Abstract
Background
Several rare surfactant-related gene (SRG) variants associated with interstitial lung disease are suspected to be associated with lung cancer, but data are missing. We aimed to study the epidemiology and phenotype of lung cancer in an international cohort of SRG variant carriers.
Methods
We conducted a cross-sectional study of all adults with SRG variants in the OrphaLung network and compared lung cancer risk with telomere-related gene (TRG) variant carriers.
Results
We identified 99 SRG adult variant carriers (SFTPA1 (n=18), SFTPA2 (n=31), SFTPC (n=24), ABCA3 (n=14) and NKX2-1 (n=12)), including 20 (20.2%) with lung cancer (SFTPA1 (n=7), SFTPA2 (n=8), SFTPC (n=3), NKX2-1 (n=2) and ABCA3 (n=0)). Among SRG variant carriers, the odds of lung cancer was associated with age (OR 1.04, 95% CI 1.01–1.08), smoking (OR 20.7, 95% CI 6.60–76.2) and SFTPA1/SFTPA2 variants (OR 3.97, 95% CI 1.39–13.2). Adenocarcinoma was the only histological type reported, with programmed death ligand-1 expression ≥1% in tumour cells in three samples. Cancer staging was localised (I/II) in eight (40%) individuals, locally advanced (III) in two (10%) and metastatic (IV) in 10 (50%). We found no somatic variant eligible for targeted therapy. Seven cancers were surgically removed, 10 received systemic therapy, and three received the best supportive care according to their stage and performance status. The median overall survival was 24 months, with stage I/II cancers showing better survival. We identified 233 TRG variant carriers. The comparative risk (subdistribution hazard ratio) for lung cancer in SRG patients versus TRG patients was 18.1 (95% CI 7.1–44.7).
Conclusions
The high risk of lung cancer among SRG variant carriers suggests specific screening and diagnostic and therapeutic challenges. The benefit of regular computed tomography scan follow-up should be evaluated.
Shareable abstract
Among 99 adult patient carriers of surfactant-related gene variants, 20 patients developed lung cancer. Regarding the risk of lung cancer in this population, systematic evaluation of familial pulmonary fibrosis relatives by CT scan should be considered. https://bit.ly/48xJKwq
Introduction
The high prevalence of lung cancer in patients with fibrotic interstitial lung disease (fILD), particularly idiopathic pulmonary fibrosis (IPF), has specific diagnostic and therapeutic challenges [1–7]. Survival is poorer with lung cancer and fILD than with either disease alone [1–7]. Smoking is a confirmed risk factor for both diseases and an ideal culprit, but genetic risk factors for both diseases may also be involved [8].
The main known monogenic causes of fILD are variants in telomere-related genes (TRGs) or surfactant-related genes (SRGs) [9]. Germline TRG variants account for 25–35% of familial pulmonary fibrosis (FPF) cases and are also associated with high risk of haematological malignancy, but the reported prevalence of lung cancer is <5% [10, 11].
Germline SRG variants account for 1–5% of FPF cases [12–15]. Six SRGs (SFTPA1, SFTPA2, SFTPB, SFTPC, NKX2-1 and ABCA3) have been implicated in fILD [9]. SFTPA1, SFTPA2, SFTPB, SFTPC and SFTPD code for the surfactant proteins A–D [16]. ABCA3, coding for an ATP-binding cassette family A, member 3 (ABCA3) membrane transporter involved in surfactant alveolar homeostasis, and the alveolar lineage transcription factor NKX2-1, regulating transcription of surfactant encoding genes, are usually included in SRGs despite their different pathophysiology. Surfactant proteins B and C are secreted into the alveolar space to the cell membrane after being processed through the lamellar bodies in which ABCA3 plays a critical function [17]. Thyroid transcription factor 1 (TTF-1, encoded by NKX2-1) is involved in regulating surfactant protein B and C expression in addition to having a double-edged role in tumour suppression [18] and tumour carcinogenesis [19, 20]. SFTPA1 and SFTPA2 variants found in adult ILD are rare in childhood fILD [14]. SFTPB, SFTPC, NKX2-1 and ABCA3 variants are mostly identified in childhood fILD and rarely found in adult fILD [21–23]. The pathophysiology of fILD remains unclear but may involve endoplasmic reticulum stress and altered intracellular trafficking in alveolar type II epithelial cells [24, 25].
In addition to ILD, increased risk of lung cancer in SRG variant carriers was suggested by the high prevalence of lung cancer in the first reported families with SFTPA1 and SFTPA2 variants (14 cases among 37 SRG variant carriers) [13, 14, 26]. Moreover, NKX2-1 variants are rare in adult ILD, but at least four cases of lung cancer in adult variant carriers have been reported [27]; of note, NKX2-1 codes for TTF-1, a homeobox-containing transcription factor specifically involved in terminal respiratory unit differentiation and adenocarcinoma lineage, showing amplification in 7% of lung adenocarcinomas [28].
This study aimed to study the epidemiology of lung cancer in SRG variant carriers in comparison with TRG variant carriers as the main monogenic cause of fILD and to describe lung cancer characteristics occurring in SRG variant carriers.
Methods
SRG cohort patient recruitment
In this retrospective, observational, non-interventional study, all centres from the OrphaLung network identified adults (>18 years old) with a pathogenic or likely pathogenic variant in one of the SRG genes that was diagnosed during 2008–2022, including 1) proband adult ILD patients with an SRG variant, 2) adult family member carriers of the SRG variant with or without ILD and 3) previously identified children with ILD associated with a TRG variant who reached the age of 18 years during this period (supplementary figure S1). Genetic analyses of SRG in adults began in 2008 in France. Cases were cross-identified by the three molecular diagnostic laboratories in France.
The fILD pattern was classified locally in each OrphaLung centre, without central reviewing, according to the 2022 official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Asociación Latinoamericana de Tórax statement for IPF and the revised classification for idiopathic interstitial pneumonia [29, 30].
We collected clinical characteristics, genetic analyses, age at diagnosis and survival. Age at diagnosis refers to age at ILD or lung cancer diagnosis for patients with ILD or lung cancer (no patient exhibited ILD during follow-up) or age at genetic diagnosis for asymptomatic relatives in the framework of pre-symptomatic testing. Environmental exposure refers to environmental exposure known to be associated with risk of fILD that was queried during the ILD initial assessment: metal dust, wood dust, farming, raising birds, hair dressing, stone cutting/polishing, and exposure to livestock and to vegetable dust/animal dust [31].
Signed informed consent was obtained from all patients. The study was approved by local ethics committees (CPP IDF1, 0811760 and CERC-SFCTCV-2016-3-23-16-35-3-MoPi).
Surfactant protein gene molecular analysis and DNA sequencing
Genomic DNA was obtained from whole-blood EDTA samples. Exons and intron–exon junctions of SFTPA1, SFTPA2, SFTPB, SFTPC, NKX2-1 and ABCA3 were sequenced by Sanger or next-generation sequencing (NGS) and compared with the reference sequences in one of the three molecular diagnosis laboratories. All variants were centrally reviewed (M. Legendre and P. Fanen) and were interpreted as pathogenic or likely pathogenic (hereafter called a variant) according to international guidelines [32].
TRG variant comparative group and general population
To compare the cancer incidence in SRG variant carriers with relevant control groups, we assessed lung cancer incidence in a group of 233 TRG likely pathogenic/pathogenic variant carriers from the Bichat database with follow-up data censored as of July 2023 (190 previously reported [11, 33–36]). Furthermore, we estimated the French national lung cancer incidence from the open-access French national cancer database for 2018 [37].
Lung cancer histology, molecular analysis and treatment
All histological samples were reviewed by an expert thoracic pathologist (A. Cazes). Tumour samples underwent histological diagnosis with a standard immunohistochemistry panel for lung cancer (TTF-1, cytokeratin 7 (CK7), cytokeratin 20 (CK20) and p40) with additional staining for anaplastic lymphoma kinase (ALK), ROS and programmed death ligand-1 (PD-L1) expression. Molecular diagnosis for lung cancer genes was available for 18 out of 20 patients (supplementary material). Cancer treatments were left to the discretion of the investigators, with therapeutic decisions validated in local multidisciplinary tumour boards according to international European Society for Medical Oncology/American Society of Clinical Oncology guidelines and retrospectively retrieved from patients’ electronic files.
Statistical analysis
Data are expressed as median (interquartile range (IQR)) for quantitative variables or number (percentage) for categorical variables. Because the phenotype associated with SFTPA1 and SFTPA2 variants is similar, SFTPA1 and SFTPA2 variant carriers were merged for analysis and compared with non-SFTPA variant carriers. Survival curves were plotted using the Kaplan–Meier method from the date of diagnosis and the date of birth and compared using the log-rank test. Hazard ratios (HRs) for factors associated with overall survival after ILD or lung cancer diagnosis and their 95% confidence intervals were studied with a Cox proportional hazards model. Comparison of age of onset of ILD among the genotypes involved one-way ANOVA. We used a univariable and multivariable logistic regression procedure to calculate odd ratios for factors associated with the onset of lung cancer with or without a random intercept at the family level. In multivariable analysis, we included variables according to the strength of association on univariable analysis and their medical relevancy with or without a random intercept at the family level (package lme4). We plotted the cumulative incidence function of the cancer onset by genotype, using “death without cancer” as a competitive risk. The Fine–Gray model [38] was used to calculate subdistribution HRs to determine the competitive risk of lung cancer in the SRG group versus the TRG group. Two-sided p<0.05 was considered statistically significant. Statistical analyses were performed with R version 3.1.0 (www.r-project.org).
Results
SRG cohort patient characteristics
In total, 101 adults with a diagnosis of SRG-associated ILD during 2008–2022 were referred for inclusion; we included 99 adults with a pathogenic SRG variant (SFTPA1, SFTPA2, SFTPC, ABCA3 or NKX2-1) (table 1), including 74 who were previously reported (supplementary figure S1) [14, 21, 23, 27, 39]. One variant in SFTPC (c.500G>A, p.(Arg167Gln)) and one in ABCA3 (c.1502C>A, p.(Ala501Glu)) were reclassified as likely benign, and the cases were excluded from the study.
TABLE 1.
Main characteristics of patients with surfactant-related gene (SRG) variants including characteristics of subpopulations with and without lung cancer
| Cohort | Lung cancer | No lung cancer | |
| Population characteristics | |||
| Patients | 99 | 20 | 79 |
| Families | 69 | 15 | 54 |
| Male | 52 (52.5) | 11 (55) | 41 (52) |
| Smoking >5 pack-years | 25 (25.3) | 15 (75) | 10 (12.6) |
| Mean pack-years | 0 (0.0–5.0) | 14 (8.75–20.0) | 0 (0.0–0.0) |
| Environmental exposure# | 6 (6.1) | 2 (10) | 5 (6.3) |
| Long-term oxygen therapy | 4 (20) | ||
| mMRC dyspnoea scale ≤2 | 18 (90) | ||
| Performance status ≤2 | 17 (85) | ||
| SRG variants | |||
| SFTPA1 | 18 (18.2) | 7 (35.0) | 11 (13.9) |
| SFTPA2 | 31 (31.3) | 8 (40.0) | 23 (29.1) |
| SFTPC | 24 (24.3) | 3 (15.0) | 21 (26.6) |
| ABCA3 | 14 (14.1) | 0 (0.0) | 14 (17.7) |
| NKX2-1 | 12 (12.1) | 2 (10.0) | 10 (12.7) |
| ILD | |||
| Patients | 82 | 18 | 64 |
| Age at ILD diagnosis | 41.4 (29.6–49.1) | 48.7 (42.5–54.5) | 35.5 (28.0–47.7) |
| CT pattern | |||
| Indeterminate | 48 (58.5) | 8 (44.0) | 40 (62.5) |
| UIP (definite or probable) | 16 (19.5) | 5 (28.0) | 11 (17.2) |
| Non-UIP | 18 (24.4) | 5 (28.0) | 13 (20.3) |
| Histological pattern | 32 | 7 | 25 |
| UIP (definite or probable) | 14 (17.0) | 2 (11.0) | 12 (18.7) |
| Non-UIP | 18 (21.9) | 5 (27.7) | 13 (20.3) |
| ILD diagnosis | |||
| Unclassifiable | 42 (51.2) | 8 (44.0) | 34 (53.5) |
| IPF | 23 (28.0) | 5 (28.0) | 18 (28.1) |
| Non-IPF | 17 (20.8) | 5 (28.0) | 12 (18.4) |
| Pulmonary function tests | |||
| FVC % pred | 63 (52–78) | 66 (53–82) | 62 (51–77) |
| DLCO % pred | 44 (28–59) | 45 (35–64) | 37 (28–56) |
| Cancer-related information | |||
| Age at cancer diagnosis (years) | 50.3 (47.2–58.2) | ||
| Diagnostic circumstances | |||
| Fatigue | 2 (10) | ||
| Genetic counselling | 3 (15) | ||
| ILD follow-up | 15 (75) | ||
| After lung transplantation | 2 (10) |
Data are presented as n, n (%) or median (interquartile range). mMRC: modified Medical Research Council dyspnoea scale; ILD: interstitial lung disease; CT: computed tomography; UIP: usual interstitial pneumonia; IPF: idiopathic pulmonary fibrosis; FVC: forced viral capacity; DLCO: diffusing capacity of the lung for carbon monoxide. #: patients were questioned about their potential exposure to metal dust, wood dust, farming, raising birds, hair dressing, stone cutting/polishing, and exposure to livestock and to vegetable dust/animal dust known to be associated with risk of fibrotic ILD [31].
Median (IQR) age at ILD, lung cancer or genetic diagnosis was 41.2 (29.8–49.2) years, with a sex ratio (male/female) of 1.1. 82 patients were initially referred for an ILD diagnosis, two for a lung cancer diagnosis with genetic analyses performed according to the recommended French diagnosis algorithm [40] and 15 after genetic counselling in a context of familial ILD. The 99 patients belonged to 69 families with 1–10 members per family. Variants were identified in SFTPA1 (n=18), SFTPA2 (n=31), SFTPC (n=24), ABCA3 (n=14) and NKX2-1 (n=12), and all were classified as pathogenic or likely pathogenic (table 1 and supplementary table S1).
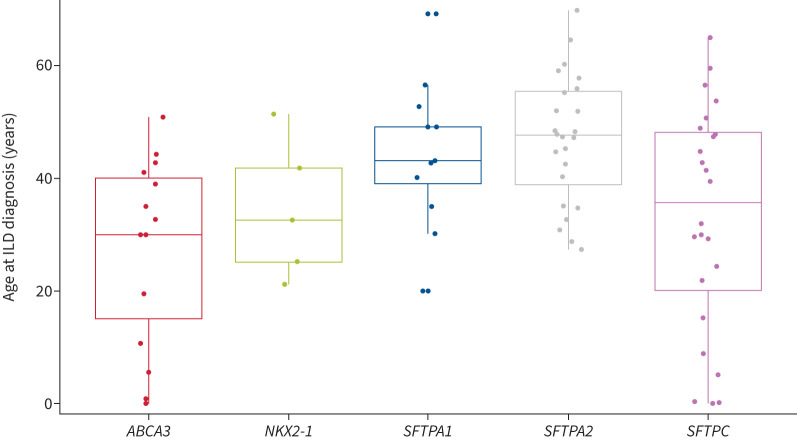
Overall, 82 (82.8%) patients had ILD at diagnosis; no new ILD case occurred during follow-up. Median (IQR) age at ILD diagnosis was 41.9 (30.0–49.2) years. Compared with other genes, SFTPA1 and SFTPA2 were associated with older age at ILD diagnosis (median age 47.6 years) (p<0.001) (figure 1). Computed tomography (CT) pattern was classified as indeterminate (n=48 (58.5%)), usual interstitial pneumonia (UIP) (n=16 (19.5%)) or suggestive of another diagnosis: non-specific interstitial pneumonia (NSIP) (n=10 (12.2%)), pleuro-pulmonary fibro-elastosis (PPFE) (n=2 (2.5%)), desquamative interstitial pneumonia (DIP) (n=2 (2.5%)), fibrotic hypersensitivity pneumonitis (fHP) (n=1 (1.2%)), lymphoid interstitial pneumonia (LIP) (n=1 (1.2%)), organising pneumonia (OP) (n=1 (1.2%)) or multiple cystic lung disease (n=1 (1.2%)).
FIGURE 1.
Age at interstitial lung disease (ILD) diagnosis by surfactant-related gene variant. p<0.001 SFTPA versus non-SFTPA.
Lung histology was available for 32 patients after video-assisted thoracoscopic surgical biopsy (n=21 (25.6%)) or lung transplantation (n=11 (13.4%)) and showed a pattern of UIP (n=14 (17.0%)), NSIP (n=9 (10.9%)), indeterminate (n=5 (6.0%)), fHP (n=2 (2.4%)), LIP (n=1 (1.2%)) or OP (n=1 (1.2%)).
The pulmonary diagnosis was unclassifiable pulmonary fibrosis (n=42 (51.2%)), IPF (n=23 (28.0%)), NSIP (n=9 (11.0%)), PPFE (n=2 (2.5%)), DIP (n=2 (2.5%)), fHP (n=2 (2.4%)), LIP (n=1 (1.2%)) or OP (n=1 (1.2%)).
The median (IQR) follow-up was 7.0 (3.0–11.7) years. Eight (9.8%) patients received antifibrotic treatment and 27 (32.9%) underwent lung transplantation, with a median (IQR) time from ILD diagnosis to lung transplantation of 4.8 (2.4–8.2) years.
Lung cancer characteristics in SRG patients
Lung cancer was diagnosed in 20 patients (20.2%) at a median (IQR) age of 50.3 (47.2–58.3) years with a sex ratio (male/female) of 1.22 (table 1). Lung cancer was the reason for consultation for two (10%) patients, revealed by fatigue in both patients. Three (15%) patients were referred for genetic counselling and lung cancer was diagnosed after pre-symptomatic testing. Lung cancer was diagnosed during ILD follow-up in 15 (75%) patients (table 1), including two with lung cancer diagnosed after lung transplantation (one single lung transplantation with lung cancer on the native lung and one double lung transplantation with prior infra-clinic lymph node invasion).
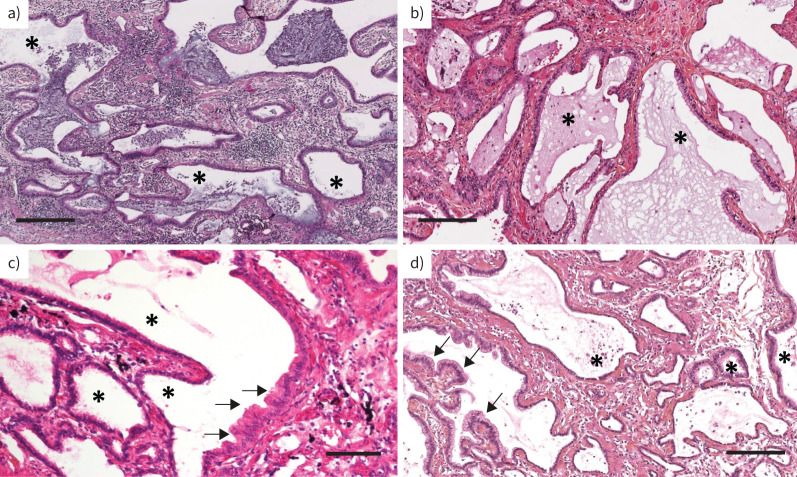
Lung cancer was associated with overt ILD in 18 (90%) patients, but two had no ILD (diagnosed after genetic counselling). Lung cancer was located in lower zones in 13 (65%) patients. Tobacco smoking history was reported in 15 (75%) patients with a mean cumulative exposure of 15 pack-years (table 1). We reviewed the lung tissue from 10 patients: nine with ILD and one without radiological ILD (SFTPA1 (n=5), SFTPA2 (n=3), NKX2-1 (n=1) and ABCA3 (n=1)), including four patients (all with SFTPA1 variants) who had cancer. Bronchiolar metaplasia was observed in all 10 lung samples (figure 2). Of note, the four cancers were located in close proximity to bronchiolar metaplasia, which supports a link between bronchiolar metaplasia and oncogenesis.
FIGURE 2.
Representative lung histology of four surfactant-related gene variant carriers: a) patient 4, b) patient 20, c) patient 8 and d) patient 11 (supplementary table S1). All patients showed bronchiolar metaplasia (asterisks), including one patient (c) without overt radiological interstitial lung disease. Haematoxylin eosin staining. Arrows: adenocarcinoma. Scale bars: a, b, d) 250 µm; c) 100 µm.
Compared with patients in whom lung cancer did not develop during follow-up, those with lung cancer were older (median age 48.7 versus 37.5 years; p=0.02), were more frequently smokers (75% versus 12.6%; p<0.001) and carried an SFTPA1 or SFTPA2 variant versus other SRG variants (75% versus 43%; p=0.01). The risk of lung cancer was significantly lower with SFTPC (12%; p=0.05) and ABCA3 (0%; p=0.04) than SFTPA2 (table 2 and supplementary table S2). In addition, we ran a generalised linear model with a random intercept at the family level to study the factors associated with lung cancer in the SRG mutation carriers. Univariable and multivariable analysis showed comparable results without a random intercept at the family level; the odds ratios for STFPA versus non-SFTPA genes were 3.97 (95% CI 1.39–13.2; p=0.014) on univariable analysis and 2.89 (95% CI 0.78–12.16; p=0.12) on multivariable analysis. The SFTPA1 and SFTPA2 variants identified in patients with lung cancer span valine 178 to cysteine 238, with no obvious hotspot.
TABLE 2.
Factors associated with lung cancer in surfactant-related gene (SRG) variant carriers
| No lung cancer | Lung cancer | Univariable analysis | Multivariable analysis | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Patients | 79 | 20 | ||||
| Age at diagnosis (years) | 37.5 (28.9–47.9) | 48.70 (42.5–53.3) | 1.04 (1.01–1.08) | 0.029 | 1.02 (0.98–1.07) | 0.31 |
| Male sex | 41 (51.9) | 11 (55) | 1.13 (0.42–3.10) | 0.80 | ND | |
| SRG variant | ||||||
| SFTPC/NKX2-1/ABCA3 | 45 (57.0) | 5 (25) | Reference | Reference | Reference | Reference |
| SFTPA1/SFTPA2 | 34 (43.0) | 15 (75) | 5.24 (1.20–22.8)) | 0.03 | 2.74 (0.45–16.69) | 0.27 |
| Smoking | 10 (12.7) | 15 (75) | 20.7 (6.60–76.2) | <0.001 | 17.5 (5.28–68.6) | <0.0001 |
| Environmental exposure | 6 (7.6) | 2 (10) | 1.35 (0.19–6.45) | 0.72 | ND | |
| ILD | 64 (81.0) | 18 (90) | 2.29 (0.58–15.3) | 0.30 | 3.14 (0.58–26.1) | 0.22 |
| Antifibrotic treatment | 4 (5.1) | 7 (35) | 10.1 (2.68–43.4) | <0.001 | ND# | |
| Lung transplantation | 22 (27.8) | 4 (20) | 0.65 (0.17–2.00) | 0.48 | ND | |
| FVC % pred | 62 (51–77) | 65 (53–84) | 1.01 (0.99–1.04) | 0.31 | 0.98 (0.92–1.03) | 0.39 |
| DLCO % pred | 37 (28–57) | 44 (32–64) | 1.01 (0.98–1.04) | 0.45 | 1.03 (0.97–1.11) | 0.30 |
Data are presented as n, median (interquartile range) or n (%), unless otherwise stated. ILD: interstitial lung disease; FVC: forced viral capacity; DLCO: diffusing capacity of the lung for carbon monoxide; ND: not done (variable not included in the multivariable analysis). #: antifibrotic treatment was not included in the multivariate analysis because this variable is a proxy for ILD. Bold indicates p<0.05.
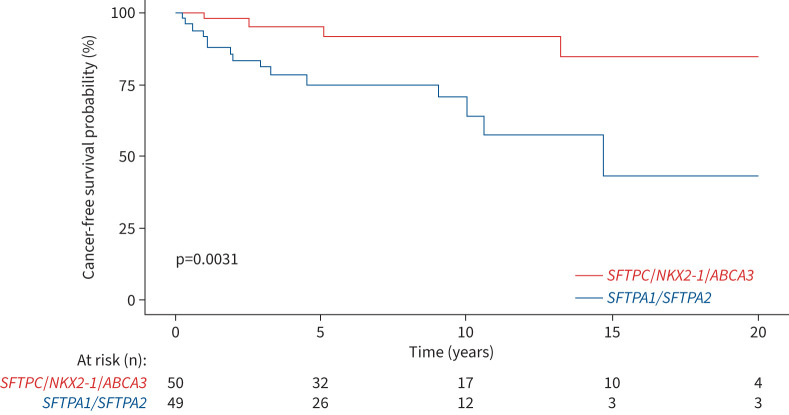
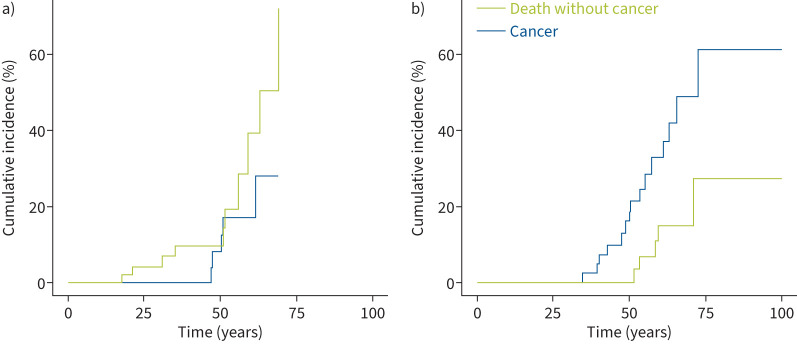
The median cancer-free survival was not reached but was significantly lower among patients carrying SFTPA1 or SFTPA2 variants than non-SFTPA variants (15-year cancer survival rate: 0.43 versus 0.85; log-rank p=0.003) (figure 3). The cumulative incidence of lung cancer and death without cancer was significantly increased in the SFTPA1 or SFTPA2 variant carriers (p=0.02) (figure 4).
FIGURE 3.
Cancer-free survival from diagnosis in surfactant-related gene variant carriers.
FIGURE 4.
Cumulative incidence of lung cancer and death without cancer in a) non-SFTPA (SFTPC/NKX2-1/ABCA3) surfactant-related gene variant carriers and b) SFTPA1/SFTPA2 variant carriers. p=0.2 non-SFTPA versus SFTPA.
The pathological diagnosis of lung cancer was obtained by transthoracic needle biopsy (n=10 (50%)), surgical biopsy (n=5 (25%)), endoscopic transbronchial biopsy (n=3 (15%)), endobronchial ultrasound biopsy (n=1 (5%)) or thoracocentesis (n=1 (5%)). Adenocarcinoma was the only histological subtype diagnosed, with a majority of invasive mucinous adenocarcinoma (35%) and acinar adenocarcinoma (35%) cases (table 3). TTF-1 staining was positive for five samples. PD-L1 expression was available for 20 samples and was positive (≥1%) for only three. NGS for somatic variants associated with lung cancer was available for 18 out of 20 patients. Nine (45%) of the lung cancer patients had at least one somatic variant (supplementary table S3). The KRAS variant (six out of nine patients with NGS-detected variant) was the most frequent, without the KRAS G12C variant. None of the variants was eligible for a currently approved targeted therapy.
TABLE 3.
Lung cancer characteristics in the surfactant-related gene (SRG) and telomere-related gene (TRG) populations
|
SRG population
(n=20 cases) |
TRG population
(n=6 cases) |
|
| Tumour localisation | ||
| Upper zones | 7 (45) | 3 (50) |
| Lower zones | 13 (65) | 3 (50) |
| Stage at diagnosis | ||
| Stage I | 7 (35) | 0 (0) |
| Stage II | 1 (5) | 0 (0) |
| Stage III | 2 (10) | 1 (17) |
| Stage IV | 10 (50) | 5 (83) |
| Histopathology | ||
| Adenocarcinoma | 20 (100) | 3 (50) |
| Invasive mucinous | 7 (35) | 0 (0) |
| Acinar | 7 (35) | 0 (0) |
| Invasive lepidic | 2 (10) | 0 (0) |
| Non-specific | 2 (10) | 2 (66) |
| Solid | 1 (5) | 0 (33) |
| Mixed acinar and papillary | 1 (5) | 0 (0) |
| Micropapillary infiltration >5% | 0 (0) | 0 (0) |
| Squamous cell carcinoma | 0 (0) | 2 (33) |
| Small cell carcinoma | 0 (0) | 1 (17) |
| Immunohistochemistry | ||
| PD-L1 <1% | 17 (85) | 6 (100) |
| PD-L1 1–50% | 2 (10) | 0 (0) |
| PD-L1 ≥50% | 1 (5) | 0 (0) |
| Somatic molecular alteration | n=18 9 (45) |
n=3 0 (0) |
Data are presented as n (%). PD-L1: programmed death ligand-1.
Lung cancer treatments and specific survival
Among the eight patients with a localised cancer, seven (35%) underwent surgery as first-line treatment. Because of chronic respiratory insufficiency, one patient received only chemotherapy. None of the seven patients presented acute ILD exacerbation after surgery. The median (IQR) follow-up was 62.4 (2–130) months; one patient (pT1cN0) experienced relapse 50 months after surgery and one patient died of chronic respiratory failure 70 months after surgery.
10 (50%) patients received platinum-based chemotherapy (pemetrexed (n=6), paclitaxel (n=3) and vinorelbine (n=1)) and one patient carrying a KRAS G12F variant was included in a randomised clinical trial (docetaxel±selumetinib [41]). The first-line chemotherapy response rate was 50%. Three (30%) patients experienced major adverse effects (grade 3 or 4: fatigue, chronic kidney disease and thrombocytopenia); all received platinum-pemetrexed treatment.
Three patients (two ILD and one non-ILD) received anti-PD1 immunotherapy (nivolumab) and two received bevacizumab, including two during the first-line regimen with carboplatin and pemetrexed. The overall response rate was 0%. After a median (IQR) follow-up of 3 (1–10) months, no adverse effects (including fibrosis exacerbation) were reported. None of the patients had radiotherapy. Three (15.0%) patients received only best supportive care: two due to impaired lung function and one the patient's decision.
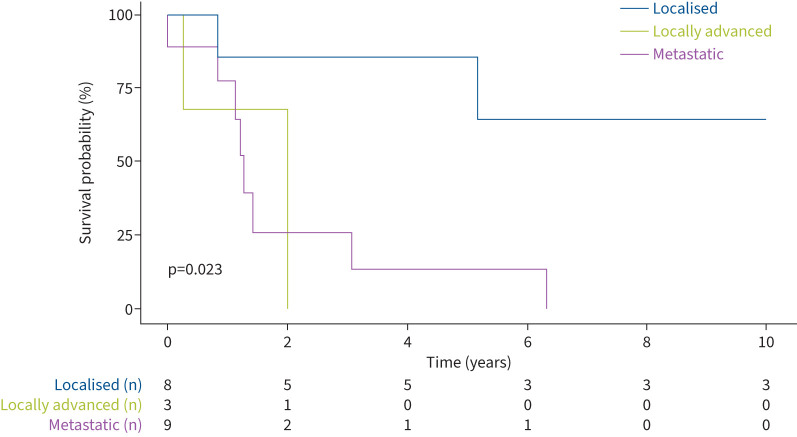
Median overall survival was 24 months. As expected, survival was longer with stage I/II lung cancer than other stages and this was the only variable associated with survival (5-year survival 0.86 for stage I/II versus 0.13 for stage IV; p=0.023) (figure 5 and supplementary table S4).
FIGURE 5.
Overall survival from lung cancer diagnosis.
Overall outcome
28 (28.3%) patients, including 13 (18.2%) with a diagnosis of lung cancer, died during follow-up. The most frequent causes of death were chronic respiratory failure (n=12) and post-operative complications after lung transplantation (n=2); medical data were not available for 15 patients.
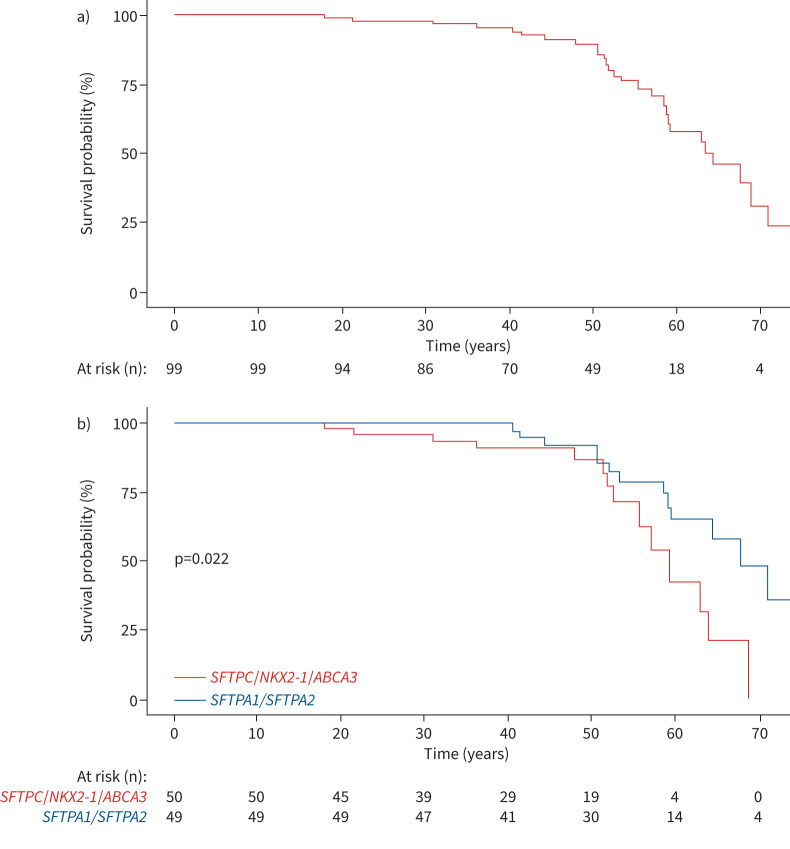
Median overall survival after an ILD diagnosis was not reached. Median (IQR) 5- and 10-year overall survival was 0.83 (0.75–0.91) and 0.73 (0.63–0.83), respectively (figure 6 and supplementary material). Among patients with ILD or lung cancer at diagnosis, older age, male sex, smoking and occurrence of lung cancer at diagnosis were significantly associated with decreased survival (table 4). ILD characteristics were not associated with survival (table 4). Survival was lower for patients with non-SFTPA SRG variants versus SFTPA1/SFTPA2 variants (p=0.022) (figure 6 and supplementary material).
FIGURE 6.
Overall survival from birth a) in the whole cohort and b) stratified by surfactant-related gene variant.
TABLE 4.
Factors associated with overall survival from the date of interstitial lung disease (ILD) or lung cancer diagnosis, whichever was first (n=82)
| Univariable analysis | Multivariable analysis | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.05 (1.02–1.09) | 0.0006 | 1.04 (1.00–1.08) | 0.03 |
| Male sex | 3.16 (1.28–7.84) | 0.013 | 3.22 (1.25–8.30) | 0.015 |
| SRG mutation | ||||
| SFTPC/NKX2-1/ABCA3 | Reference | Reference | Reference | Reference |
| SFTPA1/SFTPA2 | 1.39 (0.61–3.16) | 0.43 | 0.73 (0.28–1.89) | 0.52 |
| Smoking | 6.36 (2.70–15.0) | <0.0001 | 2.32 (0.92–5.90) | 0.076 |
| Diagnosis mode | ||||
| ILD | Reference | Reference | Reference | Reference |
| Lung cancer | 14.1 (4.49–44.1) | <0.0001 | 5.57 (1.64–18.9) | 0.006 |
| IPF versus non-IPF ILD | 1.24 (0.52–2.95) | 0.62 | ND | |
| FVC % pred | 1.01 (0.98–1.03) | 0.57 | ND | |
| DLCO % pred | 0.99 (0.97–1.02) | 0.93 | ND | |
HR: hazard ratio; SRG: surfactant-related gene; IPF: idiopathic pulmonary fibrosis; FVC: forced vital capacity; DLCO: diffusing capacity of the lung for carbon monoxide; ND: not done (variable not included in the multivariable analysis). Bold indicates p<0.05.
Lung cancer in SRG variant carriers, TRG variant carriers and the French general population
In SRG variant carriers, the lung cancer incidence was 1492.24 per 100 000 patient-years for women and 2043.84 per 100 000 patient-years for men (p=0.8).
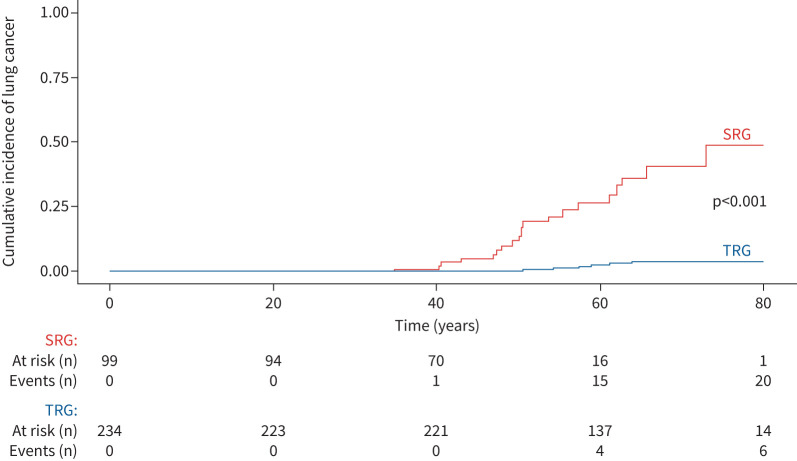
We examined a comparative group: a cohort of 233 adults with a pathogenic/likely pathogenic TRG variant (TERT (n=124), RTEL1 (n=42), PARN (n=41), TERC (n=25) and DKC1 (n=1)) (table 5). Compared with SRG variant carriers, TRG variant carriers were older at diagnosis (age at ILD or genetic diagnosis 57.3 versus 40.2 years; p<0.001) and more frequently current or ex-smokers (52.0% versus 25.3%; p<0.001). The prevalence of ILD was similar in the SRG and TRG groups (89% versus 83%; p=0.10), but an IPF diagnosis was more frequent in the TRG than SRG group (64% versus 28%; p<0.001) and disease was less severe at ILD diagnosis (table 5). In the TRG cohort, after a median (IQR) follow-up of 4.9 (2.2–8.3) years, lung cancer developed in six (2.5%) patients (three adenocarcinoma, two squamous cell carcinoma and one small cell carcinoma). The observed incidence of lung cancer in the TRG cohort was 190.1 per 100 000 patient-years for women and 679.6 per 100 000 patient-years for men (p=0.24 between women and men). After adjusting for sex, lung cancer incidence was higher in the SRG than TRG group (HR 18.1, 95% CI 7.1–44.7; p<0.001) (figure 7).
TABLE 5.
Clinical characteristics of surfactant-related gene (SRG) and telomere-related gene (TRG) patients
| SRG cohort (n=99) | TRG cohort (n=233) | p-value | |
| Patients | |||
| Age at diagnosis (years) | 40.2 (29.3–49.1) | 57.3 (50.1–65.4) | <0.001 |
| Male | 52 (52.5) | 134 (57.5) | 0.4 |
| Smoking >5 pack-years | 25 (25.3) | 122 (52.0) | <0.001 |
| Environmental exposure | 6 (6.1) | 23 (9.8) | 0.36 |
| ILD | 82 | 208 | 0.11 |
| ILD diagnosis | <0.001 | ||
| Unclassifiable | 42 (51.2) | 14 (6.7) | |
| IPF | 23 (28.0) | 133 (63.9) | |
| Non-IPF | 17 (20.8) | 61 (29.4) | |
| Pulmonary function tests | |||
| FVC % pred | 63 (52–78) | 76 (56–91) | 0.001 |
| DLCO % pred | 44 (28–59) | 45 (22–61) | 0.038 |
Data are presented as median (interquartile range), n (%) or n, unless otherwise stated. ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis; FVC: forced viral capacity; DLCO: diffusing capacity of the lung for carbon monoxide. Bold indicates p<0.05.
FIGURE 7.
Cumulative incidence of cancer and death without cancer in surfactant-related gene (SRG) and telomere-related gene (TRG) variant carriers. The subdistribution hazard ratio for comparative risk for lung cancer in the SRG cohort compared with the TRG cohort was 18.1 (95% CI 7.1–44.7); p<0.001.
As a second comparative group, we evaluated lung cancer incidence in the French general population aged 45–55 years from the French national population cancer register available in open access [37]. The active smoker rate in France in the same period was 23.5% for men and 21.6% for women aged 45–55 years [42]. In this population, lung cancer incidence was 46.7 per 100 000 patient-years for women and 56.1 per 100 000 patient-years for men. The standardised incidence ratio (SIR) was 47.9 for women and 50.1 for men among SRG variant carriers compared with the general population.
Discussion
Since the first report of SFTPA2 variants in two families with both fILD and lung cancer, the involvement of SFTPA1 and SFTPA2 variants in ILD has been repeatedly confirmed, although the exact mechanism has yet to be understood [43]. However, the increased risk of lung cancer in SRG variant carriers remains hypothetical. By reporting 20 cases of lung cancer in a cohort of 99 adult SRG variant carriers, a competitive over-risk of more than 18.1 compared with TRG variant carriers and an SIR of 50 compared with the general population, these data indicate an increased risk of lung cancer in patients with SRG rare variants, particularly for carriers of SFTPA1/SFTPA2 variants.
In this series, as in the general population, lung cancer occurrence was highly associated with smoking, i.e. a 20-fold increased risk compared with never-smoker variant carriers, which is important to emphasise for patients and their family members.
Several arguments suggest a specific risk of lung cancer in SRG variant carriers. SRG variants are associated with fILD and the relative risk of lung cancer is increased in fILD patients, estimated at almost 10% throughout life [9]. However, lung cancer is most frequently diagnosed after age 60 years in fILD patients and patients with SRG would conceivably have a lower risk of lung cancer because of earlier fILD-related mortality [44]. A 20.2% incidence of lung cancer at a median age of 50 years in this series suggests that SRG variants could directly contribute to the development of lung cancer. In addition, the presence of ILD was not significantly associated with the incidence of cancer in this cohort (table 2). Lack of sensitivity due to the relatively small number of patients cannot be excluded, but this finding supports a specific role for SRG variants in the development of lung cancer independent of associated fILD.
Of note, the pathophysiology of lung cancer may vary depending on the SRG. Indeed, SFTPA1, SFTPA2 and SFTPC are thought to cause ILD via gain-of-toxic-function mechanisms [14, 24]. NKX2-1-related disease is thought to be due to haploinsufficiency, although there are some reports of possible gain-of-function variants. Most importantly, in addition to its role in lung development, NKX2-1 also acts as a tumour suppressor gene [18, 20, 45]. ABCA3 deficiency-associated ILD is a recessive disorder due to loss-of-function variants [17], which may explain why we found no individuals with ABCA3 deficiency and lung cancer in this series. However, we studied few patients for a relatively short time and the real risk of lung cancer in ABCA3 biallelic variant carriers remains to be determined. Further research is needed to determine whether lung cancer is related to a common mechanism in all SRG variant carriers or involves different pathways and processes, opening potential routes for more appropriate treatments or prevention. However, the presence of bronchiolar metaplasia of the alveolar epithelium, a potential pre-cancerous lesion, may indicate common terminal pathways.
As expected, univariate and multivariable analyses indicated that tobacco smoking was an independent risk factor for lung cancer (HR 17.7 (95% CI 5.34–69.4); p<0.001). This risk factor could be specifically deleterious in patients with ILD and germline variants. Of note, the 24.7% rate of SRG variant carriers with a smoking history is consistent with the 25.5% rate of reported daily smoking in the French population in 2020 [42]. Patients with SRG variants and/or fILD should be advised to quit smoking to prevent lung cancer.
All patients with germline SRG variants and lung cancer had adenocarcinoma, which contrasts with a rate of 29–68% of squamous cell carcinoma among non-small cell lung cancer cases in the general and ILD populations [2]. Lung cancer was located in lower lobes in more than half of the IPF patients and in 65% of this cohort; such localisation could reflect the predominance of fibrotic lesions in the lower zones, suggesting a pro-tumoural environment of ILD [2]. Overall, histopathological and molecular characteristics in lung cancer suggested that specific pathogenesis may occur in people with SRG variants with or without fILD.
Our work has several limitations, including its limited sample size despite representing the largest series of patients with fILD, SRG germline variants and lung cancer. A second limitation is its retrospective and observational design. Therefore, we were not able to calculate the penetrance of lung cancer in SRG variant carriers. In addition, this descriptive work focused on lung cancer, and fILD cases were not centrally reviewed. The lack of an association between survival and ILD severity assessed by lung function alteration could be explained by the large number of cancer-related deaths in this cohort (11 out of 27) and the diversity of the patients included in the cohort, with five SRGs and different patterns of ILD.
The association of SRG mutations and lung cancer in this cohort may be driven by a few highly penetrant variants in addition to unmeasured environmental exposures because four out of seven SFTPA1-associated lung cancer cases belonged to the same family. However, statistical analysis stratified by gene or family did not provide clarification on this point (table 2 and supplementary table S2). We grouped the SFTPA1 and SFTPA2 variants. From a pathophysiological standpoint, including BRICHOS variants of SFTPC in this group could make sense. Dominant-negative SFTPC BRICHOS variants as well as SFTPA1 and SFTPA2 variants may be considered toxic gain-of-function variants resulting in endoplasmic reticulum stress, which may pathophysiologically link SRG variants, lung fibrosis and risk of lung cancer [46–48]. The biological mechanism of ILD is likely different for heterozygous non-BRICHOS-domain SFTPC and NKX2-1 variants, and homozygous ABCA3 variants, resulting in a different or potentially no increased risk of lung cancer [49]. Additional retrospective and prospective studies in addition to translational research are necessary to address these questions and determine whether certain SRG variants confer a higher risk of lung cancer than others and provide answers to patients and their relatives.
Despite these limitations, our findings of a high incidence of lung cancer in relatively young adults support a proactive policy of avoiding or quitting tobacco smoking. Considering the other well-known environmental risk factors for lung cancer, we also suggest paying particular attention to occupational and personal air contaminants. In addition, a programme dedicated to lung cancer and ILD screening could be proposed to SRG variant carriers, the modalities of which remain to be determined. Low-dose annual chest CT scan is now recommended for lung cancer screening in high-risk populations in several countries [40, 50] and might be evaluated for SRG variant carriers. The age at the first CT scan and the frequency of repeated assessments need to be carefully considered because of the unknown long-term risk associated with irradiation in this relatively young population.
In conclusion, unlike ABCA3 variants, SRG variants, especially SFTPA1 and SFTPA2, may be associated with a high risk of lung cancer, which warrants scrutiny when interpreting chest CT scans in these patients and a specific screening programme for both fILD and lung cancer in this relatively young adult population.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01809-2023.Supplement (605.7KB, pdf)
Shareable PDF
Acknowledgements
We express our sincere gratitude to all patients, relatives and professionals who contributed to this research paper, especially the members of the OrphaLung network. We acknowledge Anna Karakatsani (National and Kapodistrian University of Athens, Athens, Greece) for patient care.
Footnotes
Ethics statement: This work was based on anonymous patient data and required the approval of the local ethics committees (CPP IDF1, 0811760 and CERC-SFCTCV-2016-3-23-16-35-3-MoPi).
Author contributions: A. Brudon, M. Legendre and R. Borie collected and analysed the data, and drafted the original manuscript. A. Mageau performed the statistical analysis, analysed the data, and participated in the review and editing of the final manuscript. G. Defossez performed the statistical analysis of the national database of lung cancer. M. Legendre, P. Fanen, C. Kannengiesser, I. Ba, N. Nathan and S. Amselem performed the genetic analysis of surfactant variants and analysed the data. N. Théou-Anton performed molecular analysis of cancer, and participated in the review and editing of the final manuscript. A. Cazes performed the centralised histological analysis. G. Zalcman, V. Cottin, J. Bermudez, P. Bonniaud, J. Cadranel, D. Bouvry, T. Dégot, C. Delestrain, R. Diesler, R. Epaud, M-P. Debray, E. Manali, S. Papiris, A. Gondouin, A. Guillaumot, S. Hirschi, S. Leroy, S. Marchand-Adam, H. Nunes, C. Picard, G. Prévot, Q. Philippot, M. Reynaud-Gaubert, P. De Vuyst, L. Wemeau, C. Andréjak, S. Jouneau, G. Beltramo, Y. Uzunhan, F. Galodé, V. Westeel and A. Mehdaoui collected data for the study, and participated in the review and editing of the final manuscript.
Conflict of interest: P. Bonniaud reports grants from AstraZeneca, lecture honoraria from Sanofi and AstraZeneca, travel support from AstraZeneca, Novartis, Sanofi, Boehringer and Stallergenes, and advisory board membership with AstraZeneca, Novartis, Sanofi, GSK and Boehringer. J. Cadranel had a patent planned, received consulting fees and participated on a data safety monitoring board or advisory board for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Daichi, Lilly, Pfizer, Novartis, MSD, Roche and Takeda. A. Cazes reports lecture honoraria and travel support from Boehringer Ingelheim. B. Crestani reports grants from Boehringer Ingelheim, consulting fees from Apellis, BMS, Boehringer Ingelheim and Sanofi, lecture honoraria from Apellis, AstraZeneca, BMS, Boehringer Ingelheim, Novartis and Sanofi, support for attending meetings or travel from AstraZeneca, BMS, Boehringer Ingelheim and Sanofi, participated on a data safety monitoring board or advisory board for Apellis, BMS, Boehringer Ingelheim and Sanofi, and had a leadership role as President of the Board of Trustees of the Fondation du Souffle. R. Epaud reports consulting fees from AstraZeneca, lecture honoraria from GSK, AstraZeneca and Menarini, travel support from GSK and AstraZeneca, and advisory board membership with AstraZeneca and Novartis. M-P. Debray reports lecture honoraria and travel support from Boehringer Ingelheim. E. Manali reports lecture honoraria from Boehringer Ingelheim, CLS Behring and Hoffman-La Roche, support for attending meetings or travel from Boehringer Ingelheim, CLS Behring, Hoffman-La Roche and Elpen, and had a leadership role as a Chair in the ERS Task Force for transition of chILD to adult care. S. Papiris reports lecture honoraria from Boehringer Ingelheim and Hoffmann-La Roche, and travel support from Boehringer Ingelheim and Elpen. N. Nathan reports grants from Legs poix de la Chancellerie des Universités 2022 (number 2022000594). C. Andréjak participated on a data safety monitoring board or advisory board for the EVER-ILD2 study (rituximab in diffuse interstitial pneumonia) and received funding via a grant from the French Research Ministry. S. Jouneau reports grants from AIRB, Boehringer Ingelheim and Roche, lecture honoraria from AIRB, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Chiesi, Genzyme, GSK, LVL, Novartis, Pfizer, Roche and Sanofi, travel support from Boehringer Ingelheim, Roche and AIRB, and advisory board participation for Boehringer Ingelheim, GSK and Sanofi. G. Beltramo reports lecture honoraria from Bristol Myers Squibb, and support for attending meetings or travel from Sanofi Aventis France and Boehringer Ingelheim France. S. Hirschi reports research grants from Agence de la Biomedécine, CSL Behring and Adiral medical assistance, lecture honoraria from Boehringer Ingelheim, travel support from CSL Behring, Boehringer Ingelheim and ISIS Medical, and received medical equipment from ISIS Medical. C. Picard reports lecture honoraria and consulting fees from Boehringer Ingelheim. G. Prévot reports honoraria for presentations and educational event from Boehringer Ingelheim, Sanofi, Jansen and MSD. G. Zalcman reports consulting fees from AstraZeneca, BMS, Pfizer and Sanofi, lecture honoraria from BMS, AstraZeneca and Sanofi, support for attending meetings or travel from AstraZeneca and BMS, and participated on a data safety monitoring board or advisory board for AstraZeneca and BMS. V. Cottin reports grants from Boehringer Ingelheim, consulting fees from AstraZeneca, Boehringer Ingelheim, Celgene/BMS, CSL Behring, Ferrer/United Therapeutics, GSK, Pliant, Pure Tech, RedX, Roche, Sanofi and Shionogi, lecture honoraria from Boehringer Ingelheim, Ferrer/United Therapeutics and Roche, support for attending meetings or travel from Boehringer Ingelheim and Roche, participated on a data safety monitoring board or advisory board for Galapagos, Galecto and GSK, and had a leadership role in an adjudication committee for Fibrogen. R. Borie reports consulting fees from Boehringer Ingelheim, Ferrer and Sanofi, lecture honoraria from Boehringer Ingelheim and Roche, travel support from Boehringer Ingelheim, Roche and Chiesi, and advisory board participation for Savara. The remaining authors have no potential conflicts of interest to disclose.
References
- 1.Choi W-I, Park SH, Park BJ, et al. Interstitial lung disease and lung cancer development: a 5-year nationwide population-based study. Cancer Res Treat 2018; 50: 374–381. doi: 10.4143/crt.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzouvelekis A, Spagnolo P, Bonella F, et al. Patients with IPF and lung cancer: diagnosis and management. Lancet Respir Med 2018; 6: 86–88. doi: 10.1016/S2213-2600(17)30478-2 [DOI] [PubMed] [Google Scholar]
- 3.Brown S-AW, Dobelle M, Padilla M, et al. Idiopathic pulmonary fibrosis and lung cancer. A systematic review and meta-analysis. Ann Am Thorac Soc 2019; 16: 1041–1051. doi: 10.1513/AnnalsATS.201807-481OC [DOI] [PubMed] [Google Scholar]
- 4.Lee HY, Lee J, Lee C-H, et al. Risk of cancer incidence in patients with idiopathic pulmonary fibrosis: a nationwide cohort study. Respirology 2021; 26: 180–187. doi: 10.1111/resp.13911 [DOI] [PubMed] [Google Scholar]
- 5.Tomassetti S, Gurioli C, Ryu JH, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest 2015; 147: 157–164. doi: 10.1378/chest.14-0359 [DOI] [PubMed] [Google Scholar]
- 6.Kato E, Takayanagi N, Takaku Y, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ Open Res 2018; 4: 00111-2016. doi: 10.1183/23120541.00111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naccache J-M, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018; 10: 3829–3844. doi: 10.21037/jtd.2018.05.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan WP, Sutherland JC, Fulton JK, et al. Acute diffuse interstitial fibrosis of the lungs. AMA Arch Intern Med 1952; 90: 468–482. doi: 10.1001/archinte.1952.00240100045005 [DOI] [PubMed] [Google Scholar]
- 9.Borie R, Kannengiesser C, Antoniou K, et al. European Respiratory Society statement on familial pulmonary fibrosis. Eur Respir J 2023; 61: 2201383. doi: 10.1183/13993003.01383-2022 [DOI] [PubMed] [Google Scholar]
- 10.Schratz KE, Haley L, Danoff SK, et al. Cancer spectrum and outcomes in the Mendelian short telomere syndromes. Blood 2020; 135: 1946–1956. doi: 10.1182/blood.2019003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philippot Q, Kannengiesser C, Debray MP, et al. Interstitial lung diseases associated with mutations of poly(A)-specific ribonuclease: a multicentre retrospective study. Respirology 2022; 27: 226–235. doi: 10.1111/resp.14195 [DOI] [PubMed] [Google Scholar]
- 12.Borie R, Kannengiesser C, de Fontbrune FS, et al. Management of suspected monogenic lung fibrosis in a specialised centre. Eur Respir Rev 2017; 26: 160122. doi: 10.1183/16000617.0122-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet 2009; 84: 52–59. doi: 10.1016/j.ajhg.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legendre M, Butt A, Borie R, et al. Functional assessment and phenotypic heterogeneity of SFTPA1 and SFTPA2 mutations in interstitial lung diseases and lung cancer. Eur Respir J 2020; 56: 2002806. doi: 10.1183/13993003.02806-2020 [DOI] [PubMed] [Google Scholar]
- 15.van Moorsel CHM, van der Vis JJ, Grutters JC. Genetic disorders of the surfactant system: focus on adult disease. Eur Respir Rev 2021; 30: 200085. doi: 10.1183/16000617.0085-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med 2010; 61: 105–119. doi: 10.1146/annurev.med.60.041807.123500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flamein F, Riffault L, Muselet-Charlier C, et al. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum Mol Genet 2012; 21: 765–775. doi: 10.1093/hmg/ddr508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winslow MM, Dayton TL, Verhaak RGW, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 2011; 473: 101–104. doi: 10.1038/nature09881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillot L, Carré A, Szinnai G, et al. NKX2-1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “brain-lung-thyroid syndrome”. Hum Mutat 2010; 31: E1146–E1162. doi: 10.1002/humu.21183 [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Hosono Y, Yanagisawa K, et al. NKX2-1/TTF-1: an enigmatic oncogene that functions as a double-edged sword for cancer cell survival and progression. Cancer Cell 2013; 23: 718–723. doi: 10.1016/j.ccr.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 21.Manali ED, Legendre M, Nathan N, et al. Bi-allelic missense ABCA3 mutations in a patient with childhood ILD who reached adulthood. ERJ Open Res 2019; 5: 00066-2019. doi: 10.1183/23120541.00066-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottin V, Cordier JF. SFTPC mutations in patients with familial pulmonary fibrosis: combined with emphysema? Am J Respir Crit Care Med 2011; 183: 1113. doi: 10.1164/ajrccm.183.8.1113 [DOI] [PubMed] [Google Scholar]
- 23.Nattes E, Lejeune S, Carsin A, et al. Heterogeneity of lung disease associated with NK2 homeobox 1 mutations. Respir Med 2017; 129: 16–23. doi: 10.1016/j.rmed.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 24.Thurm T, Kaltenborn E, Kern S, et al. SFTPC mutations cause SP-C degradation and aggregate formation without increasing ER stress. Eur J Clin Invest 2013; 43: 791–800. doi: 10.1111/eci.12107 [DOI] [PubMed] [Google Scholar]
- 25.Zhong Q, Zhou B, Ann DK, et al. Role of endoplasmic reticulum stress in epithelial–mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am J Respir Cell Mol Biol 2011; 45: 498–509. doi: 10.1165/rcmb.2010-0347OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Moorsel CHM, Hoffman TW, van Batenburg AA, et al. Understanding idiopathic interstitial pneumonia: a gene-based review of stressed lungs. Biomed Res Int 2015; 2015: 304186. doi: 10.1155/2015/304186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borie R, Funalot B, Epaud R, et al. NKX2.1 (TTF1) germline mutation associated with pulmonary fibrosis and lung cancer. ERJ Open Res 2021; 7: 00356-2021. doi: 10.1183/23120541.00356-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Wan L, Shen H, et al. Thyroid transcription factor-1 amplification and expressions in lung adenocarcinoma tissues and pleural effusions predict patient survival and prognosis. J Thorac Oncol 2012; 7: 76–84. doi: 10.1097/JTO.0b013e318232b98a [DOI] [PubMed] [Google Scholar]
- 29.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips-Houlbracq M, Mal H, Cottin V, et al. Determinants of survival after lung transplantation in telomerase-related gene mutation carriers: a retrospective cohort. Am J Transplant 2021; 22: 1236–1244. doi: 10.1111/ajt.16893 [DOI] [PubMed] [Google Scholar]
- 34.Borie R, Kannengiesser C, Gouya L, et al. Pilot experience of multidisciplinary team discussion dedicated to inherited pulmonary fibrosis. Orphanet J Rare Dis 2019; 14: 280. doi: 10.1186/s13023-019-1256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyard A, Gaudemer, A, Kannengiesser C, et al. Pathology and radiology of interstitial lung disease in patients with telomere-related genes mutations. Eur Respir J 2023; 62: Suppl. 67, PA1153. doi: 10.1183/13993003.congress-2023.PA1153 [DOI] [Google Scholar]
- 36.Camboulive A, Borie R, Latrasse M, et al. Impact of familial character and/or the existence of a mutation in telomere-related genes in patients with pleuroparenchymal fibroelastosis. Eur Respir J 2023; 62: Suppl. 67, PA3953. doi: 10.1183/13993003.congress-2023.PA3953 [DOI] [Google Scholar]
- 37.Santé Publique France . Cancers. 2023. www.santepubliquefrance.fr/maladies-et-traumatismes/cancers Date last accessed: 3 March 2024.
- 38.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 39.Bermudez J, Nathan N, Coiffard B, et al. Outcome of lung transplantation for adults with interstitial lung disease associated with genetic disorders of the surfactant system. ERJ Open Res 2023; 9: 00240-2023. doi: 10.1183/23120541.00240-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cottin V, Bonniaud P, Cadranel J, et al. Recommandations pratiques pour le diagnostic et la prise en charge de la fibrose pulmonaire idiopathique – Actualisation 2021. Version courte. [French practical guidelines for the diagnosis and management of IPF – 2021 update, short version.] Rev Mal Respir 2022; 39: 275–312. doi: 10.1016/j.rmr.2022.01.005 [DOI] [PubMed] [Google Scholar]
- 41.Jänne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer: the SELECT-1 randomized clinical trial. JAMA 2017; 317: 1844–1853. doi: 10.1001/jama.2017.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquereau A, Andler R, Guignard R, et al. Consommation de tabac parmi les adultes en 2020: résultats du Baromètre de Santé publique France. [Tobacco use among adults in 2020: results from the Sante Publique France Health Barometer.] Bull Epidemiol Hebd 2021; 8: 132–139. [Google Scholar]
- 43.Liu Q, Zhou Y, Cogan JD, et al. The genetic landscape of familial pulmonary fibrosis. Am J Respir Crit Care Med 2023; 207: 1345–1357. doi: 10.1164/rccm.202204-0781OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borie R, Kannengiesser C, Nathan N, et al. Familial pulmonary fibrosis. Rev Mal Respir 2015; 32: 413–434. doi: 10.1016/j.rmr.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 45.Mu D. The complexity of thyroid transcription factor 1 with both pro- and anti-oncogenic activities. J Biol Chem 2013; 288: 24992–25000. doi: 10.1074/jbc.R113.491647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girard N, Marchand-Adam S, Naccache J-M, et al. Lung cancer in combined pulmonary fibrosis and emphysema: a series of 47 Western patients. J Thorac Oncol 2014; 9: 1162–1170. doi: 10.1097/JTO.0000000000000209 [DOI] [PubMed] [Google Scholar]
- 47.Katzen J, Wagner BD, Venosa A, et al. An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight 2019; 4: e126125. doi: 10.1172/jci.insight.126125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maitra M, Wang Y, Gerard RD, et al. Surfactant protein A2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J Biol Chem 2010; 285: 22103–22113. doi: 10.1074/jbc.M110.121467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nureki S-I, Tomer Y, Venosa A, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest 2018; 128: 4008–4024. doi: 10.1172/JCI99287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanoue LT, Tanner NT, Gould MK, et al. Lung cancer screening. Am J Respir Crit Care Med 2015; 191: 19–33. doi: 10.1164/rccm.201410-1777CI [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01809-2023.Supplement (605.7KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01809-2023.Shareable (1MB, pdf)