Abstract
Study Objectives:
The association of in-hospital medical emergency team activation (META) among patients with atrial fibrillation (AF) at risk for obstructive sleep apnea (OSA) is unclear. This study evaluates the performance of the DOISNORE50 sleep questionnaire as an OSA screener for patients with AF and determines the prevalence of META among perioperative patients with underlying AF who have a diagnosis or are at risk for OSA.
Methods:
A prospective perioperative cohort of 2,926 patients with the diagnosis of AF was assessed for DOISNORE50 questionnaire screening. Propensity-score matching was used to match patients’ physical characteristics, comorbidities, length of stay, and inpatient continuous positive airway pressure device usage. META and intensive care unit admissions during the surgical encounter, 30-day hospital readmissions, and 30-day emergency department visits were evaluated.
Results:
A total of 1,509 out of 2,926 AF patients completed the DOISNORE50 questionnaire and were enrolled in the OSA safety protocol. Following propensity-score matching, there were reduced adjusted odds of META in the screened group of 0.69 (95% confidence interval: 0.48–0.98, P < .001) in comparison to the unscreened group. The adjusted odds of intensive care unit admissions and emergency department visits within 30 days of discharge were statistically lower for the screened group compared with the unscreened group.
Conclusions:
Among perioperative AF patients, evidence supports DOISNORE50 screening and implementation of an OSA safety protocol for reduction of META. This study identified decreased odds of META, intensive care unit admissions, and emergency department visits among the screened group. The high-risk and known OSA group showed reduced odds of META following the implementation of an OSA safety protocol.
Citation:
Saha AK, Sheehan KN, Xiang KR, et al. Preoperative sleep apnea screening protocol reduces medical emergency team activation in patients with atrial fibrillation. J Clin Sleep Med. 2024;20(5):783–792.
Keywords: obstructive sleep apnea, atrial fibrillation, complications, perioperative, DOISNORE50, OSA safety protocol
BRIEF SUMMARY
Current Knowledge/Study Rationale: Undiagnosed and untreated obstructive sleep apnea is associated with increased in-hospital morbidity and serves as a risk factor for cardiac complications, including atrial fibrillation. This study assessed the utility of using the DOISNORE50 screening questionnaire with an obstructive sleep apnea safety protocol to predict and mitigate adverse hospital events.
Study Impact: The DOISNORE50 questionnaire was predictive of obstructive sleep apnea in a perioperative population with underlying atrial fibrillation. Implementation of this screening tool and sleep safety interventions were associated with a reduction in medical emergency team activations, intensive care unit admissions, and emergency department visits within 30 days of hospital discharge across all obstructive sleep apnea risk categories.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep disorder with an increasing incidence and prevalence.1 Despite these trends, OSA remains an underrecognized and underdiagnosed condition, with some estimating that only 1 in 50 patients with symptoms consistent with OSA undergo an appropriate diagnostic evaluation.2 OSA is associated with multiple comorbidities, including obesity, stroke, Alzheimer’s disease, hypertension, and diabetes mellitus. Its presence correlates with all-cause mortality in the general population.1,3–8 Specifically, among perioperative patients, unrecognized OSA is associated with higher rates of adverse cardiovascular events and is an independent risk factor for the development of atrial fibrillation (AF).9–11 The association between AF, OSA risk, and medical emergency team activation (META) among vulnerable postoperative patients remains unclear.
In the postoperative period, both patients with existing OSA and those at risk for OSA have increased rates of META.12 These include rapid response team activation (RRT), code blue (CB), code stroke, and reintubation. META events often lead to patient escalation to a higher level of care, result in longer hospital stays and increased health care costs.9,13–18 In-hospital patients diagnosed with AF account for a high percentage of META and have been associated with increased in-hospital mortality.18–20 Currently, little is known about the prevalence of META among patients with AF who have OSA or are at risk for OSA. Routine perioperative screening for OSA has been proposed as a means of identifying patients who may benefit from interventions such as automatic titrating positive airway pressure therapy.12,21,22 These measures are of particular interest in patients with underlying AF, because existing evidence suggests that positive airway pressure may improve the efficacy of antiarrhythmic drug therapy and mitigate arrhythmic burden.23
Previously published studies showed perioperative patients who were screened with an OSA risk questionnaire and followed an “OSA safety protocol” had improved OSA recognition.24 One such OSA risk questionnaire is the DOISNORE50 sleep screening questionnaire, developed at Atrium Health Wake Forest Baptist Medical Center (AHWFBMC) and derived from ISNORED and META analyses.12 DOISNORE50 was created as a simple means to screen perioperative patients and stratify them as low-risk, at-risk, or high-risk for OSA. It was previously validated among 64,949 patients in the AHWFBMC perioperative assessment clinic from 2016–2020 and found to be predicative of OSA, confirmed by a polysomnogram. Its area under the receiver operating curve was 0.78, outperforming other sleep apnea screening questionnaires (STOP-BANG and ISNORED).12 This study further investigates the patient characteristics and risk of META in perioperative patients with underlying AF who were screened for OSA, as well as the impact of the previously published OSA safety protocol.12
METHODS
Participants
This is a single-center, retrospective analysis of a prospective cohort study conducted at AHWFBMC, a tertiary care, 900-bed university hospital in Winston-Salem, North Carolina. Patients undergoing elective procedures performed between January 2020 and April 2022 with hospital stays of 2 or more days were included in the study. Of the total 60,219 patients, 2,926 had a diagnosis of AF, identified by using International Classification of Diseases 10th Revision billing codes available in the electronic medical record (EMR). The diagnosis of AF was validated by the presence of AF on the patient’s problem list and a detailed review of provider documentation noting AF as both an admission and a discharge diagnosis. A consort diagram of the study cohort is shown in Figure 1.
Figure 1. Consort diagram for the study population.
Of a cohort of preoperative patients (60,129), 2,926 had underlying atrial fibrillation and 51.6% underwent screening for obstructive sleep apnea with the DOISNORE50 questionnaire. OSA = obstructive sleep apnea.
DOISNORE50 screening and OSA safety protocol
Validation of the DOISNORE50 questionnaire was previously done using the apnea-hypopnea index as obtained from polysomnographic testing using SomnoStar software (Vyaire Medical, Inc., Mettawa, Illinois), WatchPAT ONE (Itamar Medical, Caesarea, Israel), a home sleep testing device, or Alice 3 (Philips, Andover, Massachusetts). Sensitivity, specificity, positive predictive values, and negative predictive values of self-administered questionnaires were calculated based on apnea-hypopnea index ≥ 5 events/h or respiratory event index ≥ 5 events/h, per Centers for Medicare & Medicaid Services/American Academy of Sleep Medicine guidelines.12,25
The presence or absence of an underlying OSA diagnosis was documented during screening with the DOISNORE50 questionnaire via patient report and International Classification of Diseases 10th Revision billing codes in the EMR (Figure 2A). As part of the institutionally designed OSA Safety Protocol (Figure 2B), patients answered 2 questions: “Do you have OSA?” and “Are you on CPAP?” The patient was identified as having known OSA if he or she answered “yes” to either question. Otherwise, the patient was screened with the DOISNORE50 questionnaire. The questionnaire contains 9 questions, and each is weighted equally to establish a score from 0–9 (Figure 2A). Those patients who affirmed 0–3 questions were considered low- risk, 4–5 questions at-risk, and 6–9 questions high-risk for OSA.
Figure 2. DOISNORE50 action items and OSA safety protocol illustration.
(A) DOISNORE50 action items. The 9 questions are weighted equally, to establish a score from 0–9. Patients with scores of 0–3 were considered “low-risk,” those with scores 4–5 “at-risk,” and those with scores 6–9 “high-risk” for OSA. (B) OSA safety protocol illustration. Patients are risk-stratified with the DOISNORE50 questionnaire in preoperative clinic. Safety interventions include EMR alert, safety wrist band, empiric autoPAP, sleep consultation, and outpatient follow-up. *EMR alert for OSA status remains present during present and future admission until patient undergoes sleep study outpatient. AutoPAP = automatic titrating positive airway pressure, BMI = body mass index, CPAP = continuous positive airway pressure, EMR = electronic medical record, OSA = obstructive sleep apnea.
Of 2,926 patients with AF, 1,509 underwent preoperative DOISNORE50 questionnaire. Patients who are categorized as known OSA, at-risk for OSA, or high-risk for OSA were added to the existing Atrium Health Wake Forest Sleep Registry. Thereafter, patients were automatically entered into the “OSA safety protocol,” which included 5 essential actions: an EMR OSA risk alert banner, sleep alert wrist band, a clinical decision support pop-up alert (termed best practice advisory [BPA]) recommending sleep consultation, empiric automatic titrating positive airway pressure on high-risk OSA inpatients, and postdischarge sleep referral and polysomnography. Upon entry into a chart, the EMR identifies the patient’s OSA, or risk of OSA, status. On the day of surgery or admission, a sleep alert wristband is placed on the patient to alert various disciplined providers to the patient’s OSA status. A BPA with safety recommendations is sent to admitting providers based on their patient’s OSA status. If a patient has known OSA and is on a continuous positive airway pressure (CPAP) device at home, the BPA suggests use of the home CPAP device. If a patient has known OSA and is not on CPAP, the BPA suggests an inpatient sleep consultation. If the patient is at-risk for OSA, the BPA recommends an inpatient sleep consultation. If the patient is high-risk for OSA or at-risk with documented desaturations < 85% for 90 seconds, the BPA recommends sleep consultation and a trial of automatic titrating positive airway pressure with predefined setting (5–15 cmH2O with ability to adjust). Patients who are at risk or high risk for OSA also received recommendations for outpatient orders for follow-up with a sleep provider and sleep study after discharge. The remaining 1,417 patients were not screened with DOISNORE50 either because they refused, because they were not evaluated in preoperative assessment clinic, or because they had not had a surgical navigator call prior to hospitalization. Electronic collection of patients’ responses to recommendations, including declined treatment, were collected and documented by physicians and respiratory therapists. The AHWFB sleep screening safety protocol is illustrated in Figure 2B.
Confounders
Because adverse outcomes are potentially influenced by a wide variety of factors, we have extracted potential confounders for each patient that included age, sex, race, body mass index, hospital length of stay in days, inpatient CPAP usage, measures of comorbid conditions using the Charlson Comorbidity Index, patient comorbidity of congestive heart failure, heart failure with preserved ejection fraction, chronic obstructive pulmonary disease, hypercapnia, diabetes mellitus, and myocardial infarction.
Outcomes
Our primary outcome was the number of METAs, a composite of 1 or more of 4 component events consisting of RRT activation, code stroke, CB, and reintubation. Secondary outcomes included each of the 4 individual adverse component events. Additional study outcomes include the incidence of intensive care unit admission during the hospital encounter, hospital readmission within 30 days of discharge, and emergency department visits within 30 days of discharge.
The methods for identification of META have been published previously.17 Briefly, billing dates for META in patients who underwent preoperative screening were obtained from the EMR. META was validated by blinded reviewers who searched the corresponding medical record number of each patient and opened the encounter corresponding to the admission associated with the billed META. Confirmation of META was achieved first by finding documentation describing the event leading to META and second by screening the institution’s electronic paging system to ensure that there existed a cataloged page associated with the reported META.
Statistical approach
Statistical analysis was performed in R v3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) using the RStudio environment v1.1.456 (RStudio, Boston, Massachusetts). Continuous covariates were initially evaluated for normalcy using Shapiro–Wilk normality test and the Kolmogorov–Smirnov goodness-of-fit test. Continuous data were compared at the univariate level using an independent, 2-sample, 2-tailed t test if normally distributed and Mann–Whitney U test if nonnormally distributed. Normally distributed data are presented as means with a standard deviation and data that were not normally distributed are presented as medians with interquartile ranges. All categorical covariates were evaluated initially using the chi-squared test to determine their association at a univariate level. We estimated the association of OSA group status with the primary composite outcome and secondary outcomes using logistic regression. We constructed a series of models: (1) We conducted univariate logistic regression with all confounding variables vs primary and secondary outcomes and (2) OSA groups and all confounders associated with outcomes in the univariate analyses were combined to construct an adjusted multivariable logistic regression model. Collinearity was assessed using variance inflation factor and condition index, with values less than 10 indicating acceptability. For each model, we calculated the odds ratio and 95% confidence interval (CI) for each variable including OSA risks group. A 2-tailed P value of < .05 was considered statistically significant.
This study was approved by the Atrium Health Wake Forest Baptist Institutional Review Board and was deemed an exempt investigation, so consent of individual participants was waived. This report was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.26
RESULTS
The demographics and comorbidities of the study cohort of 2,927 patients with underlying AF are depicted in Table 1. The median age was 73 years (interquartile range: 65, 79); 60.7% of patients were male and 84.4% were White; 47.2% carried an underlying diagnosis of congestive heart failure, 19.1% of patients were diagnosed with heart failure with preserved ejection fraction, and 34.4% of screened patients carried a diagnosis of chronic obstructive pulmonary disease. Of our study cohort 51.6% had DOISNORE50 screening, and among them 24.3% scored low-risk for OSA, 29.9% at-risk for OSA, and 14.9% high-risk for OSA.
Table 1.
Patient demographics, CPAP usage, and comorbidities.
| Demographics | Unmatched Cohort | Propensity-Matched Cohort | ||||
|---|---|---|---|---|---|---|
| Unscreened | Screened | SMD | Unscreened | Screened | SMD | |
| (n = 1,417) | (n = 1,509) | (n = 711) | (n = 711) | |||
| Age, median [IQR] | 73.00 [65.00, 74.00] | 73.00 [65.00, 79.00] | 0.014 | 73.00 [65.00, 79.00] | 73.00 [66.00, 78.00] | 0.024 |
| Male, n (%) | 819 (57.8%) | 957 (63.4%) | 0.115 | 412 (57.9%) | 444 (62.4%) | 0.092 |
| BMI, median [IQR] | 28.94 [24.98, 25.33] | 29.20 [25.13, 34.24] | 0.085 | 28.48 [24.31, 34.73] | 29.10 [25.37, 33.42] | 0.015 |
| Race, n (%) | 0.04 | 0.063 | ||||
| Caucasian | 1,202 (84.8%) | 1,267 (84.0%) | 612 (86.1%) | 596 (83.8%) | ||
| African American | 167 (11.8%) | 196 (13.0%) | 80 (11.3%) | 92 (12.9%) | ||
| Hispanic | 35 (2.5%) | 14 (0.9%) | 19 (2.7%) | 23 (3.2%) | ||
| Other | 48 (3.4%) | 46 (3.0%) | 8 (1.1%) | 11 (1.5%) | ||
| CPAP (%) | 63 (4.4%) | 276 (18.3%) | 0.39 | 55 (7.7%) | 72 (10.1%) | 0.084 |
| Sleep consult, n (%) | 11 (0.80%) | 84 (5.5%) | 0.439 | 7 (0.98%) | 55 (7.8%) | 0.044 |
| LOS (median [IQR]) | 7.27 [3.88, 13.89] | 5.74 [3.19, 10.41] | 0.451 | 6.10 [3.17, 10.82] | 5.28 [3.10, 9.30] | 0.029 |
| Comorbidity | ||||||
| CHF, n (%) | 720 (50.8%) | 660 (43.7%) | 0.142 | 179 (25.2%) | 194 (27.3%) | 0.048 |
| HFpEF, n (%) | 47 (3.3%) | 511 (33.9%) | 0.854 | 47 (6.6%) | 59 (8.3%) | 0.064 |
| COPD, n (%) | 339 (23.9%) | 667 (44.2%) | 0.438 | 142 (20.0%) | 152 (21.4%) | 0.035 |
| Hypercapnia, n (%) | 88 (6.2%) | 532 (35.3%) | 0.767 | 63 (8.9%) | 65 (9.1%) | 0.01 |
| DM, n (%) | 438 (30.9%) | 511 (33.9%) | 0.063 | 48 (6.8%) | 59 (8.3%) | 0.059 |
| MI, n (%) | 246 (17.4%) | 636 (42.1%) | 0.563 | 97 (13.6%) | 95 (13.3%) | 0.027 |
| CCI (median [IQR]) | 5.00 [4.00, 6.00] | 5.00 [4.00, 6.00] | 0.447 | 5.00 [3.00, 6.00] | 5.00 [4.00, 6.00] | 0.094 |
| DOISNORE50 group | ||||||
| Low risk, n (%) | 366 (24.3%) | 189 (26.6%) | ||||
| At risk, n (%) | 451 (29.9%) | 225 (31.6%) | ||||
| High risk, n (%) | 255 (16.9%) | 93 (13.1%) | ||||
| Known OSA, n (%) | 467 (30.9%) | 204 (28.7%) | ||||
Various patient demographics and comorbidities stratified into an unscreened group, screened group, and those screening low risk, at risk, and high risk for OSA based on DOISNORE50 questionnaire scores. BMI = body mass index, CCI = Charlson Comorbidity Index, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, CPAP = continuous positive airway pressure, DM = diabetes mellitus, HFpEF = heart failure with preserved ejection fraction, IQR = interquartile range, LOS = length of stay, MI = myocardial infarction, OSA = obstructive sleep apnea, SMD = standardized mean difference.
Among our study cohort, META occurred on 413 occasions, with an event rate of 14.1%. The overall rates of RRT, CB, code stroke, and reintubation in our study cohort were 10.6%, 8.3%, 0.6%, and 2.9%, respectively. Overall, META occurred at a rate of 16.8% in patients not screened with DOISNORE50. The rates of META subtypes stratified by unscreened DOISNORE50 and screened groups, along with prior diagnosis of OSA and DOISNORE50 risk categories, are shown in Figure S1 (242KB, pdf) in the supplemental material.
Using propensity-score matching, we were able to match 711 (51%) cases from the unscreened group with an equivalent number of controls from the screened group. All patient characteristics between the screened group and unscreened group were balanced after propensity-score matching with absolute standardized differences for all covariates between the 2 groups, propensity matching being < 0.1 (Table 1).
Figure 3 depicts the adjusted odds of META subtypes along with intensive care unit admissions, 30-day hospital readmissions, and emergency department visits within 30 days of hospital discharge between unscreened and DOISNORE50-screened groups for both the propensity-score-matched and unmatched cohort. The propensity-matched cohort of patients screened with DOISNORE50 demonstrated significantly reduced odds of META 0.69 (95% CI: 0.48–0.98, P < .001) in comparison to unscreened groups after adjustment for all covariates. Apart from code stroke and 30-day readmission, all other secondary outcomes for screened groups were statistically significantly lower in the screened group compared with the unscreened cohort (Figure 3).
Figure 3. Adjusted odds of study outcomes for unscreened groups against DOISNORE50-screened groups.
CI = confidence interval, ED = emergency department, ICU = intensive care unit, META = medical emergency team activation, OR = odds ratio.
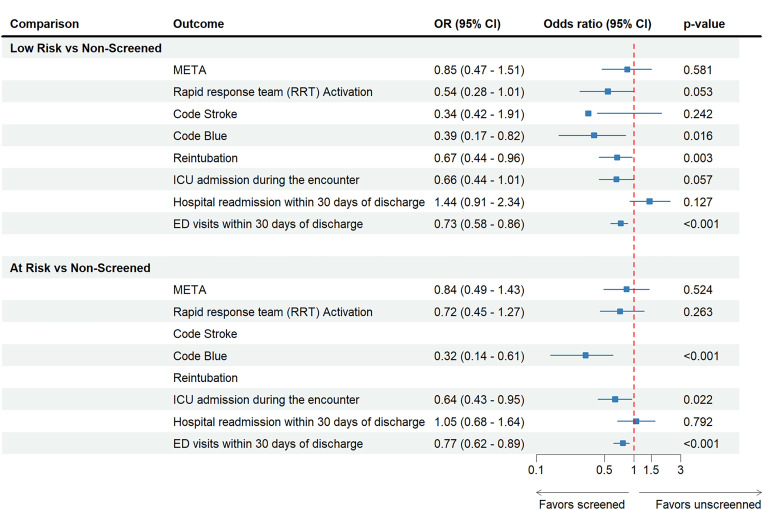
Figure 4 shows decreased odds of META in the screened group compared with the unscreened group after adjustment for all other covariates for the propensity-matched cohort. Adjusted odds of intensive care unit admission were lower among the high-risk group (0.48; 95% CI: 0.27–0.84, P < .001) and among the known-OSA group (0.68; 95% CI: 0.53–0.86, P < .001), in comparison to unscreened patients. This association of 30-day hospital readmissions is less clear in the low-risk patients (0.66; 95% CI: 0.44–1.01, P = .057) compared with unscreened patients.
Figure 4. Adjusted odds of study outcomes for unscreened groups against DOISNORE50-screened groups among a propensity-matched cohort.
CI = confidence interval, ED = emergency department, ICU = intensive care unit, META = medical emergency team activation, OR = odds ratio.
DISCUSSION
With rising rates of OSA, there is a need for quick and effective screening tools that patients can self-administer. This need is particularly apparent in the perioperative setting where OSA is associated with complications often leading to META.9,17,18 We demonstrated that the DOISNORE50 screening tool that contains AF as a question, when completed by patients with underlying AF, contains predictive operational characteristics necessary to identify patients with sleep-disordered breathing.27,28
In a population of patients with AF, interventions with an OSA safety protocol guided by the results of DOISNORE50 screening were associated with improved outcomes among perioperative patients. Consistent with prior literature, we demonstrated that patients with AF and a preexisting diagnosis of OSA have an increased risk of META.12,29 They were more likely to have RRT, CB, reintubation, and total META in comparison to all perioperatively screened patients. Within OSA risk categories, those patients who scored low-risk for OSA (< 4 positive responses) by DOISNORE50 had the lowest rates of RRT and CB, as well as the lowest rates of total META. Furthermore, those patients who scored high-risk for OSA (≥ 6 positive responses) by DOISNORE50 displayed significantly higher rates of RRT, CB, and total META, suggesting that OSA risk by DOISNORE50 may play a role in the prediction of META. In our previous publication, DOISNORE50 was predictive of META across at-risk and high-risk groups. However, among this cohort of patients with AF, the questionnaire performed best in identifying patients at risk for META among patients at high risk for OSA. Further, the impact of the AHWFBMC OSA screening using DOISNORE50 and the subsequent OSA safety protocol reduced META among AF patients (Table 2).
Table 2.
Adjusted odds ratio of META between unscreened groups against DOISNORE50 groups among propensity-score-matched cohort.
| Outcome | Low Risk (Ref: Unscreened) | At Risk (Ref: Uncreened) | High Risk (Ref: Unscreened) | Known OSA (Ref: Unscreened) |
|---|---|---|---|---|
| Medical emergency team activation, OR (95% CI) | 0.85 (0.47–1.51) | 0.84 (0.49–1.43) | 0.51 (0.23–0.97) | 0.42 (0.24–0.65) |
| P = .581 | P = .524 | P = .046* | P < .001* | |
| Rapid response team activation | 0.54 (0.28–1.01) | 0.72 (0.45–1.27) | 0.43 (0.19–0.93) | 0.31 (0.25–0.57) |
| P = .053 | P = .263 | P = .039* | P < .001* | |
| Code stroke | 0.34 (0.42–1.91) | — | — | 0.19 (0.07–2.16) |
| P = .242 | P = .219 | |||
| Code blue | 0.39 (0.17–0.82) | 0.32 (0.14–0.61) | 0.26 (0.09–0.67) | 0.24 (0.15–0.32) |
| P = .016* | P < .001* | P < .001* | P < .001* | |
| Reintubation | 0.67 (0.44–0.96) | — | 0.68 (0.46–0.92) | 0.59 (0.18–0.71) |
| P = .003* | P = .028* | P < .001* | ||
| ICU admission during the encounter, OR (95% CI) | 0.66 (0.44–1.01) | 0.64 (0.43–0.95) | 0.48 (0.27–0.84) | 0.68 (0.53–0.86) |
| P = .057 | P = .022* | P < .001* | P < .001* | |
| Hospital readmission within 30 days of discharge, OR (95% CI) | 1.44 (0.91–2.34) | 1.05 (0.68–1.64) | 0.83 (0.72–0.86) | 0.57 (0.24–1.13) |
| P = .127 | P = .792 | P = .028 * | P = .256 | |
| ED visits within 30 days of discharge, OR (95% CI) | 0.73 (0.58–0.86) | 0.77 (0.62–0.89) | 0.55 (0.42–0.96) | 0.75 (0.61–0.84) |
| P < .001* | P < .001* | P = .026* | P < .001* |
META includes rapid response team activation, code stroke, code blue, and reintubation between patients screened low risk, at risk, high risk for OSA, and with previous OSA diagnosis among a propensity-score-matched cohort. Patients not screened with DOISNORE50 questionnaire are used as references (Ref). *Statistically significant. CI = confidence interval, ED = emergency department, ICU = intensive care unit, META = medical emergency team activation, OR = odds ratio, OSA = obstructive sleep apnea.
This validated, patient-completed sleep questionnaire (DOISNORE50) in conjunction with the AHWFBMC OSA safety protocol incorporated EMR-initiated safety measures and reminders that led to fewer major hospital events. Although other questionnaires and protocols have been able to identify OSA risk among AF patients (and in some cases requiring modification to improve diagnostic performance), reporting on favorable outcomes such as reduced META is lacking.30,31 Other studies have demonstrated fewer events in hospitalized patients during a retrospective records analysis among patients who received in-hospital sleep consultations using the STOP questionnaire.30 Our prospective patient cohort who were perioperatively screened were provided with a postoperative inpatient sleep safety protocol in which a retrospective analysis identified reduced META among AF patients. Sleep consultation, although recommended in the sleep safety pathway (Figure 2B), was not always requested by the admissions provider, thus suggesting a possible benefit of knowing a patient’s OSA status by multiple modalities (EMR and “wrist bands”). Among the unmatched cohort, 11 patients had inpatient sleep consultation, compared with 84 of the screened patients (Table 1). Further study is needed to identify those actions (passive or active) that have the greatest influence on the patient and/or provider interface and influence a favorable outcome of reduced META. Additional research is also needed to assess the effect of positive airway pressure device usage as a mitigating factor for META.
The limitations of this study include its retrospective design, therefore limiting the ability to draw cause-and-effect inferences from these results. The results represent those of a single regional academic health system. This patient population had a median age of 73 years, 63.7% of patients were male, and 84% were White. Although age and sex may be representative of the regional population usually diagnosed with OSA, the percentage of non-Whites appears below expectations and may reflect bias. Lack of polysomnography results to confirm a prior OSA diagnosis is also a drawback. This study also has several strengths, including a large sample and detailed data collection strategies involving low-incident events associated with significant clinical impact (META). This study has shown that a patient-administered and validated sleep questionnaire, along with sleep safety interventions that incorporate EMR alerts, wrist bands, automatic titrating positive airway pressure, and inpatient sleep consultation is associated with fewer METAs among AF patients. We found the lowest-risk group had the fewest number of events, supporting that the DOISNORE50 questionnaire appropriately stratified patient risk status. This study also shows the association between known-OSA or high-risk OSA screening with META rates in patients with AF. These findings support the implementation of screening questionnaires and OSA safety protocols that can be generalized to other at-risk populations.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AF
atrial fibrillation
- AHWFBMC
Atrium Health Wake Forest Baptist Medical Center
- BPA
best practice advisory
- CB
code blue
- CI
confidence interval
- CPAP
continuous positive airway pressure
- EMR
electronic medical record
- META
medical emergency team activation
- OSA
obstructive sleep apnea
- RRT
rapid response team
REFERENCES
- 1. Heinzer R, Vat S, Marques-Vidal P, et al . Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study . Lancet Respir Med. 2015. ; 3 ( 4 ): 310 – 318 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veasey SC, Rosen IM . Obstructive sleep apnea in adults . N Engl J Med. 2019. ; 380 ( 15 ): 1442 – 1449 . [DOI] [PubMed] [Google Scholar]
- 3. Fleming WE, Ferouz-Colborn A, Samoszuk MK, et al . Blood biomarkers of endocrine, immune, inflammatory, and metabolic systems in obstructive sleep apnea . Clin Biochem. 2016. ; 49 ( 12 ): 854 – 861 . [DOI] [PubMed] [Google Scholar]
- 4. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J . Longitudinal study of moderate weight change and sleep-disordered breathing . JAMA. 2000. ; 284 ( 23 ): 3015 – 3021 . [DOI] [PubMed] [Google Scholar]
- 5. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V . Obstructive sleep apnea as a risk factor for stroke and death . N Engl J Med. 2005. ; 353 ( 19 ): 2034 – 2041 . [DOI] [PubMed] [Google Scholar]
- 6. Ancoli-Israel S, Palmer BW, Cooke JR, et al . Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study . J Am Geriatr Soc. 2008. ; 56 ( 11 ): 2076 – 2081 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vgontzas AN, Li Y, He F, et al . Mild-to-moderate sleep apnea is associated with incident hypertension: age effect . Sleep. 2019. ; 42 ( 4 ): zsy265 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M . Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep . Hypertension . 2007. ; 49 ( 6 ): 1235 – 1241 . [DOI] [PubMed] [Google Scholar]
- 9. Chan MTV, Wang CY, Seet E, et al. Postoperative Vascular Complications in Unrecognized Obstructive Sleep Apnea (POSA) Study Investigators . Association of unrecognized obstructive sleep apnea with postoperative cardiovascular events in patients undergoing major noncardiac surgery . JAMA. 2019. ; 321 ( 18 ): 1788 – 1798 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong JK, Mariano ER, Doufas AG, Olejniczak MJ, Kushida CA . Preoperative treatment of obstructive sleep apnea with positive airway pressure is associated with decreased incidence of atrial fibrillation after cardiac surgery . J Cardiothorac Vasc Anesth. 2017. ; 31 ( 4 ): 1250 – 1256 . [DOI] [PubMed] [Google Scholar]
- 11. Zimetbaum P . Atrial fibrillation . Ann Intern Med. 2017. ; 166 ( 5 ): ITC33 – ITC48 . [DOI] [PubMed] [Google Scholar]
- 12. Namen AM, Forest D, Saha AK, et al . DOISNORE50: a perioperative sleep questionnaire predictive of obstructive sleep apnea and postoperative medical emergency team activation. A learning health system approach to sleep questionnaire development and screening . J Clin Sleep Med. 2022. ; 18 ( 8 ): 1909 – 1919 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herod R, Frost SA, Parr M, Hillman K, Aneman A . Long term trends in medical emergency team activations and outcomes . Resuscitation. 2014. ; 85 ( 8 ): 1083 – 1087 . [DOI] [PubMed] [Google Scholar]
- 14. Kurita T, Nakada TA, Kawaguchi R, Shinozaki K, Abe R, Oda S . Timing and location of medical emergency team activation is associated with seriousness of outcome: an observational study in a tertiary care hospital . PLoS One. 2016. ; 11 ( 12 ): e0168729 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernando SM, Reardon PM, Scales DC, et al . Prevalence, risk factors, and clinical consequences of recurrent activation of a rapid response team: a multicenter observational study . J Intensive Care Med. 2019. ; 34 ( 10 ): 782 – 789 . [DOI] [PubMed] [Google Scholar]
- 16. Tran A, Fernando SM, McIsaac DI, et al . Predictors of mortality and cost among surgical patients requiring rapid response team activation . Can J Surg. 2020. ; 63 ( 6 ): E598 – E605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang K, Spilman SK, Hahn KD, McCann DA, Purtle MW . Prevalence of risk for sleep apnea among hospitalized patients who survived a medical emergency team activation . J Clin Sleep Med. 2020. ; 16 ( 1 ): 91 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Namen AM, Forest DJ, Ahmad ZN, et al . Preoperative sleep questionnaires identify medical emergency team activation in older adults . J Am Med Dir Assoc. 2019. ; 20 ( 10 ): 1340 – 1343.e2 . [DOI] [PubMed] [Google Scholar]
- 19. Thein PM, Ong J, Crozier TM, et al . Predictors of acute hospital mortality and length of stay in patients with new-onset atrial fibrillation: a first-hand experience from a medical emergency team response provider . Intern Med J. 2019. ; 49 ( 8 ): 969 – 977 . [DOI] [PubMed] [Google Scholar]
- 20. Schneider A, Calzavacca P, Jones D, Bellomo R . Epidemiology and patient outcome after medical emergency team calls triggered by atrial fibrillation . Resuscitation. 2011. ; 82 ( 4 ): 410 – 414 . [DOI] [PubMed] [Google Scholar]
- 21. Delesie M, Knaepen L, Hendrickx B, et al . The value of screening questionnaires/scoring scales for obstructive sleep apnoea in patients with atrial fibrillation . Arch Cardiovasc Dis. 2021. ; 114 ( 11 ): 737 – 747 . [DOI] [PubMed] [Google Scholar]
- 22. Verbraecken J, Hedner J, Penzel T . Pre-operative screening for obstructive sleep apnoea . Eur Respir Rev. 2017. ; 26 ( 143 ): 160012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linz D, McEvoy RD, Cowie MR, et al . Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review . JAMA Cardiol. 2018. ; 3 ( 6 ): 532 – 540 . [DOI] [PubMed] [Google Scholar]
- 24. Namen AM, Forest D, Saha AK, et al . Reduction in medical emergency team activation among postoperative surgical patients at risk for undiagnosed obstructive sleep apnea . J Clin Sleep Med. 2022. ; 18 ( 8 ): 1953 – 1965 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kapur VK, Auckley DH, Chowdhuri S, et al . Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline . J Clin Sleep Med. 2017. ; 13 ( 3 ): 479 – 504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies . J Clin Epidemiol. 2008. ; 61 ( 4 ): 344 – 349 . [DOI] [PubMed] [Google Scholar]
- 27. Chung F, Yegneswaran B, Liao P, et al . STOP questionnaire: a tool to screen patients for obstructive sleep apnea . Anesthesiology. 2008. ; 108 ( 5 ): 812 – 821 . [DOI] [PubMed] [Google Scholar]
- 28. Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y . High STOP-Bang score indicates a high probability of obstructive sleep apnoea . Br J Anaesth. 2012. ; 108 ( 5 ): 768 – 775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma S, Chowdhury A, Tang L, Willes L, Glynn B, Quan SF . Hospitalized patients at high risk for obstructive sleep apnea have more rapid response system events and intervention is associated with reduced events . PLoS One. 2016. ; 11 ( 5 ): e0153790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jensen MH, Dalgaard F, Laub RR, et al . Protocol for detecting unrecognized sleep apnea in patients with atrial fibrillation by a home-monitoring device: the DAN-APNO study . BMC Cardiovasc Disord. 2022. ; 22 ( 1 ): 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Betz K, Verhaert DVM, Gawalko M, et al . Atrial fibrillation-specific refinement of the STOP-Bang sleep apnoea screening questionnaire: insights from the Virtual-SAFARI study . Clin Res Cardiol. 2023. ; 112 ( 6 ): 834 – 845 . [DOI] [PMC free article] [PubMed] [Google Scholar]