Abstract
BACKGROUND
The last decade has seen increased research on the relationship between diet and male fertility, but there are no clearly defined nutritional recommendations for men in the preconception period to support clinical fertility outcomes.
OBJECTIVE AND RATIONALE
The purpose of this scoping review is to examine the extent and range of research undertaken to evaluate the effect(s) of diet in the preconception period on male clinical fertility and reproductive outcomes.
SEARCH METHODS
Four electronic databases (MEDLINE and EMBASE via Ovid, CAB Direct, and CINAHL via EBSCO) were searched from inception to July 2023 for randomized controlled trials (RCTs) and observational studies (prospective/retrospective, case–control, and cross-sectional). Intervention studies in male participants or couples aiming to achieve dietary or nutritional change, or non-intervention studies examining dietary or nutritional components (whole diets, dietary patterns, food groups or individual foods) in the preconception period were included. Controls were defined as any comparison group for RCTs, and any/no comparison for observational studies. Primary outcomes of interest included the effect(s) of male preconception diet on clinical outcomes such as conception (natural or via ART), pregnancy rates and live birth rates. Secondary outcomes included time to conception and sperm parameters.
OUTCOMES
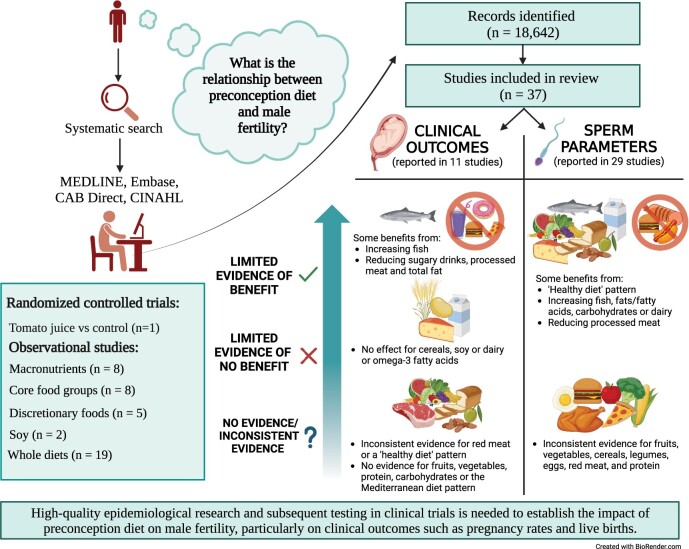
A total of 37 studies were eligible, including one RCT and 36 observational studies (prospective, cross-sectional, and case–control studies; four studies in non-ART populations) published between 2008 and 2023. Eight reported clinical outcomes, 26 reported on secondary outcomes, and three reported on both. The RCT did not assess clinical outcomes but found that tomato juice may benefit sperm motility. In observational studies, some evidence suggested that increasing fish or reducing sugar-sweetened beverages, processed meat or total fat may improve fecundability. Evidence for other clinical outcomes, such as pregnancy rates or live birth rates, showed no relationship with cereals, soy and dairy, and inconsistent relationships with consuming red meat or a ‘healthy diet’ pattern. For improved sperm parameters, limited evidence supported increasing fish, fats/fatty acids, carbohydrates and dairy, and reducing processed meat, while the evidence for fruits, vegetables, cereals, legumes, eggs, red meat and protein was inconsistent. Healthy diet patterns in general were shown to improve sperm health.
WIDER IMPLICATIONS
Specific dietary recommendations for improving male fertility are precluded by the lack of reporting on clinical pregnancy outcomes, heterogeneity of the available literature and the paucity of RCTs to determine causation or to rule out reverse causation. There may be some benefit from increasing fish, adopting a healthy dietary pattern, and reducing consumption of sugar-sweetened beverages and processed meat, but it is unclear whether these benefits extend beyond sperm parameters to improve clinical fertility. More studies exploring whole diets rather than singular foods or nutritional components in the context of male fertility are encouraged, particularly by means of RCTs where feasible. Further assessment of core fertility outcomes is warranted and requires careful planning in high-quality prospective studies and RCTs. These studies can lay the groundwork for targeted dietary guidelines and enhance the prospects of successful fertility outcomes for men in the preconception period. Systematic search of preconception diet suggests that increasing fish and reducing sugary drinks, processed meats and total fat may improve male fertility, while consuming healthy diets, fish, fats/fatty acids, carbohydrates and dairy and reducing processed meat can improve sperm health.
Keywords: preconception diet, male infertility, sperm, seminal plasma, dietary patterns, macronutrients
Graphical Abstract
Systematic search of preconception diet suggests that increasing fish and reducing sugary drinks, processed meats and total fat may improve male fertility, while consuming healthy diets, fish, fats/fatty acids, carbohydrates and dairy and reducing processed meat can improve sperm health.
Introduction
The World Health Organization (WHO) defines infertility as a disease of the male or female reproductive system resulting in the failure to achieve a pregnancy after 12 or more months of regular unprotected sexual intercourse (WHO, 2023). Importantly, of all couples diagnosed with infertility, a male factor is identified as a primary or contributing cause in up to 50% of cases (Agarwal et al., 2021). There are multiple factors that may contribute to male infertility, including genetic influences, hormonal disorders, and testicular dysfunction, as well as injuries, infections or chronic conditions that impair normal sperm production (Agarwal et al., 2021). More recently, unhealthy lifestyle practices have been associated with male infertility and are thought to contribute to the marked decline in sperm counts (∼1% per year) observed in the Western world over the past 60 years (Levine et al., 2017). Infertility poses a significant psychological and economic burden; thus, improving male health in the preconception period is important to alleviate the public health impact of infertility.
Increasing research has examined the impact of modifiable lifestyle factors on male fertility during the preconception period. There is emerging evidence linking paternal nutrition with semen quality (count, motility, and morphology) and, to a lesser extent, with clinical outcomes such as time to pregnancy and live birth rate. For example, compelling evidence suggests that chronic heavy alcohol consumption (Van Heertum and Rossi 2017; Finelli et al., 2021), tobacco smoking (Sansone et al., 2018), and obesity (Campbell et al., 2015; Leisegang and Dutta 2021) are associated with an increased risk of fetal loss and decreased chances of live birth, as well as poorer sperm parameters. Previous meta-analyses examining the impact of dietary patterns (Cao et al., 2022), nutrient profiles, or dietary supplements (Salas-Huetos et al., 2018) on a range of sperm parameters have reported generally positive findings. However, these meta-analyses are limited by a small number of studies, small sample sizes of included studies, inter-study heterogeneity, and/or a lack of real-life applicability given the focus on single nutrients or supplements. A comprehensive exploration of dietary patterns, whole foods and macronutrients, examining both clinical trials and observational studies, can offer a broad assessment of the existing literature. In turn, this approach can discern the effects of preconception nutrition on clinical outcomes of male fertility as well as on sperm quality, generating insights that are more clinically relevant and have greater applicability in a real-world context.
To map the breadth of research that has been conducted to date, the primary aim of this scoping review is to examine the available evidence evaluating the influence of preconception diet on clinical male fertility outcomes. Measures of reproductive health (sperm parameters) are assessed as secondary outcomes. This review focuses on studies of men in the preconception period (i.e. planning/attempting pregnancy), rather than men of reproductive age in general, to clarify which foods/dietary patterns may be associated with improved clinical fertility outcomes at the time of preconception planning.
Methods
Research question
This scoping review is underpinned by the following broad research question:
What is the relationship between preconception diet and male fertility?
To adequately address this research question, we focused on randomized controlled trials (RCTs) and observational studies that assessed dietary exposures among men who are planning a pregnancy or undergoing ART.
Eligibility criteria
The eligibility criteria were determined using the Participant-Intervention/Exposure-Comparison-Outcome (PICO or PECO) framework, which follows a protocol defined a priori, available on the open science framework (10.17605/OSF.IO/FBV6W).
Participants (P)
Participants included men in the preconception period, that is those who were planning a pregnancy and/or those who were between pregnancies (inter-conception).
Intervention (I) or exposure (E)
Intervention included intervention studies that aimed to produce dietary or nutritional change or non-intervention (observational) studies examining dietary or nutritional components (i.e. whole diets, dietary patterns, food groups, or individual foods). Trials based solely on micronutrients, caffeine, or alcohol and studies with a stated goal of weight loss were excluded.
Comparison (C)
Comparison included any comparison group for intervention trials or no comparison for observational studies.
Outcomes (O)
To be included in this review, the study must have reported at least one fertility-related outcome. The primary outcomes of interest as prespecified in our protocol are natural conception, conception via ART, pregnancy rate, and live birth rate. Time to conception and semen parameters were secondary outcomes, including semen volume, sperm count, sperm concentration, sperm morphology, and/or sperm motility.
Study selection
Search strategy
The search strategy, including database selection and search terms, was developed via consultation with experts in fertility and nutrition, systematic review methodology, and an expert medical librarian. The following databases were searched: MEDLINE (Ovid), EMBASE (Ovid), CAB Direct, and CINAHL Plus (EBSCO). All sources were searched from inception to 27 September 2021 and updated on 8 July 2023. There were no limits on language. A variety of keywords relating to preconception/pre-pregnancy, diet, and fertility were used in the search strategy (Supplementary Table S1).
Screening process
Screening was undertaken using Covidence web-based software (www.covidence.org). Titles, abstracts, and keywords were assessed by several reviewers (S.A., D.S., X.T.T., C.T.T., C.P., J.B., L.M., and N.M.) in duplicate. Full text screening was conducted by several reviewers (S.A., D.S., X.T.T., C.T.T., L.M., N.M., and C.T.) and reasons for exclusion were listed. Disagreements were resolved by discussion in consultation with another reviewer where necessary (J.G. or A.M.).
Data extraction and synthesis
Data were extracted by reviewers (S.A., X.T.T., C.T.T., T.R., L.M., N.M., and C.T.) with 20% duplicate extraction (C.T., A.M., and J.G.) completed to ensure accuracy. Data extracted included: study details (year of publication, country, study design and setting, sample size(s), number of study arms, comparators, intervention or exposure form, duration, and frequency), participants (population characteristics including age, fertility status, and relevant history), and outcomes (pregnancy rate, live birth rate, IVF or ART outcomes, time to pregnancy, semen volume, sperm concentration, sperm count, sperm motility, sperm morphology). As scoping reviews are intended to characterize the broader scope or coverage of a body of literature over time, and to identify relevant gaps, assessments of study-level quality are not required (Munn et al., 2018).
Results
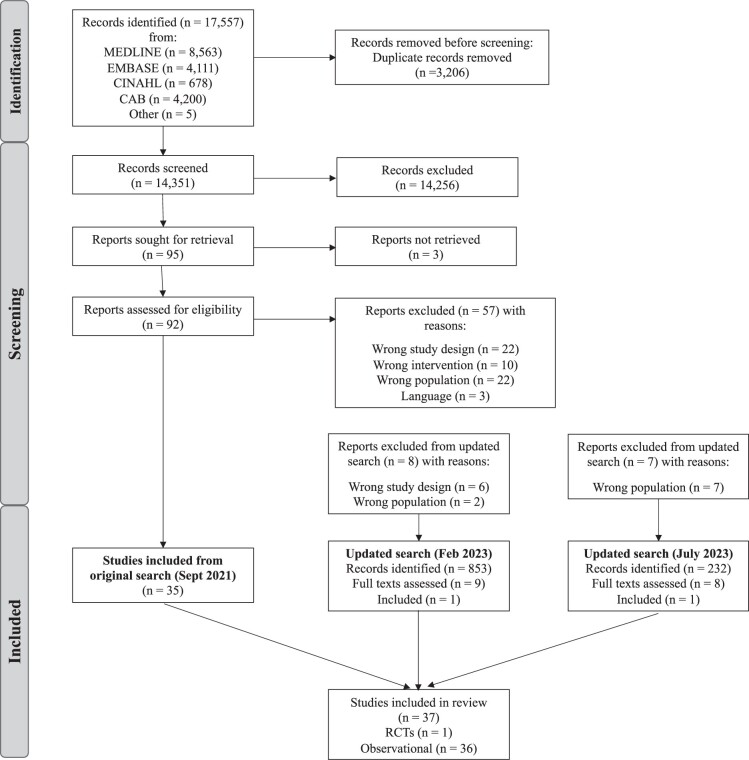
From 17 557 results from the initial search (September 2021), 3206 duplicates were removed, and 14 351 articles titles and abstracts were screened, leaving 95 articles to be assessed in full text (Fig. 1). Of these, 35 articles met the inclusion criteria. An updated search conducted in July 2023 yielded 1085 titles and abstracts, from which 17 full texts were screened. Of these, two additional eligible studies were identified, bringing the total to 37 articles that met the inclusion criteria.
Figure 1.
PRISMA diagram for the literature search process for a systematic scoping review of the influence of preconception diet on male fertility. RCT, randomized controlled trial.
The included studies comprised one RCT (Yamamoto et al., 2017) and 36 observational studies; 14 prospective (Chavarro et al., 2008; Vujkovic et al., 2009; Braga et al., 2012; Twigt et al., 2012; Afeiche et al., 2014a; Afeiche et al., 2014b; Minguez-Alarcon et al., 2015; Xia et al., 2015, 2016; Oostingh et al., 2017; Gaskins et al., 2018; Hatch et al., 2018; Salas-Huetos et al., 2022a; Salas-Huetos et al., 2022b), 16 cross-sectional (Attaman et al., 2012; Karayiannis et al., 2017; Danielewicz et al., 2018; Efrat et al., 2018; Jurewicz et al., 2018; Danielewicz et al., 2019; Ricci et al., 2019, 2020; Shirani et al., 2020; Mitsunami et al., 2021a; Mitsunami et al., 2021b; Abdollahi et al., 2022; Leilami et al., 2022; Nouri et al., 2022a; Nouri et al., 2022b; Geller et al., 2023), and six case–control (Mendiola et al., 2009, 2010; Eslamian et al., 2015, 2016, 2017; Haeri et al., 2021) studies. Study characteristics are outlined in Supplementary Table S2, with a summary of results (stratified by food groups/patterns) provided in Supplementary Table S3.
Study characteristics
Of the 37 studies, 11 reported on clinical outcomes. Table 1 outlines the results for each outcome assessed in the studies, stratified by nutritional component, including macronutrients, core food groups, discretionary foods, phytoestrogens, and whole diet approaches. The single included RCT assessed the addition of tomato juice to the usual diet in comparison to a control group. Eight individual observational studies reported on macronutrients, which included fats and fatty acids (n = 7), protein (n = 3), carbohydrates (n = 2), and fibre (n = 1), with two of the eight reporting on more than one macronutrient. Eight studies assessed one or more core food groups, such as dairy (n = 5), meat (n = 5), fish and seafood (n = 5), vegetables (n = 3), fruit (n = 2), whole grains and cereals (n = 2), legumes (n = 2), and eggs (n = 1). Five studies assessed discretionary food intake, including sugar sweetened beverages (n = 2), sweet foods (n = 2), and processed meat (n = 3). Two studies examined soy food intake. Nineteen studies investigated a whole diet approach; eight a priori dietary patterns were reported, including the Mediterranean Diet (MedDiet) (n = 4), Healthy Eating Index (HEI/alternative HEI) (n = 3), the Dietary Approaches to Stop Hypertension (DASH) diet (n = 3), and the American Heart Association diet (n = 1); and there were a range of posteriori dietary patterns identified (traditional/healthy/prudent/plant-based/antioxidant/empirical diet score).
Table 1.
Results for the influence of preconception diet on male fertility outcomes based on randomized controlled trials and observational studies, stratified by different food groups/patterns.
| Category | Studies | Semen volume | Sperm concentration | Sperm count | Sperm motility | Sperm morphology | Clinical Outcomes |
|---|---|---|---|---|---|---|---|
|
Randomized Controlled Trials | |||||||
|
1 | −ve: reduced semen volume in the antioxidant group and in the tomato juice group compared to control at 12 weeks | NS | NS | +ve: increased sperm motility in tomato juice group compared to control at 6 weeks | NS | — |
|
Observational studies | |||||||
|
Energy and macronutrients | |||||||
| Fats/Fatty acids | 7 |
|
|
|
|
|
|
| Protein | 3 |
|
|
— |
|
|
— |
| Carbohydrates | 2 | NS: (Nouri et al., 2022b #35; Mendiola et al., 2010 #22) |
|
— |
|
|
— |
| Fibre | 1 | NS: (Mendiola et al., 2010 #22) | +ve: lower sperm concentration associated with lower fibre intake (Mendiola et al., 2010 #22) | — | +ve: lower sperm motility associated with lower fibre intake (Mendiola et al., 2010 #22) | +ve: lower sperm morphology associated with lower fibre intake (Mendiola et al., 2010 #22) | — |
|
Core food groups | |||||||
| Dairy | 5 | NS: (Mendiola et al., 2009 #23; Afeiche et al., 2014a #9; Ricci et al., 2020 #10) |
|
NS: (Afeiche et al., 2014a #9; Ricci et al., 2020 #10) |
|
|
NS: fertilization, implantation, clinical pregnancy, live birth rate (Xia et al., 2016 #32) |
| Meat | 5 |
|
|
NS: (Afeiche et al., 2014b #6; Ricci et al., 2020 #10) | NS: (Mendiola et al., 2009 #23; Braga et al., 2012 #12; Afeiche et al., 2014b #6; Ricci et al., 2020 #10) | NS: (Mendiola et al., 2009 #23; Braga et al., 2012 #12) |
|
| Fish/Seafood | 5 |
|
|
|
|
|
|
| Vegetables | 3 | NS: (Mendiola et al., 2009 #23; Ricci et al., 2020 #10) |
|
+ve: vegetable intake (Ricci et al., 2020 #10) |
|
|
NS: implantation, fertilization or pregnancy rate with ICSI (Braga et al., 2012 #12) |
| Fruit | 2 | — |
|
— | +ve: higher sperm motility with higher fruit intake (Mendiola et al., 2009 #23; Braga et al., 2012 #12) |
|
NS: implantation, fertilization or pregnancy rate with ICSI (Braga et al., 2012 #12) |
| Cereals | 2 | −ve: lower semen volume with higher cereal consumption (Ricci et al., 2020 #10) |
|
NS: (Ricci et al., 2020 #10) |
|
NS: (Braga et al., 2012 #12) | NS: implantation, fertilization or pregnancy rate with ICSI (Braga et al., 2012 #12) |
| Legumes | 2 | NS: (Mendiola et al., 2009 #23) |
|
— | NS: (Mendiola et al., 2009 #23; Braga et al., 2012 #12) | NS: (Mendiola et al., 2009 #23; Braga et al., 2012 #12) | NS: implantation, fertilization or pregnancy rate with ICSI (Braga et al., 2012 #12) |
| Eggs | 1 | NS: (Mendiola et al., 2009 #23) | NS: (Mendiola et al., 2009 #23) | — | NS: (Mendiola et al., 2009 #23) | NS: (Mendiola et al., 2009 #23) | — |
|
Discretionary foods | |||||||
| Processed meat | 3 | NS: (Afeiche et al., 2014b #6) | NS: (Afeiche et al., 2014b #6) | NS: (Afeiche et al., 2014b #6) | NS: (Afeiche et al., 2014b #6) | −ve: increasing intake of processed red meat associated with poor sperm morphology (Afeiche et al., 2014b #6) | −ve: lower fertilization rate with standard IVF but not ICSI (Xia et al., 2015 #31) |
| Sweet foods | 2 | — |
|
— |
|
|
NS: implantation, fertilization or pregnancy rate with ICSI (Braga et al., 2012 #12) |
| Sugar-sweetened beverages | 2 | — | NS: (Braga et al., 2012 #12) | — | NS: (Braga et al., 2012 #12) | NS: (Braga et al., 2012 #12) |
|
|
Phytoestrogens | |||||||
| Soy | 2 | NS: (Chavarro et al., 2008 #79) | NS: (Chavarro et al., 2008 #79) | NS: (Chavarro et al., 2008 #79) | NS: (Chavarro et al., 2008 #79) | NS: (Chavarro et al., 2008 #79) | NS: fertilization rate, embryo quality, embryo cleavage, implantation, clinical pregnancy, live birth rate (Minguez-Alarcon et al., 2015 #24) |
|
Whole diets | |||||||
| ‘Healthy’/‘Prudent’ diet vs Western diet | 5 |
|
|
|
|
|
NS: fertilization rate, implantation, clinical pregnancy, live birth (Mitsunami et al., 2021b #150) |
| MedDiet/aMedDiet | 4 | NS: (Ricci et al., 2019 #27; Salas-Huetos et al., 2022b #153) |
|
|
|
|
NS: fertilization rate, implantation, clinical pregnancy, live birth (Salas-Huetos et al., 2022b #153) |
| Antioxidant-rich | 2 | NS: (Mendiola et al., 2010 #22) | +ve: lower concentration with lower intake of folate, vitamin C and lycopene (Mendiola et al., 2010 #22) | — |
|
+ve: lower morphology with lower antioxidant intake (Mendiola et al., 2010 #22) | — |
| HEI/AHEI | 3 | NS: (Leilami et al., 2022 #36; Salas-Huetos et al., 2022b #153) |
|
|
NS: (Leilami et al., 2022 #36; Salas-Huetos et al., 2022b #153) |
|
NS: fertilization rate, implantation, clinical pregnancy, live birth (Salas-Huetos et al., 2022b #153) |
| DASH | 3 | NS: (Danielewicz et al., 2019 #88; Salas-Huetos et al., 2022b #153) |
|
|
NS: (Efrat et al., 2018 #96; Danielewicz et al., 2019 #88; Salas-Huetos et al., 2022b #153) |
|
NS: fertilization rate, implantation, clinical pregnancy, live birth (Salas-Huetos et al., 2022b #153) |
| Prudent/Traditional Iranian/Vegetable/Mixed/Dietary Diversity Score | 2 |
|
|
— |
|
|
— |
| Healthy/Traditional Dutch | 1 | — | +ve: higher sperm concentration with adherence to traditional Dutch pattern (Vujkovic et al., 2009 #30) | NS: (Vujkovic et al., 2009 #30) | NS: (Vujkovic et al., 2009 #30) | NS: (Vujkovic et al., 2009 #30) | — |
| Plant-based diet | 2 |
|
|
NS: (Salas-Huetos et al., 2022b #153) |
|
NS: (Salas-Huetos et al., 2022b #153) | NS: fertilization rate, implantation, clinical pregnancy, live birth (Salas-Huetos et al., 2022b #153) |
| American Heart Association diet | 1 | NS: (Salas-Huetos et al., 2022b #153) | NS: (Salas-Huetos et al., 2022b #153) | NS: (Salas-Huetos et al., 2022b #153) | NS: (Salas-Huetos et al., 2022b #153) | NS: (Salas-Huetos et al., 2022b #153) |
|
| Preconception diet | 1 | — | — | — | — | — | NS: chance of pregnancy (Twigt et al., 2012 #51) |
| Empirical diet score | 1 | — | — | — | — | — | NS: fertilization rate, probability of implantation, clinical pregnancy or live birth (Mitsunami et al., 2021a #149) |
aHEI, alternative healthy eating index; aMED, alternative Mediterranean diet; CHO, carbohydrate; DASH, Dietary Approaches to Stop Hypertension; HEI, healthy eating index; LA, linoleic acid; LNA, linolenic acid; MedDiet, Mediterranean diet; MUFA, monounsaturated fatty acid; NS, not significant; PDI, plant-based diet index; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; TTP, time to pregnancy.
Randomized controlled trials
In a 12-week RCT among 44 infertile Japanese men (aged 26–50 years), no clinical outcomes were reported and there were no statistically significant differences in semen parameters at 6 or 12 weeks between the group taking tomato juice (190 g/day containing 30 mg lycopene) and the control group (avoiding lycopene-rich foods containing tomatoes) (Yamamoto et al., 2017). However, the change in semen volume between baseline and 12 weeks was significantly greater in the tomato juice group compared with controls (mean ± SE: tomato juice group −0.21 ± 0.20 versus control group 0.40 ± 0.41; P = 0.037), and the tomato juice group had a significant increase in sperm motility at 6 weeks, in comparison to the control group (5.87 ± 3.26 versus −3.24 ± 3.35, respectively, P = 0.019) (Table 1).
Observational studies
Macronutrients
Fats and fatty acids
Seven studies assessed the influence of total fat and fatty acid intake on male fertility outcomes (Mendiola et al., 2010; Attaman et al., 2012; Eslamian et al., 2015; Ricci et al., 2020; Nouri et al., 2022b; Salas-Huetos et al., 2022a; Geller et al., 2023). Two prospective US studies examined fatty acid intake and fecundability (Salas-Huetos et al., 2022a; Geller et al., 2023). In the first study among 697 couples trying to conceive without the use of fertility treatment, intake of total fat among male participants was weakly positively associated with fecundability, defined as the per-cycle probability of conception (Geller et al., 2023) (Table 1). The second study of 229 couples undergoing IVF treatment (Salas-Huetos et al., 2022a) found that higher intake of omega-3 fatty acids was not associated with implantation, clinical pregnancy or live birth rates, but was associated with higher total sperm count, sperm concentration, and motility (highest quartile 4.77–9.49 g/day versus lowest quartile 1.06–3.12 g/day, Ptrend= 0.008, 0.01, and 0.02, respectively) (Table 1).
In the remaining five studies, relationships between higher total fat intake and poorer sperm parameters were reported, while higher intake of polyunsaturated fatty acids (PUFAs) or omega-3 tended to be associated with improved motility and normospermia. One of these was a Spanish case control study of 61 men with poor versus normal semen parameters, which found that higher total fat consumption was associated with poor sperm quality (odds ratio [OR]: 11.81; 95% CI: 1.87–74.65) (Mendiola et al., 2010) (Table 1). Similarly, in a cross-sectional study of 99 men from a US fertility clinic, men in the highest versus lowest tertile of total fat consumption (37% versus 26% of daily energy intake) had a 43% lower total sperm count (95% CI: 62–14%) and a 38% lower sperm concentration (95% CI: 58–10%) (Attaman et al., 2012) (Table 1). There were no associations of fat intake or specific fatty acids with sperm motility, but men in the highest tertile of omega-3 PUFA intake had a 1.9% (95% CI: 0.4–3.5%) higher normal sperm morphology than men in the lowest tertile (Ptrend = 0.01, across the median intake tertiles) (Table 1). An Italian study of 323 men undergoing fertility treatment found that sperm concentrations <15 million/ml were more frequent in those with a higher intake of PUFA (linoleic and linolenic acids) and a lower monounsaturated fatty acid/saturated fatty acid ratio (Ricci et al., 2020) (Table 1). In a case–control study of men attending infertility clinics in Iran (n = 342), the highest tertile of intake of total saturated fatty acids, total trans fatty acids, and palmitic acid had increased odds of asthenozoospermia (low motility) by 85% (OR: 1.85; 95% CI: 1.24–2.96), 153% (OR: 2.53; 95% CI: 1.54–3.92), and 90% (OR: 1.90; 95% CI: 1.26–2.74), respectively, while higher intakes of omega-3 PUFAs reduced the odds of asthenozoospermia by 34% (OR: 0.68; 95% CI: 0.58–0.94) (Eslamian et al., 2015) (Table 1). Finally, a cross-sectional study of 260 infertile men in an Iranian fertility clinic reported that abnormal sperm concentrations were 5.6 times (95% CI: 1.39–22.82) higher in the third quartile of dietary cholesterol intake and 4.8 times (95% CI: 1.30–17.87) higher in the third quartile of saturated fatty acid intake in comparison to the first (lowest) quartile (g/day) (P = 0.01) (Nouri et al., 2022b) (Table 1).
Protein
Three studies reported on protein intake and semen parameters, with none reporting on clinical outcomes. In the Spanish case–control study of 61 men undergoing fertility treatment, higher protein consumption was associated with poorer sperm quality (concentration, motility, and morphology; highest versus lowest tertile, OR: 8.27 [95% CI: 1.58–43.34], P = 0.011), but not with semen volume (Mendiola et al., 2010) (Table 1). Conversely, a cross-sectional study of 260 infertile men in Iran found a significant positive association between protein intake and semen volume (r = 0.18, P < 0.001), but not with other measures of sperm quality (Nouri et al., 2022b) (Table 1). Another study in the same Iranian population reported that, compared with the lowest tertile, those in the highest tertile of animal protein consumption had a higher risk of abnormal sperm concentration (OR: 2.42; 95% CI: 1.13–5.19) and morphology (OR: 3.68; 95% CI: 0.97–13.91), while those in the highest tertile of plant protein consumption had a lower risk of abnormal sperm concentration (OR: 0.31; 95% CI: 0.14–0.65), but no difference in other sperm parameters (Abdollahi et al., 2022) (Table 1).
Carbohydrates and fibre
In the same study of 260 infertile Iranian men, carbohydrate intake (g/day) was positively associated with sperm concentration (P = 0.01) and motility (P = 0.03) (Nouri et al., 2022b) (Table 1). The Spanish case–control study of 61 men also found that those with poor sperm parameters had lower carbohydrate consumption (highest versus lowest tertile: OR 0.09 [95% CI: 0.02–0.59], P = 0.01) and the lowest intake of fibre (highest versus lowest tertile: OR 0.13 [95% CI: 0.02–0.68]) (Mendiola et al., 2010) (Table 1).
Core food groups
Dairy
The relationship between dairy intake and male fertility was reported in five studies (Mendiola et al., 2009; Braga et al., 2012; Afeiche et al., 2014a; Xia et al., 2016; Ricci et al., 2020). Of these, only one study reported on clinical ART outcomes, finding no association between dairy intake and fertilization, implantation, clinical pregnancy or live birth rates (n = 141; 246 ART cycles) (Xia et al., 2016). For semen/sperm parameters, the Spanish case–control study of 61 men reported that those with poor sperm quality were more likely to consume higher intakes of high fat dairy (Mendiola et al., 2009) (Table 1). A prospective study of 155 men attending a US fertility clinic reported that total dairy intake was not associated with semen volume or sperm morphology. However, men in the highest quartile of low-fat dairy intake (1.22–3.54 servings/day) had 33.3% (95% CI: 0.6–55.2) higher sperm concentration and 9.3% (95% CI: 1.4–17.2) higher progressive motility, compared with the lowest quartile (<0.28 servings/day) (Afeiche et al., 2014a) (Table 1). The remaining studies reported no significant associations with dairy consumption (Braga et al., 2012; Ricci et al., 2020) (Table 1).
Meat, legumes, and eggs
Five studies assessed whether male fertility was influenced by meat intake; two of these examined clinical outcomes with conflicting results. The first was a Brazilian prospective study of 250 couples undergoing ICSI, which found that red meat but not legume consumption in men was negatively associated with implantation rate and chance of pregnancy, but not with semen parameters (Braga et al., 2012) (Table 1). The second was a prospective US study of 141 couples undergoing ART, which reported no significant relationship between meat intake and implantation, clinical pregnancy, or live birth rates, but found a positive association between poultry intake and fertilization rate (Ptrend = 0.02) (Xia et al., 2015) (Table 1).
All five studies assessed semen/sperm parameters, but only the study by Ricci et al., (2020) found a significant relationship, where men with lower semen volume consumed meat less frequently, while those with a sperm count <15 million/ml reported more frequent meat and meat product consumption (P = 0.03) (Ricci et al., 2020) (Table 1). Four studies found no associations with semen or sperm parameters (Mendiola et al., 2009; Braga et al., 2012; Afeiche et al., 2014b; Xia et al., 2015).
In the same Brazilian study of 250 ICSI patients, higher legume consumption was associated with increased sperm concentration (Braga et al., 2012), while the case–control study of 61 men in Spain found no relationship between legume or egg consumption and any sperm parameters (Mendiola et al., 2009) (Table 1).
Soy
A prospective US study of 184 men undergoing IVF reported no association between soy intake and any clinical/ART outcome, including embryo development, implantation, clinical pregnancy or live birth rates (Minguez-Alarcon et al., 2015) (Table 1). In a second prospective US study of 99 male partners of subfertile couples, men in the highest category of soy consumption (≥0.30 servings/day) had on average 41 million/ml less sperm than men who did not consume soy foods (95% CI: −74 to −8; P = 0.02) (Chavarro et al., 2008).
Fish/seafood
Five studies examined fish or seafood intake and fertility outcomes (Mendiola et al., 2009; Braga et al., 2012; Afeiche et al., 2014b; Gaskins et al., 2018; Ricci et al., 2020). For clinical outcomes, one study found no relationship between less than weekly fish consumption and fertilization, implantation, pregnancy, or miscarriage rates (Braga et al., 2012) (Table 1). In contrast, a prospective US study found that fecundity (measured by time to pregnancy) was increased by 47% (95% CI: 7–103%) in couples attempting to conceive naturally when men consumed ≥8 servings of seafood per cycle, compared with those who consumed <1 serving per cycle (n = 501). Fecundity was increased by 61% (95% CI: 17–122%) when both male and female partners were consuming ≥8 serves of seafood per cycle (Gaskins et al., 2018) (Table 1).
Sperm parameters were assessed in a prospective US study of 155 male patients undergoing IUI or ART, which found that higher total fish intake (≥2.4 servings/week) was associated with higher total sperm count (Ptrend = 0.005) and percentage of morphologically normal sperm (Ptrend = 0.01) compared with those who consumed <0.8 servings/week (Afeiche et al., 2014b) (Table 1). The Spanish case–control study of 61 men also reported that those with oligo- or teratozoospermia had lower intake of shellfish (P = 0.006) compared with normospermic controls (Mendiola et al., 2009). However, two studies among men undergoing ART or ICSI found no association between fish consumption and various sperm parameters (Braga et al., 2012; Ricci et al., 2020).
Cereals
Two studies reported on consumption of cereals and fertility outcomes. The prospective Brazilian study by Braga et al., (2012) of 250 men undergoing ICSI cycles reported that cereal consumption was positively related to sperm concentration and motility (P < 0.01), but not to clinical outcomes (fertilization, implantation, pregnancy, or miscarriage rates) (Braga et al., 2012) (Table 1). Cereal consumption in the second study (Ricci et al., 2020) was inversely associated with semen volume in multivariable analysis (P = 0.012), but not with any other semen/sperm parameter measured (Table 1).
Fruits and vegetables
No clinical outcomes were assessed in relation to fruit and vegetable intake, with the exception of one study by Braga et al., (2012), reporting no association with fertility treatment outcomes. For semen/sperm parameters, three studies reported on vegetable intake (Mendiola et al., 2009; Braga et al., 2012; Ricci et al., 2020), of which two also examined fruit intake (Mendiola et al., 2009; Braga et al., 2012). Braga et al. (2012) found a positive association between higher fruit consumption and higher sperm motility, but no relationships with other sperm measures (Braga et al., 2012) (Table 1). Mendiola et al. (2009) reported lower consumption of apricots and peaches in men with poor sperm quality (low sperm concentration, motility, and/or morphology). Raw vegetable consumption was positively associated with sperm quality, while higher potato consumption was associated with poorer sperm parameters (Mendiola et al., 2009) (Table 1). Ricci et al. (2020) reported a positive association between vegetable intake and sperm concentration and total sperm count, but not with sperm motility or semen volume (Ricci et al., 2020) (Table 1).
Discretionary foods
Sugar-sweetened beverages and foods
A large US study of 1045 male partners of couples planning pregnancy found that fecundity, measured by time to pregnancy, was reduced in those who consumed ≥7 sugar-sweetened beverages per week compared with none (fecundability ratio = 0.78, 95% CI = 0.63–0.95) (Table 1). Fecundity was further reduced among those who consumed ≥7 sugar-sweetened sodas per week (fecundability ratio = 0.67, 95% CI = 0.51–0.89) (Hatch et al., 2018) (Table 1). The Brazilian study by Braga et al. (2012) found no significant association between intake of sugar-sweetened beverages or sweet foods and clinical outcomes (fertilization, implantation, pregnancy, or miscarriage rates) or sperm concentration, motility, or morphology (Braga et al., 2012). The smaller Spanish case–control study of 61 men found that those with a higher intake of sweets were more likely to have normal sperm parameters (P = 0.027) (Mendiola et al., 2009) (Table 1).
Processed meat
Three studies reported on processed meat intake and fertility outcomes (Mendiola et al., 2009; Afeiche et al., 2014b; Xia et al., 2015). Clinical outcomes were reported in one study among couples undergoing IVF, with a 28% lower fertilization rate among men in the highest quartile of processed meat intake (0.62–2.79 servings/day) compared with the lowest quartile (<0.22 servings/day) (Xia et al., 2015) (Table 1).
Sperm parameters were assessed in the US prospective study of 155 men attending a fertility clinic. Men in the highest quartile of processed meat intake (0.56–2.79 servings/day) had 23.2% fewer morphologically normal sperm than those in the lowest quartile (<0.23 servings/day), with no differences in other semen quality indicators (total sperm count, sperm concentration, progressive motility, or semen volume) (Afeiche et al., 2014b) (Table 1). Finally, Mendiola et al., (2009) reported that men with oligo- or teratazoospermia had a higher intake of processed meat products than normozoospermic controls (Mendiola et al., 2009) (Table 1).
Dietary patterns
Nineteen studies examined whole diet patterns, which included eight a priori patterns: MedDiet, alternative Mediterranean diet (aMedDiet), Trichopoulou Mediterranean diet, Panagiotakos Mediterranean diet (Karayiannis et al., 2017; Efrat et al., 2018; Ricci et al., 2019; Salas-Huetos et al., 2019, Salas-Huetos et al., 2022b), HEI, alternative HEI (aHEI) (Efrat et al., 2018; Leilami et al., 2022; Salas-Huetos et al., 2022b), DASH diet (Efrat et al., 2018; Danielewicz et al., 2019; Salas-Huetos et al., 2022b), American Heart Association diet (Salas-Huetos et al., 2022b), and seven posteriori patterns: ‘prudent’ or ‘healthy/health conscious’ diet (Eslamian et al., 2016; Oostingh et al., 2017; Danielewicz et al., 2018; Jurewicz et al., 2018; Mitsunami et al., 2021b), ‘traditional’ (Vujkovic et al., 2009; Shirani et al., 2020; Haeri et al., 2021), ‘plant-based’ (Nouri et al., 2022a; Salas-Huetos et al., 2022b), ‘antioxidant rich’ (Mendiola et al., 2010; Eslamian et al., 2017), ‘empirical diet score’ (Mitsunami et al., 2021a), ‘preconception’ (Twigt et al., 2012).
Mediterranean diet
No clinical outcomes were reported for the MedDiet, but two cross-sectional studies (Karayiannis et al., 2017; Ricci et al., 2019) reported a positive relationship with sperm parameters, with greater compliance (assessed by the validated MedDietScore) associated with better semen quality measures. The first study was in 255 men attending a fertility clinic in Greece, where those in the lowest tertile of the MedDietScore were 2.6 times more likely to have abnormal semen parameters (Karayiannis et al., 2017) (Table 1). The second study by Ricci et al. (2019) reported an association between MedDietScore and semen parameters in 390 men undergoing ART at an Italian fertility clinic. Men in the lowest MedDietScore category were more likely to have lower sperm concentration (OR: 2.42; 95% CI: 1.21–4.83, Ptrend = 0.011) and total sperm count (OR: 2.08; 95% CI: 1.05–4.12, Ptrend = 0.034) compared with the highest category (Ricci et al., 2019) (Table 1).
Mixed dietary patterns
Salas-Huetos et al. (2022b) examined eight different a priori patterns (various MedDiet scores, aMED, HEI, aHEI, DASH, American Heart Association, and Plant-based) in a prospective study of 245 men and their female partners who underwent ART cycles from 2007 to 2020. There were no associations between any of the dietary patterns and clinical outcomes, including live birth, fertilization, implantation, or clinical pregnancy, or with sperm parameters (Salas-Huetos et al., 2022b) (Table 1). In a cross-sectional study of 280 men attending an Israeli fertility clinic from 2012 to 2015, dietary patterns including the HEI, aHEI, aMed, and DASH, were compared (Efrat et al., 2018). Compared with the lowest quartiles, men in the highest quartiles of any of these patterns had significantly higher adjusted mean sperm concentration (by 10%, 49%, and 24% for HEI, aHEI, and DASH, respectively), normal sperm morphology (by 21% and 8% for aHEI and DASH, respectively), and sperm motility (by 6% and 11% for aMed and HEI, respectively) (Table 1). In 260 infertile Iranian men, those in the highest tertile of HEI score had a 61% lower risk of abnormal sperm concentration than those in the lowest tertile (95% CI: 0.15–0.99, P = 0.04) (Leilami et al., 2022) (Table 1). Adherence to the DASH diet was also associated with higher sperm count (ΔT3–T1 = 82.1 million) and concentration (ΔT3–T1 = 24.6 million/ml), but not with other sperm parameters in a Polish cross-sectional study of 207 men attending a reproductive medicine centre (Danielewicz et al., 2019).
Healthy, prudent, or traditional diets
Nine studies examined adherence to ‘traditional’ and ‘healthy’ or ‘prudent’ diets (the latter two defined as having high intakes of fruits, vegetables, whole grains, seafood, and poultry), with some comparing these with ‘unhealthy’ or ‘Western-style’ diets (defined as having a high intake of red and processed meats, fats, refined grains, added sugar, and soft drinks) (Vujkovic et al., 2009; Twigt et al., 2012; Eslamian et al., 2016; Oostingh et al., 2017; Danielewicz et al., 2018; Jurewicz et al., 2018; Shirani et al., 2020; Haeri et al., 2021; Mitsunami et al., 2021b).
In a Polish prospective study (n = 336), the prudent dietary pattern was associated with decreased percentage of sperm with DNA damage, higher testosterone, and higher sperm concentration, but not sperm motility or morphology compared with the Western dietary pattern (Jurewicz et al., 2018) (Table 1). A second Polish cross-sectional study (n = 114) (Danielewicz et al., 2018) reported a significantly higher risk of abnormal progressive sperm motility in the middle (OR: 2.89, 95% CI: 1.03–8.09) and upper (OR: 7.78, 95% CI: 1.52–15.06) tertiles of adherence to a Western diet compared to the bottom tertile, but found no association with other measures of sperm quality (Table 1).
Two studies used principal component analysis, the first of which was a US prospective cohort study of 231 couples undergoing fertility treatment. This study showed no associations between any dietary patterns and clinical outcomes, and reported that an empirical diet score, representing the relationship of a man’s diet with semen quality, was also not associated with any clinical outcome (fertilization rate, probability of implantation, clinical pregnancy, or live birth) (Mitsunami et al., 2021a). However, adherence to the Western diet was positively associated with sperm concentration and inversely associated with sperm morphology (Mitsunami et al., 2021b) (Table 1). In the second study of 129 male partners of pregnant women from the Rotterdam Periconception Cohort (Predict study) (Oostingh et al., 2017), strong adherence to the ‘healthy’ diet pattern, but not the ‘unhealthy’ diet pattern, was associated with sperm concentration (β = 0.278; 95% CI: 0.112–0.444), total sperm count (β = 1.369; 95% CI: 0.244–2.495), progressive motility (β = 4.305; 95% CI: 0.675–7.936), and total motile sperm count (β = 0.319; 95% CI: 0.113–0.526).
Three studies were case–control designs conducted in Iran. The first (n = 342) found that the highest tertiles of adherence to a prudent diet or a Western diet saw a 54% lower risk and 186% higher risk of asthenozoospermia, respectively, compared with the lowest tertile (Eslamian et al., 2016) (Table 1). The second study examined 400 newly diagnosed infertile men and 537 healthy men without infertility (Haeri et al., 2021). Compared with those below the median adherence, men above the median for a healthy diet pattern had a lower risk of infertility (OR: 0.52; 95% CI: 0.33–0.83), while men above the median for Western and mixed dietary patterns had higher risks of infertility (OR: 2.66; 95% CI: 1.70–4.17 and OR: 2.82; 95% CI: 1.75–4.56, respectively) (Table 1). The third study of 260 infertile men (Shirani et al., 2020) found that those in the highest quartile of the traditional Iranian dietary pattern had higher odds of abnormal sperm volume, compared with the lowest quartile (adjusted odds ratio [aOR]: 2.69, 95% CI: 1.06–6.82). The second quartile of the prudent dietary pattern had higher odds of abnormal semen volume in comparison to the lowest quartile (aOR: 4.36, 95% CI: 1.75–10.86). No significant associations were found with dietary diversity scores and semen parameters (Table 1).
Finally, two studies examined traditional Dutch dietary patterns. Vujkovic et al. (2009) reported that following a traditional Dutch eating pattern (higher consumption of meat, potatoes, and whole grains) was correlated with higher sperm concentration (β = 0.06, P = 0.04), while following to a ‘health conscious’ eating pattern was associated with a lower percentage of sperm DNA fragmentation (β = −2.81, P = 0.05) (Vujkovic et al., 2009) (Table 1). Twigt et al. (2012) found no association between adherence to a preconception diet, which met Dutch guidelines, and the chance of ongoing pregnancy in couples undergoing IVF treatment (β = −0.054; OR: 0.95, 95% CI: 0.48–1.86) (Table 1). However, men in the highest quartile of adherence to a prudent eating pattern had 83% lower odds of having abnormal sperm morphology on multivariable analysis (OR: 0.17, 95% CI: 0.02–1.18) (Twigt et al., 2012) (Table 1).
Plant-based or antioxidant-rich diets
Salas-Huetos et al. (2022b) found no association between men’s adherence to a plant-based diet and any clinical ART outcomes, including fertilization, implantation, clinical pregnancy or live birth rates or semen/sperm parameters (Salas-Huetos et al., 2022b) (Table 1). In the cohort of 260 infertile Iranian men, Nouri et al. (2022a) found that greater dietary adherence to the healthful plant-based index (high levels of refined grains, fruits, vegetables, nuts, and vegetable oils) was associated with improved sperm density (T3–T1, P = 0.03), but not other sperm parameters. Adherence to the unhealthful plant-based index (fruit juices, sugar-sweetened beverages, refined grains, potatoes, sweets/desserts, and dairy) was associated with improved sperm motility (T3–T1, P = 0.009) (Nouri et al., 2022a) (Table 1). The Iranian case–control study by Eslamian et al. (2017) (n = 342) reported 51% lower odds of asthenozoospermia (P = 0.004) among men in the highest compared with the lowest tertile of an antioxidant dietary pattern (rich in vitamins E, D and C, zinc, selenium, folate, total fibre, and PUFAs) (Eslamian et al., 2017) (Table 1). Similarly, the Spanish case–control study of 61 men found that those with severe or moderate oligozoospermia (<5 or 5–20 million sperm/ml, respectively) and severe teratozoospermia (<6% normal forms) reported significantly lower consumption of antioxidant nutrients (Mendiola et al., 2010) (Table 1).
Discussion
This scoping review provides the most up to date and comprehensive summary of the literature regarding preconception diet and male fertility, assessed by both clinical outcomes and sperm parameters. The included RCT did not report on clinical outcomes but found that tomato juice may benefit sperm motility. In observational studies, some evidence suggested that increasing fish or reducing sugar-sweetened beverages, processed meat or total fat may improve fecundability. Cereals, soy and dairy showed no associations with clinical outcomes such as pregnancy rates or live birth rates, while there was inconsistent evidence for these outcomes in relation to reducing red meat or consuming a healthy diet pattern. For improving sperm parameters, limited evidence supported increasing fish, fats/fatty acids, carbohydrates and dairy and reducing processed meat, while the evidence for the consumption of fruits, vegetables, cereals, legumes, eggs, red meat, and protein was inconsistent. Healthy diet patterns in general were shown to improve sperm health.
Although improved sperm quality was demonstrated with holistic a priori (e.g. MedDiet, HEI, and aHEI) and posteriori diet patterns (e.g. DASH), associations with clinical outcomes were seldom reported and thus remain unclear. Before recommendations can be made regarding the impact of healthy diet patterns on fertility, clinical outcomes such as time to pregnancy and live birth need to be consistently assessed. The MedDiet, HEI, and aHEI dietary patterns all typically involve increased intake of core food groups such as fruits, vegetables, nuts, legumes, soy, whole grains, fish or seafood, with limited intake of red or processed meat and trans and saturated fats. Similarly, the DASH diet is rich in vegetables, fruits and whole grains, and includes fat-free or low-fat dairy products, fish, poultry, beans and nuts, while limiting foods that are high in saturated fat, such as fatty meats and full-fat dairy products. Despite consistent associations between these dietary patterns and improved sperm parameters, the precise mechanisms remain unclear. It is proposed that higher consumption of anti-inflammatory and antioxidant-rich nutrients in these dietary patterns leads to reduced low-grade inflammation (Alesi et al., 2022). Oxidative stress resulting from inflammation has increasingly been shown to be deleterious for male fertility (Agarwal et al., 2018). This is because the high concentrations of long-chain PUFAs in the plasma membrane of sperm, together with their reduced antioxidant capabilities owing to cytoplasmic shedding during spermiogenesis, make them highly susceptible to oxidative stress and associated damage (Aitken et al., 2022). Although some studies have shown that high-dose oral antioxidant supplementation can improve male fertility by reducing sperm DNA damage (a consequence of oxidative stress) (Tremellen et al., 2007, 2021; Tunc et al., 2009), recent systematic reviews have concluded that evidence in support of antioxidant use for male subfertility is inadequate (Smits et al., 2019; de Ligny et al., 2022). An increased intake of antioxidant-rich foods in the diet (such as through consumption of healthier dietary patterns) may be ideal to optimize the synergistic effects of other nutrients in food, rather than using single or multi-supplements; however, further studies examining clinical outcomes are needed.
Similarly, recommendations for the intake of fats and fatty acids cannot be made owing to the lack of clinical outcomes assessed, although some evidence suggests benefits to sperm parameters. Here, the type of fat appears to be an important influence on sperm health, with higher mono- and polyunsaturated fat consumption generally associated with improved sperm motility and morphology (Attaman et al., 2012; Eslamian et al., 2015; Salas-Huetos et al., 2022a). Several roles for PUFAs have been proposed, including in sperm assembly, anti-apoptosis, eicosanoid formation, hormonal activity, and gene expression alterations including downregulation of the peroxisome proliferator-activated receptor-γ in sperm (Esmaeili et al., 2015). Given that fatty acids can only be obtained through nutritional intake, it is not surprising that a higher intake of mono- and polyunsaturated fats is associated with better sperm quality. On the other hand, diets high in trans fats have been consistently found to decrease sperm counts and testosterone concentrations, thereby reducing chances of conception, but the mechanisms of these actions remain unknown (Chavarro et al., 2014; Çekici and Akdevelioglu 2019). Given the limited evidence, further investigations of these fats and their subtypes in relation to both clinical outcomes and semen parameters are warranted.
In terms of discretionary choices, the association between intake of sugar-sweetened beverages and reduced fecundability is of interest. Increased consumption of simple sugars may lead to higher circulating blood glucose concentrations. This, in turn, increases the production of reactive oxygen species potentially via glucose auto-oxidation, metabolism, the polyol pathway, and formation of advanced glycosylation end products, thereby reducing sperm quality (Bonnefont-Rousselot 2002). Although several studies in women have demonstrated associations between intake of sugar-sweetened beverages and discretionary food choices with fertility (Alesi et al., 2023), further studies in men are needed, along with delineating potential differences in ART versus non-ART populations.
Associations reported between fish/seafood consumption and clinical outcomes are mixed, but higher fish intake appears to benefit both sperm parameters and clinical outcomes. These findings appear to be related to intake, with studies reporting positive clinical outcomes involving consumption at or above recommended intakes of seafood (≥2 servings/week), whereas studies reporting no association involved seafood intake below this amount. The high levels of omega-3 fatty acids and zinc found in seafood may improve sperm measures, since they both have an essential role in spermatogenesis (Fallah et al., 2018; Salas-Huetos et al., 2018), but further studies are needed in both ART and non-ART populations, with clinical outcomes measured to support these findings.
Studies focusing on individual food groups were less convincing, in part, owing to the small number of included studies for each food group and the lack of reporting on clinical outcomes. Higher consumption of fruit and vegetables was associated with better sperm quality, with a protective effect on sperm. This is likely because of their antioxidant, vitamin, and fibre content (Salas-Huetos et al., 2018). While total dairy intake was not consistently associated with male fertility, closer examination of the type of dairy product consumed showed that fertility outcomes may be influenced by saturated fat content. Similarly, associations with fertility measures differed by the type of meat consumed, with meats containing higher amounts of saturated fats and trans fatty acids being associated with poorer sperm parameters. However, it should be noted that each outcome was only reported in a single study.
Finally, carbohydrates and protein have been associated with male fertility through modulation of testosterone production and sperm oxidative stress (Ferramosca and Zara 2022). In the current evidence, no clinical outcomes were reported for protein or carbohydrate studies, and relationships with sperm parameters cannot be established from the few available studies.
Strengths and limitations
This scoping review provides the most current and comprehensive synthesis of the literature exploring the influence of male preconception diet on fertility. We highlight the paucity of RCTs and provide an overview of the observational data in this area. Broad limitations of the literature have been identified in this review and include the heterogeneity of the studies in terms of design, exposures, outcomes assessed, types and ranges of foods, as well as the observational nature of most studies, which can demonstrate associations but not causation. Given that many of the studies are in ART populations, there is also potential for reverse causation in the presented evidence (i.e. knowledge of infertility impacting diet) and results should be interpreted in light of this, since direct biological effects of diet on fertility cannot be ascertained. Notably, several studies were derived from the same prospective cohort (the Environment and Reproductive Health [EARTH] study) (Messerlian et al., 2018) and over one-third of the included studies are from the same author group, raising questions regarding the generalizability of the available data. Further, the majority of studies only examined dietary associations with semen parameters and not with clinical outcomes, and only three assessed both. This highlights a key finding of our review; that is, the need to extend male fertility research beyond sperm parameters, to include pregnancy and other clinical outcomes. Indeed, live birth has been recommended as a core outcome to be included in fertility research (Duffy et al., 2021), including in the context of male fertility.
Limitations of our review are also acknowledged. Owing to the research question and nature of the evidence collated, we opted to conduct a systematic scoping review which does not include quantitative synthesis (meta-analysis) or study-level quality appraisal. Although this is standard practice for scoping reviews, we acknowledge that results drawn from narrative synthesis are not as definitive as those derived from meta-analysis. Moreover, the review focuses on men at preconception, encompassing both pre- or inter-conception periods. The rationale for selecting this population was driven by our predefined primary outcomes, which are clinical in nature, including pregnancy rates, live birth rates, and ART outcomes. Clinical outcomes are not explored in studies of general male populations (i.e. pregnancy rate would not be assessed in men not trying/planning to conceive). Hence, the inclusion of broader population studies (e.g., in university students) was deemed incompatible with our objectives, as this would not capture the outcomes of interest and would likely introduce heterogeneity through variations in age, reproductive intention and the use of contraception, medications or substances, etc. Consequently, the review is overrepresented by preconception men undergoing ART, with only four studies in non-ART populations. The ART population may inherently possess a higher motivation to conceive, greater health consciousness and an increased likelihood of fertility problems, restricting the relevance of our findings to broader populations. While this reduces the generalizability of our results, there is evidence to suggest that many men do not make lifestyle changes in preparation for trying to conceive (Bodin et al., 2017), and although some men attempt to make lifestyle changes after a diagnosis of infertility, these tend to be in relation to reducing alcohol and smoking rather than modifying diet (Hanna and Gough 2022). Finally, we acknowledge that, although the effects of individual food groups or macronutrients on male fertility are of interest, in reality, food is not consumed in isolation. Whole diets, incorporating a combination of nutrients and interactions, are likely to have a greater influence on fertility and sperm quality than single foods or nutrients. This could partially explain the variable outcome responses found with single foods compared with the relatively consistent outcomes reported for dietary patterns. Further studies exploring whole diets rather than singular foods or nutritional components in the context of male fertility are encouraged, particularly by means of RCTs where feasible.
Challenges and future directions
A key limitation emerging in the literature is the reliance on semen analysis as a singular means of determining male fertility status. Semen analysis has been the cornerstone for assessing male fertility and includes assessments of semen volume and sperm count, motility, morphology, and vitality (Patel et al., 2018; World Health Organization 2021). While these parameters are generally considered to be indicative of male fertility status, and total progressive motile sperm count (volume × concentration × progressive motile sperm) is associated with earlier time to conception (Keihani et al., 2021), it is important to recognize that a ‘normal’ semen analysis does not necessarily equate to fecundity and pregnancy success (Sakkas et al., 2015). To establish the latter, clinical outcomes, such as pregnancy and live birth rates, are needed. Yet, less than one-third of the studies (n = 11) reported associations between diet and clinical pregnancy or ART outcomes, and the dietary components or patterns that were associated with semen parameters did not generally show the same association with clinical outcomes. Similarly, dietary factors which were not associated with semen parameters may in fact be clinically relevant. Clinical fertility outcomes should be a key focus of future studies to facilitate the translation of evidence into clinically relevant dietary recommendations for men who are attempting to conceive, with or without fertility treatment.
However, investigating clinical outcomes, such as time to pregnancy and live birth, demands a longitudinal approach, which can be resource-intensive and faces challenges in retaining participants over an extended period. Clinical outcomes also involve both male and female contributions, complicating the assessment of changes solely attributable to male diet. Shared dietary habits among couples pose further challenges in isolating the individual impact of the male diet on fertility, with shared lifestyle factors potentially confounding results. Early consideration of these factors in study design and planning is crucial to maximize participant compliance with assigned dietary interventions and address potential biases.
There is also a clear need for RCTs, which were noticeably lacking in the literature, but these too present unique challenges. The impossibility of blinding of diet interventions can lead to potential contamination and under- or over-estimation of effects, while the highly controlled environment of RCTs limits the external validity of results to broader populations or real-world contexts. Including diverse populations (e.g., both ART and general populations) is important, but adds further complexity, requiring careful consideration of the cultural, socioeconomic, and lifestyle differences which may influence outcomes. To navigate these complex challenges, the design and conduct of future longitudinal studies and RCTs requires rigorous, advance planning around logistics, ethics, study design and recruitment protocols, and robust data collection and analysis methods to enhance the reliability and validity of research findings. Such well-designed studies are now needed to inform dietary guidelines for men in the preconception period and maximize their chances of achieving a healthy pregnancy.
Conclusion
This comprehensive review has identified the dearth of studies, particularly RCTs, focusing on the pre-conception male diet and fertility outcomes. Limited available evidence suggests that increasing fish and reducing sugar-sweetened beverages, processed meat and total fat may improve clinical fertility outcomes. Sperm parameters may be improved by increasing fish, fats/fatty acids, carbohydrates, and dairy, reducing processed meat and following a generally healthy diet pattern. However, specific dietary recommendations for improving male fertility are precluded primarily by the lack of reporting on clinical fertility outcomes, as well as the small number of studies, observational nature of the available evidence, focus on ART populations and the potential for reverse causation, and the heterogeneity in methods and study designs. Before any recommendations for specific food groups, macronutrients, or dietary patterns can be made, rigorous studies including RCTs are needed to improve our understanding of male fertility, beyond semen analysis. Such studies can lay the groundwork for targeted dietary guidelines and enhance the prospects of successful conception for men in the preconception period.
Supplementary Material
Abbreviations
- AHA
American Heart Association
- aHEI
Alternative Healthy Eating Index
- aMedDiet
Alternative Mediterranean Diet
- ART
Assisted reproductive technology
- BMI
Body mass index
- CHO
Carbohydrate
- CI
Confidence interval
- DASH
Dietary Approaches to Stop Hypertension
- DNA
Deoxyribonucleic acid
- HEI
Healthy Eating Index
- IQR
Interquartile range
- IVF
In vitro fertilization
- LA
Linoleic acid
- LNA
Linolenic acid
- MDS
Mediterranean Diet Score
- MedDiet
Mediterranean diet
- MUFA
Monounsaturated fatty acid
- NS
Not significant
- OR
Odds ratio
- PDI
Plant-based diet index
- PUFA
Polyunsaturated fatty acid
- SD
Standard deviation
- SFA
Saturated fatty acid
- TTP
Time to pregnancy
Contributor Information
Cathryn A Tully, Adelaide Medical School, The University of Adelaide, Adelaide, SA, Australia; Repromed, Dulwich, Adelaide, SA, Australia.
Simon Alesi, Monash Centre for Health Research and Implementation (MCHRI), Monash University, Clayton, VIC, Australia.
Nicole O McPherson, Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia; Freemasons Center for Male Health and Wellbeing, The University of Adelaide, Adelaide, SA, Australia; School of Biomedicine, Discipline of Reproduction and Development, The University of Adelaide, Adelaide, SA, Australia.
David J Sharkey, Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia; School of Biomedicine, Discipline of Reproduction and Development, The University of Adelaide, Adelaide, SA, Australia.
Xiao Tong Teong, Adelaide Medical School, The University of Adelaide, Adelaide, SA, Australia; Lifelong Health Theme, South Australian Health and Medical Research Institute, Adelaide, SA, Australia.
Chau Thien Tay, Monash Centre for Health Research and Implementation (MCHRI), Monash University, Clayton, VIC, Australia.
Thais Rasia Silva, Postgraduate Program in Endocrinology and Metabolism, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Carolyn Puglisi, School of Agriculture, Food and Wine, Faculty of Sciences, University of Adelaide, Waite Campus, Urrbrae, SA, Australia.
Jacqueline P Barsby, Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia; School of Agriculture, Food and Wine, Faculty of Sciences, University of Adelaide, Waite Campus, Urrbrae, SA, Australia.
Lisa J Moran, Monash Centre for Health Research and Implementation (MCHRI), Monash University, Clayton, VIC, Australia; Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia.
Jessica A Grieger, Adelaide Medical School, The University of Adelaide, Adelaide, SA, Australia; Robinson Research Institute, The University of Adelaide, Adelaide, SA, Australia.
Aya Mousa, Monash Centre for Health Research and Implementation (MCHRI), Monash University, Clayton, VIC, Australia.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
No new data were generated or analysed in support of this work. All data are available via published manuscripts cited in this article.
Authors’ roles
S.A., N.M., D.S., X.T.T., C.T.T., T.R., C.P., J.B., L.M., J.G., and A.M. assisted in title and abstract screening. S.A., D.S., X.T.T., C.T.T., T.R., L.M., N.M., and J.G. assisted in full-text screening and data extraction. C.T., J.G., and A.M. provided intellectual input. C.T. and S.A. reviewed and synthesized the extracted data and C.T. wrote the first draft of the manuscript with assistance from S.A., J.G., and A.M. J.G., A.M., and L.M. conceptualized and determined the scope of the manuscript and supervised the review process. All authors meet ICMJE criteria for authorship and all authors reviewed and approved the final version for publication.
Funding
This work received no specific funding. C.T. is supported by a Monash IVF Group PhD Scholarship. S.A. is supported by a Monash University Faculty Graduate Research Stipend. A.M. is supported by a Peter Doherty Biomedical Research Fellowship provided by the National Health and Medical Research Council (NHMRC) of Australia. J.G. is supported by a National Health and Medical Research Council (NHMRC) grant (APP2000905). N.M. is supported by an Australian Research Council (ARC) DECRA (DE220101449). T.R.S. is a recipient of a Fellowship provided by the Fundação de Apoio à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/Brazilian National Institute of Hormones and Women’s Health/Conselho Nacional de Desenvolvimento Científico e Tecnológico) (FAPERGS INCT 17/2551-0000519-8).
Conflict of interest
All authors declare no conflicts of interest.
References
- Abdollahi N, Nouri M, Leilami K, Mustafa YF, Shirani M.. The relationship between plant and animal based protein with semen parameters: a cross-sectional study in infertile men. Clin Nutr ESPEN 2022;49:372–377. [DOI] [PubMed] [Google Scholar]
- Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE.. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 2014a;101:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE.. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr 2014b;144:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner Selvam MK, Shah R.. Male infertility. Lancet 2021;397:319–333. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R.. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018;50:e13126. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Drevet JR, Moazamian A, Gharagozloo P.. Male infertility and oxidative stress: a focus on the underlying mechanisms. Antioxidants (Basel) 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alesi S, Habibi N, Silva TR, Cheung N, Torkel S, Tay CT, Quinteros A, Winter H, Teede H, Mousa A. et al. Assessing the influence of preconception diet on female fertility: a systematic scoping review of observational studies. Hum Reprod Update 2023;29:811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alesi S, Villani A, Mantzioris E, Takele WW, Cowan S, Moran LJ, Mousa A.. Anti-inflammatory diets in fertility: an evidence review. Nutrients 2022;14(19):3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE.. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin M, Kall L, Tyden T, Stern J, Drevin J, Larsson M.. Exploring men's pregnancy-planning behaviour and fertility knowledge: a survey among fathers in Sweden. Ups J Med Sci 2017;122:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 2002;5:561–568. [DOI] [PubMed] [Google Scholar]
- Braga DPdAF, Halpern G, Figueira RdCS, Setti AS, Iaconelli A, Borges E Jr. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril 2012;97:53–59. [DOI] [PubMed] [Google Scholar]
- Campbell JM, Lane M, Owens JA, Bakos HW.. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod Biomed Online 2015;31:593–604. [DOI] [PubMed] [Google Scholar]
- Cao LL, Chang JJ, Wang SJ, Li YH, Yuan MY, Wang GF, Su PY.. The effect of healthy dietary patterns on male semen quality: a systematic review and meta-analysis. Asian J Androl 2022;24:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çekici H, Akdevelioğlu Y.. The association between trans fatty acids, infertility and fetal life: a review. Hum Fertil (Camb) 2019;22:154–163. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Minguez-Alarcon L, Mendiola J, Cutillas-Tolin A, Lopez-Espin JJ, Torres-Cantero AM.. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod 2014;29:429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Sadio SM, Hauser R.. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod 2008;23:2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielewicz A, Morze J, Przybyłowicz M, Przybyłowicz KE.. Association of the dietary approaches to stop hypertension, physical activity, and their combination with semen quality: a cross-sectional study. Nutrients 2019;12(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielewicz A, Przybyłowicz KE, Przybyłowicz M.. Dietary patterns and poor semen quality risk in men: a cross-sectional study. Nutrients 2018;10(9):1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ligny W, Smits RM, Mackenzie-Proctor R, Jordan V, Fleischer K, de Bruin JP, Showell MG.. Antioxidants for male subfertility. Cochrane Database Syst Rev 2022;5:CD007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JMN, AlAhwany H, Bhattacharya S, Collura B, Curtis C, Evers JLH, Farquharson RG, Franik S, Giudice LC, Khalaf Y. et al. ; Core Outcome Measure for Infertility Trials (COMMIT) Initiative. Developing a core outcome set for future infertility research: an international consensus development study. Fertil Steril 2021;115:191–200. [DOI] [PubMed] [Google Scholar]
- Efrat M, Stein A, Pinkas H, Unger R, Birk R.. Dietary patterns are positively associated with semen quality. Fertil Steril 2018;109:809–816. [DOI] [PubMed] [Google Scholar]
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Baghestani AR, Hekmatdoost A.. Dietary fatty acid intakes and asthenozoospermia: a case-control study. Fertil Steril 2015;103:190–198. [DOI] [PubMed] [Google Scholar]
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Baghestani AR, Hekmatdoost A.. Adherence to the Western pattern is potentially an unfavorable indicator of asthenozoospermia risk: a case-control study. J Am Coll Nutr 2016;35:50–58. [DOI] [PubMed] [Google Scholar]
- Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A.. Nutrient patterns and asthenozoospermia: a case-control study. Andrologia 2017;49(3):e12624. [DOI] [PubMed] [Google Scholar]
- Esmaeili V, Shahverdi AH, Moghadasian MH, Alizadeh AR.. Dietary fatty acids affect semen quality: a review. Andrology 2015;3:450–461. [DOI] [PubMed] [Google Scholar]
- Fallah A, Mohammad-Hasani A, Colagar AH.. Zinc is an essential element for male fertility: a review of Zn roles in men's health, germination, sperm quality, and fertilization. J Reprod Infertil 2018;19:69–81. [PMC free article] [PubMed] [Google Scholar]
- Ferramosca A, Zara V.. Diet and male fertility: the impact of nutrients and antioxidants on sperm energetic metabolism. Int J Mol Sci 2022;23(5):2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli R, Mottola F, Agarwal A.. Impact of alcohol consumption on male fertility potential: a narrative review. Int J Environ Res Public Health 2021;19(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Sundaram R, Buck Louis GM, Chavarro JE.. Seafood intake, sexual activity, and time to pregnancy. J Clin Endocrinol Metab 2018;103:2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller RJ, Wesselink AK, Koenig MR, Eisenberg ML, Tucker KL, Hatch EE, Wise LA.. Association of male fatty acid intake with fecundability among couples planning pregnancy. Hum Reprod 2023;38:1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri F, Pourmasoumi M, Ghiasvand R, Feizi A, Salehi-Abargouei A, Marvast LD, Clark CCT, Mirzaei M.. The relationship between major dietary patterns and fertility status in Iranian men: a case-control study. Sci Rep 2021;11:18861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna ES, Gough B.. Are Men Modifying Their Lifestyles to Optimise Fertility Success? (in)Fertile Male Bodies. Bingley: Emerald Publishing Limited, 2022, 49–60. [Google Scholar]
- Hatch EE, Wesselink AK, Hahn KA, Michiel JJ, Mikkelsen EM, Sorensen HT, Rothman KJ, Wise LA.. Intake of sugar-sweetened beverages and fecundability in a north American preconception cohort. Epidemiology 2018;29:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Radwan M, Sobala W, Radwan P, Bochenek M, Hanke W.. Dietary patterns and their relationship with semen quality. Am J Mens Health 2018;12:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N.. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod 2017;32:215–222. [DOI] [PubMed] [Google Scholar]
- Keihani S, Verrilli LE, Zhang C, Presson AP, Hanson HA, Pastuszak AW, Johnstone EB, Hotaling JM.. Semen parameter thresholds and time-to-conception in subfertile couples: how high is high enough? Hum Reprod 2021;36:2121–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leilami K, Zareie A, Nouri M, Bagheri M, Shirani M.. The association between healthy eating index score with semen parameters in infertile men: a cross-sectional study. Int J Reprod Biomed 2022;20:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisegang K, Dutta S.. Do lifestyle practices impede male fertility? Andrologia 2021;53:e13595. [DOI] [PubMed] [Google Scholar]
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH.. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R.. Food intake and its relationship with semen quality: a case-control study. Fertil Steril 2009;91:812–818. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Torres-Cantero AM, Vioque J, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R.. A low intake of antioxidant nutrients is associated with poor semen quality in patients attending fertility clinics. Fertil Steril 2010;93:1128–1133. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, Braun JM, Gaskins AJ, Meeker JD, James-Todd T. et al. ; EARTH Study Team. The Environment and Reproductive Health (EARTH) study: a prospective preconception cohort. Hum Reprod Open 2018;2018(2):hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Afeiche MC, Chiu YH, Vanegas JC, Williams PL, Tanrikut C, Toth TL, Hauser R, Chavarro JE.. Male soy food intake was not associated with in vitro fertilization outcomes among couples attending a fertility center. Andrology 2015;3:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunami M, Salas-Huetos A, Minguez-Alarcon L, Attaman JA, Ford JB, Kathrins M, Souter I, Chavarro JE.. A dietary score representing the overall relation of men's diet with semen quality in relation to outcomes of infertility treatment with assisted reproduction. F S Rep 2021a;2:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunami M, Salas-Huetos A, Minguez-Alarcon L, Attaman JA, Ford JB, Kathrins M, Souter I, Chavarro JE; E.S. Team. Men's dietary patterns in relation to infertility treatment outcomes among couples undergoing in vitro fertilization. J Assist Reprod Genet 2021b;38:2307–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E.. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri M, Abdollahi N, Leilami K, Shirani M.. The relationship between plant-based diet index and semen parameters of men with infertility: a cross-sectional study. Int J Fertil Steril 2022a;16:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri M, Mehrabani S, Firoozbakht H, Vataniyan E, Abbasi H, Shirani M.. The association between dietary fat and mineral intake with semen parameters: a cross-sectional study in infertile men. Int J Reprod Biomed 2022b;20:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostingh EC, Steegers-Theunissen RP, de Vries JH, Laven JS, Koster MP.. Strong adherence to a healthy dietary pattern is associated with better semen quality, especially in men with poor semen quality. Fertil Steril 2017;107:916–923.e2. [DOI] [PubMed] [Google Scholar]
- Patel AS, Leong JY, Ramasamy R.. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: a systematic review. Arab J Urol 2018;16:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci E, Bravi F, Noli S, Ferrari S, De Cosmi V, Vecchia IL, Cavadini M, La Vecchia C, Parazzini F.. Mediterranean diet and the risk of poor semen quality: cross-sectional analysis of men referring to an Italian Fertility Clinic. Andrology 2019;7:156–162. [DOI] [PubMed] [Google Scholar]
- Ricci E, Noli S, Ferrari S, La Vecchia I, Castiglioni M, Cipriani S, Somigliana E, Parazzini F, Agostoni C.. Fatty acids, food groups and semen variables in men referring to an Italian Fertility Clinic: cross-sectional analysis of a prospective cohort study. Andrologia 2020;52:e13505. [DOI] [PubMed] [Google Scholar]
- Sakkas D, Ramalingam M, Garrido N, Barratt CL.. Sperm selection in natural conception: what can we learn from Mother Nature to improve assisted reproduction outcomes? Hum Reprod Update 2015;21:711–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Arvizu M, Minguez-Alarcon L, Mitsunami M, Ribas-Maynou J, Yeste M, Ford JB, Souter I, Chavarro JE; E.S. Team. Women's and men's intake of omega-3 fatty acids and their food sources and assisted reproductive technology outcomes. Am J Obstet Gynecol 2022a;227:246.e1–246.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Babio N, Carrell DT, Bullo M, Salas-Salvado J.. Adherence to the Mediterranean diet is positively associated with sperm motility: a cross-sectional analysis. Sci Rep 2019;9:3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Minguez-Alarcon L, Mitsunami M, Arvizu M, Ford JB, Souter I, Yeste M, Chavarro JE; E.S. Team. Paternal adherence to healthy dietary patterns in relation to sperm parameters and outcomes of assisted reproductive technologies. Fertil Steril 2022b;117:298–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Rosique-Esteban N, Becerra-Tomas N, Vizmanos B, Bullo M, Salas-Salvado J.. The effect of nutrients and dietary supplements on sperm quality parameters: a systematic review and meta-analysis of randomized clinical trials. Adv Nutr 2018;9:833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone A, Di Dato C, de Angelis C, Menafra D, Pozza C, Pivonello R, Isidori A, Gianfrilli D.. Smoke, alcohol and drug addiction and male fertility. Reprod Biol Endocrinol 2018;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirani M, Saneei P, Nouri M, Maracy MR, Abbasi H, Askari G.. Associations of major dietary patterns and dietary diversity score with semen parameters: a cross-sectional study in Iranian infertile men. Int J Fertil Steril 2020;14:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG.. Antioxidants for male subfertility. Cochrane Database Syst Rev 2019;3:CD007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K, Miari G, Froiland D, Thompson J.. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol 2007;47:216–221. [DOI] [PubMed] [Google Scholar]
- Tremellen K, Woodman R, Hill A, Shehadeh H, Lane M, Zander-Fox D.. Use of a male antioxidant nutraceutical is associated with superior live birth rates during IVF treatment. Asian J Androl 2021;23:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc O, Thompson J, Tremellen K.. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online 2009;18:761–768. [DOI] [PubMed] [Google Scholar]
- Twigt JM, Bolhuis ME, Steegers EA, Hammiche F, van Inzen WG, Laven JS, Steegers-Theunissen RP.. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum Reprod 2012;27:2526–2531. [DOI] [PubMed] [Google Scholar]
- Van Heertum K, Rossi B.. Alcohol and fertility: how much is too much? Fertil Res Pract 2017;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP.. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod 2009;24:1304–1312. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Fact Sheet: Infertility. https://www.who.int/news-room/fact-sheets/detail/infertility (14 April 2023, date last accessed).
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: World Health Organization, 2021. [Google Scholar]
- Xia W, Chiu YH, Afeiche MC, Williams PL, Ford JB, Tanrikut C, Souter I, Hauser R, Chavarro JE; E.S. Team. Impact of men's dairy intake on assisted reproductive technology outcomes among couples attending a fertility clinic. Andrology 2016;4:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Chiu YH, Williams PL, Gaskins AJ, Toth TL, Tanrikut C, Hauser R, Chavarro JE.. Men's meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil Steril 2015;104:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Aizawa K, Mieno M, Karamatsu M, Hirano Y, Furui K, Miyashita T, Yamazaki K, Inakuma T, Sato I.. The effects of tomato juice on male infertility. Asia Pac J Clin Nutr 2017;26:65–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this work. All data are available via published manuscripts cited in this article.