Abstract
Complications occurring after lymphodepleting chemotherapy (LDC) may delay chimeric antigen receptor (CAR) T-cell infusion. The effect of these delays on clinical outcomes is unclear. We performed a retrospective analysis of 240 patients with relapsed/refractory large B-cell lymphoma treated with standard-of-care axicabtagene ciloleucel (axi-cel) and identified 40 patients (16.7%) who had delay in axi-cel infusion. Of these, 85% had delay due to infection. At time of LDC initiation, patients with delayed infusion had lower absolute neutrophil count (P=0.006), lower platelets (P=0.004), lower hemoglobin (P<0.001) and higher C-reactive protein (P=0.001) than those with on-time infusion. Patients with delayed infusion had lower day 30 overall response rates (59.0% vs. 79.4%; P=0.008) and shorter median progression-free survival (PFS) (3.5 vs. 8.2 months; P=0.002) and overall survival (7.8 vs. 26.4 months; P=0.046) than those with on-time infusion. The association with PFS was maintained on multivariate analysis. There was also an association between extent of delay and survival, with shorter median PFS in patients who had delays of 2-5 days (1.8 vs. 8.2 months; P=0.001) and >5 days (4.6 vs. 8.2 months; P=0.036), but not 1 day (5.7 vs. 8.2 months; P=0.238). Following propensity score matching, patients with delayed infusion continued to have shorter median PFS (3.5 vs. 6.0 months; P=0.015). Levels of pro-inflammatory cytokines on day of infusion were significantly higher in patients with delayed infusion. Together, these findings suggest that delays in CAR T-cell administration after initiation of LDC are associated with inferior outcomes. Further studies are needed to guide strategies to improve efficacy in such patients.
Introduction
Prior to chimeric antigen receptor (CAR) T-cell infusion, a conditioning regimen of lymphodepleting chemotherapy (LDC) is typically administered. This LDC regimen is critical for CAR T-cell efficacy and functions through multiple mechanisms, including alterations in circulating cytokine levels and effects on recipient lymphocytes, myeloid-derived suppressor cells (MDSC) and other regulatory cells.1-4 While the importance of LDC is well-established, the optimal dose and timing of LDC have not been conclusively determined and guidelines for LDC administration in the clinical setting vary by product and indication. The guidelines for axicabtagene ciloleucel (axi-cel) are most stringent, dictating that fludarabine and cyclophosphamide be administered on days -5, -4 and -3 prior to CAR T-cell infusion.5 In contrast, the guidelines for lisocabtagene maraleucel (liso-cel) and tisagenlecleucel (tisa-cel) provide a range of acceptable infusion dates, with liso-cel infusion recommended to occur 2-7 days after LDC completion and tisa-cel infusion recommended to occur 2-11 days after LDC completion for diffuse large B-cell lymphoma and 2-6 days after LDC completion for follicular lymphoma.6,7 Despite best efforts, clinical and logistical complications occurring after LDC administration may delay CAR T-cell infusion. The effect of these delays on clinical outcomes is unclear and no guidelines are available regarding how long of a delay is permissible before additional LDC is required. Previous studies have reported that longer time from leukapheresis to cell infusion (vein-to-vein time) is associated with worse outcomes following axi-cel therapy.8 However, vein-to-vein time includes product manufacturing time as well as logistical delays preceding LDC and is not a sensitive measure to assess the impact of delays after LDC on CAR T-cell efficacy. Thus, we performed a single-center retrospective study to examine the impact of delays in cell infusion after LDC administration on clinical outcomes and cytokine levels in large B-cell lymphoma (LBCL) patients treated with axi-cel.
Methods
Patient selection and assessment
This is a retrospective cohort analysis of 240 consecutive patients with relapsed or refractory LBCL treated with standard-of-care (SOC) axi-cel at our institution between January 2018 and December 2021. SOC was defined as administration of commercial product outside of a clinical trial. The study was approved by the Institutional Review Board of MD Anderson Cancer Center and conducted in accordance with our institutional guidelines and the principles of the Declaration of Helsinki. Delayed cell infusion was defined as axi-cel infusion occurring ≥ 6 days after the initiation of LDC (i.e., after the originally scheduled day 0) and the extent of delay was calculated accordingly (e.g., axi-cel infusion the day following the originally scheduled day 0 represents a 1-day delay). Baseline characteristics for all patients were collected on the day of initiation of LDC. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded for up to 30 days after axi-cel infusion, according to the CARTOX grading system from January 2018 to April 2019, and according to ASTCT criteria from May 2019 onward.9,10 Performance status was defined according to the Eastern Cooperative Oncology Group (ECOG).11 Response status was determined by the Lugano 2014 classification.12
Quantification of cytokine levels
Cytokine levels were quantified by immunoassay of plasma samples from the day of axi-cel infusion collected prior to cell infusion. Quantification was performed using MSD V-Plex Cytokine Panel 1 Human and Proinflammatory Panel 1 Human kits (Meso Scale Diagnostics, Rockville, MD).
Statistical methods
Association between categorical variables was evaluated using a χ2 test or Fisher’s exact test. Differences in continuous variables between patient groups were evaluated by the Mann-Whitney U test (2 groups) or Kruskal-Wallis test (3 or more groups). For cytokine analyses, false discovery rate (FDR) q values were calculated to account for multiple comparisons. Progression-free survival (PFS) was defined as the time from the start of axi-cel infusion to progression of disease, death, or last follow-up (whichever occurred first). Overall survival (OS) was defined as the time from the start of axi-cel infusion to death or last follow-up. PFS and OS were calculated for all patients in the study and for subgroups of patients using Kaplan-Meier estimates and were compared between subgroups using the log-rank test. Only factors significant (P≤0.05) on univariate analysis were included in multivariate models. Propensity score matching of patients with on-time infusion to those with delayed infusion was performed based on variables which differed significantly between the two groups. A propensity score was calculated using logistic regression and patients with on-time infusion were matched 3:1 with patients who had delayed infusion. Statistical analyses were completed using SPSS 24, GraphPad Prism 8, and R version 4.1.1.
Results
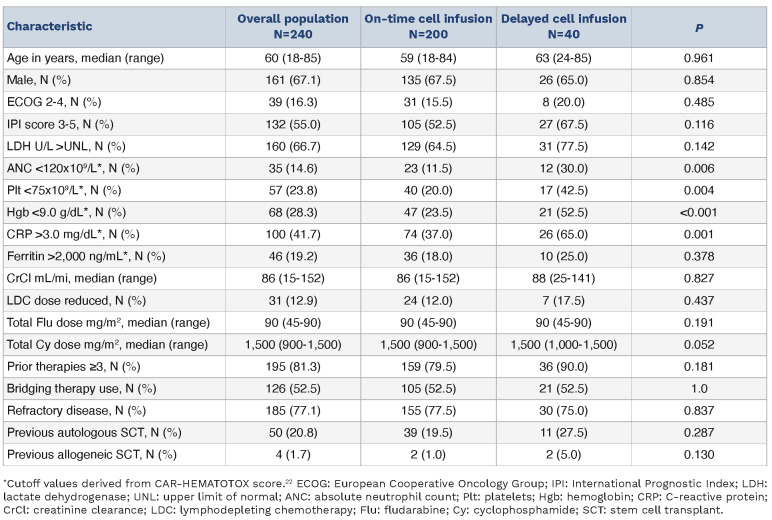
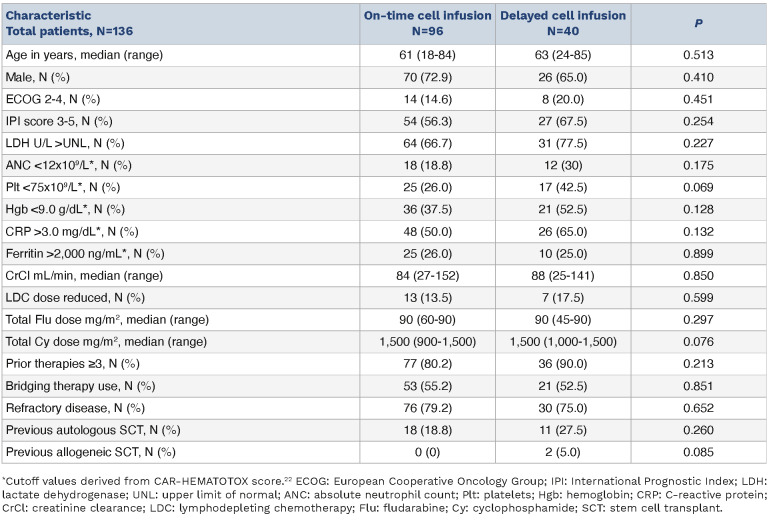
Two hundred and forty patients with relapsed or refractory LBCL received SOC axi-cel between January 2018 and December 2021. Of these, 40 (16.7%) had a delay in axi-cel infusion, defined as infusion occurring ≥6 days following initiation of LDC. The extent of infusion delay in these patients is shown in Figure 1. The reasons for delay included concern for active infection (e.g., fever, pneumonia, sepsis) in 34 patients (85%), need for disease-related procedures (e.g., thoracentesis, radiation therapy) in three patients (7.5%), and logistical reasons in three patients (7.5%). Baseline characteristics at time of LDC initiation in patients with on-time and delayed cell infusion are shown in Table 1. On univariate analysis, patients with delayed cell infusion had lower absolute neutrophil count (ANC; P=0.006), lower platelets (P=0.004), lower hemoglobin (P<0.001) and higher C-reactive protein (CRP; P=0.001) than patients with on-time cell infusion. On multivariate analysis, low ANC (P=0.025) and elevated CRP (P=0.037) remained associated with infusion delay. No difference in baseline ferritin levels was noted between the two groups (P=0.378). At the time of CAR T-cell infusion, median absolute lymphocyte count (ALC) was 30 cells/mL (range, 0-1,900) in patients with ontime infusion and 0 cells/mL (range, 0-100) in patients with delayed cell infusion, demonstrating a lack of significant lymphocyte recovery by the time of cell infusion in both populations.
Figure 1.
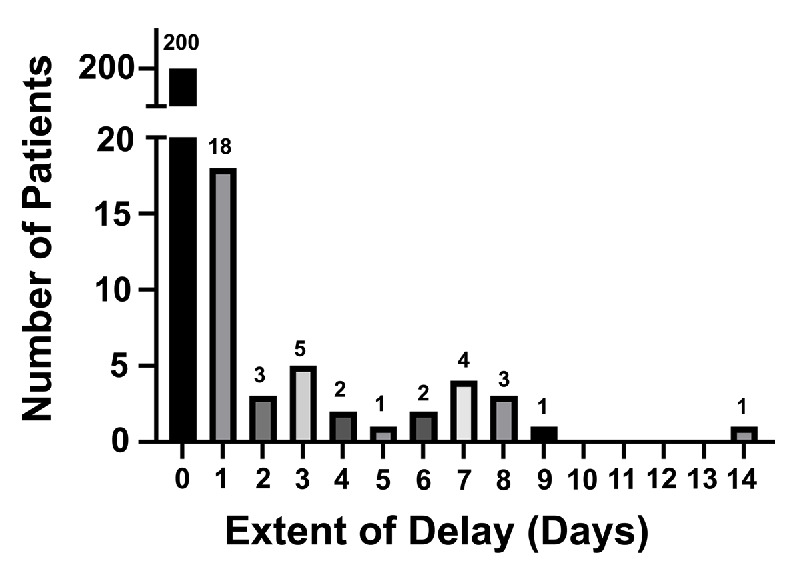
Extent of infusion delay in patients receiving axicabtagene ciloleucel.
Table 1.
Baseline characteristics of overall populations.
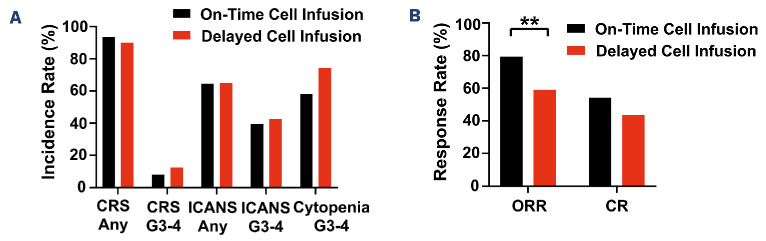
Patients with delayed cell infusion had similar rates of any-grade CRS (90% vs. 93.5%), grade 3-4 CRS (12.5% vs. 8.0%), any-grade ICANS (65% vs. 64.5%) and grade 3-4 ICANS (42.5% vs. 39.5%) compared to patients with ontime cell infusion (Figure 2A). However, there was a trend toward increased rate of grade 3-4 cytopenias at day 30 in patients with delayed cell infusion compared to those with on-time cell infusion (74.3% vs. 58.0%; P=0.09). Patients with delayed cell infusion had a significantly lower day 30 overall response rate (59.0% vs. 79.4%; P=0.008) and numerically lower complete response rate (43.6% vs. 54.3%) than those with on-time cell infusion (Figure 2B).
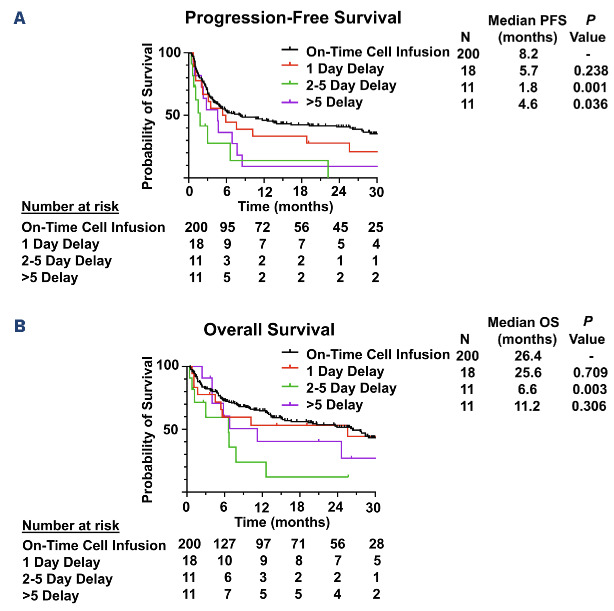
After a median follow-up of 25.7 months (95% confidence interval [CI]: 22.6-28.8), patients with delayed infusion had significantly shorter median PFS (3.5 vs. 8.2 months; P=0.002) and OS (7.8 vs. 26.4 months; P=0.046) compared to those with on-time infusion. The majority of deceased patients in both groups had disease recurrence/progression documented as their cause of death (Online Supplementary Table S1).
An association between extent of delay and survival was observed, with significantly shorter median PFS in patients who had delay of 2-5 days (1.8 vs. 8.2 months; P=0.001) and >5 days (4.6 vs. 8.2 months; P=0.036) but no significant difference in median PFS for patients with a delay of 1 day (5.7 vs. 8.2 months; P=0.238) compared to those with on-time infusion (Figure 3). Patients with a delay of 2-5 days also had significantly shorter median OS (6.6 vs. 25.6 months; P=0.003) compared to those with on-time infusion. The association between delayed infusion and shorter PFS was maintained on multivariate analysis including age, International Prognostic Index score, lactate dehydrogenase (LDH) and CRP (hazard ratio [HR]=1.567; 95% CI: 1.045-2.351; P=0.03). When comparing patients with a delay of 1 day, 2-5 days, and >5 days, the only differences in baseline characteristics were that patients with a delay of >5 days were more likely to have low ANC (P=0.010) and patients with a delay of 1 day were more likely to have a prior autologous stem cell transplant (P=0.027) than patients in the other two groups (Online Supplementary Table S2). Concern for infection remained the predominant reason for infusion delay in all three groups (Online Supplementary Table S3). All logistical delays resulted in only 1 day infusion delay and all delays >5 days were due to infection.
Figure 2.
Toxicity and response rates of patients with on-time (N=200) and delayed (N=40) cell infusion. (A) Toxicity of patients with on-time and delayed cell infusion. (B) Response rates of patients with on-time and delayed cell infusion. CRS: cytokine release syndrome; ICANS: immune effector cell-associated neurotoxicity syndrome; ORR: overall response rate; CR: complete response; **P<0.01
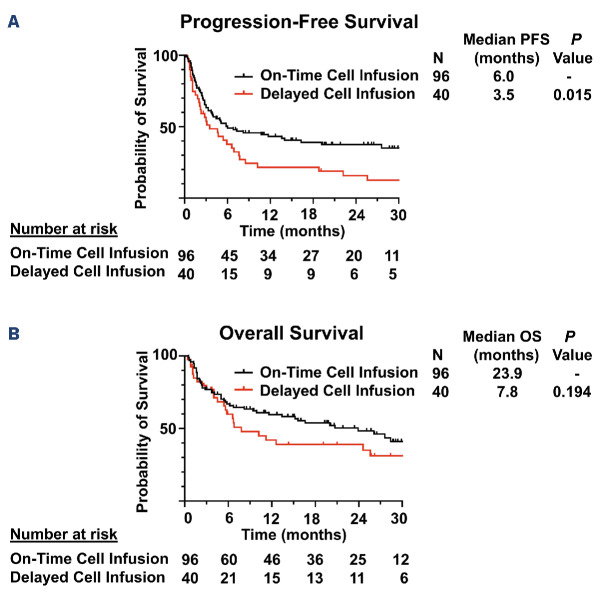
As the baseline characteristics of patients with delayed infusion were noted to differ from those with on-time infusion, propensity score matching was performed based on variables which differed significantly between the two groups: baseline ANC, platelets, hemoglobin and CRP. No significant differences in the characteristics of the matched cohorts were identified (Table 2). In the matched cohorts, patients with delayed infusion had significantly shorter median PFS (3.5 vs. 6.0 months; P=0.015) and a trend towards shorter OS (7.8 vs. 23.9 months; P=0.194) compared to those with on-time infusion (Figure 4).
Figure 3.
Progression-free and overall survival of all patients with on-time and delayed cell infusion. (A) Progression-free survival (PFS) of all patients with on-time and delayed cell infusion. (B) Overall survival (OS) of all patients with on-time and delayed cell infusion.
Table 2.
Baseline characteristics of matched populations.
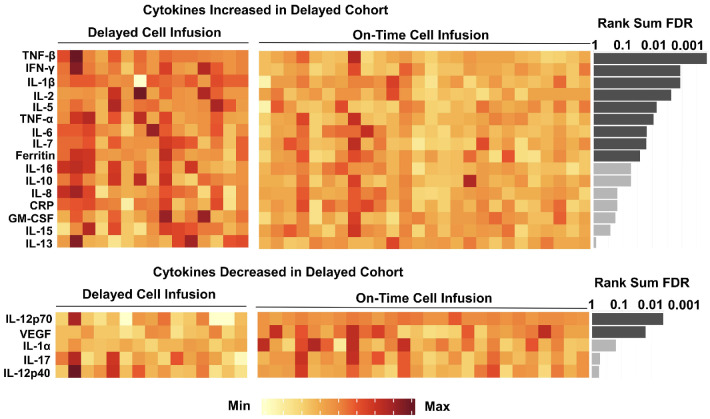
In order to further investigate the impact of delayed cell infusion on biological factors known to influence CAR T-cell efficacy, plasma cytokine levels on the day of cell infusion from 41 patients (15 with delayed infusion [6 with 1 day delay, 6 with 2-5 day delay and 3 with >5 day delay], 26 with on-time infusion) were measured and compared between groups (Figure 5). Levels of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), TNF-|3, interferon-y (IFN-y), interleukin-1|3 (IL-1|3), IL-2, IL-5, IL-6, IL-7 and ferritin were significantly higher in patients with delayed cell infusion than in patients with on-time cell infusion (q<0.05). In contrast, levels of IL-12p70 and vascular endothelial growth factor (VEGF) were significantly lower in patients with delayed cell infusion (q<0.05).
Discussion
In this study, we showed that delays in cell infusion occurring after initiation of LDC were associated with inferior outcomes in patients receiving standard-of-care axi-cel for LBCL. These delays were relatively common, occurring in 16.7% of patients in our institutional cohort, and were most frequently due to concern for infection. Despite infection being the most common reason for delay, the most common cause of death for patients with either ontime or delayed infusion remained disease recurrence/ progression. Unlike previous studies that examined the association between vein-to-vein time and outcomes,8 our study focused on the time period between initiation of LDC and cell infusion. This period excludes the time required for CAR T-cell manufacturing as well as logistical delays in obtaining the CAR T-cell product and scheduling CAR T-cell administration. Instead, this relatively short period represents a time in which patients are actively receiving chemotherapy and in which unexpected clinical deterioration may force providers to decide whether to proceed with CAR T-cell infusion under suboptimal conditions or delay until the patient’s clinical status improves. Little evidence has been available regarding the consequences associated with either strategy. Our study is the first to show that patients with delayed cell infusion have inferior survival outcomes.
Due to the retrospective nature of this study, it is difficult to determine how much of this difference in outcomes is driven by differences in the baseline characteristics of our study populations. Previous studies examining the impact of bridging therapy prior to CAR T-cell administration have faced similar challenges as patients requiring bridging therapy typically have more aggressive disease, which predisposes them to worse outcomes.13,14 In our case, there were no differences in disease-related characteristics such as IPI score, elevated LDH, use of bridging therapy or refractory disease between patients with on-time and delayed infusion. Instead, patients with delayed cell infusion had lower ANC, lower platelets, lower hemoglobin and higher CRP at time of LDC initiation compared to those with ontime infusion. These findings are consistent with a pro-inflammatory state and compromised bone marrow function which could predispose patients to develop infections which, in turn, lead to CAR T-cell infusion delays. In order to adjust for these differences, we conducted a propensity score matched analysis based on these variables which continued to demonstrate shorter PFS in patients receiving delayed cell infusion. While prospective data are still needed, this finding suggests that the inferior outcomes noted in this patient population are not entirely due to differences in their baseline characteristics.
Figure 4.
Progression-free and overall survival of propensity score matched cohorts. (A) Progression-free survival (PFS) of propensity score matched cohorts. (B) Overall survival (OS) of propensity score matched cohorts.
Our study also identified an association between extent of delay and survival, with significantly shorter median PFS noted in patients who had delay of 2-5 days and >5 days, but not delay of 1 day. These results must be interpreted with caution given the small number of patients involved.
This limitation also complicates efforts to understand the differences between these subgroups. For instance, although the general characteristics of each subgroup were broadly similar, only the 1-day delay group contained patients with logistical delays. Nevertheless, only three patients were delayed for this reason and 77% of the patients in this subgroup were delayed due to concern for infection. Similarly, patients with delays >5 days were more likely to have low ANC than patients in the other two subgroups but patients with delays of 1 day and 2-5 days had similar rates of low baseline ANC. The limitations of subgroup analysis are especially important to note with our cytokine analysis, where sample availability has further decreased the number of patients in each group. For these reasons, we are unable to draw any conclusions regarding differences in cytokine levels among these subgroups. Nevertheless, it remains possible that time-dependent changes in cytokine profiles may impact CAR T-cell efficacy. The time course of cytokine changes induced by LDC is particularly important given the disparate recommendations for timing of CAR T-cell administration by product.57 Larger studies are needed to elucidate the relationship between timing of CAR T-cell infusion, systemic cytokine milieu, and clinical outcomes.
Our analysis of plasma cytokine levels on the day of cell infusion demonstrated significantly higher levels of pro-inflammatory cytokines (e.g., TNF-α, TNF-|3, IFN-y, IL-1|3, IL-2, IL-5, IL-6, IL-7, ferritin) in patients with delayed cell infusion compared to those with on-time infusion. These results are suggestive of a systemic pro-inflammatory state and are consistent with the finding that most infusion delays were due to concern for infection. Although some cytokines, such as IL-7, have been shown to improve CAR T-cell function in isolation, pro-inflammatory states manifested by high levels of plasma IL-6 and ferritin have previously been associated with poor CAR T-cell expansion and lower durable response rates after administration of axi-cel.4,15 Multiple mechanisms have been proposed to explain this association, including increased numbers of circulating MDSC and increased intra-tumoral expression of immune checkpoint ligands driven by inflammatory signaling pathways. In particular, macrophages and the cytokines they produce play a complex role in determining CAR T-cell efficacy.16 Macrophage gene expression within the tumor microenvironment has been closely tied to intra-tumoral IFN signaling, and the presence of high numbers of intra-tumoral macrophages has been associated with poor CAR T-cell expansion, impaired tumor infiltration and inferior outcomes with a variety of CAR T-cell products.4,17,18
Figure 5.
Cytokine levels on the day of axi-cel infusion in patients with delayed (N=15) and on-time (N=26) cell infusion. Each column represents a single patient. Significance level was tested by Mann-Whitney U test. False discovery rate (FDR) q value was calculated for multiple testing correction. Dark grey bars are statistically significant at a level of q<0.05. Light grey bars are not statistically significant at a level of q<0.05.
Due to the key role IFN-γ plays in inflammatory signaling, several groups have examined the impact of IFN-γ blockade on CAR T-cell efficacy and toxicity. Although large-scale clinical trials have not yet been performed, preclinical data and case reports suggest that IFN-γ blockade with the antibody emapalumab may ameliorate CAR T-cell toxicity without compromising efficacy.19-21 Furthermore, systemic pro-inflammatory states and IFN-γ signaling in particular have been associated with the development of prolonged cytopenia after CAR T-cell therapy.22,23 This finding may explain the trend toward increased rates of grade 3-4 cytopenias at day 30 in patients receiving delayed cell infusion. Given the increased level of circulating IFN-γ in patients with delayed cell infusion, the impact of IFN-γ blockade in these patients would be another interesting area to explore.
In the event of unavoidable delays, it is important to carefully consider whether to proceed with cell infusion immediately or to further delay infusion until all active issues have resolved and the patient is able to receive another course of LDC. Although our data suggest that delays in cell infusion are associated with inferior outcomes, proceeding with cell infusion in the setting of active infection has also been associated with poor outcomes and is not recommended.24-26 Further studies are needed to inform this decision-making process, which will require consideration of the reason for delay as well as the patient’s clinical characteristics, disease course and laboratory findings. Additional studies are also needed to determine whether delayed cell infusion has a similar impact on outcomes with other CAR T-cell products or lymphodepleting agents (such as bendamustine). Furthermore, as novel conditioning strategies and interventions targeting inflammatory pathways (such as IFN-γ blockade) are developed, their application in patients experiencing unavoidable delays in cell infusion would be a promising area of investigation.
We acknowledge multiple limitations of this study, including its single-center and retrospective nature, its focus on a single CAR T-cell product and lymphodepleting regimen, the small size of certain subgroups, and the lack of data regarding CAR T-cell expansion and natural killer cell reconstitution.
In conclusion, our data suggest that delays in axi-cel infusion following administration of LDC, particularly those lasting ≥2 days, are associated with inferior survival outcomes and increased pro-inflammatory milieu. Further studies are needed to guide management of this patient population and determine whether additional conditioning strategies or other interventions directed at improving CAR T-cell function in these patients would be beneficial.
Supplementary Material
Funding Statement
Funding: This research was supported in part by the University of Texas MD Anderson Cancer Center support grant from the National Institutes of Health (P30 CA016672) and by the University of Texas MD Anderson Cancer Center B-cell Lymphoma Moonshot (SSN). PS salary is supported by the Lymphoma Research Foundation Career Development Award, the Leukemia Lymphoma Society Scholar in Clinical Research Career Development Program, the Sabin Fellowship Award, and by an R21 NIH grant. APJ is supported by a Conquer Cancer Foundation Young Investigator Award, a Lymphoma Scientific Research Mentoring program grant from the Lymphoma Research Foundation, and a training grant from the National Institutes of Health (T32 CA009666). The MD Anderson Lymphoma Tissue Bank was utilized in this study and is supported by KW Cares.
Data-sharing statement
De-identified data are available upon request by e-mail to the corresponding authors.
References
- 1.Neelapu SS. CAR-T efficacy: is conditioning the key? Blood. 2019;133(17):1799-1800. [DOI] [PubMed] [Google Scholar]
- 2.Hirayama AV, Gauthier J, Hay KA, et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood. 2019;133(17):1876-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strati P, Jallouk AP, Sun R, et al. Impact of conditioning chemotherapy on lymphocyte kinetics and outcomes in LBCL patients treated with CAR T-cell therapy. Leukemia. 2022;36(11):2669-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain MD, Zhao H, Wang X, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood. 2021;137(19):2621-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.YESCARTA (axicabtagene ciloleucel) package insert: https://www.fda.gov/media/108377/download. Accessed Aug 15, 2023. [Google Scholar]
- 6.BREYANZI (lisocabtagene maraleucel) package insert: https://www.fda.gov/media/145711/download. Accessed Aug 15, 2023 [Google Scholar]
- 7.KYMRIAH (tisagenlecleucel) package insert: https://www.fda.gov/media/107296/downloaded. Accessed Aug 15, 2023 [Google Scholar]
- 8.Locke F, Hu ZH, Siddiqi T, et al. Real-world impact of time from leukapheresis to infusion (vein-to-vein time) in patients with relapsed or refractory (r/r) large B-cell lymphoma (LBCL) treated with axicabtagene ciloleucel. Blood. 2022;140(Suppl 1):7512-7515. [Google Scholar]
- 9.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American J Clin Oncol. 1982;5(6):649. [PubMed] [Google Scholar]
- 12.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano Classification. J Clin Oncol. 2014;32(27):3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amini L, Silbert SK, Maude SL, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19(5):342-355. [DOI] [PubMed] [Google Scholar]
- 14.Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4(13):2871-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Zaki A, McCurry D, Strati P. CAR T-cells and macrophages in large B-cell lymphoma: impact on toxicity and efficacy. Leuk Lymphoma. 2023;64(4):808-815. [DOI] [PubMed] [Google Scholar]
- 17.Reiss DJ, Do T, Kuo D, et al. Multiplexed immunofluorescence (IF) analysis and gene expression profiling of biopsies from patients with relapsed/refractory (R/R) diffuse large B cell lymphoma (DLBCL) treated with lisocabtagene maraleucel (liso-cel) in transcend NHL 001 reveal patterns of immune infiltration associated with durable response. Blood. 2019;134(Suppl 1):202. [Google Scholar]
- 18.Yan Z-X, Li L, Wang W, et al. Clinical efficacy and tumor microenvironment influence in a dose-escalation study of anti-CD19 chimeric antigen receptor T cells in refractory B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 2019;25(23):6995-7003. [DOI] [PubMed] [Google Scholar]
- 19.Bailey SR, Vatsa S, Larson RC, et al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov. 2022;3(2):136-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manni S, Del Bufalo F, Merli P, et al. Neutralizing IFNγ improves safety without compromising efficacy of CAR-T cell therapy in B-cell malignancies. Nat Commun. 2023;14(1):3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainone M, Ngo D, Baird JH, et al. Interferon-γ blockade in CAR T-cell therapy–associated macrophage activation syndrome/ hemophagocytic lymphohistiocytosis. Blood Adv. 2023;7(4):533-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell–related hematologic toxicity in relapsed/ refractory large B-cell lymphoma. Blood. 2021;138(24):2499-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strati P, Li X, Deng Q, et al. Prolonged cytopenia following CD19 CAR T cell therapy is linked with bone marrow infiltration of clonally expanded IFNγ-expressing CD8 T cells. Cell Rep Med. 2023;4(8):101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood. 2018;131(1):121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grupp S, Hu Z-H, Zhang Y, et al. Tisagenlecleucel chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory children and young adults with acute lymphoblastic leukemia (ALL): real world experience from the Center for International Blood and Marrow Transplant Research (CIBMTR) and Cellular Therapy (CT) Registry. Blood. 2019;134(Suppl 1):2619. [Google Scholar]
- 26.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data are available upon request by e-mail to the corresponding authors.