Abstract
Fibronectin receptor integrin-mediated cell adhesion triggers intracellular signaling events such as the activation of the Ras/mitogen-activated protein (MAP) kinase cascade. In this study, we show that the nonreceptor protein-tyrosine kinases (PTKs) c-Src and focal adhesion kinase (FAK) can be independently activated after fibronectin (FN) stimulation and that their combined activity promotes signaling to extracellular signal-regulated kinase 2 (ERK2)/MAP kinase through multiple pathways upstream of Ras. FN stimulation of NIH 3T3 fibroblasts promotes c-Src and FAK association in the Triton-insoluble cell fraction, and the time course of FN-stimulated ERK2 activation paralleled that of Grb2 binding to FAK at Tyr-925 and Grb2 binding to Shc. Cytochalasin D treatment of fibroblasts inhibited FN-induced FAK in vitro kinase activity and signaling to ERK2, but it only partially inhibited c-Src activation. Treatment of fibroblasts with protein kinase C inhibitors or with the PTK inhibitor herbimycin A or PP1 resulted in reduced Src PTK activity, no Grb2 binding to FAK, and lowered levels of ERK2 activation. FN-stimulated FAK PTK activity was not significantly affected by herbimycin A treatment and, under these conditions, FAK autophosphorylation promoted Shc binding to FAK. In vitro, FAK directly phosphorylated Shc Tyr-317 to promote Grb2 binding, and in vivo Grb2 binding to Shc was observed in herbimycin A-treated fibroblasts after FN stimulation. Interestingly, c-Src in vitro phosphorylation of Shc promoted Grb2 binding to both wild-type and Phe-317 Shc. In vivo, Phe-317 Shc was tyrosine phosphorylated after FN stimulation of human 293T cells and its expression did not inhibit signaling to ERK2. Surprisingly, expression of Phe-925 FAK with Phe-317 Shc also did not block signaling to ERK2, whereas FN-stimulated signaling to ERK2 was inhibited by coexpression of an SH3 domain-inactivated mutant of Grb2. Our studies show that FN receptor integrin signaling upstream of Ras and ERK2 does not follow a linear pathway but that, instead, multiple Grb2-mediated interactions with Shc, FAK, and perhaps other yet-to-be-determined phosphorylated targets represent parallel signaling pathways that cooperate to promote maximal ERK2 activation.
Cell adhesion to extracellular matrix (ECM) proteins can generate transmembrane signals important for cell survival and can promote directed cell migration events. In a variety of cell types, integrin stimulation by ECM proteins such as fibronectin (FN) leads to changes in intracellular protein tyrosine phosphorylation events. In fibroblasts, the focal adhesion kinase (FAK), a nonreceptor protein-tyrosine kinase (PTK), colocalizes with integrin receptors at sites of cell attachment to ECM proteins. FAK may associate directly with β integrin cytoplasmic domains (44) or may cocluster with integrin receptors through interactions with other integrin-associated proteins (4, 8, 22). FAK tyrosine phosphorylation is stimulated by cell binding to ECM proteins (for a review, see reference 50), by overexpression of the β integrin cytoplasmic domains (52) and also by other growth factor or serum mitogens (for a review, see reference 24). Since integrin receptors lack catalytic activity, FAK association and activation may be important for integrin-mediated signal transduction events (for a review, see reference 20). This hypothesis is supported by gene knockout results in which both the FN- and FAK-null mice die as a result of similar developmental gastrulation defects (15, 25).
In addition to integrin stimulation of FAK, ECM protein binding to cells can lead to changes in the tyrosine phosphorylation of a number of different signaling proteins, including p130Cas, Shc, and Cbl, as well as structural proteins such as paxillin and tensin. Integrin stimulation can also promote increases in intracellular calcium levels (51), protein kinase C activity (32, 56), and phosphatidylinositol (PI) 3-kinase activity (7, 28). One downstream target for integrin-initiated signaling events is the activation of the extracellular signal-regulated kinase 2/mitogen-activated protein (ERK2/MAP) kinase pathway (9, 38, 39, 47, 59). Although integrin-initiated signaling to ERK2 is dependent on the integrity of the actin cytoskeleton and involves the activation of both the Rho and the Ras families of small GTPase proteins (12, 40), the integrin signaling pathways upstream of Ras have not been clearly defined.
Attempts to delineate the molecular mechanisms of integrin-stimulated signaling to ERK2 have yielded potentially conflicting results. In NIH 3T3 fibroblasts, Grb2 transiently binds to a motif surrounding FAK Tyr-925 after FN stimulation (47), with the binding of Src-family PTKs to the motif surrounding the FAK autophosphorylation site (Tyr-397) being important for Src-mediated phosphorylation of FAK Tyr-925 in vivo (48). Direct Grb2 binding to FAK and association with the Sos GDP-GTP exchange protein for the small G protein Ras is one pathway through which integrins may activate ERK2. However, partial ERK activation in NIH 3T3 fibroblasts can be achieved by antibody-mediated clustering of a chicken β1 integrin subunit lacking the cytoplasmic domain without concomitant activation of FAK (35). Although the components of this pathway remain to be defined, other studies also find that antibody-mediated clustering of α1β1, α5β1, and αvβ3 integrins in suspended cells can stimulate Shc tyrosine phosphorylation, Grb2 binding to Shc, and ERK2 activation without detectable tyrosine phosphorylation of FAK (57).
The PTK responsible for integrin antibody-mediated Shc tyrosine phosphorylation in suspended cells has not been defined, but overexpression of Shc mutated at the Tyr-317 Grb2 binding site can block integrin-stimulated ERK2 activation (57), a finding that underscores the importance of Grb2-Shc interactions in integrin signaling events. Interestingly, subsequent studies have shown that full integrin and Shc-mediated activation of Ras requires physical attachment and/or cell spreading on ECM ligands (37). Cell attachment and spreading on ECM ligands are conditions that stimulate FAK activation, suggesting that FAK also could play a role in Shc phosphorylation. Overexpression of FAK in 293T cells enhances FN-stimulated ERK2 activation, and this signaling event is dependent upon FAK autophosphorylation at Tyr-397 (49). However, FAK-enhanced FN-stimulated signaling to ERK2 is not dependent on Grb2 binding to FAK Tyr-925. Significantly, an alternate FAK-mediated signaling route is through enhanced c-Src PTK activation, Shc tyrosine phosphorylation, and Grb2 binding to Shc after FN stimulation of cells (49). Overexpression of the c-Src binding site mutant of FAK (Phe-397) inhibits FN-stimulated signaling to ERK2, implying that Src-family PTK binding may be essential for FAK-mediated signaling events (49).
Studies performed with Src-deficient fibroblasts show that FN-stimulated signaling to ERK2 is significantly reduced compared to the same cells reexpressing normal mouse c-Src (46). Although FAK is activated normally in Src-deficient cells, the FN-stimulated tyrosine phosphorylation of targets such as p130Cas and Shc are reduced compared to cells reexpressing c-Src. Surprisingly, expression of a dominant-negative fragment of c-Src in the Src-deficient cells enhanced FAK and p130Cas phosphotyrosine (P.Tyr) levels and did not block FN-stimulated signaling to ERK2 (46). This result supports the existence of an integrin-activated and Src-independent signaling pathway. In this current study, we extend these findings by showing that FAK and c-Src can be regulated independently after FN stimulation of NIH 3T3 fibroblasts and that in the absence of Src PTK activity, FAK can associate and directly phosphorylate Shc at Tyr-317 to promote Grb2 binding and low-level signaling to ERK2. Moreover, through the combined use of pharmacological inhibitors with NIH 3T3 fibroblasts and protein overexpression studies in 293T epithelial cells, we find that FN-stimulated signaling to ERK2 is dependent upon PTK activity, involves signaling inputs from protein kinase C, and is not blocked by Phe-317 Shc overexpression but instead may be mediated by multiple Grb2-mediated interactions with Shc, FAK, or other yet-to-be-determined phosphorylated targets. Our findings support the conclusion that multiple and potentially parallel signaling pathways can regulate the extent and duration of FN receptor integrin-stimulated ERK2 activation.
MATERIALS AND METHODS
Reagents.
Calphostin C, cytochalasin D, enolase, bovine plasma-purified FN, herbimycin A, PP1, and tyrphostin A47 (AG213) were purchased from Calbiochem (San Diego, Calif.). With calphostin C, cell treatments were performed in the presence of a fluorescent light source to photoactivate the drug. All other reagents or chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless stated otherwise. Purified recombinant mouse c-Src was a generous gift from M. Broome (The Salk Institute).
Cells, fusion proteins, plasmids, and antibodies.
Mouse NIH 3T3 fibroblasts and human kidney epithelial 293T cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% bovine calf serum (CS), penicillin (50 U/ml), and streptomycin (50 μg/ml). Glutathione S-transferase (GST) fusion proteins encompassing either the c-Src or the Grb2 SH2 domains were produced and purified as described previously (47). Epitope-tagged wild-type (WT), Phe-397, Arg-454, and Phe-925 mouse FAK cloned by using pCDNA3 (Invitrogen, La Jolla, Calif.) and epitope-tagged ERK2 cloned by using pLNC were used as described previously (49). Epitope-tagged WT and Phe-317 human Shc (54) cloned by using pCDNA3 were kindly provided by P. van der Geer (University of California, San Diego, Calif.). WT and an SH3 domain-inactivated mutant of human Grb2 (Leu-49 and Leu-206) cloned by using pSLX were used as described (14). 293T cells attached to cell culture dishes precoated with 25 μg of poly-l-lysine (PLL) per ml were transfected by standard calcium phosphate methods with either 5 μg (FAK, Shc, and Grb2) or 1 μg of ERK2 plasmid constructs in growth medium containing chloroquine (25 μM). Control pCDNA3 vector was added as necessary to equalize the total amount of DNA transfected. The cells were incubated at 37°C (5% CO2) for 8 h, the precipitate was removed by washing with phosphate-buffered saline (PBS), and the cells were incubated with DMEM containing 10% CS for 24 h. Cells were serum starved in DMEM containing 0.5% CS for 24 h prior to cell lysis or FN-replating experiments.
Polyclonal rabbit anti-FAK antiserum was produced against a FAK GST–C-terminal fusion protein and affinity-purified as previously described (47); affinity-purified antibody to ERK2 (C-14) was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Polyclonal antiserum to Grb2 was produced as described previously (48), and antisera to p130Cas and to Shc were generously provided by H. Hirai (University of Tokyo) and P. van der Geer, respectively. Affinity-purified rabbit antibody to Shc was purchased from Upstate Biotechnology (Lake Placid, N.Y.). Monoclonal antibodies to c-Src (MAb 2-17) and to ERK2 (MAb B3B9) were kindly provided by S. Simon (The Salk Institute) and by M. Weber (University of Virginia), respectively. Monoclonal antibodies to paxillin and phosphotyrosine (4G10) were purchased from Transduction Laboratories (Lexington, Ky.) and Upstate Biotechnology. Monoclonal antibody to the hemagglutinin antigen epitope tag (12CA5) was kindly provided by J. Meisenhelder (The Salk Institute).
Cell stimulation with FN or adherence to PLL.
Cells were serum-starved in DMEM containing 0.5% CS for 24 h and harvested by limited trypsin-EDTA treatment (0.05% trypsin and 2 mM EDTA in PBS). The trypsin was inactivated by soybean trypsin inhibitor (0.5 mg/ml) with 0.25% bovine serum albumin (BSA; Fraction V; ICN Biomedicals, Aurora, Ohio) in DMEM, and cells were collected by centrifugation, resuspended in DMEM containing no serum with 0.1% BSA, and held in suspension for 1 h at 37°C (2 × 105 cells/ml). Cell culture dishes (10 cm diameter) were precoated with FN purified from bovine plasma (10 to 20 μg/ml) or PLL (100 μg/ml) in PBS overnight at 4°C, rinsed with PBS, and warmed to 37°C for 1 h prior to replating. Suspended cells were distributed onto ligand-coated dishes (106 cells per dish) and incubated at 37°C; at various times following plating, the attached cells were rinsed in PBS (4°C) and lysed in 0.75 ml of modified radioimmunoprecipitation assay (RIPA) lysis buffer (see below). Total cell protein in lysates from serum-starved, suspended, or replated cells was determined by a colorimetric assay (Bio-Rad Laboratories, Hercules, Calif.) and standardized prior to further analyses.
Cell lysis, immunoprecipitation, and immunoblotting.
Unless otherwise described, cells in suspension or on 10-cm-diameter culture dishes were lysed at 4°C with 0.75 ml of modified RIPA lysis buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM HEPES [pH 7.4], 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, leupeptin [10 μg/ml], aprotinin [100 U/ml], and 1 mM phenylmethylsulfonyl fluoride) and insoluble material was removed by centrifugation (16,000 × g, 10 min at 4°C). Antibodies or purified GST fusion proteins (5 μg) were added to the cell lysates and incubated for 2 h at 4°C, and the antibodies were collected on protein A (Repligen, Cambridge, Mass.) or protein G-plus (Calbiochem, La Jolla, Calif.) agarose beads, whereas the GST fusion proteins were collected by binding to glutathione-agarose beads. The precipitated protein complexes were washed at 4°C in Triton-only lysis buffer (1% Triton X-100, 50 mM HEPES [pH 7.4], 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM NaF, leupeptin [10 μg/ml], aprotinin [100 U/ml], and 1 mM phenylmethylsulfonyl fluoride) followed by washing in HNTG buffer (50 mM HEPES [pH 7.4], 150 mM NaCl, 0.1% Triton X-100, 10% glycerol) prior to direct analysis by SDS-polyacrylamide gel electrophoresis (PAGE) or in vitro 32P labeling.
For Triton-insoluble cell fractionation, 0.75 ml of Triton-only lysis buffer at 4°C was added to each dish (106 cells), the dishes were scraped, and the lysates were clarified by centrifugation (16,000 × g, 5 min at 4°C). The cell supernatant (Triton soluble) was removed and the insoluble cell material (Triton insoluble) was extracted with 0.5 ml of RIPA cell lysis buffer and repeat pipetting and was clarified by centrifugation (16,000 × g, 5 min at 4°C). FAK immunoprecipitates (IPs) were made from 1 ml of Triton cell extract (∼106 cells) or RIPA-extracted material (insoluble pellet from 2 × 106 cells). FAK IPs were analyzed by protein immunoblotting and compared for differences in in vitro kinase activity as described below.
For immunoblotting, proteins were transferred to membranes overnight at 30 V. The membranes were stained with Coomassie blue to visualize molecular weight standards, washed in TBST (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, 0.05% Tween 20), and placed in blocking buffer (TBST containing 2% BSA) for 2 h at room temperature. The blots were incubated with 1 μg of antiphosphotyrosine (4G10) per ml; a 1:5,000 dilution of anti-ERK2, anti-paxillin, or 12CA5 monoclonal antibodies; or a 1:1,000 dilution of anti-FAK, anti-p130Cas, anti-Shc, or anti-Grb2 polyclonal antibodies for 2 h at room temperature. Bound primary antibody was visualized by enhanced chemiluminescent detection with horseradish peroxidase-conjugated anti-mouse antibody or protein A at a 1:5,000 dilution (Amersham, Arlington Heights, Ill.). Membranes were stripped of bound antibodies by incubation in 70 mM Tris-HCl (pH 6.8), 1% SDS, and 150 mM β-mercaptoethanol at 75°C for 30 min. Prior to reprobing with different primary antibodies, stripped membranes were washed extensively in TBST and placed in blocking buffer overnight.
Immune complex 32P-labeled kinase reactions.
FAK or c-Src immunoprecipitates (IPs) were washed in Triton-only lysis buffer, followed by HNTG buffer, and then kinase buffer (20 mM HEPES [pH 7.4], 10% glycerol, 10 mM MgCl2, 10 mM MnCl2, 150 mM NaCl). To initiate kinase reactions, excess buffer was removed from the IPs, 2.5 μl of [γ-32P]ATP (3,000 Ci/mmol, 10 μCi/μl) was added, and the IPs were incubated for 15 min at 37°C (∼30 μl, total volume). For direct analyses, reactions were stopped by the addition of 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. To dissociate protein complexes for subsequent immunoprecipitation steps, kinase reactions were stopped by the addition of 100 μl of kinase-SDS stop solution (1% SDS, 1 mM EDTA, 5 mM dithiothreitol, 20 mM NaPO4 [pH 7.4]) and boiled for 2 min; the agarose beads were then removed by centrifugation. The supernatant was divided in half and analyzed directly by SDS-PAGE or diluted 15-fold with Triton-only lysis buffer and subjected to antibody reimmunoprecipitation for 2 h at 4°C. The secondary IPs were washed in HNTG buffer and analyzed directly by SDS-PAGE.
To measure ERK2 kinase activity, polyclonal ERK2 IPs were made from 500 μg of total cell lysate, washed in Triton lysis buffer and HNTG buffer, and then ERK2 kinase buffer (25 mM HEPES [pH 7.4], 10 mM MgCl2). Myelin basic protein (MBP; 2.5 μg) was added to each IP as a substrate. Kinase reactions (∼35 μl, total volume) were initiated by the addition of an ATP mix (5 μl; final concentration of 20 μM ATP and 20 μCi of [γ-32P]ATP per nmol), incubated at 37°C for 15 min, and stopped by the addition of a 2× SDS-PAGE sample buffer. The phosphorylated MBP was resolved on a 17.5% acrylamide gel and visualized by autoradiography, and the amount of 32P incorporated into MBP was determined by Cerenkov counting.
To measure quantitatively c-Src kinase activity, IPs (monoclonal antibody 2-17 covalently coupled to protein G agarose [47]) from ∼500 μg of total NIH 3T3 cell protein in RIPA lysis buffer were washed in Triton lysis buffer, HNTG buffer, and then enolase kinase buffer (20 mM PIPES [pH 7.0], 10 mM MnCl2, 1 mM dithiothreitol). Acid-denatured enolase (2.5 μg) was added to each IP. Kinase reactions (∼35 μl, total volume) were initiated by the addition of an ATP mix (5 μl; final concentration of 20 μM ATP and 10 μCi [γ-32P]ATP per nmol), incubated at 30°C for 10 min, and stopped by the addition of 2× SDS-PAGE sample buffer, and the products were resolved on a 10% acrylamide gel. The enolase band was visualized by autoradiography and cut from the gel, and the amount of 32P incorporated was determined by Cerenkov counting. FAK kinase activity in Triton-only cell lysates was measured by autophosphorylation as described above in the presence of 20 μM ATP (10 μCi of [γ-32P]ATP per nmol) incubated at 30°C for 10 min.
Phosphopeptide mapping.
32P-labeled FAK was digested with trypsin and subjected to two-dimensional phosphopeptide mapping as described previously (3). Electrophoresis in pH 1.9 buffer (2.2% formic acid, 7.8% acetic acid) was performed for 1 h at 1,000 V, and ascending chromatographic separation was performed overnight in phosphochromo buffer (37.5% n-butanol, 25% pyridine, and 7.5% acetic acid in water).
RESULTS
FAK, p130Cas, paxillin, and Shc are tyrosine phosphorylated in quiescent as well as FN-stimulated NIH 3T3 fibroblasts.
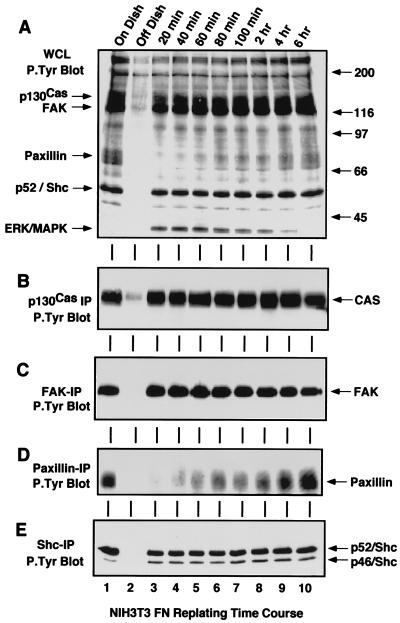
Several studies have shown that integrin receptor activation, mediated either by antibody clustering or through cell interactions with ECM proteins, can lead to the activation of the Ras/ERK2 MAP kinase pathway. However, the critical initial signaling events involved and the regulatory factors that modulate these pathways still remain relatively undefined. To investigate the role of tyrosine phosphorylation in FN-stimulated signaling events to ERK2, whole-cell lysates (WCLs) (Fig. 1A) or specific IPs of p130Cas (Fig. 1B), FAK (Fig. 1C), paxillin (Fig. 1D), or Shc (Fig. 1E) were made from either serum-starved NIH 3T3 fibroblasts, starved NIH 3T3 fibroblasts detached by limited trypsin-EDTA treatment and held in suspension for 30 min, or suspended NIH 3T3 fibroblasts that were replated onto either FN (10 μg/ml)- or PLL (100 μg/ml)-coated cell culture dishes for the times indicated (Fig. 1 and 2). This assay activates integrin receptors by presenting cells to an immobilized ECM substrate.
FIG. 1.
Tyrosine phosphorylation of p130Cas, FAK, paxillin, and Shc is not always associated with FN-stimulated signaling events. NIH 3T3 fibroblasts were either serum-starved “On Dish” (lane 1), held in suspension for 30 min “Off Dish” (lane 2), or replated onto FN-coated dishes for the times indicated (lanes 3 to 10). RIPA buffer-treated cell lysates were equalized for protein content and ∼100 μg of WCL (A), or IPs from ∼500 μg of WCL were made with p130Cas (B), FAK (C), paxillin (D), or Shc (E) antibodies. The samples were resolved by SDS-PAGE, analyzed by anti-P.Tyr blotting, and visualized by enhanced chemiluminescence (ECL) detection. Arrows indicate the positions of p130Cas, FAK, paxillin, Shc, and ERK2. ECL exposure time for panel A was 30 s.
FIG. 2.
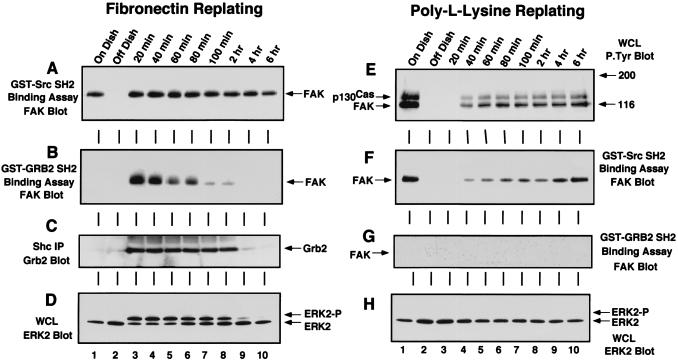
Grb2 SH2 domain binding to FAK and Shc is stimulated by FN replating of NIH 3T3 cells and correlates with the period of ERK2 activation. NIH 3T3 fibroblasts were either serum-starved “On Dish” (lane 1), held in suspension for 30 min “Off Dish” (lane 2), or replated onto FN-coated (A to D) or PLL-coated (E to H) dishes for the times indicated (lanes 3 to 10). FN-stimulated cell lysates were analyzed by GST-Src SH2 (A) or GST-Grb2 SH2 (B) binding assays, and FAK associated with the SH2 domains was visualized by anti-FAK blotting after SDS-PAGE. (C) Grb2 associated with polyclonal Shc IPs from FN-stimulated cell lysates was visualized by anti-Grb2 blotting after SDS-PAGE. (D) A 100-μg sample of FN-stimulated WCL was resolved by SDS-PAGE and analyzed by ERK2 blotting. (E) A 100-μg sample of PLL-stimulated WCL was resolved by SDS-PAGE and analyzed by anti-P.Tyr blotting. GST-Src SH2 (F) and GST-Grb2 (G) binding assays were performed with PLL-stimulated cell lysates, and FAK associated with the SH2 domains was visualized by anti-FAK blotting after SDS-PAGE. (H) A 100-μg sample of PLL-stimulated WCL was resolved by SDS-PAGE and analyzed by ERK2 blotting.
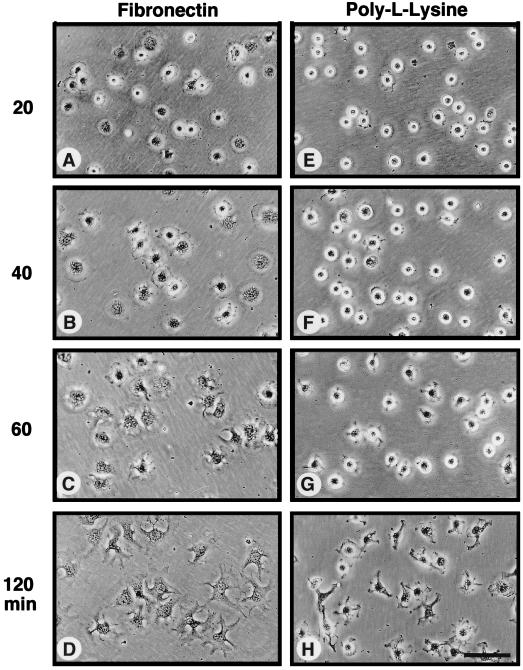
Many studies have shown that integrin stimulation can enhance the tyrosine phosphorylation of p130Cas, FAK, paxillin, and Shc. Antiphosphotyrosine (anti-P.Tyr) blotting analyses of IPs made from serum-starved NIH 3T3 cells showed that these proteins all contained significant levels of P.Tyr in quiescent cells when ERK2 was not activated (Fig. 1A to E, lane 1). Detachment of the NIH 3T3 cells led to the rapid loss of P.Tyr from these proteins (Fig. 1A to E, lane 2) and by itself did not activate signaling pathways to ERK2 (Fig. 2D). When the suspended cells were plated onto dishes precoated with the ECM protein FN, which binds to the α5β1 integrin receptor, P.Tyr levels in p130Cas, FAK, and Shc were rapidly and maximally elevated within 20 min (Fig. 1 and 2) as the cells started to spread (see Fig. 3A to D). Maximal ERK2 phosphorylation (ERK2-P) and activation also occurred within 20 min after FN plating as detected by anti-P.Tyr blotting of WCLs (Fig. 1A, lanes 3 to 8) or monitored by the appearance of a second protein band with decreased SDS-PAGE mobility by ERK2 blotting (Fig. 2D). Control experiments were performed by plating suspended cells onto PLL-coated dishes, where the cells “stick” independently of integrin activation. On PLL-coated plates, the NIH 3T3 fibroblasts remained rounded for the first 60 min (Fig. 3E to H) and began to spread slowly after 60 min, at which time p130Cas and FAK become weakly tyrosine phosphorylated (Fig. 2E). Significantly, the cell spreading and tyrosine phosphorylation events on PLL-coated plates after 60 min did not stimulate signaling to ERK2 (Fig. 2H).
FIG. 3.
Time course of FN-stimulated cell spreading compared to NIH 3T3 cell adhesion to PLL. Serum-starved NIH 3T3 fibroblasts were held in suspension for 30 min and plated (1.3 × 104 cells/cm2) onto either FN-coated (A to D) or PLL-coated (E to H) cell culture dishes. At the times indicated, phase-contrast micrographs were taken with a Nikon inverted microscope (Nikon, Inc., Melville, N.Y.) equipped with a 40× objective lens and photographed with T-MAX 400 film (Kodak). Scale bar, ca. 100 μm.
FAK and Shc “activation” as measured by Grb2 SH2-dependent binding.
Anti-P.Tyr blotting analyses of p130Cas, FAK, and Shc IPs showed that these proteins were rapidly rephosphorylated after FN plating and that their P.Tyr levels remained elevated beyond the period of maximal ERK2 activation (Fig. 1B, C, and E). In contrast, paxillin tyrosine phosphorylation was low during the period of maximal signaling to ERK2 (Fig. 1D, lanes 3 to 6) and became elevated during the formation of actin stress fibers and focal contacts 1 to 2 h after FN stimulation (data not shown). Although the tyrosine phosphorylation of FAK, p130Cas, and Shc is important for these proteins to interact with Src homology 2 (SH2) domain-containing signaling proteins, measures other than P.Tyr blotting analyses are required to determine the “activation” states of these proteins. With respect to FAK, it has been shown that Tyr-397 is phosphorylated in vivo in serum-starved as well as in FN-plated cells (48). In a finding consistent with this observation, in vitro binding of the c-Src SH2 domain to FAK was observed in quiescent serum-starved cells (Fig. 2A, lane 1) within 20 min of FN stimulation (Fig. 2A, lane 3) and within 60 to 120 min of PLL plating (Fig. 2F) and was therefore directly correlated with the extent of Tyr-397 phosphorylation. However, in vivo SH2-dependent c-Src PTK binding to this site is tightly regulated and occurs transiently only after FN stimulation of NIH 3T3 cells (47) (see Fig. 4).
FIG. 4.
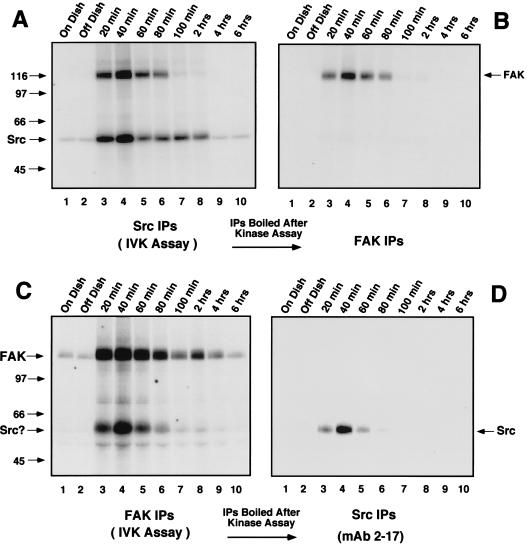
Transient associations between FAK and c-Src detected during the time course of FN-stimulated cell spreading and signaling to ERK2. NIH 3T3 fibroblasts were either serum-starved “On Dish” (lane 1), held in suspension for 30 min “Off Dish” (lane 2), or replated onto FN-coated dishes for the times indicated (lanes 3 to 10). Cell lysates were equalized for protein content (∼1 mg of total cell protein) and either c-Src (A) or FAK (C) IPs were labeled by the addition of [γ-32P]ATP in an in vitro kinase assay. Aliquots of the labeled c-Src or FAK IPs were either resolved by SDS-PAGE and visualized by autoradiography (A and C) or boiled, diluted, and reimmunoprecipitated with antibodies to FAK (B) or c-Src (D). The secondary IPs were resolved by SDS-PAGE and visualized by autoradiography. Arrows indicate the positions of c-Src and FAK.
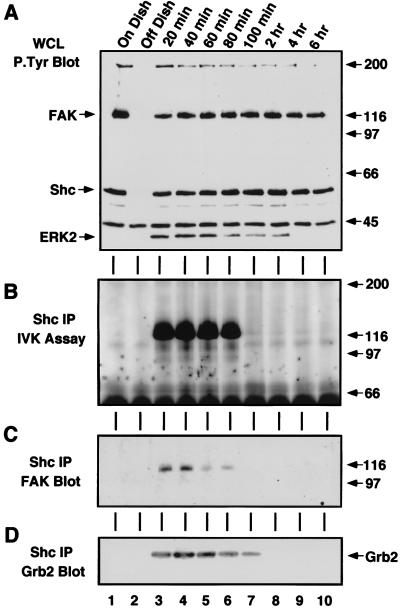
Even though in vitro c-Src SH2 domain binding to FAK does not necessarily correlate with FN-stimulated ERK2 activation events, FAK phosphorylation at Tyr-397 is necessary for the recruitment of Src family PTKs in the formation of an integrin-stimulated signaling complex (49). The formation of an FAK–c-Src complex permits c-Src to phosphorylate FAK within the catalytic domain (5) and at Tyr-925 (47), thus enhancing FAK kinase activity and promoting Grb2 binding, respectively. The Grb2 SH2 domain, which specifically binds to the FAK peptide containing phosphorylated Tyr-925 (48), only bound to FAK in vitro during the initial period (20 to 80 min) of FN-stimulated signaling to ERK2 (Fig. 2B). Control plating of NIH 3T3 cells onto PLL did not promote Grb2 SH2 binding to FAK (Fig. 2G), indicating that this binding assay can be used as an indirect measure of FAK “activation.”
As with FAK, Grb2 binding to Shc has been shown to be an integrin-stimulated signaling event upstream of ERK2 (37, 46, 57). In vitro, the GST-Grb2 SH2 domain bound to Shc from lysates made after serum starvation and FN replating but not from lysates of suspended or PLL-plated NIH 3T3 cells (data not shown). Although Shc is tyrosine phosphorylated under serum-starved conditions (Fig. 1E), ERK2 is not activated (Fig. 2D) and in vitro Grb2 binding to Shc under these conditions may not reflect in vivo signaling complexes. To determine conditions in which Grb2 binding to Shc parallels signaling events in NIH 3T3 fibroblasts, Shc IPs were made from serum-starved and FN-plated NIH 3T3 fibroblasts and blotted for the presence of associated Grb2 (Fig. 2C). Grb2 did not coimmunoprecipitate with Shc in lysates from serum-starved cells or suspended cells (Fig. 2C, lanes 1 and 2), whereas Grb2 was associated with Shc within 20 min of FN plating (Fig. 2C, lane 3). Interestingly, this Grb2-Shc association paralleled the time course of FN-stimulated ERK2 activation (20 min to 2 h) and persisted beyond the period of Grb2 binding to FAK (20 to 80 min). The Grb2-Shc association declined after 2 h (Fig. 2C, lanes 9 and 10) even though Shc remained tyrosine phosphorylated at these later time points (Fig. 1E).
Activated c-Src associates with FAK during the period of FN-stimulated signaling to ERK2.
Activation of Src family PTKs is required for the phosphorylation of FAK at Tyr-925 to promote Grb2 binding, but the kinase(s) responsible for integrin-stimulated Shc tyrosine phosphorylation has not been clearly defined. Since FAK overexpression enhances both Shc tyrosine phosphorylation and c-Src PTK activity after FN stimulation (49), experiments were performed to measure the kinetics c-Src PTK activation after FN stimulation of NIH 3T3 fibroblasts. In vitro kinase assays in the presence of [γ-32P]ATP were performed from RIPA cell lysates containing equal amounts of immunoprecipitated c-Src (data not shown) from either serum-starved, suspended, or FN-plated cells for the times indicated (Fig. 4). Assays were performed in either the presence (see Fig. 6A; data not shown) or absence (Fig. 4A) of added acid-denatured enolase protein substrate, and the labeled proteins were resolved by SDS-PAGE. Enhanced 32P incorporation into c-Src (Fig. 4A) or into enolase (data not shown) was observed 20 to 40 min following FN stimulation. Significantly, a labeled ∼120-kDa protein coimmunoprecipitated with c-Src at the early time points after FN stimulation (Fig. 4A). This band was subsequently identified as FAK by denaturation of duplicate 32P-labeled c-Src IPs prior to analyzing secondary IPs with antibody specific for FAK (Fig. 4B). This transient FAK association with c-Src correlated with the period of GST-Grb2 binding to FAK (Fig. 2B) and supports the hypothesis that FAK autophosphorylation after FN stimulation is a signal to recruit Src family PTKs to sites of integrin clustering (43, 47).
FIG. 6.
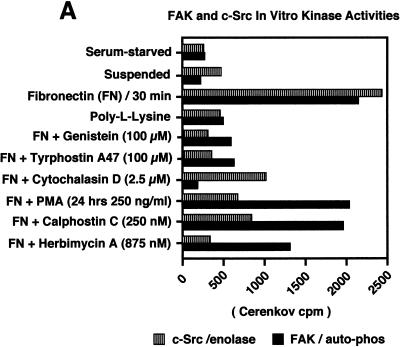
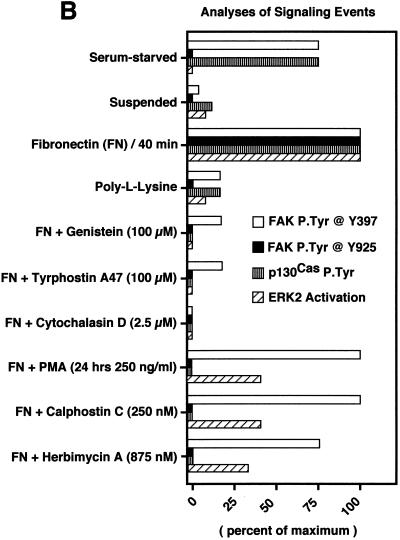
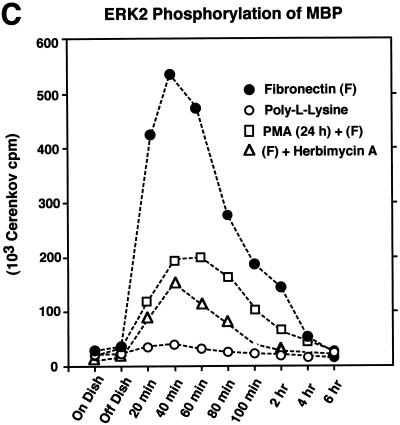
FAK and c-Src activities are regulated independently after FN stimulation. The effects of pharmacological inhibitors on FN-stimulated signaling events and ERK2 activation are demonstrated. Cell lysates were prepared from serum-starved, suspended, PLL- and FN-plated NIH 3T3 cells (30 min) in the presence of the indicated pharmacological inhibitors. (A) Quantitative analyses are shown of c-Src in vitro kinase activity toward acid-denatured enolase (striped boxes) or FAK (black boxes) in vitro autophosphorylation activity. The total cell protein was normalized prior to the immunoprecipitation and in vitro kinase assays. The 32P-labeled enolase or FAK bands were quantitated by Cerenkov counting, and the values represent the average of three separate experiments. (B) Comparisons of FAK tyrosine phosphorylation at Tyr-397 as measured by GST-Src SH2 binding and FAK blotting (white boxes), FAK phosphorylation at Tyr-925 as measured by GST-Grb2 SH2 binding and FAK blotting (black boxes), p130Cas tyrosine phosphorylation as measured by p130Cas IP and anti-P.Tyr blotting (striped boxes), and ERK2 activation as measured by band shifts in ERK2 blotting of WCLs (hatched boxes). The extent of positive values was quantitated by scanning ECL-derived images on a flatbed scanner and performing densitometric analyses with the software program NIH Image. Values, averaged from two separate experiments, are expressed as the percentage of maximum as determined by the FN plating control. PLL- or FN-plated-cell analyses were performed at 30 min, and qualitative ERK2 activation was performed by measuring the intensity of the slower-migrating phosphorylated ERK2 band (see Fig. 2D). (C) Cell lysates, made at the time points indicated from NIH 3T3 fibroblasts replated on FN (closed circles), on PLL (open circles), or on FN after PKC down-regulation by PMA treatment for 24 h (open squares) or by 24-h herbimycin A treatment (open triangles), were equalized for protein content prior to ERK2 immunoprecipitation and kinase assays. ERK2 in vitro kinase activity was measured by the phosphorylation of MBP. The amount of 32P incorporated into MBP was determined by Cerenkov counting, and the points represent the average of two separate experiments.
Although FN-stimulated increases of FAK in vitro kinase activity occur in Src-deficient fibroblasts (46), c-Src binding to and phosphorylation of FAK catalytic domain residues can further enhance FAK kinase activity (5). To determine the time course of FN-stimulated FAK in vitro kinase activity, IPs were made from RIPA lysates containing equal amounts of immunoprecipitated FAK (data not shown) and were labeled in vitro by the addition of [γ-32P]ATP (Fig. 4C). Whereas FAK kinase activity as measured by autophosphorylation was low in serum-starved and suspended cells, FAK in vitro phosphorylation and association with a strongly labeled protein at ∼60 kDa, as well as with a weakly labeled band at ∼52 kDa, were significantly enhanced 20 to 60 min after FN stimulation (Fig. 4C, lanes 3 to 6).
The FAK-associated ∼60-kDa band was identified as c-Src by denaturation of duplicate 32P-labeled FAK IPs prior to performing secondary immunoprecipitations with a monoclonal antibody specific for c-Src (Fig. 4D). Phosphopeptide mapping of the FAK-associated 52-kDa protein were similar to those of in vitro-phosphorylated Shc (data not shown, see Fig. 8). No significant c-Src association with FAK could be detected by immunoblotting in FAK IPs from serum-starved cell lysates (data not shown), even though the c-Src binding site at FAK Tyr-397 is phosphorylated under these conditions (Fig. 2A and F) (48). These results confirm that the association between c-Src and FAK after FN stimulation of NIH 3T3 cells is tightly regulated. Although these in vitro kinase assays (Fig. 4) cannot discriminate FN-stimulated FAK autophosphorylation from c-Src transphosphorylation of FAK, the time course of enhanced FAK kinase activity between 20 min and 2 h in the FN-stimulated NIH 3T3 cells was similar to the extended time course of FN-stimulated FAK in vitro kinase activity from Src-deficient fibroblasts (46). In addition, our NIH 3T3 fibroblast results show that the time course of enhanced PTK activity of both c-Src and FAK parallels the period of maximal FN-stimulated signaling to ERK2.
FIG. 8.
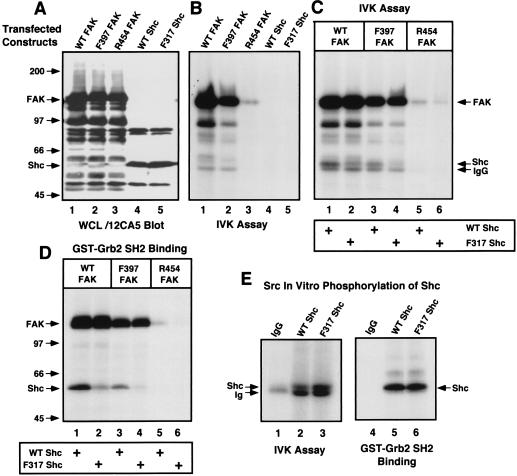
Direct phosphorylation of Shc by FAK or c-Src in vitro promotes Grb2 binding. (A) Human 293T cells were transiently transfected with either hemagglutinin epitope-tagged WT FAK, Phe-397 FAK, Arg-454 FAK, WT Shc, or Phe-317 Shc, and recombinant protein expression in WCLs was analyzed by immunoblotting with the 12CA5 monoclonal antibody to the hemagglutinin epitope tag. (B) Serum-starved 293T cells transfected with the indicated FAK or Shc constructs were stimulated (lanes 1 to 3) with PMA (250 ng/ml, 10 min), and kinase activity associated with 12CA5 IPs was measured by the addition of [γ-32P]ATP in an in vitro kinase (IVK) assay. (C) As shown in panel B, 12CA5 IPs of the indicated FAK proteins expressed in 293T cells stimulated with PMA were mixed with 12CA5 IPs of the indicated Shc proteins from serum-starved cells, and the proteins were labeled by the addition of [γ-32P]ATP in an in vitro kinase assay. (D) The products of duplicate reactions shown in panel C were denatured by the addition of SDS and boiling, diluted, and incubated with purified GST-Grb2 SH2 domain (5 μg). The labeled proteins associated with the Grb2 SH2 domain were visualized by autoradiography. The identities of FAK and Shc were confirmed by 12CA5 immunoblotting (data not shown). (E) Purified mouse c-Src (25 ng) was incubated with 12CA5 IPs from nontransfected 293T cells (lane 1) and from 293T cells transfected with WT (lane 2) or Phe-317 (lane 3) Shc, and the proteins were labeled by the addition of [γ-32P]ATP in an in vitro kinase assay. Grb2 SH2 domain-bound proteins (lanes 4 to 6) were isolated from duplicate in vitro kinase reactions. The identity of Shc was confirmed by 12CA5 immunoblotting (data not shown).
FAK and c-Src associate with the Triton-insoluble cell fraction after FN-stimulation of NIH 3T3 fibroblasts.
The fact that the dissociation of the FAK–c-Src complex (∼80 min after FN stimulation) occurred prior to the complete down-regulation of FAK or c-Src in vitro kinase activities (both occur at ∼2 h after FN stimulation) (Fig. 4A and C) suggests that the regulation of both FAK and c-Src association and PTK activity is complex and partly independent. Significantly, if NIH 3T3 fibroblasts were extracted with 1% Triton cell lysis buffer instead of RIPA lysis buffer, both FAK and c-Src IPs exhibited lower levels of FN-stimulated in vitro kinase activity and the amount of coimmunoprecipitation of the other PTK was significantly reduced (data not shown), suggesting that this PTK complex may be predominantly localized to the Triton-insoluble cell fraction.
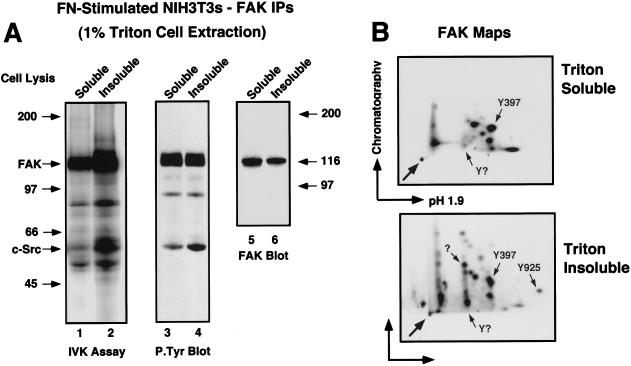
Previous studies have shown that activated c-Src partitions to Triton-insoluble cytoskeletal complexes after platelet stimulation (11) and fibroblast stimulation (27). To determine if FAK partitions to a Triton-insoluble fraction after FN stimulation (30 min) of NIH 3T3 fibroblasts, cells were extracted with 1% Triton lysis buffer, and the remaining insoluble cell material was reextracted with RIPA lysis buffer prior to FAK immunoprecipitation analyses (Fig. 5). Although greater amounts of FAK protein were recovered from the Triton-soluble fraction, FAK extracted from the insoluble pellet fraction was highly tyrosine phosphorylated and exhibited a two- to threefold-higher specific in vitro kinase activity than Triton-soluble FAK (Fig. 5A). In serum-starved or suspended cells, FAK was not associated with the Triton-insoluble fraction (data not shown). Significantly, phosphopeptide mapping and immunoblotting (data not shown) confirmed that the labeled (Fig. 5A, lane 2) and the tyrosine-phosphorylated (Fig. 5A, lane 4) 60-kDa protein in the Triton-insoluble FAK IP was c-Src.
FIG. 5.
Localization of activated FAK and c-Src to the Triton-insoluble fraction of NIH 3T3 fibroblasts after FN stimulation. (A) NIH 3T3 cells were plated onto FN for 30 min and extracted with 1% Triton lysis buffer, and the Triton-insoluble cell material was secondarily extracted with RIPA lysis buffer as described in Materials and Methods. FAK IPs made from the Triton-soluble or insoluble fractions were labeled in vitro by the addition of [γ-32P]ATP and resolved by SDS-PAGE (lanes 1 and 2) or separately analyzed by anti-P.Tyr (lanes 3 and 4) and anti-FAK immunoblotting (lanes 5 and 6). (B) The 32P-labeled FAK bands in A were digested with trypsin and processed for phosphopeptide mapping analyses (pH 1.9, 1,000 V, 1 h). Arrows within the panels indicate known positions of FAK tryptic phosphopeptides. The horizontal and vertical arrows indicate the directions of electrophoretic (arrow points to cathode) and chromatographic separation, respectively. The arrow in the lower left corner of each panel indicates the sample origin.
These results support the model that FN stimulation promotes the activation, association, and translocation of both c-Src and FAK to specific intracellular sites as represented by the redistribution of these PTKs to the Triton-insoluble cell fraction. Phosphopeptide mapping analyses of in vitro-labeled FAK were used to investigate whether FAK may be subject to additional phosphorylation events in the Triton-insoluble fraction (Fig. 5B). Phosphopeptide maps of Triton-soluble FAK revealed that it was phosphorylated at two or three sites in vitro, with Tyr-397 being the primary location (48). Mapping analyses of Triton-insoluble FAK showed that it was also phosphorylated at Tyr-397 in vitro but that, in addition, it was strongly labeled at additional sites not detected in the Triton-soluble FAK. Since the FAK Tyr-925 phosphopeptide could be positively identified in the Triton-insoluble FAK sample by Grb2 SH2 domain affinity binding (47) and since similar complex maps have been obtained with epitope-tagged FAK isolated from RIPA cell lysates (48), we conclude that the additional spots in the Triton-insoluble FAK maps represent additional sites of FAK tyrosine phosphorylation. These results support the hypothesis that FN receptor integrin-stimulated signaling complexes involving FAK and c-Src may be preferentially localized to the Triton-insoluble cell fraction.
FAK and c-Src can be activated independently after FN stimulation.
Although our results show that FAK and c-Src transiently associate (Fig. 4) and that these signaling complexes are localized to the Triton-insoluble cell fraction after FN stimulation, Triton-soluble FAK also exhibited increased FN-stimulated in vitro kinase activity (Fig. 5, lane 1) compared to the low levels of FAK PTK activity in serum-starved cells (Fig. 4C, lane 1). Since only very low levels of c-Src are associated with Triton-soluble FAK and since FN stimulation of Src-deficient fibroblasts activates FAK (46), it is possible that integrin signals may be able to promote FAK in vitro kinase activation without significant input from c-Src. Similarly, studies in platelets have shown that integrin-stimulated increases in c-Src PTK activity occur prior to detectable FAK activation (23).
To explore the regulation of FAK and c-Src PTK activities, in vitro kinase measurements were performed on either FAK or c-Src IPs made from either serum-starved, suspended, or FN-plated NIH 3T3 fibroblasts over a time course of 20 min to 6 h (Fig. 6; data not shown). FAK PTK activity was measured by autophosphorylation in IPs after 1% Triton cell lysis. Measurements of c-Src PTK activity after RIPA cell lysis were performed with added acid-denatured enolase as a substrate. The input of associated FAK PTK activity in the c-Src IPs is minimal, since FAK does not efficiently transphosphorylate enolase (49). Both FAK and c-Src exhibited low levels of in vitro kinase activity in lysates from serum-starved and suspended cells (Fig. 6A), and these results are consistent with the results of previous phosphorylation assays (Fig. 4). Maximal activities for both FAK and c-Src (four- to eightfold over basal levels) were observed 20 to 40 min after FN but not after PLL stimulation (Fig. 6A and data not shown).
To investigate the regulatory factors that affect FN-stimulated FAK and c-Src activation, as well as the downstream integrin signaling events leading to ERK2, various pharmacological inhibitors were added to the NIH 3T3 cells in suspension prior to FN plating for 30 min (Fig. 6A and B). The addition of the PTK inhibitors genistein (100 μM) or tyrphostin A47 (100 μM) inhibited both FAK and c-Src activation (Fig. 6A), as well as FN-stimulated signaling to ERK2 (Fig. 6B). These results support the hypothesis that integrin-stimulated PTK activation events regulate signaling to ERK2. Interestingly, the addition of cytochalasin D (2.5 μM), which binds to the barbed ends of actin filaments and prevents actin polymerization, completely inhibited FN-stimulated FAK tyrosine phosphorylation and signaling to ERK2 (Fig. 6A and B).
Significantly, cytochalasin D treatment of NIH 3T3 fibroblasts did not inhibit platelet-derived growth factor-stimulated tyrosine phosphorylation events and signaling to ERK2 (data not shown), indicating that the cytochalasin D inhibition of FN receptor integrin signaling events is specific. Surprisingly, FN stimulation of NIH 3T3 fibroblasts in the presence of cytochalasin D reduced but did not block increases in c-Src activity (Fig. 6A) and the tyrosine phosphorylation of cellular proteins such as Shc (data not shown). Interestingly, previous studies have shown that cytochalasin D does not block FN-stimulated integrin clustering and FAK recruitment to these sites, whereas cytochalasin D blocks the translocation of Src family PTKs to integrin clustering sites (38). Our data support a role for FAK tyrosine phosphorylation and the coordinated recruitment of signaling proteins to a cytoskeletal scaffold as important in the transmission of integrin but not growth factor-generated signals to ERK2. Our results also suggest that potentially diffusible integrin-generated signals may facilitate c-Src activation and phosphorylation events independent of FAK.
Inhibitors of protein kinase C decrease FN-stimulated c-Src activity and reduce but do not block signaling to ERK2.
Previous studies have shown that down-regulation of phorbol ester-sensitive isoforms of protein kinase C (PKC) and pharmacological PKC inhibitors such as calphostin C result in reduced integrin-stimulated FAK phosphorylation (32, 56). To test the role of PKC in FN-stimulated FAK and c-Src activation, as well as in the activation of ERK2, NIH 3T3 cells were either treated with phorbol myristate acetate (PMA; 250 ng/ml) for 24 h to down-regulate PKC or calphostin C (250 nM) was added to suspended cells prior to FN plating. Surprisingly, these treatments did not significantly affect FN-stimulated FAK in vitro kinase activity as measured by autophosphorylation (Fig. 6A) or FAK Tyr-397 phosphorylation as measured by c-Src SH2 domain binding to FAK (Fig. 6B), but these treatments reduced both FAK tyrosine phosphorylation at Tyr-925 and FN-stimulated signaling to ERK2 (Fig. 6B and C). Interestingly, PMA or calphostin C treatments reduced FN-stimulated c-Src in vitro kinase activity toward enolase approximately threefold (Fig. 6A) and also resulted in a two- to threefold reduction of peak FN-stimulated ERK2 activity (Fig. 6C).
Both PMA and calphostin C treatments of FN-stimulated NIH 3T3 fibroblasts also resulted in the inhibition of p130Cas tyrosine phosphorylation (Fig. 6B). Since Src family PTK activity has been shown to be responsible for integrin-stimulated p130Cas tyrosine phosphorylation (46, 55), these results support the conclusion that a PKC-mediated pathway is important for integrin-stimulated c-Src PTK activation and subsequent p130Cas tyrosine phosphorylation. Although direct PKC phosphorylation of c-Src does not affect c-Src in vitro kinase activity (16), PMA stimulation of 293T cells can transiently activate both c-Src and FAK in vitro kinase activities (see Fig. 8), and PMA stimulation of fibroblasts can affect Src-family PTK activity in the absence of FAK tyrosine phosphorylation potentially through the activation of protein-tyrosine phosphatases (42). These results provide further evidence that FAK and c-Src can become activated independently after FN stimulation.
Elucidation of a Src-independent signaling pathway involving FAK, Shc, and Grb2.
In Src-deficient fibroblasts, FAK is activated by FN stimulation and signaling to ERK2 is reduced but not totally abolished (46). It is possible that Fyn or c-Yes partially compensates for the loss of c-Src in Src-deficient fibroblasts, or it is also possible that FAK may be able to promote low-level signaling to ERK2 in the absence of Src family PTK activity. To extend the results from the PKC inhibitor studies and explore the possibility of a direct FAK-mediated signaling pathway to ERK2, Src family PTKs were inhibited in NIH 3T3 fibroblasts by extended treatment with herbimycin A (875 nM, 24 h) or by the addition of the PP1 (19) Src PTK-specific inhibitor (10 μM) to suspended cells prior to FN stimulation. Herbimycin A treatment resulted in reduced levels of c-Src but not FAK protein expression (data not shown). FN-stimulated c-Src kinase activity (Fig. 6A) and tyrosine phosphorylation of FAK Tyr-925 and p130Cas (Fig. 6B) were inhibited in the presence of herbimycin A, but these treatments had only minor effects on FN-stimulated FAK autophosphorylation activity (Fig. 6A). Similar results were obtained with PP1 addition to suspended NIH 3T3 cells prior to FN stimulation (data not shown).
Significantly, extended herbimycin A treatment reduced but did not block FN-stimulated activation of ERK2 (Fig. 6B and C), a finding similar to the results obtained with PKC inhibitors or PP1 treatment of suspended cells. Combined IP–anti-P.Tyr blotting analyses identified FAK, Shc, and ERK2 as three of the five major P.Tyr-containing proteins in the FN-stimulated and herbimycin A-treated fibroblasts (Fig. 7A). To identify the PTK that phosphorylates Shc under these conditions, Shc IPs were made from herbimycin A-treated and FN-stimulated NIH 3T3 cells and incubated in the presence of [γ-32P]ATP to detect associated kinase activity (Fig. 7B). At the early time points after FN stimulation, a ∼116-kDa phosphorylated protein coimmunoprecipitated Shc (Fig. 7B), and immunoblotting of the same membrane identified this protein as FAK (Fig. 7C). Interestingly, Grb2 was found to coimmunoprecipitate with Shc during the same period as the FAK-Shc association in the herbimycin A-treated and FN-stimulated NIH 3T3 cells (Fig. 7D). Therefore, FAK may be able to promote signaling to ERK2 through Shc tyrosine phosphorylation in NIH 3T3 fibroblasts. Although this FAK-Shc association was best visualized under conditions of reduced c-Src expression and PTK activity, phosphopeptide mapping comparisons (data not shown) identified the labeled 52-kDa protein associated with FAK after FN stimulation of NIH 3T3 fibroblasts as Shc (Fig. 4C). These results show that FAK can promote the recruitment of signaling proteins such as Shc, as well as Src family PTKs after FN stimulation of cells.
FIG. 7.
Detection of in vivo FN-stimulated FAK, Shc, and Grb2 associations in the absence of Src family PTK activity. Cell lysates were made at the time points indicated from NIH 3T3 fibroblasts serum starved and treated with herbimycin A (875 nM) for 24 h prior to and during FN plating. (A) RIPA cell lysates were equalized for protein content, and ∼250 μg of WCL was analyzed by anti-P.Tyr blotting; ECL exposure time was ∼2 min. The relative levels of both FAK and Shc tyrosine phosphorylation in the herbimycin A-treated cells are reduced compared to levels in the control (∼100 μg) cell lysates (see Fig. 1). (B) Polyclonal Shc IPs from ∼1 mg of RIPA lysate were labeled by the addition of [γ-32P]ATP in an in vitro kinase (IVK) assay, and the proteins associated with Shc were resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and visualized by autoradiography. (C) Anti-FAK blotting was performed on the Shc IP-containing membrane shown in panel B. (D) Transient Grb2 association with polyclonal Shc IPs from ∼500 μg of RIPA lysates was visualized by anti-Grb2 blotting.
FAK can directly phosphorylate Shc to promote Grb2 binding.
FAK autophosphorylation at Tyr-397 creates a binding site (P.Tyr-Ala-Glu-Ile) for SH2-containing proteins such as Src family PTKs. Since Shc coimmunoprecipitation with WT but not Phe-397 FAK after FN stimulation of 293T cells and a GST-Shc SH2 domain fusion protein binds to WT but not Phe-397 FAK in vitro (49), experiments were performed to determine whether FAK could directly phosphorylate Shc to promote Grb2 binding (Fig. 8). In vitro experiments were performed with epitope-tagged WT, Phe-397, and kinase-inactive (Arg-454) FAKs or WT and Phe-317 Shc constructs individually expressed in human 293T cells (Fig. 8A). Grb2 binding to Shc Tyr-317 has been characterized as an integrin-stimulated signaling pathway to ERK2 (37, 57).
FAK in vitro kinase activity measurements were made after PMA treatment (250 ng/ml, 10 min) of serum-starved 293T cells and both WT and Phe-397 FAKs but not Arg-454 FAK exhibited enhanced autophosphorylation activity (Fig. 8B, lanes 1 to 3). In serum-starved 293T cells transfected with either WT or Phe-317 Shc, no significant kinase activity was detected with IPs to the epitope-tagged Shc constructs (Fig. 8B, lanes 4 and 5). Upon mixing FAK IPs from PMA-stimulated 293T cells with either WT or Phe-317 Shc IPs from serum-starved 293T cells, WT and Phe-397 but not Arg-454 FAK transphosphorylated WT Shc (Fig. 8C). Phe-397 FAK phosphorylated WT but not Phe-317 Shc (Fig. 8C, lanes 3 and 4).
To determine whether FAK phosphorylation of Shc created binding sites for the Grb2 SH2 domain, aliquots of the in vitro kinase assays (Fig. 8C) were heat denatured, diluted in 1% Triton lysis buffer, and incubated with the GST-Grb2 SH2 domain (Fig. 8D). Both WT and Phe-317 Shc bound to the Grb2 SH2 domain after phosphorylation by WT FAK, whereas the Grb2 SH2 domain bound only WT Shc after phosphorylation by Phe-397 FAK (Fig. 8D, lanes 1 to 3). These results show that FAK can directly phosphorylate Shc at Tyr-317 to promote Grb2 binding since Phe-397 FAK phosphorylation of WT Shc most likely represents a direct event. Since WT FAK can promote low-level Grb2 binding to Phe-317 Shc, it is possible that Src family PTKs associated with FAK (through SH2 binding to FAK Tyr-397) could promote the phosphorylation of additional Grb2 binding sites on Shc. Previous studies have shown that v-Src can phosphorylate Shc at three sites: Tyr-239, Tyr-240, and Tyr-317 (54). Shc Tyr-239 is conserved in evolution and constitutes a second Grb2 SH2 binding site on Shc (54), and phosphorylation of Shc at Tyr-239 and Tyr-317 facilitates the formation of in vivo Shc-Grb2-Sos signaling complexes (21).
To determine the role of Src family PTK phosphorylation of Shc, purified normal mouse c-Src (25 ng) was incubated with WT or Phe-317 Shc IPs from serum-starved 293T cells (Fig. 8E). Purified c-Src readily phosphorylated both WT and Phe-317 Shc, and the phosphorylated forms of both WT and Phe-317 Shc bound to the Grb2 SH2 domain. In vitro, c-Src phosphorylated Phe-317 Shc as well as or better than WT Shc (Fig. 8C, lane 3). These in vitro results with c-Src are consistent with in vivo v-Src results showing that Src may preferentially phosphorylate Shc Tyr-239/240 (54). Therefore, in signaling complexes involving FAK it is possible that FAK-associated Src phosphorylates Shc Tyr-239 to promote Grb2 binding, whereas FAK promotes Grb2 binding to Shc through phosphorylation of Tyr-317.
FN-stimulated ERK2 activation is dependent upon Grb2-mediated signals.
Previous studies have shown that Grb2 binding to Shc Tyr-317 is important for integrin activation of the MAP kinase pathway since Phe-317 Shc overexpression can block integrin-stimulated ERK2 activation (37, 57). Since FAK overexpression can potentiate FN-stimulated Shc tyrosine phosphorylation in 293T cells (49), experiments were performed to test the effects of FAK, Shc, and Grb2 overexpression on FN-stimulated signaling to ERK2 (Fig. 9A to F).
FIG. 9.
Shc Phe-317 overexpression does not block FN-stimulated or FAK-enhanced Grb2-mediated signaling to ERK2. 293T cells were transiently transfected with hemagglutinin-tagged ERK2 in addition to the indicated expression constructs, and RIPA cell lysates were prepared after cell stimulation with FN in the absence of serum (20 min). FAK (A) and ERK2 (C) expression as detected by 12CA5 immunoblotting of ∼100 μg of WCL is shown. (B) Shc expression as detected by 12CA5 IPs followed by anti-Shc polyclonal immunoblotting. (D) Endogenous and recombinant Grb2 expression as determined by polyclonal Grb2 immunoblotting of ∼100 μg of WCL as resolved by SDS–17.5% PAGE. (E) Hemagglutinin-tagged ERK2 activity toward MBP as determined by 12CA5 IP in vitro kinase (IVK) assays in the presence of [γ-32P]ATP. The amount of 32P incorporated into MBP was determined by Cerenkov counting. The values shown represent the average of two separate experiments. (F) Analyses of FAK (lanes 1 to 6) or Shc (lanes 7 to 14) tyrosine phosphorylation in vivo as determined by monoclonal antibody anti-P.Tyr immunoblotting analyses of 12CA5 IPs. Cross-reactivity of the 12CA5 immunoglobulin chain with anti-mouse ECL detection reagents is indicated (lanes 7 to 14). Lanes 1 to 6, 7 to 12, and 13 to 15 are from separate experiments.
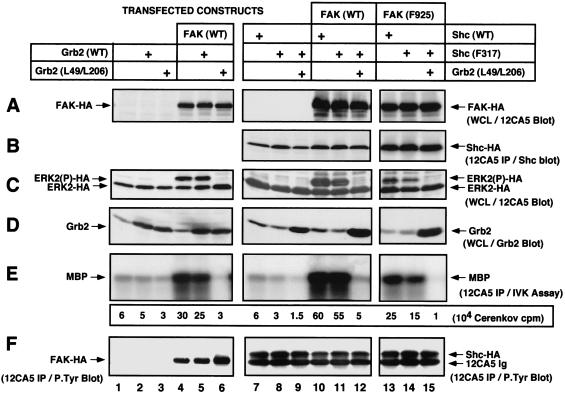
FN stimulation of 293T cells promoted only low-level activation of epitope-tagged ERK2, whereas FAK coexpression greatly enhanced FN-stimulated ERK2 activity (Fig. 9, lanes 1 and 4). Cotransfection of 293T cells with WT human Grb2 did not affect FN-stimulated ERK2 activation, but expression of SH3 domain-inactivated Grb2 (Leu-49/Leu-206) significantly reduced both endogenous and FAK-enhanced activation of ERK2 after FN stimulation (Fig. 9, lanes 1 to 6). Interestingly, expression of Leu-49/Leu-206 Grb2 but not WT Grb2 resulted in a slight increase in the tyrosine phosphorylation of transfected FAK (Fig. 9F, lane 6), indicating that the Grb2 block is downstream of FN-stimulated FAK activation events. Since Leu-49/Leu-206 Grb2 does not bind to the Ras-specific Sos GDP-GTP exchange protein (14) and since expression of similar Grb2 SH3 domain inactivation mutants inhibit growth factor stimulation of ERK2 (53), we conclude that Grb2 SH3-mediated coupling to downstream targets such as Sos are essential for enhanced FN-stimulated ERK2 activation.
Contrary to previous studies (57), overexpression of WT Shc did not enhance FN-stimulated ERK2 activation (Fig. 9, lanes 1 and 7) and Phe-317 Shc expression reduced but did not block endogenous FN-mediated ERK2 activation in 293T cells (Fig. 9, lane 8). Interestingly, overexpression of WT FAK in combination with either WT or Phe-317 Shc (Fig. 9, lanes 10 and 11) promoted greater FN-stimulated ERK2 activation than FAK overexpression alone (Fig. 9, lane 4). Expression of Leu-49/Leu-206 Grb2 blocked the FAK-Shc-enhanced signaling to ERK2 (Fig. 9, lane 12). Compared to serum-starved 293T cells (data not shown), FN stimulation enhanced both WT and Phe-317 Shc tyrosine phosphorylation in 293T cells and Leu-49/Leu-206 Grb2 expression did not block these events (Fig. 9F). Since FAK overexpression enhances FN-stimulated c-Src PTK activity (49) and since c-Src phosphorylation of Shc can promote Grb2 binding to Phe-317 Shc in vitro (Fig. 8), it is not surprising that Phe-317 Shc was tyrosine phosphorylated after FN stimulation and that its expression did not block signaling to ERK2.
Multiple Grb2-mediated signaling pathways to ERK2.
To determine whether FAK overexpression could enhance FN-stimulated signaling to ERK2 in the absence of direct Grb2 binding to FAK at Tyr-925 or to Shc at Tyr-317, coexpression experiments were performed with Phe-925 FAK and Phe-317 Shc in 293T cells. Expression of Phe-925 FAK with WT Shc enhanced signaling to ERK2 (Fig. 9, lane 13) to a level comparable to that stimulated by WT FAK alone (Fig. 9, lane 4) but less than that after combined expression of WT FAK and WT Shc (Fig. 9, lane 10). This result shows that FAK Tyr-925 contributes to maximal integrin-stimulated signaling to ERK2, but as previous results have documented (49), Grb2 binding to FAK Tyr-925 is not essential.
Coexpression of Phe-317 Shc with Phe-925 FAK resulted in reduced FN-stimulated activation of ERK2 compared to that obtained with Phe-925 FAK coexpressed with WT Shc (Fig. 9, lanes 13 and 14). FN-stimulated ERK2 activation in the presence of Phe-925 FAK and Phe-317 Shc was blocked by the coexpression of Leu-49/Leu-206 Grb2, but this Grb2 mutant did not block the FN-stimulated tyrosine phosphorylation of Shc Phe-317 (Fig. 9, lane 15). The level of FN-stimulated ERK2 activity with Phe-925 FAK and Phe-317 Shc expression was still elevated compared to the level of endogenous 293T ERK2 activation after FN stimulation (Fig. 9, lane 1). These results show that although FN-stimulated signaling to ERK2 in 293T cells is dependent upon Grb2 function, neither direct Grb2 binding to FAK Tyr-925 nor direct Grb2 binding to Shc Tyr-317 is essential for signaling. But since the combined expression of Phe-925 FAK with Phe-317 Shc led to reduced levels of FN-stimulated signaling to ERK2 compared to that of the WT FAK and Shc controls, our results support the hypothesis that Grb2 binding to these sites contributes to maximal FN-stimulated ERK2 activation.
What targets other than FAK Tyr-925 and Shc Tyr-317 may mediate integrin signaling to ERK2? Grb2 was present in 12CA5 IPs in 293T cells expressing Phe-925 FAK and Phe-317 Shc (data not shown), and therefore it is possible that FAK-enhanced and Src-mediated phosphorylation of Shc at sites such as Tyr-239 may also promote Grb2 binding and signaling to ERK2. In addition, other Grb2 binding proteins such as Gab1 are tyrosine phosphorylated after FN stimulation and therefore could also contribute to the overall integrin-stimulated signal (45a). In summary, our studies support the hypothesis that multiple Grb2-mediated interactions with Shc, FAK, and other yet-to-be-determined phosphorylated targets represent parallel signaling pathways that cooperate to promote maximal ERK2 activation.
DISCUSSION
A number of different signaling pathways have been proposed to mediate integrin activation of ERK2. As summarized in a working model (Fig. 10), our kinetic and pharmacological studies with NIH 3T3 fibroblasts in combination with studies of protein function by coexpression analyses in human 293T cells show that FN-stimulated signaling to ERK2 is dependent on PTK activity and that both FAK and c-Src can promote ERK2 activation through more than one Grb2-mediated signaling pathway. Our studies also show that there are multiple and potentially parallel FN receptor integrin-stimulated signaling pathways upstream of Ras. Src-mediated phosphorylation of FAK Tyr-925 and both FAK and Src-mediated phosphorylation of Shc Tyr-317 promote Grb2 binding and signaling to ERK2. Importantly, although we find that these pathways contribute to maximal integrin-stimulated ERK2 activation, neither pathway is essential. Whereas conditions can be found such that one particular Grb2 pathway may be sufficient, our studies show that it is the summation of Grb2-mediated signals that regulate the extent and duration of ERK2 activation after integrin stimulation as performed by cell replating assays.
FIG. 10.
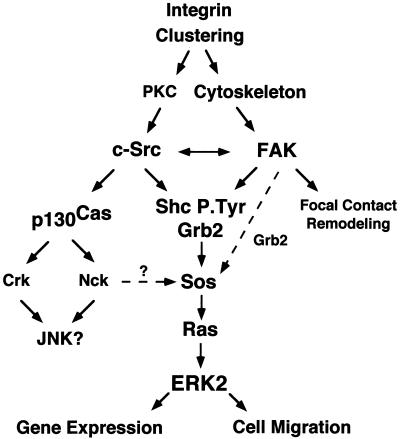
Model of the integrin signaling network to ERK2. FN stimulation of cells promotes integrin clustering and signals that can independently activate FAK or Src family PTKs. Integrin-stimulated c-Src activation may be downstream of PKC-mediated events, whereas integrin-stimulated FAK activation is dependent upon the integrity of the actin cytoskeleton. FAK and c-Src transiently associate and translocate to the Triton-insoluble fraction of cells after FN stimulation, and this association may mutually enhance and extend the time course of their integrin-stimulated PTK activity. Both FAK and c-Src can phosphorylate Shc at multiple sites to promote Grb2 adapter protein binding, whereas c-Src phosphorylation of FAK at Tyr-925 also promotes Grb2 binding. Grb2 SH3 domain association with the Sos GDP-GTP exchange protein can activate Ras, and ERK2/MAP kinase is one downstream target of Ras-mediated signaling events. It is proposed that maximal signaling to ERK2 would result from the stimulation of multiple pathways, whereas lower levels of integrin-stimulated signaling to ERK2 can occur in the absence of either Src family or FAK PTK activity. Integrin-stimulated c-Src PTK activity promotes p130Cas tyrosine phosphorylation and Crk and Nck adaptor protein binding to p130Cas, which may link integrins to the activation JNK or ERK2/MAP kinase pathways. Integrin-activated ERK2 may function to promote gene transcription by phosphorylation of targets in the nucleus or ERK2 may promote cell migration through phosphorylation and enhanced activation of myosin light-chain kinase (29). FAK may play multiple roles as a scaffold for the recruitment of signaling proteins and function in the processes of cell substratum remodeling events during cell spreading or migration.
Our study also provides evidence that FAK and c-Src may become independently activated after integrin stimulation of fibroblasts. This result may explain how integrin-generated signals in the absence of detectable FAK tyrosine phosphorylation can activate signaling pathways to ERK2 (35, 57) and are consistent with studies with FAK-deficient mouse embryonic fibroblasts whereby integrin-generated signals can activate Src-family PTKs (45b). How Src family PTKs are activated by integrin stimulation in the absence of FAK is not known, but it is possible that integrins could stimulate the activity of a protein-tyrosine phosphatase leading to the dephosphorylation of C-terminal Src family tyrosine residues and resulting in the conformational activation of Src family PTKs (41, 58). Another potential link between integrins and Src family PTK activation also involves PKC, which is also rapidly activated upon FN stimulation of cells (56).
Although previous studies have shown that integrin-stimulated PKC activation precedes α5β1 FN-stimulated cell spreading (56) and that inhibitors of PKC decrease αvβ5-dependent FAK tyrosine phosphorylation (32), our results show that some of these effects may be mediated through the prevention of full c-Src PTK activation after FN stimulation. By evaluating known targets of c-Src PTK activity, such as p130Cas and FAK Tyr-925 phosphorylation, and by measurements of in vitro kinase activities, we show that these PKC inhibitors prevented c-Src but not FAK activation after FN stimulation and resulted in decreased but not abolished signaling to ERK2. Although the use of pharmacological inhibitors is an indirect means for assessing protein function, we also found that cytochalasin D treatment of cells prevented FN-stimulated FAK activation and signaling to ERK2. These results with cytochalasin D are consistent with previous studies (9, 36) and support the hypothesis that FN receptor-stimulated FAK activation is indirect and mediated by actin cytoskeletal associations or through binding interactions with other integrin-associated proteins. The fact that cytochalasin D did not completely inhibit increases in FN-stimulated c-Src activity further supports our model that integrin signals can activate Src-family PTKs in a FAK-independent manner.
Although FAK and Src-family PTKs respond differentially to specific integrin-generated signals downstream of the actin polymerization or PKC, we found that maximal c-Src and FAK activation after FN stimulation may require a physical association between these two PTKs. Previous studies have shown that c-Src preferentially partitions to the Triton-insoluble fraction of platelets (13) and localizes to cell substratum adhesion contact sites after integrin stimulation of fibroblasts (26). Our results here show that after FN stimulation of NIH 3T3 fibroblasts, activated FAK and c-Src are both associated with the Triton-insoluble fraction of cells. This is the first demonstration of FAK redistribution after integrin stimulation that reinforces the importance of using strong detergent cell lysis conditions such as RIPA buffer containing 1% Triton, 1% sodium deoxycholate, and 0.1% SDS to analyze FAK tyrosine phosphorylation events. Previous studies with such lysis conditions have demonstrated that FN-stimulated FAK tyrosine phosphorylation occurs early within 10 min (17) and that FAK tyrosine phosphorylation can occur prior to integrin-stimulated cell spreading events (30). Other studies with milder cell lysis conditions have failed to detect early FAK tyrosine phosphorylation events after integrin stimulation (35, 57).
Since we show that FAK and c-Src transiently associate after FN stimulation, this FAK-Src association may prolong the activation of both PTKs. Although recent structural determinations have confirmed that the non-ligand-bound SH2 and SH3 domains of c-Src can act to constrain c-Src kinase domain activity (58), much less is known about the regulation of FAK kinase activity. In this study, we show that although FAK is highly tyrosine phosphorylated in serum-starved NIH 3T3 fibroblasts, it exhibits only minimal in vitro kinase activity. FN but not PLL replating of NIH 3T3 cells promoted both FAK tyrosine phosphorylation and in vitro kinase activity compared to that measured in suspended cells. Interestingly, FN-stimulated FAK in vitro kinase activity decreased over time without significant alterations in total FAK P.Tyr content. This decrease in FAK activity began at the same time as that observed for actin stress fiber and focal contact formation in the spreading NIH 3T3 fibroblasts. We speculate that FAK incorporation into these mature focal contact structures may promote c-Src dissociation and a down-regulation of FAK kinase activity through conformational changes or serine phosphorylation events (6, 48) without a concomitant reduction in observable FAK P.Tyr content. Under these circumstances FAK may be held in an inactive but “ready” (autophosphorylated at Tyr-397) conformation.
This model accommodates the fact that FAK exhibits high levels of tyrosine phosphorylation in quiescent serum-starved NIH 3T3 fibroblasts and is consistent with observations that mechanical forces such as contractility (10), physical stretching (18), or fluid flow shear stress (34) can stimulate FAK kinase activity. Under these circumstances, FAK may become activated through a conformational release of inhibitory factors, and exposure of phosphorylated FAK Tyr-397 could promote the specific recruitment of Src family PTKs or other SH2 domain-containing proteins to promote signaling events. The integrin stimulated association of Src family PTKs could further enhance FAK PTK activity through Src-mediated phosphorylation of residues within the FAK catalytic domain activation loop (5).
In a finding consistent with our previous studies (46), we found that maximal FN-stimulated signaling to ERK2 required the coordinated activation of both FAK and c-Src PTK activities. We show that the transient association of FAK and c-Src after FN stimulation (from 20 to 80 min) was correlated with the kinetics of maximal in vitro kinase activities and the phosphorylation of FAK Tyr-925 to promote Grb2 binding. Interestingly, we found that FN-stimulated Grb2 binding to Shc in NIH 3T3 cells paralleled the extended time course of ERK2 activation for up to 2 h, but that the amount of Grb2 associated with Shc was constant during the period of maximal ERK2 activation (20 to 40 min) at early times after FN stimulation. Therefore, we speculate that it is the summation of signals generated by Grb2 binding to FAK and Grb2 binding to Shc at early times during FN-stimulated cell spreading that may be responsible for maximal ERK2 activation. Our direct studies in 293T cells support this hypothesis and also show that there may be additional integrin-stimulated Grb2-mediated signaling pathways in cells.
Significantly, we also provide evidence in this study of an Src-independent FN receptor-stimulated signaling pathway to ERK2. As shown previously with Src-deficient fibroblasts (46) and in the current study with NIH 3T3 fibroblasts treated with Src-specific PTK inhibitors, FN-stimulated FAK autophosphorylation can promote low-level signaling to ERK2. We show that in the absence of Src family PTK activity, FN stimulation can promote the transient association of Shc with FAK and that FAK PTK activity can directly phosphorylate Shc at Tyr-317 to promote Grb2 binding. In vitro, FAK exhibited phosphorylation site specificity on Shc since it did not phosphorylate other known sites such as Shc Tyr-239 and Tyr-240 (21, 54). Interestingly, the aspartate amino acid residues preceding Shc Tyr-317 (Asp-Asp-Pro-Ser-P.Tyr) match the positions of two acidic glutamate residues preceding the identified FAK phosphorylation sites on paxillin at Tyr-31 (Glu-Glu-Thr-Pro-P.Tyr) and on paxillin at Tyr-118 (Glu-Glu-His-Val-P.Tyr) (1, 45), and it is possible that these acidic residues may direct FAK kinase specificity. Although both WT and Phe-397 FAK were able to directly phosphorylate Shc in vitro, Shc associates with WT but not with Phe-397 FAK in vivo (49).
This result supports the hypothesis that FAK may interact with multiple signaling proteins through binding interactions at the motif surrounding the Tyr-397 autophosphorylation site. FAK Tyr-397 is the direct SH2 binding site for Src family PTKs (43), and mutation of this site indirectly regulates Grb2 binding to FAK Tyr-925 (48). Mutation of FAK Tyr-397 also disrupts the association of the p85 subunit of PI 3-kinase with FAK (7). Since the Shc SH2 domain binds directly to WT but not to Phe-397 FAK in vitro (49) and the Shc SH2 domain binding consensus (P.Tyr-Ile-X-Ile) matches the motif surrounding FAK Tyr-397 (P.Tyr-Ala-Glu-Ile), Tyr-397 is likely to be the direct Shc binding site on FAK. Interestingly, only low levels of FAK-Shc associations after FN stimulation can be detected without overexpression. This may be a result of competition between Shc and Src family PTKs for the same binding site on FAK. Nevertheless, although direct FAK tyrosine phosphorylation of Shc in normal cells occurs, it is also likely that Shc tyrosine phosphorylation after integrin stimulation is mediated by other PTKs such as c-Src.
It has been postulated that Shc tyrosine phosphorylation at Tyr-317 is critically important for integrin signaling to ERK2 (57). We show that c-Src can phosphorylate both WT and Phe-317 Shc to promote Grb2 binding in vitro. Since FAK and c-Src are activated after integrin stimulation and since FAK overexpression can also potentiate c-Src PTK activity after FN stimulation (49), it is not surprising that Phe-317 Shc is tyrosine phosphorylated after FN stimulation of 293T cells and that its expression did not block WT FAK-enhanced FN-stimulated ERK2 activation. Our results also are consistent with findings that Phe-317 Shc overexpression does not block middle T antigen-stimulated and potentially Src family PTK-mediated Grb2 binding to Shc and cell transformation events (2). Therefore, what may be the mechanism by which Phe-317 Shc could block integrin signaling in some cells? As shown for FAK, other integrin-activated PTKs such as c-Abl (31) may preferentially phosphorylate Shc Tyr-317 and thus limit the number of Grb2 binding sites on Shc. It is also possible that the documented association of integrin subunits with caveolin (57) may negatively regulate integrin-stimulated c-Src PTK activation and signaling events (33).
Our results do not discount the importance of Grb2 binding to Shc Tyr-317 in mediating integrin-stimulated signals to ERK2, but in the case with Grb2 binding to FAK Tyr-925 (49) our studies show that Grb2 binding to Shc Tyr-317 is not essential for FN-stimulated signaling to ERK2. Significantly, we find that the expression of a SH3 domain-inactivated Grb2 protein, which does not bind to downstream targets such as the Sos GDP-GTP exchange protein for Ras, blocked FN-stimulated signaling to ERK2. The fact that coexpression of FAK Phe-925 and Shc Phe-317 results in lower ERK2 activation shows that these sites on the WT proteins do indeed function to promote FN-stimulated signaling events and therefore we conclude that signaling upstream of Ras does not proceed through one linear pathway (Fig. 10). Moreover, our results support the hypothesis that FN-stimulated signals to ERK2 proceed through multiple Grb2-mediated binding interactions with FAK, Shc, or perhaps other unidentified tyrosine-phosphorylated proteins. This multiplicity of signaling events affecting ERK2 activation is consistent with other observations that PI 3-kinase activity can also modulate integrin signaling pathways downstream of Ras to regulate ERK2 activation (28).
Most studies on integrin-based signaling have used acute cell stimulation via antibodies or synthetic ligands to induce integrin receptor clustering or via plating detached cells on insoluble ECM proteins. Conditions of this sort will be found rarely in humans, except perhaps for cells leaving the circulation or at the leading edge of migrating cells. But with acute stimulation it has been possible to find assay conditions in which one signaling pathway predominates and may be sufficient for ERK2 activation. Whereas this information is important to the mechanistic aspects of integrin signaling, our results support the hypothesis that multiple inputs and pathways may act in combination to regulate the extent and duration of ERK2 activation in vivo. Future studies will be directed at better understanding the role of integrin-stimulated ERK2 activation as it may pertain to cell survival, gene transcription, or cell migration.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R29 CA75240 (D.D.S.) and in part by grants CA14195 (T.H.) and CA39780 (T.H.) from the National Cancer Institute. Tony Hunter is an American Cancer Society Research Professor.
Footnotes
Paper 10863-IMM of The Scripps Research Institute, La Jolla, Calif.
REFERENCES
- 1.Bellis S L, Perrotta J A, Curtis M S, Turner C E. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem J. 1997;325:375–381. doi: 10.1042/bj3250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaikie P A, Fournier E, Dilworth S M, Birnbaum D, Borg J P, Margolis B. The role of the Shc phosphotyrosine interaction/phosphotyrosine binding domain and tyrosine phosphorylation sites in polyoma middle T antigen-mediated cell transformation. J Biol Chem. 1997;272:20671–20677. doi: 10.1074/jbc.272.33.20671. [DOI] [PubMed] [Google Scholar]
- 3.Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 4.Brown M C, Perrotta J A, Turner C E. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calalb M, Polte T, Hanks S K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for the Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calalb M B, Zhang X, Polte T R, Hanks S K. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- 7.Chen H C, Appeddu P A, Isoda H, Guan J L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 8.Chen H C, Appeddu P A, Parsons J T, Hildebrand J D, Schaller M D, Guan J L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 10.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark E A, Brugge J S. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark E A, Hynes R O. Ras activation is necessary for integrin-mediated activation of extracellular signal regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- 13.Clark E A, Shattil S J, Brugge J S. Regulation of protein tyrosine kinases in platelets. Trends Biochem Sci. 1994;19:464–469. doi: 10.1016/0968-0004(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 14.den Hertog J, Hunter T. Tight association of GRB2 with receptor protein-tyrosine phosphatase α is mediated by the SH2 and C-terminal SH3 domains. EMBO J. 1996;15:3016–3027. [PMC free article] [PubMed] [Google Scholar]
- 15.George E L, Georges-Labouesse E N, Patel-King R S, Rayburn H, Hynes R O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 16.Gould K L, Woodgett J R, Cooper J A, Buss J E, Shalloway D, Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985;42:849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- 17.Guan J-L, Trevithick J E, Hynes R O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamasaki K, Mimura T, Furuya H, Morino N, Yamazaki T, Komuro I, Yazaki Y, Nojima Y. Stretching mesangial cells stimulates tyrosine phosphorylation of focal adhesion kinase pp125FAK. Biochem Biophys Res Commun. 1995;212:544–549. doi: 10.1006/bbrc.1995.2004. [DOI] [PubMed] [Google Scholar]
- 19.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 20.Hanks S K, Polte T R. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 21.Harmer S L, DeFranco A L. Shc contains two Grb2 binding sites needed for efficient formation of complexes with SOS in B lymphocytes. Mol Cell Biol. 1997;17:4087–4095. doi: 10.1128/mcb.17.7.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildebrand J D, Schaller M D, Parsons J T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang M M, Lipfert L, Cunningham M, Brugge J S, Ginsberg M H, Shattil S J. Adhesive ligand binding to integrin αIIbβ3 stimulates tyrosine phosphorylation of novel protein substrates before phosphorylation of pp125FAK. J Cell Biol. 1993;122:473–83. doi: 10.1083/jcb.122.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilic D, Damsky C H, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- 25.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan K B, Bibbins K B, Swedlow J R, Arnaud M, Morgan D O, Varmus H E. Association of the amino-terminal half of c-Src with focal adhesions alter their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 1994;13:4745–4756. doi: 10.1002/j.1460-2075.1994.tb06800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan K B, Swedlow J R, Morgan D O, Varmus H E. c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 28.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong L, Hughes P E, Schwartz M A, Ginsberg M H, Shattil S J. Integrin signaling: roles for the cytoplasmic tails of αIIbβ3 in the tyrosine phosphorylation of pp125FAK. J Cell Sci. 1995;108:3817–3825. doi: 10.1242/jcs.108.12.3817. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J M, Baskaran R, Taagepera S, Schwartz M A, Wang J Y J. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic nuclear transport. Proc Natl Acad Sci USA. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis J M, Cheresh D A, Schwartz M A. Protein kinase C regulates αvβ5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Couet J, Lisanti M P. Src tyrosine kinase, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein caveolin. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Kim M, Hu Y-L, Jalali S, Schlaepfer D D, Hunter T, Chien S, Shyy J Y. Fluid shear stress activation of focal adhesion kinase. Linking to mitogen-activated protein kinases. J Biol Chem. 1997;272:30455–30462. doi: 10.1074/jbc.272.48.30455. [DOI] [PubMed] [Google Scholar]
- 35.Lin T H, Aplin A E, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano R L. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mainiero F, Murgia C, Wary K K, Curatola A M, Pepe A, Blumemberg M, Westwick J K, Der C J, Giancotti F G. The coupling of α6β4 integrin to Ras-MAP kinase pathways mediated by Shc controls keratinocyte proliferation. EMBO J. 1997;16:2365–2375. doi: 10.1093/emboj/16.9.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44Erk-1 and p42Erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- 40.Renshaw M W, Toksoz D, Schwartz M A. Involvement of the small GTPase Rho in integrin-mediated activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:21691–21694. doi: 10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- 41.Roach T, Slater S, Koval M, White M, Cahir-McFarland E, Okumura M, Thomas M, Brown E. CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:407–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Fernandez J L, Rozengurt E. Bombesin, bradykinin, vasopressin, and phorbol esters rapidly and transiently activate Src family tyrosine kinases in Swiss 3T3 cells. J Biol Chem. 1996;271:27895–27901. doi: 10.1074/jbc.271.44.27895. [DOI] [PubMed] [Google Scholar]
- 43.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaller M D, Otey C A, Hildebrand J D, Parsons J T. Focal adhesion kinase and paxillin bind peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaller M D, Parsons J T. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Schlaepfer, D. D. Unpublished data.
- 45b.Schlaepfer, D. D., et al. Submitted for publication.
- 46.Schlaepfer D D, Broome M A, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase–c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor [sic] proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 48.Schlaepfer D D, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlaepfer D D, Hunter T. FAK overexpression enhances Ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 51.Sjaastad M D, Angres B, Lewis R S, Nelson W J. Feedback regulation of cell-substratum adhesion by integrin-mediated intracellular Ca2+ signaling. Proc Natl Acad Sci USA. 1994;91:8214–8218. doi: 10.1073/pnas.91.17.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tahiliani P D, Singh L, Auer K L, LaFlamme S E. The role of conserved amino acid motifs within the integrin β3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J Biol Chem. 1997;272:7892–7898. doi: 10.1074/jbc.272.12.7892. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka M, Gupta R, Mayer B J. Differential inhibition of signaling pathways by dominant-negative SH2/SH3 adapter proteins. Mol Cell Biol. 1995;15:6829–6837. doi: 10.1128/mcb.15.12.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Geer P, Wiley S, Gish G D, Pawson T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol. 1996;6:1435–1444. doi: 10.1016/s0960-9822(96)00748-8. [DOI] [PubMed] [Google Scholar]
- 55.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Induction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for the Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- 57.Wary K K, Mainiero F, Isakoff S J, Marcantonio E E, Giancotti F G. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 58.Xu W, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 59.Zhu X, Assoian R K. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]