Abstract
To release transcription factor NF-κB into the nucleus, the mammalian IκB molecules IκBα and IκBβ are inactivated by phosphorylation and proteolytic degradation. Both proteins contain conserved signal-responsive phosphorylation sites and have conserved ankyrin repeats. To confer specific physiological functions to members of the NF-κB/Rel family, the different IκB molecules could vary in their specific NF-κB/Rel factor binding activities and could respond differently to activation signals. We have demonstrated that both mechanisms apply to differential regulation of NF-κB function by IκBβ relative to IκBα. Via alternative RNA processing, human IκBβ gives rise to different protein isoforms. IκBβ1 and IκBβ2, the major forms in human cells, differ in their carboxy-terminal PEST sequences. IκBβ2 is the most abundant species in a number of human cell lines tested, whereas IκBβ1 is the only form detected in murine cells. These isoforms are indistinguishable in their binding preferences to cellular NF-κB/Rel homo- and heterodimers, which are distinct from those of IκBα, and both are constitutively phosphorylated. In unstimulated B cells, however, IκBβ1, but not IκBβ2, is found in the nucleus. Furthermore, the two forms differ markedly in their efficiency of proteolytic degradation after stimulation with several inducing agents tested. While IκBβ1 is nearly as responsive as IκBα, indicative of a shared activation mechanism, IκBβ2 is only weakly degraded and often not responsive at all. Alternative splicing of the IκBβ pre-mRNA may thus provide a means to selectively control the amount of IκBβ-bound NF-κB heteromers to be released under NF-κB stimulating conditions.
Transcriptional induction of genes driven by members of the NF-κB/Rel family of activators plays a crucial role in many processes of the immune response and under a number of cellular stress conditions including viral infection (2, 5, 40, 44). The activation of NF-κB through different pathways is controlled primarily by its release from the interaction with inhibitory IκB molecules. Five different mammalian NF-κB/Rel proteins are known, p50, p52, p65, c-Rel, and RelB, which form homo- and heterodimers. These are sequestered by the IκB molecules IκBα, IκBβ, and IκBɛ and by p105 and p100, the precursor molecules for p50 and p52, respectively (2, 42). Best understood is the activation of NF-κB from complexes with IκBα by diverse inducers like tumor necrosis factor alpha (TNF-α), lipopolysaccharide (LPS), phorbol myristate acetate, (PMA) or human T-cell leukemia virus type 1 Tax (7, 8, 38, 43). These lead to phosphorylation at Ser 32 and 36. Phosphorylated IκBα is then polyubiquitinated and subsequently degraded by the proteasome (3, 9, 34). In addition to the amino-terminal signal response box containing Ser 32 and 36, signal-dependent degradation is more efficient in the presence of the carboxy-terminal PEST sequence of IκBα (7, 8, 38, 43), although the mechanistic need of the latter is not understood. The high turnover of IκBα in the resting state is also driven by the proteasome but is independent of the amino-terminal serines and of the PEST sequence and does not involve ubiquitin conjugation of the molecule (19). Processing of p105 is, at least in some cell lines, signal inducible and involves the same steps as IκBα breakdown, including phosphorylation, ubiquitin conjugation, and degradation by the proteasome (14, 29, 31). An endoprotease activity initially cleaves p105 into p50 and the carboxy-terminal IκB domain (21). A multisubunit kinase, which phosphorylates IκBα at Ser 32 and 36 and which requires ubiquitination of one of its subunits, has been identified biochemically (10). Recently, two IκBα kinases which phosphorylate IκBα at Ser 32 and 36 and mediate NF-κB activation in response to TNF-α have been cloned (24, 35).
The overlapping functions of the various IκB and NF-κB proteins contrast with their specific physiological activities, as revealed by gene-targeting experiments (2). These specific physiological activities imply that functional specificity may be imposed by the differential response of individual IκB or NF-κB/Rel proteins to particular signaling pathways. Therefore, potential regulatory differences between diverse IκBs, such as IκBα and IκBβ, are of interest.
IκBβ was first identified by Zabel and Baeuerle (45), and murine IκBβ was cloned with probes derived from peptides of the purified protein (37). Human cDNAs encoding IκBβ were isolated by using the two-hybrid system (20) (see below). A notable difference between IκBβ and IκBα is the basal phosphorylation of IκBβ, which is needed for interaction with NF-κB (22). Recent reports have shown that IκBα and IκBβ have properties in common. Murine IκBβ is proteolytically degraded after stimulation with LPS or interleukin-1 (IL-1) (37). In contrast to IκBα, IκBβ is not resynthesized immediately after stimulation and is degraded with delayed kinetics compared to IκBα (37). The degradation of murine IκBβ after stimulation with TNF-α, IL-1, or human T-cell leukemia virus type 1 Tax depends on Ser 19 and Ser 23 (12, 25). These residues are embedded in a sequence similar to the one surrounding Ser 32 and 36 in IκBα, and a recently identified IκB kinase phosphorylates both proteins at these residues (13, 33). Furthermore, the carboxy-terminal PEST sequence of IκBβ is needed for efficient proteolysis (16, 41). Inducible degradation of IκBβ is blocked by inhibitors of the proteasome and possibly requires ubiquitination (12, 25). Unphosphorylated recombinant IκBα, but not IκBβ, can associate with Rel factors, and latent cytoplasmic complexes of IκBβ and Rel factors are sensitive to phosphatase treatment. Major sites of constitutive phosphorylation of IκBβ have been mapped to Ser 313 and Ser 315, which are phosphorylated by casein kinase II (CKII) (11). Phosphorylation at these residues is required for efficient complex formation between IκBβ and c-Rel (11). Recombinant unphosphorylated IκBβ can bind to p65 but not to c-Rel (11). Carboxy-terminal phosphorylation by CKII of IκBβ increases its affinity to NF-κB (39). Tax-induced degradation of IκBβ leads to the induction of c-Rel-containing complexes (15), further indicating that c-Rel–IκBβ complexes are physiologically important.
Newly synthesized IκBβ first accumulates as an underphosphorylated species, which forms a stable complex with NF-κB p65 (36). It has been shown that in contrast to the phosphorylated form, underphosphorylated IκBβ interacts with p65 without shielding the nuclear localization signal region. De novo-translated, underphosphorylated IκBβ may sequester NF-κB, thereby protecting it from being withdrawn into cytoplasmic complexes with IκBα, and it can be transported with NF-κB into the nucleus (36).
We show here that in human cells the IκBβ gene gives rise to different gene products, which elicit differential responsiveness to several inducers tested. Two of these, termed IκBβ1 and IκBβ2, have been analyzed in detail. Compared to IκBα, IκBβ1 is most similar in responsiveness to inducing agents whereas IκBβ2, which has a carboxy-terminal sequence substitution truncating a PEST domain, is either degraded incompletely or inert in response to some inducers. Both IκBβ1 and IκBβ2 bind with highest affinity to p65 homodimers and with lower affinity to heterodimers containing c-Rel or p65. The affinity depends largely on constitutive phosphorylation at the carboxy-terminal PEST sequences of both molecules. IκBβ1, but not IκBβ2, is partially localized in the nucleus in B cells, indicating different roles of the two isoforms for modulating or maintaining constitutive NF-κB activation. The resistance of IκBβ2, the predominant form in human cells, to a number of inducers tested suggests that this isoform may serve to limit the fraction of cytoplasmic NF-κB released into the nucleus upon cellular stimulation.
MATERIALS AND METHODS
Cell culture.
Namalwa and IM9 cells were grown in RPMI 1640 (BRL/GIBCO)–4 mM glutamine–100 U of penicillin per ml–100 μg of streptomycin per ml–7.5% fetal calf serum; suspension HeLa cells were kept in Joklik modified minimum essential medium (BRL/GIBCO)–1% nonessential amino acids, 100 U of penicillin per ml–100 μg of streptomycin per ml–10% fetal calf serum. Jurkat cells were grown in RPMI 1640 (BRL/GIBCO)–4 mM glutamine–100 U of penicillin per ml–100 μg of streptomycin per ml–10% fetal calf serum. Where indicated, cells were stimulated with 10 ng of TNF-α (Biomol) per ml, 50 ng of PMA (Sigma) per ml–5 μg of ionomycin (Sigma) per ml, or 50 U of IL-1β (Biomol) per ml for the indicated times.
Genomic PCR and reverse transcription-PCR.
PCR was performed by standard methods with IκBβ-specific primers and HeLa cell-derived nucleic acids.
Library screening.
A HeLa cell cDNA library was screened with the insert of the TRIP9 clone isolated by two-hybrid screening (20) as a probe. Positive cDNAs were sequenced on both strands by the dideoxynucleotide method.
DNA constructs for prokaryotic expression.
pETIκBβ2 (encoding amino acids 1 to 338) was generated from pCDM8IκBβ(351/21) by PCR with the appropriate oligonucleotides with the insert cloned into the BamHI site of pET3c (Novagen). Protein expression in Escherichia coli BL21(DE3)pLysS, and purification was carried out as described previously (27).
DNA constructs for eukaryotic cell expression.
pECEp50, pECEp65, pECEc-rel, and pcDNA3IκBα have been described previously (19, 28). FLAG-tagged pcDNA1IκBβ2 (amino acids 1 to 338), FLAG-tagged pcDNA1IκBβ2ΔN (amino acids 55 to 338), FLAG-tagged pcDNA1IκBβ2ΔC (amino acids 1 to 307), and FLAG-tagged pcDNA1IκBβ2ΔNC (amino acid 55 to 307) were generated from pCDM8IκBβ(351/21) by PCR and cloning into the BamHI site of FLAG-tagged pcDNA1, which contained a FLAG epitope inserted into the HindIII and BamHI sites of pcDNA1. FLAG-tagged pcDNA1IκBβ1 was generated by cloning the IκBβ1 cDNA from pCDM8IκBβ(351/1) into pcDNA1. The NF-κB reporter plasmid 2X κB-Luc contained the annealed tandem Igκ enhancer sequence 5′-CAGTTGAGGGGACTTTCCCAGATCTAGTTGAGGGGACTTTCCCAG-3′ and the −45 to +83 fragment of the human immunodeficiency virus type 1 promoter (EcoRI and HindIII sites) inserted into KpnI-HindIII of pGV-B (Toyo Ink Co., Tokyo, Japan).
Antibodies for immunoblots and immunoprecipitation.
We used anti-p65 (rabbit) (Santa Cruz Biotechnology Inc., sc-109), anti-p50 (rabbit) (Rockland), anti-c-Rel (rabbit) (Santa Cruz Biotechnology Inc., sc-272), anti-relB (rabbit) (Santa Cruz Biotechnology Inc., sc-226), anti-p52 (mouse) (Upstate Biotechnology Inc., 05-361), anti-IκBα/MAD3 (rabbit) (Santa Cruz Biotechnology Inc., sc-203), anti-FLAG (rabbit) (Santa Cruz Biotechnology Inc., sc-807), anti-IκBβ (rabbit) (Santa Cruz Biotechnology Inc., sc-969, against the N-terminal sequence of IκBβ1 and IκBβ2 [N-20]), anti-IκBβ (rabbit) (Santa Cruz Biotechnology Inc., sc-945, against the C-terminal sequence of IκBβ1 [C-20]), and the antibody against the C-terminal sequence of IκBβ2 [b2(C)], raised against the peptide VSQEERQGSPAGGSG (amino acids 324 to 338 of IκBβ2, synthesized by Eurogentec S.A.).
Preparation of cell extracts.
For whole-cell extracts, cells were washed with phosphate-buffered saline (PBS) twice and incubated on ice for 15 min in 20 mM HEPES (pH 7.9)–350 mM NaCl–1 mM MgCl2–0.5 mM EDTA–0.1 mM EGTA–1% Nonidet P-40 (NP-40)–0.5 mM dithiothreitol–0.4 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Boehringer Mannheim)–50 mM sodium fluoride–1 mM sodium orthovanadate. After centrifugation at 14,000 rpm for 20 min in an Eppendorf centrifuge, the supernatant was used as a whole-cell extract. For preparation of cytoplasmic and nuclear extracts, the cells were washed and resuspended in buffer A and 0.125% NP-40 was added. The cells were left for 5 min on ice and centrifuged at 1,000 × g for 10 min. The supernatant was used as the cytoplasmic extract, and the pellet was treated with buffer C for 15 min to yield the nuclear extract as described previously (27).
Immunoblots.
Cell extracts (40-μg samples) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and prior to transfer, the gels were equilibrated in ice-cold blotting buffer (25 mM Tris-HCl [pH 8.3], 0.01% SDS, 20% methanol). The proteins were transferred to a polyvinylidene difluoride membrane (Millipore) as described previously (28). Western blots were analyzed by chemiluminescence (Tropix) as described previously (19).
Immunoprecipitation.
Antibodies (10-μl samples) were coupled to protein A-Sepharose (Pharmacia) in PBS for 2 h. The whole-cell extract or cytoplasmic extract was incubated for 2 h with antisera or antibodies coupled to protein A-Sepharose. The precipitated proteins were washed several times with ice-cold PBS, boiled, separated by SDS-PAGE, and transferred to a PVDF membrane as described previously (27).
Electrophoretic mobility shift assay (EMSA).
Gel retardation assays were performed with an H2K oligonucleotide probe. DNA binding reactions were performed in 20 μl of binding buffer [20 mM HEPES (pH 8.4), 60 mM KCl, 4% Ficoll, 5 mM dithiothreitol 1 μg of bovine serum albumin, 2 μg of poly(dI-dC)] for 20 min at 30°C. The reaction mixture was loaded onto a 4% nondenaturing polyacrylamide gel containing 1× Tris-borate-EDTA (TBE). The gels were run at 250 V for 1 h, dried, and visualized by autoradiography.
Immunoprecipitation-EMSA (IP-shift) assay.
The precipitated proteins, coupled to protein A beads, were washed several times with ice-cold PBS and incubated in 20 μl of 0.8% deoxycholate on ice for 10 min. After centrifugation, NP-40 (final concentration, 1%) was added to the supernatant, which was then used for EMSA.
Phosphatase treatment of cytoplasmic extracts.
Cell extract proteins (40 μg) were incubated with 2 U of shrimp alkaline phosphatase (United States Biochemical Co.) at 37°C for 30 min. As a control, shrimp alkaline phosphatase was inactivated at 65°C for 30 min.
Transient transfection.
Transfection of suspension HeLa cells was performed by electroporation with a Bio-Rad Gene Pulser. Cells were grown at 5 × 105 cells/ml, harvested 5 to 6 h after the last feeding, and resuspended at 2 × 107 cells/ml. Then 0.4 ml of cells was mixed with 20 μg of DNA at room temperature, electroporated at 250 V and 950 μF, and immediately transferred to small culture flasks containing 10 ml of prewarmed medium. At 48 h later, the transfected cells were harvested. For reporter gene assays, HeLa cells were transfected by electroporation with 2× κB-Luc (10 μg), p65 expression vector (5 μg), and expression vectors containing IκBα, IκBβ1, or IκBβ2 (1 or 5 μg). Luciferase activities were measured with a Luminometer Lumat LB9501 (Berthold).
RESULTS
Human IκBβ is expressed as a set of differentially spliced isoforms.
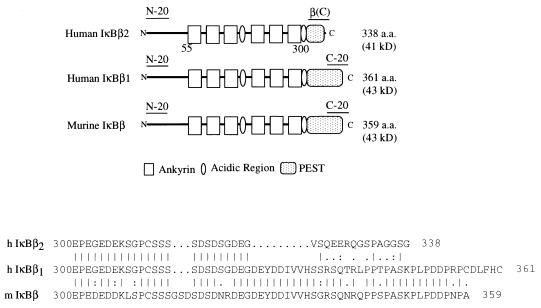
We previously isolated a human homolog of IκBβ by using the human thyroid receptor as a bait in the two-hybrid system (20). Rescreening HeLa cell cDNA libraries with the original yeast isolate yielded additional clones with two distinct 3′ ends. Sequence alignment with murine IκBβ (37) demonstrates that one of these encodes a protein, IκBβ1, that is most similar to the murine protein. The variant IκBβ2 isoform differs from IκBβ1 by a substitution of new C-terminal and 3′ untranslated sequences, which results in truncation of the C-terminal PEST motif (Fig. 1).
FIG. 1.
(Top) Schematic presentation of human IκBβ1 and IκBβ2 compared to murine IκBβ. The position of the peptides used for raising the antibodies is indicated. (Bottom) Sequence alignment of the carboxy-terminal PEST sequences of human IκBβ1 and IκBβ2 and murine IκBβ. a.a., amino acids.
Northern blot analysis of various human tissues with a common probe confirms the existence of two major mRNAs of approximately 1.8 and 2.8 kb (20). The divergence between IκBβ1 and IκBβ2 occurs at a consensus 5′ splice site present only in the IκBβ2 sequence, suggesting that the two transcripts are generated by alternative mRNA processing. Analysis by reverse transcription-PCR and genomic PCR confirms the existence of two transcripts and demonstrates that IκBβ2 retains intronic sequences extending beyond the exon that encodes the sixth ankyrin repeat (data not shown). Since a poly(A) tract is present in the IκBβ1 sequence approximately 150 nucleotides downstream of the site of divergence, while the IκBβ2 sequence extends much further, the former presumably corresponds to the shorter transcript and the latter corresponds to the longer transcript. Overall, these results indicate that the IκBβ gene encodes at least two transcripts as a consequence of alternative mRNA splicing.
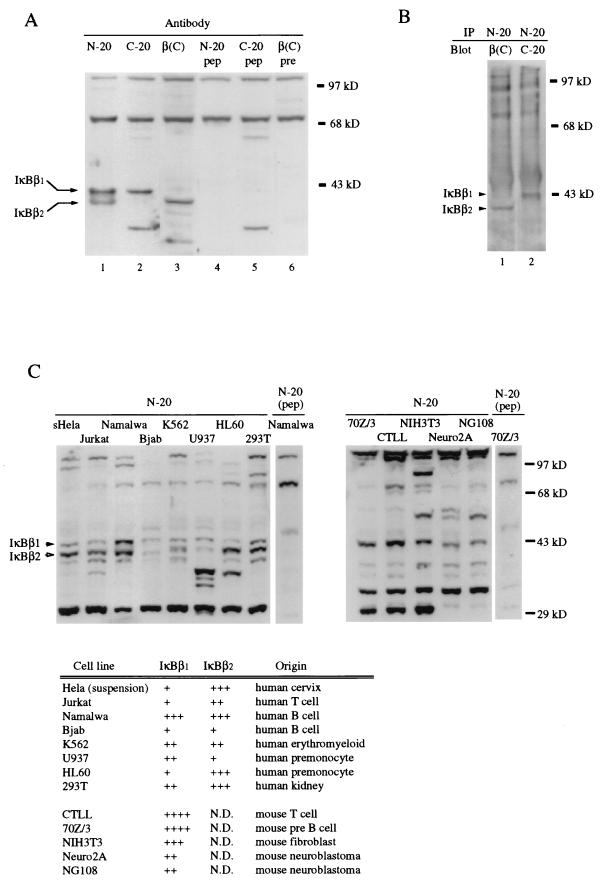
IκBβ1 and IκBβ2 encode proteins of 38 and 35.5 kDa, respectively. To investigate the protein expression of both forms, a Western blot analysis of Namalwa cell cytosol was performed (Fig. 2A). The blot was probed either with an antibody recognizing an amino-terminal sequence common to both forms (N-20, lane 1) or with antibodies raised against the isoform-specific carboxy-terminal sequences (lanes 2 and 3). Two specific bands of 43 and 41 kDa, respectively (IκBβ1 and IκBβ2), were detected. The specificity of the IκBβ1 and IκBβ2 signals was confirmed by peptide competition (lanes 4 to 6). Immunoprecipitation with the N-20 antibody and immunostaining with the isotype-specific antibodies further confirmed the expression of both splicing variants (Fig. 2B). The larger sizes of both isoforms compared to the predicted molecular masses suggest posttranslational modifications of both forms. To compare the relative amounts of IκBβ1 and IκBβ2 in several human and murine cell lines, Western analysis was carried out with the amino-terminal antibody (Fig. 2C). All human cell lines expressed both isoforms (Fig. 2C, left panel). The relative abundance varied (a summary is given in Fig. 2C, bottom panel), but IκBβ2 was the most abundant isoform in most human cell types. In contrast, only IκBβ1 was detected in all the murine cell types tested (Fig. 2C, right panel). In some human cell lines, further signals were detected, some of which may represent further splicing isoforms (data not shown). We conclude that both of the cloned IκBβ transcripts are expressed as proteins and that IκBβ2 is the predominant isoform in human cell lines.
FIG. 2.
Protein expression of IκBβ isoforms. (A) Western blot analysis of Namalwa cell cytosol with an amino-terminal antibody recognizing a common epitope (N-20) or carboxy-terminal antibodies raised against isoform-specific sequences [C-20 for IκBβ1 and β(C) for IκBβ2], as indicated (lanes 1 to 3). Specific bands at 43 and 41 kDa were absent after peptide competition (lane 4 and 5) or probing with preimmune serum (lane 6). (B) Immunoprecipitation. Namalwa cell cytosolic extracts were subjected to immunoprecipitation with N-20, and the precipitates were immunoblotted with C-20 or β(C) antibodies, as indicated. (C) Equal cell equivalents of human and murine cell lines were analyzed by Western blotting for IκBβ isoform expression with the amino-terminal antibody, as indicated. (Left) Human cell lines; (right), murine cell lines; (bottom), summary of the relative expression levels of IκBβ1 and IκBβ2. N.D., not detectable. The signals around 29 kDa are cross-reacting proteins. The positions of IκBβ1 and IκBβ2 are indicated by solid and open arrowheads, respectively.
Both cellular IκBβ isoforms associate predominantly with p65 homodimers but also with heteromers containing c-Rel or p65; equal inhibition of NF-κB-dependent transcription.
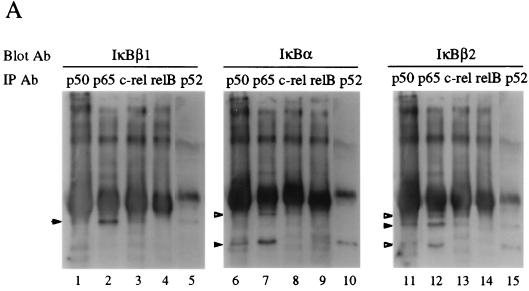
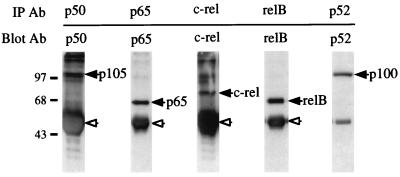
To investigate the preferential association of IκBβ1, IκBβ2, and IκBα with Rel factors, cellular proteins immunoprecipitated with antibodies against the five mammalian NF-κB/Rel subunits were blotted and subsequently probed with IκB-specific antibodies (Fig. 3A). IκBβ1 and IκBβ2 associated predominantly with p65 and only weakly with p50 or p52, presumably through their heteromeric complexes with p65 (lanes 1, 2, 5, 11, 12, and 15). In contrast, almost no RelB and only little c-Rel associated with IκBβ1 or IκBβ2 (lanes 3, 4, 13, and 14), although both Rel proteins were efficiently precipitated (Fig. 3A, bottom). Similarly, IκBα interacted predominantly with complexes containing p65 (lanes 6 to 10). IκBα was found to a larger extent in complexes with p50 or p52 than were IκBβ1 and IκBβ2 (lanes 6 and 10). Similarly, in primary and transformed murine T cells, IκBα and IκBβ (IκBβ1) were associated primarily with p65 and to a lesser extent with c-Rel (11).
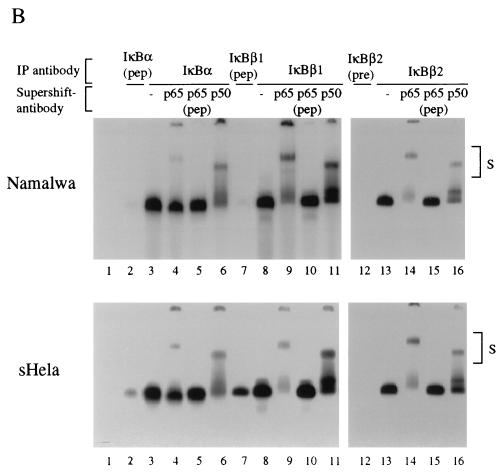
FIG. 3.
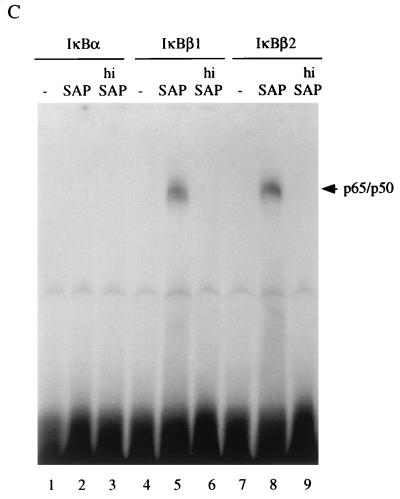
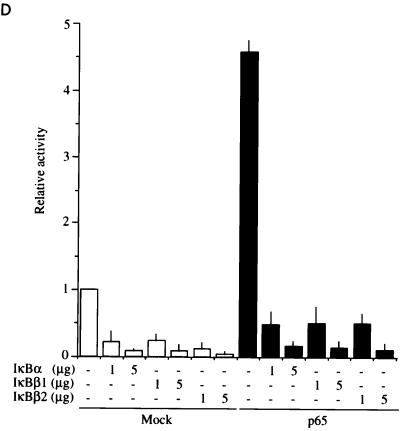
Interaction and functional interference of IκBβ1, IκBβ2, and IκBα with NF-κB/Rel factors (A) Coimmunoprecipitation of IκBs with NF-κB/Rel. Whole-cell Namalwa extracts were subjected to immunoprecipitation with antibodies (Ab) directed against p50, p65, c-Rel, RelB, or p52. (Top) Precipitates were separated by SDS-PAGE, and the blot was sequentially probed with antibodies against IκBβ1 (lanes 1 to 5), IκBα (lanes 6 to 10), and IκBβ2 (lanes 11 to 15). Specific signals are indicated by solid arrowheads; open arrowheads indicate residual signals from the preceding staining. (Bottom) Precipitates from anti-Rel immunoprecipitations were probed with anti-Rel antibodies, as indicated. The position of precipitated immunoglobulin is indicated by open arrowheads. (B) Coimmunoprecipitation/supershift (IP-shift) analysis of IκB-bound Rel factors. (Top) Namalwa cells; (bottom) HeLa cells. Immunoprecipitation was carried out with whole-cell extracts and antibodies directed against IκBα, IκBβ1, or IκBβ2. Bound NF-κB/Rel factors were eluted from the pellets by deoxycholate treatment and analyzed for subunit composition by EMSA and supershifting/inhibition with NF-κB/Rel-specific antibodies. Antibodies against IκBα, IκBβ1, or IκBβ2 precipitated NF-κB/Rel DNA binding activity (lanes 3, 8, and 13), which was strongly reduced or absent when the epitope-containing peptides were included in the precipitation reaction mixture or when the preimmuneserum was used (lanes 2, 7, and 12). DNA binding activity obtained from IκBα (lanes 2 to 6), IκBβ1 (lanes 7 to 11), or IκBβ2 (lanes 12 to 16) was challenged with anti-p65 antibody (lanes 4, 9, and 14), with anti-p65 antibody and peptide competition (lanes 5, 10, and 15), or with anti-p50 antibody (lanes 6, 11, and 16), as indicated. In lanes 1, no protein was added. Free DNA is not shown. Supershifted complexes are indicated (S). (C) Phosphorylation-dependent interaction of IκBβ1 and IκBβ2 with NF-κB in HeLa cells. Whole-cell extracts were subjected to immunoprecipitation with antibodies against IκBα, IκBβ1, or IκBβ2 (lanes 1 to 3, 4 to 6, and 7 to 9, respectively). The beads were treated with shrimp alkaline phosphatase (SAP; lanes 2, 5, and 8), heat-inactivated phosphatase (hi SAP; lanes 3, 6, and 9), or buffer alone (lanes 1, 4, and 7), and the supernatants were tested by EMSA with the H2K binding-site probe. (D) IκBα, IκBβ1, and IκBβ2 equally inhibit NF-κB reporter gene activation by p65. HeLa cells were transfected with 2×κB-Luc reporter without (open bars) or with (solid bars) p65 expression construct. IκBα, IκBβ1, or IκBβ2 expression constructs were cotransfected in increasing amounts, as indicated. The mean values from four independent transfections are shown.
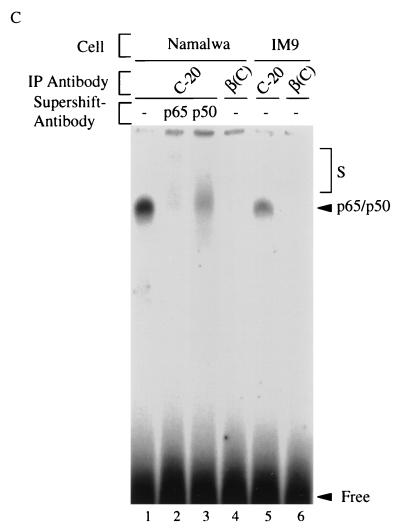
Conversely, Rel factors bound to the three IκB molecules were analyzed by immunoprecipitation with IκB-specific antibodies, followed by detergent elution of the precipitated NF-κB/Rel factors and their identification by antibody supershifting in gel retardation assays (Fig. 3B). In Namalwa cells (upper panel) and HeLa cells (lower panel), the DNA binding complexes retrieved from an anti-IκBα-immunoprecipitation were inhibited and supershifted only partially with an anti-p65 antibody but almost completely with an anti-p50 antibody (compare lanes 3 with lanes 4 and 6). In striking contrast, DNA binding activity derived from IκBβ1 complexes was inhibited or supershifted completely with an anti-p65 antibody but only faintly with an anti-p50 antibody (compare lanes 8 with lanes 9 and 11). The same was observed for DNA binding complexes derived from IκBβ2 (lanes 13 to 16). In no case were there any significant differences between Namalwa or HeLa cells. Thus, IκBβ1 and IκBβ2 are associated almost exclusively with p65-containing heteromeric complexes and presumably to a larger extent with p65 homodimers, whereas IκBα associates predominantly with heteromers containing p50.
The association of cellular IκBβ activity with NF-κB depends on constitutive phosphorylation (22). The subunit composition of the NF-κB DNA binding activity released from HeLa cell cytosol by alkaline phosphatase treatment was similar to that observed with the IκBβ-coprecipitated NF-κB activity. Phosphatase, but not heat-inactivated enzyme, released a κB site-specific band shift activity, which was completely supershifted or inhibited by anti-p65 antibody but was only very weakly affected by anti-p50, anti-p52, or anti-c-Rel antibodies (data not shown). To assess possible differences in the role of basal phosphorylation for the Rel/NF-κB association of both IκBβ isoforms, immune complexes of IκBα, IκBβ1, or IκBβ2 precipitated from HeLa cells were treated with alkaline phosphatase and the supernatants were tested by EMSA (Fig. 3C). Dephosphorylation released NF-κB activity from both IκBβ1 or IκBβ2 but not from IκBα complexes (lanes 2, 5, and 8). No DNA binding activity was obtained with heat-inactivated enzyme (lanes 6 and 9). Therefore, the structural difference between the IκBβ isoforms does not affect the phosphorylation-dependent interaction with NF-κB.
The similar interaction of cellular IκBβ1 or IκBβ2 with NF-κB suggests that they should interfere equally with NF-κB-dependent gene activation. When IκBα, IκBβ1, or IκBβ2 was cotransfected into HeLa cells with p65 and an NF-κB dependent reporter, all three inhibitors strongly reduced p65-driven activation in an almost identical fashion (Fig. 3D). The low-level reporter gene activation by endogenous NF-κB in the absence of transfected p65 was also similarly decreased in the presence of the three IκBs. Thus, IκBβ2 is functional and has the same NF-κB/Rel specificity as IκBβ1.
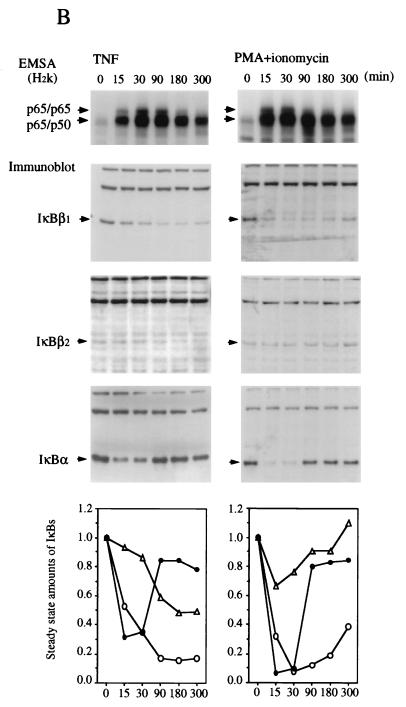
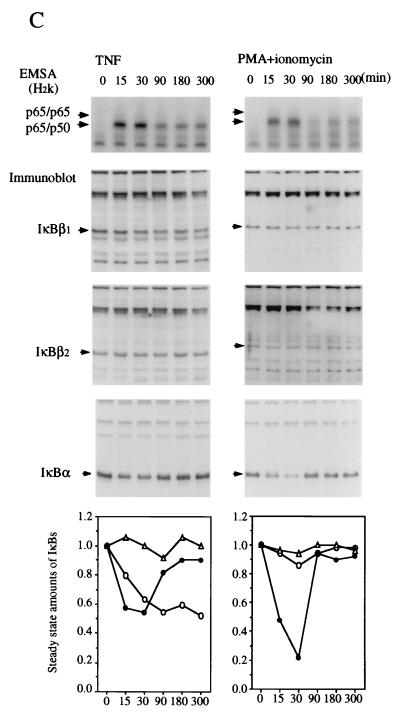
IκBβ1 and IκBα elicit a similar signal responsiveness, whereas IκBβ2 is less responsive or even resistant to signal-induced degradation.
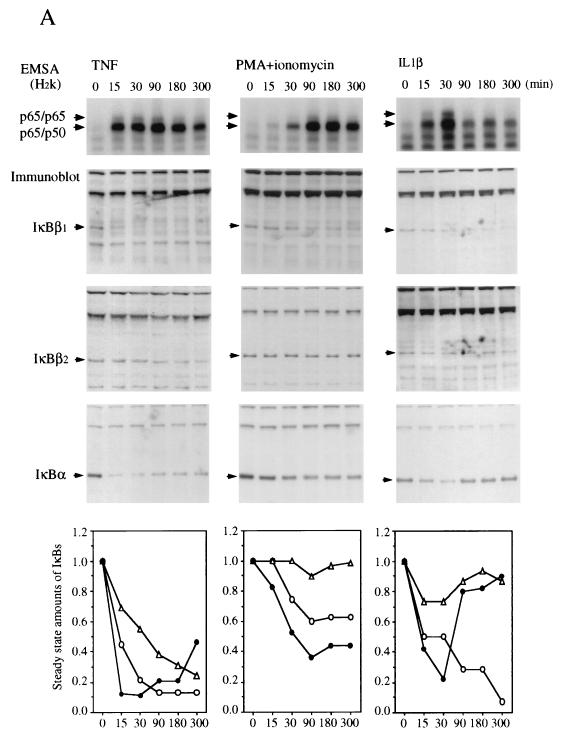
The sequence difference between IκBβ1 and IκBβ2 affects the PEST sequence, which plays a role in signal-dependent degradation in human IκBα (7, 8, 38, 43). To investigate potential differences in signal inducibility, HeLa, Namalwa, or Jurkat cells were stimulated with TNF-α, PMA/ionomycin, or IL-1β for various times and analyzed for nuclear NF-κB accumulation and degradation of IκBα, IκBβ1, and IκBβ2 (Fig. 4). An image quantitation of the steady-state amounts of IκB shown in the Western blots is depicted graphically (Fig. 4, bottom panels). TNF-α or IL-1β stimulation led to a rapid translocation of NF-κB p50-p65 and of p65 homodimers, irrespective of the cell type. In contrast, PMA acted rapidly in Namalwa and Jurkat cells but only slowly in HeLa cells, indicating cell type differences in the PMA/Ca2+ induction mechanism. Similarly, the induction of NF-κB by PMA required 30 to 45 min and was dependent on de novo protein synthesis in Hl60 cells but was fast in 70Z/3 cells (18). The kinetics of nuclear translocation of NF-κB was most often paralleled by a synchronous degradation of IκBα and of IκBβ1. The relative decrease in steady-state amounts, however, was greatest for IκBα, closely followed by IκBβ1. In contrast, IκBβ2 was much less responsive and in some cases even resisted degradation. The identical degradation kinetics suggest that all three inhibitors were degraded by the same mechanism. In line with this conclusion, the delayed activation of NF-κB in PMA-stimulated HeLa cells coincided with simultaneous degradation of IκBα and IκBβ1 at the same delayed time point, while IκBβ2 seemed not to respond (Fig. 4A). Thus, the relative amount of IκBβ2 produced by alternative splicing provides a means of limiting the fraction of IκBβ-bound NF-κB that is released after cellular stimulation.
FIG. 4.
Different efficiencies of signal-induced proteolytic degradation of IκBβ1 and IκBβ2. EMSA (top) and Western blots (whole-cell extracts) (middle) are shown. (A) HeLa cells; (B) Namalwa cells; (C) Jurkat cells. Stimulation of cells with TNF-α, PMA plus ionomycin, or IL-1β was carried out for 0, 15, 30, 90, 180, or 300 min, as indicated. The positions of IκBα, IκBβ1, and IκBβ2 in the Western blots are indicated. (Bottom) Densitometric quantitation of IκBβ1 (open circles), IκBβ2 (triangles), and IκBα (dots). The steady-state amounts of the three IκBs after increasing times of stimulation are plotted, with the amounts at 0 min arbitrarily set at 1.0. Activated NF-κB consisted of p65 homodimers and p50-p65 as determined by antibody supershifting (data not shown).
Only IκBα was significantly and consistently resynthesized within the experimental period (Fig. 4). Resynthesis of IκBβ1 and IκBβ2 was only partially observed in PMA-stimulated Namalwa cells (Fig. 4B). Similarly, murine IκBβ1 was partially resynthesized after initial degradation following LPS stimulation of 70Z/3 pre-B cells (36). The longer time required to fill up the cytosolic IκBβ pool by de novo synthesis may therefore explain the persistent activation of nuclear NF-κB, as has been proposed by Thompson et al. (37). In line with this, the slow and persistent activation of NF-κB by 2-deoxyglucose or brefeldin A, agents that promote the accumulation of proteins in the endoplasmic reticulum (30), is caused by reduced levels of both IκBβ1 and IκBβ2, but not of IκBα, at 24 h after drug administration (data not shown).
IκBβ1 and IκBβ2 are basally phosphorylated but differ in their nuclear accumulation in B cells.
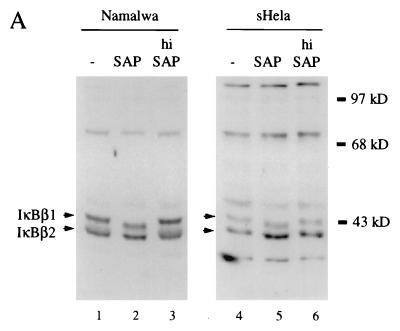
Since IκBβ1 and IκBβ2 differ in their PEST sequence, which is a target of constitutive phosphorylation of IκBα and of IκBβ (4, 11), we analyzed the effect of alkaline phosphatase treatment on the electrophoretic mobility of both isoforms in SDS-PAGE (Fig. 5A). Both IκBβ1 and IκBβ2 revealed increased mobility after treatment with alkaline phosphatase but not after treatment with heat-inactivated enzyme. The same result was obtained with HeLa or Namalwa cell-derived proteins. Thus, both IκBβ isoforms are constitutively phosphorylated. This finding is also in agreement with the phosphatase-mediated release of NF-κB activity from both isoforms (Fig. 3C).
FIG. 5.
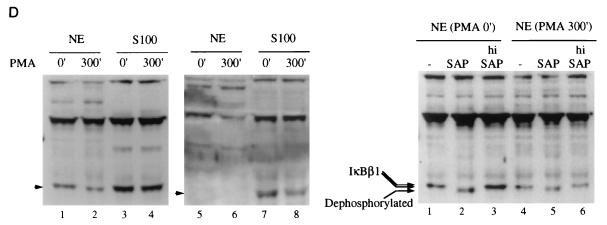
(A) Constitutive phosphorylation of IκBβ1 and IκBβ2. Cytosolic extracts of Namalwa (lanes 1 to 3) or HeLa (lanes 4 to 6) cells were either untreated (lanes 1 and 4), treated with shrimp alkaline phosphatase (SAP; lanes 2 and 5), or heat-inactivated phosphatase (hi SAP; lanes 3 and 6) and subjected to Western blot analysis with anti-IκBβ (N-20) antibody. (B) Nuclear localization of IκBβ1, but not of IκBβ2, in B cells. Whole-cell extracts (WCE), S100 extracts (S100), or nuclear extracts (NE) of Namalwa cells (top) or HeLa cells (bottom) were analyzed in Western blots with antibodies against IκBβ1 or IκBβ2 as indicated. (C) Nuclear IκBβ1 in B cells is bound to dimers containing p65. IP-shift analysis (as described in the legend to Fig. 3B) with nuclear extracts of Namalwa (lanes 1 to 4) or IM9 (lanes 5 and 6) B cells. An antibody specific for IκBβ1 (C-20) precipitated an NF-κB activity which was challenged with antibodies directed against p65 or p50 (lanes 2 and 3). An antibody directed against IκBβ2 [β(C)] did not precipitate NF-κB (lane 4). C-20, but not β(C), precipitated NF-κB activity from IM9 cells (lanes 5 and 6). (D) Nuclear IκBβ1 is constitutively phosphorylated. (Left) Namalwa cells, either untreated or stimulated with PMA for 5 h, were fractionated into S100 extracts and nuclear extracts (NE) and immunoblotted with anti-IκBβ1 (lanes 1 to 4) or anti-IκBβ2 antibodies (lanes 5 to 8), as indicated. (Right) Nuclear extracts of unstimulated (lanes 1 to 3) or PMA-stimulated (lanes 4 to 6) Namalwa cells were treated with shrimp alkaline phosphatase (SAP; lanes 2 and 5) or heat-inactivated SAP (hi SAP; lanes 3 and 6) or left untreated (lanes 1 and 4) and analyzed for IκBβ1 migration in a Western blot.
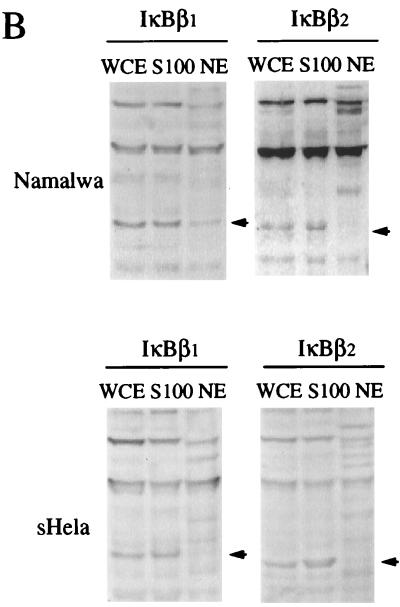
IκB molecules are normally confined to the cytoplasm, except for the transient poststimulation nuclear accumulation of IκBα (1, 46) or murine IκBβ (36). When the subcellular distribution of IκBβ1 and IκBβ2 in unstimulated HeLa cells was compared, both forms were detected exclusively in the cytoplasm (Fig. 5B, bottom panel). In unstimulated Namalwa B cells however, IκBβ1, but not IκBβ2 was found to some extent in the nucleus (top panel). Since IκBβ1 and IκBβ2 are almost equally abundant in Namalwa cells (Fig. 2C), the observed differential nuclear accumulation should be due to the difference in the PEST sequence of the two forms.
We analyzed NF-κB/Rel proteins bound to nuclear IκBβ1 by IP-shift analysis (Fig. 5C). From both Namalwa and IM9 B cells, the antibody specific for IκBβ1 (lanes 1 and 5) but not the antibody specific for IκBβ2 (lanes 4 and 6) precipitated an NF-κB DNA binding activity. This activity was completely inhibited by anti-p65 antibody and to a large extent also by anti-p50 antibody (compare lane 1 with lanes 2 and 3). Thus, nuclear IκBβ1 in B cells is associated predominantly with p65-containing hetero- and homodimers. In contrast to the two B-cell lines, nonlymphoid cell lines, devoid of constitutive NF-κB/Rel activity, contain exclusively cytoplasmic IκBβ1 and IκBβ2. As observed with HeLa cells, HL60 promyelocytes, SCH and MKN-1 stomach carcinoma cells, and T98G glioblastoma cells did not have nuclear IκBβ1 (data not shown).
Hypophosphorylated murine IκBβ1, generated after a long stimulation, migrates to the nucleus, presumably because it does not shield the nuclear localization signal of p65 (36). To investigate potential differences in the phosphorylation status of nuclear and cytosolic IκBβ isoforms, subcellular extracts of untreated or PMA-stimulated Namalwa cells were treated with alkaline phosphatase (Fig. 5D, left panel). Nuclear IκBβ1 revealed a faster migration after PMA stimulation, indicating hypophosphorylation (lanes 1 and 2), whereas cytoplasmic IκBβ1 was observed as a double band before and after stimulation (lanes 3 and 4). In contrast, IκBβ2 was completely retained in the cytoplasm (lanes 7 and 8) and could not be detected in the nucleus after PMA stimulation (lanes 5 and 6). Alkaline phosphatase treatment of nuclear IκBβ1 before stimulation caused a mobility shift in extracts of nonstimulated cells (Fig. 5D, right panel), whereas after PMA stimulation a weaker mobility shift was observed (lanes 1 to 6). Thus, nuclear IκBβ1 in Namalwa cells is constitutively phosphorylated before and after a long stimulation but the extent of phosphorylation decreases after PMA treatment. Nuclear localization of IκBβ1 in Namalwa and IM9 cells but not in HeLa cells could be functionally coupled to the mechanism of maintaining constitutive NF-κB activity in B cells.
Role of the amino-terminal and carboxy-terminal domains of IκBβ for NF-κB/Rel interaction, DNA binding inhibition, and basal phosphorylation.
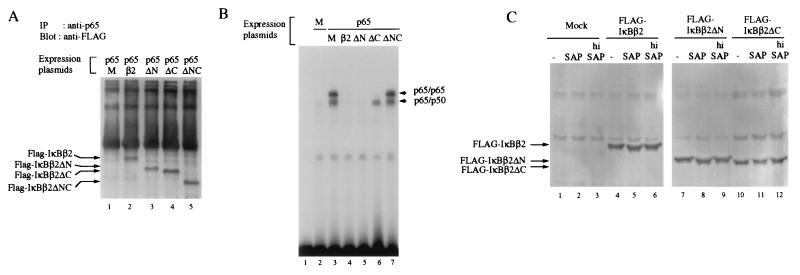
In both IκBα and NF-κB–p105, an acidic sequence, which is part of the PEST sequence, is required for a high-affinity interaction with NF-κB (17). IκBβ has two acidic regions, one after the third ankyrin repeat and one as part of the PEST sequence (20, 37). To address the role of the carboxy-terminal PEST sequence in the interaction with NF-κB, FLAG-tagged wild-type IκBβ or IκBβ mutants lacking either amino-terminal, carboxy-terminal, or both sequences (ΔN, ΔC, and ΔNC) were cotransfected with p65 into HeLa cells (Fig. 6A). Immunoprecipitation with an anti-p65 antibody and blotting with an anti-FLAG antibody revealed that none of the sequences before the first and after the sixth ankyrin repeat is required for binding to NF-κB (lanes 1 to 5). This is in contrast to IκBα, which requires carboxy-terminal sequences for high-affinity interaction (19). Despite equal affinities under immunoprecipitation conditions, marked differences were observed in the ability of the IκBβ mutants to inhibit the DNA binding of NF-κB (Fig. 6B). Whereas wild-type IκBβ and IκBβΔN effectively inhibited DNA binding of p65 homodimers and heterodimers of transfected p65 and endogenous p50 (compare lane 3 with lanes 4 and 5), IκBβΔC inhibited only p65 dimers but not heteromeric NF-κB (lane 6). Surprisingly, IκBβΔNC was even unable to inhibit either homodimeric or heterodimeric NF-κB (lane 7). Since IκBβΔC and IκBβΔNC efficiently bound to NF-κB (Fig. 6A), a folding problem in these mutants is unlikely. None of the IκBβ mutants was able to inhibit the DNA binding of transfected p50 homodimers (data not shown). IκBβ or IκBβΔN, but not IκBβΔC, was able to inhibit the DNA binding of transfected p50–c-Rel (data not shown). Thus, efficient DNA binding inhibition with less favored NF-κB heteromers requires the presence of the carboxy-terminal sequences of IκBβ.
FIG. 6.
(A) Interaction of IκBβ deletion mutants with p65 in transfected HeLa cells as shown by immunoprecipitation. p65 was transfected alone (lane 1) or together with wild-type IκBβ2 or IκBβ2 mutants lacking either the sequences before the first ankyrin repeat (ΔN) or after the sixth repeat (ΔC) or both (ΔNC) (lanes 2 to 5). All constructs were equally well expressed (data not shown). After immunoprecipitation with an anti-p65 antibody, coprecipitated IκBβ molecules were detected by immunoblotting. (B) DNA binding inhibition assay. Whole-cell extracts of mock-transfected (M) HeLa cells (lane 2), cells transfected with p65 alone (lane 3), or cells cotransfected with p65 and either wild-type IκBβ2, IκBβ2ΔN, IκBβ2ΔC, or IκBβ2ΔNC (lanes 4 to 7) were subjected to EMSA. Lane 1 contains DNA probe assayed alone. The position of DNA complexes containing p65 homodimers and p50-p65 is indicated. (C) IκBβ2 is constitutively phosphorylated in the carboxy-terminal domain. S100 extracts of HeLa cells that were either mock transfected (lanes 1 to 3), transfected with FLAG-tagged IκBβ2 (lanes 4 to 6), IκBβ2ΔN (lanes 7 to 9), or IκBβ2ΔC (lanes 10 to 12) were left untreated (lanes 1, 4, 7, and 10) or incubated with shrimp alkaline phosphatase (SAP; lanes 2, 5, 8, and 11) or heat-inactivated enzyme (hi SAP; lanes 3, 6, 9, and 12) and blotted with anti-FLAG antibody.
The ability of IκBβ to interact with NF-κB depends on its constitutive phosphorylation (22), and recombinant underphosphorylated IκBβ1 is impaired in its ability to inhibit DNA binding of p65 or c-Rel (11, 36, 39). When adding bacterially expressed IκBβ2 to transfected NF-κB, we observed that only p65 homodimers, but not p65-p50 or c-Rel–p50, were partially inhibited from binding to DNA (data not shown). Unphosphorylated bacterial IκBβ2 is thus similar to transfected IκBβΔC (Fig. 5B). In addition, IκBβ1 was shown to be basally phosphorylated in the carboxy-terminal PEST sequence at residues Ser 313 and Ser 315 (11), both of which are conserved in IκBβ2 (there is one more CKII site in the PEST sequence of IκBβ2 at Ser 325). Both transfected FLAG-tagged IκBβ and IκBβ2ΔN revealed an increased mobility in SDS-PAGE after phosphatase treatment but not after incubation with inactivated phosphatase (Fig. 6C, compare lanes 4 to 6 with lanes 7 to 9). In contrast, IκBβ2ΔC did not show a mobility shift (lanes 10 to 12). We conclude, therefore, that IκBβ2 (like IκBβ1) is constitutively phosphorylated at its carboxy-terminal PEST sequence.
DISCUSSION
One possibility to account for specific physiological activities of NF-κB/Rel transcription factors is differential regulation of IκB proteins. In this study, we show that transcripts generated from the human IκBβ gene are alternatively processed, yielding products which are distinct in their signal responsiveness. Two isoforms, IκBβ1 and IκBβ2, are found in human cells, whereas the murine cell lines tested express only IκBβ1. IκBβ2 differs from IκBβ1 by a substitution that replaces the carboxy-terminal PEST sequences with intron-derived sequences. We have analyzed the functional consequence of this difference in protein structure for binding specificity and affinity to NF-κB Rel proteins, for signal-responsive degradation, basal phosphorylation, nuclear localization, and inhibition of NF-κB-dependent transcription.
All human cell lines tested expressed IκBβ1 and IκBβ2, the latter often as the predominant form, whereas murine cells expressed only IκBβ1. We cannot fully exclude the possibility that IκBβ2 is expressed in murine cells at a low level or is confined to specific cell types. We detected IκBβ1 but not IκBβ2 in murine liver, lung, lymph node, thymus, ovary, and brain by Western blotting (data not shown). It is possible that the IκBβ2 carboxy-terminal sequence is posttranslationally modified in murine cells and thus not recognized by the antibody. Alternatively, splicing variants in murine cells may contain less highly conserved intron-derived sequences not recognized by the antibody directed against the human protein. Immunodepletion and precipitation studies indicate that murine cells may contain additional IκBβ isoforms distinct from IκBβ1 and IκBβ2 (data not shown). As a further possibility, specific functions of IκBβ splicing isoforms in human cells could be compensated for in murine cells by other modifications of IκB proteins yet to be identified.
We have previously shown that IκBβ mRNA is expressed at some level as two distinct signals (1.8 and 2.8 kb) in several human tissues analyzed, such as pancreas, kidney, skeletal muscle, liver, lung, placenta, brain, and heart tissues (20). Based on the lengths of the cloned cDNAs, IκBβ2 should represent the longer of the two transcripts. The N-terminal anti-IκBβ antibody recognized further signals in the transformed cell lines (Fig. 2C), suggesting the existence of still further isoforms. Several additional, independently isolated cDNA clones lacking an internal coding segment are being investigated further.
Our results suggest that IκBβ2 binds to the same cellular NF-κB/Rel hetero- and homodimers as IκBβ1, which are predominantly p65 homodimers and heterodimers containing p65. In contrast, IκBα interacted predominantly with heterodimers containing p50, such as p50-p65. Heterodimers that were bound with lower affinity, however, such as c-Rel containing dimers, are possibly bound more efficiently by IκBβ1 than by IκBβ2. p65 homodimers were observed by EMSA after cellular stimulation and degradation of IκBβ1 and/or IκBβ2 and are probably liberated from complexes with IκBβ (Fig. 4). Preferential association with p65 homodimers was also evident when the affinity of IκBβ to NF-κB was weakened by truncation of the carboxy terminus. In DNA binding inhibition assays (Fig. 6B), only p65 homodimers, not p65 heterodimers, were affected. Therefore, we assume that the carboxy-terminal sequences are needed to increase the binding affinity of IκBβ to NF-κB whereas the specific recognition of NF-κB is achieved by the ankyrin repeat domain. As observed for IκBα (17), the binding affinity of IκBβ may depend on negatively charged residues contained in the PEST sequence. Basal phosphorylation by CKII in the PEST sequence increases the negative charge and could therefore enhance the interaction strength of IκBβ with less favored heterodimers containing c-Rel (11). Band shift assays are more stringent than immunoprecipitations under mild detergent conditions, possibly due to a competition between NF-κB–DNA and NF-κB–IκB complex formation. This can provide the explanation why some interactions seen in one assay (precipitation) may not be detected in the other (band shift). Different stringencies in the experimental conditions may also explain conflicting results obtained by Chu et al. (11) and Suyang et al. (36). The former study reported that unphosphorylated IκBβ can inhibit the DNA binding of p65 homodimers, whereas the latter reported that p65 forms a complex with IκBβ that still binds to DNA. Recombinant, underphosphorylated IκBβ cannot bind to c-Rel and inhibit its DNA binding (11). In this study, we showed that a carboxy-terminal deletion mutant of IκBβ can bind to p65 homodimers and inhibit DNA binding but cannot inhibit the DNA binding of heterodimers. We propose that the affinity of IκBβ to NF-κB/Rel dimers decreases from p65 homodimers to p65 heterodimers or c-Rel heterodimers and that stable binding to the latter requires the negatively charged carboxy-terminal sequences of IκBβ, which are most optimal after phosphorylation.
Previously, it has been reported that IκBα and IκBβ elicit distinct responses to different inducers of NF-κB and that IκBβ is degraded only in response to LPS or IL-1 but not in response to TNF-α or PMA (37). In IκBα-deficient mice, constitutive NF-κB activity was observed only in cells of hematopoietic origin, not in fibroblasts (6). Furthermore, NF-κB could still be stimulated by TNF-α in fibroblasts, presumably involving degradation of IκBβ (6). We show that TNF-α, PMA/Ca2+ ionophore, and IL-1 can all equally induce the degradation of IκBα and IκBβ1 in three different human cell lines. The extent of responsiveness varied among cell lines and stimuli, but the fact that time points of the onset of proteolysis are the same for IκBα and IκBβ1 suggests that both are degraded by the same mechanism. It is therefore possible that both are phosphorylated by the same kinase(s) and degraded by a ubiquitin-dependent proteasome pathway (12, 25). In contrast, IκBβ2 was barely degraded. This refractory response is consistent with the truncated PEST sequence in IκBβ2, since this motif promotes efficient inducible degradation of IκBα. Thus, IκBβ2 can be seen as a naturally occurring dominant negative IκBβ isoform whose expression could generate a state of NF-κB unresponsiveness to a variety of inducing agents.
An interesting and unexpected observation is the constitutive nuclear accumulation of IκBβ1 in Namalwa and IM9 B cells which was not found in five different nonlymphoid cell lines tested. In these experiments, equal cellular ratios of cytoplasmic and nuclear fractions were used and the same fractions were analyzed for IκBβ1 and IκBβ2. Since IκBβ2 was not found in the nucleus, leakage or cross-contamination of the fractions can be excluded. Nuclear IκBβ was associated predominantly with p65, whereas the main constitutive NF-κB activity in B cells consists of c-Rel–p50 (23, 26, 27). It is therefore possible that nuclear IκBβ1 in B cells masks p65 which might have been activated constitutively by the same mechanism as c-Rel–p50. Nuclear IκBβ has also been detected recently in mature murine WEHI 231 B cells (32). It is therefore likely that B lymphocytes in general contain nuclear IκBβ. Consistent with the model proposed by Suyang et al. and Phillips and Ghosh (32, 36), the nuclear accumulation of IκBβ could not only account for the prolonged NF-κB activation after stimulation of non-B cells with agents such as LPS but could also directly contribute to the constitutive Rel activity in B cells.
The fact that IκBβ2 is localized exclusively to the cytoplasm indicates that the specific carboxy-terminal sequences of IκBβ1 are required for nuclear accumulation. Since both IκBβ1 and IκBβ2, are constitutively phosphorylated and contain the CKII phosphorylation sites at Ser 313 and Ser 315 (11), it is unlikely that hypophosphorylation of IκBβ1 but not of IκBβ2 accounts for their differential nuclear uptake. The latter mechanism has been proposed for nuclear translocation of IκBβ1 after LPS stimulation of 70Z/3 pre-B cells (36). Therefore, mechanisms other than basal phosphorylation may account for nuclear uptake of IκBβ1 in human cells. These may include the association and import with (modified) Rel factors or the presence of a conditional nuclear import signal in IκBβ1.
REFERENCES
- 1.Arenzana Seisdedos F, Thompson J, Rodriguez M S, Bachelerie F, Thomas D, Hay R T. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol Cell Biol. 1995;15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 3.Baldi L, Brown K, Franzoso G, Siebenlist U. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of IκBα. J Biol Chem. 1996;271:376–379. doi: 10.1074/jbc.271.1.376. [DOI] [PubMed] [Google Scholar]
- 4.Barroga C F, Stevenson J K, Schwarz E M, Verma I M. Constitutive phosphorylation of IκBα by casein kinase II. Proc Natl Acad Sci USA. 1995;92:7637–7641. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg A A, Baldwin A S., Jr The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Sha W C, Bronson R T, Baltimore D. Constitutive NF-κB activation, enhanced granulopoiesis, and neonatal lethality in IκBα-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 7.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 11.Chu Z L, McKinsey T A, Liu L, Qi X, Ballard D W. Basal phosphorylation of the PEST domain in IκBβ regulates its functional interaction with the c-rel proto-oncogene product. Mol Cell Biol. 1996;16:5974–5984. doi: 10.1128/mcb.16.11.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiDonato J, Mercurio F, Rosette C, Wu Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 14.Fan C M, Maniatis T. Generation of p50 subunit of NF-κB by processing of p105 through an ATP-dependent pathway. Nature. 1991;354:395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- 15.Good L, Sun S C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 tax involves degradation of IκBβ. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harhaj E W, Sun S C. The serine/threonine phosphatase inhibitor calyculin A induces rapid degradation of IκBβ. Requirements of both the N- and C-terminal sequences. J Biol Chem. 1997;272:5409–5412. doi: 10.1074/jbc.272.9.5409. [DOI] [PubMed] [Google Scholar]
- 17.Hatada E N, Naumann M, Scheidereit C. Common structural constituents confer IκB activity to NF-κB p105 and IκB/MAD-3. EMBO J. 1993;12:2781–2788. doi: 10.1002/j.1460-2075.1993.tb05939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohmann H P, Remy R, Scheidereit C, van Loon A P. Maintenance of NF-κB activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol. 1991;11:259–266. doi: 10.1128/mcb.11.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krappmann D, Wulczyn F G, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-κB inhibitor IκBα in vivo. EMBO J. 1996;15:6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J W, Choi H S, Gyuris J, Brent R, Moore D D. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol Endocrinol. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 21.Lin L, Ghosh S. A glycine-rich region in NF-κB p105 functions as a processing signal for the generation of the p50 subunit. Mol Cell Biol. 1996;16:2248–2254. doi: 10.1128/mcb.16.5.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link E, Kerr L D, Schreck R, Zabel U, Verma I, Baeuerle P A. Purified IκBβ is inactivated upon dephosphorylation. J Biol Chem. 1992;267:239–246. [PubMed] [Google Scholar]
- 23.Liou H C, Sha W C, Scott M L, Baltimore D. Sequential induction of NF-κB/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maniatis T. Catalysis by a multiprotein IκB kinase complex. Science. 1997;278:818–819. doi: 10.1126/science.278.5339.818. [DOI] [PubMed] [Google Scholar]
- 25.McKinsey T A, Brockman J A, Scherer D C, Al Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by the tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto S, Schmidt M J, Verma I M. Qualitative changes in the subunit composition of κB-binding complexes during murine B cell differentiation. Proc Natl Acad Sci USA. 1994;91:5056–5060. doi: 10.1073/pnas.91.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naumann M, Scheidereit C. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naumann M, Wulczyn F G, Scheidereit C. The NF-κB precursor p105 and the proto-oncogene product Bcl-3 are IκB molecules and control nuclear translocation of NF-κB. EMBO J. 1993;12:213–222. doi: 10.1002/j.1460-2075.1993.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orian A, Whiteside S, Israel A, Stancovski I, Schwartz A L, Ciechanover A. Ubiquitin-mediated processing of NF-κB transcriptional activator precursor p105. Reconstitution of a cell-free system and identification of the ubiquitin-carrier protein, E2, and a novel ubiquitin-protein ligase, E3, involved in conjugation. J Biol Chem. 1995;270:21707–21714. doi: 10.1074/jbc.270.37.21707. [DOI] [PubMed] [Google Scholar]
- 30.Pahl H L, Baeuerle P A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-κB. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 32.Phillips R J, Ghosh S. Regulation of IκBβ in WEHI 231 mature B cells. Mol Cell Biol. 1997;17:4390–4396. doi: 10.1128/mcb.17.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Régnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 34.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of IκBα requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stancovski I, Baltimore D. NF-κB activation: the IκB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 36.Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson J E, Phillips R J, Erdjument Bromage H, Tempst P, Ghosh S. IκBβ regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 38.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran K, Merika M, Thanos D. Distinct functional properties of IκBα and IκBβ. Mol Cell Biol. 1997;17:5386–5399. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 41.Weil R, Laurent-Winter C, Israel A. Regulation of IκBβ degradation. Similarities to and differences from IκBα. J Biol Chem. 1997;272:9942–9949. doi: 10.1074/jbc.272.15.9942. [DOI] [PubMed] [Google Scholar]
- 42.Whiteside S T, Epinat J C, Rice N R, Israel A. IκBɛ, a novel member of the IκB family, controls RelA and c-Rel NF-κB activity. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteside S T, Ernst M K, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Mol Cell Biol. 1995;15:5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wulczyn F G, Krappmann D, Scheidereit C. The NF-κB/Rel and IκB gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–769. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- 45.Zabel U, Baeuerle P A. Purified human IκB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell. 1990;61:255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- 46.Zabel U, Henkel T, Silva M S, Baeuerle P A. Nuclear uptake control of NF-κB by MAD-3, an IκB protein present in the nucleus. EMBO J. 1993;12:201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]