic refractory UC, TFB seems to be effective in acute severe UC (ASUC) as well.6-9 This is supported by previous findings, in which TFB was proved to be effective in steroid-refractory ASUC in biologic-experienced patients.6,7,10,11 However, safety concerns have been raised,12 notably the higher incidence of deep vein thrombosis and pulmonary embolism compared with tumor necrosis factor α inhibitors13; nonetheless, the higher incidence of thrombotic events in patients with rheumatoid arthritis was not reproduced in UC studies.14 Both safety and efficacy data are lacking in real-life clinical studies.
For this reason, we organized an international collaboration to conduct the largest-ever retrospective study to collect patient numbers with targeted outcome data. We aimed to study steroid-free remission (SFR), colectomy-free survival, primary nonresponse, and loss of response in different indications of UC (ASUC and chronic activity [CA]). In addition, we aimed to identify predictive factors of efficacy in different outcomes and to assess the prevalence of adverse events (AEs).
Methods
Study Design and Settings
A retrospective, international, multicenter cohort study was conducted including 23 tertiary referral centers in Europe, Canada, and Israel. Data collection with collaborating centers was performed between February 1 and July 31, 2022. All investigators had to complete the unified database via medical record systems. The treatment protocols differ between the centers due to various funding protocols in different countries; consequently, tofacitinib is not allowed as a rescue treatment in ASUC or as a first-line treatment in UC in some of the involved centers. All centers were tertiary referral centers.
Participants
Consecutive patients with UC ≥18 years of age receiving tofacitinib were enrolled in our cohort. The indication of TFB was classified in to ASUC according to the Truelove-Witts criteria15 or CA/steroid dependence. Patients with a follow-up duration of <6 weeks were excluded. In addition, patients with previous colectomy + ileal pouch–anal anastomosis or not obvious indication (marked as other) were excluded from further analysis.
Data Collections
The baseline demographic and clinical information comprised gender, age at UC diagnosis, date of UC diagnosis, disease extension, and severity at diagnosis. The disease phenotype was evaluated based on the Montreal classification.16 Clinical data comprised previous colectomy, extraintestinal manifestations, previous biological treatments (infliximab [IFX], adalimumab, golimumab, vedolizumab, ustekinumab [UST]), concomitant corticosteroid or thiopurine therapy, and indication of tofacitinib induction. Clinical (partial Mayo score [pMayo]), endoscopic (Mayo endoscopic score [eMayo]), and biochemical activity (C-reactive protein [CRP] and fecal calprotectin) and laboratory parameters (hemoglobin, serum iron, ferritin, transferrin saturation, thrombocyte count, cholesterol, triglyceride, and liver enzymes [aspartate aminotransferase, alanine aminotransferase, γ-glutamyl transferase [GGT], and alkaline phosphatase]) were collected at week 0, weeks 2 to 6, weeks 8 to 14, weeks 22 to 30, and weeks 48 to 56.
The dose of TFB, the number and the type of AEs, the need of corticosteroid and/or immunomodulator treatment, hospitalization, and colectomy rates were obtained during the follow-up period as well.
Outcomes
The primary outcome was SFR rates at weeks 12 and 52. SFR was defined as a pMayo <2 and CRP <5 mg/L with no rectal bleeding and with no concomitant corticosteroid therapy.
Secondary outcomes were colectomy rates at weeks 12 and 52, primary nonresponse (PNR) rates, loss-of-response (LOR) rates, and persistence of the treatment. PNR was defined as <30% decrease in pMayo or ≥2 bleeding score at week 12, and LOR was defined as need of dose optimization or prolonged induction dose. We compared the secondary outcomes in ASUC and CA. The predictors of SFR, PNR, LOR, and colectomy-free survival rates were analyzed.
The change in the clinical endoscopic activity index (pMayo, eMayo [endoscopic remission was defined as eMayo ≤1]), laboratory parameters monitoring disease activity, iron homeostasis, lipid metabolism, and liver enzymes were compared between visits during the admission of TFB. Liver enzyme elevation was considered as a level at least 1.5 times as high as normal.
The severity of infections was graded by the need of antibiotic or antiviral therapy. Severity of an AE was classified by the need of cessation of the treatment or dose reduction of therapy. AEs were also analyzed in the relation with concomitant steroid therapy. Definitions are shown in the Supplementary Appendix.
Statistical Methods
Statistical tests were performed using R statistical software version 4.1.1 (R Foundation for Statistical Computing) and SPSS software version 24 (IBM Corporation). Descriptive statistics are interpreted as median and interquartile range (IQR) or mean ± SD for continuous variables or numbers with percentage for categorical variables. Handling missing variables, the outcomes were analyzed with the intention-to treat viewpoint. Change in continuous variables during the follow-up period was assessed with repeated-measures analysis of variance. Pearson’s chi-square tests were performed to determine difference in frequency of categorical data. After identification of possible predictive factors of primary outcomes and secondary outcomes (colectomy, and PNR and LOR rates), multivariate logistic regression models were constructed (overall model fit was assessed by the Nagelkerke R2 and goodness of fit was determined by performing the Hosmer-Lemeshow test). Kaplan-Meyer analysis was performed to describe persistence on therapy and to compare the week 52 colectomy-free survival rate between ASUC and CA groups. Patients with incomplete follow-ups were included in the analysis as censored data. Values of P < .05 were considered statistically significant.
Ethical Approval
Ethical approval for the study was obtained from the Regional and Institutional Human Medical Biological Research Ethics Committee, University of Szeged (39/2022-SZTE RKEB; 5153). The research was carried out according to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Results
Baseline Characteristics
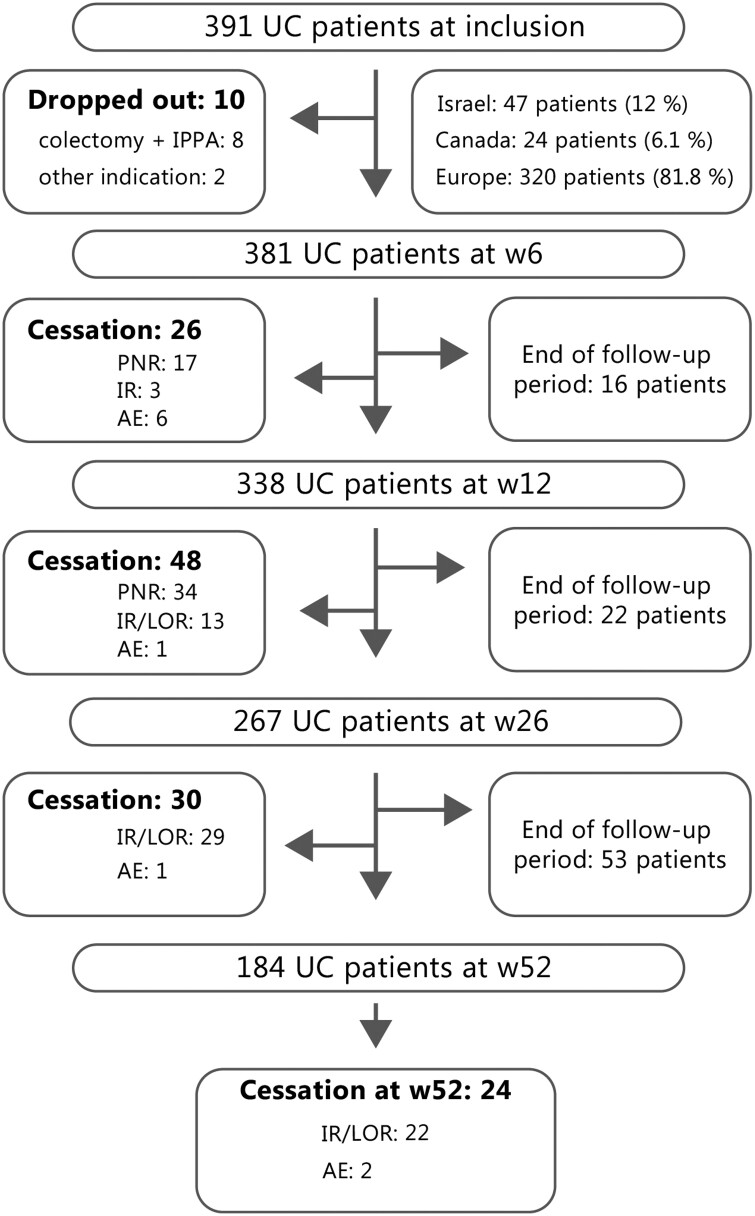
In total, 391 UC patients (male-to-female ratio of 208:183; median follow-up period of 26 [IQR, 14-52] weeks) were included. A total of 47 (12.0%) patients were from Israel, 24 (6.1%) were from Canada, and 320 (81.8%) were from Europe. The median age was 38 (IQR, 28-47) years and the median duration of disease was 7 (IQR, 4-12) years. The flowchart of included patients is presented in Figure 1.
Figure 1.
Study flowchart of included patients. AE, adverse event; IPAA, ileal pouch–anal anastomosis; IR, ineffective response; LOR, loss of response; PNR, primary nonresponse; UC, ulcerative colitis; w, week.
Almost two-thirds of the patients had pancolitis (64.7%). The main indications of TFB were CA (70.1%) and ASUC (27.1%). Further 2.8% of patients received TFB marked as other indication, specified as colectomy + ileal pouch–anal anastomosis surgeries in 2.6% of the patients; thus, they received TFB due to pouchitis, and a further 0.2% was not specified. All patients received an induction dose of 20 mg/d. The vast majority of the cohort had received anti-tumor necrosis factor treatment previously (83.6%; IFX: 74.9%, adalimumab: 39.1%, and golimumab: 0.8%), and two-thirds of the patients had received vedolizumab (64.2%). TFB was used in biologic-naïve patients in 11.8% of patients. Baseline demographic characteristics by groups are summarized in Table 1.
Table 1.
Baseline demographic and clinical data of the cohort.
| Characteristics | Total number of patients (n = 391) | ASUC (n = 106) | Chronic activity (n = 274) |
|---|---|---|---|
| Age, y | 38 (28-47) | 38 (29-48) | 37 (28-46) |
| Male | 208 (53.2) | 45 (42.5) | 156 (56.9) |
| pMayo | 7 (5-8) | 7 (5-8) | 6 (5-8) |
| eMayo | 3 (2-3) | 3 (3-3) | 3 (2-5) |
| CRP, mg/L | 19.3 ± 26.6 | 35.3 ± 36.82 | 13.5 ± 18.72 |
| Fecal calprotectin, µg/g | 1559.7 ± 1345.9 | 2046.5 ± 1282.7 | 1361.9 ± 1351.8 |
| Disease duration, y | 7 (4-12) | 8 (5-12) | 7 (3-12) |
| Follow-up period, wk | 26 (14-52) | 26 (14-52) | 39 (14-52) |
| Disease extensiona | |||
| Proctitis | 10 (2.6) | 3 (2.8) | 7 (2.6) |
| Left-sided colitis | 125 (32.7) | 34 (32.1) | 90 (32.8) |
| Pancolitis | 247 (64.7) | 69 (65.1) | 177 (64.6) |
| Previous colectomy + IPAA | 9 (2.3) | — | — |
| Follow-up period, wk | 26 (14-52) | 26 (14-52) | 39 (17-52) |
| Baseline concomitant treatment | |||
| 5-ASA | 206 (52.6) | 55 (51.9) | 148 (54.0) |
| Budesonide-MMX | 58 (14.8) | 20 (18.9) | 36 (13.1) |
| Azathioprine | 17 (4.3) | 5 (4.7) | 11 (4.0) |
| Methotrexate | 7 (1.8) | 2 (1.9) | 5 (1.8) |
| Cyclosporine | 6 (1.5) | 1 (0.9) | 5 (1.8) |
| Prednisolone/methylprednisolone | 179 (45.7) | 52 (49.1) | 123 (44.9) |
| Previous biologic treatments | |||
| IFX | 293 (74.9) | 74 (69.8) | 209 (76.3) |
| ADA | 153 (39.1) | 37 (34.9) | 109 (39.8) |
| GOL | 3 (0.8) | 1 (0.9) | 2 (0.7) |
| VDZ | 251 (64.2) | 64 (60.4) | 179 (65.3) |
| UST | 25 (6.4) | 9 (8.5) | 13 (4.7) |
| Therapeutic line | |||
| Biologic naive | 46 (11.8) | 21 (19.8) | 25 (9.1) |
| Second line | 89 (22.8) | 22 (20.8) | 65 (23.7) |
| Third line | 147 (37.6) | 31 (29.2) | 113 (41.2) |
| Fourth line | 97 (24.8) | 28 (26.4) | 65 (23.7) |
| Fifth line | 12 (3.1) | 4 (3.8) | 6 (2.2) |
| Extraintestinal manifestation | 85 (21.7) | 22 (20.8) | 63 (23.0) |
| Arthritis | 57 (14.6) | 15 (14.2) | 40 (14.6) |
| Erythema nodosum/pyoderma gangrenosum | 7 (1.8) | 1 (0.9) | 4 (1.5) |
| Uveitis | 2 (0.5) | 1 (0.9) | 1 (0.4) |
| PSC | 16 (4.1) | 1 (0.9) | 13 (4.7) |
Values are median (interquartile range), n (%), or mean ± SD.
Abbreviations: ADA, adalimumab; ASUC, acute severe ulcerative colitis; CRP, C-reactive protein; eMayo, Mayo endoscopic score; GOL, golimumab; IFX, infliximab; IPAA, ileal pouch–anal anastomosis; pMayo, partial Mayo score; PSC, primary sclerosing cholangitis; UST, ustekinumab; VDZ, vedolizumab; 5-ASA, 5-acetylsalicylic acid.
aMontreal classification.
Baseline clinical activity indexes were the following: median pMayo was 7 (IQR, 5-8) and median eMayo was 3 (IQR, 2-3). Biochemical markers showed elevated CRP (19.3 ± 26.6 mg/L) and fecal calprotectin (measured in 189 [48.3%] patients; mean 1559.7 ± 1356.0 µg/g). At the initiation of TFB, 45.7% of patients were on systemic corticosteroids. The change in clinical indexes and laboratory parameters are summarized in the Supplementary Appendix.
Corticosteroid-Free Remission Rates
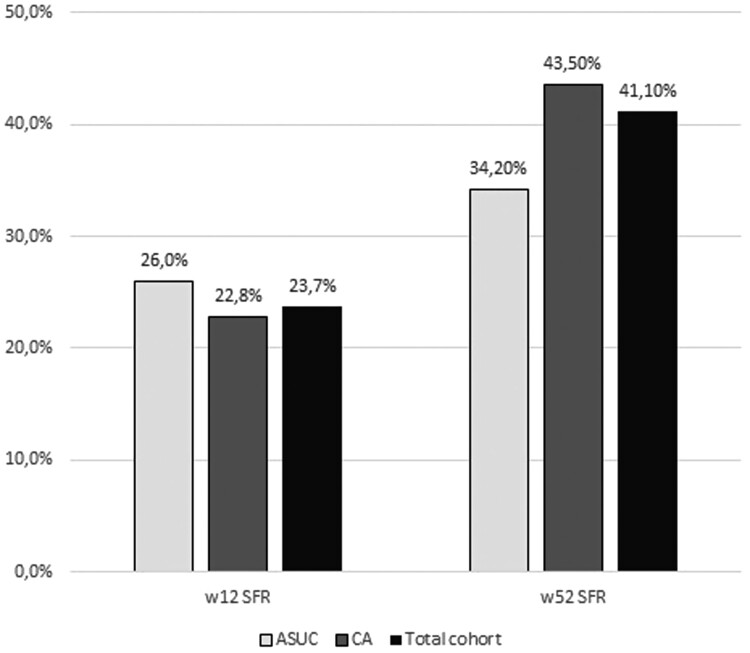
In total, 81 (23.7%) patients achieved SFR at week 12 and 117 (41.1%) patients achieved SFR at week 52. The SFR rates at week 12 were 26.0% in the ASUC group and 22.8% in the CA group, and no difference was observed between groups (P = .522). The SFR rates at week 52 were 34.2% in the ASUC group and 43.5% in the CA group, and no difference was observed between groups (P = .352). The sustained steroid-free clinical remission rate was 17.9% (Figure 2).
Figure 2.
Week 12 (w12) and w52 steroid-free remission (SFR) rates in our cohort. ASUC, acute severe ulcerative colitis; CA, chronic activity.
In the total cohort, a higher pMayo at baseline was negatively associated with the week 12 SFR (odds ratio [OR], 0.850; P = .006), such as in the ASUC group (OR, 0.765; P = .012). In the CA group, male sex (OR, 0.503; P = .04) and baseline CRP (OR, 0.962; P = .031) decreased the week 12 SFR rates (Table 2).
Table 2.
Logistic regression to predict week 12 and 52 SFR, and week 52 colectomy rate in the cohorta.
| OR | SE | z | P value | 95% CI | |||
|---|---|---|---|---|---|---|---|
| SFR predictive factors | |||||||
| Week 12 | ASUC | Constant | 1.658 | 0.682 | 0.741 | .459 | 0.426-6.394 |
| Baseline pMayo | 0.765 | 0.107 | -2.502 | .012b | 0.616-0.940 | ||
| CA | Constant | 0.589 | 0.300 | -1.763 | .078 | 0.324-1.058 | |
| Male | 0.503 | 0.334 | -2.058 | .040b | 0.260-0.967 | ||
| Baseline CRP | 0.962 | 0.018 | -2.163 | .031b | 0.926-0.992 | ||
| Total cohort | Constant | 0.785 | 0.367 | -0.658 | .510 | 0.377-1.602 | |
| Baseline pMayo | 0.850 | 0.059 | -2.764 | .006b | 0.756-0.954 | ||
| Week 52 | ASUC | Constant | 0.341 | 0.299 | -3.599 | <.001b | 0.190-0.613 |
| Biologic naive | 5.378 | 0.589 | 2.856 | .004b | 1.695-17.061 | ||
| CA | Constant | 0.290 | 0.434 | -2.855 | .004b | 0.124-0.678 | |
| Age | 1.026 | 0.011 | 2.415 | .016b | 1.005-1.047 | ||
| Total cohort | Constant | 0.632 | 0.130 | -3.525 | <.001b | 0.489-0.815 | |
| Biologic naive | 2.078 | 0.357 | 2.052 | .040b | 1.033-4.180 | ||
| Colectomy rate predictive factors | |||||||
| Week 52 | Total cohort | Constant | 0.374 | 0.763 | -1.288 | .198 | 0.084-1.670 |
| ASUC | 4.829 | 0.501 | 3.144 | .002b | 1.810-12.886 | ||
| Age | 0.946 | 0.022 | -2.482 | .013b | 0.906-0.988 | ||
Abbreviations: ASUC, acute severe ulcerative colitis; CA, chronic activity; CI, confidence interval; eMayo, Mayo endoscopic score; OR, odds ratio; pMayo, partial Mayo score; SFR, steroid-free remission.
aAfter identification of possible predictive factors, multivariate logistic regression models were constructed.
b P < .05 was considered as statistically significant result.
Both in the ASUC group (OR, 5.378; P = .004) and in the total cohort (OR, 2.078; P = .04), biologic-naïve patients more likely achieved week 52 SFR, while in the CA group older age (OR, 1.026; P = .016) seemed to be beneficial (Table 2). A higher proportion of patients achieved 52-week SFR who received TFB in lower therapeutic line, close to the significance level (P = .061) (Supplementary Appendix).
Colectomy Rates, PNR, LOR, and Persistence of Therapy
In total, the colectomy rate was 4.6% at week 12, and no predictive factor was identified. In the ASUC group, the colectomy rate was higher (7.5% compared with 3.5% in the CA group); however, the difference was not significant (P = .115). No factor was found to be associated with the colectomy rate either in the ASUC or in the CA group.
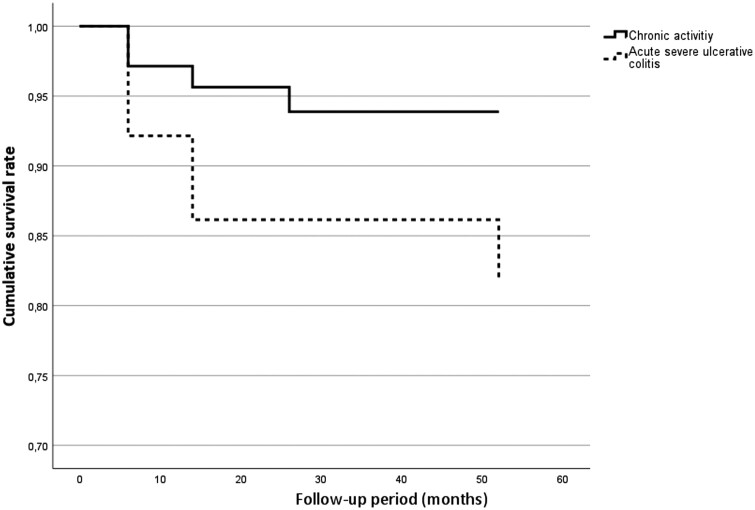
The week 52 colectomy rate was 8.0% in total, and the colectomy rate was significantly higher in the ASUC group compared with the CA group (17.6% vs 5.7%; P = .005). Based on the survival analysis (Figure 3), more patients had colectomy during the follow-up period in the ASUC group (P = .008). In the total cohort, ASUC indication (OR, 4.829; P < .001) increased the colectomy rate at week 52, whereas older age decreased it (OR, 0.946; P = .013). No specific predictive factors were identified in the CA and ASUC groups (Table 2).
Figure 3.
Survival analysis regarding week 52 colectomy rates between the chronic activity and acute severe ulcerative colitis groups (P = .06).
The PNR rate was 21.5% in the total cohort. The frequency of PNR was higher in the ASUC group (36.5% compared with 24.5%); however, it was not significant (P = .175). No marker was found to be associated with higher a PNR rate.
In total, the prevalence of LOR was 54.1%, and it was more common in the CA group (58.5%) compared with the ASUC group (41.0%) (P = .006). In the LOR group, dose optimization was effective in 69 (37.5%) patients. No predictive factor was identified. At week 52, the dosage was distributed as follows: 5 mg/d in 7 (3.9%) patients, 10 mg/d in 94 (52.8%) patients, 20 mg/d in 75 (42.1%) patients, 25 mg/d in 1 (0.6%) patient, and 30 mg/d 1 in (0.6%) patient.
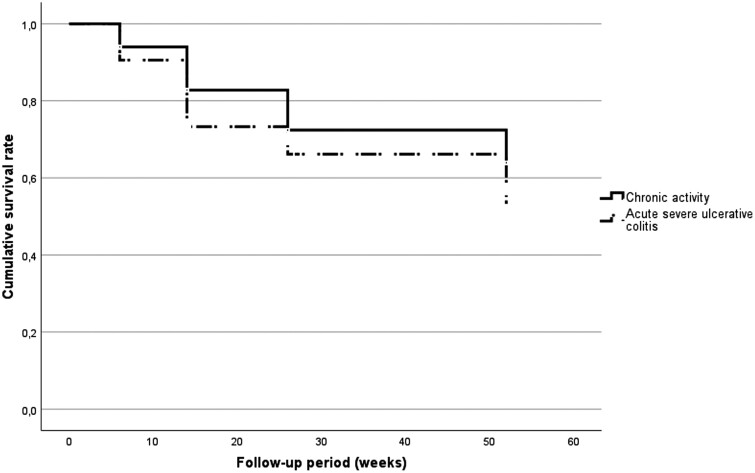
Patients with a CA indication seemed to remain on TFB for a longer period of time (35.3 ± 17.7 weeks) compared with patients with ASUC (29.6 ± 18.8 weeks) (P = .07) (Figure 4).
Figure 4.
Drug persistence between various indications of tofacitinib (P = .056).
Change in Clinical and Biochemical Activity
The clinical activity index decreased significantly (P < .001) from a baseline level of 6.2 ± 2.2 to 3.5 ± 2.7 at week 12 and 1.8 ± 2.1 at week 52. Changes in pMayo are presented in the Supplementary Appendix.
Markers of biochemical activity decreased significantly during the treatment period. Baseline CRP was 19.34 ± 26.59 µg/mL, and it decreased to 10.15 ± 18.86 µg/mL at week 12 and 6.42 ± 11.46 µg/mL at week 52 (P < .001). Fecal calprotectin decreased from a baseline level of 1559.7 ± 1355.96 mg/g to 663.73 ± 654.08 mg/g at week 12 and 313.76 ± 401.47 mg/g at week 52 (P < .001).
The level of serum albumin increased (P < .001) as well during the treatment, from a baseline level of 38.6 ± 8.7 g/L to 43.1 ± 6.1 g/L at week 52. An increase was already observed after 6 weeks (P < .001), as albumin was 41.2 ± 5.4 g/L. Furthermore, the platelet level altered during the treatment (P = .002), as it decreased from a baseline level of 372.9 ± 136.0 g/L to 311.2 ± 109.8 g/L at week 52. The changes in laboratory parameters are presented in Supplementary Appendix.
Endoscopic Response
Colonoscopy was performed in 312 (79.8%) cases before and in 242 cases after the admission of TFB (mean 22.5 ± 16.1 weeks). In total, 12 (3.9%) patients were in endoscopic remission before TFB treatment, whereas 92 (43.0%) patients were in remission at the follow-up endoscopy, based on eMayo (P < .001). Changes in eMayo are presented in the Supplementary Appendix.
Adverse Events
In total, 67 (17.1%) AEs were reported during the study. Based on our definition, 17.9% of them (12 cases) were severe AEs (3.1% of the total cohort). In 6 (50.0%) cases, the cessation of TFB was due to severe infection (2 herpes zoster, 1 pneumonia, 1 perianal abscess, 1 carbuncle, and 1 Clostridioides difficile infection), while in the other half of the cases, it was dyspnea (with no evidence of thromboembolic complications), vertigo, nausea, gastric pain, and skin rash (not specified as urticaria).
A total of 25 (6.4%) patients had an infection during TFB. The 3 most common infectious diseases were herpes zoster (24.0%), pneumonia (16.0%), and Clostridioides difficile infection (16.0%). The infection rate was not influenced by concomitant steroid therapy (P = .847).
Two (0.5%) patients were reported to have malignant diseases (melanoma and cholangiohepatic adenocarcinoma) during the administration of TFB therapy. Only 1 (0.25%) patient had a pulmonary venous thromboembolic event; however, this patient had cholangiohepatic adenocarcinoma as well besides primary sclerosing cholangitis, which was diagnosed nearly after 1 year of treatment. This patient ultimately passed away due to the carcinoma. No more fatal events were reported.
The level of serum cholesterol was altered during the treatment (P = .006), as baseline 4.8 ± 1.2 mmol/L increased to 5.5 ± 3.6 mmol/L. However, no similar change was observed in the triglyceride level (baseline 1.26-1.30 mmol/L at week 52; P = .229).
In addition, abnormal liver functions were also observed in a few cases, as 12 (3.1%) patients had novum glutamate oxaloacetate transaminase/glutamate pyruvate transaminase liver enzyme elevation, while 6 (1.5%) patients had novum GGT elevation. There was no overlap between these patients. Liver enzyme elevation was above 1.5-fold in 16 (88.9%) cases, and in 2 cases it was above 3-fold (in 1 patient, the dose of TFB was reduced, while in the other case, statin therapy was initiated). Concomitant corticosteroid administration was not associated with elevated liver enzymes (P = .621). In total, no significant change was observed regarding the level of liver enzymes during the treatment period.
The AEs were mostly self-limiting, although in some cases, certain interventions were needed. We wish to highlight that all AEs were reversible. Moderate AEs resulting in dose reduction or admission of specific therapeutic agent occurred in 6 (14.3%) cases and moderate infections occurred in 13 (52.0%) cases. Cessation of the TFB treatment was needed in 12.0% and dose reduction was needed in 8.0%, or with specific treatment for the AE (Table 3).
Table 3.
All adverse events in the cohort.
| Adverse events | Severe | Moderate | |
| Infectious diseases | |||
| Total | 25 (6.4) | — | — |
| Pneumonia | 4 (1.0) | 1 (25.0) | 3 (75.0) |
| Herpes zoster | 6 (1.5) | 2 (33.3) | 4 (66.7) |
| Herpes labialis | 2 (0.5) | — | — |
| Clostridioides difficile | 4 (1.0) | 1 (25.0) | 3 (75.0) |
| COVID-19 | 1 (0.26) | — | — |
| Perianal abscess | 3 (0.8) | 1 (33.3) | 2 (66.7) |
| Acne | 2 (0.5) | 1 (50.0) | 1 (50.0) |
| Gastroenteritis | 3 (0.8) | - | - |
| Malignant disorder | |||
| Total | 2 (0.5) | — | — |
| Melanoma | 1 (0.26) | — | — |
| Cholangiohepatic adenocarcinoma | 1 (0.26) | — | 1 (100.0) |
| Cardiovascular/hemorheology | |||
| Total | 2 (0.5) | — | — |
| Hypertension | 1 (0.26) | — | 1 (100.0) |
| Pulmonary embolism | 1 (0.26) | — | 1 (100.0) |
| Liver functions | |||
| Total | 18 (4.9) | — | — |
| Elevated GOT/GPT | 12 (3.1) | — | 2 (16.7) |
| Elevated GGT | 6 (1.5) | — | — |
| Other | |||
| Total | 20 (5.1) | — | — |
| Dyspnea | 1 (0.26) | 1 (100.0) | — |
| Arthralgia | 8 (2.0) | — | 1 (12.5) |
| Skin rash | 5 (1.3) | 2 (40.0) | — |
| Headache | 1 (0.26) | — | — |
| Gastric pain/dyspepsia | 3 (0.8) | 1 (33.3) | — |
| Nausea | 1 (0.26) | 1 (100.0) | — |
| Vertigo | 1 (0.26) | 1 (100.0) | — |
Values are n (%).
Abbreviations: GGT, γ-glutamyl transferase; GOT, glutamate oxaloacetate transaminase; GPT, glutamate pyruvate transaminase.
Discussion
Our international, retrospective, multicenter study has the largest study population so far: it is comparable to the Global Clinical Programme with 1157 patients17 and to a real-life meta-analysis with 1162 patients.2 To our knowledge, our study is the first to compare the efficacy of TFB in different indications of UC (CA, rescue therapy); furthermore, it investigated predictive factors for SFR, colectomy, PNR, and LOR as well. So far, only few adult and pediatric patients have been reported to receive TFB treatment with rescue therapy indication.18-20
In our study, the week 12 SFR rate was 23.7%, in accordance with the week 16 to 26 SFR rate of 25.0% in the meta-analysis conducted by Lucaciu et al,21 which was lower than the real-life meta-analysis of 44.3% conducted by Taxonera et al2 but exceeded the week 8 remission result of the OCTAVE 1 and 2 trials of 18.5% and 16.6%, respectively.22 In our study, early efficacy did not differ between the CA and ASUC groups. Higher clinical activity reduced the chance of achieving week 12 SFR, whereas in CA group, higher CRP and male sex were negative predictors, in addition, older age was associated with increased chance of achieving week 52 SFR in CA. Male sex has previously been associated with reduced SFR rates in a meta-analysis.21 The ENEIDA registry (Estudio Nacional en Enfermedad Inflamatoria intestinal sobre Determinantes genéticos y Ambientales) has identified baseline pMayo as the only short-term predictor.23
Based on our findings, TFB is remarkably efficient as a rescue therapy in ASUC, as the week 12 colectomy rate was 7.5%, and the week 52 was 17.6%. Compared with international data, both IFX and cyclosporine had poorer short and long term outcome. Based on the study conducted by Laharie et al,24 the 98-day colectomy rates were 17% in the cyclosporine and 21% in the IFX group, while Williams et al25 found the 3-month colectomy rates to be 29% in IFX and 30% in cyclosporine. In this study, the 12-month outcome was also higher than experienced in our cohort, as in IFX it was 35%, and in cyclosporine it was 41%. These findings can be confirmed as well by a South Korean cohort,26 in which the 3-month colectomy rate was 26.1% in cyclosporine and 13.3% in IFX. However, the 12-month colectomy rate was comparable to our study in the case of IFX (18.4%). Nevertheless, we emphasize the favorable effect of TFB knowing that the majority of the patients (almost 90%) did not receive it as a first-line treatment. In addition, our study found that biologic-naïve patients more likely achieved SFR in the total cohort, especially in patients with ASUC, and TFB admission in lower therapeutic line seemed to be more effective in achieving week 52 SFR. These results are confirmed by a meta-analysis2 in which biologically naive patients had better response to therapy. Our findings did not confirm previous findings that low albumin level and higher eMayo may be associated with higher colectomy rate. Furthermore, we found that the chance of colectomy is 4.8 times higher in ASUC. In accordance with the meta-analyses conducted by Steenholdt et al,27 TFB could be a promising therapeutic agent in ASUC to prevent colectomy.
In the total cohort, the 8.0% week 52 colectomy rate is almost the same as in the meta-analysis.2 Our study confirmed the findings of Sandborn et al28 that older age increases the chance of remission, as we found it to be a protective factor both in colectomy and SFR (in the CA group); however, we would like to highlight the observations of Lichtenstein et al29 that serious AEs, opportunistic infections, herpes zoster, malignancies, and major cardiovascular events are more frequent in elderly patients. We suggest further investigations to clarify the role of age as a protective factor, as previous studies have shown similar or worse outcomes in case of other biological therapies.30-32
Primary nonresponse rates were slightly lower (21.5%) compared with previous data (26.0%).33 No predictive factor was identified, in contrast with the retrospective observational cohort study conducted by Honap et al33 in which higher baseline CRP and younger age were associated with increased PNR rates. We found no link between the number of previous biologic therapies and PNR, in accordance with existing data.33,34
Almost 1 in 2 patients had LOR, and it was more common in cases of CA, which is probably due to the fact that PNR was more frequent and that the follow-up time was shorter in case of ASUC. However, an extended induction dose and an increase in dose were effective in one-third of the patients. We wish to highlight that LOR is the least studied outcome of TFB admission in UC. All patients received TFB as 20-mg/d induction dose, and the maintenance dose was 10 mg/d, while the treatment escalation was mostly identical to the induction dose; however, in selected cases, a higher dosage was used at the decision of the physician as well (25 and 30 mg/d, respectively).
Both AE and serious AE rates were comparable with international data.2 In our cohort, herpes zoster was the most common infection in addition to pneumonia, C. difficile, and perianal abscess. In 1 in 4 patients, the infection was severe, and the affected patients made up half of the cases of cessation of TFB, and 1 patient passed away due to it. A thromboembolic event occurred in only 1 case; additionally, this patient had concurrent pancreas carcinoma. In accordance with our results, patients in the OCTAVE induction 1 and 2 trials and in the OCTAVE-Sustain trails have had thromboembolic events.35,36 Five thromboembolic events were reported (1 deep vein thrombosis and 4 pulmonary embolisms) and they had concomitant risk factors as well (eg, anamnestic deep venous thrombosis/pulmonary embolisms or thrombophlebitis, oral contraceptive, traumatic event, or concomitant metastatic cholangiocarcinoma).36 In addition to pancreas tumor, in 1 case, melanoma was reported. Furthermore, we experienced both aminotransferase37 and GGT38 elevation as well in a few cases, but these results were mild, temporary, and reversible. Furthermore, in accordance with previous data,39 cholesterol level was increased in our study as well without the elevation of triglyceride level. Based on our previous results, similar changes in lipid metabolism were also observed in 2 other rescue therapeutic agents, cyclosporine40 and systemic steroids,41 as a significant increase was observed in the level of cholesterol but not in the level of triglyceride. In summary, it can be concluded that AEs were predominantly mild and ceased spontaneously or with a reduction in dose or providing specific treatment.
The major strengths of the study are the large number of patients included, the nationwide setup, and that there are no previous data and comparisons of TFB in different indications. The limitations of this study were retrospective data collection and analysis and that the 52-week follow-up period was reached by only 184 of a total of 391 patients, and thus the extrapolation of the data could be narrowed. In addition, due to the multicenter setting, the analysis was impacted by data collection bias.
Conclusions
Our results in a larger cohort proved that TFB may be effective in both moderate-to-severe UC and in patients with ASUC as a rescue therapy. In addition, our observations suggest better colectomy rates in ASUC compared with IFX or cyclosporine. TFB treatment resulted in high rates of SFR and mucosal healing in both short and long terms even after anti-tumor necrosis factor and vedolizumab failure. Higher baseline disease activity and the number of previous biological therapies negatively influenced efficacy. TFB admission could be more effective as a first-line treatment in biologic-naïve patients as a rescue therapy or in a lower therapeutic line in CA due to its outstanding efficacy among available therapeutic options and its reassuring safety profile. Serious AEs were rare, and our results seem to support the assumption that thromboembolic events are associated with other risk factors.
The TFB Study Group comprises Simone Saibeni, Kristyna Kastylova, Jakob Benedict Seidelin, Johan Burisch, and Helga Hajdú.
Supplementary Material
Contributor Information
Tamás Resál, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Péter Bacsur, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Csilla Keresztes, Department for Medical Communication and Translation Studies, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Anita Bálint, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Renáta Bor, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Anna Fábián, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Bernadett Farkas, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Kostas Katsanos, Division of Gastroenterology, Department of Internal Medicine, Faculty of Medicine, School of Health Sciences, University of Ioannina, Ioannina, Greece.
George Michalopoylos, Gastroenterology Department, General Hospital of Athens G. Gennimatas, Athens, Greece.
Davide Giuseppe Ribaldone, Department of Medical Sciences, University of Turin, Turin, Italy.
Mohamed Attauabi, Department of Gastroenterology and Hepatology, Copenhagen University Hospital - Herlev and Gentofte, Herlev, Denmark.
Mirabella Zhao, Gastrounit, Copenhagen University Hospital - Amager and Hvidovre, Hvidovre, Denmark.
Hadar Amir Barak, Division of Gastroenterology, Rabin Medical Center, Petah Tikva, Israel.
Henit Yanai, Division of Gastroenterology, Rabin Medical Center, Petah Tikva, Israel.
Cristina Bezzio, IBD Unit/Gastroenterology Unit, Rho Hospital, ASST Rhodense, Rho, Italy.
Antonio Rispo, IBD Unit Department, Clinical Medicine and Surgery, Azienda Ospedaliera Universitaria Federico II of Naples, Naples, Italy.
Fabiana Castiglione, IBD Unit Department, Clinical Medicine and Surgery, Azienda Ospedaliera Universitaria Federico II of Naples, Naples, Italy.
Ariella Bar-Gil Shitrit, Digestive Diseases Institute, Shaare Zedek Medical Center, Hebrew University of Jerusalem, Jerusalem, Israel.
Daniela Pugliese, IBD Center, Centro Malattie Apparato Digerente, Unità Operativa Complessa di Medicina Interna e Gastroenterologia, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Alessandro Armuzzi, IBD Center, IRCCS Humanitas Research Hospital, Rozzano, Italy; Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy.
Edoardo Vincenzo Savarino, Department of Surgery, Oncology and Gastroenterology, University of Padua, Padua, Italy; Gastroenterology Unit, Azienda Ospedale Università di Padova, Padua, Italy.
Martin Kolar, IVth Medical Department, Charles University in Prague, Prague, Czech Republic.
Milan Lukáš, IVth Medical Department, Charles University in Prague, Prague, Czech Republic.
Elena Chashkova, Irkutsk Scientific Center of Surgery and Traumatology, Irkutsk, Russia.
Rafał Filip, Department of Gastroenterology with IBD, Unit of Clinical Hospital No. 2 im. Sw. Jadwigi Królowej, Rzeszow, Poland.
Aurore Rozieres, Department of Gastroenterology, Lyon-Sud Hospital, Hospices Civils de Lyon, Lyon, France; Centre International de Recherche et Infectologie, INSERM U1111, Lyon, France.
Stéphane Nancey, Department of Gastroenterology, Lyon-Sud Hospital, Hospices Civils de Lyon, Lyon, France; Centre International de Recherche et Infectologie, INSERM U1111, Lyon, France.
Željko Krznarić, Department of Gastroenterology and Hepatology, University Hospital Centre Zagreb, Zagreb, Croatia; Department of Nutrition, School of Medicine, University of Zagreb, Zagreb, Croatia.
Eszter Schäfer, Department of Gastroenterology, Military Hospital Medical Centre, State Health Centre, Budapest, Hungary.
Tamás Szamosi, Department of Gastroenterology, Military Hospital Medical Centre, State Health Centre, Budapest, Hungary.
Patrícia Sarlós, Division of Gastroenterology, First Department of Medicine, Medical School, University of Pécs, Pécs, Hungary; Institute for Translational Medicine, Medical School, University of Pécs, Pécs, Hungary.
Matej Franko, Department of Gastroenterology, University Medical Centre Ljubljana, Ljubljana, Slovenia.
David Drobne, Department of Gastroenterology, University Medical Centre Ljubljana, Ljubljana, Slovenia; Department of Gastroenterology, University Medical Centre Ljubljana, Medical Faculty, University of Ljubljana, Ljubljana, Slovenia.
Oleg V Knyazev, Moscow Clinical Scientific Center named after A. S. Loginov, Moscow, Russia; National Medical Research Center of Coloproctology named after A. N. Ryzhykh, Moscow, Russia.
Anna V Kagramanova, Moscow Clinical Scientific Center named after A. S. Loginov, Moscow, Russia; Research Institute of Health Organization and Medical Management, Moscow, Russia.
Jimmy Limdi, Department of Gastroenterology, Pennine Acute Hospitals NHS Trust, Manchester, United Kingdom.
Panu Wetwittayakhlang, Division of Gastroenterology, McGill University Health Centre, Montreal, Quebec, Canada; Gastroenterology and Hepatology Unit, Division of Internal Medicine, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand.
Peter L Lakatos, Division of Gastroenterology, McGill University Health Centre, Montreal, Quebec, Canada; Department of Internal Medicine and Oncology, Semmelweis University, Budapest, Hungary.
Nitsan Maharshak, Department of Gastroenterology and Liver Diseases, Tel Aviv Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Lian Bannon, Department of Gastroenterology and Hepatology, Copenhagen University Hospital - Herlev and Gentofte, Herlev, Denmark.
Tibor Nyári, Department of Medical Physics and Informatics, Albert Szent-Györgyi Medical School University of Szeged, Szeged, Hungary.
Zoltán Szepes, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Klaudia Farkas, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
Tamás Molnár, Division of Gastroenterology, Department of Medicine, Albert Szent-Györgyi Medical School, University of Szeged, Szeged, Hungary.
TFB Study Group:
Simone Saibeni, Kristyna Kastylova, Jakob Benedict Seidelin, Johan Burisch, and Helga Hajdú
Author Contribution
T.R.: Original Draft Preparation, Review & Editing, Data Curation, Investigation, Methodology, Formal Analysis. P.B.: Review & Editing, Data Curation, Conceptualization, Project Administration. C.K.: Supervision, Review & Editing. A.B., R.B., A.F., B.F., K.K., G.M., D.G.R., M.A., M.Z., H.A.B., H.Y., C.B., A.R., F.C., A.B.-G.S., D.P., A.A., E.V.S., M.K., M.L., E.C., R.F., A.R., S.N., Ž.K., E.S., T.S., P.S., M.F., D.D., O.V.K., A.V.K., J.K., N.M., L.B.: Review & Editing, Supervision, Data Curation. T.N.: Software, Formal analysis, Visualisation. P.W., P.L.L., Z.S., K.F.: Review & Editing, Supervision, Data Curation, Conceptualization, Project Administration, Methodology. T.M.: Conceptualization, Investigation, Methodology, Project Administration, Resources, Supervision, Original Draft Preparation, Review & Editing.
Funding
This work was supported by the research grants of Hungarian Scientific Research Fund (K22-143549), the National Research, Development and Innovation Office (Grant IDs: 125377, 129266, and 134863), the New National Excellence Program of the Ministry of Human Capacities (UNKP-22-3-SZTE-278 to T.R., UNKP-22-5-SZTE-545 to R.B., UNKP-22-4 -SZTE-296 to A.F., UNKP-21-5-SZTE-552 to K.F., UNKP-22-3-SZTE-233 to P.B.), and Janos Bolyai Research Grant (BO/00598/19/5 to K.F. and BO/00723/22 to R.B.) and the Géza Hetényi Research Grant (to K.F., M.R., and A.B.) by the Albert Szent-Györgyi Medical School, University of Szeged.
Conflicts of Interest
G.M. has received speaker fees from AbbVie, Takeda, Janssen, and Pfizer. N.M. has received speaking and/or consulting fees from Pfizer, Takeda, Janssen, Ferring, BiomX, BMS, Nestlé, and Trobix; and grant support from Takeda, Janssen, Abbott, AbbVie, Pfizer, BMS, Corundum Innovation Ltd, and Nestlé. L.M. has served as a speaker for AbbVie, Takeda, Celltrion, Biogen, and Janssen Cilag. C.B. has received lecture fees and served as a consultant for Takeda, MSD, Ferring, Galapagos, and Janssen. S.S. has received lecture fees and served as a consultant and advisory board member for AbbVie, Arena, Ferring, Gilead, Janssen, MSD, and Takeda. D.D. has served as a speaker, consultant, and an advisory board member for MSD, AbbVie, Takeda, Pfizer, Janssen, Amgen, Biogen, and Krka. E.V.S. has served as a speaker for AbbVie, AGPharma, Alfasigma, Dr Falk, EG Stada Group, Fresenius Kabi, Grifols, Janssen, Innovamedica, Malesci, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Unifarco; served as a consultant for Alfasigma, Amgen, Biogen, Bristol-Myers Squibb, Celltrion, Diadema Farmaceutici, Dr Falk, Fresenius Kabi, Janssen, Merck & Co, Reckitt Benckiser, Regeneron, Sanofi, Shire, SILA, Sofar, Synformulas GmbH, Takeda, and Unifarco; and received research support from Pfizer, Reckitt Benckiser, SILA, Sofar, and Unifarco. D.P. has received speaker fees from MSD, Takeda, Galapagos, and Janssen, Pfizer; advisory board fees from Pfizer. A.A. has received consulting and/or advisory board fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, Takeda; lecture and/or speaker bureau fees from AbbVie, Amgen, Arena, Biogen, Ferring, Galapagos, Gilead, Janssen, MSD, Mitsubishi-Tanabe, Nikkiso, Novartis, Pfizer, Sandoz, Samsung Bioepis, Takeda; and research grants from MSD, Pfizer, Takeda and Biogen. P.L.L. has served as a speaker and/or advisory board member for AbbVie, Amgen, BioJamp, Bristol Myers Squibb, Fresenius Kabi, Genetech, Gilead, Janssen, Merck, Mylan, Organon, Pendopharm, Pfizer, Roche, Sandoz, Takeda, Tillots, and Viatris; and received unrestricted research grant support from AbbVie, Gilead, Takeda, and Pfizer. K.F. has received speaker honoraria from AbbVie, Janssen, Ferring, Takeda, and Goodwill Pharma. T.M. has received speaker’s honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer, Janssen, Sandoz, MundiPharma, Phytotec, Roche, Fresenius, and Teva.
References
- 1. Fernández-Clotet A, Castro-Poceiro J, Panés J.. Tofacitinib for the treatment of ulcerative colitis. Expert Rev Clin Immunol. 2018;14(11):881-892. doi: 10.1080/1744666X.2018.1532291 [DOI] [PubMed] [Google Scholar]
- 2. Taxonera C, Olivares D, Alba C.. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. 2022;28(1):32-40. doi: 10.1093/ibd/izab011 [DOI] [PubMed] [Google Scholar]
- 3. O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A.. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111-ii115. doi: 10.1136/annrheumdis-2012-202576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lohan C, Diamantopoulos A, LeReun C, Wright E, Bohm N, Sawyer LM.. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterology 2019;6(1):e000302. doi: 10.1136/bmjgast-2019-000302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paschos P, Katsoula A, Giouleme O, et al. Tofacitinib for induction of remission in ulcerative colitis: systematic review and meta-analysis. Ann Gastroenterol. 2018;31(5):572-582. doi: 10.20524/aog.2018.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilmore R, Hilley P, Srinivasan A, Choy M, De Cruz P.. Sequential use of high-dose tofacitinib after infliximab salvage therapy in acute severe ulcerative colitis. J Crohns Colitis. 2022;16(1):166-168. doi: 10.1093/ecco-jcc/jjab109 [DOI] [PubMed] [Google Scholar]
- 7. Berinstein JA, Steiner CA, Regal RE, et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2019;17(5):988-990.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Honap S, Pavlidis P, Ray S, et al. Tofacitinib in acute severe ulcerative colitis-a real-world tertiary center experience. Inflamm Bowel Dis. 2020;26(11):e147-e149. [DOI] [PubMed] [Google Scholar]
- 9. Xiao Y, Benoit N, Sedano R, et al. Effectiveness of tofacitinib for hospitalized patients with acute severe ulcerative colitis: case series. Dig Dis Sci. 2022;67(11):5213-5219. [DOI] [PubMed] [Google Scholar]
- 10. Kotwani P, Terdiman J, Lewin S.. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis 2020;14(7):1026-1028. [DOI] [PubMed] [Google Scholar]
- 11. Jena A, Mishra S, Sachan A, Singh H, Singh AK, Sharma V.. Tofacitinib in acute severe ulcerative colitis: case series and a systematic review. Inflamm Bowel Dis. 2021;27(9):e101-e103. doi: 10.1093/ibd/izab087 [DOI] [PubMed] [Google Scholar]
- 12. Taxonera C, Olivares D, Alba C.. Tofacitinib in acute severe ulcerative colitis: case series and a systematic review-authors’ reply. Inflamm Bowel Dis. 2021;27(9):e107. [DOI] [PubMed] [Google Scholar]
- 13. Desai RJ, Pawar A, Weinblatt ME, Kim SC.. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol. 2019;71(6):892-900. doi: 10.1002/art.40798 [DOI] [PubMed] [Google Scholar]
- 14. Kochar BD, Cheng D, Cai T, Ananthakrishnan AN.. Comparative risk of thrombotic and cardiovascular events with tofacitinib and anti-TNF agents in patients with inflammatory bowel diseases. Dig Dis Sci. 2022;67(11):5206-5212. doi: 10.1007/s10620-022-07404-z [DOI] [PubMed] [Google Scholar]
- 15. Truelove SC, Witts LJ.. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J 1954;2(4884):375-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satsangi J, Silverberg MS, Vermeire S, Colombel JF.. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749-753. doi: 10.1136/gut.2005.082909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sandborn WJ, D’Haens GR, Sands BE, et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical program. J Crohns Colitis. 2023;17(3):338-351. doi: 10.1093/ecco-jcc/jjac141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Constant BD, Baldassano R, Kirsch J, Mitchel EB, Stein R, Albenberg L.. Tofacitinib salvage therapy for children hospitalized for corticosteroid- and biologic-refractory ulcerative colitis. J Pediatr Gastroenterol Nutr. 2022;75(6):724-730. doi: 10.1097/MPG.0000000000003616 [DOI] [PubMed] [Google Scholar]
- 19. Berinstein JA, Sheehan JL, Dias M, et al. Tofacitinib for biologic-experienced hospitalized patients with acute severe ulcerative colitis: a retrospective case-control study. Clin Gastroenterol Hepatol. 2021;19(10):2112-2120.e1. doi: 10.1016/j.cgh.2021.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uzzan M, Bresteau C, Laharie D, et al. ; GETAID-TALC Study Group. Tofacitinib as salvage therapy for 55 patients hospitalised with refractory severe ulcerative colitis: A GETAID cohort. Aliment Pharmacol Ther. 2021;54(3):312-319. doi: 10.1111/apt.16463 [DOI] [PubMed] [Google Scholar]
- 21. Lucaciu LA, Constantine-Cooke N, Plevris N, et al. Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2021;14:17562848211064004. doi: 10.1177/17562848211064004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723-1736. doi: 10.1056/NEJMoa1606910 [DOI] [PubMed] [Google Scholar]
- 23. Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35-42. doi: 10.1093/ecco-jcc/jjaa145 [DOI] [PubMed] [Google Scholar]
- 24. Laharie D, Bourreille A, Branche J, et al. ; Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380(9857):1909-1915. doi: 10.1016/S0140-6736(12)61084-8 [DOI] [PubMed] [Google Scholar]
- 25. Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016;1(1):15-24. doi: 10.1016/S2468-1253(16)30003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Song EM, Oh EH, Hwang SW, et al. Comparison of outcomes of cyclosporine A and infliximab for steroid-refractory acute severe ulcerative colitis. J Gastroenterol Hepatol. 2021;36(9):2463-2470. doi: 10.1111/jgh.15508 [DOI] [PubMed] [Google Scholar]
- 27. Steenholdt C, Ovesen PD, Brynskov J, Seidelin JB.. Tofacitinib for acute severe ulcerative colitis: a systematic review. J Crohns Colitis. Published online March 1, 2023. doi: 10.1093/ecco-jcc/jjad036 [DOI] [PubMed] [Google Scholar]
- 28. Sandborn WJ, Armuzzi A, Liguori G, et al. Predictors of sustained response with tofacitinib therapy in patients with ulcerative colitis. Inflamm Bowel Dis. 2022;28(9):1338-1347. doi: 10.1093/ibd/izab278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lichtenstein GR, Bressler B, Francisconi C, et al. Assessment of safety and efficacy of tofacitinib, stratified by age, in patients from the ulcerative colitis clinical program. Inflamm Bowel Dis. 2023;29(1):27-41. doi: 10.1093/ibd/izac084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen NA, Plevris N, Kopylov U, et al. Vedolizumab is effective and safe in elderly inflammatory bowel disease patients: a binational, multicenter, retrospective cohort study. United European Gastroenterol J. 2020;8(9):1076-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mak JW, Lok Tung Ho C, Wong K, et al. Epidemiology and natural history of elderly‐onset inflammatory bowel disease: results from a territory-wide Hong Kong IBD registry. J. Crohns Colitis. 2021;15(3):401-408. [DOI] [PubMed] [Google Scholar]
- 32. Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(2):309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Honap S, Chee D, Chapman TP, et al. ; LEO [London, Exeter, Oxford] IBD Research Consortium. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14(10):1385-1393. doi: 10.1093/ecco-jcc/jjaa075 [DOI] [PubMed] [Google Scholar]
- 34. Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. Real-world experience with tofacitinib in IBD at a tertiary center. Dig Dis Sci. 2019;64(7):1945-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17(8):1541-1550. doi: 10.1016/j.cgh.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 36. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther. 2019;50(10):1068-1076. doi: 10.1111/apt.15514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tofacitinib. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK547848/ [Google Scholar]
- 38. Valenzuela F, Korman NJ, Bissonnette R, et al. Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol. 2018;179(4):853-862. doi: 10.1111/bjd.16798 [DOI] [PubMed] [Google Scholar]
- 39. Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(1):123-132.e3. doi: 10.1016/j.cgh.2019.04.059 [DOI] [PubMed] [Google Scholar]
- 40. Bálint A, Farkas K, Szűcs M, et al. Long-term increase in serum cholesterol levels in ulcerative colitis patients treated with cyclosporine: an underdiagnosed side effect frequently associated with other drug-related complications. Scand J Gastroenterol. 2014;49(1):59-65. doi: 10.3109/00365521.2013.848231 [DOI] [PubMed] [Google Scholar]
- 41. Farkas K, Bálint A, Valkusz Z, et al. Bolus administration of steroid therapy is more favorable than the conventional use in preventing decrease of bone density and the increase of body fat percentage in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8(9):992-997. doi: 10.1016/j.crohns.2014.01.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.