Abstract
Several specific cell cycle activities are dependent on cell-substratum adhesion in nontransformed cells, and the ability of the Ras oncoprotein to induce anchorage-independent growth is linked to its ability to abrogate this adhesion requirement. Ras signals via multiple downstream effector proteins, a synergistic combination of which may be required for the highly altered phenotype of fully transformed cells. We describe here studies on cell cycle regulation of anchorage-independent growth that utilize Ras effector loop mutants in NIH 3T3 and Rat 6 cells. Stable expression of activated H-Ras (12V) induced soft agar colony formation by both cell types, but each of three effector loop mutants (12V,35S, 12V,37G, and 12V,40C) was defective in producing this response. Expression of all three possible pairwise combinations of these mutants synergized to induce anchorage-independent growth of NIH 3T3 cells, but only the 12V,35S-12V,37G and 12V,37G-12V,40C combinations were complementary in Rat 6 cells. Each individual effector loop mutant partially relieved adhesion dependence of pRB phosphorylation, cyclin E-dependent kinase activity, and expression of cyclin A in NIH 3T3, but not Rat 6, cells. The pairwise combinations of effector loop mutants that were synergistic in producing anchorage-independent growth in Rat 6 cells also led to synergistic abrogation of the adhesion requirement for these cell cycle activities. The relationship between complementation in producing anchorage-independent growth and enhancement of cell cycle activities was not as clear in NIH 3T3 cells that expressed pairs of mutants, implying the existence of either thresholds for these activities or additional requirements in the induction of anchorage-independent growth. Ectopic expression of cyclin D1, E, or A synergized with individual effector loop mutants to induce soft agar colony formation in NIH 3T3 cells, cyclin A being particularly effective. Taken together, these data indicate that Ras utilizes multiple pathways to signal to the cell cycle machinery and that these pathways synergize to supplant the adhesion requirements of specific cell cycle events, leading to anchorage-independent growth.
Ras proteins are small guanine nucleotide binding proteins that play a central role in signal transduction pathways that regulate cell proliferation (2). Wild-type Ras proteins are activated transiently, via guanine nucleotide exchange mechanisms, in response to a wide variety of extracellular signalling agents (3). When in the GTP-bound state, Ras is capable of binding to several different established and potential effector proteins, including members of the Raf, phosphatidylinositol 3 (OH)-kinase [PI(3)K], and Ral guanine nucleotide dissociation stimulator (RalGDS) families, Rin, protein kinase Cζ, AF6, and the GTPase-activating proteins p120GAP and neurofibromin (17, 28). Binding to Ras leads, directly or indirectly, to activation of these effectors, which in turn activate downstream signalling cascades. Thus, Ras may be viewed as a hub from which multiple pathways radiate. Activating mutations in Ras result in constitutive signalling to these downstream elements, and such mutations are observed with high frequency in human tumors (4).
Expression of mutated, oncogenic Ras in cultured rodent fibroblast cell lines induces a highly pleiotropic response, including alterations in cell morphology, loss of contact inhibition, changes in gene expression, decreased dependence on serum growth factors, and the ability to proliferate in the absence of adhesion to a substratum (i.e., anchorage-independent growth). Many of these phenotypes can be dissociated from one another. For example, introduction of Ras oncoprotein into quiescent Swiss 3T3 cells led to both morphological transformation and DNA synthesis, but only the induction of DNA synthesis required activation of protein kinase C (27). Furthermore, a Rat 6 fibroblast-derived mutant cell line, ER-1-2, responded to stable expression of the v-H-ras oncogene with alterations in morphology and gene expression that were nearly indistinguishable from those observed with a matched control cell line yet failed to form colonies in soft agar in response to ras (11, 22). Similarly, expression of a dominant negative form of Rac1 in Ras-transformed Rat 1 cells inhibited anchorage-independent growth of these cells but had only marginal effects on their transformed morphology (34).
The studies cited above raise the possibility that different aspects of the transformed phenotype might be controlled by distinct combinations of Ras-regulated pathways. Evidence for this notion has been elegantly provided through the use of Ras effector loop mutants. Certain point mutations in this region (amino acids 32 to 40 in H-Ras) render Ras defective for binding specific effector proteins while remaining competent for binding and activating others, albeit at lower than wild-type efficiency (39, 46). Several individual mutants were defective in transformation assays but, when coexpressed, complemented each other (19, 39, 46). These and additional studies have implicated at least three effector proteins as potential synergistic mediators of transformation by Ras: Raf, PI(3)K, and RalGDS (15, 19, 20, 33, 39, 46, 47). Raf stimulates the mitogen-activated protein kinase (MAPK)/Erk cascade, leading to phosphorylation and activation of transcription factors and other proteins (29). PI(3)K stimulates cortical actin rearrangement via the small GTP binding protein Rac (39). Ras also activates the c-Jun N-terminal kinase cascade in a Rac-dependent manner (30), but the role of PI(3)K in Ras-mediated activation of this pathway is not yet established. The mechanisms by which RalGDS contributes to the transformed phenotype are not known but may involve both Ral-dependent and -independent pathways (33, 45, 47). It should be mentioned that Raf, MAPK, PI(3)K, and Rac are each required for Ras to exert its full powers of transformation (18, 21, 35, 39) and, under certain conditions, transform fibroblasts on their own (6, 9, 18, 44).
We have undertaken an analysis of the mechanisms by which Ras inappropriately drives cell cycle events to induce anchorage-independent growth. Anchorage-independent growth is the best in vitro correlate of tumorigenicity (42), but the effector pathway or pathways utilized by Ras to produce this phenotype are still largely unknown. We and others demonstrated that several important cell cycle events were dependent on cell-substratum adhesion of nontransformed fibroblast cell lines, including (i) activation of G1 cyclin-dependent kinases (Cdks; as measured by cyclin D1- and E-dependent kinase activities and phosphorylation of pRB family members) and (ii) expression of the cyclin A gene (5, 10, 13, 16, 40, 51). Certain other aspects of the cell cycle, such as expression of cyclin E, were not regulated by adhesion. Strikingly, all of these cell cycle activities occurred in the absence of adhesion in Ras-transformed cell lines (5, 16). In contrast, ER-1-2 cells that expressed v-H-ras (ER-1-2/ras cells), which failed to proliferate in soft agar, possessed G1 Cdk activities when cultured without adhesion but remained almost completely adhesion dependent for expression of cyclin A (16). Importantly, ectopic expression of cyclin A rescued anchorage-independent growth of ER-1-2/ras cells but did not induce anchorage-independent growth of control or ER-1-2 cells, presumably because these cells still lacked G1 Cdk activities in the absence of adhesion (16). Anchorage-independent activation of G1 Cdks and expression of cyclin A are therefore likely to be functionally relevant endpoints in determination of the transformed phenotype.
Expression of the cyclin A gene is dependent on G1 Cdk activity (50). Results from the ER-1-2 cell system and other studies (7, 8, 16, 51) indicate, however, that there is an additional, adhesion- and Ras-regulated function that is also required for cyclin A expression and that this function is at least partly dissociable from the mechanisms by which adhesion and Ras regulate G1 Cdk activity. It is possible, therefore, that multiple Ras effector pathways are required to supplant adhesion-mediated cell cycle regulation and induce growth in soft agar. To test this hypothesis more directly, we have stably expressed Ras effector loop mutants in Rat 6 and NIH 3T3 fibroblasts and analyzed the cells for (i) growth in soft agar and (ii) the ability to drive specific cell cycle events in the absence of cell-substratum adhesion. These studies indicate that Ras signals to the cell cycle machinery via several pathways and that a combination of pathways is required to produce efficient anchorage-independent growth.
MATERIALS AND METHODS
Cell culture.
Rat 6- and NIH 3T3-derived cell lines were cultured in Dulbecco modified Eagle medium (Gibco) plus 10% bovine calf serum as previously described (16, 22). NIH 3T3 cells were obtained from T. Hei (Columbia University). This strain of NIH 3T3, which has a very low frequency of spontaneous focus formation, derives originally from a deposit by S. A. Aaronson to the Japanese Cancer Research Resources Bank. Soft agar assays were performed as described by Kang and Krauss (16). To recover cells cultured under nonadherent conditions, preparative methylcellulose cultures were used in place of soft agar cultures (16). Briefly, 105 cells were inoculated into 10 ml of Dulbecco modified Eagle medium containing 5% calf serum and 1.3% methylcellulose in a 50-ml conical tube. The tubes were then placed in a water-jacketed CO2 incubator at 37°C. Three days later, the medium was diluted with 40 ml of ice-cold phosphate-buffered saline (to solubilize the methylcellulose), and the cells were recovered by gentle centrifugation.
Transfections and retroviral infections.
pDCR-ras(12V), pDCR-ras(12V35S), pDCR-ras(12V37G), and pDCR-ras(12V40C) encode the indicated mutant Ras alleles under the control of the cytomegalovirus promoter and also contain the selectable marker gene neo. These reagents were originally developed by White and colleagues (46) and were kindly provided by D. Bar-Sagi (State University of New York at Stony Brook). Rat 6 and NIH 3T3 cells were transfected with a total of 20 μg of these plasmids, either singly or in paired combination, by the calcium phosphate technique (48). Transfected cultures were selected in G418-containing medium (400 μg per ml for Rat 6-derived cell lines and 700 μg per ml for NIH 3T3-derived cell lines), and drug-resistant colonies were pooled and analyzed as described in Results. Two independent, paired combination transfections were performed for the Rat 6 cells, with indistinguishable results. Additionally, because cotransfection of the 12V,35S and 12V,40C constructs did not produce macroscopic colonies in Rat 6 cells, these plasmids were also transfected serially. The pooled transfectants that stably expressed the 12V,35S mutant were then retransfected with the 12V,40C construct along with a plasmid encoding hygromycin resistance. Transfected cultures were selected in hygromycin-containing medium (200 μg/ml), and resistant colonies were pooled and tested for growth in soft agar. The serial transfectants gave results that were very similar to those for the cotransfectants.
Production of pBabePuro-based recombinant retroviruses that encode human cyclin D1, E, or A and infection of Rat 6 and NIH 3T3 cell lines were performed as previously described (16). Infected cultures were selected in puromycin-containing medium (5 μg per ml for Rat 6-derived cell lines and 7.5 μg per ml for NIH 3T3-derived cell lines), and drug-resistant colonies were pooled and analyzed as described in Results.
Immunoblotting.
Cells from monolayer or methylcellulose suspension cultures were harvested in lysis buffer (50 mM Tris-HCl [pH 8.0], 250 mM NaCl, 1% Nonidet P-40, 2 mM EDTA) containing 1 mM phenylmethylsulfonyl fluoride, 10 ng of leupeptin per ml, 50 mM NaF, and 1 mM sodium orthovanadate. Total proteins were then separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes (Amersham), and the membranes were probed with specific antibodies. After extensive washing (with 40 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM EDTA), the blots were reprobed with horseradish peroxidase-conjugated secondary antibody, and specific protein bands were visualized with the Amersham ECL chemiluminescence detection system. Immunoblotting was performed with the following antibodies, from the indicated sources; anti-H-Ras (SC-35; Santa Cruz Biotechnology), anti-Erk2 (SC-154; Santa Cruz Biotechnology), anti-cyclin D (06-13T; UBI), anti-cyclin E (SC-481; Santa Cruz Biotechnology), anti-cyclin A (M. Pagano, New York University), anti-human cyclin A (clone 6E6; Novocastra Laboratories), anti-human cyclin E (06-134; UBI), anti-pRB (14001A; PharMingen), anti-p27kip1 (A. Koff, Memorial Sloan-Kettering Cancer Center), and anti-p21cip1 (SC-397; Santa Cruz Biotechnology).
Erk2 assays.
Cells from methylcellulose suspension cultures were harvested in lysis buffer (50 mM HEPES [pH 7.6], 100 mM NaCl, 1% Nonidet P-40, 2 mM EDTA) containing 1 mM phenylmethylsulfonyl fluoride, 10 ng of leupeptin per ml, 50 mM NaF, and 1 mM sodium orthovanadate; 400 μg of cell lysate was immunoprecipitated with anti-Erk2 antibody (SC-154; Santa Cruz Biotechnology), and the precipitates were washed repeatedly and incubated in reaction buffer (10 mM HEPES, 10 mM magnesium acetate) containing 50 μM ATP, 5 μCi of [γ-32P]ATP, and 40 μg of myelin basic protein in a final volume of 40 μl for 30 min at 30°C. The products of the reaction were separated on an SDS–15% polyacrylamide gel. The gel was then dried and exposed to X-ray film.
Cyclin E-dependent kinase assays.
For in vitro cyclin E-dependent kinase assays, cyclin E was immunoprecipitated from 500 μg of total cellular protein (from lysates prepared as described above) with polyclonal anti-cyclin E antibody (SC-481; Santa Cruz Biotechnology), and the immunoprecipitates were washed three times with lysis buffer and twice with kinase buffer (20 μM Tris-HCl [pH 7.4], 4 μM MgCl2). The washed immunoprecipitates were then incubated with kinase buffer, 2 μg of histone H1, 1 μM ATP, and 5 μCi of [γ-32P]ATP in a final volume of 16 μl for 30 min at 37°C. The products of the reaction were separated on an SDS–12% polyacrylamide gel. The gel was then dried and exposed to X-ray film.
RESULTS
Phenotypic effects of Ras effector loop mutants on NIH 3T3 and Rat 6 cells.
To test the hypothesis that multiple Ras effector pathways might contribute to the induction of anchorage-independent growth, the NIH 3T3 and Rat 6 cell lines were transfected with expression vectors for fully activated H-Ras (12V), three H-Ras effector loop mutants that also contained the activating 12V mutation (12V,35S, 12V,37G, and 12V,40C), or an empty vector as a control. Of the three effector proteins for which there is evidence of a role in Ras-mediated transformation, the 12V,35S mutant binds to Raf but not PI(3)K or RalGDS, the 12V,37G mutant binds to RalGDS but not Raf or PI(3)K, and the 12V,40C mutant binds to PI(3)K but not Raf or RalGDS (33, 39, 46, 47). The expression vector also contained the selectable marker gene neo; multiple G418-resistant colonies were selected, pooled, and tested for expression of Ras protein by Western blotting with anti-Ras antibody. As shown in Fig. 1A, endogenous levels of Ras protein were at the limit of detection in empty vector controls (designated neo), but all Ras mutants were expressed at similar, easily detectable levels.
FIG. 1.
Expression of H-Ras 12V and effector loop mutant proteins in NIH 3T3 and Rat 6 cells. (A) NIH 3T3 and Rat 6 cells were stably transfected with expression vectors for the indicated proteins. The identity of the more rapidly migrating band of variable intensity is unknown, but it is not endogenous Ras. (B) NIH 3T3 and Rat 6 cells were transfected with combinations of expression vectors for the indicated proteins. Cell extracts (100 μg) were analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting with anti-Ras antibodies. The higher signal strength of immunoreactive Ras proteins in panel B than in panel A is due to a longer film exposure in panel B. Quantitation of the respective Ras proteins by densitometry gave the following ratios (in parentheses): for NIH 3T3 cells, 12V (1.0), 12V,35S (2.0), 12V,37G (3.2), 12V,40C (2.1), 12V,35S plus 12V,37G (1.5), 12V,35S plus 12V,40C (2.0), 12V,37G plus 12V,40C (1.6); for Rat 6 cells, 12V (1.0), 12V,35S (1.0), 12V,37G (0.7), 12V,40C (1.0), 12V,35S plus 12V,37G (1.9), 12V,35S plus 12V,40C (1.1), 12V,37G plus 12V,40C (1.5). Note that the same 12V-expressing cells and the same amounts of extract were used for panels A and B.
The various transfectants were then tested for the ability to form colonies in soft agar. As shown in Table 1 and Fig. 2A, the neo controls of both NIH 3T3 and Rat 6 cells remained as single cells when cultured in suspension. As expected, expression of H-Ras 12V led to production of large, macroscopic colonies in both cell lines. In contrast, each effector loop mutant was severely impaired at inducing anchorage-independent growth, producing no macroscopic colonies at all in either NIH 3T3 or Rat 6 cells. The effector loop mutants were not completely devoid of activity, however, as all three mutants led to the formation of very small colonies (∼8 to 12 cells) in both cell types.
TABLE 1.
Induction of anchorage-independent growth by H-Ras and various H-Ras effector loop mutants
| Vector(s) | Colony-forming efficiency (%)a
|
|
|---|---|---|
| NIH 3T3 | Rat 6 | |
| neo | 0 | 0 |
| 12V | 2.2 | 1.9 |
| 12V,35S | 0b | 0b |
| 12V,37G | 0b | 0b |
| 12V,40C | 0b | 0b |
| 12V,35S + 12V,37G | 1.9 | 1.7 |
| 12V,35S + 12V,40C | 1.4 | 0b |
| 12V,37G + 12V,40C | 0.6 | 0.5 |
A total of 10,000 cells were seeded into 0.3% agar. Macroscopic colonies were scored after 2 weeks of growth. Values represent averages of duplicate determinations that differed by less than 5%. The experiment was repeated at least twice for each transfection; data from several experiments are normalized to a single value from the 12V transfectant; the range for 12V over numerous experiments was from 1 to 5%.
While no macroscopic colonies were formed, microscopic colonies of 8 to 12 cells were formed with an efficiency of ∼1.5% (see text for details).
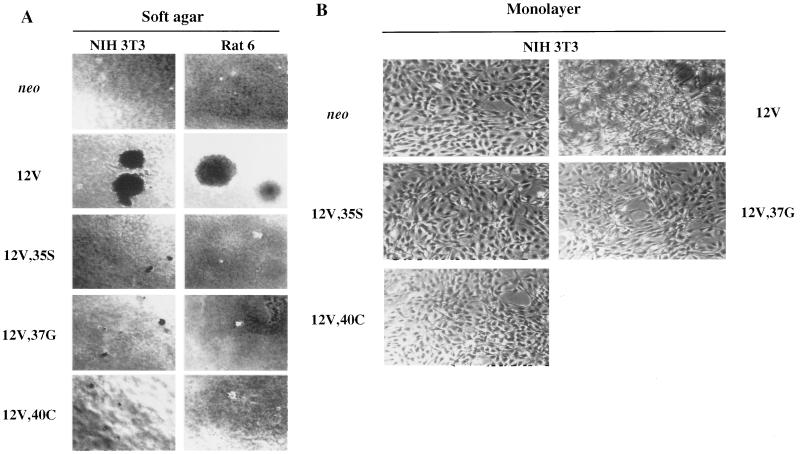
FIG. 2.
Soft agar colony formation and morphology in adherent cultures of NIH 3T3 and Rat 6 cells that express H-Ras 12V and effector loop mutants. NIH 3T3 and Rat 6 cells that expressed neo or the indicated H-Ras constructs were cultured in soft agar or on tissue culture dishes (monolayer) as described in Materials and Methods and photographed. (A) Soft agar cultures. The photomicrographs demonstrate relative colony size; for colony-forming efficiencies, see Table 1. (B) Monolayer cultures of NIH 3T3 cells. Original magnification, ×40.
The data described above suggested that more than one Ras-regulated signalling pathway may be required for efficient induction of anchorage-independent growth. We therefore tested the ability of pairwise combinations of Ras effector loop mutants to stimulate soft agar colony formation by NIH 3T3 and Rat 6 cells. Combinations of 12V,35S plus 12V,37G, 12V,35S plus 12V,40C, and 12V,37G plus 12V,40C were transfected into each cell line and, again, G418-resistant colonies were pooled, analyzed for expression of H-Ras, and tested for growth in soft agar. Figure 1B demonstrates that the double transfectants produced similar amounts of immunoreactive H-Ras protein, presumably a mixture of the two transfected mutants.
Coexpression of all three pairwise combinations of effector loop mutants in NIH 3T3 cells led to production of macroscopic colonies in soft agar (Table 1). The combination of 12V,35S plus 12V,37G was nearly as efficient as the fully transforming 12V, while the 12V,35S-12V,40C combination was somewhat less effective and the 12V,37G-12V,40C combination was significantly less effective. Additionally, in each case the colonies formed by coexpression of two effector loop mutants were only about one-half of the diameter of those formed by 12V (0.5 mm versus 1.0 mm). These data indicate that Ras can induce the formation of macroscopic colonies in soft agar with reasonable efficiency in the absence of efficient binding to Raf, or PI(3)K, or RalGDS but not, most likely, in the absence of binding to any two of these effectors.
Similar to NIH 3T3 cells, the combined expression of 12V,35S plus 12V,37G in Rat 6 cells produced a number of colonies that was only slightly lower than that observed with 12V (Table 1), and these colonies were, on average, half of the diameter of those formed with 12V (0.5 mm versus 1.0 mm). The combination of 12V,37G and 12V,40C also exhibited complementation in colony formation but, again, was not as effective as 12V,35S-12V,37G; furthermore, these colonies averaged only about 0.3 mm in diameter. In contrast to NIH 3T3 cells, the combination of 12V,35S and 12V,40C was ineffective at inducing colony formation in Rat 6 cells, beyond the microcolonies that each mutant produced individually. These data suggest that a function supplied by the 12V,37G mutant, but not the other two mutants, may be necessary but not sufficient for induction of anchorage-independent growth of Rat 6 cells.
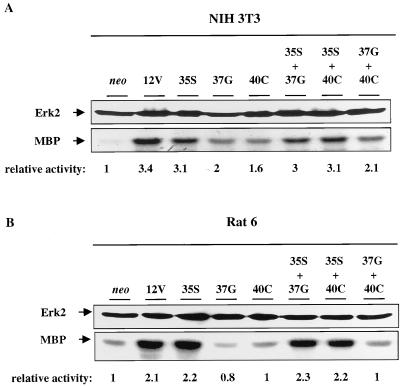
As a test for the various Ras effector loop mutants to display the predicted specificity under the conditions in which the cells were phenotypically analyzed, MAPK/Erk activity was assessed in anti-Erk2 immunoprecipitates from suspension cultures of each of the NIH 3T3 and Rat 6 cell derivatives described above (Fig. 3). Expression of 12V in NIH 3T3 cells increased Erk2 activity three- to fourfold. As would be predicted from its ability to bind to Raf, 12V,35S also led to an ∼3-fold increase, and this was not increased further in double transfectants containing 12V,35S. Expression of 12V,37G or 12V,40C, alone or in combination, led to a 1.5- to 2-fold increase in Erk2 activity. It is not clear whether this reflects residual binding of these mutants to Raf, an ability of other effectors to couple inefficiently to the Erk2 cascade in these cells, or indirect actions resulting from the weak but detectable proliferative capacity of these transfectants. In Rat 6 cells, expression of 12V led to an ∼2-fold increase in Erk2 activity. Expression of 12V,35S led to a similar increase, as did each double transfectant that included 12V,35S. In contrast, expression of 12V,37G or 12V,40C or a combination of the two failed to activate Erk2 above levels observed in the neo controls. Thus, cells that expressed various effector loop mutants displayed Erk2 activity that is consistent with the known effector binding properties of these mutants.
FIG. 3.
Analysis of Erk2 activity in NIH 3T3 and Rat 6 cells that express H-Ras 12V and various effector loop mutants. Cells expressing the indicated constructs were cultured in methylcellulose and harvested. Erk2 was immunoprecipitated and assessed for activity with myelin basic protein (MBP) as a substrate as described in Materials and Methods. Erk2 levels in each cell type were also assessed by Western blotting with anti-Erk2 antibodies. The numbers below the MBP kinase assay represent relative activities derived by densitometric analysis of the signal, with the neo lane designated 1.
The effects of the different H-Ras proteins on cell morphology were also examined. NIH 3T3 cells that expressed 12V displayed a typical transformed phenotype: the cells were rounded, refractile, and disorganized compared to the neo control transfectants, which grew as a flat monolayer (Fig. 2B). Cells that expressed each of the effector loop mutants resembled the neo controls much more closely than the 12V-expressing cells, but each also exhibited somewhat higher cell density and refractility. The effect of the H-Ras proteins on Rat 6 cell morphology was less pronounced than the effect on NIH 3T3 cells. The 12V-expressing Rat 6 cells grew in a swirling pattern and to a density higher than that observed with the neo control cells, but the effector loop mutant-expressing cells were indistinguishable from these control transfectants (data not shown).
Effects of various Ras mutants on adhesion-mediated expression and regulation of cell cycle proteins.
Phosphorylation of pRB, cyclin E-dependent kinase activity, and expression of cyclin A are all fully dependent on cell anchorage in several different nontransformed cell types, including NIH 3T3 and Rat 6 (5, 10, 13, 16, 40, 51). Oncogenic Ras abrogates the adhesion dependence of these activities in both cell types, and its ability to do so is apparently linked to its ability to drive anchorage-independent growth (5, 16). We therefore asked to what extent, if any, expression of the Ras effector loop mutants might relieve adhesion dependence of these events. Because it is not possible to recover viable, nonadherent cells from soft agar cultures, we used a methylcellulose culture system that allows for nearly quantitative recovery of intact cells cultured under nonadherent conditions (16). It has been demonstrated previously that the growth properties in soft agar and methylcellulose of the fibroblast lines used in these studies are nearly identical (16, 49). Furthermore, the proliferative behavior of the effector loop mutant-expressing cells in methylcellulose culture was very similar to that in soft agar (data not shown).
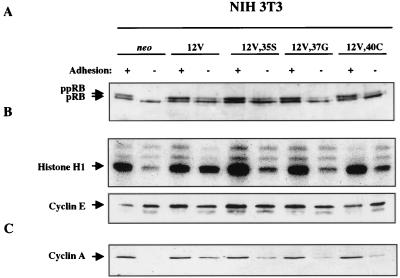
Similar to results of previous studies (16, 51), pRB phosphorylation, cyclin E-dependent kinase activity, and cyclin A expression were all adhesion dependent in neo control cells and adhesion independent in the 12V-expressing NIH 3T3 cells (Fig. 4). Interestingly, each of the three effector loop mutants induced detectable levels of pRB phosphorylation in the absence of adhesion (Fig. 4A). The level of activity was in the order 12V,35S > 12V,40C > 12V,37G, with the 12V,35S mutant displaying activity nearly as robust as that seen with the fully transforming 12V. All three mutants also induced cyclin E-dependent kinase activity under anchorage-independent culture conditions (Fig. 4B). In this case, none of the effector loop mutants were as effective as 12V, but 12V,35S and 12V,40C were each more effective than 12V,37G. Finally, all three mutants also induced expression of cyclin A in an adhesion-independent manner but at a level significantly lower than that for 12V (Fig. 4C). In particular, expression of 12V,37G or 12V,40C in NIH 3T3 cells led to production of only trace levels of cyclin A. It is concluded that in NIH 3T3 cells, Ras proteins that are defective in binding any two of the three effectors implicated in transformation are still able to abrogate, in part, adhesion-mediated regulation of the cell cycle. The remaining signalling capabilities of such defective Ras proteins are not, however, sufficient to override fully the adhesion requirement for cell proliferation, as reflected by molecular markers (Fig. 4) and colony formation in soft agar (Table 1 and Fig. 2A).
FIG. 4.
Analysis of adhesion-regulated cell cycle activities in NIH 3T3 cells that express H-Ras 12V and effector loop mutants. Cells expressing neo or the indicated H-Ras constructs were grown on tissue culture plates (+ adhesion) or in preparative methylcellulose cultures (− adhesion) and were analyzed by immunoblotting or immunoprecipitation and kinase assay as follows. (A) pRB phosphorylation. Blots were probed with a specific anti-pRB antibody. The hyperphosphorylated (ppRB) and hypophosphorylated (pRB) forms are distinguished by their mobilities and are indicated by arrows. (B) Cyclin E-dependent kinase activity. Cyclin E was immunoprecipitated and analyzed for associated histone H1 kinase activity. The bottom panel shows a Western blot of cyclin E, demonstrating approximately equivalent levels of cyclin E in the different cell types under the various culture conditions. (C) Expression of cyclin A. Blots were probed with a specific anti-cyclin A antibody. Each experiment was performed at least twice on two independent extracts from each cell line. Representative data are shown. See Materials and Methods for additional details.
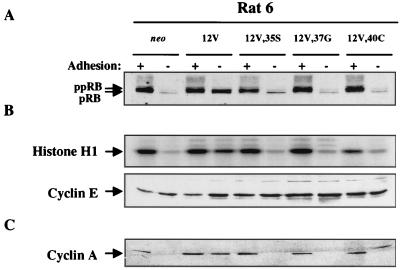
The ability of individual effector loop mutants to abrogate, even partially, the anchorage dependence of cell cycle events was much less pronounced in Rat 6 cells than it was in NIH 3T3 cells. As with NIH 3T3 cells, pRB phosphorylation, cyclin E-dependent kinase activity, and cyclin A expression were all adhesion dependent in neo controls and adhesion independent in the 12V-expressing cells (Fig. 5). Expression of each of the three effector loop mutants in Rat 6 cells, however, led to just trace levels of pRB phosphorylation and cyclin E-dependent kinase activity when the cells were cultured in suspension (Fig. 5A and B). Furthermore, these cells remained completely dependent on adhesion for production of cyclin A (Fig. 5C).
FIG. 5.
Analysis of adhesion-regulated cell cycle activities in Rat 6 cells that express H-Ras 12V and effector loop mutants. Cells expressing neo or the indicated H-Ras constructs were grown on tissue culture plates (+ adhesion) or in preparative methylcellulose cultures (− adhesion) and were analyzed by immunoblotting or immunoprecipitation and kinase assay as follows: pRB phosphorylation (A), cyclin E-dependent kinase activity (B), and expression of cyclin A (C). The more slowly migrating band in the minus adhesion lane of the 12V,40C-expressing cells is of unknown origin but has not been observed in other experiments and is not cyclin A. Details are as described in the legend to Fig. 4. Each experiment was performed at least twice on two independent extracts from each cell line. Representative data are shown.
Cells transfected with combinations of different effector loop mutants, most of which showed complementation in inducing colony formation in soft agar (Table 1), were also assessed for pRB phosphorylation, cyclin E-dependent kinase activity, and expression of cyclin A under adherent and nonadherent culture conditions. In NIH 3T3 cells transfected with 12V,35S plus 12V,37G or 12V,35S plus 12V,40C, both of which gave a significant level of agar colony formation, anchorage-independent phosphorylation of pRB and cyclin E-dependent kinase activity were not substantially different from those observed with single mutants (Fig. 6A and B). Production of cyclin A in these two pairwise combinations, however, was close to that observed with the fully transforming 12V (Fig. 6C). The 12V,37G-12V,40C transfectant, which was a less effective complementation pairing, was also less able to overcome the adhesion dependence of these activities.
FIG. 6.
Analysis of adhesion-regulated cell cycle activities in NIH 3T3 cells that express H-Ras 12V and various combinations of effector loop mutants. Cells expressing the indicated H-Ras constructs were grown on tissue culture plates (+ adhesion) or in preparative methylcellulose cultures (− adhesion) and were analyzed by immunoblotting or immunoprecipitation and kinase assay as follows: pRB phosphorylation (A), cyclin E-dependent kinase activity (B), and expression of cyclin A (C). See Fig. 3 for typical expression pattern of cyclin A in neo-expressing NIH 3T3 cells. Details are as described in the legend to Fig. 4. Each experiment was performed at least twice.
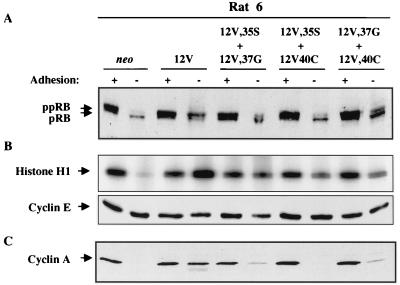
A related but distinct pattern was observed in Rat 6 cells. The 12V,35S-12V,37G and 12V,37G-12V,40C double transfectants both produced colonies in soft agar and, unlike the single transfectants, displayed significant levels of pRB phosphorylation, cyclin E-dependent kinase activity, and cyclin A expression in the absence of adhesion (Fig. 7). In contrast, the 12V,35S-12V,40C combination, which did not synergize to form colonies in soft agar in Rat 6 cells, produced detectable levels of phosphorylated pRB and cyclin E-dependent kinase activity but did not lead to expression of cyclin A (Fig. 7).
FIG. 7.
Analysis of adhesion-regulated cell cycle activities in Rat 6 cells that express H-Ras 12V and various combinations of effector loop mutants. Cells expressing the indicated H-Ras constructs were grown on tissue culture plates (+ adhesion) or in preparative methylcellulose cultures (− adhesion) and were analyzed by immunoblotting or immunoprecipitation and kinase assay as follows: pRB phosphorylation (A), cyclin E-dependent kinase activity (B), and expression of cyclin A (C). Details are as described in the legend to Figure 4. Each experiment was performed at least twice.
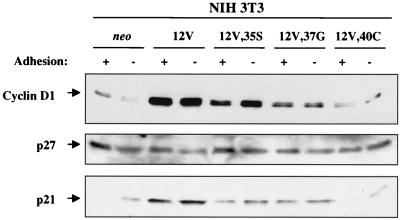
Several different mechanisms play a role in regulation of G1 Cdk activity by cell-substratum adhesion (5, 10, 13, 16, 40, 51). It was reported previously that suspension of NIH 3T3 cells led to decreased amounts of cyclin D1 and elevated amounts of the Cdk inhibitor p27kip1 (40, 51). We therefore assessed the effects of 12V and effector loop mutants on adhesion-mediated regulation of the levels of these proteins in NIH 3T3 cells. As shown in Fig. 8, expression of cyclin D1 was partially adhesion dependent in the neo controls. In contrast, 12V-expressing cells possessed very high levels of cyclin D1, regardless of their state of adhesion. A significant fraction of the 12V-induced increase in cyclin D1 levels was observed with 12V,35S, suggesting a role for Raf in mediating this response. This result is consistent with those of previous reports implicating MAPK/Erk in transcriptional regulation of the cyclin D1 promoter (24). 12V,37G-expressing cells displayed somewhat elevated levels of cyclin D1 that were also unaltered by loss of adhesion, but the 12V,40C mutant was largely without effect on cyclin D1.
FIG. 8.
Effects of adhesion and various Ras proteins on expression of cyclin D1, p27kip1, and p21cip1 in NIH 3T3 cells. Cells expressing neo or the indicated H-Ras constructs were grown on tissue culture plates (+ adhesion) or in preparative methylcellulose cultures (− adhesion) and were analyzed by immunoblotting with specific antibodies to cyclin D1, p27kip1, or p21cip1, as indicated. Each experiment was performed twice on two independent extracts from each cell line. Representative data are shown.
In contrast to a previous report (40), we found that levels of p27kip1 in NIH 3T3 cells were essentially unaffected by either adhesion or Ras (Fig. 8). We therefore investigated the effects of adhesion and Ras on the levels of a different Cdk inhibitor, p21cip1. p21cip1 was induced by loss of adhesion in neo control cells but was present at even higher levels in 12V-transformed cells, whether these cells were cultured in monolayer or suspension (Fig. 8). p21cip1 levels were influenced by effector loop mutants in a fashion similar to that observed with cyclin D1. 12V,35S and 12V,37G each led to elevated levels of p21cip1, regardless of adhesive state, whereas 12V,40C was again without significant effect. p21cip1, like cyclin D1, is encoded by a mitogen-inducible gene (25), and its constitutive expression by Ras-transformed cells is therefore not surprising. The results presented in Fig. 6 suggest that similar pathways might regulate production of cyclin D1 and p21cip1. This is of interest since p21cip1 has been implicated in assembly of cyclin D-Cdk4 complexes (23).
We previously reported that the levels of cyclin D1, p27kip1, and p21cip1 were not regulated by adhesion in Rat 6-derived cell lines (16), and therefore the effects of the various Ras mutants on production of these proteins were not investigated in suspension cultures of this cell line. (The effects of Ras mutants on production of cyclin D1 in adherent cultures are, however, presented below.)
Cooperation between Ras effector loop mutants and cyclins in inducing anchorage-independent growth.
Expression of cyclins D1 and A is adhesion dependent in several cell types (13, 16, 51). Ectopic expression of each of these cyclins influenced adhesion-mediated regulation of cell cycle progression, although in most cases, it was not sufficient to induce colony formation in soft agar (16, 36, 51). The ras-mediated increase in cyclin D1 levels, observed in this and other studies (Fig. 8) (16 and 26), is thought to be important for transformation by this oncogene. Furthermore, ectopic expression of cyclin A rescued anchorage-independent growth of transformation-resistant ER-1-2/ras cells (16) (see the introduction). Thus, it was of interest to test whether ectopic expression of cyclin D1, E, or A could complement individual Ras effector loop mutants in the induction of anchorage-independent growth. NIH 3T3 and Rat 6 cells that expressed neo, 12V, or each of the three effector loop mutants were infected with recombinant retroviruses that drive expression of human cyclin D1, E, or A cDNA from the viral long terminal repeat (16). The parental vector, pBabePuro (31), also confers resistance to the drug puromycin. The cells were infected with one of the cyclin-expressing viruses or, as a control, a virus that lacked a cDNA insert. The infectants were selected in puromycin-containing medium, and puromycin-resistant colonies were then pooled and analyzed for expression of the various exogenous cyclins and colony formation in soft agar.
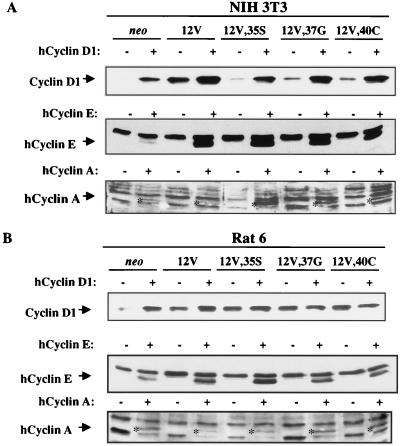
As we have reported previously, it was more difficult to achieve ectopic expression of cyclin A than of cyclin D1 or E (16); many of the colonies that initially emerged following infection of either the NIH 3T3 or Rat 6 cell derivatives with the cyclin A virus died. Nevertheless, ectopic producers of each of the three cyclins were obtained from the pooled infectants. As shown in Fig. 9A, NIH 3T3 derivatives infected with the cyclin D1 virus displayed enhanced levels of cyclin D1 when analyzed with an antibody that recognized both the endogenous mouse protein and the exogenous human protein. The 12V derivative had only about twofold more cyclin D1 immunoreactivity than its matched control infectant population, but the neo cells and each of the effector loop mutant-expressing cells all produced significantly enhanced levels of cyclin D1 (Fig. 9A; the low level of cyclin D1 seen in the control virus-infected, 12V,35S-expressing cells, relative to that observed with these cells in Fig. 6, was due to underloading of this lane in this particular experiment). Ectopic expression of cyclins E and A was detected with antibodies that were specific for the exogenous human proteins (Fig. 9A).
FIG. 9.
Analysis of ectopic expression of cyclins D1, E, and A in NIH 3T3 and Rat 6 cell derivatives. NIH 3T3 or Rat 6 cells that express neo, H-Ras 12V, or various effector loop mutants were infected with recombinant retroviruses that either lacked a cDNA insert (−) or harbored a human cyclin D1 (hCyclin D1), human cyclin E, or human cyclin A cDNA (+). Cell extracts were analyzed by immunoblotting with antibodies specific for cyclin D1, human cyclin E, or human cyclin A. Specific bands are indicated by the arrows. The human cyclin A band migrates between two nonspecific, cross-reactive bands and is also indicated by asterisks in the appropriate lanes.
The NIH 3T3 infectants were then tested for the ability to form macroscopic colonies in soft agar. As shown in Table 2, ectopic expression of cyclin D1, E, or A was not sufficient to induce anchorage-independent growth of the neo control cells. Infection of the 12V cells with the control retrovirus (pBabePuro) did not increase the efficiency of colony formation of these cells (compare Tables 1 and 2). In contrast, infection of the 12V cells with any of the cyclin-expressing viruses led to enhanced growth in soft agar: the 12V/cyclin D1, 12V/cyclin E, and 12V/cyclin A cells displayed 5.4-, 2.1-, and 2.3-fold-higher colony-forming efficiencies, respectively. These data indicate that the levels of each cyclin are rate limiting for anchorage-independent growth, even in cells that express abundant oncogenic Ras protein.
TABLE 2.
Cooperation between Ras effector loop mutants and cyclins in inducing anchorage-independent growth of NIH 3T3 cells
| Cell line | Colony-forming efficiency (% ± SD)a
|
|||
|---|---|---|---|---|
| +pBabePuro | +Cyclin D1 | +Cyclin E | +Cyclin A | |
| neo | 0 | 0 | 0 | 0 |
| 12V | 2.2 ± 0.1 | 12.1 ± 1.0 | 4.7 ± 0.5 | 5.1 ± 0.6 |
| 12V,35S | 0b | 1.0 ± 0.1 | 0.4 ± 0.1 | 2.0 ± 0.2 |
| 12V,37G | 0b | 2.5 ± 0.1 | 1.2 ± 0.1 | 1.7 ± 0.4 |
| 12V,40C | 0b | 1.7 ± 0.2 | 0b | 3.7 ± 0.3 |
A total of 10,000 cells were seeded into 0.3% agar. Macroscopic colonies were scored after 2 weeks of growth. Each infection with cyclin-expressing viruses was performed twice; each pool of infectants was then analyzed twice in duplicate. Values represent averages of all determinations ± 1 standard deviation. See text for further details. +pBabePuro, cells infected with a retroviral vector lacking a cDNA insert; +cyclin D1, E, or A, cells infected with a retroviral vector that drives expression of a human cyclin of the indicated type.
Formation of microscopic colonies (see footnote b to Table 1).
Stable overproduction of cyclin D1 in the various Ras effector loop mutant-expressing NIH 3T3 cells led to a low but significant level of colony formation in soft agar relative to the number of colonies observed with the 12V/cyclin D1 cells, which are the most appropriate cell type for comparison (Table 2). The order of sensitivity to cyclin D1-induced colony formation was 12V,37G > 12V,40C > 12V,35S, with these infectants achieving 21, 14, and 9% the efficiency seen with V12/cyclin D1 cells, respectively. Ectopic production of cyclin E had a similar effect on the 12V,37G- and 12V,35S-expressing cells, yielding 26 and 9% the number of colonies seen with V12/cyclin E cells. In contrast, the 12V,40C-expressing NIH 3T3 cells were not complemented by infection with the cyclin E virus, despite production of easily detectable levels of human cyclin E (Fig. 9A). Ectopic expression of cyclin A had a much more pronounced effect on anchorage-independent growth of the effector loop mutant-expressing cells than did cyclin D1 or E. The order of sensitivity to cyclin A-induced colony formation was V12,40C > 12V,35S > 12V,37G, with these infectants producing 71, 39, and 34% the number of colonies produced by V12/cyclin A cells, respectively (Table 2). These data are consistent with the idea that cyclin A plays a key role in the induction of anchorage-independent growth (13, 16, 49). It should be noted that the level of ectopic cyclin A expression achieved in the various cell lines did not correlate with their respective colony-forming efficiencies; e.g., the 12V,35S-expressing cells produced larger quantities of exogenous cyclin A than did the 12V,40C-expressing cells, yet the latter cells formed colonies more efficiently. Likewise, the levels of exogenous cyclins D1 and E were not significantly different between the various effector loop mutant-expressing lines, but quantitative differences in formation of colonies in response to these cyclins were observed. These data suggest there is specificity to the cyclin-mediated complementation and that individual cyclins may rescue the absence of some signalling pathways more effectively than other pathways.
We performed a similar set of experiments with the various Ras-expressing Rat 6 cell derivatives. In contrast to NIH 3T3 cells, expression of each effector loop mutant in Rat 6 cells led to an increase in cyclin D1 levels that was similar to that seen with expression of 12V (Fig. 9B; see Fig. 8 for data on NIH 3T3 cells). Infection with the cyclin D1-expressing virus failed to increase significantly the production of cyclin D1 by these cell lines, perhaps as a result of this already high level. Infection with the cyclin E- and A-expressing viruses did lead to production of the human proteins in all of the Rat 6 cell-derived lines, at levels comparable to that observed with the NIH 3T3 infectants (Fig. 9B). In contrast to the NIH 3T3 cells, however, coexpression of any of the three Ras effector loop mutants with any of the three cyclins failed to result in formation of macroscopic colonies (data not shown). Thus, cyclins D1, E, and A were able to complement, with some degree of specificity, the Ras effector loop mutants in the induction of anchorage-independent growth of NIH 3T3, but not Rat 6, cells.
DISCUSSION
Stable expression of oncogenic Ras in rodent fibroblast cell lines induces a wide array of responses referred to collectively as the transformed phenotype. Such responses are thought to be of relevance to the role of Ras in human cancers and include alterations in cell morphology, growth factor requirements, and gene expression. A response that shows an excellent correlation with tumorigenicity is anchorage-independent growth, measured as the ability to form colonies in semisolid medium (42). Recent studies have indicated that Ras functions by binding to multiple effector proteins that, in turn, activate distinct downstream signalling pathways (17, 28). A major task is to discern which effector pathways contribute to which aspects of the transformed phenotype. Ras effector loop mutants, which are defective for binding specific effector proteins while remaining competent for binding and activating others, are particularly well suited to such investigations (46). In this study, we have exploited Ras effector loop mutants to examine whether multiple Ras-regulated pathways are involved in the induction of anchorage-independent growth. It has previously been shown that several cell cycle events, including activation of G1 Cdks and expression of cyclin A, are dependent on cell adhesion in nontransformed fibroblasts (5, 10, 13, 16, 40, 51) and that anchorage-independent growth most likely depends on the ability of Ras to supplant this requirement for adhesion (5, 16, 49). The effector loop mutants were therefore also used to assess whether multiple pathways might signal to the cell cycle machinery in cells cultured in suspension. We report here that although oncogenic Ras (12V) induced formation of colonies in soft agar by both NIH 3T3 and Rat 6 cells, each of three effector loop mutants (12V,35S, 12V,37G, and 12V,40C) had almost completely lost this ability. Pairwise combinations of these mutants, however, synergized to produce growth in soft agar by both cell types. The most likely explanation for these observations is that multiple Ras-mediated pathways are required for efficient induction of anchorage-independent growth. Synergy between specific effector loop mutants has also been observed previously in assays of focus formation and of induction of DNA synthesis following microinjection (15, 19, 39, 46). This report is, as far as we are aware, the first to demonstrate such synergy in stably transfected cell lines.
The ability of individual Ras effector loop mutants, and combinations of such mutants, to abrogate the adhesion dependence of specific cell cycle events is consistent with the conclusion drawn above. In Rat 6 cells, expression of individual mutants produced only trace levels of pRB phosphorylation and cyclin E-dependent kinase activity in the absence of cell-substratum adhesion. Furthermore, none led to expression of cyclin A under nonadherent conditions. In contrast, coexpression of 12V,35S plus 12V,37G or 12V,37G plus 12V,40C led to both colony formation in soft agar and loss of the anchorage requirement for these cell cycle activities. This correlation also held for the combination of 12V,35S and 12V,40C, which did not lead to significant levels of either colony formation or adhesion-independent expression of cyclin A in Rat 6 cells. These data suggest that an effector that binds to 12V,37G, but not the other two mutants, may be required for induction of anchorage-independent growth of this cell line. Two potential effectors that fit this description are RalGDS and Rin1 (14, 47).
NIH 3T3 cells responded to the effector loop mutants in a manner roughly similar to that observed with Rat 6 cells. The NIH 3T3 line was, however, different from Rat 6 in two important ways. First, in NIH 3T3 cells, all three mutants were partially able to drive pRB phosphorylation, cyclin E-dependent kinase activity, and expression of cyclin A in the absence of cell-substratum adhesion. This is striking and indicates that, at least in these cells, Ras may signal to the cell cycle machinery without binding to any two of the three effectors thus far implicated in transformation: Raf, PI(3)K, and RalGDS. It is not yet clear whether signalling by any of these pathways alone is sufficient to drive partial, anchorage-independent activation of the cell cycle machinery, because the effector mutants used in this study may bind to additional proteins that could also participate in these actions. For example, when assayed in the yeast two-hybrid system, 12V,37G bound to Rin1, and all three mutants bound to AF6 (14, 19).
NIH 3T3 cells that expressed 12V,37G or 12V,40C produced only trace levels of cyclin A in suspension, providing a reasonable explanation for their inability to form macroscopic colonies in soft agar (16, 49, 51). The 12V,35S-expressing cells, however, displayed higher levels of hyperphosphorylated pRB and cyclin A in suspension culture than did cells that expressed the other two mutants, yet they were equally impaired at forming colonies. The synergy in soft agar colony formation seen with NIH 3T3 cells that expressed combinations of mutants was, however, reflected in enhanced adhesion-independent expression of cyclin A by these cells. It is possible, therefore, that there is a tightly regulated threshold effect for cyclin A levels and transformation. Thus, the relatively small differences in cyclin A expression seen between NIH 3T3 cells that expressed 12V,35S and the effective paired combinations may have been sufficient to trigger an all-or-none response in macroscopic colony formation. Alternatively, there may be additional requirements for efficient induction of anchorage-independent growth that are synergistically regulated by the various effector loop mutants. Whether these putative requirements represent additional cell cycle events, or metabolic activities such as anaerobic glycolysis (41), is not known.
A second difference between NIH 3T3 and Rat 6 cells was that in the former cell line but not the latter, Ras effector loop mutants synergized with ectopic expression of various cyclins in the formation of colonies in soft agar. Cyclins D1, E, and A are each required for the G1-to-S phase transition, and the levels of these proteins are rate limiting for G1 phase progression (1, 12, 32, 36–38). Taken together, these data strongly suggest one important reason individual effector loop mutants are impaired at inducing anchorage-independent growth is that in the absence of adhesion-mediated signals, they are insufficient to activate fully the cell cycle machinery that controls G1 phase progression and the G1-to-S phase transition. Overall, cyclin A was much more effective at synergizing with the effector loop mutants than were cyclins D1 and E. This is consistent with our previous conclusion that the ability of ras to drive expression of cyclin A in the absence of cell adhesion is likely to be critical to the induction of anchorage-independent growth by this oncogene (16, 49). The failure of ectopic cyclin expression to complement individual effector loop mutants in Rat 6 cells may be due to the much weaker ability of these mutants to supplant the adhesion requirements of all of the cell cycle activities investigated in these cells relative to NIH 3T3 cells.
The conclusions drawn in this report are consistent with those derived from most other recent studies; i.e., multiple pathways contribute to, and may be required for, full transformation by Ras (15, 19, 20, 33, 39, 46, 47). There are, however, some exceptions worth mentioning. Khosravi-Far et al. reported that stable expression of the 12V,35S, 12V,37G, and 12V,40C mutants each led to growth in soft agar and tumorigenicity (19). These results were obtained with one strain of NIH 3T3 cells but not, apparently, with a second strain (19, 46). In another study Stang et al. investigated an exhaustive panel of effector loop mutants and concluded that the Raf pathway alone may be sufficient for transformation of Rat 2 cells (43). This may be due to a particular sensitivity of this cell line to this pathway or, possibly, to the fact that transformation was scored only by examination of the morphology of cells in drug-resistant colonies and not by more stringent assays such as growth in soft agar. The failure of the 12V,40C mutant to induce morphological transformation of NIH 3T3 or Rat 6 cells is also noteworthy. Microinjection of this construct into several different cell lines caused rearrangement of cortical actin and membrane ruffling to a degree similar to that observed with 12V alone (15, 39). This finding suggests that the requirements for stable morphological transformation and for the cytoskeletal alterations measured in these short-term assays may not be identical.
Taken together, the results of this study are most consistent with the interpretation that individual Ras effector pathways do not control individual aspects of the transformed phenotype; rather, multiple effector pathways are required for each aspect of the transformed phenotype. With regard to anchorage-independent growth, these pathways collaborate to supplant the adhesion requirements of specific cell cycle events which presumably drive cell proliferation in the absence of cell-substratum adhesion. It is hoped that the cell lines developed in this study will facilitate identification of the Ras-mediated pathways involved in anchorage-independent growth and the biochemical mechanisms by which they connect to the cell cycle machinery.
ACKNOWLEDGMENTS
We thank Michele Pagano and Andy Koff for gifts of antibodies and Mitch Goldfarb and Andrew Chan for comments on the manuscript.
This work was supported by a grant from the NIH (CA59474) and a Sinsheimer Scholar’s Award to R.S.K. R.S.K. is a Career Scientist of the Irma T. Hirschl Trust.
REFERENCES
- 1.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 2.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 3.Boguski M S, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 4.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 5.Carstens C-P, Kramer A, Fahl W E. Adhesion-dependent control of cyclin E/cdk2 activity and cell cycle progression in normal cells but not in Ha-ras transformed NRK cells. Exp Cell Res. 1996;229:86–92. doi: 10.1006/excr.1996.0346. [DOI] [PubMed] [Google Scholar]
- 6.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Knudsen E S, Wang J Y J. Cells arrested in G1 by the v-Abl tyrosine kinase do not express cyclin A despite the hyperphosphorylation of RB. J Biol Chem. 1996;271:19637–19640. doi: 10.1074/jbc.271.33.19637. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Knudsen E S, Wang J Y J. The RB/p107/p130 phosphorylation pathway is not inhibited in rapamycin-induced G1-prolongation of NIH3T3 cells. Oncogene. 1996;13:1765–1771. [PubMed] [Google Scholar]
- 9.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 11.Feinleib J L, Krauss R S. Dissociation of ras oncogene-induced gene expression and anchorage-independent growth in a series of somatic cell mutants. Mol Carcinog. 1996;16:139–148. doi: 10.1002/(SICI)1098-2744(199607)16:3<139::AID-MC4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Girard F, Strausfeld U, Fernandez A, Lamb N J C. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 13.Guadagno T M, Ohtsubo M, Roberts J M, Assoian R K. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- 14.Han L, Wong D, Dhaka A, Afar D, White M, Xie W, Herschman H, Witte O, Colicelli J. Protein binding and signaling properties of RIN1 suggest a unique effector function. Proc Natl Acad Sci USA. 1997;94:4954–4959. doi: 10.1073/pnas.94.10.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 16.Kang J-S, Krauss R S. Ras induces anchorage-independent growth by subverting multiple adhesion-regulated cell cycle events. Mol Cell Biol. 1996;16:3370–3380. doi: 10.1128/mcb.16.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz M E, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 18.Khosravi-Far R, Solski P A, Clark G J, Kinch M S, Der C J. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khosravi-Far R, White M A, Westwick J K, Solski P A, Chrzanowska-Wodnicka M, Van Aelst L, Wigler M H, Der C J. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolch W, Heidecker G, Lloyd P, Rapp U R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;349:426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- 22.Krauss R S, Guadagno S N, Weinstein I B. Novel revertants of H-ras oncogene-transformed R6-PKC3 cells. Mol Cell Biol. 1992;12:3117–3129. doi: 10.1128/mcb.12.7.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaBaer J, Garrett M D, Stevenson L F, Slingerland J M, Sandhu C, Chou H S, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 24.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Jenkins C W, Nichols M A, Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994;9:2261–2268. [PubMed] [Google Scholar]
- 26.Liu J-J, Chao J-R, Jiang M-C, Ng S-Y, Yen J J-Y, Yang-Yen H-F. Ras transformation results in an elevated level of cyclin D1 and acceleration of G1 progression in NIH 3T3 cells. Mol Cell Biol. 1995;15:3654–3663. doi: 10.1128/mcb.15.7.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd A C, Paterson H F, Morris J D H, Hall A, Marshall C J. p21H-ras-induced morphological transformation and increases in c-myc expression are independent of functional protein kinase C. EMBO J. 1989;8:1099–1104. doi: 10.1002/j.1460-2075.1989.tb03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall C J. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- 29.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 30.Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcription activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 31.Morganstern J P, Land H. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okazaki M, Kishida S, Hinoi T, Hasegawa T, Tamada M, Kataoka T, Kikuchi A. Synergistic activation of c-fos promoter activity by Raf and Ral GDP dissociation stimulator. Oncogene. 1997;14:515–521. doi: 10.1038/sj.onc.1200860. [DOI] [PubMed] [Google Scholar]
- 34.Qiu R-G, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 36.Quelle D E, Ashmun R A, Schurtleff S A, Kato J, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 37.Resnitzky D, Gossen M, Bujard H, Reed S I. Accelation of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resnitzky D, Hengst L, Reed S I. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 40.Schulze A, Zerfass-Thome K, Berges J, Middendorf S, Jansen-Durr P, Henglein B. Anchorage-dependent transcription of the cyclin A gene. Mol Cell Biol. 1996;16:4632–4638. doi: 10.1128/mcb.16.9.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shim H, Dolde C, Lewis B C, Wu C-S, Dang G, Jungmann R A, Dalla-Favera R, Dang C V. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin S-I, Freedman V H, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci USA. 1975;72:4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stang S, Bottorff D, Stone J C. Interaction of activated Ras with Raf-1 alone may be sufficient for transformation of rat2 cells. Mol Cell Biol. 1997;17:3047–3055. doi: 10.1128/mcb.17.6.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanton V P, Nichols D W, Laudano A P, Cooper G M. Definition of the Raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989;9:639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 46.White A M, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 47.White M A, Vale T, Camonis J H, Schaefer E, Wigler M H. A role for Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 48.Wigler M, Pellicer A, Silverstein S, Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978;14:725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- 49.Yang J-J, Krauss R S. Extracellular ATP induces anchorage-independent expression of cyclin A and rescues the transformed phenotype of a Ras-resistant mutant cell line. J Biol Chem. 1997;272:3103–3108. doi: 10.1074/jbc.272.5.3103. [DOI] [PubMed] [Google Scholar]
- 50.Zerfass-Thome K, Schulze A, Zwerschke W, Vogt B, Helin K, Bartek J, Henglein B, Jansen-Durr P. p27KIP1 blocks cyclin E-dependent transactivation of cyclin A gene expression. Mol Cell Biol. 1997;17:407–415. doi: 10.1128/mcb.17.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu X, Ohtsubo M, Bohmer R M, Roberts J M, Assoian R K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]