Abstract
Teriparatide has been effective in treating people diagnosed with medication-related osteonecrosis of the jaw (MRONJ). However, its efficacy is not well established to be accepted as a standard of care. The objective of this paper was to investigate the efficacy of recombinant human parathyroid hormone for the treatment of MRONJ. We report three cases of MRONJ patients with osteoporosis as the primary disease who were treated with a teriparatide agent along with other adjunctive measures. Each patient was administered a teriparatide injection subcutaneously for 16 weeks, 36 weeks, or 60 weeks. Surgical intervention including partial resection, sequestrectomy, decortication, and saucerization took place during the teriparatide administration. Complete lesion resolution was identified clinically and radiographically in all three patients. In patients diagnosed with MRONJ, teriparatide therapy is an efficacious and safe therapeutic option to improve healing of bone lesions. These findings demonstrate that teriparatide in combination with another therapy, especially bone morphogenetic protein, platelet-rich fibrin, or antibiotic therapy, can be an effective protocol for MRONJ.
Keywords: Teriparatide, Human parathyroid hormone (1-34), Forteo, Bisphosphonate-associated osteonecrosis of the jaw, Osteoporosis
I. Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is an infrequent but morbid and potentially debilitating condition associated with antiresorptive and antiangiogenic therapies.
Although MRONJ can be prevented with optimal oral health, management of an established lesion is challenging. In aggressive cases, MRONJ does not always respond to routine treatments and may persist or progress to an advanced stage, hindering treatment.
Interventions used to treat MRONJ are diverse, controversial, and largely empirical. One adjunctive management modality is recombinant human parathyroid hormone (1-34) [rhPTH(1-34), teriparatide], which has shown promise as an adjunct for treating MRONJ in osteoporotic patients1-3. However, the efficacy of this treatment is not verified for acceptance as a standard of care for MRONJ patients. Teriparatide is a molecule that comprises the first 34 amino acids of intact parathyroid hormone4. It is an anabolic bone hormone and has been shown to be effective in treating postmenopausal osteoporosis5,6. Teriparatide is able to reverse the anti-resorptive effects of bisphosphonates by promoting osteoblast activity and enhancing the metabolic function of osteoclasts.
Harper and Fung7 reported the first case in which teriparatide was successfully used to treat MRONJ. Subsequently, there have been multiple studies of teriparatide for treating MRONJ, and the International Task Force on Osteonecrosis of the Jaw currently considers teriparatide an option for treating MRONJ in osteoporotic patients. In this case report, we describe three cases that were treated successfully with teriparatide accompanied with surgical intervention2.
The study was approved by the Institutional Review Board (IRB) of at Ewha Womans University Mokdong Hospital (IRB No. EUMC 2018-01-046-028). The written informed consent was waived due to the retrospective nature of the study.
II. Cases Report
1. Case 1
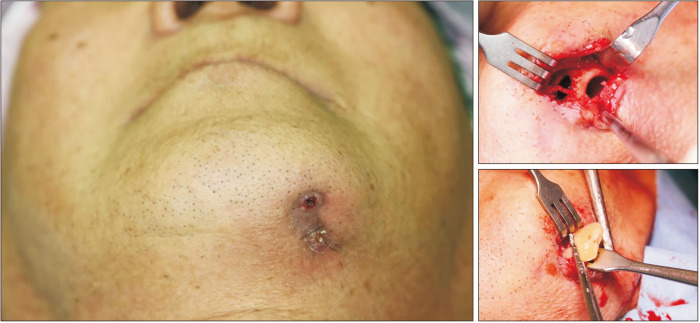
A 68-year-old male with a two-year history of alendronate therapy from 2016 through 2018 due to osteoporosis was referred for treatment for intra-oral pus discharge and an extra-oral fistula with purulent discharge on the left mandibular area.(Fig. 1, left) The patient had undergone decortication and saucerization twice and received antibiotics therapy for the lesion at a local hospital before visiting our clinic with an unhealed lesion. The initial bone scan revealed an active bone lesion in the mandible, associated with inflammatory change. He was diagnosed with stage-3 MRONJ according to the American Association of Oral and Maxillofacial Surgeons guidelines (2022). Considering the refractory lesion, experimental teriparatide therapy was recommended. Conservative treatment including oral hygiene instructions, antibiotics, antimicrobial mouth gargles, and local irrigation was enforced concurrently. An early-morning fasting blood sample was collected for analysis of bone turnover markers: carboxy-terminal type-1 collagen crosslinks (CTXs; 0.113 ng/mL), Pyrilinks-D (3.5), C-terminal cross-linking telopeptide (CTX; 0.113 ng/mL), osteocalcin (10.40 nL/mL), bone specific alkaline phosphatase (ALP) (8.7 μg/L), serum parathyroid hormone (50.60 pg/mL), and total calcium (8.7 mg/dL) were within normal limits during this time period. Teriparatide therapy by subcutaneous injection (Teribone, 56.5 μg, weekly) was continued from the initial visit for 12 weeks and then replaced with daily subcutaneous injection (Forsteo, 20 μg, daily) for 24 weeks. During this time, the patient did not complain of any side effects associated with the injections. Extraoral pus discharge was observed beyond the 14th injection, which led to surgical intervention at the 17th week. Sequestrum on the left mandibular margin was removed with an extra-oral approach under local sedation.(Fig. 1, right) Bone morphogenetic protein and platelet-rich fibrin were inserted to promote bone healing on the operation site. The culture report was positive for Staphylococcus hominis.
Fig. 1.
Clinical photo of an extra-oral fistula with purulent discharge in the left mandibular area on initial visit (left). Clinical intraoperative photos of a left mandibular cortical defect (upper right) and removal of sequestrum (lower right). The sequestrum was isolated from the mandible.
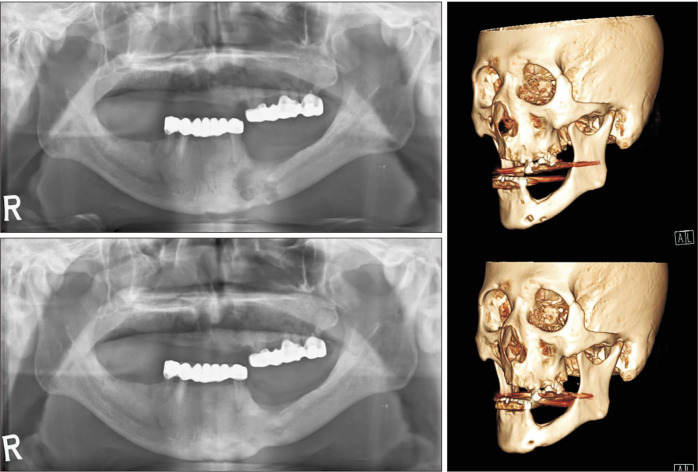
Three weeks after sequestrectomy, epithelial closure of the MRONJ lesion was confirmed clinically. The necrotic bone had been replaced with healthy bone, and there was complete coverage with normal epithelium and no sign of inflammation. Panoramic radiography and postoperative follow-up computed tomography (CT) reconstructed in a three-dimensional image showed resolution of the osteolytic lesion extending to the inferior border of the mandible.(Fig. 2)
Fig. 2.
Preoperative panoramic view (upper left) shows invasion of the inferior border of the mandible. Postoperative panoramic view (lower left) at seven months shows recovery at the inferior border of the mandible. Reconstructed views of the preoperative (upper right) and postoperative (lower right) lesion.
2. Case 2
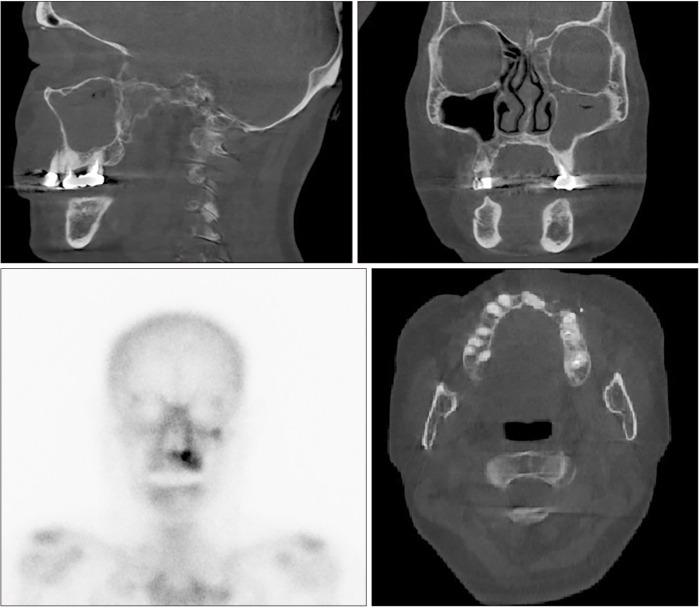
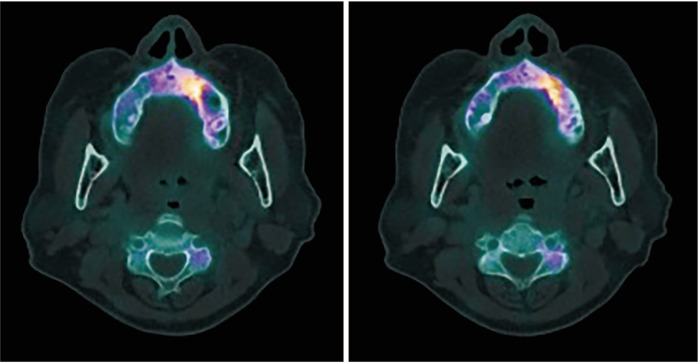
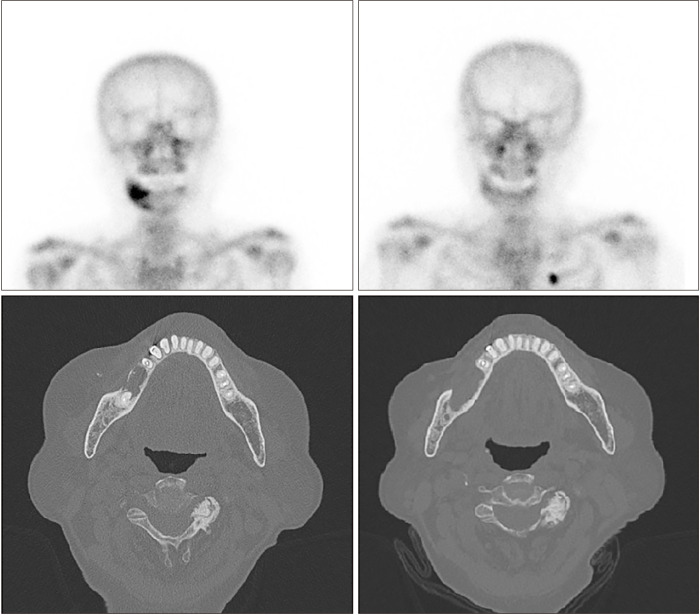
A 73-year-old female with a 10-year history of antiresorptive therapy (oral alendronate for six years, oral risedronate for three years, denosumab subcutaneous injection for one year, from October 2016 through February 2021) due to osteoporosis visited us complaining of swelling in the left maxillary area. At her initial visit, the panoramic view showed diffuse sclerosis of the left maxillary alveolar bone, suggestive of chronic periodontitis, which might have prompted MRONJ onset. Additionally, CT revealed a localized bone lesion with thin peripheral radiolucency at the left maxilla paracentral alveolar bone sequestrum and oro-antral communication that resulted in sinusitis in the left sinus area, which led to a diagnosis of stage 3 MRONJ.(Fig. 3) Initial bone scintigraphy showed increased uptake on the left maxillary alveolar process along with mild uptake on the left zygomatic arch.(Fig. 3, lower left) The patient was admitted for MRONJ treatment on the left maxillary area and underwent conservative treatment including oral hygiene instructions, antibiotics, antimicrobial mouth gargles, and local irrigation. Surgical intervention was performed after admission; the left maxillary posterior area was partially resected for complete removal of necrotic bone, and the left maxillary premolars and second molar were extracted. Collagen sponge sections with recombinant human bone morphogenetic protein-2 (rhBMP-2) were placed in direct contact with the bone surface, followed by leucocyte- and platelet-rich fibrin (L-PRF) application on the extraction site. Functional endoscopic sinus surgery on the left sinus was performed. Since the initial visit, teriparatide therapy with subcutaneous injection (Teribone, 56.5 μg, weekly) was continued for 18 weeks and then replaced with daily subcutaneous injections (Forsteo, 20 μg, daily) for 32 weeks. The patient’s bone turnover markers including total procollagen type-1 N-terminal propeptide (P1NP, 21.37 nM BCE/mM Cr), Pyrilinks-D (5.8), serum calcitonin (3.48 pg/mL), C-terminal cross-linking telopeptide (CTX, 0.162 ng/mL), osteocalcin (11.90 nL/mL), bone specific ALP (10.3 μg/L), serum parathyroid hormone (46.90 pg/mL), and total calcium (8.6 mg/dL) stayed within normal limits during this period, and the patient did not complain of any side effects from the injections. The patient presented with full mucosal coverage of the lesion two weeks after her postoperative visit. The patient was followed with bone single-photon emission computed tomography (SPECT)/CT at four months and eight months postoperatively, which showed marked decrease in uptake intensity.(Fig. 4) At the final follow-up, the lesion was completely covered with healthy mucosa without any signs of inflammation.
Fig. 3.
Sagittal view of the left maxillary sinusitis area (upper right) shows oro-antral communication (stage 3). Coronal view of the left maxillary sinusitis area (upper left) with trabecular sclerosis. Axial view of cortical erosion and trabecular sclerosis in the left maxillary anterior area (lower right). Initial bone scintigraphy shows increased uptake in the left maxillary alveolar process along with mild uptake in the left zygomatic arch (lower left).
Fig. 4.
Patient follow up with bone SPECT/CT (single-photon emission computed tomography/computed tomography) at four (right) and eight months postoperatively (left), showing marked decrease in uptake intensity.
3. Case 3
An 87-year-old female with a four-year history of antiresorptive therapy (oral ibandronate) due to osteoporosis was referred for bone exposure with necrotic change on the right mandibular posterior area one week after extraction of the right mandibular first molar at a local dental clinic. The clinical findings revealed swelling and pus discharge in the extraction socket of the right mandible. CT showed cortical erosion and trabecular sclerosis at the extraction site. The patient also had comorbid uncontrolled diabetes mellitus (HbA1c at 10.3%). The patient was diagnosed with stage 2 MRONJ in the right mandibular area. Surgical resection of the lesion was performed, and the operation site had completely healed at her postoperative three-week check-up. Teriparatide therapy was recommended at this point to prevent MRONJ on the other site and to treat osteoporosis, but the patient refused. Bone scintigraphy was followed up at two-months and eight-months postoperatively and confirmed improvement in osteonecrosis of the right mandible.(Fig. 5, upper) Two years after resolution of the lesion, the patient re-visited, complaining of pain in her right mandibular premolar area. CT showed cortical erosion extending to the mesial surface of the right first premolar area.(Fig. 5, lower) Surgical resection and decortication of the lesion were performed, and the right mandibular premolars were extracted. rhBMP-2 within a collagen plug was applied to the extraction site. Daily subcutaneous teriparatide injection (Forsteo, 20 μg, daily) was started and continued for 16 weeks. The patient did not report any side effects associated with teriparatide injections. Bone turnover markers including serum parathyroid hormone (19.30 pg/mL), osteocalcin (1.34 ng/mL), C-terminal cross-linking telopeptide (CTX, 0.075 ng/mL), and bone specific ALP (10.1 μg/L) remained within normal limits. Postoperative bone SPECT/CT at three months showed a decrease of uptake at the preexisting inflammatory necrotic lesion. Full mucosal coverage was achieved, and there were no signs or symptoms of the previous lesion at the final follow-up.
Fig. 5.
Bone scintigraphy at two (upper right) and eight (upper left) months postoperatively. Improved osteonecrosis on the right mandible was confirmed. Computed tomography (CT) showed cortical erosion extending to the mesial surface of the right first premolar area (lower right). Postoperative two-month CT shows normal trabecular bone healing on the debrided area (lower left).
III. Discussion
Since the first case of successful teriparatide therapy for MRONJ8, it has been widely used as an effective therapeutic modality for MRONJ in combination with another therapy9-12.
Some patients refuse teriparatide therapy, mainly for economic reasons, the inconvenience of low-dose daily self-injections, or the time and effort needed for weekly visits to the clinic for high-dose injections. Although no significant side effects were observed in this case series, adverse side effects of teriparatide injections are often encountered with varying degrees of symptoms. The U.S. Food and Drug Administration (FDA) has reported multiple adverse side-effects in 8.5% of patients including arthralgia, malaise, nausea, vomiting, renal impairment, and psychological problems13. Additionally, although teriparatide is an effective treatment for osteoporosis14, there are potential challenges to its use in patients with malignant bone disease, patients at risk of malignant bone tumors such as Paget’s disease, pediatric and young adult patients with open epiphyses, and patients with prior external beam or implant radiation of the skeleton due to osteosarcoma risk15. Initially, the FDA did not recommend teriparatide for longer than 24 months for these reasons. However, in 2020, the revised teriparatide label now states that use “for more than two years during a patient’s lifetime should only be considered if a patient remains at or has returned to having a high risk for fracture.” Further studies showed no increase in osteosarcoma in patients using teriparatide compared with unexposed groups nor did they find an increased risk compared with the expected population-based background disease incidence16.
In this case report, the longest period of teriparatide administration was approximately 12.5 months. Repeated teriparatide injection had a sequestration effect, clarifying the border between normal and necrotic bone, facilitating complete surgical removal of necrotic bone. Hence, the duration of therapeutic administration was individually determined to generate the optimal state for surgical removal of the bone, which was based on follow up imaging studies such as cone-beam CT and bone SPECT.
Since long-term use of teriparatide has not been well investigated, it should be used with close surveillance of periodic bone marker follow-ups and clinical signs and symptoms. Reassuringly, no causal relationship between teriparatide and osteosarcoma has been demonstrated in humans in post-marketing surveillance. Sim et al.1 presented a placebo-controlled randomized trial in which teriparatide was used effectively and safely in patients with bone metastasis and osteoporosis.
There were significant variations in defining treatment outcomes and measuring treatment response. The follow-up periods in this case report were 18 months, 16 months, and 34 months, and all patients were followed until complete resolution and absence of recurrence. The overall quality of evidence is weak, with high potential for bias, making it difficult to determine the efficacy of teriparatide and its long-term effects. However, teriparatide may play a role in treating intractable MRONJ in osteoporotic patients or those unfit for surgery. Therefore, randomized clinical trials with larger patient cohorts and long-term follow-up are required to confirm efficacy and safety and to inform treatment indications for teriparatide for MRONJ. Additionally, whether comorbid diseases such as cancer without bone metastasis are absolute contraindications to teriparatide use in treating MRONJ must also be investigated in future studies.
In this case series, all three patients underwent surgical treatment, from minimal debridement to partial resection, concurrent with teriparatide therapy. Previous studies found that patients who underwent treatment with teriparatide in association with another therapeutic modality were more likely to experience total resolution of osteonecrosis than those who received only teriparatide treatment12. Surgical management has been reported to have superior results, with success rates greater than 80%17,18. However, surgical protocols remain controversial, and surgical interventions with a high degree of invasiveness may be difficult, particularly in elderly patients. Generally, surgical intervention is indicated when necrotic bone is persistent even after conservative measures. Unless a patient’s general health is not a contraindication to surgery, lesions unresponsive to conservative treatment are subject to surgical removal.
Once the surgical approach was decided, both BMP-2 and L-PRF were administered to the first two patients, while BMP-2 alone was administered to the third patient. The mechanism of the effects of these agents on MRONJ resolution was not evaluated.
Teriparatide treatment was associated with a favorable resolution of established MRONJ lesions in this case series. However, further studies are required for teriparatide therapy to be validated as an evidence-based modality with an established regimen for treating MRONJ patients.
Funding Statement
Funding This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI22C1377).
Footnotes
Authors’ Contributions
S.Y.C. participated in data collection and wrote the manuscript. K.M.K., D.Y., and H.Y.K. participated in the study design and data collection. J.H.P., S.J.K., and J.W.K. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Sim IW, Borromeo GL, Tsao C, Hardiman R, Hofman MS, Papatziamos Hjelle C, et al. Teriparatide promotes bone healing in medication-related osteonecrosis of the jaw: a placebo-controlled, randomized trial. J Clin Oncol. 2020;38:2971–80. doi: 10.1200/JCO.19.02192. https://doi.org/10.1200/jco.19.02192. [DOI] [PubMed] [Google Scholar]
- 2.Khan AA, Morrison A, Kendler DL, Rizzoli R, Hanley DA, Felsenberg D, et al. ; International Task Force on Osteonecrosis of the Jaw. Case-based review of osteonecrosis of the jaw (ONJ) and application of the international recommendations for management from the International Task Force on ONJ. J Clin Densitom. 2017;20:8–24. doi: 10.1016/j.jocd.2016.09.005. https://doi.org/10.1016/j.jocd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Rubin MR, Bilezikian JP. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med. 2003;19:415–32. doi: 10.1016/S0749-0690(02)00074-5. https://doi.org/10.1016/s0749-0690(02)00074-5. [DOI] [PubMed] [Google Scholar]
- 4.Eastell R, Walsh JS. Anabolic treatment for osteoporosis: teriparatide. Clin Cases Miner Bone Metab. 2017;14:173–8. doi: 10.11138/ccmbm/2017.14.1.173. https://doi.org/10.11138/ccmbm/2017.14.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. https://doi.org/10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- 6.Frost ML, Siddique M, Blake GM, Moore AE, Schleyer PJ, Dunn JT, et al. Differential effects of teriparatide on regional bone formation using (18)F-fluoride positron emission tomography. J Bone Miner Res. 2011;26:1002–11. doi: 10.1002/jbmr.305. https://doi.org/10.1002/jbmr.305. [DOI] [PubMed] [Google Scholar]
- 7.Harper RP, Fung E. Resolution of bisphosphonate-associated osteonecrosis of the mandible: possible application for intermittent low-dose parathyroid hormone [rhPTH(1-34)] J Oral Maxillofac Surg. 2007;65:573–80. doi: 10.1016/j.joms.2006.10.076. https://doi.org/10.1016/j.joms.2006.10.076. [DOI] [PubMed] [Google Scholar]
- 8.Narongroeknawin P, Danila MI, Humphreys LG, Jr, Barasch A, Curtis JR. Bisphosphonate-associated osteonecrosis of the jaw, with healing after teriparatide: a review of the literature and a case report. Spec Care Dentist. 2010;30:77–82. doi: 10.1111/j.1754-4505.2009.00128.x. https://doi.org/10.1111/j.1754-4505.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwamoto J, Yago K, Sato Y, Matsumoto H. Teriparatide therapy for bisphosphonate-associated osteonecrosis of the jaw in an elderly Japanese woman with severe osteoporosis. Clin Drug Investig. 2012;32:547–53. doi: 10.1007/BF03261908. https://doi.org/10.1007/bf03261908. [DOI] [PubMed] [Google Scholar]
- 10.Kim KM, Park W, Oh SY, Kim HJ, Nam W, Lim SK, et al. Distinctive role of 6-month teriparatide treatment on intractable bisphosphonate-related osteonecrosis of the jaw. Osteoporos Int. 2014;25:1625–32. doi: 10.1007/s00198-014-2622-8. https://doi.org/10.1007/s00198-014-2622-8. [DOI] [PubMed] [Google Scholar]
- 11.Kakehashi H, Ando T, Minamizato T, Nakatani Y, Kawasaki T, Ikeda H, et al. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: preliminary findings. Int J Oral Maxillofac Surg. 2015;44:1558–64. doi: 10.1016/j.ijom.2015.07.018. https://doi.org/10.1016/j.ijom.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Dos Santos Ferreira L, Abreu LG, Calderipe CB, Martins MD, Schuch LF, Vasconcelos ACU. Is teriparatide therapy effective for medication-related osteonecrosis of the jaw? A systematic review and meta-analysis. Osteoporos Int. 2021;32:2449–59. doi: 10.1007/s00198-021-06078-z. https://doi.org/10.1007/s00198-021-06078-z. [DOI] [PubMed] [Google Scholar]
- 13.Eli Lilly and Company, author. FORTEO (teriparatide [rDNA origin] injection) for subcutaneous use [Internet] U.S. Food and Drug Administration; Silver Spring (MD): 2024. [cited 2024 Feb 7]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021318s012lbl.pdf . [Google Scholar]
- 14.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. https://doi.org/10.1056/nejm200105103441904. [DOI] [PubMed] [Google Scholar]
- 15.Morishita K, Yamada SI, Kawakita A, Hashidume M, Tachibana A, Takeuchi N, et al. Treatment outcomes of adjunctive teriparatide therapy for medication-related osteonecrosis of the jaw (MRONJ): a multicenter retrospective analysis in Japan. J Orthop Sci. 2020;25:1079–83. doi: 10.1016/j.jos.2020.01.012. https://doi.org/10.1016/j.jos.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Krege JH, Gilsenan AW, Komacko JL, Kellier-Steele N. Teriparatide and osteosarcoma risk: history, science, elimination of boxed warning, and other label updates. JBMR Plus. 2022;6:e10665. doi: 10.1002/jbm4.10665. https://doi.org/10.1002/jbm4.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):85–95. doi: 10.1016/j.joms.2009.01.006. https://doi.org/10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Stockmann P, Burger M, von Wilmowsky C, Ebker T, Lutz R, Bauersachs A, et al. The outcome after surgical therapy of bisphosphonate-associated osteonecrosis of the jaw--results of a clinical case series with an average follow-up of 20 months. Clin Oral Investig. 2014;18:1299–304. doi: 10.1007/s00784-013-1092-2. https://doi.org/10.1007/s00784-013-1092-2. [DOI] [PubMed] [Google Scholar]