Data from the Surveillance, Epidemiology, and End Results (SEER) database shows that chronic lymphocytic leukemia (CLL) patients with higher social economic status have better outcomes but the reasons underlying this observation remain undetermined.1
This single center study (ethical approval granted by South East Wales Research Ethics Committee -02/4806) of 665 prospectively diagnosed participants between August 2005 and December 2019 i.e., prior to the Covid-19 pandemic, included 413 (62.1%) males and 252 (37.9%) females who were overwhelmingly white Caucasian (98%). There were two (2005-2010) and then a single specialist consultant (2010-2019) managing primary, secondary and tertiary patients through two specialist CLL clinics. Patients were diagnosed according to the five-parameter immunophenotyping scoring system.2 Prognostic markers included clinical stage, lymphocyte doubling time (LDT), CD38 expression, β-2 microtubulin (B2M), immunoglobulin heavy-chain gene variable region (IGHV) mutation status at diagnosis with cytogenetic analysis (fluorescence in situ hybridization) routinely performed prior to treatment initiation. Patients were managed in accordance with the prevailing national/international guidelines with all eligible patients offered access to all licensed drugs and various open clinical trials, Online Supplementary Table S1.3
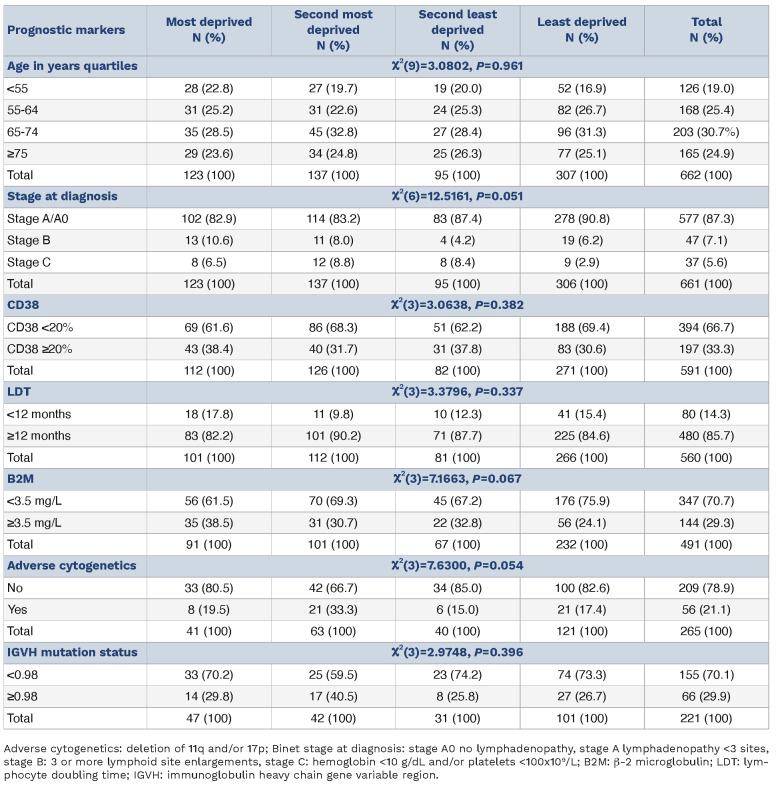
The Welsh Government’s Welsh Index of Multiple Deprivation (WIMD - last update 2019) provides a weighted (%) relative deprivation scoring system derived from eight domains -income (22%), employment (22%), health (15%), education (14%), access to services (10%), housing (7%), community safety (5%) and physical environment (5%), https://www.gov. wales/sites/default/files/statistics-and-research/2020-06/ welsh-index-multiple-deprivation-2019-results-report.pdf -providing each individual household with a deprivation score. Patients were assigned into categories of relative deprivation based on quartiles with Cox regression for univariate and multivariable analyses used for primary outcomes, Pearson’s χ2 for relative deprivation and prognostic markers and Mann-Whitney U test for age. Analysis was conducted in Stata version 17. The Kaplan-Meier plots were generated using R version 4.2.1. The median age at diagnosis was 67 years with the overwhelming majority of patients (87.3%) presenting with early-stage disease (stage A/A0) and good prognostic markers: lymphocyte doubling time (LDT) >12 months (85.7%), low CD38 expression (66.7%), low B2M (70.7%), mutated IGHV genes (70.1%) and no adverse cytogenetics (78.9%), Table 1.
In keeping with the published literature, age, stage, LDT, CD38, IGHV status and B2M at diagnosis were all identified as poor prognostic markers as was adverse cytogenetics (11q and/or 17p deletions). The likelihood of a poorer outcome was predicted by level of deprivation independently and after adjusting for other explanatory covariates, Online Supplementary Table S1. There was significantly better overall survival with the least deprived having improved survival compared to the most deprived group (2nd most deprived hazard ratio [HR]=0.85, 95% confidence interval [CI]: 0.59-1.25, P=0.413; 2nd least deprived HR=0.8, 95% CI: 0.52-1.22, P=0.294; least deprived HR=0.59, 95% CI: 0.42-0.82, P=0.002), Figure 1.
Progression-free survival (PFS) and time-to-first treatment (TTFT) showed no statistically significant difference between the various deprivation quartiles (P=0.084 and P=0.23, respectively) and the most deprived versus the least deprived quartile (P=0.087 and P=0.236, respectively). However, analyzing just those patients requiring treatment, survival from the time of first treatment, was significantly worse in the more deprived when comparing the four quartiles (P<0.001), Figure 2.
The overall cohort had a median age at diagnosis of 67 years, but those with advanced-stage disease (stages B or C) presented at a significantly earlier age (60 vs. 67 years, P<0.001), Online Supplementary Table S2. Although there was no substantial difference in age at diagnosis for patients presenting with early-stage disease (65 vs. 67 years - most vs. least deprived, P=0.153) across deprivation quartiles, the least deprived group with advanced-stage disease presented 10 years younger (57 vs. 67 years; P=0.074), Online Supplementary Table S2. Interestingly, the age at diagnosis for the most deprived is almost identical whether they presented with early- or advanced-stage disease (65 vs. 67 years, P=0.835) whereas there was a significant 10-year difference in the least deprived quartile (67 vs. 57 years, P=<0.001), Online Supplementary Table S2. On average, the most deprived patients presenting with advanced-stage disease showed a median survival of 10.5 years from diagnosis whereas the least deprived lived 15 years, Online Supplementary Table S2.
Analyzing only the 263 patients who died, as expected advanced-stage disease was associated with significantly earlier death (81 vs. 71 years, P<0.001) with earlier death also associated with increasing deprivation (P=0.052). The most deprived quartile early-stage patients died 1.5 years earlier (P=0.077) but the age of death for advanced-stage disease was similar (P=0.529), Online Supplementary Table S2.
There were no significant differences in the baseline characteristics of the four deprivation quartile groups in age at presentation, LDT and CD38 expression but less deprived patients presented with earlier stage (stage A/A0) disease (P=0.051) - least deprived 90.9% versus most deprived 82.9%. There was also some albeit weak evidence for higher B2M levels in the more deprived quartile groups compared to the least deprived (P=0.067), Table 1. Somewhat surprisingly, in the least deprived quartile, there were fewer patients with adverse cytogenetics (P=0.054). There was also some scant evidence that the two most deprived quartiles had a higher frequency of unmutated IGHV genes (31/89-34.8% vs. 35/132-26.5%, P=0.185), Table 1.
Sixteen clinical therapeutic trials were open during 2005-2019 with 87 patients (38.2% of 228 patients requiring treatment) eligible and offered entry into a therapeutic clinical trial but ten declined. There was no significant difference in the offering of clinical trials by clinicians or trial entry across the four WIMD quartiles (P=0.917), Online Supplementary Table S3. This is the first study to assess not only the impact of deprivation on CLL outcomes but explore the possible underlying reasons. The major advantages of this single center study, is that all patients were managed in a free universal health care system, in specialist CLL clinics, according to national guidelines with patients having access to global clinical trials, by a maximum of two specialists as type of care (primary, secondary or tertiary center) and access to specialists and clinical trials are known to impact CLL patient survival.4 This study shows for the first time that deprivation leads to more advanced-stage disease at presentation and worse survival once therapy is initiated leading to a significantly worse overall survival and a possible link between deprivation and the well-established and very important CLL prognostic markers of high B2M and adverse cytogenetics (11q and 17p deletions).
Table 1.
Prognostic marker frequency in the deprivation quartiles.
Why deprivation should impact the stage and age at presentation is unknown. Given the age at diagnosis for the most deprived is almost identical whether they presented with early- or advanced-stage disease (65 vs. 67 years) and that the least deprived group with advanced-stage disease presented 10 years younger (57 vs. 67 years) than the most deprived group, strongly suggests deprivation for whatever reason(s) leads to delayed presentation. Higher risk lifestyle behaviors e.g., smoking, obesity leading to other symptomatic illnesses increase with deprivation.5-9 Comorbidities are very common in CLL patients and may mimic the vague symptoms of CLL and hence reassure the patient there is not another pathology, leading to a delay in presentation.10 Alternatively, deprivation may increase fear of cancer or its treatments, alter the willingness of patients to take time off work to have their symptoms investigated, or perhaps may lead to inequalities in accessing health care, all of which would contribute to advanced-stage disease at presentation.5-9
Why deprivation should lead to worse overall clinical outcomes and worse outcomes once therapy has been initiated is also unknown. Comorbidities e.g., heart or lung disease etc. may contribute to a worse outcome once therapy is required by reducing the choices of available therapies or by reducing the duration or doses of therapy a patient may be able to tolerate. Furthermore, deprivation has been shown to impact patient compliance e.g., not attending hospital (routinely or urgently when unwell with infections) due to concerns about missing work and/or travel costs.5-9 Deprivation is not routinely formally assessed in CLL patients requiring treatment but given the significant impact deprivation has on overall survival and survival from treatment, increased medical staff awareness, additional patient education and increased attention to any potential compliance issues an individual patient may have, may improve outcomes.
The higher B2M and the frequency of adverse cytogenetics may be due to more advanced-stage disease at presentation but the trend for deprivation to impact the frequency of unmutated IGHV genes (34.8% vs. 26.5%) perhaps suggests alternative possibilities that warrant larger studies to assess if deprivation alters the frequency of clinically impactful stereotypes (especially those associated with 11q and/or 17p deletions) or driver mutations.10-12 For example, we know that as in other parts of the world, in Wales deprivation directly impacts air quality and that poor air quality can alter driver mutation expression.13,14
Figure 1.
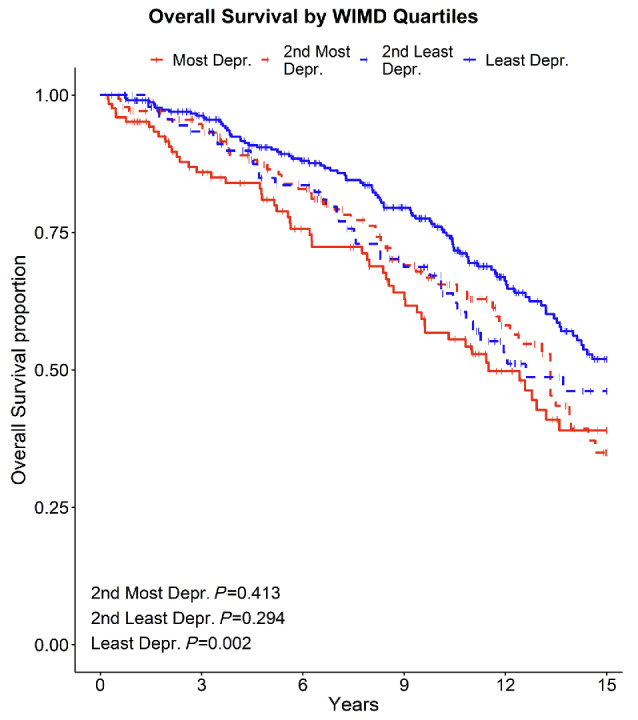
Overall survival between four quartile deprivation groups. WIMD: Welsh Index of Multiple Deprivation; Depr.: deprived.
Figure 2.

Survival since time of first treatment between four quartile deprivation groups. WIMD: Welsh Index of Multiple Deprivation; Depr.: deprived.
Finally, medical staff bias can also not be ruled out although this seems unlikely given that TTFT and access/participation into clinical trials is not significantly different between the deprivation groups.15
Great strides are being made in the treatment and outcomes of CLL patients with B-cell receptor and BCL-2 inhibitors, but if we wish to improve overall survival in all our CLL communities, then further research aimed at understanding and addressing the clinical impacts of social deprivation will be essential.
Supplementary Material
Funding Statement
Funding: This study was sponsored by Cardiff University with support from Swansea University (to GF and TD) and Cancer Research Wales (to CF) with financial support from a donation from J. Parker.
Data-sharing statement
The datasets generated during and/or analyzed during the current study are not publicly available as they contain personal patient information but are available from the corresponding author on reasonable request.
References
- 1.Nabhan C, Aschebrook-Kilfoy B, Chiu BC, et al. The impact of race, ethnicity, age and sex on clinical outcome in chronic lymphocytic leukemia: a comprehensive surveillance, epidemiology, and end results analysis in the modern era. Leuk Lymphoma. 2014;55(12):2778-2784. [DOI] [PubMed] [Google Scholar]
- 2.Moreau EJ, Matutes E, A’Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 1997;108(4):378-382. [DOI] [PubMed] [Google Scholar]
- 3.Schuh AH, Parry-Jones N, Appleby N, et al. Guideline for the treatment of chronic lymphocytic leukaemia: a British Society for Haematology Guideline. Br J Haematol. 2018;182(3):344-359. [DOI] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Kay NE, Rabe KG, et al. Hematologist/oncologist disease-specific expertise and survival: lessons from chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Cancer. 2012;118(7):1827-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCutchan GM, Wood F, Edwards A, Richards R, Brain KE. Influences of cancer symptom knowledge, beliefs and barriers on cancer symptom presentation in relation to socioeconomic deprivation: A systematic review. BMC Cancer. 2015;15:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118(8): 1130-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaife SL, Winstanley K, Robb KA, et al. Socioeconomic inequalities in attitudes towards cancer: an international cancer benchmarking partnership study. Eur J Cancer Prev. 2015;24(3):253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009;101(Suppl 2):S92-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49(1):49-56. [DOI] [PubMed] [Google Scholar]
- 10.Stamatopoulos K, Belessi C, Moreno C. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: pathogenetic implications and clinical correlations. Blood. 2007;109(1):259-270. [DOI] [PubMed] [Google Scholar]
- 11.Jaramillo S, Agathangelidis A, Schneider C, et al. Prognostic impact of prevalent chronic lymphocytic leukemia stereotyped subsets: analysis within prospective clinical trials of the German CLL Study Group (GCLLSG). Haematologica. 2020;105(11):2598-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landau DA, Wu CJ. Chronic lymphocytic leukemia: molecular heterogeneity revealed by high-throughput genomics. Genome Med. 2013;5(5):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton A, Jones SJ, Brunt H. Air pollution and public health vulnerabilities, susceptibilities and inequalities in Wales, UK. J Public Health (Oxf). 2023;45(2):432-441. [DOI] [PubMed] [Google Scholar]
- 14.Hill W, Lim EL, Weeden CE, et al. Lung adenocarcinoma promotion by air pollutants. Nature. 2023;616(7955):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014; 111(9):1684-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available as they contain personal patient information but are available from the corresponding author on reasonable request.