Abstract
This study aimed to estimate genetic parameters for feeding behavior (FB) traits and to assess their genetic relationship with performance traits in group-housed broilers. In total, 99,472,151 visits were recorded for 95,711 birds between 2017 and 2022 using electronic feeders. The visits were first clustered into 2,667,617 daily observations for ten FB traits: daily feed intake (DFI), daily number of visits (NVIS), time spent at the feeders (TSF), number of visited feeders (NVF), visiting activity interval (VAI), feeding rate (FR), daily number of meals (NMEAL), average intake per meal (INTMEAL), number of visits per meal (VISMEAL) and interval between meals (MEALIVL). All FB traits were then considered as the average per bird across the feeding test period. Three growth traits (body weight at the start – SBW and at the end of the feeding test – FBW, and weight gain over the test period – BWG), and 2 feed efficiency (FE) traits (Feed Conversion Rate - FCR and Residual Feed Intake - RFI) were also recorded. The (co)variance components were estimated using multitrait animal mixed models. For growth and FE, the heritability (h2) estimates were moderate, ranging from 0.20 ± 0.01 (BWG) to 0.32 ± 0.02 (RFI). Overall, the h2 estimates for FB traits were higher than for productive traits, ranging from 0.31 ± 0.01 (DFI) to 0.56 ± 0.02 (TSF). DFI presented high genetic correlations (0.53–0.86) with all performance traits. Conversely, the remaining FB traits presented null to moderate genetic correlations with these traits, ranging from –0.38 to 0.42 for growth traits and between –0.14 and 0.25 for FE traits. Genetic selection for favorable feeding behavior is expected to exhibit a fast genetic response. The results suggest that it is possible to consider different feeding strategies without compromising the genetic progress of FE. Conversely, breeding strategies prioritizing a higher bird activity might result in lighter broiler lines in the long term, given the negative genetic correlations between visit-related traits (NV, NVF, and NMEAL) and growth traits (SBW and FBW).
Key words: broiler, feeding behavior, feed efficiency, genetic parameter, radio frequency identification
INTRODUCTION
Feeding costs comprise a major component of meat-type poultry production expenses (Donohue and Cunningham 2009; Abasht et al., 2020). This circumstance has motivated modern breeding programs to incorporate feed efficiency (FE) related traits as a selection criterion for improving the relative ability of the animals to convert feed into growth. Improving FE is also expected to decrease nitrogen waste and gas emissions, hence, selecting energy-efficient animals might affect positively both the productivity and environmental footprint of broiler production systems in the mid to long term (Willems et al., 2013; Reyer et al., 2015).
Computing FE-related traits depends on measuring individually the animal feed intake (FI), which is generally recorded in broilers and laying hens using individual feed cages (Aggrey et al., 2010; Li et al, 2020a; Marchesi et al., 2021). However, cage-based rearing systems impose limitations on large-scale phenotype recording. Moreover, such systems modify the animal's natural feeding activity and prevent social interactions with other birds. Accordingly, measuring FI in individual cages raises welfare concerns and does not reflect commercial conditions of broiler production, which may create bias during the selection process of FE-related traits. The advent of electronic feeding systems with sensor-based identification has allowed measuring accurately the daily individual feed intake of a large number of group-housed animals (Mendes et al., 2011; Lu et al., 2017). In poultry species, different technologies for automatic feed intake recording have been described (Picard et al., 1992; Bley and Bessei, 2008; Tu et al., 2011; Yan et al., 2019). However, to our knowledge, limited information on the genetic basis of traits measured through such systems has been reported in the literature for large purebred broiler populations (Howie et al., 2011).
Electronic feeders equipped with sensor technology allow the continuous recording of information on a visit basis, which opens opportunities to unveil important biological information on the individual feeding behavior (FB) patterns associated with the feed intake in group-housed animals (Lu et al., 2017). Investigating FB traits in broiler chickens provides relevant data for guiding selection programs toward favorable feeding strategies according to different management conditions. For instance, selecting animals that visit feeders less frequently and have high consumption per visit could help prevent potential feed competition in conventional production systems that use fast-growing broiler lines. Moreover, some FB traits (e.g., feeding time) may correlate with animal welfare and health status in different species (Cornou et al., 2008; González et al., 2008; Llonch et al., 2018; Alves et al., 2022).
A question of major interest is how FB traits correlate genetically with production and FE traits since the latter are directly relevant for breeding programs and commercial poultry farms. Conversely, it is crucial to understand how selecting high-performance individuals might affect their natural feeding behavior and feed intake regulation. This aspect holds significant implications for animal welfare, as changes in these behaviors could impact the well-being of poultry (Bokkers et al., 2004; Honda 2021). Therefore, estimating (co)variance components for FB traits and their genetic association with FE and production traits is key for guiding future decisions on the genetic selection of FB traits in broilers.
The aims of this study were: 1) to characterize different criteria used for assessing feeding behavior in broilers based on daily visit data collected with automatic feeders, 2) to investigate if the individual variation observed for feeding behavior traits in modern broilers is heritable, and 3) to assess the genetic relationship between FB traits and productive traits of economic importance.
MATERIAL AND METHODS
Housing and Data Recording
The data used in this study comprised phenotypic records for broiler chickens from a male line under selection pressure for growth. The pedigree data for this line included 208,286 birds from 42 overlapping mating generations (MG). All birds were from the same pedigree line and hatched at the same farm. The birds were kept in experimental pens in environmentally controlled houses located in Oklahoma. Each house had up to 3 individual pens (typically of 56 ft length x 13 ft width) which had at least 6 feeding units containing either 8 or 16 stations (electronic feeders) of 1 ft2 each, and water lines equipped with nipple drinkers. The pens were housed with an average density of 1.5 birds/ft2. The adaptation period lasted 14 d, for which no data was recorded from the electronic feeders. The feeding trials started 21 d after the birds hatched, all animals were the same age at the start of the trials. All management procedures were performed according to the rearing stage, following the recommendations described in the Cobb Broiler management guide (Cobb-Vantress Inc., 2021).
In total, 99,472,151 visit logs were obtained for 95,711 birds from both sexes during 146 experimental feeding tests of 28 d in length, occurring between the years 2017 and 2022. The number of observations for male and female birds was approximately equal, with birds reared in separate pens according to sex. The visit logs were recorded with an electronic feeding system developed by Cobb Vantress Inc. During the experimental period, the birds wore wing-band transponders that could be individually identified by the electronic feeder sensors using a radio frequency antenna system. All birds were tagged several days before the feeding trial so that the birds' handling did not impact the results observed. The proportion of transponders that were lost was minimal, as less as 1% (Cobb-Vantress, Inc., Siloam Springs, AR, personal communication). The feeders were weekly adjusted to allow the access of only 1 bird at a time. During each visit to the feeder, the feeding system recorded the identification codes for the wing band (animal), house, pen, unit, and station, the date and time of the visit, the total time spent at the feeder (seconds), the weight of feed consumed (g), and the estimated competition level at the feeder during the visit (light, normal or heavy competition). The competition level was estimated based on the captured RFID signals when multiple birds attempted to feed from the same feeder. The time spent at the feeder and the amount of food consumed was recorded with resolutions of 1 second and 0.1 g, respectively. The visit data were automatically transferred and stored on a local server. The body weight at the start (SBW) and ending (FBW) of the feeding test were also recorded for the birds. The FBW was recorded for all animals from this line, including those that did not participate in the feeding test, totaling 154,654 birds. The FBW averages for males and females enrolled in the feeding trials were 11.08 g and 37.98 g lower (P-value < 0.001) than the average FBW observed for birds that did not participate in the trials. However, these differences are not large enough to imply practical significance.
Data Editing and Traits Definition
Records of animals that were withdrawn from the test (found dead or due to welfare culling, N = 8,813) were removed from the database. Further, animals that did not have visit data available for all days (N = 7,856) of the experimental period were also removed. This last exclusion criterion does not necessarily indicate failures in the electronic feeding system as there were human interventions for routine management during different stages of the feeding tests, which might impair an animal from visiting the feeders. All records with feed consumption of at least 0.2 g were considered valid intake events. The sequential visit logs were first clustered into 2,667,617 daily observations for ten feeding behavior (FB) traits: the daily feed intake (g/d), the daily number of visits (NVIS, count/d), time spent at the feeders (TSF, h/d), number of visited feeders (NVF, count/d), visiting activity interval (VAI, h/d), feeding rate (FR, g/h), the daily number of meals (NMEAL, count/d), average intake per meal (INTMEAL, g), number of visits per meal (VISMEAL, count) and the interval between consecutive meals (MEALIVL, min).
The meal-based traits were computed according to the biological concept of hunger and satiety (Yeates et al., 2001). Reoccurring consecutive visits to the feeder in short-time intervals suggest that the satiety for that individual has not been achieved yet. We analyzed the bimodal log distribution of these visit intervals to find a meal criterion that represented the shortest visit interval separating meal events. In our dataset, the meal criterion was estimated in 1200 s. Visits that occurred within an interval equal to or less than the meal criterion were considered as the same meal event (Howie et al., 2009). The identified meal events were counted individually in daily intervals to find NMEAL. The INTMEAL considered the total feed intake within each meal, whereas VISMEAL accounted for the total number of short-interval visits clustered into each meal event.
The FR considered the daily feed intake divided by the total time spent at the feeder (TSF). For NVF, the feeding unit and station codes were concatenated to yield the specific identification of each feeder, and we considered how many individual feeders were visited by the bird during the day, that is, multiple visits to the same feeder only accounted for one unique feeder. The VAI considered the time interval between the first and last visiting event of the bird during the day. All FB traits were considered as the average per bird across the feeding test period. The daily feed intake records were summed to compute the total feed intake (TFI) throughout the test period. The body weight gain (BWG) during the feeding test was computed as the difference between the weights at the end and the start of the feeding test. The TFI, BWG, and FBW were then used to compute the FE traits (Feed Conversion Rate - FCR and Residual Feed Intake - RFI) for the whole feeding test interval considering the following equations:
where is the intercept, and are the partial regression coefficients of TFI on and , respectively, and sex accounts for the average effect of sex on TFI. RFI was then formulated to reflect the difference between the observed feed intake and the expected feed intake given the bird's size, growth, and sex.
The contemporary groups (CG) were defined as the combination of the effects of the hatch, source, and MG. The effect of the pen was not considered since all animals from the same MG were (in most cases) reared in the same pen; therefore, this effect was already accounted for in the CG. For all traits, animals exceeding 3.5 standard deviations from the CG average were excluded from the database. This exclusion criteria was applied individually for each trait. For variables computed as a combination of other traits (e.g., FCR and FR), the resultant trait was only computed if there were no outliers in all composing traits. After editing procedures, the number of birds with valid observations was 122,822 for FBW and ranged between 73,530 and 80,044 for the remaining traits.
Statistical Analyses
As an initial step in data exploration, we investigated the influence of the feed-efficiency groups and size on the feeding behavior of the birds. Two divergent groups for RFI were defined, that is, the top 10% of animals with the highest values for RFI were considered as lowly efficient (Low), and animals expressing the 10% lowest values for RFI were classified as highly efficient (High). The animal size category was defined according to the within-sex FBW quantiles. Animals with FBW below the were considered as extra light (EL), between and as light (L), between and as heavy (H), and above as extra-heavy (EH). We performed an unpaired two-sample Wilcoxon test to assess the statistical significance of the efficiency and size groups on the studied FB traits. This is a non-parametric alternative to the t-test that can better handle deviations from the normality assumption (Figure S1).
The (co)variance components and breeding values for the growth, FE, and FB traits were estimated using a multitrait animal model, written in matrix form as follows:
in which is the vector of observations, is the vector of fixed effects (sex and CG), and u, p, and e are the vectors of additive genetic, maternal permanent environment, and residual random effects, respectively; , , and are incidence matrices linking the observations to the respective fixed and random effects. It is assumed for this model that
and , where , P = and R = , with , , and representing the covariance matrices of dimension t x t (t = number of traits analyzed) for the additive, maternal permanent environment, and residual effects; is the p x p additive numerator relationship matrix (where p is the number of individuals in the pedigree file) with elements representing the expected additive genetic relationship between individuals i and j given their common ancestors in the pedigree data; and are identity matrices of proper dimensions, and represent the Kronecker product.
The genetic associations between the FB and performance traits were estimated using a bi-trait animal model, by pairwise combining the observations of 1 FB trait with all remaining traits. We also performed an additional analysis, considering jointly all performance traits (SBW, FBW, BWG, FCR, RFI), using a 5-trait mixed model. The (co)variance components were estimated using the residual maximum likelihood (REML) approach. A first solution was obtained via the expectation-maximization (EM) algorithm with a convergence criterion of 10−12. Subsequently, these solutions were used as initial guesses for the Average Information (AI) algorithm. The estimates of heritability (h2) and proportion of phenotypic variance due to the maternal permanent environment effects (p2) for the FB traits were computed using the results from the bi-trait analyses with FBW, whereas for the productive traits, the 5-trait analysis was used to compute the estimates for the h2 and p2 parameters. The genetic correlations (rg) were estimated as , where is the genetic covariance between traits x and y, and and are their respective genetic additive variances. The empirical phenotypic correlations ( were simply computed as Pearson's correlation coefficient between the raw phenotypes. All mixed model analyses were performed using the BLUPF90 suite programs (Misztal et al., 2018).
RESULTS
Feeding Behavior Traits Description
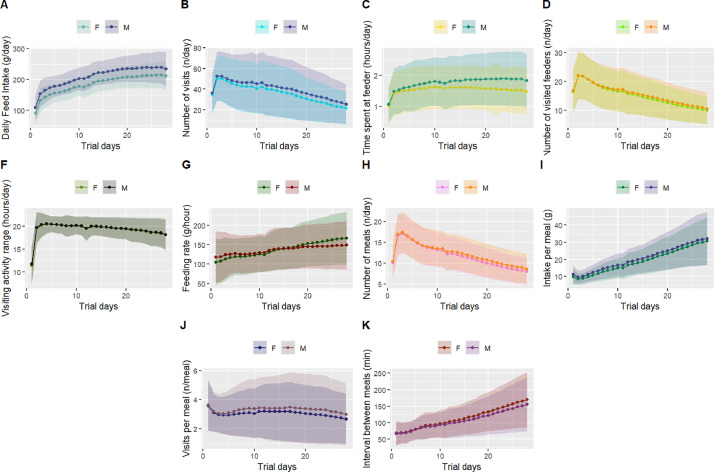
Figure 1 shows the temporal trend for each FB trait according to sex. The DFI followed a similar trend in both sexes, although the male birds presented consistently higher intake throughout the studied period. The averages for NV, NVF, and NMEAL tended to decrease over the test period, indicating less visiting activity as the birds became older (Figures 1B, 1D, and 1H). Conversely, the FR and INTMEAL increased considerably during the same time interval (Figures 1G and 1I).
Figure 1.
Line charts according to sex for different feeding behavior traits measured in broilers using a real-time radio-frequency feeding system. Shaded colors represent the observations' standard deviation for each day during the feeding test.
The visiting activity interval (i.e., the time difference between the very first and last visits of a bird during the day) presented a slight decrease since the start of the test, with the observations varying around 20 h for almost the whole period (Figure 1F). TSF showed divergent trends according to sex, with the differences observed between sexes increasing as the birds got older (Figure 1C). There was also observed a time-by-sex interaction for FR, with males eating faster at the beginning of the feeding test and the opposite being observed as the test moved onward (Figure 1G).
For all statistical analyses, we considered the individual observations for FB and FE traits as the average per bird across the test period; Table 1 provides basic descriptive statistics for these traits. The birds had on average 37.19 ± 19.69 daily visits, clustered into 11.83 ± 2.79 meals across the test period. These animals spent on average 1.66 ± 0.61 h per day at the feeders, achieving an FR of 138.94 ± 44.45 g/h with an average intake per meal of 20.51 ± 6.39 g (Table 1). Except for DFI and VAI, all FB traits presented high coefficients of variation (>20%), with a special highlight for the NV and VISMEAL, which presented standard deviations surpassing 50% of the mean value. Those high coefficients of variation are mostly due to the right-skewed distributions observed for both NV and VISMEAL (Figure S1), which are consistent with count data behavior.
Table 1.
Number of observations (N), mean ± standard deviation (SD), minimum (Min), maximum (Max), and coefficient of variation (CV) for growth, feed efficiency, and feeding behavior traits measured in group-housed broilers using electronic feeders.
| Traita | N | Mean ± SD | Min | Max | CV (%) |
|---|---|---|---|---|---|
| SBW (g) | 80,044 | 1121.57 ± 200.29 | 460.0 | 1925.0 | 17.86 |
| FBW (g) | 122,822 | 4386.58 ± 573.10 | 1945.0 | 6295.0 | 13.06 |
| BWG (g) | 79,971 | 3297.28 ± 496.11 | 1113.0 | 5050.0 | 15.05 |
| FCR | 78,495 | 1.78 ± 0.13 | 1.26 | 2.55 | 6.99 |
| RFI | 78,781 | 0.16 ± 287.83 | −2354.192 | 3101.94 | - |
| DFI (g/d) | 77,129 | 199.68 ± 23.30 | 41.83 | 339.16 | 11.67 |
| NVIS (n/d) | 77,129 | 37.19 ± 19.69 | 5.21 | 176.89 | 52.94 |
| TSF (h/d) | 77,129 | 1.66 ± 0.61 | 0.29 | 4.99 | 36.92 |
| NVF (n/d) | 77,129 | 15.04 ± 4.61 | 3.64 | 37.84 | 30.68 |
| VAI (h/d) | 77,129 | 19.34 ± 1.03 | 10.97 | 23.08 | 5.33 |
| FR (g/h) | 77,129 | 138.94 ± 44.45 | 28.28 | 458.29 | 31.99 |
| NMEAL (n/day) | 77,129 | 11.83 ± 2.79 | 3.71 | 26.42 | 23.62 |
| INTMEAL (g) | 77,129 | 20.51 ± 6.39 | 5.53 | 64.89 | 31.13 |
| VISMEAL (n) | 77,129 | 3.16 ± 1.61 | 1.03 | 16.99 | 50.93 |
| MEALIVL (min) | 73,530 | 113.61 ± 33.56 | 34.05 | 336.89 | 29.54 |
SBW: body weight measured at the start of the feeding test; FBW: body weight measured at the end of the feeding test; BWG: total weight gain during the test; FCR: feed conversion rate; RFI: residual feed intake; DFI: daily feed intake; NVIS: number of daily visits; TSF: time spent at the feeder; NVF: number of visited feeders; VAI: visiting activity interval; FR: feeding rate; NMEAL: number of meals; INTMEAL: intake per meal; VISMEAL: number of visits per meal; MEALIVL: meals interval.
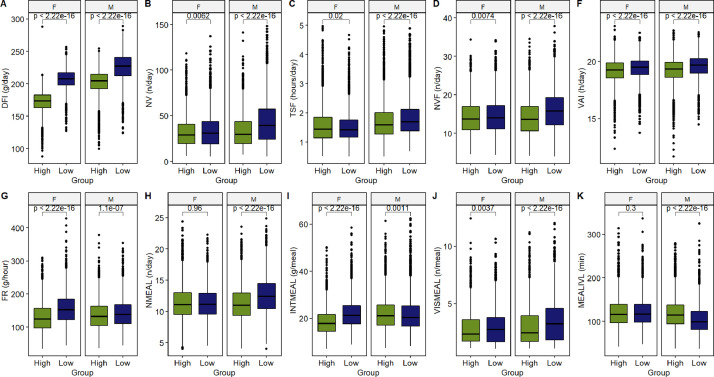
We tested the null hypothesis that the distributions for the FB traits were identical regardless of the feed-efficiency group (High or Low) using an unpaired 2-sample Wilcox test (Figure 2). The computed p-values indicate that the observed data presented a low agreement with the pattern predicted by the null hypothesis. The main exceptions were for NMEAL and MEALIVL, for which the data suggested similar distributions for the female birds according to the feed-efficiency group (Figures 2H and 2K). The DFI presented the most prominent differences, with higher intakes generally associated with the low feed-efficient group in both sexes (Figure 2A). Other noticeable patterns were that low feed-efficient males tended to have more daily visits and meals (NV and NMEAL), spent more time at the feeders (TSF), and presented higher rates of visited feeders (NVF) and shorter inter-meal intervals (Figures 2B-D and 2H). For the females, besides the DFI, the most remarkable differences were found for FR and INTMEAL, with low feed-efficient animals tending to feed faster and presenting higher intake per meal (Figures 2G and -2H).
Figure 2.
Boxplots for different feeding behavior traits according to the feed-efficiency group (Low and High were composed of the animals with the top 10% highest and lowest values for residual feed intake) and sex. The unpaired two-sample Wilcox test was used to compute the P-values under the null hypothesis of equivalence between the distributions of 2 populations.
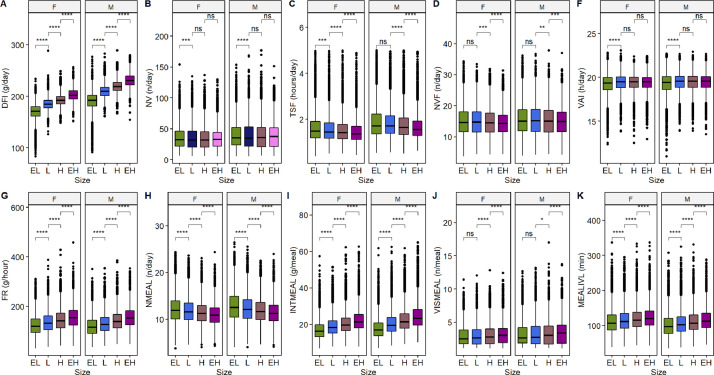
Figure 3 shows the boxplots for different FB traits according to the size groups and sex; we also tested the null hypothesis of no differences between the distributions of the FB traits according to the size group. There were notable differences for the DFI (Figure 3A), with animals from the EH and EL groups having an average daily intake difference of 41.37 g and 33.18 g for the males and females, respectively. The computed P-values also indicate a high degree of incompatibility with the null hypothesis for FR, NMEAL, INTMEAL, and MEALIVL. The boxplots for these traits and the Wilcox test suggested that heavier animals from both sexes tend to have higher feeding rates, intake per meal, and meal intervals, but fewer daily meals (Figures 3G–3I and 3K). Conversely, for NV and VAI, the observed data were consistent with the null hypothesis in most cases, which means that the observations for these traits are likely to derive from the same distribution within the same sex category (Figures 3B and 3F).
Figure 3.
Boxplots for different feeding behavior traits according to the size group (EL – Extra Light, L – Light, H – Heavy, and EH – Extra Heavy) and sex. The P-values were computed under the null hypothesis for an unpaired two-sample Wilcox test; *, ** and **** represent P-values lower than 0.05, 0.01, and 0.001 respectively; ns represent P-values higher than 0.05.
Genetic Parameter Estimates
Table 2 shows the estimates for the variance components, heritability, and proportion of phenotypic variance due to the maternal permanent environment effects for all analyzed traits. For the growth and FE traits, the heritability estimates were moderate, ranging from 0.20 ± 0.01 (BWG) to 0.32 ± 0.02 (RFI). Overall, the heritability estimates were higher for the FB traits than for the productive traits, ranging from moderate to high magnitudes (Table 2). The highest h2 estimates were observed for MEALIVL (0.42 ± 0.02), INTMEAL (0.44 ± 0.02), FR (0.47 ± 0.02), NMEAL (0.49 ± 0.02), and TSF (0.56 ± 0.02). Grouping the daily number of visits into meals increased the h2 estimate from 0.35 ± 0.02 to 0.49 ± 0.02 (Table 2). The proportion of variance due to the maternal permanent environment was low for all traits, ranging between 0.02 and 0.03 in most cases, except for SBW, which presented a slightly higher estimate for this parameter (0.07 ± 0.01).
Table 2.
Variance components and genetic parameter estimates of growth, feed efficiency, and feeding behavior traits measured in group-housed broilers using electronic feeders.
| Trait | h2 ± s.e | mpe2± s.e | |||
|---|---|---|---|---|---|
| SBW | 4236.1 | 1467.8 | 14385.0 | 0.21 ± 0.01 | 0.07 ± 0.01 |
| FBW | 35527.0 | 4509.4 | 106450.0 | 0.24 ± 0.01 | 0.03 ± 0.00 |
| BWG | 21046.0 | 1609.8 | 83632.0 | 0.20 ± 0.01 | 0.02 ± 0.00 |
| FCR | 0.0022 | 0.0001 | 0.0065 | 0.25 ± 0.01 | 0.02 ± 0.00 |
| RFI | 21395.0 | 1062.3 | 44676.0 | 0.32 ± 0.02 | 0.02 ± 0.00 |
| DFI | 98.20 | 6.52 | 211.68 | 0.31 ± 0.01 | 0.02 ± 0.00 |
| NV | 47.83 | 2.25 | 84.56 | 0.35 ± 0.02 | 0.02 ± 0.00 |
| TSF | 0.15 | 0.007 | 0.11 | 0.56 ± 0.02 | 0.02 ± 0.00 |
| NVF | 4.19 | 0.25 | 6.28 | 0.39 ± 0.02 | 0.02 ± 0.00 |
| VAI | 0.29 | 0.02 | 0.53 | 0.35 ± 0.02 | 0.02 ± 0.00 |
| FR | 712.8 | 34.9 | 755.6 | 0.47 ± 0.02 | 0.02 ± 0.00 |
| NMEAL | 3.26 | 0.15 | 3.23 | 0.49 ± 0.02 | 0.02 ± 0.00 |
| INTMEAL | 14.47 | 0.95 | 17.12 | 0.44 ± 0.02 | 0.03 ± 0.00 |
| VISMEAL | 0.25 | 0.014 | 0.52 | 0.32 ± 0.01 | 0.02 ± 0.00 |
| MEALIVL | 398.1 | 20.9 | 514.7 | 0.42 ± 0.02 | 0.02 ± 0.00 |
SBW: body weight measured at the start of the feeding test; FBW: body weight measured at the end of the feeding test; BWG: total weight gain during the test; FCR: feed conversion rate; RFI: residual feed intake; DFI: daily feed intake; NVIS: number of daily visits; TSF: time spent at the feeder; NVF: number of visited feeders; VAI: visiting activity interval; FR: feeding rate; NMEAL: number of meals; INTMEAL: intake per meal; VISMEAL: number of visits per meal; MEALIVL: meals interval. : genetic additive variance; : maternal permanent environment variance; : residual variance; h2: narrow-sense heritability; mpe2: proportion of phenotypic variance due to the maternal permanent environment effects; s.e standard error.
Phenotypic and Genetic Correlations
The phenotypic and genetic correlations among growth, FB, and FE traits are depicted in Figure 4. The raw phenotypic correlations among growth traits (SBW, FBW, and BWG) were proportional to the genetic correlations, with estimates in the same direction (positive) and with similar magnitudes (Figure 4). The highest correlation coefficient was observed between BWG and FBW, 0.94 for both phenotypic and genetic correlation coefficients. The correlation estimates between FE and growth traits varied considerably, for instance, the phenotypic correlation between FCR and BWG was moderate and negative (–0.65), but it was not nominally different from zero for the additive genetic component (–0.02), which implies that the association between these 2 traits is only due to environmental or nonadditive effects. We also observed a similar result for the correlation estimates between FCR and FBW (Figure 4). By construction, the RFI presented a null correlation with the growth traits (SBW, FBW, and BWG); although the genetic correlations indicate that there is a positive and moderate correlation between the additive genetic effects of RFI and the growth traits, ranging from 0.27 to 0.32 (Figure 4). Additionally, the FE traits presented a strong and positive correlation with each other on both scales (raw phenotypes and estimated breeding values).
Figure 4.
Phenotypic and genetic correlations among growth, feed efficiency, and feeding behavior traits, measured in group-housed broilers using electronic feeders. Blank squares indicate nonsignificant correlations; SBW: body weight measured at the start of the feeding test; FBW: body weight measured at the end of the feeding test; BWG: total weight gain during the test; FCR: feed conversion rate; RFI: residual feed intake; DFI: daily feed intake; NVIS: number of daily visits; TSF: time spent at the feeder; NVF: number of visited feeders; VAI: visiting activity interval; FR: feeding rate; NMEAL: number of meals; INTMEAL: intake per meal; VISMEAL: number of visits per meal; MEALIVL: meals interval.
The correlation coefficients among the FB traits showed a broad range in both directions and magnitudes. In general, the directions were the same for the phenotypic and genetic correlations among FB traits. Nonetheless, the genetic correlations tended to present higher magnitudes (Figure 4). The estimated genetic correlation between DFI and all remaining FB traits was null to weak (Figure 4). An important exception is for intake per meal (INTMEAL), which presented a moderate and positive genetic correlation with DFI (0.40). There was also observed a negative correlation between DFI and NVF (–0.23). There are strong antagonist genetic relationships among some FB traits. For instance, the genetic selection for increasing the number of daily visits, number of meals, number of visited feeders, or the visiting activity range would reduce the intake per meal and the interval between meals as these 2 latter traits presented genetic correlations ranging from –0.75 to –0.96 with the 4 traits mentioned before (Figure 4). We also observed a high and negative genetic correlation between TSF and FR (–0.91). On the other hand, there were observed moderate to high positive genetic correlations among NV, TSF, NMEAL, and VISMEAL, with estimates ranging from 0.51 to 0.77 (Figure 4). Likewise, the positive genetic correlation between NMEAL and VAI (0.88) indicates that animals genetically prone to have a higher number of meals also tend to have wider intervals between the very first and last daily visits.
Except for DFI, most of the FB traits were null to weakly correlated with the growth and FE traits, with the phenotypic correlation coefficients ranging from –0.25 to 0.40 and between –0.38 and 0.42 for the genetic correlation estimates (Figure 4). The DFI presented high genetic correlations (0.53–0.86) with all performance traits. The estimated breeding values of NV, NVF, NMEAL, and VAI were negatively correlated with those of SBW and FBW ( ranging from –0.38 to –0.14) while INTMEAL, FR, and MEALIVL presented positive genetic correlations with the same growth traits (Figure 4). The time spent at feeders did not have any significant genetic associations with all performance traits (growth and FE traits), although it was weakly correlated with SBW in the raw phenotypic scale (0.21). The FE traits (FCR and RFI) presented null to low genetic correlations with all FB traits, except for DFI, which presented high and positive genetic correlations with these traits. Disregarding DFI, RFI only presented significant genetic correlations with INTMEAL (0.25 ± 0.03), FR (0.21 ± 0.03), and VISMEAL (0.12 ± 0.04), while FCR presented low and negative genetic correlations with NVF, VAI, and NMEAL (ranging from –0.14 to –0.11) and it was (also in low magnitudes) positively correlated with FR, INTMEAL, VISMEAL, and MEALIVL ( ranging from 0.11 to 0.21).
Selection Strategies Considering Feeding Behavior Traits
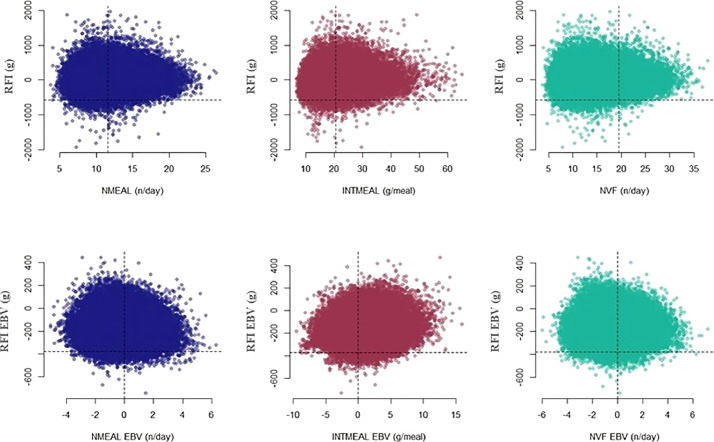
Figure 5 shows the dispersion plots for the FBW (y-axis) with different FB traits (x-axis) on both phenotypic and EBV scales. Phenotypically, it can be seen that animals demonstrate a wide range of strategies in terms of the number of meals, intake per meal, and the number of visited feeders for achieving relatively the same high-weight performances (Figure 5), especially for NMEAL and NVF, which presented null or close to null phenotypic correlations with FBW (Figure 4). Conversely, setting a high selection intensity for the FBW breeding values (2% of the best-ranked animals) would tend to increase the selection pressure toward a higher intake per meal and a lower number of meals and visited feeders. Nevertheless, it would be possible to consider different selection strategies for NMEAL, as considerable genetic variation still exists in the right tail of the EBV distribution considering solely the observations above the truncation point for the FBW breeding values (Figure 5).
Figure 5.
Dispersion plots for the phenotypic observations and breeding values of body weight at the final of the feeding test (FBW) and different feeding behavior traits (NMEAL – number of meals, INTMEAL – intake per meal, NVF – number of visited feeders) measured in group-housed broilers. Dashed lines in the y-axis were set at the threshold of 2% of the distribution upper-tail whereas the variable median was set for the x-axis.
The scatter plots in Figure 6 indicate that different levels of NMEAL, INTMEAL, and NVF can result in comparable FE across animals. In addition, it can be noticed that there is much more flexibility in selecting animals with high FE considering different FB strategies than there is for selecting high FBW (Figure 5, Figure 6). Animals presenting a wide range of EBV for NMEAL and NVF surpass the truncation point for RFI (Figure 6), as one would expect given the null correlations estimated between these traits and the FE trait. This is also true for INTMEAL, which presented a weak genetic correlation with RFI (Figure 4).
Figure 6.
Dispersion plots for the phenotypic observations and breeding values of residual feed intake (RFI) and different feeding behavior traits (NMEAL – number of meals, INTMEAL – intake per meal, NVF – number of feeders visited), measured in group-housed broilers. Dashed lines in the y-axis were set at the threshold of 2% of the distribution lower tail whereas the variable median was set for the x-axis.
DISCUSSION
Feed efficiency largely influences the production costs of animal protein (Herd et al., 2003; Waller, 2007). Accordingly, different animal breeding schemes have been focused on improving FE-related traits (Herd et al., 2003; Willems et al., 2013; Patience et al., 2015). With the adoption of sensor technologies for automatic intake recording, it is currently possible to measure feed efficiency massively across different livestock species (Lu et al., 2017; Li et al., 2020b; Cavani et al., 2022). The use of electronic feeders also opens novel possibilities to investigate behavioral patterns that can be associated with feed intake in group-housed animals (Howie et al., 2009). Recording FB data has many practical implications, such as understanding the regulation mechanisms of spontaneous feeding and investigating the effects of drugs, diets, feeding equipment, and housing systems, among others (Hyun et al, 1997; Fouad et al., 2008; O'Driscoll et al., 2009; Neves et al., 2010). The feeding behavior is also associated with the animal's health status and could be used for illness detection (Cornou et al., 2008; González et al., 2008; Alves et al., 2022). Additionally, different behavior parameters might be used as selection criteria in breeding schemes for achieving genetic improvement toward desired feeding behavior (Howie et al., 2011). In this study, we aimed mainly to characterize the genetics of FB traits measured in group-housed broilers and their relationships with important production traits. The genetic parameters were estimated for visit and meal-based behavior traits, vastly recorded in a purebred line that has been intensively selected for growth.
The results have pointed out that feeding behavior at the individual level presents a high potential for selection response given the moderate to high heritability estimates for all FB traits investigated (Table 2). The highest h2 values were estimated for the TSF (0.56 ± 0.02), followed by NMEAL (0.49 ± 0.02) and FR (0.47 ± 0.02). In general, we observed that transforming the number of visits into a meal-based unit better translated the individuals’ variation in terms of genetic additive effects, besides smoothing the data distribution towards normality (Figure S1). Nonetheless, the models were also able to capture a sizable portion of the phenotypic variance attributable to the genetic additive component when considering the FB traits on a visit basis.
Our results are rather consistent with those previously reported in poultry and pig species, which also presented moderate to high heritability values for FB traits measured with electronic feeding systems (Howie et al., 2011; Lu et al., 2017; Li et al., 2020b). For instance, Howie et al. (2011) found h2 values ranging between 0.45 and 0.57 for number of meals, intake per meal, and feeding rate. Furthermore, these authors found heritability estimates around 0.35 for the number of visits per meal, which are very similar to the observed in the present study (0.32 ± 0.01). Similarly, in Pekin ducks, Li et al. (2020b) reported h2 values ranging between 0.31 (DFI) and 0.65 (meal duration) for different FB traits. However, we also found important differences for some traits within and across poultry species, as illustrated by the higher heritability value estimated for time spent at the feeder (0.56 ± 0.02) in this study compared to the values lying between 0.30 and 0.38 found in Howie et al. (2011). Furthermore, the meal-based traits reported here presented considerably higher heritability estimates than the values previously reported in turkeys, with estimates ranging between 0.15 and 0.31 for number of meals, meal duration, and meal size (Willems, 2015).
In this study, we used the averaged observations across the feeding tests as the FB indicator per bird, which is a more reliable measure than the individual daily observations. This might partially explain the high heritability values for the FB traits. Nonetheless, considerable changes in the average trends of FB traits are expected to occur throughout the feeding test, although the relative variability seems to remain constant for most FB traits (Figure 1). Future work modeling the daily FB observations as longitudinal data using appropriate statistical techniques (e.g., repeatability and random regression mixed models) could shed light on the relative importance of the permanent environment component on the FB traits variation.
There is also a substantial genetic variation for FE traits measured in group-housed broilers, with moderate h2 estimates of 0.25 ± 0.01 and 0.32 ± 0.02 for FCR and RFI, respectively. These results are within the interval (0.10–0.49) reported by previous studies for FE-related traits measured in caged broiler chickens (Pakdel et al., 2005; Aggrey et al., 2010; Begli et al., 2016; Mebratie et al., 2019) and are slightly lower than those reported for feed conversion rate in cage-free broilers (Howie et al., 2011). Broiler chickens are more commonly reared in floor pens as this housing system generally favors growth performance and is associated with lower mortality rates (Reece et al., 1971; Fouad et al., 2008; Idrus et al., 2021; Yan et al., 2021). The cage-based rearing system also raises several welfare concerns as it limits the broilers' natural behavior (e.g., ground scratching), and alters feeding patterns, besides preventing social interaction among animals (Fouad et al., 2008; Shields and Greger, 2013). Nonetheless, most efforts to understand the genetics of FE in broilers have been performed using experimental feeding tests with animals housed in individual cages (Pakdel et al., 2005; Aggrey et al., 2010; Begli et al., 2016; Li et al, 2020a; Mebratie et al., 2019; Marchesi et al., 2021). This is mostly due to the inherent difficulty of measuring individual feed intake in large samples of group-housed animals. The genetic selection for FE measured in broilers under individual caging assumes the lack of genotype-by-environment interaction, which implies that there is no re-ranking in the breeding values of selection candidates from experimental rearing systems to commercial alike conditions (Mulder and Bijma, 2005). This is hardly the case as birds reared in different housing systems present differences in key components associated with digestive metabolisms, such as gastrointestinal development and enteric microflora (Idrus et al., 2021; Wang et al., 2021; Yan et al., 2021). It has been demonstrated that the genetic correlations between FE traits measured in turkeys reared under individual and group housing systems were only moderate, suggesting the presence of G x E effects according to the rearing system (Willems, 2015).
At the phenotypic level, we found pieces of evidence supporting differences in feeding behavior between extremely contrasting groups for FE (Figure 2). The results presented here indicate an association between high FE in male broilers with less frequent visits and meals per day, which overall also reflected in longer meal intervals, less time spent at the feeders, and less wandering across feeding stations. Our findings replicate partially those found in Yan et al. (2019) and Li et al. (2020b), which reported statistical associations between groups expressing low residual feed intake (hence higher feed efficiency) and lower values for their equivalent definitions of NV, NMEAL, and TSF in slow-growing yellow broilers and Pekin ducks, respectively. Intriguingly, the FB traits presenting the strongest associations with FE differed according to the sex in this study (Figure 2). For the female birds, the feeding rate and intake per meal presented the most remarkable associations with contrasting groups of FE while relevant traits in the males such as NMEAL and VISMEAL (P-value < 2.22 × 10−16) were not important at all for distinguishing low and high FE females (P-values = 0.96 and 0.30, respectively). This illustrates how the sex of broilers could influence the different feeding strategies responsible, on average, for higher FE.
Despite the significant differences at the phenotypic level between contrasting FE groups for some FB traits, these differences are not translated into substantial genetic associations as indicated by the low genetic correlation estimates between most FB traits and FE indicators, except for DFI, which presented moderate to high (unfavorable) positive correlations with FCR and RFI (Figure 4). Accordingly, the selection for FE in this line would have little implications for the feeding behavior and vice versa, this aligns with the evidence provided so far, which suggests that the feeding behavior has little or no influence on the genetic factors affecting the nutrient efficiency in poultry systems (Howie et al., 2011, Proulx, 2016; Li et al., 2020). On the other hand, in the long term, the selection for high growth performance is more likely to cause correlated genetic changes in different feeding behavior indicators (Figure 4, Figure 5). The moderate and negative correlations between body weight (SBW and FBW), NVF, and NMEAL indicate that the genetic background involved in growth development could partially present pleiotropic effects responsible for promoting a reduced ability to search for feed on the floor. As a compensating mechanism, heavier animals generally present higher genetic potential to have larger meal sizes and longer meal intervals (Figure 4), which favors a tendency to reach only for readily available food at the feeders. Howie et al. (2011) demonstrated this phenomenon in 4 broiler lines selected with different intensities for growth performance.
To the best of our knowledge, the current study was the first to use NVF as a feeding behavior parameter in poultry species. One could interpret this trait as an indicator of the bird's activity and curiosity. Furthermore, it is expected that the number of unique feeders visited is correlated with the walked distance wandered by a bird during the day. Hence, NVF could be used as a proxy for the walking ability in broilers, which in turn is associated with welfare and leg health in poultry species (Santos et al., 2022). Selecting for higher NVF is expected to cause a high positive correlated response in the number of meals ( = 0.76) and, to a lesser extent, reduce the body weight at the beginning ( = -0.38) and the ending ( = -0.28) of the feeding test. On the other hand, animals that visit more feeders, strongly tend to feed more frequently and have smaller meals (Figure 4). We also observed that selecting animals that tend to visit several different feeders (NVF) would also negatively affect their daily feed intake, as suggested by the low and negative genetic correlation (–0.23) between these 2 traits (Figure 4). These patterns might reflect the behavior of subordinate animals in the social hierarchy, as proposed for sows (Brouns and Edwards, 1994). This hypothesis gets strength when one observes the phenotypic correlation between NVF and VISMEAL ( = 0.62), suggesting that animals with a higher number of visits during a meal would be those chased away more often from the current feeder, being forced to search for the next available feeders. The genetic correlations between NVF and VISMEAL were much smaller ( = 0.25), indicating that the correlation between these 2 traits is mainly due to environmental factors. However, caution must then be exercised when interpreting the implications of genetic selection for NVF, especially regarding its impact on the social interaction dynamics of broiler populations.
Harmful social interactions escalate with feed restriction (Brouns and Edwards, 1994; Piles et al., 2017). Integrating indirect social effects into mixed models helps differentiate genetic variation from interactions among competing individuals within the same pen (Muir, 2005). While effective for small groups (Lu et al., 2017), this approach assumes uniform interaction among pen mates, an assumption easily violated in larger populations with hundreds or thousands of birds in a single pen. Additionally, strong collinearity between group and pen effects in our dataset complicates their separation without confounding. Therefore, we chose to ignore the indirect social effects in this study. Nevertheless, alternative methods such as social network modeling (Foister et al., 2018) could provide valuable insights into future research.
Although the selection focus must be on productive traits, the inclusion of feeding behavior traits in breeding programs opens possibilities to explore different strategies for feeding management in broiler production systems. There is no optimal solution as the goals may vary with the rearing environment. For instance, in rearing systems with high-density rates, one may want to focus on animals that utilize larger but less frequent meals to reduce competition for food. In environmental conditions where the birds might be subject to heat stress, it might be advantageous to select animals that feed on a higher number of meals, thus reducing the intake per meal. This strategy would favor a reduced diet-induced thermogenesis per meal (Swennen et al., 2007). Finally, traits such as VAI could be useful to explore individual variations in circadian rhythms, favoring adaptation to different light regimens.
CONCLUSIONS
The different feeding behavior traits analyzed in this study have a sizable genetic component, presenting moderate to high heritability estimates. Hence, future selection for favorable FB is expected to exhibit a fast genetic response. The genetic correlations between FB and productive traits suggest that is possible to consider different feeding strategies without compromising the genetic progress for feed efficiency. On the other hand, breeding strategies that prioritize a higher bird activity (e.g., NMEAL, NVS, and NVF) might result in lighter broiler lines in the long term. Lastly, one must highlight that the use of electronic feeders is extremely important for the genetic improvement of FE traits measured in group-housed animals, contributing to reducing the differences between elite and commercial environments in meat-type breeding schemes.
ACKNOWLEDGMENTS
The authors would like to thank Cobb Vantress for providing the dataset used in this study.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103737.
Appendix. Supplementary materials
REFERENCES
- Abasht B., Mignon-Grasteau S., Bottje W., Lake J. In: Pages 183–220 in Advances in poultry Genetics and Genomics. Aggrey S.E., Zhou H., Tixier-Boichard M., Rhoads D.D., editors. Burleigh Dodds Series in Agricultural Science; 2020. Genetics and genomics of feed utilization efficiency in poultry species. [Google Scholar]
- Aggrey S.E., Karnuah A.B., Sebastian B., Anthony N.B. Genetic properties of feed efficiency parameters in meat-type chickens. Genet. Sel. Evol. 2010;42:25. doi: 10.1186/1297-9686-42-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves A.A.C., Fernandes A.F.A., Lopes F.B., Breen V., Hawken R., Rosa G.J.M. Prediction of culling and mortality risks in group-housed broilers using machine learning methods trained with time-series data of feeding behavior traits. J. Anim. Sci. 2022;100:2. [Google Scholar]
- Begli E.H., Vaez Torshizi R., Masoudi A.A., Ehsani A., Jensen J. Longitudinal analysis of body weight, feed intake, and residual feed intake in F2 chickens. Livest. Sci. 2016;184:28–34. [Google Scholar]
- Bley T.A.G., Bessei W. Recording of individual feed intake and feeding behavior of pekin ducks kept in groups. Poult. Sci. 2008;87:215–221. doi: 10.3382/ps.2006-00446. [DOI] [PubMed] [Google Scholar]
- Bokkers E.A.M., Koene P., Rodenburg T.B., Zimmerman P.H., Spruijt B.M. Working for food under conditions of varying motivation in broilers. Anim. Behav. 2004;68:105–113. [Google Scholar]
- Brouns F., Edwards S.A. Social rank and feeding behaviour of group-housed sows fed competitively or ad libitum. Appl. Anim. Behav. Sci. 1994;39:225–235. [Google Scholar]
- Cavani L., Brown W.E., Kristen L., Gaddis P., Tempelman R.J., VandeHaar M.J., White H.M., Peñagaricano F., Weigel K.A. Estimates of genetic parameters for feeding behavior traits and their associations with feed efficiency in Holstein cows. J. Dairy. Sci. 2022;105:7564–7574. doi: 10.3168/jds.2022-22066. [DOI] [PubMed] [Google Scholar]
- Cobb-Vantress Inc. 2021. Cobb broiler management guide. Accessed Feb. 2024. https://www.cobb-vantress.com/assets/Cobb-Files/4d0dd628b7/Broiler-Guide_English-2021-min.pdf
- Cornou C., Vinther J., Kristensen A.R. Automatic detection of oestrus and health disorders using data from electronic sow feeders. Livest. Sci. 2008;118:262–271. [Google Scholar]
- Donohue M., Cunningham D.L. Effects of grain and oilseed prices on the costs of US poultry production. J. Appl. Poult. Res. 2009;18:325–337. [Google Scholar]
- Foister S., Doeschl-Wilson A., Roehe R., Arnott G., Boyle L., Turner S. Social network properties predict chronic aggression in commercial pig systems. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad M.A., Abeer H., Razek A., Badawy E.S.M. Broilers welfare and economics under two management alternatives on commercial scale. Int. J. Poult. Sci. 2008;7:1167–1173. [Google Scholar]
- González L.A., Tolkamp B.J., Coffey M.P., Ferret A., Kyriazakis L. Changes in feeding behavior as possible indicators for the automatic monitoring of health disorders in dairy cows. J. Dairy. Sci. 2008;97:1017–1028. doi: 10.3168/jds.2007-0530. [DOI] [PubMed] [Google Scholar]
- Herd R.M., Archer J.A., Arthur P.F. Reducing the cost of beef production through genetic improvement of feed intake: opportunity and challenges to application. J. Anim. Sci. 2003;81:9–17. [Google Scholar]
- Honda K. Peripheral regulation of food intake in chickens: adiposity signals, satiety signals and others. Worlds Poult. Sci. J. 2021;77:301–312. [Google Scholar]
- Howie J.A., Tolkamp B.J., Avendano S., Kyriazakis I. A novel flexible method to split feeding behavior into bouts. Appl. Anim. Behav. Sci. 2009;116:101–109. [Google Scholar]
- Howie J.A., Avendano S., Tolkamp B.J., Kyriazakis I. Genetic parameters of feeding behavior traits and their relationship with live performance traits in modern broiler lines. Poult. Sci. 2011;90:1197–1205. doi: 10.3382/ps.2010-01313. [DOI] [PubMed] [Google Scholar]
- Hyun Y., Ellis M., McKeith F.K., Wilson E.R. Feed intake pattern of group-housed growing-finishing pigs monitored using a computerized feed intake recording system. J. Anim. Sci. 1997;75:1443–1451. doi: 10.2527/1997.7561443x. [DOI] [PubMed] [Google Scholar]
- Idrus Z., Norsam N.S., Silahuddin M.F., Awad E.A. Growth performance, well-being, and gut microbial population of broilers raised in cages and floor pens under the hot and humid tropical climate. Ital. J. Anim. Sci. 2021;20:383–394. [Google Scholar]
- Li W., Liu R., Zheng M., Feng F., Liu D., Guo Y., Zhao G., Wen J. New insights into the associations among feed efficiency, metabolizable efficiency traits and related QTL regions in broiler chickens. J. Anim. Sci. Biotechnol. 2020;11:65. doi: 10.1186/s40104-020-00469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.-S., Zhu F., Yang F.-X., Hao J.-P., Hou Z.-C. Selection response and genetic parameter estimation of feeding behavior traits in Pekin ducks. Poult. Sci. 2020;99:2375–2384. doi: 10.1016/j.psj.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llonch P., Mainau E., Ipharraguerre I.R., Bargo F., Tedó G., Blanch M., Manteca X. Chicken or the egg: the reciprocal association between feeding behavior and animal welfare and their impact on productivity in dairy cows. Front. Vet. Sci. 2018;5:305. doi: 10.3389/fvets.2018.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Jiao S., Tiezzi F., Knauer M., Huang Y., Gray K.A., Maltecca C. The relationship between different measures of feed efficiency and feeding behavior traits in Duroc pigs. J. Anim. Sci. 2017;95:3370–3380. doi: 10.2527/jas.2017.1509. [DOI] [PubMed] [Google Scholar]
- Marchesi J.A.P., Ono R.K., Cantão M.E., Ibelli A.M.G., Peixoto J.O., Moreira G.C.M., Godoy T.F., Coutinho L.H., Munari D.P., Ledur M.C. Exploring the genetic architecture of feed efficiency traits in chickens. Sci. Rep. 2021;11:4622. doi: 10.1038/s41598-021-84125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebratie W., Madsen P., Hawken R., Romé H., Marois D., Henshall J., Bovenhuis H., Jensen J. Genetic parameters for body weight and different definitions of residual feed intake in broiler chickens. Genet. Sel. Evol. 2019;51:53. doi: 10.1186/s12711-019-0494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes E.D.M., Carstens G.E., Tedeschi L.O., Pinchak W.E., Friend T.H. Validation of a system for monitoring feeding behavior in beef cattle. J. Anim. Sci. 2011;89:2904–2910. doi: 10.2527/jas.2010-3489. [DOI] [PubMed] [Google Scholar]
- Misztal, I., S. Tsuruta, D. A. L. Lourenco, Y. Masuda, I. Aguilar, A. Legarra, and Z. Vitezica. 2018. Manual for BLUPF90 family programs. University of Georgia. Accessed Jun. 2022. http://nce.ads.uga.edu/wiki/doku.php?id=documentation
- Muir W.M. Incorporation of competitive effects in forest tree or animal breeding programs. Genetics. 2005;170:1247–1259. doi: 10.1534/genetics.104.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder H.A., Bijma P. Effects of genotype × environment interaction on genetic gain in breeding programs. J. Anim. Sci. 2005;83:49–61. doi: 10.2527/2005.83149x. [DOI] [PubMed] [Google Scholar]
- Neves D.P., Nääs I.A., Vercellino R.A., Moura D.J. Do broilers prefer to eat from a certain type of feeder? Braz. J. Poult. Sci. 2010;12:179–187. [Google Scholar]
- O'Driscoll K., Boyle L., Hanlon A. The effect of breed and housing system on dairy cow feeding and lying behavior. Appl. Anim. Behav. Sci. 2009;116:156–162. [Google Scholar]
- Pakdel A., van Arendonk J.A.M., Vereijken A.L., Bovenhuis H. Genetic parameters of ascites-related traits in broilers: correlations with feed efficiency and carcass traits. Br. Poult Sci. 2005;46:43–53. doi: 10.1080/00071660400023805. [DOI] [PubMed] [Google Scholar]
- Patience J.F., Rossoni-Serao M.C., Gutierrez N.A. A review of feed efficiency in swine: biology and application. J. Anim. Sci. Biotechnol. 2015;6:33. doi: 10.1186/s40104-015-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, M., I. Turro, F. Launay, A. D. Mills, J. M. Melin, and J. M. Faure. 1992. Food intake patterns of three week old broilers caged individually or in groups. Pages 429–434 in 19th World's Poult. Congr., Amsterdam, The Netherlands.
- Piles M., David I., Ramon J., Canario L., Rafel O., Pascual M., Ragab M., Sánchez J.P. Interaction of direct and social genetic effects with feeding regime in growing rabbits. Gen. Sel. Evol. 2017;49:58. doi: 10.1186/s12711-017-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx J.H. University of Guelph; Ontario: 2016. Ph.D. Diss. [Google Scholar]
- Reece F.N., Deaton J.W., May J.D., May N.K. Cage versus floor rearing of broiler chickens. Poult. Sci. 1971;50:1786–1790. [Google Scholar]
- Reyer H., Hawken R., Murani E., Ponsuksili S., Wimmers K. The genetics of feed conversion efficiency traits in a commercial broiler line. Sci. Rep. 2015;5:16387. doi: 10.1038/srep16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M.N., Widowski T.M., Kiarie E.G., Guerin M.T., Edwards A.M., Torrey S. In pursuit of a better broiler: walking ability and incidence of contact dermatitis in conventional and slower growing strains of broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S., Greger M. Animal welfare and food safety aspects of confining broiler chickens to cages. Animals. 2013;3:386–400. doi: 10.3390/ani3020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swennen Q., Delezie E., Collin A., Decuypere E., Buyse J. Further investigations on the role of diet-induced thermogenesis in the regulation of feed intake in chickens: comparison of age-matched broiler versus layer cockerels. Poult. Sci. 2007;86:895–903. doi: 10.1093/ps/86.5.895. [DOI] [PubMed] [Google Scholar]
- Tu X., Du S., Tang L., Xin H., Wood B. A real-time automated system for monitoring individual feed intake and body weight of group housed turkeys. Comput. Electron. Agric. 2011;75:313–320. [Google Scholar]
- Waller A. Take a fresh look at broiler nutrition. Poult. Int. 2007;46:12–15. [Google Scholar]
- Wang L.-D., Zhang Y., Kong L.-L., Wang Z.-X., Bai H., Jiang Y., Bi Y.-L., Chang G.-B., Chen G.-H. Effects of rearing system (floor vs. cage) and sex on performance, meat quality and enteric microorganism of yellow feather broilers. J. Integr. Agric. 2021;20:1907–1920. doi: 10.1016/S2095-3119(20)63420-7. [DOI] [Google Scholar]
- Willems O.W. University of Guelph; Ontario: 2015. PhD Diss. [Google Scholar]
- Willems O.W., Miller S.P., Wood B.J. Aspects of selection for feed efficiency in meat producing poultry. Worlds Poult. Sci. J. 2013;69:77–88. doi: 10.1017/S004393391300007X. [DOI] [Google Scholar]
- Yan W., Sun C., Wen C., Ji C., Zhang D., Yang N. Relationships between feeding behaviors and performance traits in slow-growing yellow broilers. Poult. Sci. 2019;98:548–555. doi: 10.3382/ps/pey424. [DOI] [PubMed] [Google Scholar]
- Yan L., Lv Z.Z., An S., Xing K., Wang Z.G., Lv M.B., Choct M., Guo Y.M., Zhou G.L. Effects of rearing system and narasin on growth performance, gastrointestinal development, and gut microbiota of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates M.P., Tolkamp B.J., Allcroft D.J., Kyriazakis I. The use of mixed distribution models to determine bout criteria for analysis of animal behaviour. J. Theoret. Biol. 2001;213:413–425. doi: 10.1006/jtbi.2001.2425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.