Abstract
Sebaceous glands (SGs), as holocrine-secreting appendages, lubricate the skin and play a central role in the skin barrier. Large full-thickness skin defects cause overall architecture disruption and SG loss. However, an effective strategy for SG regeneration is lacking. Organoids are 3D multicellular structures that replicate key anatomical and functional characteristics of in vivo tissues and exhibit great potential in regenerative medicine. Recently, considerable progress has been made in developing reliable procedures for SG organoids and existing SG organoids recapitulate the main morphological, structural and functional features of their in vivo counterparts. Engineering approaches empower researchers to manipulate cell behaviors, the surrounding environment and cell–environment crosstalk within the culture system as needed. These techniques can be applied to the SG organoid culture system to generate functionally more competent SG organoids. This review aims to provide an overview of recent advancements in SG organoid engineering. It highlights some potential strategies for SG organoid functionalization that are promising to forge a platform for engineering vascularized, innervated, immune-interactive and lipogenic SG organoids. We anticipate that this review will not only contribute to improving our understanding of SG biology and regeneration but also facilitate the transition of the SG organoid from laboratory research to a feasible clinical application.
Keywords: Sebaceous glands, Stem cells, Organoid engineering, Organoid maturation, Skin appendage regeneration, Regenerative medicine
Highlights.
Lrig1+ stem cells in SGs may offer the nearest hope for an ideal source of SG organoid engineering.
Precise regulation of Wnt signaling spatially and temporally appear to be the key point to trigger SG organoid self-assembly appropriately.
Synthetic polymer hydrogels are the most common source of matrix for the ease with which their biological and physical properties can be manipulated.
Induced pluripotent stem cells and skin-specific endothelial progenitor cells are the ideal sources for SG organoid vascularization.
Human SG organoids provide a near-authentic platform to study pathophysiology and discover potential drug targets of acne vulgaris.
Background
The sebaceous gland (SG), an important epidermal appendage, produces a lipid-rich mixture, which is crucial for maintaining the physiological structure and function of the skin [1]. Numerous pieces of evidence have suggested that SG dysregulation and sebum composition disturbances contribute to certain skin disorders, including skin aging, acne vulgaris, rosacea, androgenic and scarring alopecia, and sebaceous tumors. Among these disorders, acne vulgaris is an exceedingly common skin disease, and sebaceous gland carcinomas are aggressive, malignant tumors prone to metastasis, resulting in high mortality [2,3]. Particularly, large full-thickness skin defects resulting from severe injuries and following scar formation usually disrupt overall skin architecture and cause loss of appendages. Loss of SGs makes skin prone to dermatologic diseases, with xerosis cutis becoming the most common diagnosis, frequently accompanied by itching, redness and desquamation [4]. Although not life-threatening, these symptoms cause both physical and psychological distress and seriously affect patients’ life quality. Consequently, SG regeneration, accompanied by repairing large full-thickness skin defects, is important for patients’ health and well-being.
Current strategies to repair large skin areas play a major role in rapidly covering wound beds, accelerating epidermis healing rate and preventing infections without SG regeneration, suggesting that new approaches are required to address the unmet clinical need. Recent advances in organoid technologies have provided powerful tools for tissue repair and organ regeneration. The organoids, as mini organ-like 3D spheres derived from definite stem cells, resemble in vivo organs both structurally and functionally [5]. As the smallest functional unit of an organ, the organoid is resilient to environmental variations and apt to survive in vitro. Therefore, organoid technologies combined with existing strategies are anticipated to solve the technical problem that SGs fail to regenerate after severe skin injuries using the present methodology. Engineering approaches empower researchers to manipulate cell behaviors, the surrounding environment and cell–environment crosstalk within the organoid culture system as needed. These approaches have been applied to diverse organoid culture systems to generate organoids with more complex structures, longer lifespans and refined functions. These methodologies can be utilized to produce SG organoids closely resembling in vivo tissue and more suitable for regenerative medicine.
This review aims to provide an overview of SG organoid engineering, functionalization and their potential application. First, we summarize key aspects of SG biology, including SG physiology and cellular and molecular mechanisms of SG morphogenesis. Second, we discuss the key factors of engineering SG organoids, focusing on SG stem cell clusters and environmental modeling. Afterward, we explore possible strategies to achieve fully-functional SG organoids, including vascularization, innervation, immune communication and establishment of lipid profiles. Finally, we discuss the applications of SG organoids for both basic science research and translational medicine.
Review
SG biology
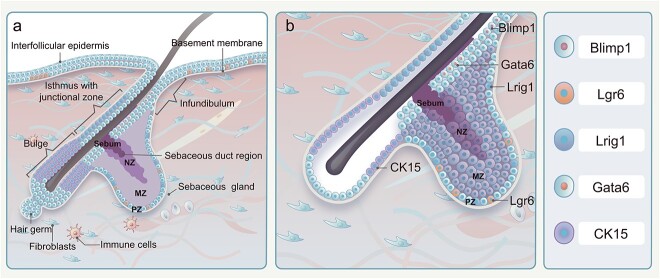
Except for the palms of the hands and soles of the feet, SGs are distributed throughout the body, with the majority being associated with hair follicles (HFs). However, some specialized SGs develop independently from the HF, like the meibomian glands of the eyelids [6]. One or two SGs are connected to the isthmus of the HF, termed the junctional zone (JZ), via a short, keratinized excretory duct, namely the sebaceous duct, and open into a pilary canal (Figure 1a). The SGs and HFs form the pilosebaceous unit (PSU), surrounded by a connective tissue sheath composed of mesenchymal fibroblasts and collagen [7] (Figure 1a).
Figure 1.
Schematic representation of PSU and SG stem cell clusters. (a) The SG is anatomically linked to the isthmus of the HF, known as the JZ, through the sebaceous duct. The SG and HF collectively constitute the PSU, which is encompassed by vascular networks, fibroblasts, immune cells and other associated components. (b) The pool of stem cells in the SG consists of multi-cell clusters. Specifically, Blimp1-positive cells are situated at the base of the SG, Lrg6-positive cells are found at the isthmus of the HF, Lrig1-positive stem cells are located at the JZ of the HF, CK15-positive cells reside in the bulge of the HF and Gata6-positive cells are present in the duct of the SG. PSU pilosebaceous unit, SG sebaceous gland, HF hair follicle, JZ junctional zone, Blimp1 B-lymphocyte-induced nuclear maturation protein 1, Lrg6 leucine-rich repeat-containing G protein-coupled receptor 6, Lrig1 leucine-rich repeats and immunoglobulin-like domains protein 1, CK15 cytokeratin 15, Gata6 GATA binding protein 6
SG structure
While the elementary structure of SGs remains consistent, their size and lobular quantity differ. The SG is composed of two parts: the acinus gland and the excretory duct, which extends to the lateral wall of the pilary canal and the epidermis. The SG accordingly comprises two cell types: lipid-producing cells in the acinus gland (sebocytes) and squamous epithelial cells in the excretory duct (duct cells) [8].
Sebocytes have three distinct stages of differentiation and are located in three different zones, including the peripheral zone (PZ), maturation zone (MZ) and necrosis zone (NZ) (Figure 1a). The PZ is a basal layer attached to the basement membrane and is composed of outermost sebocytes, which are undifferentiated and proliferative (Figure 1a, b). These cells differentiate into lipid-secreting cells through symmetric and asymmetric division [9]. The MZ, adjacent to the PZ, has multiple layers housing lipid-secreting cells undergoing differentiation (Figure 1a, b). The size of sebocytes within the MZ is considerably increased due to the accumulation of lipid droplets. The NZ is where sebocytes aggregate and release lipid-containing sebum into the secretory duct (Figure 1a, b). Sebocytes in the NZ exhibit pyknotic nuclei and undergo holocrine secretion, a process resembling autophagic cell death [10]. The transition cycle from undifferentiated sebocytes to terminally differentiated sebocytes within human and rodent SGs lasts ~2 two weeks [8].
The excretory duct of the SG exhibits some features of squamous epithelium, although it lacks the stratum granulosum and the stratum corneum. Notably, lipid droplets can also be observed within the cells of the excretory duct, although they are smaller than those in the sebocytes. The basal cells attached to the basement membrane contribute to maintaining excretory duct homeostasis. In addition, excretory duct cells generate a thin horny layer composed of fragile and loose corneocytes that shed directly into the lumen, are subsequently secreted along with lipid droplets and eventually reach the skin surface [8].
SG function
Studies have revealed a pivotal role of SGs in the mammalian skin barrier. The sebum secreted continuously through the excretory duct of SGs deposited on the hair shaft and the skin surface contributes to lubricating and waterproofing the hair and skin. Certain evidence has indicated that sebum loss correlates with scarring alopecia and increased risks of UVB-induced epidermal apoptosis and severe ocular surface pathology, resembling meibomian gland dysfunction in mice [11,12]. Alterations in sebum content or composition disrupt the structural and functional integrity of the skin barrier by affecting lipid film structure and composition, elevating skin pH, decreasing water content, etc. SGs, combined with apocrine glands, also play an important thermoregulatory role and prevent liquid loss from the body [13]. In addition, SGs execute immunoregulatory functions, producing peptides and lipids with antimicrobial activity [14,15].
Distinct variations exist in sebum lipid contents among different species [16]. Human sebum is mainly a non-polar lipid mixture, including triglycerides, free fatty acids, wax esters, squalene, cholesterol esters and free cholesterol [13]. Triglycerides and fatty acids constitute the majority, accounting for 57.5% of the lipids, followed by wax esters (26%) and squalene (12%). Cholesterol is the rarest, representing 4.5% of the lipids with its esters [13]. Some lipid composition in human skin surface sebum, including free fatty acids, differs from that found in isolated human SG sebum [17]. Additional free fatty acids are believed to originate from the breakdown of triglycerides by commensal microorganisms, including Propionibacterium acnes and yeasts of the genus Malassezia [18]. Squalene and Δ6 monounsaturated fatty acids, like cis-6-hexadecenoic acid, are exclusively produced by the human SGs [19]. Interestingly, the lipid composition of sebum is tissue-specific and varies with age in humans [20].
During the final trimester of fetal development, fetal SGs produce vernix caseosa, a waxy white substance that shields the skin from amniotic fluid [21]. Postnatally, SG activity decreases almost entirely from ages 2–6 years, then increases to peak levels during puberty in response to elevated hormonal levels [22].
Stem cells involved in SG organogenesis
The evolutionary origins of SGs remain unclear. Human SGs develop around the 13th to 14th week of gestation [23]. The first sebocytes in mice emerge around embryonic day 18, covering the majority of the back skin by postnatal day 3–4 [24]. SG development is coupled to the PSU morphogenesis, initiated by interactions between the non-neuroectoderm and mesoderm during embryogenesis. HF development is classified into eight distinct stages based on fundamental morphological criteria [25]. The details of the classification of the morphogenesis stage and the related characterization of stages 1–8 have been described previously [25]. As a critical event during stage 5, the first sebocytes emerge at the distal part of the HF epithelium and later form glandular structures [26]. Post-morphogenesis, SGs undergo constant renewal to maintain tissue homeostasis [27].
Recent research has attempted to characterize SG stem cells by the expression of specific genes and proteins. During embryonic and early postnatal life, SRY-box transcription factor 9 (Sox9) expression was first observed in a few suprabasal cells of the developing hair germ [28]. As PSU morphogenesis preceded, leucine-rich repeats and immunoglobulin-like domains protein 1 (Lrig1) and Sox9 were co-expressed in progenitors during the hair peg stage [26]. Subsequently, two distinct stem cell pools comprising Sox9+ and Lrig1+ cells were generated from the Lrig1 + Sox9+ progenitor cells. The Sox9+ cells were confined to the presumed HF bulge, while Lrig1+ cells were localized to the distal area of the follicle where mature sebocytes were about to emerge. Dynamic analysis suggested that Lrig1+ cells produced SGs through asymmetric cell division [26]. Following SG development, Lrig1+ stem cells migrated to the JZ and sustained long-term homeostasis of both SG and infundibulum (Figure 1a, b).
In embryonic hair placodes, leucine-rich repeat-containing G protein-coupled receptor 6 (Lgr6) is another HF placode marker emerging around embryonic day 14.5 [29]. Lgr6+ stem cells, identified as a distinct stem cell cluster separate from other HF bulge stem cells, can generate HFs, SGs and interfollicular epidermis (IFE) prenatally. Adjacent to the JZ, Lgr6+ stem cells located in the HF isthmus persistently renew SGs and IFE [30] (Figure 1a, b).
GATA binding protein 6 (Gata6) + cells may originate from Sox9+ cell clusters in stage 5 during HF morphogenesis, eventually developing into SG ducts and upper SGs [31]. Gata6+ stem cell localization is restricted to the sebaceous ducts, and they also maintain the homoeostasis of the upper SGs [32,33] (Figure 1a, b).
B-lymphocyte-induced nuclear maturation protein 1 (Blimp1) + cells found at the SG base are identified as unipotent stem cells controlling cellular contributions within the SG; however, their specific origin within the SG remains unknown [34]. Interestingly, a recent study regarding cellular dynamics during SG morphogenesis suggested that ~11 equipotent and lineage-segregated progenitors equally contributed to SG development [35] (Figure 1a, b).
HF bulge is believed to harbor bipotent stem cells, which can generate both HFs and SGs. Among them are CK15+ multipotent stem cells of a higher hierarchy that transited several stem cell clusters, including Lgr6+ and Lrig1+ cells, before differentiating into sebocytes during PSU homeostasis [36] (Figure 1a, b). Genetic lineage-tracing experiments showed that the Gata6+ cell cluster is one of the progenies of Lgr6+ stem cells and Gata6 co-expressed Lrig1 in neonatal and adult mice. This suggests that Lgr6+ and Lrig1+ stem cells maintain the entire SG’s homeostasis, while Gata6+ stem cells, as the progeny of the two aforementioned stem cells, exclusively contribute to the cellular turnover of sebaceous ducts and upper SGs [31]. Furthermore, the enriched expression of Blimp1 in purified Lrig1+ stem cells implies that unipotent Blimp1+ stem cells may derive from Lrig1+ stem cell clusters [37].
Molecular mechanism regulating SG organogenesis
In recent decades, extensive research has focused on understanding the underlying molecular signals governing SG development and homeostasis. Although the mechanisms remain incompletely understood, it has been well-documented that numerous factors are involved, including signaling pathways, hormones, transcription factors and growth factors [38]. In this review, we highlight crucial factors involved in SG development and their potential for SG organoid engineering (Figure 2).
Figure 2.

Major signaling pathways and factors regulating SG identity and homeostasis. The ΔLef1 mutation leads to the inhibition of Wnt signaling, causing a transition from HFSCs to SG progenitors. Reactivation of TCF3 converts both SG and ORS lineages into HFSCs. Gli1 accumulates in the nuclei of SG progenitors which can be achieved through the forced expression of PPARγ or SMO in IFE stem cells. Activation of c-Myc, IHH, FGFR2b or EGFR promotes the proliferation of SG progenitors. Notch signaling plays a dual role in SG differentiation, promoting sebocyte differentiation within the SG stem cell niche and inhibiting sebocyte proliferation outside of the niche. The orange stripes in the nuclei of SG progenitors indicate Gli1 accumulation. Arrows represent stimulatory effects and bar represents an inhibitory effect. SG sebaceous gland, Lef1 lymphoid enhancer binding factor 1, Wnt wingless/integrated, TCF3 transcription factor 3, ORS outer root sheath, HFSCs hair follicle stem cells, Gli1 GLI family zinc finger 1, PPARγ peroxisome proliferator-activated receptor gamma, SMO smoothened, frizzled class receptor, IFE interfollicular epidermis, c-Myc c-MYC proto-oncogene, BHLH transcription factor, IHH Indian hedgehog, FGFR2b fibroblast growth factor receptor 2b, EGFR epidermal growth factor receptor
Signaling pathways
As mentioned above, SG development is coupled with HF morphogenesis. Numerous reviews exist on signaling involving HF development, and we will not reiterate those in this review. Among the related signaling pathways, wingless/integrated (Wnt) signaling is significantly crucial for PSU induction, organogenesis and cytodifferentiation [39]. β-Catenin serves as a transcriptional co-activator interacting with the transcription factor (TCF)/lymphoid enhancer binding factor (LEF) complex, which is central to Wnt signaling activation [40]. ΔNLef1 without β-catenin-interacting domain blocked HF lineage specification and induced SG lineage differentiation in mice [41] (Figure 2). Conversely, without Wnt signals, TCF3 reactivation repressed both HF and SG fate specification to maintain skin stem cells in an undifferentiated state [42] (Figure 2). Even postnatally, TCF3 induction blocked SG terminal differentiation by repressing peroxisome proliferator-activated receptor gamma (PPARγ) [42]. Petersson et al. [36] further confirmed that Lef1 inactivation induced sebocyte differentiation instead of HF lineage specification. They also demonstrated cell fate determination between HF and SG controlled by TCF/Lef1 activity regulation in HF bulge stem cells [36] (Figure 2). These studies illustrated that skin progenitors regulate the lineage switch from HF to SG destiny during PSU development by precisely regulating Wnt signaling activity.
The second important pathway regarding the proliferation and differentiation of SG lineage is hedgehog (Hh) signaling. Hh signaling as a ligand-dependent signaling pathway is activated by three ligands, including sonic, desert and Indian Hh (IHH) protein, and the GLI zinc finger family is the downstream transcription factor of the pathway [43]. Immunohistochemical staining of human skin samples showed that IHH is expressed in differentiated sebocytes and GLI1 in SG progenitors [44] (Figure 2). The IHH notably stimulates sebocyte precursor proliferation [44] (Figure 2). Gain- and loss-of-function experiments in transgenic mouse models suggested that inhibiting Hh signaling activity in epidermal keratinocytes selectively blocks sebocyte formation while stimulating Hh pathway promotes sebocyte development [45]. Ectopic SGs emerged from the footpad epidermis in epidermal Hh signaling constitutively activating K5-M2SMO mice, suggesting that Hh signaling activation can induce lineage conversion from epidermal keratinocytes to sebocyte lineage [45] (Figure 2). Besides, mice constitutively expressing GLI2 generate prominent SG ducts, indicating that the Hh pathway significantly influences the fate decisions of the SG duct [46].
The Notch signaling pathway, a key mediator of epithelial differentiation, is also essential for SG morphogenesis. γ-Secretase-mediated proteolytic cleavage of the Notch receptor transmembrane domain is required for Notch signaling activation. Before the onset of PSU morphogenesis, γ-secretase deletion in mice resulted in SG loss [47]. JunB-K14-deficient mice demonstrated de novo SG regeneration during wound healing, linked to Notch signaling hyperactivation. This was further confirmed by de novo SG loss in the wound after inhibiting Notch signaling [48]. Veniaminova et al. [49] revealed a dual role of Notch signaling in SG differentiation, promoting sebocyte differentiation within the SG stem cell niche and inhibiting sebocyte proliferation outside the niche (Figure 2).
Hormones, transcription factors and growth factor receptors
SG homeostasis is tightly regulated by steroid hormone levels, with androgens prominently influencing SG biology. Androgens exert direct effects on androgen receptors expressed in early differentiated sebocytes within human and rat SGs [50]. Androgens are well-defined regulators with pronounced effects on SG physiology. Previous data have demonstrated that androgen treatment promoted the proliferation and terminal differentiation of sebocytes and the synthesis of specific sebaceous lipids [51].
Several key transcription factors are also required for SG homeostasis. The transcription factor c-Myc is a key regulator affecting SG formation. It has been suggested that the expansion of SGs and ectopic sebocytes found in the HF and IFE are due to c-Myc activation in the cutaneous epithelium [52] (Figure 2). Other relevant transcription factors, including PPARs, also regulate sebocyte development and differentiation by governing the expression of lipogenic genes [53]. All known PPAR isoforms have been identified in human sebocytes, whereas PPARγ is mainly expressed in differentiating sebocytes. A previous study demonstrated that PPARγ-null mice showed a complete loss of SG, suggesting that PPARγ may exert a crucial role in SG patterning [54]. Besides, our recent research has shown that PPARγ overexpression in human epidermal keratinocytes plays an important role in SG fate conversion [55] (Figure 2).
Additionally, during morphogenesis, SG-associated growth factors regulate SG number and size by binding with the corresponding receptors. In this way, these growth factor receptors serve as important mediators in SG development. Among these are fibroblast growth factor receptor 2b (FGFR2b) [56] and epidermal growth factor receptor (EGFR) [57], both of which are expressed in SGs. Epidermis-specific deletion mice lacking FGFR2b could generate SGs; however, the SGs subsequently underwent atrophy [56], suggesting that FGFR2b is required for SG homeostasis (Figure 2). Manipulating EGFR expression in the epidermis showed EGFR-dependent SG enlargement and increased sebum production in mice, suggesting the role of EGFR in SG biology [58] (Figure 2).
SG organoid engineering
SG organoid engineering integrates classic developmental biology, stem cell technology and engineering principles. While SG stem cells function as ‘seeds’ in the organoid system, the microenvironment serves as ‘soil’ controlling the SG stem cell behavior. The structure and physiological function of SG organoids are tightly regulated by the cell–microenvironment interactions. Suitable stem cell resources for the SG organoid and an appropriate microenvironment akin to the SG stem cell niche are the two key elements of SG organoid engineering.
Stem cell clusters for SG organoids
SG organoids can be derived from two types of stem cells, i.e. primary SG adult stem cells (ASCs) and pluripotent stem cells (PSCs), due to their ability to self-organize into structures that mimic the fundamental properties of in vivo SGs [59].
ASC-derived SG organoids
Recent SG organoids were derived from mouse SG Blimp1+ stem cells, consistent with a previous study in that Blimp1 defined a progenitor population governing cellular input to the SG [34,60]. Before being used for organoid generation, these cells were sorted by flow cytometry and purified by in vitro expansion (Figure 3a, b). Subsequently, the proliferative cells were embedded in Matrigel, the most widely used matrix in organoid culture, to achieve 3D growth. Particularly, the 3D cultures were placed in a specific medium containing SG morphogenesis-associated molecules, including fibroblast growth factor (FGF), EGF, R-spondin 1 and Noggin (Figure 3b, c). After 7–12 days of culture, SG-like structures were generated. The SG organoids were composed of an outer single layer of proliferating cells and an inner mass of cells in varying degrees of differentiation. Interestingly, the outer layer cells underwent asymmetric divisions, moving toward the center of the SG organoids. These features are in accordance with SG cellular kinetics. Moreover, the SG organoids were able to generate SG-specific lipids, exhibiting functions akin to their in vivo counterparts.
Figure 3.
Workflow of SG organoid culture. (a) The surrounding in vivo environment of the SG. The SG is situated within the dermis, where it coexists with various types of cells, including fibroblasts and immune cells. The SG is highly vascularized, which potentially facilitates its communication with the neighboring environment. (b) The isolation of Blimp1+ stem cells. Briefly, epidermal cells were obtained from Blimp1-YFP reporter mice and subsequently subjected to flow sorting to collect α6 + Sca1-Blimp1-YFP+ cells. Afterwards, Blimp1-positive stem cells were screened out owing to their self-renewal ability. (c) The SG organoid culture system. Blimp1-positive stem cells were embedded in a 3D scaffold Matrigel and cultured in a modified medium supplemented with R-spondin1, Noggin, EGF and FGF2. Following a designated period of culture, the formation of sphere structures was observed. SG sebaceous gland, Blimp1 B-lymphocyte-induced nuclear maturation protein 1, EGF epidermal growth factor, FGF2 fibroblast growth factor 2, YFP yellow fluorescent protein
Based on the success of Blimp1+ cell-derived organoids, other SG stem cell clusters hold great promise for establishing SG organoids using fluorescent labeling and sorting technologies. Given that Gata6 controls the sebaceous duct lineage identity, the lack of ductal structures in Blimp1+ cell-derived organoids aligns with expectations. This problem could be addressed by co-culturing Gata6 SG stem cells with Blimp1+ cells. Other stem cell clusters, including Lrig1+ stem cells, Lgr6+ stem cells and CK15+ bulge stem cells, possess the potential to differentiate into both gland and duct cells of SG, and these can be utilized to generate SG organoids containing both glandular and ductal structures.
PSC-derived SG organoids
Human skin organoids bearing SGs and HFs were derived from the WA25 human embryonic stem cell line [61,62]. Before induction, the cells were first separated into individual cells and cultured on U-bottom 96-well plates to generate uniform cell aggregates. Subsequently, the cell aggregates were transferred into the differentiation medium containing Matrigel, bone morphogenetic protein 4 (BMP4) and SB431542 (a transforming growth factor beta inhibitor) to induce ectoderm epithelium [61]. Afterward, the epithelium was further differentiated into the surface ectoderm in another differentiation medium containing BMP inhibitor and FGF. Meanwhile, the surface ectoderm was enveloped by cranial neural crest-like cells through co-treatment with basic FGF (fibroblast growth factor 2, FGF2) and LDN-193189 (a BMP inhibitor). After 120 days, HFs associated with SGs were observed in the skin organoids.
Adding defined soluble factors—appropriate and precise doses of proteins at the right time—is crucial for achieving 3D structures and the intended lineages [63]. For example, EGF timing in the sequential differentiation protocol was crucial to generating epithelial stem cells [64]. Theoretically, the surface ectoderm-derived human PSCs can differentiate into sebocyte lineage in a defined induction medium. Optimizing the cultivation system with PSC-derived epithelial stem cells shows promise in establishing SG-like organoids that mirror key features of SGs. Human PSCs are an ideal cell resource for modeling SG organoids possessing glandular and tubular structures resembling normal SGs.
ASC-derived SG organoids vs PSC-derived SG organoids
Although SG organoids could be obtained from both ASCs and PSCs, as demonstrated above, some differences could, however, be observed. To facilitate comparisons between ASC-derived SG organoids and PSC-derived SG organoids, the details of the differentiation between the two and their current structural and functional limitations compared to in vivo SGs are shown in Table 1.
Table 1.
Comparisons between ASC- and PSC-derived SG organoids
| ASC-derived SG organoids | PSC-derived SG organoids | |
|---|---|---|
| Advantages | Culture duration (1–2 weeks) is relatively short. Culture protocol is relatively simple (no co-induction of surface ectoderm and CNC cells). Epigenetic alterations and transcriptomic features of ASC can be retained by organoids to some extent. |
Sources of PSCs are extensive. Protocols of PSC induction have been standardized. Culture methods are easy to reproduce, convenient to handle and amenable to large-scale use. Duration of in vitro maintenance of organoids is relatively long (several months). |
| Disadvantages | Sources of ASCs are limited. Origins of ASCs are heterogeneous. ASCs are difficult to isolate, culture and manipulate. Standardizing culture protocols for large-scale use is also difficult. Duration of in vitro maintenance of organoids is relatively short (several weeks). |
Time needed to acquire organoid-containing SGs (>100 days) is too long. There are too many steps (co-induction of surface ectoderm and CNC cells) involved in the protocols. |
| Structure limitation (vs. in vivo SGs) |
Size of organoids is much smaller. The organoids merely share a similar structure with their fetal in vivo counterparts, but they lack an identical structure. The sebaceous duct structure is lacking. |
Size of organoids is smaller. The organoids merely share a similar structure with their fetal in vivo counterparts, but they lack an identical structure. |
| Function limitation (vs. in vivo SGs) |
Whether the organoids produce antimicrobial peptides is unknown. The organoids are, at most, functionally equal to the fetal in vivo SGs. |
The lipid files of the organoids are unknown. Whether the organoids produce anti-microbial peptides is unknown. The organoids are, at most, functionally equal to the fetal in vivo SGs. |
ASC adult stem cell, PSC pluripotent stem cell, SG sebaceous gland, CNC cranial neural crest
Microenvironmental modelling for SG organoids
The microenvironment of SG organoids mainly includes matrix and signaling molecules. Matrix engineering and the orchestration of soluble factors are required to mimic the native SG environment physically, chemically and eventually functionally.
Matrix engineering for SG organoid establishment
The extracellular matrix (ECM) provides structural support to surrounding stem cells and modulates their behaviors, including proliferation, mobilization and differentiation. Similarly, the most common way to develop organoids involves using materials that mimic the scaffolding support provided by the in situ ECM of the tissue. Three main factors must be considered when choosing a material for organoid culture: component origin, mechanical properties and geometry [65]. Based on component origin, the current matrices can be categorized as: decellularized tissue matrices, mixture-component natural polymer matrices, single-component natural polymer matrices, synthetic polymer hydrogels, engineered hydrogels and a combination of the above.
Decellularized ECM shares a similar structure and composition with the native tissue matrix and retains the unique advantages of organ-transplanted scaffolds due to its intact vascular networks ready for vascular endothelial cell repopulation [66]. Hydrogels derived from decellularized ECM have been used to develop human gastric organoids and more [67]. Acellular adipose-derived mesenchymal stromal cell matrix has been utilized to cover scaffold surfaces for engineered meibomian glands, suggesting that acellular adipose tissue matrix-derived hydrogels may be a candidate resource of materials for SG organoid culture [68].
Matrigel, extracted from Engelbreth–Holm–Swarm tumors, is a widely utilized mixture-component natural polymer matrix in organoid development [69]. SG organoids are examples of successfully developed organoids using Matrigel that mimics basement membranes [60]. Though Matrigel contains diverse ECM components and bioactive factors, harboring abundant cell attachment sites, it is degradable and remodelable during organoid development. However, Matrigel is unsuitable for clinical use due to its murine tumor origin, batch–batch differences and limited adjustability.
Compared to Matrigel, the single-component natural biopolymer matrices like collagen I, laminin, gelatin, fibrin and hyaluronic acid are derived from the normal ECM and offer high reproducibility [66]. Among these, collagen I hydrogel has been applied to multiple types of organoid systems, including human and murine intestinal organoids [70]. Notably, collagen I is the most abundant matrix protein within the dermis where SGs reside. In addition, the stiffness in normal skin is ~4 kPa Young’s modulus, which is in the range of stiffness of collagen I hydrogel. Considering this, collagen I hydrogel can be used as an alternative matrix for SG organoid construction.
Synthetic hydrogels have been increasingly utilized in organoid culture for their biofouling resistance, high cost-effectiveness, customizable topology and molecular weight, and easy crosslinking with desired biological ligands or signaling molecules for precise control over physical and biochemical properties [66]. Among the synthetic hydrogels, polyethylene glycol (PEG) hydrogels are widely employed. For instance, 4-arm PEG-maleimide hydrogel is suitable for embedding cells and serves as an injection vehicle for in vivo organoid delivery, providing valuable insights that may be applied to SG organoid transplantation [71].
Bioengineered matrices can be alternative options for SG organoid engineering due to their modifiability, well-defined chemical structures, ease of synthesis and minimal batch variation. To modulate crucial SG stem cell niche cues, other factors also require consideration, including oxygen distribution, pH levels and nutrient transportation. Given this, techniques like 3D cell aggregates in suspension and air–liquid interface methods have been employed for the optic cup and kidney organoids, respectively [72,73]. Likewise, the air–liquid interface method can be used to develop SG organoids with a multi-layered duct structure resembling in vivo microarchitecture. Additionally, sensors and 3D microfluidic devices can be incorporated into the system to achieve precise control over culture conditions, including oxygen and nutrient delivery.
Signaling pathway manipulation for building SG organoids
As mentioned above, SG organogenesis is orchestrated by sequential activation or inhibition of critical signaling pathways, including Wnt, Hh and Notch signaling pathways, among others. Wnt signaling activation was demonstrated to stimulate self-renewal and long-term expansion in various gland organoids like mammary and salivary gland organoids [74,75]. Accordingly, for ASC-derived SG organoids, R-spondin1—a potent Wnt signaling pathway activator—was included in the culture medium. While Wnt signaling finely regulates SG and HF fates by assigning low-level preference to SG lineage and high-level guidance to HF specification, R-spondin1 in the SG organoid system may be mainly involved in SG stem cell self-renewal. Considering this, R-spondin1 and Wnt signaling agonists (e.g. Chir99021) can be applied to SG organoids derived from other stem cell clusters like Lrig1+ stem cells.
Besides R-spondin1, ASC-derived SG organoids have been developed using exogenous signals to stimulate the self-organization response [60] (Figure 3b). An exogenous small molecule, the Rho associated coiled-coil containing protein kinase (ROCK) inhibitor Y27632, which can prevent anoikis induced by cell–cell detachment, is crucial to promote BLIMP1+ stem cells to generate organoid structures; further exogenous stimulation with Noggin, EGF and FGF2 is then required to guide cells in developing large spheroid structures. Noggin, the BMP signaling inhibitor, was administered because inhibiting the BMP signaling pathway has been reported to facilitate sebocyte differentiation. Specifically, FGF2 was used for its mitogenic activity on sebocytes. Additionally, EGF was administered, as activating the EGF signaling pathway has been demonstrated to promote sebocyte proliferation and lipid production.
The Hippo pathway is one of the important growth- and proliferation-regulating signaling pathways. A small molecule, GA-017, promotes cell proliferation and enhances intestinal organoid growth by inhibiting large tumor suppressor kinase 1/2 (LATS1/2), subsequently activating yes1 associated transcriptional regulator (YAP)/tafazzin (TAZ), which are both key effectors of the Hippo pathway [76]. Considering this, GA-017 may be used to accelerate SG organoid expansion in the culture system. Notch signaling, identified as a feedforward circuit governing progeny cell differentiation [77], was reported to be involved in SG morphogenesis, suggesting that Notch signaling activation may be applied in SG organoid culture. In addition, the Hh pathway is crucial in SG development and fate decision; hence, it is theoretically possible to include proteins like IHH or small molecules to optimize the SG organoid system.
Our understanding of the relevant development mechanisms of SGs in vivo, guides the identification of factors and the timing of their addition. Subsequently, as the underlying molecular mechanisms involving SG development and homeostasis are elucidated, the additional signaling components added to the SG organoid system can be optimized, which further enhances our understanding of SG organogenesis.
SG organoid functionalization
The lack of cell maturation is a major shortcoming of current SG organoid methodologies. SG organoids exhibit certain characteristics resembling their fetal counterparts and lack mature structural and functional properties [78], which may be due to the absence of multiple cell types (e.g. blood vessels, proper innervation and immune cells) that promote organoid maturation in the culture system [79]. Similarly, the paradigm of normal organogenesis can be applied to develop SG organoids with structure and function closely resembling native SGs (Figure 4a).
Figure 4.
Schematic diagram of functionalization and applications of SG organoids. (a) Utilizing engineering approaches holds promise for enhancing the complexity and maturation of SG organoids, including vascularization, innervation, immune-epithelial crosstalk and lipid profile characterization. (b) Utilization of transcriptomics, single-cell sequencing and biobank in the field of SG organoids is expected to greatly enhance their application in basic research, disease modeling, drug discovery and regenerative medicine. SG sebaceous gland
Vascularized SG organoids
Vascularization, beyond oxygen, nutrients and circulating factors, also delivers hematopoietic cells like macrophages, which contribute to organ development and maturation [80]. In vivo, SGs are surrounded by a complex vascular network [81] (Figure 4a), as blood supply in SGs is essential for maintaining normal structure and function. Despite significant advancements in organoid culture, the lack of vascularization in most organoids, including SG organoids, remains a major limitation in achieving organoids with in vivo-like matured structural organization and tissue size. Therefore, there is a requirement for vascularized organoids, which will lead to larger and more functional SG organoids. Generally, organoid vascularization needs a combination of three main factors: endothelial progenitor cells (EPCs), engineering technologies and matrices.
Blood vessel generation requires EPCs, and induced PSCs are the ideal source of EPCs, thereby providing a potentially abundant supply of EPCs from the same donor [80]. Besides, numerous protocols have been developed to generate EPCs from induced PSCs. Considering the varied vascularization among different organs, skin-specific EPCs could be a viable alternative due to their ample availability and proximity to SG EPCs. As an understanding of mechanical and chemical cues improves the mimicking of in vivo SG conditions (e.g. providing physical support for EPCs to form tubule-like structures or introducing specific soluble factors), the self-organization and appropriate differentiation of EPCs will be further enabled, thereby enhancing the feasibility of developing vascularized SG organoids in the future.
Emerging bioengineering approaches have played an important role in organoid vascularization. For example, microfluidic systems, such as sacrificial molds and laser ablation, have been used to develop vascularized tissue-like structures [82,83]. Microvasculature can be created in vitro by embedding endothelial cells within a hydrogel culturing scaffold introduced into a microfluidic device. This configuration facilitates the self-organization of endothelial cells into perfusable vascular networks [84]. 3D bioprinting is an alternative strategy to create vascular-like structures to fabricate perfusable channels comprising endothelial cells [85]. Regardless of the approaches used to develop vascularized systems, the primary technical challenge exists in maintaining the stability and function of microvascular networks within organoids.
The ECM is another critical factor of vascularization [80]. Along with the development of organoid technologies and engineering methods, researchers have established multiple approaches to achieve functional vasculature in vitro with diverse kinds of hydrogels, ranging from natural materials like Matrigel, collagen and fibrin to synthetic biomaterials, including PEG and its derivatives. Microvascular networks are influenced by hydrogel properties, including density, degradability, viscoelasticity and integrin binding sites [80]. Natural ECM enables stable vascular network formation but exhibits batch-to-batch variability. Synthetic biodegradable polymers offer modifiable biophysical and biochemical properties but possess limited paracrine morphogen permeability. Besides, fabricating and maintaining stable vessel networks using synthetic biodegradable polymers is challenging. Decellularized ECM of skin may be a promising option with the increasing optimization of acellular methods.
Innervated SG organoids
Innervation is critical in regulating organ development and determining organ functional control and modulation [86]. Despite extensive research, it remains unclear whether SGs, as a part of PSUs, are directly innervated by cutaneous nerves [87]. One hypothesis suggests that SG innervation is established through cutaneous nerve fibers that control the cellular input of SGs [87]. The HF bulge, as a putative location of SG stem cells, is intensively innervated, which is considered the basis of innervating and regulating SGs. However, previous studies demonstrated that specialized SGs, which are independent of HFs, including meibomian and preputial glands, are intensively innervated by a fine nerve-fiber network [88]. Therefore, innervation should be meticulously considered throughout the process of SG organoid functionalizion independent of HFs (Figure 4a).
Limited studies on organoid innervation have been reported to date. Recent attempts have been made to co-culture human PSC-derived neural crest cells with various organoids to generate innervated organoids. These studies might shed light on SG organoid innervation [86]. For example, the co-culture method has been applied to intestinal organoids [89,90]. Alternatively, advancements in 3D bioprinting have allowed researchers to develop a 3D bio-fabrication system to generate innervated epithelial organoids like salivary gland organoids from neural crest-derived mesenchymal stem cells [91]. These studies stress the importance of selectively considering specific neural crest cell populations to achieve proper innervation in SG organoids.
Immune-interactive SG organoids
Immune cells and skin epithelium constitute a sophisticated barrier system in symbiotic relationships with microbes [92]. SGs regulate the skin barrier by modulating lipid composition and amount of sebum, a complex formation of lipids produced by SGs, thereby affecting the growth of diverse bacteria [93]. Moreover, sebum composition has been demonstrated to be crucial in regulating immune responses against microbes by regulating macrophage polarization. Additionally, skin-resident innate lymphoid cells have been shown to regulate the skin microbiota by controlling SG function [92]. This interaction between SGs and immune cells is essential for the homeostasis of skin immunological and structural functions (Figure 4a). Therefore, signals from the immune system are pivotal factors for SG organoid functional maturation.
Various protocols exist for co-culturing organoids with different immune cells, including innate lymphoid cells, macrophages and neutrophils, among others [94]. Autologous immune cells are suitable for translational application, while their in vitro expansion post-isolation from patients requires a lengthy duration. This can be resolved by pre-cryopreserving peripheral blood lymphocytes, followed by isolating and expanding specific cell lineages as needed. Apart from 3D co-culture, other methods like monolayer culture (facilitated by an air–liquid interface) have been established [95,96]. Monolayer culture generates a luminal, basal side, which can directly contact with immune cells, thereby facilitating the study of host reactions to microbes and immune–epithelial crosstalk in the skin barrier. A similar approach is likely suitable for SG organoids.
However, the requirements for growth factors supplemented to culture media vary across cell types. Co-culture of organoids with immune cells necessitates solutions to sustain the survival and functionality of distinct cell types within the same culture compartment. Addressing this challenge may benefit from insights and knowledge gained from other disciplines.
Lipogenic SG organoids
SG differentiation, maturation and lipid production are closely interrelated (Figure 4a). SG functional maturation is often accompanied by lipid synthesis, which is regulated by several signaling pathways. Notably, the PPAR pathway is among the most important lipogenic signaling pathways [97]. The PPAR family members, including PPARα, PPARβ and PPARγ, form heterodimers with retinoid X receptors (RXRs) to regulate lipogenic gene transcription and can be activated by unsaturated fatty acids like linoleic acid and eicosanoids [98]. Treatment with PPAR agonists increased sebum secretion in both SGs and sebocytes. Accordingly, the PPARγ activator rosiglitazone and PPARβ activator linoleic acid stimulated SG organoid lipogenesis [60].
The liver X receptors (LXRs) belong to the nuclear receptor family and contribute to cellular cholesterol homeostasis and lipid metabolism [99]. Activation of LXRs by their ligands resulted in increased expression of critical fatty acid synthase and sterol regulatory element-binding protein-1 and the accumulation of lipid droplets [100].
Retinoids are also involved in regulating SG proliferation and lipogenesis [101]. Human sebocytes express both retinoic acid receptors and RXRs, and their ligands, including all-trans retinoic acid (atRA) and 9-cis-retinoic acid (9cRA), have been demonstrated to inhibit sebocyte proliferation and lipid synthesis [101]. Treatment with atRA (Tretinoin), formulated atRA (Ret-Avit) and 13-cis RA (Isotretinoin) led to significantly smaller SG organoids compared to the control group, which is consistent with their clinical effectiveness.
Low-glycemic diets result in a decrease in acne severity, suggesting the involvement of insulin/insulin-like growth factor (IGF)1 signaling in acne pathogenesis [102]. Both insulin and IGF promote lipid production via phosphatidylinositol-3-kinase/protein kinase B signaling [103]. Additionally, insulin administration stimulates lipid accumulation in adipocytes, suggesting a potential enhancement of lipogenesis in SG organoids upon insulin treatment.
Applications of SG organoids
As previously described, the organoid systems, including the classical organoid culture methods and novel engineering strategies, have achieved significant progress in replicating some of the critical structural and functional features of in vivo organs. This makes SG organoids promising tools for basic research, therapeutic innovations and clinical applications (Figure 4b).
SG organoids for skin appendage organogenesis
SGs and HFs are usually considered together as the PSUs. Defining sebaceous-autonomous mechanisms is difficult due to the potentially confounding signals from nearby HFs. Consequently, knowledge about SG development, homeostasis and regeneration has been mostly derived from studies that primarily focused on HFs. SG organoids hold the potential for modeling SG development and homeostasis because of their ability to recapitulate major processes of self-organization during SG morphogenesis. Moreover, SG organoids can exist as a simplified minimal system, contributing to determining the respective roles of distinct components in the system. Therefore, SG organoids could be used to reveal mechanisms of SG-autonomous self-organization during morphogenesis.
ASC-derived SG organoids exhibited the same molecular profiles and homeostatic kinetics as in vivo SGs [60]. Moreover, experiments on SG organoids have provided insights into the pathways regulating SG homeostasis. Previous studies showed that transcription factor c-Myc is an essential regulator of sebocyte differentiation [104]. Treating SG organoids with c-Myc inhibitory drugs showed a decrease in organoid size. Further study demonstrated that c-Myc regulates SG organoid homeostasis, which further confirmed the role of c-Myc in SG homeostasis.
Compared to mammalian animal models, organoids are more accessible and offer more insights into homeostasis and organ biology. Besides, considering the potential limitations of animal models in accurately simulating human SG organogenesis, human stem cell-derived SG organoids provide a more faithful reflection. Current SG organoids are equivalent to in vivo SGs in early developmental stages. The key mechanisms regulating SG development and maturation might be revealed by comprehensive and comparative transcriptome analyses to compare the transcriptomes between SG organoids on different days and their in vivo counterparts at multiple developmental stages. Understanding these mechanisms can optimize the SG organoid systems, and subsequent developments in the research will enhance our understanding of skin appendage organogenesis.
SG organoids for disease modeling, drug discovery and personalized medicine
Some SG disorders are associated with dysregulated SG homeostasis. Acne vulgaris, which is among the most common skin disorders, affects millions globally [105]. Multiple factors contribute to acne vulgaris, including SG dysregulation and the host–P. acnes interaction. Additionally, SG dysregulation can cause SG carcinomas with high metastatic rates and mortality [106]. Organoid cultures can mimic pathologies at a 3D organ-like level, which is a clear advantage over traditional 2D cell cultures. Furthermore, organoids developed from human ASCs or iPSCs can mimic specific human features that animal models like acne vulgaris may not fully replicate. Therefore, the organoid is an ideal platform for disease modeling, drug discovery and personalized medicine.
ASC-derived SG organoids have been used as a platform to simulate the initial stage of acne vulgaris pathophysiological processes [60]. The platform was generated by PPAR signaling pathway activation in SG organoids through its combination with dihydrotestosterone, a potent androgen demonstrated to synergistically promote sebocyte proliferation and lipogenesis [97]. After treatment, the SG organoid exhibited enlarged size, increased lipid accumulation and active proliferation, which, to some extent, reflected certain characteristics of acne vulgaris. Considering this, the model was administered atRA, the current first-line therapy for acne vulgaris, to study its response to the drugs. The results demonstrated that the proliferative activity, differentiation process and organoid volume of SG organoid cells were all affected by the drugs, which was consistent with the clinical findings. Additionally, the model suggested that c-Myc may be a key regulatory factor associated with acne vulgaris pathology. These data indicated that SG organoids can potentially serve as a drug discovery platform.
Organoid biobanks with different pathologies are an emerging application of organoid research [79]. These biobanks provide a powerful platform for genetic variance screening of patient-derived tumors. Therefore, targeted drugs designed based on the screening results can be used for personalized treatment. For precision medicine, establishing ex vivo model systems to predict patient-specific responses is essential [63]. Cancer stem cells, which can self-renew, can self-assemble organoids like patient-derived organoids (PDOs) [63]. Recently, a PDO biobank has been established from metastatic gastrointestinal cancer patients enrolled in phase 1 or 2 clinical trials [107]. This has enabled researchers to compare the responses of patients and those of PDOs when treated with the same drugs. Interestingly, the drug response of cancer organoids has been shown to be similar to that observed in clinics, enhancing the potential of PDOs as a drug screening platform [108]. However, studies focusing on SG carcinoma organoids are lacking, and PDOs from SG carcinoma may provide new therapeutic target cues for individualized precision treatment.
In particular, it is crucial to note that certain structural and functional differences still exist between SG organoids and their in vivo counterparts, as described in Table 1. These disparities render them inadequate as near-physiological substitutes for actual SGs in disease modeling. The reliable production of high-quality SG organoids requires further improvement of the culture system to address prevailing gaps.
SG organoids for functional skin regeneration
SG organoids, beyond their role as preclinical drug test models, also hold promise as graft sources for functional skin regeneration. Compared to conventional skin graft or cell therapy, autologous organoids offer numerous advantages. First, SG organoids generated from patient-derived iPSCs and ASCs circumvent ethical concerns associated with allografts. Second, SG organoids, as the smallest element of SGs, possess superior survival ability compared to individual cells. Given the uncertainty of stem cell differentiation, SG organoids of directed differentiation are relatively safer. Besides, site-directed delivery of SG organoids via microneedles can achieve SG regeneration and ameliorate fibrosis even in the scarring skin. In a previous study, an organoid culture system that generated complex skin from human PSCs was developed [61]. When grafted onto nude mice, the skin organoids formed hair-bearing skin, providing a basis for future research into human reconstructive surgery and skin regeneration.
Recent methods combining organoid technology with cell-fate reprogramming hold promise for skin regeneration. A previous study showed that induced sweat gland organoids (SwGs) reprogrammed from human epidermal keratinocytes (HEKs) successfully developed into competently functional SwGs in a murine skin damage model after transplantation. This highlighted the great translational potential for personalized SwG regeneration in patients with large skin defects [109]. SG loss occurs in skin injury, especially full-thickness wounds in adult mammals, resulting in scar formation, skin appendage damage and impaired barrier function. SG organoids derived from ASCs of specific SGs, such as Lrig1+ or Lgr6+ stem cells, with bipotent characteristics, hold promise in facilitating wound healing and SG regeneration. In addition, cellular reprogramming technologies may represent an alternative platform for generating SG stem cells via direct conversion from other cell types, such as HEKs [55].
Identifying the therapeutic time window for regeneration is critical. As previously mentioned, the cultivation period for SG organoids is notably lengthy. Developing ASC-derived SG organoids takes 1–2 weeks, encompassing cell isolation, culture, flow sorting and expansion. Conversely, PSC-derived skin organoids containing SGs demand >100 days. New optimized protocols with a shorter period of cultivation of SG organoids are urgently needed to expand the potential clinical applications.
Conclusions
In this review, we summarized the main aspects of SG biology and SG organoid engineering, as well as the potential applications of SG organoids. Although considerable progress has been made in recent years in developing organoid culture systems for various organs, including the intestine, brain, liver and others, the organoid development of certain tissues, like SG organoid generation, is comparatively slow, which limits its translational application. Considering the essential role of SGs in the skin barrier, as ‘the brain of skin’, this area of study should receive greater attention. However, there are many reasons to believe that the prospect of SG organoids is a tangible reality. The application of transcriptomics and single-cell sequencing offers the potential to deeply comprehend SG morphogenesis and stem cell niches, which can be applied to SG organoid development. Engineering approaches like microwell arrays, droplet-based microfluidics and 3D bioprinting, as emerging domains in translational medicine, enable greater control over the initial size, composition and spatial organization of SG organoids. Therefore, the complexity and maturation of SG organoids will be enhanced, which will expand the application of SG organoids in disease models, drug discovery and individualized treatment. We believe that as SG organoid research advances, it holds a promising future for the transition from laboratory research to clinical applications.
Contributor Information
Yiqiong Liu, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Huanhuan Gao, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Huating Chen, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Shuaifei Ji, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Lu Wu, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Hongliang Zhang, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Yujia Wang, Queen Mary School of Nanchang University, Nanchang University, Nanchang, Jiangxi 330006, P. R. China.
Xiaobing Fu, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Xiaoyan Sun, Research Center for Tissue Repair and Regeneration affliated to the Medical Innovation Research Department and 4th Medical Center, PLA General Hospital and PLA Medical College; PLA Key Laboratory of Tissue Repair and Regenerative Medicine and Beijing Key Research Laboratory of Skin Injury, Repair and Regeneration; Research Unit of Trauma Care, Tissue Repair and Regeneration, Chinese Academy of Medical Sciences, 2019RU051, Beijing 100048, P. R. China.
Funding
This work was supported in part by the National Natural Science Foundation of China (92268206, 81830064), the CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-059), the Military Medical Research Projects (145AKJ260015000X, 2022-JCJQ-ZB-09600, 2020-JCJQ-ZD-256-021), the Military Medical Research and Development Projects (AWS17J005, 2019-126), and the Specific Research Fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202317).
Authors’ contributions
YL and HG sorted the reference data, designed illustrations and wrote the manuscript. HC and SJ helped with constructive discussion. LW, HZ and YW edited the manuscript. XF and XS contributed to the conception of the study and conceived the manuscript outline. All authors discussed and commented on the whole article and read and agreed to the published version.
Conflict of interest
None declared.
References
- 1. Zouboulis CC, Yoshida GJ, Wu Y, Xia L, Schneider MR. Sebaceous gland: milestones of 30-year modelling research dedicated to the “brain of the skin”. Exp Dermatol. 2020;29:1069–79. [DOI] [PubMed] [Google Scholar]
- 2. Esler WP, Tesz GJ, Hellerstein MK, Beysen C, Sivamani R, Turner SM, et al. Human sebum requires de novo lipogenesis, which is increased in acne vulgaris and suppressed by acetyl-CoA carboxylase inhibition. Sci Transl Med. 2019;11:eaau8465. 10.1126/scitranslmed.aau8465 [DOI] [PubMed] [Google Scholar]
- 3. Cook S, Pethick J, Kibbi N, Hollestein L, Lavelle K, Vere HI, et al. Sebaceous carcinoma epidemiology, associated malignancies and lynch/Muir-Torre syndrome screening in England from 2008 to 2018. J Am Acad Dermatol. 2023;89:1129–35. [DOI] [PubMed] [Google Scholar]
- 4. Hoover E, Aslam S. Krishnamurthy K. Physiology, Sebaceous Glands. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC, 2022. [Google Scholar]
- 5. Schutgens F, Clevers H. Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol. 2020;15:211–34. [DOI] [PubMed] [Google Scholar]
- 6. Ehrmann C, Schneider MR. Genetically modified laboratory mice with sebaceous glands abnormalities. Cellular and molecular life sciences : CMLS. 2016;73:4623–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358:697–704. [DOI] [PubMed] [Google Scholar]
- 8. Thody AJ, Shuster S. Control and function of sebaceous glands. Physiol Rev. 1989;69:383–416. [DOI] [PubMed] [Google Scholar]
- 9. Epstein EH, Epstein WL, Krasnobrod H. New cell formation in human sebaceous glands. J Invest Dermatol. 1966;46:453–8. [PubMed] [Google Scholar]
- 10. Fischer H, Fumicz J, Rossiter H, Napirei M, Buchberger M, Tschachler E, et al. Holocrine secretion of sebum is a unique DNase2-dependent mode of programmed cell death. J Invest Dermatol. 2017;137:587–94. [DOI] [PubMed] [Google Scholar]
- 11. Stoffel W, Schmidt-Soltau I, Jenke B, Binczek E, Hammels I. Hair growth cycle is arrested in SCD1 deficiency by impaired Wnt3a-Palmitoleoylation and retrieved by the artificial lipid barrier. J Invest Dermatol. 2017;137:1424–33. [DOI] [PubMed] [Google Scholar]
- 12. Dahlhoff M, Camera E, Schafer M, Emrich D, Riethmacher D, Foster A, et al. Sebaceous lipids are essential for water repulsion, protection against UVB-induced apoptosis and ocular integrity in mice. Development. 2016;143:1823–31. [DOI] [PubMed] [Google Scholar]
- 13. Picardo M, Ottaviani M, Camera E, Mastrofrancesco A. Sebaceous gland lipids. Dermatoendocrinol. 2009;1:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lovaszi M, Mattii M, Eyerich K, Gacsi A, Csanyi E, Kovacs D, et al. Sebum lipids influence macrophage polarization and activation. Br J Dermatol. 2017;177:1671–82. [DOI] [PubMed] [Google Scholar]
- 15. Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010;130:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–81. [DOI] [PubMed] [Google Scholar]
- 17. Downing DT, Strauss JS, Pochi PE. Variability in the chemical composition of human skin surface lipids. J Invest Dermatol. 1969;53:322–7. [DOI] [PubMed] [Google Scholar]
- 18. Sommer B, Overy DP, Haltli B, Kerr RG. Secreted lipases from Malassezia globosa: recombinant expression and determination of their substrate specificities. Microbiology (Reading, England). 2016;162:1069–79. [DOI] [PubMed] [Google Scholar]
- 19. Nicolaides N. Skin lipids: their biochemical uniqueness. Science (New York, NY). 1974;186:19–26. [DOI] [PubMed] [Google Scholar]
- 20. Ludovici M, Kozul N, Materazzi S, Risoluti R, Picardo M, Camera E. Influence of the sebaceous gland density on the stratum corneum lipidome. Sci Rep. 2018;8:11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Míková R, Vrkoslav V, Hanus R, Háková E, Hábová Z, Doležal A, et al. Newborn boys and girls differ in the lipid composition of vernix caseosa. PLoS One. 2014;9:e99173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cotterill JA, Cunliffe WJ, Williamson B, Bulusu L. Age and sex variation in skin surface lipid composition and sebum excretion rate. Br J Dermatol. 1972;87:333–40. [DOI] [PubMed] [Google Scholar]
- 23. Kligman AM, Strauss JS. The formation of vellus hair follicles from human adult epidermis. J Invest Dermatol. 1956;27:19–23. [DOI] [PubMed] [Google Scholar]
- 24. Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. BioEssays : news and reviews in molecular, cellular and developmental biology. 2005;27:247–61. [DOI] [PubMed] [Google Scholar]
- 25. Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. [DOI] [PubMed] [Google Scholar]
- 26. Frances D, Niemann C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev Biol. 2012;363:138–46. [DOI] [PubMed] [Google Scholar]
- 27. Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13: 471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Snippert HJ, Haegebarth A, Kasper M, Jaks V, Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science (New York, NY). 2010;327:1385–9. [DOI] [PubMed] [Google Scholar]
- 30. Füllgrabe A, Joost S, Are A, Jacob T, Sivan U, Haegebarth A, et al. Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and Interfollicular epidermis. Stem cell reports. 2015;5:843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oules B, Rognoni E, Hoste E, Goss G, Fiehler R, Natsuga K, et al. Mutant Lef1 controls Gata6 in sebaceous gland development and cancer. EMBO J. 2019;38:e100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swanson JB, Vagnozzi AN, Veniaminova NA, Wong SY. Loss of Gata6 causes dilation of the hair follicle canal and sebaceous duct. Exp Dermatol. 2018;28:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oulès B, Philippeos C, Segal J, Tihy M, Vietri Rudan M, Cujba AM, et al. Contribution of GATA6 to homeostasis of the human upper pilosebaceous unit and acne pathogenesis. Nat Commun. 2020;11:5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andersen MS, Hannezo E, Ulyanchenko S, Estrach S, Antoku Y, Pisano S, et al. Tracing the cellular dynamics of sebaceous gland development in normal and perturbed states. Nat Cell Biol. 2019;21:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petersson M, Brylka H, Kraus A, John S, Rappl G, Schettina P, et al. TCF/Lef1 activity controls establishment of diverse stem and progenitor cell compartments in mouse epidermis. EMBO J. 2011;30:3004–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clayton RW, Gobel K, Niessen CM, Paus R, Steensel MAM, Lim X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br J Dermatol. 2019;181:677–90. [DOI] [PubMed] [Google Scholar]
- 39. Lim X, Nusse R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb Perspect Biol. 2013;5:a008029. 10.1101/cshperspect.a008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. [DOI] [PubMed] [Google Scholar]
- 41. Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–83. [DOI] [PubMed] [Google Scholar]
- 43. Siebel C, Lendahl U. Notch Signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97:1235–94. [DOI] [PubMed] [Google Scholar]
- 44. Niemann C, Unden AB, Lyle S, Zouboulis CC, Toftgard R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci USA. 2003;100:11873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allen M, Grachtchouk M, Sheng H, Grachtchouk V, Wang A, Wei L, et al. Hedgehog Signaling regulates sebaceous gland development. Am J Pathol. 2003;163:2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gu LH, Coulombe PA. Hedgehog signaling, keratin 6 induction, and sebaceous gland morphogenesis: implications for pachyonychia congenita and related conditions. Am J Pathol. 2008;173:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. An Y, Lin MH, Tian X, Cheng HT, Gridley T, Shen J, et al. Gamma-secretase functions through notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell. 2004;7:731–43. [DOI] [PubMed] [Google Scholar]
- 48. Singh K, Camera E, Krug L, Basu A, Pandey RK, Munir S, et al. JunB defines functional and structural integrity of the epidermo-pilosebaceous unit in the skin. Nat Commun. 2018;9:3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Veniaminova NA, Grachtchouk M, Doane OJ, Peterson JK, Quigley DA, Lull MV, et al. Niche-specific factors dynamically regulate sebaceous gland stem cells in the skin. Dev Cell. 2019;51:326–340.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426–31. [DOI] [PubMed] [Google Scholar]
- 51. Rosignoli C, Nicolas JC, Jomard A, Michel S. Involvement of the SREBP pathway in the mode of action of androgens in sebaceous glands in vivo. Exp Dermatol. 2003;12:480–9. [DOI] [PubMed] [Google Scholar]
- 52. Bull JJ, Pelengaris S, Hendrix S, Chronnell CM, Khan M, Philpott MP. Ectopic expression of c-Myc in the skin affects the hair growth cycle and causes an enlargement of the sebaceous gland. Br J Dermatol. 2005;152:1125–33. [DOI] [PubMed] [Google Scholar]
- 53. Trivedi NR, Cong Z, Nelson AM, Albert AJ, Rosamilia LL, Sivarajah S, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol. 2006;126:2002–9. [DOI] [PubMed] [Google Scholar]
- 54. Sardella C, Winkler C, Quignodon L, Hardman JA, Toffoli B, Giordano Attianese GMP, et al. Delayed hair follicle morphogenesis and hair follicle dystrophy in a lipoatrophy mouse model of Pparg Total deletion. J Invest Dermatol. 2018;138:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Y, Ji S, Gao H, Chen H, Xiang J, Cui S, et al. Sebaceous gland reprogramming with a single gene, PPARG, and small molecules. Signal Transduction and Targeted Therapy. 2023;8:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grose R, Fantl V, Werner S, Chioni AM, Jarosz M, Rudling R, et al. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J. 2007;26:1268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dahlhoff M, Angelis MH, Wolf E, Schneider MR. Ligand-independent epidermal growth factor receptor hyperactivation increases sebaceous gland size and sebum secretion in mice. Exp Dermatol. 2013;22:667–9. [DOI] [PubMed] [Google Scholar]
- 58. Dahlhoff M, Frances D, Kloepper JE, Paus R, Schafer M, Niemann C, et al. Overexpression of epigen during embryonic development induces reversible, epidermal growth factor receptor-dependent sebaceous gland hyperplasia. Mol Cell Biol. 2014;34:3086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Unagolla JM, Jayasuriya AC. Recent advances in organoid engineering: a comprehensive review. Applied. Mater Today. 2022;29:101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feldman A, Mukha D, Maor II, Sedov E, Koren E, Yosefzon Y, et al. Blimp1+ cells generate functional mouse sebaceous gland organoids in vitro. Nat Commun. 2019;10:2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee J, Valk WH, Serdy SA, Deakin C, Kim J, Le AP, et al. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat Protoc. 2022;17:1266–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gunther C, Winner B, Neurath MF, Stappenbeck TS. Organoids in gastrointestinal diseases: from experimental models to clinical translation. Gut. 2022;71:1892–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang R, Zheng Y, Burrows M, Liu S, Wei Z, Nace A, et al. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells. Nat Commun. 2014;5:3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kratochvil MJ, Seymour AJ, Li TL, Pasca SP, Kuo CJ, Heilshorn SC. Engineered materials for organoid systems. Nat Rev Mater. 2019;4:606–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Magno V, Meinhardt A, Werner C. Polymer hydrogels to guide Organotypic and organoid cultures. Adv Funct Mater. 2020;30:2000097. 10.1002/adfm.202000097. [DOI] [Google Scholar]
- 67. Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 2019;10:5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen L, Yan D, Wu N, Zhang W, Yan C, Yao Q, et al. 3D-printed poly-Caprolactone scaffolds modified with biomimetic extracellular matrices for tarsal plate tissue engineering. Frontiers in Bioengineering and Biotechnology. 2020;8:219. 10.3389/fbioe.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sachs N, Tsukamoto Y, Kujala P, Peters PJ, Clevers H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development. 2017;144:1107–12. [DOI] [PubMed] [Google Scholar]
- 71. Cruz-Acuña R, Quirós M, Huang S, Siuda D, Spence JR, Nusrat A, et al. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat Protoc. 2018;13:2102–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6. [DOI] [PubMed] [Google Scholar]
- 73. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2016;536:238. [DOI] [PubMed] [Google Scholar]
- 74. Jardé T, Lloyd-Lewis B, Thomas M, Kendrick H, Melchor L, Bougaret L, et al. Wnt and Neuregulin1/ErbB signalling extends 3D culture of hormone responsive mammary organoids. Nat Commun. 2016;7:13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BN, et al. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem cell reports. 2016;6:150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aihara A, Iwawaki T, Abe-Fukasawa N, Otsuka K, Saruhashi K, Mikashima T, et al. Small molecule LATS kinase inhibitors block the hippo signaling pathway and promote cell growth under 3D culture conditions. J Biol Chem. 2022;298:101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow RD-W, Prabhu M, et al. Parent stem cells can serve as niches for their daughter cells. Nature. 2015;523:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takebe T, Wells JM. Organoids by design. Science (New York, NY). 2019;364:956–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–87. [DOI] [PubMed] [Google Scholar]
- 80. Shirure VS, Hughes CCW, George SC. Engineering vascularized organoid-on-a-Chip models. Annu Rev Biomed Eng. 2021;23:141–67. [DOI] [PubMed] [Google Scholar]
- 81. Requena L, Sangüeza O. Embryology, anatomy, histology, and physiology of the sebaceous glands. In: Cutaneous Adnexal Neoplasms. Springer, Gewerbestrasse 11, 6330 Cham, Switzerland, 2017, 755–64. [Google Scholar]
- 82. Wang XY, Jin ZH, Gan BW, Lv SW, Xie M, Huang WH. Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template. Lab Chip. 2014;14:2709–16. [DOI] [PubMed] [Google Scholar]
- 83. Brandenberg N, Lutolf MP. In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv Mater. 2016;28:7450–6. [DOI] [PubMed] [Google Scholar]
- 84. Garreta E, Kamm RD, Sousa C, Lopes SM, Lancaster MA, Weiss R, et al. Rethinking organoid technology through bioengineering. Nat Mater. 2021;20:145–55. [DOI] [PubMed] [Google Scholar]
- 85. Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. 2017;124:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Das S, Gordián-Vélez WJ, Ledebur HC, Mourkioti F, Rompolas P, Chen HI, et al. Innervation: the missing link for biofabricated tissues and organs. NPJ Regen Med. 2020;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Clayton RW, Langan EA, Ansell DM, Vos I, Gobel K, Schneider MR, et al. Neuroendocrinology and neurobiology of sebaceous glands. Biol Rev Camb Philos Soc. 2020;95:592–624. [DOI] [PubMed] [Google Scholar]
- 88. Bründl M, Garreis F, Schicht M, Dietrich J, Paulsen F. Characterization of the innervation of the meibomian glands in humans, rats and mice. Ann Anat. 2021;233:151609. [DOI] [PubMed] [Google Scholar]
- 89. Loffet E, Brossard L, Mahe MM. Pluripotent stem cell derived intestinal organoids with an enteric nervous system. Methods Cell Biol. 2020;159:175–99. [DOI] [PubMed] [Google Scholar]
- 90. Eicher AK, Kechele DO, Sundaram N, Berns HM, Poling HM, Haines LE, et al. Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells. Cell Stem Cell. 2022;29:36–51.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Adine C, Ng KK, Rungarunlert S, Souza GR, Ferreira JN. Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials. 2018;180:52–66. [DOI] [PubMed] [Google Scholar]
- 92. Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo JH, Shih HY, et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell. 2019;176:982–97.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lovaszi M, Szegedi A, Zouboulis CC, Torocsik D. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermatoendocrinol. 2017;9:e1375636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bar-Ephraim YE, Kretzschmar K, Clevers H. Organoids in immunological research. Nat Rev Immunol. 2020;20:279–93. [DOI] [PubMed] [Google Scholar]
- 95. Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid Modeling of the tumor immune microenvironment. Cell. 2018;175:1972–88.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, et al. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep. 2017;7:45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dozsa A, Dezso B, Toth BI, Bacsi A, Poliska S, Camera E, et al. PPARgamma-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytes. J Invest Dermatol. 2014;134:910–20. [DOI] [PubMed] [Google Scholar]
- 98. Downie MM, Sanders DA, Maier LM, Stock DM, Kealey T. Peroxisome proliferator-activated receptor and farnesoid X receptor ligands differentially regulate sebaceous differentiation in human sebaceous gland organ cultures in vitro. Br J Dermatol. 2004;151:766–75. [DOI] [PubMed] [Google Scholar]
- 99. Töröcsik D, Szanto A, Nagy L. Oxysterol signaling links cholesterol metabolism and inflammation via the liver X receptor in macrophages. Mol Asp Med. 2009;30:134–52. [DOI] [PubMed] [Google Scholar]
- 100. Russell LE, Harrison WJ, Bahta AW, Zouboulis CC, Burrin JM, Philpott MP. Characterization of liver X receptor expression and function in human skin and the pilosebaceous unit. Exp Dermatol. 2007;16:844–52. [DOI] [PubMed] [Google Scholar]
- 101. Tsukada M, Schröder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115:321–7. [DOI] [PubMed] [Google Scholar]
- 102. Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247–56. [DOI] [PubMed] [Google Scholar]
- 103. Boucher J, Tseng YH, Kahn CR. Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J Biol Chem. 2010;285:17235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cottle DL, Kretzschmar K, Schweiger PJ, Quist SR, Gollnick HP, Natsuga K, et al. C-MYC-induced sebaceous gland differentiation is controlled by an androgen receptor/p53 axis. Cell Rep. 2013;3:427–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ahle CM, Feidenhansl C, Brüggemann H. Cutibacterium acnes. Trends Microbiol. 2022;31:419–20. [DOI] [PubMed] [Google Scholar]
- 106. Armijos PO, Uhlenhake E, Milman T. Secretory carcinoma of the eyelid arising in an adnexal gland. Ophthalmology. 2022;129:1218. [DOI] [PubMed] [Google Scholar]
- 107. Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science (New York, NY). 2018;359:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yan HHN, Siu HC, Ho SL, Yue SSK, Gao Y, Tsui WY, et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut. 2020;69:2165–79. [DOI] [PubMed] [Google Scholar]
- 109. Sun X, Xiang J, Chen R, Geng Z, Wang L, Liu Y, et al. Sweat gland organoids originating from reprogrammed epidermal keratinocytes functionally recapitulated damaged skin. Adv Sci (Weinh). 2021;8:e2103079. [DOI] [PMC free article] [PubMed] [Google Scholar]