Extract
Current rifampicin/ethambutol/azithromycin regimens for the treatment of Mycobacterium avium complex pulmonary disease (MAC-PD) are long, toxic and yield relatively poor outcomes [1]: a meta-analysis lumping nodular bronchiectatic disease and fibro-cavitary disease reported a 65% prolonged culture conversion rate; following initially successful treatment, recurrence rates of 30% have been reported [2].
Shareable abstract
Rifampicin is used for the treatment of Mycobacterium avium complex pulmonary disease, but pharmacokinetic and pharmacodynamic studies suggest that rifampicin cannot have therapeutic utility. We need to find better alternatives, using PK-PD science. https://bit.ly/3PUGvbV
Current rifampicin/ethambutol/azithromycin regimens for the treatment of Mycobacterium avium complex pulmonary disease (MAC-PD) are long, toxic and yield relatively poor outcomes [1]: a meta-analysis lumping nodular bronchiectatic disease and fibro-cavitary disease reported a 65% prolonged culture conversion rate; following initially successful treatment, recurrence rates of 30% have been reported [2].
The MAC-PD literature often reports an apparent disconnect between in vitro susceptibility of causative bacteria and clinical outcomes, especially for classic antituberculosis drugs such as rifampicin and ethambutol [3]. However, a review of published minimal inhibitory concentration (MIC) data, pharmacokinetic/pharmacodynamic (PK-PD) parameters and outcomes shows that there is no such disconnect for rifampicin. Three distinct lines of evidence show that rifampicin is simply inactive in MAC-PD treatment. This needs to become common knowledge amongst pulmonologists, infectious disease physicians and clinical microbiologists, as well as in the nontuberculous mycobacteria (NTM) drug discovery community.
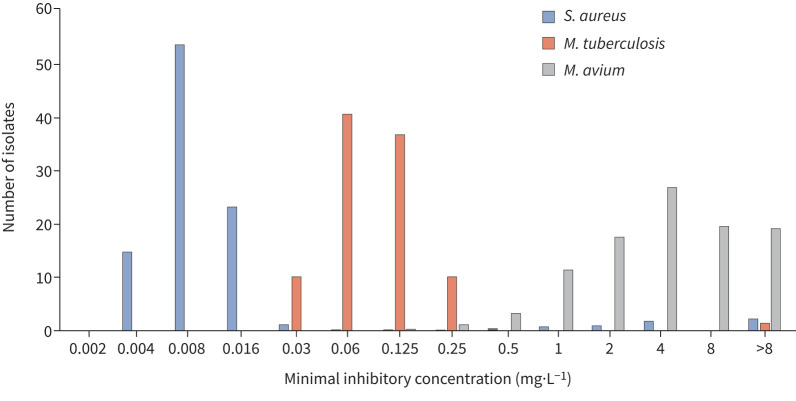
First, MIC distributions of rifampicin against Mycobacterium tuberculosis, Staphylococcus aureus and M. avium (figure 1) show that rifampicin MICs against M. avium (as a representative of MAC) are far above current clinical breakpoints for S. aureus (0.06 mg·L−1; www.eucast.org) and M. tuberculosis (0.5 mg·L−1 [4–6]). It has been suggested that this low intrinsic activity is overcome by synergy with other drugs in the regimen, i.e. ethambutol and macrolides. Yet in vitro studies that show this synergy also show that even in combinations, rifampicin MICs never come below 0.5 mg·L−1 and still preclude meeting pharmacodynamic targets [7, 8]. The clinical relevance of this synergy remains unproven.
FIGURE 1.
Rifampicin minimal inhibitory concentration distributions for Mycobacterium avium in comparison to Mycobacterium tuberculosis and Staphylococcus aureus. Breakpoints for S. aureus and M. tuberculosis are 0.06 and 0.5 mg·L−1, respectively. Data from [4–6].
Second, PK-PD studies preclude a role of rifampicin in MAC-PD treatment. Recently, a target area under the time–concentration curve (AUC)/MIC ratio of >197.3 was identified as driving efficacy of rifampicin against MAC [9]. Given the previously recorded rifampicin mean AUC of 68.42 mg·h·L−1 in MAC-PD patients [8], only MAC isolates with MICs of (68.42/197.3) 0.35 mg·L−1 or lower can be successfully treated with rifampicin. The median MIC of 4 mg·L−1 (figure 1) shows that AUCs as high as (197.3×4) 789.2 mg·h·L−1 are needed for rifampicin to become effective; even the intolerable 50 mg·kg−1 dose in tuberculosis patients failed to achieve such exposures, with a mean AUC of 571 mg·h·L−1 [10]. Even when accounting for synergy, these pharmacodynamic targets are not met [7, 8].
The hope that rifampicin accumulation at the site of disease and hypothetical physiological properties of MAC in lung lesions may compensate for the unachievable AUC/MIC ratio is unsubstantiated. In fact, a recent hollow fibre model study of M. avium pulmonary disease, which accounted for site-of-infection-specific (i.e. epithelial lining fluid) and intracellular pharmacokinetics of rifampicin, found that omission of rifampicin from the treatment regimen did not have any impact on antimycobacterial activity [11].
Third, two clinical studies in patients with nodular bronchiectatic MAC-PD have now suggested, in line with the hollow fibre model observation, that two-drug ethambutol/macrolide regimens can be as efficacious as the classic rifampicin-containing three-drug regimen [12, 13]. Both studies noted that high bacterial loads (cavities, smear positivity) increased the risk of treatment failure [12, 13], but the same is true for the rifampicin-containing regimen [14], so that is not a consequence of omitting rifampicin. Two-drug regimens did not present an increased risk for the emergence of macrolide resistance, neither in the clinical studies [12, 13], nor in the hollow fibre model [11]. For MAC-PD with high bacterial loads, three-drug regimens are likely required, but the third drug should not be rifampicin. For MAC-PD with low bacterial loads, as in most patients with nodular bronchiectatic disease, a randomised trial of ethambutol/azithromycin versus rifampicin/ethambutol/azithromycin treatment is currently ongoing (ClinicalTrials.gov identifier: NCT03672630). This will more definitively show whether rifampicin adds any activity to MAC-PD regimens.
In addition to being inactive in itself, rifampicin also negatively affects the pharmacokinetics of macrolides and other antibiotics via CYP3A4 induction; rifampicin reduces azithromycin exposure by 30% and clarithromycin exposure by 65% [8]. Azithromycin peak blood concentrations >0.4 mg·L−1 are known to be associated with good treatment outcomes in MAC-PD [15]. Concurrent administration of rifampicin leads to mean azithromycin peak concentrations of 0.27±0.18 mg·L−1, as compared to the more favourable 0.35±0.26 mg·L−1 in the absence of rifamycins in MAC-PD patients [8]. The clinical significance hereof has been contested, using azithromycin's well-known accumulation in the lung and inside macrophages as an argument. Yet this accumulation is a result of a gradient, resulting from transporters and passive diffusion, evidenced by stable blood:epithelial lining fluid and blood:alveolar macrophage concentration ratios [16]. The lower blood concentrations thus, in turn, also lead to lower azithromycin concentrations at the site of infection.
There is no rationale for NTM disease and treatment being immune to the principles of PK-PD science. Rather, we should come to the sobering realisation that rifampicin appears in MAC-PD treatment guidelines but does not add any appreciable activity to the regimens. The so-called discrepancy between in vitro and in vivo activity is no more: rifampicin is inactive in vitro and in vivo against MAC. The time is now to consider removing rifamycins from MAC-PD therapies.
Along the same lines, we should reconsider the true role and optimal dosing of all other commonly applied drugs, particularly ethambutol, clofazimine and azithromycin. We need to move forward and improve regimens using PK-PD science and rational regimen design. For azithromycin and clofazimine, peak serum levels are predictive of good treatment outcomes [15, 17], providing the incentive to explore higher dosing for MAC. Omitting rifampicin from regimens will be an important first step to optimising azithromycin exposure [8].
As a short-term solution, could any repurposed antibiotics or clinical development candidates replace rifampicin against MAC-PD? A reasonable target product profile may include oral bioavailability or delivery via inhalation, strong bactericidal and sterilising activity, acceptable tolerability, absence of pharmacokinetic interactions with other relevant antimycobacterial drugs and 6-month treatment duration to deliver cure within a combination. Several repurposed oral and inhaled drugs either are in clinical development for NTM disease or exhibit attractive PK-PD properties and could be considered: high-dose clofazimine, SPR720 by Spero Pharmaceuticals, omadacycline by Paratek Pharmaceuticals, epetraborole by AN2 Therapeutics, amikacin liposome inhalation suspension by Insmed and inhaled clofazimine by MannKind (for all trials, see https://clinicaltrials.gov/). Whether these new candidates for inclusion in regimens meet the target product profile and achieve PK-PD targets at tolerated doses remains to be determined.
As a longer-term strategy, chemical optimisation of the rifamycins to overcome intrinsic resistance could be considered to rehabilitate the class for the treatment of MAC-PD. This approach has delivered promising preclinical results for Mycobacterium abscessus [18]. A similar strategy could be applied for other bactericidal drugs, such as fluoroquinolones, suffering from unfavourable MIC distributions in MAC [4].
In summary, the role of rifampicin in the treatment of MAC-PD is questionable and not supported by PK-PD science; its in vitro activity is low, PK-PD targets cannot be attained by safe and tolerable doses and preclinical (as well as clinical) studies suggest that it does not add any activity to the ethambutol/azithromycin backbone and does not prevent the emergence of macrolide resistance. Its negative pharmacokinetic interactions with macrolides are another important reason to reconsider its place in treatment regimens. Its simple omission, thus two-drug regimens, may be suitable for MAC-PD with low bacterial loads. High failure rates suggest that three-drug regimens are required for MAC-PD with high bacterial loads and in those a replacement for rifampicin should be sought. Potential replacements should meet agreed target product profiles and adhere to PK-PD science.
Shareable PDF
Footnotes
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Support statement: This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI132374. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020; 56: 2000535. doi: 10.1183/13993003.00535-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diel R, Nienhaus A, Ringshausen FC, et al. Microbiologic outcome of interventions against Mycobacterium avium complex pulmonary disease: a systematic review. Chest 2018; 153: 888–921. doi: 10.1016/j.chest.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 3.Moon SM, Kim SY, Kim DH, et al. Relationship between resistance to ethambutol and rifampin and clinical outcomes in Mycobacterium avium complex pulmonary disease. Antimicrob Agents Chemother 2022; 66: e0202721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fröberg G, Maurer FP, Chryssanthou E, et al. Towards clinical breakpoints for non-tuberculous mycobacteria – determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin Microbiol Infect 2023; 29: 758–764. doi: 10.1016/j.cmi.2023.02.007 [DOI] [PubMed] [Google Scholar]
- 5.Kaniga K, Cirillo DM, Hoffner S, et al. A multilaboratory, multicountry study to determine MIC quality control ranges for phenotypic drug susceptibility testing of selected first-line antituberculosis drugs, second-line injectables, fluoroquinolones, clofazimine, and linezolid. J Clin Microbiol 2016; 54: 2963–2968. doi: 10.1128/JCM.01138-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Köser CU, Georghiou SB, Schön T, et al. On the consequences of poorly defined breakpoints for rifampin susceptibility testing of Mycobacterium tuberculosis complex. J Clin Microbiol 2021; 59: e02328-20. doi: 10.1128/JCM.02328-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ingen J, Hoefsloot W, Mouton JW, et al. Synergistic activity of rifampicin and ethambutol against slow-growing nontuberculous mycobacteria is currently of questionable clinical significance. Int J Antimicrob Agents 2013; 42: 80–82. doi: 10.1016/j.ijantimicag.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 8.van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med 2012; 186: 559–565. doi: 10.1164/rccm.201204-0682OC [DOI] [PubMed] [Google Scholar]
- 9.Boorgula GD, Jakkula LUMR, Gumbo T, et al. Comparison of rifamycins for efficacy against Mycobacterium avium complex and resistance emergence in the hollow fiber model system. Front Pharmacol 2021; 12: 645264. doi: 10.3389/fphar.2021.645264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Te Brake LHM, de Jager V, Narunsky K, et al. Increased bactericidal activity but dose-limiting intolerability at 50 mg·kg−1 rifampicin. Eur Respir J 2021; 58: 2000955. doi: 10.1183/13993003.00955-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schildkraut JA, Raaijmakers J, Aarnoutse R, et al. The role of rifampicin within the treatment of Mycobacterium avium pulmonary disease. Antimicrob Agents Chemother 2023; 67: e0087423. doi: 10.1128/aac.00874-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miwa S, Shirai M, Toyoshima M, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A preliminary study. Ann Am Thorac Soc 2014; 11: 23–29. doi: 10.1513/AnnalsATS.201308-266OC [DOI] [PubMed] [Google Scholar]
- 13.Moon SM, Yoo IY, Huh HJ, et al. Intermittent treatment with azithromycin and ethambutol for noncavitary Mycobacterium avium complex pulmonary disease. Antimicrob Agents Chemother 2019; 64: e01787-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danho R, Schildkraut JA, Zweijpfenning SMH, et al. Mycobacterium Growth Indicator Tube time-to-positivity can serve as an early biomarker of treatment response in Mycobacterium avium complex pulmonary disease. Chest 2022; 161: 370–372. doi: 10.1016/j.chest.2021.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong BH, Jeon K, Park HY, et al. Peak plasma concentration of azithromycin and treatment responses in Mycobacterium avium complex lung disease. Antimicrob Agents Chemother 2016; 60: 6076–6083. doi: 10.1128/AAC.00770-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodvold KA, George JM, Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 2011; 50: 637–664. doi: 10.2165/11594090-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 17.Zweijpfenning SMH, Aarnoutse R, Boeree MJ, et al. Clofazimine is a safe and effective alternative for rifampicin in Mycobacterium avium complex pulmonary disease treatment: outcomes of a randomized trial. Chest 2023; in press [ 10.1016/j.chest.2023.11.038]. [DOI] [PubMed] [Google Scholar]
- 18.Lan T, Ganapathy US, Sharma S, et al. Redesign of rifamycin antibiotics to overcome ADP-ribosylation-mediated resistance. Angew Chem Int Ed Engl 2022; 61: e202211498. doi: 10.1002/anie.202211498 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02210-2023.Shareable (330.1KB, pdf)